Abstract
Aims
Inflammation of the heart is a complex biological and pathophysiological response of the immune system to a variety of injuries leading to tissue damage and heart failure. MicroRNAs (miRNAs) emerge as pivotal players in the development of numerous diseases, suggesting their potential utility as biomarkers for inflammation and as viable candidates for therapeutic interventions. The primary aim of this investigation was to pinpoint and assess particular miRNAs in individuals afflicted by virus‐negative inflammatory dilated cardiomyopathy (DCMi).
Methods and results
The study involved the analysis of 152 serum samples sourced from patients diagnosed with unexplained heart failure through endomyocardial biopsy. Among these samples, 38 belonged to DCMi patients, 24 to DCM patients, 44 to patients displaying inflammation alongside diverse viral infections, and 46 to patients solely affected by viral infections without concurrent inflammation. Additionally, serum samples from 10 healthy donors were included. The expression levels of 754 distinct miRNAs were evaluated using TaqMan OpenArray. MiR‐1, miR‐23, miR‐142‐5p, miR‐155, miR‐193, and miR‐195 exhibited exclusive down‐regulation solely in DCMi patients (P < 0.005). These miRNAs enabled effective differentiation between individuals with inflammation unlinked to viruses (DCMi) and all other participant groups (P < 0.005), boasting a specificity surpassing 86%.
Conclusions
The identification of specific miRNAs offers a novel diagnostic perspective for recognizing intramyocardial inflammation within virus‐negative DCMi patients. Furthermore, these miRNAs hold promise as potential candidates for tailored therapeutic strategies in the context of virus‐negative DCMi.
Keywords: MiRNA, Biomarker, Inflammation, Cardiomyopathy
Introduction
The three most common causes of myocarditis and inflammatory cardiomyopathy are viral infections, autoimmune responses, and toxicity. 1 , 2 , 3 , 4 Inflammation is a complex biological, pathophysiological, and protective response involving immune cells, blood vessels, and molecular mediators to a variety of harmful stimuli and can cause infections or injuries leading to damage or disease. 5 Depending on the stimuli involved, the inflammatory reaction can be acute or chronic, local, or systemic. 6 However, it always requires the same components, such as activation of different plasma cascade systems, 7 vascular dilatation induced by cellular mediators, leucocyte activation, migration, and extravasation, 8 but to varying degrees. 5 Chronic inflammation of the cardiac muscle often leads to progressive heart failure. 9 , 10
MicroRNAs (miRNAs) are non‐coding, small (18–25 kb), relatively stable RNAs that have emerged as key regulators of inflammation 11 , 12 and control gene expression at the post‐transcriptional level. 13
Depending on the target, 14 miRNAs can either promote or suppress inflammation. 15 , 16 The regulation of inflammation is caused primarily through altered expression of specific miRNAs. 14 There is also evidence that the biogenesis of miRNAs is regulated as part of the inflammatory response, by altering the transcription level, processing or stabilization of mature or precursor miRNA transcripts. 17 , 18 The initiation, spread, and resolution steps of inflammation are subject to both positive and negative regulatory events via miRNAs. 19 The positive feedback initiates a cascade of molecular events that serve to combat invasion of microbial pathogens and successful repair of tissue damage. 15
Understanding the interaction between miRNA function and inflammatory response is important, as this interaction may contribute to a better understanding of inflammatory disease development and subsequently to better treatment options. Recent research studies have implicated miRNAs in the inflammatory networks in various tissue types. Several data indicate already that miRNAs can serve as diagnostic serological markers in inflammatory heart disease. 20 Thus, in this retrospective study, which was designed to ascertain differential miRNA patterns in human endomyocardial biopsy (EMB) samples, miRNA expression levels of biopsy‐proven patients with inflammatory cardiomyopathy (with or without viral infection) were compared with healthy controls.
In addition, miRNAs may be potential targets for novel therapeutic strategies achieved by targeting endogenous miRNA levels through, for example, the administration of miRNA inhibitors. 15 , 21 , 22 Despite the fact that miRNA sequences can be easily synthesized, 23 miRNA‐based therapies are already used in clinical trials among others 24 and also for chronic heart failure. 25 , 26 There is still much to learn about how to transform these into effective, patient‐compliant, and targeted drug delivery therapies. Until now, it is risky to invest in miRNA therapeutics due to biological challenges, the cost of production, scale‐up, and the anticipated clinical approval challenges. 27 The main barrier to miRNA‐based therapy is development of pharmaceutical strategies of low toxicity for targeted delivery to specific sites. 24 While extracellular circulating miRNAs have shown a high level of stability in human blood and other body fluids, an ideal delivery method should protect the miRNAs from the circulatory nucleases and deliver mRNAs intact to the target site. 15
Therapeutic modulation of miRNAs may have several advantages over alternative strategies as immunosuppression, notably the more personalized treatment, better monitoring, and less or even no side effects. Even one miRNA can target multiple genes, which may form a network amplification effect and be more beneficial than targeting multiple different genes individually. 28 Alongside the critical role of miRNAs in the regulation of inflammation and their potential to be targeted by new therapeutics, caution must be taken in case of excessive inhibition or overexpression of miRNAs. 15
According to statistics, more than 60 miRNA‐based therapeutic drugs are in different stages of development, some of which have completed clinical trials and have been approved. 29 Although miRNA‐based treatment methods have shown promising results in vivo, they are still in preliminary stages for their applications to wound healing. 30 It is a considerable challenge to modulate a process controlled by a complex and large network of interacting factors through single miRNAs. However, to exceed this limitation, a detailed understanding of the processes and functions of miRNAs in cardiac inflammation is required.
Thus, in this retrospective study, aimed to identify different miRNA patterns in human EMB samples, miRNA expression levels of patients with biopsy‐proven inflammatory cardiomyopathy, with or without viral infections, were compared with those of healthy controls. Subsequently, clinically relevant novel diagnostic markers in terms of miRNA expression for cardiac inflammation should be identified to select patients who need intervention.
Material and methods
Study population
In this retrospective study, miRNA expression levels were evaluated in serum samples from 152 patients with clinically unexplained heart failure diagnosed by EMB. The diagnoses of EMBs were based on different disease entities and sent from different clinics in Germany to the College of American Pathologists‐accredited Institute for Cardiac Diagnostics and Therapy (IKDT), Berlin, Germany, for further routine diagnosis (Table 1 ). Ten serum samples (500 μL) from 10 healthy donors were used as control group.
Table 1.
Clinical data, ejection fraction, and histological findings of all patients
| Characteristic | Inflammation and no virus—DCMi | No inflammation and no virus—DCM | Inflammation and virus | Virus and no inflammation | Healthy controls |
|---|---|---|---|---|---|
| Number | 38 | 24 | 44 | 46 | 10 |
| Age, mean (years) | 47 ± 25 | 52 ± 13 | 53 ± 25 | 48 ± 13 | 44.7 ± 12.5 |
| Male sex (%) | 28 (73.7) | 15 (62.5) | 32 (72.7) | 27 (61.4) | 7 (70) |
| LVEF (%), mean ± SD | 42 ± 19 | 35 ± 9 | 48 ± 19 | 45 ± 12 | — |
| EF < 55% (%) | 73 | 100 | 60 | 63 | 0 |
| LVEDD (mm), mean ± SD | 52.7 ± 21.3 | 63.3 ± 14.2 | 55.2 ± 21.0 | 48.2 ± 24.0 | — |
| Fibrosis (%) |
No fibrosis a —82% Low fibrosis b —18% |
No fibrosis—85% Low fibrosis—15% |
No fibrosis—80% Low fibrosis—20% |
No fibrosis—88% Low fibrosis—12% |
— |
DCM, dilated cardiomyopathy; DCMi, inflammatory dilated cardiomyopathy; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; SD, standard deviation.
Up to 3% of connective tissue.
5–15% of connective tissue.
Patients with coronary artery disease, other possible causes of myocardial dysfunction (e.g. valvular heart disease, hypertension, restrictive, or constrictive heart disease) diagnosed by angiography and echocardiography, and concomitant chronic inflammatory disease (e.g. rheumatological disorders) were excluded from this study. Left ventricular ejection fraction (LVEF) was determined by echocardiography.
Assessment of inflammation (inflammatory dilated cardiomyopathy) and dilated cardiomyopathy in endomyocardial biopsy samples
Using analyses on EMBs, inflammation was diagnosed immunohistochemically by detection of inflammatory infiltrates of >14.0 lymphocytes/mm2, including >7.0 CD3+ lymphocytes/mm2 according to the European Society of Cardiology guidelines. 3 Antibodies used, the immunohistochemical staining procedure, and the evaluation of EMBs were as described previously. 31 , 32
The diagnosis of dilated cardiomyopathy (DCM) was based on morphological and functional characterization with significantly impaired LVEF and/or dilated left ventricle. EMBs from DCM patients were negative for immunohistochemically detected inflammatory responses and for detection of cardiotropic viral genomes.
Detection of viral genomes in endomyocardial biopsy samples
Viral DNA and RNA were extracted from frozen heart muscle tissue probes as described previously. 33 , 34 Reverse transcriptase (RT)‐PCR was performed for the detection of enteroviruses (including coxsackieviruses), adenovirus, parvovirus B19 (B19V), and human herpesvirus type 6 (HHV6) as described previously. 34 , 35 Additionally, DNA was extracted from peripheral blood cells to exclude a systemic infection with B19V and HHV6. As a control for successful extraction of DNA and RNA from heart muscle tissue, oligonucleotide sequences were chosen from the DNA sequence of the GAPDH gene.
MicroRNAs isolation
MiRNAs from all patients were isolated using mirVANA™ PARIS™ RNA and Native Protein Purification Kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA; https://www.thermofisher.com/order/catalog/product/de/de/AM1561) according to the manufacturer's instructions.
MicroRNA reverse transcription, pre‐amplification, and expression analysis
Total RNA including miRNA fraction was initially reversely transcribed to cDNA using Megaplex stem‐loop RT primer (Thermo Fisher Scientific, Inc.) for Human Pools A and B in combination with the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Inc.) for low content samples. The entire procedure for quantification of miRNAs has been described elsewhere. 36 U6 was used as reference miRNA for data normalization. The expression of 754 unique circulating miRNAs using Human MicroRNA Panels A and B (https://www.thermofisher.com/order/catalog/product/4470187#/4470187) was analysed in each sample. All experiments were performed in minimum in duplicates.
Ethical approval
The study was performed within the CRC Transregio 19 (NCT02970227, 1 January 2004) and was approved by the local ethics committees of the participating clinical centres and by the committees of the respective federal states. The study complies with the Declaration of Helsinki. An informed written consent was obtained from each study patient.
Statistical analysis
All expression data were analysed and represented with GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). The Student's t‐test and one‐way ANOVA were used to compare miRNA expression levels between all study groups (Figure 1 ).
Figure 1.
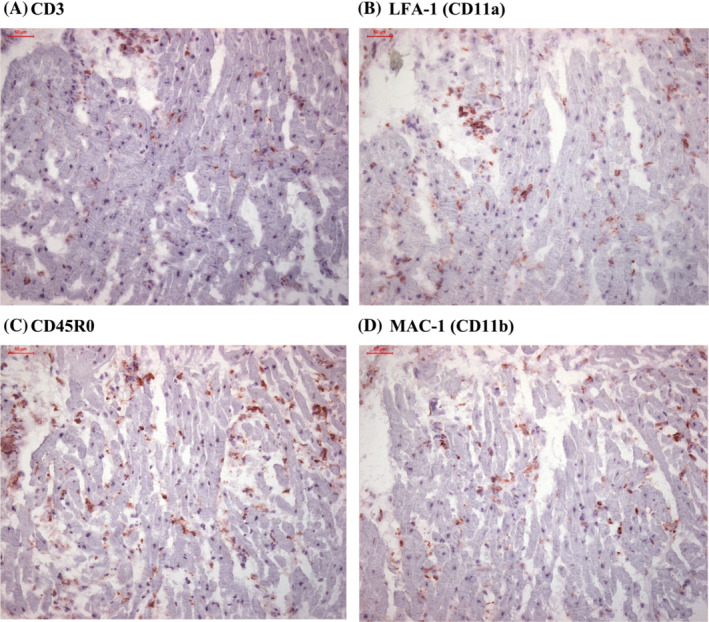
(A–D) Immunohistochemical staining for detection of endomyocardial inflammation.
The receiver operating characteristic (ROC) curves were plotted for every single miRNA, and the areas under the curve (AUCs) were calculated to prove their value and diagnostic accuracy (Figure 2 ).
Figure 2.
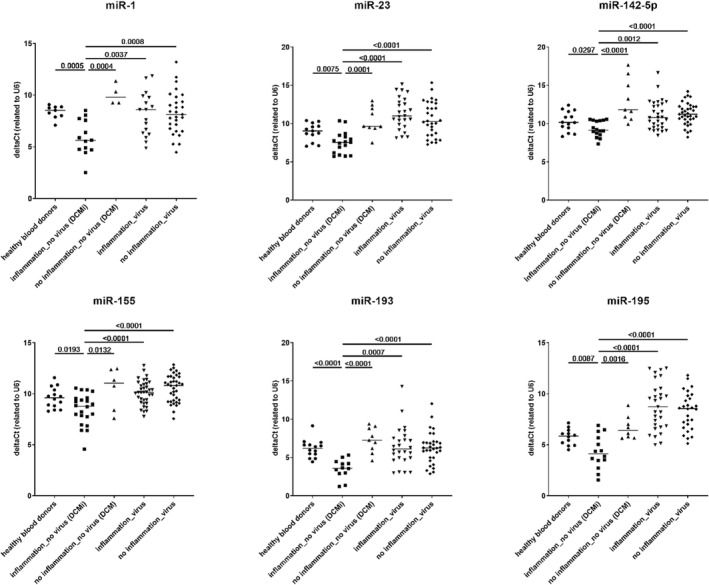
Confirmation of differentially expressed microRNAs (miRNAs) in patients with inflammatory dilated cardiomyopathy (DCMi). P‐values are shown in comparison of DCMi patients with other groups.
All data were presented as single values with mean and standard deviation, with a significance level of *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.0001.
Results
Patient characteristics
In this retrospective study, serum samples from 152 patients with clinically unexplained heart failure were analysed. EMB diagnosis showed that 38 of the 152 patients suffered from inflammation without virus infections [inflammatory dilated cardiomyopathy (DCMi); Figure 1 ], 24 of the 152 patients showed no inflammation without virus infections (DCM), 44 of the 152 patients had inflammation and various viral infections, and 46 of the 152 patients were diagnosed with viral infections without inflammation (Table 1 ).
All patients with myocardial inflammation showed a diverse pattern of inflammatory markers, ranging from mild to severe degree of inflammation as elevated infiltration of CD3+ T cells (Figure 1 A ), and increased levels of LFA‐1+ lymphocytes (Figure 1 B ), CD45R0+ T‐memory cells (Figure 1 C ), and MAC‐1+ macrophages (Figure 1 D ).
MicroRNA analyses
Based on the expression levels of selected 754 miRNAs using TaqMan® OpenArray®, six miRNAs were significantly down‐regulated in the DCMi patients group compared with all other samples (P < 0.01) (Figure 2 ). These miRNAs were miR‐1, miR‐23, miR‐142‐5p, miR‐1, miR‐155, miR‐193, and miR‐195. Considering their significantly different expression levels in EMBs, these six miRNAs (here referred to internal control U6) may have potential to be diagnostic biomarkers for myocardial inflammation.
Diagnostic value of microRNAs for dilated cardiomyopathy and cardiac diseases
To discriminate patients with inflammatory cardiomyopathy, ROC curves were plotted for every single miRNA in the U6 miRNA group (Figure 3 ). The AUCs ranged from 0.80 to 0.91, demonstrating that these miRNAs are of particular interest for the detection of myocardial inflammation.
Figure 3.
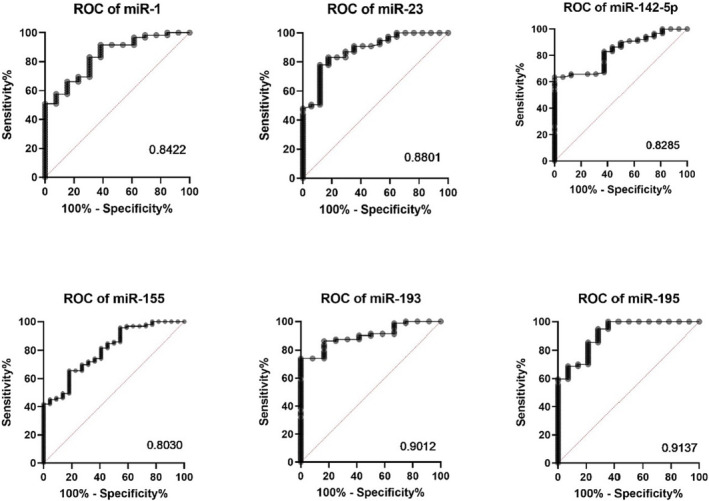
Diagnostic value of microRNAs (miRNAs) for inflammatory dilated cardiomyopathy presented in receiver operating characteristic (ROC) curves with area under the curve (AUC) compared with all other patients. The ROC curve illustrates the diagnostic ability of a binary classifier system when its discrimination threshold is varied. It was constructed by plotting the true positive rate (sensitivity) against the false positive rate (specificity). AUC is equal to the probability that a classifier will rank a randomly selected positive instance higher than a randomly selected negative one.
Discussion
Chronic inflammation of the myocardium leads to severe heart failure with progressive cardiomyocyte damage ending up in DCMi. The currently available treatment strategies for chronic heart failure often target the symptoms rather than the cause of the disease. 10 , 37
EMB is the gold standard for the correct diagnosis, as the basis for a causal, specific, and personalized therapy. 3 The effectiveness of immunosuppressive therapy in patients with EMB‐proven DCMi could be demonstrated in several studies. 38 , 39 , 40 In a randomized study, it could be shown that those patients with a myocardial viral infection were not susceptible to immunosuppressive treatment. 41 For this reason, differentiation between virus‐positive and virus‐negative patients with inflammatory cardiomyopathy is essential for the therapy decision.
MiRNAs are pivotal regulators of heart function, including myocardial injury and inflammation, opening up new approaches for a specific treatment in the future. 21 , 22 As the onset and termination of inflammation are crucial for preventing tissue damage, a number of mechanisms have evolved in nature to regulate this process. In this regard, miRNAs have emerged as important gene regulators to control inflammation. The present study is the first to identify six inflammation‐associated miRNAs (miR‐1, miR‐23, miR‐142‐5p, miR‐155, miR‐193, and miR‐195, here referred to as U6 as internal control) that are differently regulated in heart muscle biopsies of virus‐negative patients. Each of these miRNAs has previously been shown to be involved in different diseases 42 ; however, the accuracy of diagnostics can be significantly improved by combining several different miRNAs.
MicroRNA‐1 (miR‐1) is a conserved miRNA with high expression in muscle tissues (muscle‐specific miRNA), particularly in the heart muscle, which is involved in the development of this tissue. 43 MiR‐1 has been extensively investigated and confirmed as a key regulator of cardiac development and diseases. 16 , 44 The results of several studies further suggest that the patterns of miR‐1 expression are model and/or disease dependent, because miR‐1 plays a critical role in the physiological processes in the smooth and skeletal muscles, as well as in other tissues, and thus is involved in the pathogenesis of a variety of disorders. 43
Recent studies demonstrated that the expression of miR‐23 was up‐regulated in peripheral blood of patients with coronary heart diseases compared with control subjects. Overexpression of miR‐23 promoted vascular smooth muscle cells proliferation and inhibited apoptosis. 45 In addition, miR‐23 is up‐regulated in and promotes cardiac hypertrophy. Overexpression of miR‐23 significantly promoted cell growth and suppressed cell apoptosis. 46
Recent reports have shown that miR‐142‐5p is involved in the pathogenesis of myocarditis, inflammatory cardiomyopathy, and autoimmune neuroinflammation by affecting T‐cell differentiation. 16 , 47 Further studies have also demonstrated that miR‐142‐5p plays a significant role in various cancer diseases. 48 However, the role of miR‐142‐5p in the inflammatory process of the heart muscle was previously unknown.
It has been previously shown that miR‐155 has anti‐inflammatory and pro‐inflammatory functions. 49 Moreover, miR‐155 is up‐regulated in the endothelium during systemic inflammation. 50 Up‐regulation of this miRNA leads to attenuation of inflammatory pathways and adjustment to lower inflammatory intensity. 49 , 50 Overexpression of miR‐155 reduces chronic inflammation and provides, for example, protection against atherosclerosis‐associated foam cell formation. 51
When deregulated, miR‐155 contributes to the development of chronic inflammation, autoimmunity, cancer, and fibrosis. 42 , 52 Moreover, miR‐155 has been found to be involved in viral infections, particularly those caused by DNA viruses. 53 According to the analyses of Lewandowski et al., miR‐155 is among the leading candidates for a myocardial inflammation panel. 16 MiR‐155 has also been shown to be involved in the impaired development and function of B cells, T cells, and dendritic cells. 54 It represents an important therapeutic target because it is overexpressed in many tumours. 49
Among these remarkably small molecules, quite a few studies have recently revealed the important role of the miR‐193 family, consisting of miR‐193a‐3p, miR‐193a‐5p, miR‐193b‐3p, and miR‐193b‐5p, in biological processes in health and disease through interaction with specific targets and signalling pathways, which mainly contribute to as tumour suppressor. 55 MiR‐193 has been primarily associated with cancer occurrence. 56 Human miR‐193 is expressed in the heart and has been established as a biomarker to distinguish between patients with different types of heart failure, hypertrophic cardiomyopathy, ischaemia reperfusion injury, right ventricular remodelling, cardiac fibrosis, and congenital heart disease. Significant changes in heart rate, heart size, and interval time have been found with miR‐193 loss of function, which is consistent with an early aging phenotype. 57
It has been reported that miR‐195 has a significant impact on the oncogenicity of various neoplasms by binding to critical genes and signalling pathways to promote or inhibit the progression of cancers. 58 MiR‐195 inhibits the pro‐inflammatory profile of macrophages and affects the crosstalk with smooth muscle cells. 59
In conclusion, miRNAs could serve as new targets for specific treatment of heart failure. To date, specific treatments focusing on the cardiomyocyte levels are lacking, but increasing data clearly showed that miRNAs are transcriptional regulators. 60 Therefore, new data open the possibility of developing a new area of specific drugs that will enable further development of specific causal and personalized therapy. In this context, it should be pointed out that a systematic review on the role of liquid biopsy regarding the role of miRNAs as potential biomarkers for myocarditis and DCMi already identified miRNAs, which have now also been identified in blood of DCMi patients, as the best candidates for myocardial inflammation. 16 Furthermore, it is very important to emphasize that in the here presented miRNA, a differentiation between virus‐positive and virus‐negative DCMi patients could be achieved for the first time. This is extremely important, as previous clinical studies have shown that this distinction is essential from a therapeutic point of view for specific therapeutic regimens. 61 , 62 , 63
Although a future therapeutic use of miRNAs is undoubtedly appealing, there are still great practical difficulties to overcome, including the identification of proper administration routes, the control of in‐body stability, the targeting of specific tissues and cell types, and the attaining of the intended intracellular effects. Hence, only few miRNA‐based drugs have, as of now, entered a clinical test phase. 64
Further in‐depth large patient studies need to be conducted to confirm these results as a basis for a specific, causal, and personalized therapy. 65
Study limitations
There are a variety of factors that may alter the miRNA levels between cohorts and studies, including methodology and therapy of the patients, which requires careful and critical evaluation when interpreting findings and comparing results from different groups. Results may also vary depending on disease progression, including other forms of cardiomyopathies studied and other platforms (PCR devices, software, and thresholds).
Conflict of interest
None declared.
Funding
This work was supported by ProFIT grant of the Investitionsbank Berlin/EFRE (No. 10169028, Berlin, Germany).
Acknowledgements
The authors are grateful to K. Winter, S. Ochmann, and J. Klostermann (IKDT) for their excellent technical assistance.
Aleshcheva, G. , Baumeier, C. , Harms, D. , Bock, C.‐Thomas , Escher, F. , and Schultheiss, H.‐P. (2023) MicroRNAs as novel biomarkers and potential therapeutic options for inflammatory cardiomyopathy. ESC Heart Failure, 10: 3410–3418. 10.1002/ehf2.14523.
References
- 1. Schultheiss HP, Fairweather DL, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, et al. Dilated cardiomyopathy. Nat Rev Dis Primers 2019;5:32. doi: 10.1038/s41572-019-0084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leone O, Pieroni M, Rapezzi C, Olivotto I. The spectrum of myocarditis: From pathology to the clinics. Virchows Arch 2019;475:279‐301. doi: 10.1007/s00428-019-02615-8 [DOI] [PubMed] [Google Scholar]
- 3. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2648a‐2648d. doi: 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 4. Verdonschot JAJ, Merlo M, Dominguez F, Wang P, Henkens MTHM, Adriaens ME, et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur Heart J 2021;42:162‐174. doi: 10.1093/eurheartj/ehaa841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liehn E, Cabrera‐Fuentes H. Inflammation between defense and disease: Impact on tissue repair and chronic sickness. Discoveries 2015;3:3. doi: 10.15190/d.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation‐associated diseases in organs. Oncotarget 2015;9:9‐7218. doi: 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S, Wotzkow C, Bongoni AK, Shaw‐Boden J, Siegrist M, Taddeo A, et al. Role of the plasma cascade systems in ischemia/reperfusion injury of bone. Bone 2017;97. doi: 10.1016/j.bone.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 8. Pober J, Sessa W. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol 2014;7. doi: 10.1101/cshperspect.a016345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dick SA, Epelman S. Chronic heart failure and inflammation. Circ Res American Heart Association 2016;119:159‐176. doi: 10.1161/CIRCRESAHA.116.308030 [DOI] [PubMed] [Google Scholar]
- 10. Ohta‐Ogo K, Sugano Y, Ogata S, Nakayama T, Komori T, Eguchi K, et al. Myocardial T‐lymphocytes as a prognostic risk‐stratifying marker of dilated cardiomyopathy—Results of the multicenter registry to investigate inflammatory cell infiltration in dilated cardiomyopathy in tissues of endomyocardial biopsy (INDICATE study). Circ J 2022;86:1092‐1101. doi: 10.1253/circj.CJ-21-0529 [DOI] [PubMed] [Google Scholar]
- 11. Davidson‐Moncada J, Papavasiliou N, Tam W. MicroRNAs of the immune system: Roles in inflammation and cancer. Ann N Y Acad Sci 2010;1183:183‐194. doi: 10.1111/j.1749-6632.2009.05121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anglicheau D, Muthukumar T, Suthanthiran M. MicroRNAs: Small RNAs with big effects. Transplantation 2010;90:105‐112. doi: 10.1097/TP.0b013e3181e913c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: An overview of nuclear functions. Int J Mol Sci 2016; doi: 10.3390/ijms17101712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol American Heart Association 2013;33:170‐177. doi: 10.1161/ATVBAHA.112.300068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tahamtan A, Teymoori‐Rad M, Nakstad B, Salimi V. Anti‐inflammatory microRNAs and their potential for inflammatory diseases treatment. Front Immunol 2018;9:1377. doi: 10.3389/fimmu.2018.01377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewandowski P, Goławski M, Baron M, Reichman‐Warmusz E, Wojnicz R. A systematic review of miRNA and cfDNA as potential biomarkers for liquid biopsy in myocarditis and inflammatory dilated cardiomyopathy. Biomolecules 2022;12:1476. doi: 10.3390/biom12101476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol Annual Reviews 2012;30:295‐312. doi: 10.1146/annurev-immunol-020711-075013 [DOI] [PubMed] [Google Scholar]
- 18. Contreras J, Rao DS. MicroRNAs in inflammation and immune responses. Leukemia 2012;26:404‐413. doi: 10.1038/leu.2011.356 [DOI] [PubMed] [Google Scholar]
- 19. Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol 2009;9:692‐703. doi: 10.1038/nri2634 [DOI] [PubMed] [Google Scholar]
- 20. Aleshcheva G, Pietsch H, Escher F, Schultheiss H. MiRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally‐induced cardiomyopathies. ESC Hear Fail 2020;8:408‐422. doi: 10.1002/ehf2.13090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santer L, Bär C, Thum T. Circular RNAs: A novel class of functional RNA molecules with a therapeutic perspective. Mol Ther 2019;27:27‐1363. doi: 10.1016/j.ymthe.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang C‐K, Kafert‐Kasting S, Thum T. Preclinical and clinical development of noncoding RNA therapeutics for cardiovascular disease. Circ Res American Heart Association 2020;126:663‐678. doi: 10.1161/CIRCRESAHA.119.315856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kmiołek T, Paradowska‐Gorycka A. miRNAs as biomarkers and possible therapeutic strategies in rheumatoid arthritis. Cell 2022;11:452. doi: 10.3390/cells11030452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holjencin C, Jakymiw A. MicroRNAs and their big therapeutic impacts: Delivery strategies for cancer intervention. Cell 2022;11:2332. doi: 10.3390/cells11152332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, et al. Novel antisense therapy targeting microRNA‐132 in patients with heart failure: Results of a first‐in‐human Phase 1b randomized, double‐blind, placebo‐controlled study. Eur Heart J 2021;42:178‐188. doi: 10.1093/eurheartj/ehaa898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foinquinos A, Batkai S, Genschel C, Viereck J, Rump S, Gyöngyösi M, et al. Preclinical development of a miR‐132 inhibitor for heart failure treatment. Nat Commun 2020;11:633. doi: 10.1038/s41467-020-14349-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reda El Sayed S, Cristante J, Guyon L, Denis J, Chabre O, Cherradi N. MicroRNA therapeutics in cancer: Current advances and challenges. Cancers (Basel) 2021;13:2680. doi: 10.3390/cancers13112680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chipman LB, Pasquinelli AE. miRNA targeting: Growing beyond the seed. Trends Genet Elsevier 2019;35:215‐222. doi: 10.1016/j.tig.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang S, Cheng Z, Wang Y, Han T. The risks of miRNA therapeutics: In a drug target perspective. Drug Des Devel Ther 2021;15:721‐733. doi: 10.2147/DDDT.S288859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Adv Drug Deliv Rev 2018;129:308‐318. doi: 10.1016/j.addr.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 31. Kuhl U, Lassner D, Dorner A, Rohde M, Escher F, Seeberg B, et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res Cardiol 2013;108:372. doi: 10.1007/s00395-013-0372-y [DOI] [PubMed] [Google Scholar]
- 32. Escher F, Tschöepe C, Lassner D, Schultheiss H‐P. Myocarditis and inflammatory cardiomyopathy: From diagnosis to treatment. Turk Kardiyol Dern Ars 2015;43:739‐748. doi: 10.5543/tkda.2015.47750 [DOI] [PubMed] [Google Scholar]
- 33. Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005;111:887‐893. doi: 10.1161/01.CIR.0000155616.07901.35 [DOI] [PubMed] [Google Scholar]
- 34. Kühl U, Schultheiss H. Viral myocarditis. Swiss Med Wkly 2014;144:w14010. doi: 10.4414/smw.2014.14010 [DOI] [PubMed] [Google Scholar]
- 35. Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation Lippincott Williams & Wilkins 2005;111:887‐893. doi: 10.1161/01.CIR.0000155616.07901.35 [DOI] [PubMed] [Google Scholar]
- 36. Denk J, Boelmans K, Siegismund C, Lassner D, Arlt S, Jahn H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer's disease. PLoS One Public Library of Science 2015;10:e0126423. doi: 10.1371/journal.pone.0126423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat Rev Cardiol 2021;18:169‐193. doi: 10.1038/s41569-020-00435-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circ Hear Fail American Heart Association 2020;13:e00740. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schultheiss H‐P, Piper C, Sowade O, Waagstein F, Kapp J‐F, Wegscheider K, et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon‐β treatment in patients with chronic viral cardiomyopathy. Clin Res Cardiol 2016;105:763‐773. doi: 10.1007/s00392-016-0986-9 [DOI] [PubMed] [Google Scholar]
- 40. Merken J, Hazebroek M, Van PP, Verdonschot J, Van EV, Knackstedt C, et al. Immunosuppressive therapy improves both short‐ and long‐term prognosis in patients with virus‐negative nonfulminant inflammatory cardiomyopathy. Circ Hear Fail American Heart Association 2018;11:e004228. doi: 10.1161/CIRCHEARTFAILURE.117.004228 [DOI] [PubMed] [Google Scholar]
- 41. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus‐negative inflammatory cardiomyopathy: The TIMIC study. Eur Heart J 2009;30:1995‐2002. doi: 10.1093/eurheartj/ehp249 [DOI] [PubMed] [Google Scholar]
- 42. Esmailzadeh S, Mansoori B, Mohammadi A, Baradaran B. Regulatory roles of micro‐RNAs in T cell autoimmunity. Immunol Invest Taylor & Francis 2017;46:864‐879. doi: 10.1080/08820139.2017.1373901 [DOI] [PubMed] [Google Scholar]
- 43. Safa A, Bahroudi Z, Shoorei H, Majidpoor J, Abak A, Taheri M, et al. miR‐1: A comprehensive review of its role in normal development and diverse disorders. Biomed Pharmacother 2020;132:110903. doi: 10.1016/j.biopha.2020.110903 [DOI] [PubMed] [Google Scholar]
- 44. Wang Q, Chen W, Yang X, Song Y, Sun X, Tao G, et al. Inhibition of miRNA‐1‐mediated inflammation and autophagy by astragaloside IV improves lipopolysaccharide‐induced cardiac dysfunction in rats. J Inflamm Res 2022;15:2617‐2629. doi: 10.2147/JIR.S362368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu L, Cheng Z, Yang J. miR‐23 regulates cell proliferation and apoptosis of vascular smooth muscle cells in coronary heart disease. Pathol Res Pract 2018;214:1873‐1878. doi: 10.1016/j.prp.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 46. Yu R‐B, Li K, Wang G, Gao G‐M, Du J‐X. MiR‐23 enhances cardiac fibroblast proliferation and suppresses fibroblast apoptosis via targeting TGF‐β1 in atrial fibrillation. Eur Rev Med Pharmacol Sci 2019;23:4419‐4424. doi: 10.26355/eurrev_201905_17950 [DOI] [PubMed] [Google Scholar]
- 47. Talebi F, Ghorbani S, Chan WF, Boghozian R, Masoumi F, Ghasemi S, et al. MicroRNA‐142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J Neuroinflammation 2017;14:55. doi: 10.1186/s12974-017-0832-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu W, Li D, Zhang Y, Li C, Zhang C, Wang L. MiR‐142‐5p acts as a significant regulator through promoting proliferation, invasion, and migration in breast cancer modulated by targeting SORBS1. Technol Cancer Res Treat 2019;18:1533033819892264. doi: 10.1177/1533033819892264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahesh G, Biswas R. MicroRNA‐155: A master regulator of inflammation. J Interf Cytokine Res Mary Ann Liebert, Inc. 2019;39:321‐330. doi: 10.1089/jir.2018.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Etzrodt V, Idowu T, Schenk H, Seeliger B, Prasse A, Thamm K, et al. Role of endothelial microRNA 155 on capillary leakage in systemic inflammation. Crit Care 2021;25:76. doi: 10.1186/s13054-021-03500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li X, Kong D, Chen H, Liu S, Hu H, Wu T, et al. MiR‐155 acts as an anti‐inflammatory factor in atherosclerosis‐associated foam cell formation by repressing calcium‐regulated heat stable protein 1. Sci Rep 2016;6:21789. doi: 10.1038/srep39405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alivernini S, Gremese E, McSharry C, Tolusso B, Ferraccioli G, McInnes I, et al. MicroRNA‐155—At the critical interface of innate and adaptive immunity in arthritis. Front Immunol 2018;8:8. doi: 10.3389/fimmu.2017.01932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR‐155 gene: A typical multifunctional microRNA. Biochim Biophys Acta ‐ Mol Basis Dis 2009;1792:497‐505. doi: 10.1016/j.bbadis.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 54. Tao Y, Ai R, Hao Y, Jiang L, Dan H, Ji N, et al. Role of miR‐155 in immune regulation and its relevance in oral lichen planus (review). Exp Ther Med 2019;17:575‐586. doi: 10.3892/etm.2018.7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khordadmehr M, Shahbazi R, Sadreddini S, Baradaran B. miR‐193: A new weapon against cancer. J Cell Physiol John Wiley & Sons, Ltd 2019;234:16861‐16872. doi: 10.1002/jcp.28368 [DOI] [PubMed] [Google Scholar]
- 56. Izadpanah S, Shabani P, Aghebati‐Maleki A, Baghbani E, Baghbanzadeh A, Fotouhi A, et al. Insights into the roles of miRNAs; miR‐193 as one of small molecular silencer in osteosarcoma therapy. Biomed Pharmacother 2019;111:873‐881. doi: 10.1016/j.biopha.2018.12.106 [DOI] [PubMed] [Google Scholar]
- 57. Hohman AM, McNeill EM. Abstract P1109: Investigating the role of human conserved microrna‐193 in the aging Drosophila heart. Circ Res American Heart Association 2022;131:AP1109. doi: 10.1038/s41467-020-14761-8 [DOI] [Google Scholar]
- 58. Yu W, Liang X, Li X, Zhang Y, Sun Z, Liu Y, et al. MicroRNA‐195: A review of its role in cancers. Onco Targets Ther Dove Medical Press 2018;11:7109‐7123. doi: 10.2147/OTT.S183600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brás J, Silva A, Calin G, Barbosa M, Santos S, Almeida MI. miR‐195 inhibits macrophages pro‐inflammatory profile and impacts the crosstalk with smooth muscle cells. PLoS ONE 2017;12:e0188530. doi: 10.1371/journal.pone.0188530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post‐transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet 2008;9:102‐114. doi: 10.1038/nrg2290 [DOI] [PubMed] [Google Scholar]
- 61. Chimenti C, Magnocavallo M, Ballatore F, Bernardini F, Alfarano M, Della RDG, et al. Prevalence and clinical implications of COVID‐19 myocarditis. Card Electrophysiol Clin Elsevier 2022;14:53‐62. doi: 10.1016/j.ccep.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kühl U, Rohde M, Lassner D, Gross UM, Escher F, Schultheiss H‐P. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012;37:637‐643. doi: 10.1007/s00059-012-3656-3 [DOI] [PubMed] [Google Scholar]
- 63. Kuehl U, Lassner D, Gast M, Stroux A, Rohde M, Siegismund C, et al. Differential cardiac microRNA expression predicts the clinical course in human enterovirus cardiomyopathy. Circ Heart Fail 2015;8:605‐618. doi: 10.1161/CIRCHEARTFAILURE.114.001475 [DOI] [PubMed] [Google Scholar]
- 64. Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet Elsevier 2022;38:613‐626. doi: 10.1016/j.tig.2022.02.006 [DOI] [PubMed] [Google Scholar]
- 65. Shomali N, Maashi M, Baradaran B, Daei Sorkhabi A, Sarkesh A, Mohammadi H, et al. Dysregulation of survivin‐targeting microRNAs in autoimmune diseases: New perspectives for novel therapies. Front Immunol 2022;13:13. doi: 10.3389/fimmu.2022.839945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


