Abstract
Aims
The present analysis from the multicentre prospective Altshock‐2 registry aims to better define clinical features, in‐hospital course, and management of cardiogenic shock complicating acutely decompensated heart failure (ADHF‐CS) as compared with that complicating acute myocardial infarction (AMI‐CS).
Methods and results
All patients with AMI‐CS or ADHF‐CS enrolled in the Altshock‐2 registry between March 2020 and February 2022 were selected. The primary objective was the characterization of ADHF‐CS patients as compared with AMI‐CS. In‐hospital length of stay and mortality were secondary endpoints. One‐hundred‐ninety of the 238 CS patients enrolled in the aforementioned period were considered for the present analysis: 101 AMI‐CS (80% ST‐elevated myocardial infarction and 20% non‐ST‐elevated myocardial infarction) and 89 ADHF‐CS. As compared with AMI‐CS, ADHF‐CS patients were younger [63 (IQR 59–76) vs. 67 (IQR 54–73) years, P = 0.01], but presented with higher creatinine [1.6 (IQR 1.0–2.6) vs. 1.2 (IQR 1.0–1.4) mg/dL, P < 0.001], bilirubin [1.3 (IQR 0.9–2.3) vs. 0.6 (IQR 0.4–1.1) mg/dL, P = 0.01], and central venous pressure values [14 mmHg (IQR 8–12) vs. 10 mmHg (IQR 7–14),P = 0.01]. Norepinephrine was the most common catecholamine used in AMI‐CS (79.3%), whereas epinephrine was used more commonly in ADHF‐CS (65.5%); 75.8% vs. 46.6% received a temporary mechanical support in AMI‐CS and ADHF‐CS, respectively (P < 0.001). Length of hospital stay was longer in the latter [28 (IQR 13–48) vs. 17 (IQR 9–29) days, P = 0.001]. Heart replacement therapies were more frequently used in the ADHF‐CS group (heart transplantation 13.5% vs. 0% and left ventricular assist device 11% vs. 2%, P < 0.01 and 0.01, respectively). In‐hospital mortality was 41.1% (38.6% AMI‐CS vs. 43.8% ADHF‐CS, P = 0.5).
Conclusions
ADHF‐CS is characterized by a higher prevalence of end‐organ and biventricular dysfunction at presentation, a longer hospital length of stay, and higher need of heart replacement therapies when compared with AMI‐CS. In‐hospital mortality was similar between the two aetiologies. Our data warrant development of new management protocols focused on CS aetiology.
Keywords: Cardiogenic shock, Heart failure, Myocardial infarction, Mortality
Introduction
Cardiogenic shock (CS) represents the most severe form of acute heart failure with a short‐term mortality ranging between 30% and 50% despite recent diagnostic and therapeutic improvements, 1 including a larger availability and utilization of mechanical circulatory support (MCS) devices. 2 , 3 The epidemiology of CS has changed over the last few years with decreases of cases related to acute myocardial infarction (AMI‐CS ~30%) and a rise of non‐ischaemic aetiologies. 4 , 5 , 6 However, the majority of available evidence regarding patients' characterizations, diagnostic and therapeutic management is historically based on AMI‐CS, while very little is known about acute decompensated heart failure‐related CS (ADHF‐CS). 7 , 8 The definition of ADHF‐CS pathophysiology, underlying mechanisms, and patients' phenotypes, is of utmost importance to define ad hoc diagnostic and therapeutic pathways.
We present an analysis of the multicentre Altshock‐2 Registry. The aim of the present study is to describe the differences in clinical characteristics, hospital course, treatment strategies, and short‐term post‐hospital outcomes of patients with ADHF‐CS as compared with AMI‐CS patients.
Methods
Study design
The present analysis is derived from the Altshock‐2 Registry (NCT04295252), a multicentre prospective observational registry enrolling consecutive patients admitted for CS from 11 Italian centres since March 2020 (see Supporting information, Table S1 ). CS was defined as the presence of both of the following criteria:
Systolic blood pressure (SBP) <90 mmHg or mean arterial pressure (MAP) <60 mmHg, after an appropriate fluid challenge if there is no sign of overt fluid overload, or need of vasoactive agents to maintain SBP >90 mmHg or MAP >60 mmHg, or need of MCS;
At least one of the following criteria/signs of overt hypoperfusion: mixed venous oxygen saturation <60%; arterial lactates >2 mmol/L; oliguria <0.5 mL/kg/h for at least 6 h.
In accordance to the EU Regulation 536/2014, all competent patients provided written informed consent, whereas consent was waived for patients who were not competent on admission. The study was conducted in accordance with ethical principles based on the Declaration of Helsinki, 9 International Conference on Harmonization for Good Clinical Practice, and the current ethical rules. The Strengthening the Reporting of Observational Studies in Epidemiology Guidelines was followed for reporting the findings. 10 Only patients with the diagnosis of AMI‐CS or ADHF‐CS were considered for the current analysis. AMI‐CS was defined as the CS complicating an acute coronary syndrome [i.e. non‐ST‐elevated myocardial infarction (NSTEMI) or ST‐elevated myocardial infarction (STEMI)]. ADHF‐CS was defined as CS due to decompensation of heart failure in a patient with or without (de novo) a previously known history of chronic heart failure. Clinical, laboratory, procedural, pharmacological, and MCS devices used and follow‐up data of all consecutively enrolled patients were collected and registered in an electronic case report form through the RedCap® platform. For patients surviving to hospital discharge, the last available follow‐up was considered. The laboratory and haemodynamic variables as well as the Society for Cardiovascular Angiography and Interventions (SCAI) shock stages assigned according to the updated SCAI shock stages classification 6 were obtained at admission.
Endpoints
The primary objective of this study was the comparison of the clinical characteristics of ADHF‐CS and AMI‐CS patients. Secondary outcomes of interest were hospital length of stay and all‐cause mortality (both in‐hospital and at last‐available follow‐up). Left ventricular assist device (LVAD) implantation, heart transplantation, 30 day re‐hospitalizations after discharge, and bleeding events occurring during hospitalization (defined according to the Bleeding Academic Research Consortium classification) 11 were also evaluated. Worsening renal function (WRF) at 24 h was defined as an increase of creatinine levels >0.3 mg/dL and of more than 25% from the baseline value.
Statistical analysis
Continuous variables are expressed as median and interquartile range (IQR) and were compared by independent samples student T‐test or non‐parametric Mann–Whitney U‐test when normality distribution was not respected. Categorical variables are presented as counts and relative percentages on available data and were compared by χ 2 or Fisher test, as appropriate. Baseline characteristics, in‐hospital data, and outcomes were compared between the ADHF‐CS and AMI‐CS groups.
Survival analyses were performed using the Kaplan–Meyer's survival curves for each group of interest and compared using the log‐rank test. The analyses were performed with using SPSS V 25.0 (SPSS, Chicago, IL, USA). Statistical significance was set at the two‐tailed 0.05 level.
Results
Baseline characteristics and clinical presentation
A total of 238 patients with CS were included in the Altshock‐2 Registry from March 2020 to February 2022. After exclusion of those with other aetiologies (48 patients), 190 patients were considered for the present analysis: 101 patients (53%) were hospitalized for AMI‐CS [80% acute myocardial infarction with ST‐elevation (STEMI) and 20% without ST‐elevation] and 89 (47%) for ADHF‐CS (see Figure 1 ). Within the last group, 66 patients (74%) had a known history of chronic heart failure (i.e. ‘CS related to acute decompensation of chronic heart failure’), while the others (26%) were de‐novo episodes.
Figure 1.
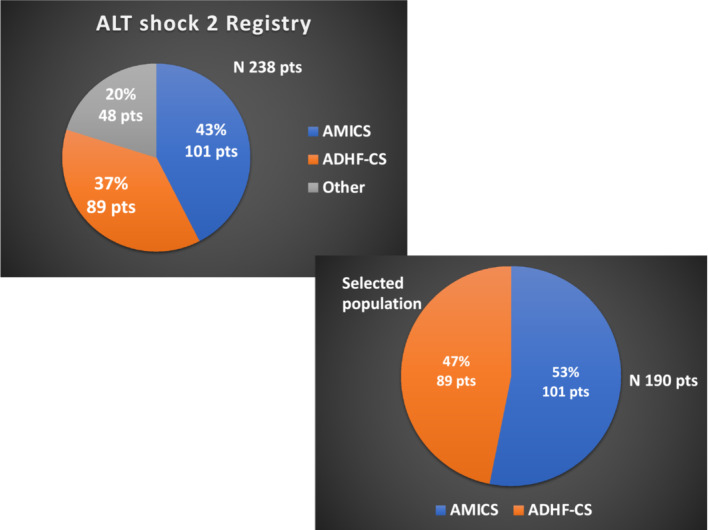
Aetiologies of cardiogenic shock (CS) patients enrolled in the Altshock‐2 Registry from March 2020 to February 2021 (above) and cohort of patients with acute myocardial infarction (AMI)‐CS or acute decompensated heart failure (ADHF)‐CS selected for the present analysis (below).
Demographic data and baseline characteristics are summarized in Table 1 . Patients with ADHF‐CS were younger as compared with AMI‐CS [63 (IQR 59–76) vs. 67 (IQR 54–73) years, P = 0.01], had a higher prevalence of comorbidities and organ dysfunction, and were more commonly receiving heart failure medications. Department of admission, invasive monitoring, biochemistry, haemodynamic, and echocardiographic parameters at presentation, including cardiac arrest incidence, are described in Table 2 . Fifty‐eight per cent of patients were admitted from the emergency department (74.5% in AMI‐CS vs. 39.1% in ADHF‐CS, P < 0.001), 24% from other wards of the same hospital (7.1% in AMI‐CS vs. 43.7% in ADHF‐CS, P < 0.001), and the remaining 18% were transferred from outside‐hospitals (18.4% in AMI‐CS vs. 17.2% in ADHF‐CS, P = 1.0).
Table 1.
Baseline characteristics of the included patients according to the main aetiology of cardiogenic shock
| Characteristic | Overall cohort | AMI‐CS | ADHF‐CS | P value |
|---|---|---|---|---|
| (N = 190) | (N = 101) | (N = 89) | ||
| Demographics | ||||
| Age, years | 65 (56–75) | 67 (59–76) | 63 (54–73) | 0.01 |
| BMI | 26 (23–29) | 26 (23–29) | 25 (23–28) | 0.3 |
| Male | 150 (78.9) | 78 (77.2) | 72 (80.9) | 0.5 |
| Ethnicity | ||||
| White | 154 (95.7) | 83 (98.8) | 71 (92.2) | 0.3 |
| Black | 1 (0.6) | 0 (0.0) | 1 (1.3) | |
| Asian | 1 (0.6) | 0 (0.0) | 1 (1.3) | |
| Hispanic | 2 (1.2) | 0 (0.0) | 2 (2.6) | |
| Other | 3 (1.9) | 1 (1.2) | 2 (2.6) | |
| Medical history | ||||
| Hypertension | 119 (62.6) | 70 (69.3) | 49 (55.1) | 0.04 |
| Diabetes | 62 (32.6) | 30 (29.7) | 32 (36.0) | 0.4 |
| Smoking | 55 (29.1) | 35 (34.7) | 20 (22.7) | 0.07 |
| Dyslipidaemia | 85 (44.7) | 49 (48.5) | 36 (40.4) | 0.3 |
| Prior PCI | 45 (23.8) | 19 (19.0) | 26 (29.2) | 0.1 |
| Prior CABG | 18 (9.5) | 8 (7.9) | 9 (11.2) | 0.4 |
| Stroke or TIA | 12 (6.3) | 6 (5.9) | 6 (6.8) | 0.9 |
| Peripheral artery disease | 33 (17.6) | 20 (19.8) | 13 (14.9) | 0.3 |
| Atrial fibrillation | 52 (27.4) | 7 (6.9) | 45 (50.6) | <0.001 |
| CKD | 45 (23.7) | 12 (11.9) | 33 (37.1) | <0.001 |
| Anaemia | 26 (13.7) | 5 (5.0) | 21 (23.6) | <0.001 |
| Liver disease | 9 (4.7) | 1 (1.0) | 8 (9.0) | 0.01 |
| Cancer history | 20 (10.6) | 8 (8.0) | 12 (13.5) | 0.2 |
| Prior known EF | 34 (21–55) | 55 (49–59) | 25 (20–40) | < 0.001 |
| Waiting list for HT | 8 (4.2) | 1 (1.0) | 7 (7.9) | 0.03 |
| Drug history | ||||
| Beta‐blocker | 82 (44.6) | 24 (25.0) | 58 (65.9) | <0.001 |
| ACE‐I | 33 (17.8) | 18 (18.6) | 15 (17.0) | 0.8 |
| ARB | 18 (9.7) | 13 (13.3) | 5 (5.7) | 0.08 |
| Sacubitril/valsartan | 25 (13.5) | 1 (1.0) | 24 (27.6) | <0.001 |
| Loop diuretics | 80 (43.0) | 17 (17.5) | 63 (70.8) | <0.001 |
| MRA | 50 (26.9) | 5 (5.1) | 45 (51.1) | <0.001 |
| Oral anticoagulant | 46 (24.9) | 10 (10.2) | 36 (41.4) | <0.001 |
| Ivabradine | 6 (3.3) | 1 (1.0) | 5 (5.8) | 0.07 |
| SAPT | 49 (26.5) | 21 (21.4) | 28 (32.2) | 0.1 |
| DAPT | 20 (10.8) | 10 (10.2) | 10 (11.5) | 0.8 |
| Oral antidiabetics | 35 (18.9) | 15 (15.3) | 20 (23.0) | 0.2 |
| Insulin therapy | 19 (10.4) | 6 (6.1) | 13 (15.3) | 0.04 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ADHF, acute decompensated heart failure; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CS, cardiogenic shock; DAPT, dual antiplatelet therapy; EF, ejection fraction; HT, heart transplant; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy; TIA, transient ischaemic attack.
Data are presented as n (% on available) and as median (IQR).
Table 2.
Department of admission, invasive monitoring, blood examination, haemodynamic, echocardiographic data, and cardiac arrest according to the aetiology of cardiogenic shock
| Variable | Overall cohort | AMI‐CS | ADHF‐CS | P value |
|---|---|---|---|---|
| (N = 190) | (N = 101) | (N = 89) | ||
| Setting and type of invasive monitoring | ||||
| ICU admission | 84 (46.7) | 46 (47.4) | 38 (45.8) | 0.8 |
| Invasive arterial pressure | 185 (98.4) | 98 (98.0) | 87 (98.9) | 0.6 |
| CVC | 171 (91.0) | 89 (89.0) | 82 (93.2) | 0.3 |
| Pulmonary artery catheter | 36 (19.3) | 17 (17.0) | 19 (21.8) | 0.4 |
| SCAI class at admission | ||||
| 0.03 | ||||
| B | 17 (9.0) | 6 (5.9) | 11 (12.5) | |
| C | 105 (55.6) | 51 (50.5) | 54 (61.4) | |
| D | 48 (25.4) | 29 (28.7) | 19 (21.6) | |
| E | 19 (10.1) | 15 (14.9) | 4 (4.5) | |
| Laboratory profile on admission | ||||
| Lactate, mmol/L | 2.7 (1.6–5.6) | 2.8 (2–5.5) | 2.4 (1.4–5.8) | 0.9 |
| Lactate ≥2 mmol/L | 124 (68.5) | 74 (77.9) | 50 (58.1) | 0.004 |
| SVcO2, % | 61 (47–71) | 63 (48–73) | 60 (44–70) | 0.2 |
| Bilirubin, mg/dL | 1.0 (0.5–1.5) | 0.6 (0.4–1.1) | 1.3 (0.9–2.3) | 0.01 |
| AST, U/L | 97 (27–377) | 161 (55–448) | 36 (24–314) | 0.1 |
| ALT, U/L | 63 (27–172) | 84 (31–165) | 47 (20–308) | 0.06 |
| INR | 1.3 (1.2–1.7) | 1.2 (1.1–1.3) | 1.5 (1.2–2.5) | <0.001 |
| GFR, mL/min | 55 (35–80) | 63 (43–81) | 51 (27–79) | 0.02 |
| Creatinine | 1.3 (1.0–2.0) | 1.2 (1.0–1.4) | 1.6 (1.0–2.6) | <0.001 |
| NT‐proBNP, ng/L | 8674 (4364–19 504) | 8674 (4009–19 504) | 8565 (4486–21 357) | 0.6 |
| Glycaemia, mg/dL | 160 (131–236) | 186 (149–267) | 142 (122–190) | 0.01 |
| Haemoglobin, g/dL | 12.7 (10.8–14.4) | 13.5 (11.9–14.9) | 11.6 (10.6–13.3) | <0.001 |
| Platelet count, 103/mm3 | 229 (165–293) | 249 (186–301) | 199 (156–254) | 0.03 |
| WBC count, 103/mm3 | 13 (9–17) | 15 (11–19) | 11 (8–15) | <0.001 |
| CRP, mg/dL | 4 (1–11) | 3 (1–10) | 5 (2–12) | 0.2 |
| SOFA score | 7 (4–9) | 7 (4–10) | 6 (4–9) | 0.6 |
| SAPS | 46 (34–59) | 47 (37–64) | 41 (30–55) | 0.06 |
| Cardiac arrest | 58/ (30.5) | 45/ (44.6) | 13/ (14.6) | <0.001 |
| Haemodynamic findings on admission | ||||
| SBP, mmHg | 93 (82–110) | 95 (80–115) | 90 (85–104) | 0.3 |
| MAP, mmHg | 70 (60–78) | 71 (60–82) | 70 (62–75) | 0.7 |
| HR | 90 (75–110) | 90 (73–110) | 90 (80–110) | 0.4 |
| CVP, mmHg | 12 (8–16) | 10 (7–14) | 14 (8–19) | 0.01 |
| CI, L/min/m2 | 1.9 (1.6–2.3) | 1.9 (1.8–2.0) | 2.2 (1.6–2.4) | 0.9 |
| Mean PAP, mmHg | 28 (25–38) | 29 (27–38) | 28 (25–38) | 0.7 |
| PCWP, mmHg | 21 (17–28) | 22 (18–28) | 21 (17–25) | 0.9 |
| Echocardiographic findings on admission | ||||
| LVEF, % | 20 (15–30) | 25 (18–34) | 20 (15–25) | 0.01 |
| Indexed LVEDV, mL/sm | 164 (131–210) | 138 (120–170) | 200 (160–257) | <0.001 |
| Severe MR | 35 (21.5) | 4 (4.8) | 31 (38.8) | <0.001 |
| Severe TR | 23 (14.4) | 2 (2.5) | 21 (26.6) | <0.001 |
| TAPSE | 15 (12–18) | 16 (14–19) | 14 (12–16) | 0.003 |
| Systolic PAP, mmHg | 46 (39–55) | 41 (33–50) | 50 (40–60) | 0.01 |
ADHF, acute decompensated heart failure; ALT, alanine aminotransferase; AMI, acute myocardial infarction; AST, aspartate aminotransferase; CS, cardiogenic shock; BNP, B‐type natriuretic peptide; CI, cardiac index; CRP, C‐reactive protein; CVP, central venous pressure; INR, international normalized ratio; IQR, interquartile range; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MR, mitral regurgitation; NT‐proBNP, N‐terminal proB‐type natriuretic peptide; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; SAPS, simplified acute physiology score; SBP, systolic blood pressure; ScvO2, central venous oxygen saturation; SOFA, sequential organ failure assessment; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell.
Data are presented as n (% on available) and as median (IQR).
Only 19.3% of patients were monitored via a pulmonary artery catheter (17.0% and 21.8% in AMI‐CS and ADHF‐CS group, respectively, P = 0.4). Echocardiographic data at admission were significantly different in ADHF‐CS as compared with AMI‐CS: left ventricular ejection fraction [20% (IQR 15–25) vs. 25% (IQR 18–34), P = 0.01], left ventricular end diastolic volume [200 (IQR 160–257) vs. 138 (IQR 120–170) mL/m2, P < 0.001], severe mitral regurgitation prevalence (38.8% vs. 4.8%, P < 0.001), and tricuspid annular plane systolic excursion [14 (IQR 12–16) vs. 16 (IQR 14–19) mm, P = 0.003].
Median values of lactate at presentation were similar in AMI‐CS and ADHF‐CS [2.8 mmol/L (IQR 2–5.5) vs. 2.4 mmol/L (IQR 1.4–5.8), P = 0.9], although values above 2 mmol/L were more commonly observed in the former (77.9% vs. 58.1%, P = 0.004). Higher invasive central venous pressure (CVP) values were noted at presentation in the ADHF‐CS group [14 (IQR 8–12) vs. 10 (IQR 7–14) mmHg, P = 0.01]. As compared with patients with AMI‐CS, those with ADHF‐CS had higher admission values of bilirubin [1.3 (IQR 0.9–2.3) vs. 0.6 (IQR 0.4–1.1) mg/dL, P = 0.01] and creatinine [1.6 (IQR 1.0–2.6) vs. 1.2 (IQR 1.0–1.4) mg/dL, P < 0.001], and lower GFR [51 (IQR 27–79) vs. 63 (IQR 43–81) ml/min, P = 0.02]. Among survivors at 24 h, incidence of WRF was similar in ADHF‐CS (13.2%) as compared with AMI‐CS (16.7%, P = 0.5) as were values of CVP [10 (IQR 5–15) vs. 8 (IQR 5–11) mmHg, P = 0.1], while bilirubin was persistently higher in the ADHF‐CS group [1.3 (IQR 0.8–2.1) vs. 0.8 (IQR 0.6–1.2) mg/dL, P < 0.001].
SCAI classes at admission and according to the two groups are described in Figure 2 . Overall, the most common class was SCAI C (55.6%), and a higher proportion of SCAI E was registered among AMI‐CS patients (14.9% vs. 4.5%, P = 0.03).
Figure 2.
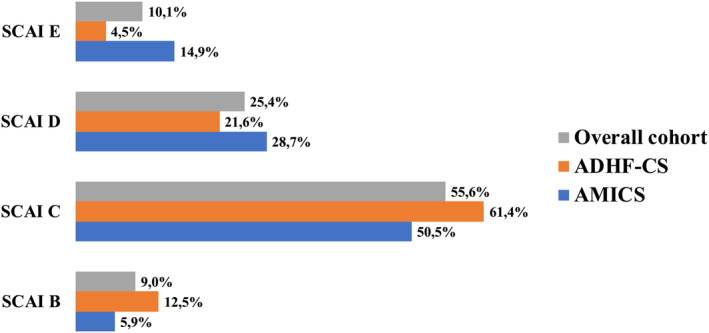
Society for Cardiovascular Angiography and Interventions (SCAI) class at admission in the whole cohort and according to cardiogenic shock (CS) aetiology. ADHF, acute decompensated heart failure; AMI, acute myocardial infarction.
Coronary artery disease characteristics and treatment within AMI‐CS group
Data on coronary artery disease characteristics and treatment are presented in Table S2 . Ninety‐four per cent of AMI‐CS patients underwent revascularization by percutaneous coronary intervention (PCI), one patient was treated with coronary artery bypass graft, and five patients did not underwent any type of revascularization. Seventy per cent of patients had multivessel disease at presentation with left anterior descendent as the most frequent involved vessel (85.6% of cases). Treatment of Culprit‐only lesion was the preferred revascularization strategy during index PCI (71.2% vs. 28.8% of complete revascularization).
Therapeutic management during hospital stay
In‐hospital management is described in Table 3 . The most commonly used adrenergic drug in AMI‐CS patients was norepinephrine (79.3%), while epinephrine was the most commonly used in the ADHF‐CS group (65.5%). Maximum inotropic score was higher in AMI‐CS population [23 (IQR 13–40) vs. 11 (IQR 6–20), P = 0.001] although ADHF‐CS had longer duration of administration of catecholamines [epinephrine: 144 (IQR 48–336) vs. 48 (IQR 24–114) hours, P = 0.04; dobutamine: 70 (IQR 31–201) vs. 47 (IQR 16–97) hours, P = 0.01; dopamine: 81 (IQR 35–636) vs. 12 (IQR 4–73) hours, P = 0.02]. The use of sodium nitroprusside was remarkably different between the two groups (20.7% in AMI‐CS and 59.3% in –ADHF‐CS, P < 0.001).
Table 3.
Therapeutic management stratified according to the CS
| Overall cohort | AMI‐CS | ADHF‐CS | P value | |
|---|---|---|---|---|
| (190 patients) | (N = 101) | (N = 89) | ||
| Vasoactive endovenous medications | ||||
| Epinephrine | 103 (57.5) | 46 (50.0) | 57 (65.5) | 0.04 |
| Max dose (y/kg/min) | 0.10 (0.05–0.12) | 0.10 (0.06–0.15) | 0.08 (0.05–0.10) | 0.04 |
| Median time (h) | 72 (30–240) | 48 (24–114) | 144 (48–336) | 0.003 |
| Norepinephrine | 107 (59.8) | 73 (79.3) | 34 (39.1) | <0.001 |
| Max dose (y/kg/min) | 0.20 (0.10–0.35) | 0.20 (0.10–0.35) | 0.19 (0.10–0.35) | 0.2 |
| Median time (h) | 48 (24–120) | 48 (15–97) | 48 (25–123) | 0.4 |
| Dobutamine | 72/ (40.2) | 30 (32.6) | 42/87 (48.3) | 0.03 |
| Max dose (y/kg/min) | 5.0 (4.0–6.0) | 5.0 (3.0–5.0) | 5.0 (4.0–7.0) | 0.2 |
| Median time (h) | 52 (24–125) | 47 (16–97) | 70 (31–201) | 0.01 |
| Dopamine | 31 (17.3) | 7 (7.6) | 24/ (27.6) | <0.001 |
| Max dose (y/kg/min) | 3.3 (2.5–5.0) | 5.0 (2.5–8.0) | 3.0 (2.5–4) | 0.3 |
| Median time (h) | 72 (12–264) | 12 (4–73) | 81 (35–636) | 0.02 |
| Milrinone | 9 (5.0) | 2 (2.2) | 7 (8.0) | 0.07 |
| Max dose (y/kg/min) | 0.33 (0.25–0.50) | 0.32 (0.30–0.33) | 0.35 (0.20–0.60) | 0.3 |
| Median time (h) | 120 (12–360) | 150 (12–288) | 120 (96–360) | 0.8 |
| Levosimendan | 68 (38.0) | 33 (35.9) | 35 (40.2) | 0.5 |
| Max dose (y/kg/min) | 0.10 (0.05–0.10) | 0.10 (0.05–0.10) | 0.10 (0.05–0.10) | 0.7 |
| Median time (h) | 29 (24–48) | 28 (24–48) | 29 (24–48) | 0.5 |
| Max inotropic score, median (IQR) | 16 (9–33) | 23 (13–40) | 11 (6–20) | 0.001 |
| Sodium nitroprusside | 66 (36.9) | 18 (19.6) | 48 (55.2) | <0.001 |
| Max dose (y/kg/min) | 0.5 (0.3–0.9) | 0.5 (0.3–0.8) | 0.5 (0.3–1.0) | 0.5 |
| Median time (h) | 158 (38–339) | 90 (12–168) | 216 (60–430) | 0.001 |
| Temporary mechanical circulatory support (tMCS) | ||||
| Overall tMCS use | 116 (62.0) | 75 (75.8) | 41 (46.6) | <0.001 |
| Number of tMCS used | ||||
| 1 | 81 (43.3) | 51 (51.5) | 30 (34.1) | <0.001 |
| 2 | 31 (16.6) | 22 (22.2) | 9 (10.2) | |
| 3 | 4 (2.1) | 2 (2.0) | 2 (2.3) | |
| IABP a | 101 (85.6) | 64 (84.2) | 37 (88.1) | 0.6 |
| IABP duration, days | 4 (2–7) | 3 (2–5) | 7 (3–16) | <0.001 |
| Impella a | 19 (16.1) | 14 (18.4) | 5 (11.9) | 0.4 |
| Impella duration, days | 5 (2–9) | 4 (2–8) | 8 (5–9) | 0.4 |
| ECMO a | 28 (23.7) | 20 (26.3) | 8 (19.0) | 0.4 |
| ECMO duration, days | 5 (2–10) | 5 (1–9) | 8 (4–12) | 0.8 |
| Other para‐corporeal assist devices a | 7 (6.0) | 3 (4.0) | 4 (9.8) | 0.2 |
| Respiratory support | ||||
| Need of ventilatory support | 129 (69.0) | 73 (73.7) | 56 (63.6) | 0.1 |
| NIV b | 66 (51.2) | 26 (35.6) | 40 (71.4) | <0.001 |
| Mechanical ventilation b | 93 (72.1) | 62 (83.6) | 32 (57.1) | 0.001 |
| Duration of respiratory support (days) | ||||
| NIV | 2 (1–4) | 1 (0.8–2) | 3 (1–6) | 0.02 |
| Mechanical ventilation | 5 (2–10) | 6 (2–10) | 5 (2–14) | 0.9 |
| CRRT | 43 (23.1) | 22 (22.4) | 21 (23.9) | 0.8 |
| Duration of CRRT (days) | 5 (3–9) | 6 (1–9) | 4 (3–9) | 0.4 |
ADHF, acute decompensated heart failure; AMI, acute myocardial infarction; CS, cardiogenic shock.
Data are presented as n (% on available) and as median (IQR). NIV, non‐invasive ventilation. Both continuous positive airway pressure and bilevel ventilation; IABP, intra‐aortic balloon pump; CRRT, continuous renal replacement therapy.
Percentage among patients treated with tMCS.
Percentage among patients treated with ventilatory support. Both NIV and VAM may be used in the same patient.
Short‐term MCS devices were implanted in 62% of patients overall, specifically in 75.8% of AMI‐CS vs. 46.6% of ADHF‐CS patients (P < 0.001). AMI‐CS patients were more likely to receive two MCS devices (22.2% vs. 10.2%, P < 0.001). Intra‐aortic balloon pump (IABP) was the most frequent MCS adopted overall (85.6%), and it was used for longer time among ADHF‐CS patients [7 (IQR 3–16) vs. 3 (IQR 2–5) days, P < 0.001].
AMI‐CS patients needed invasive mechanical ventilation more frequently as compared with ADHF‐CS (83.6% vs. 57.1%, P = 0.001), whereas the rate of continuous renal replacement therapy was similar between groups (22.4% vs. 23.9%, P = 0.8).
Events and clinical outcomes
Clinical outcomes are reported in Table 4 . A total of 78 patients died during the index hospitalization, with an overall in‐hospital mortality of 41.1%. In‐hospital mortality was similar between the AMI‐CS and ADHF‐CS groups (38.6% vs. 43.8%; P = 0.5). Details on the causes of in‐hospital death according to aetiology are presented in Table S3 . The most common type of in‐hospital death was refractory CS (62.8%) followed by septic deterioration (17.9% overall: 10.3% in AMI‐CS and 25.6% in ADHF‐CS). Furthermore, mortality at last available follow‐up of 36 days (IQR 14–110) because index event was similar between both groups (43.6% AMI‐CS vs. 46.1% ADHF‐CS; P = 0.7).
Table 4.
Outcome according to the cardiogenic shock aetiology
| Overall cohort | AMI‐CS | ADHF‐CS | P value | |
|---|---|---|---|---|
| (N = 190) | (N = 101) | (N = 89) | ||
| In‐hospital mortality | 78 (41.1) | 39 (38.6) | 39 (43.8) | 0.5 |
| Mortality at last available follow‐up a | 85 (44.7) | 44 (43.6) | 41 (46.1) | 0.7 |
| LVAD | 11 (6.0) | 2 (2.0) | 9 (11.0) | 0.01 |
| Heart transplantation | 12 (6.3) | 0 (0.0) | 12 (13.5) | <0.001 |
| Bleeding events during hospital stay b | 28 (14.7) | 13 (13.9) | 14 (15.7) | 0.7 |
| BARC minor (<3b) | 9 (33.3) | 4 (30.8) | 5 (35.7) | |
| BARC major (≥3b) | 18 (66.7) | 9 (69.2) | 9 (64.3) | |
| Follow‐up after discharge, days median (IQR) c | 56 (0–200) | 56 (0–200) | 49 (5–218) | 0.8 |
| Re‐hospitalization c | 28 (25.5) | 19 (31.6) | 9 (18.0) | 0.1 |
| 30 days re‐hospitalization c | 11 (10.0) | 8 (13.3) | 3 (6.0) | 0.2 |
Data are presented as n (%).
ADHF, acute decompensated heart failure; AMI, acute myocardial infarction; BARC, Bleeding Academic Research Consortium; CS, cardiogenic shock; IQR, interquartile range; LVAD, left ventricular assist device.
Median last available follow‐up of 36 days (interquartile range 14–110).
Missing data on details on bleeding type according to BARC for one patient in AMI‐CS group. Percentages are presented on available data.
Among patients survived after index event with available data (110 patients: 60 in AMI‐CS and 50 ADHF‐CS).
Kaplan–Meyer survival analysis is reported in Figure S1 and confirmed the similar incidence of all‐cause mortality between AMI‐CS and ADHF‐CS groups (log‐rank P value = 0.8). Survival analysis according to temporary MCS utilization during in‐hospital stay and stratified for CS aetiology are presented in Figure S2A–C .
Patients with ADHF‐CS had longer hospital stay than AMI‐CS [28 (IQR 13–48) vs. 17 (IQR 9–29) days, P = 0.001].
Eleven patients (5.8%) underwent LVAD implantation (11% ADHF‐CS vs. 2% AMI‐CS, P = 0.03) while 12 ADHF patients (13.5%) underwent urgent heart transplantation during the index admission. Survival of patients treated with LVAD was 50% in AMI‐CS and 45% in ADHF‐CS (P = 0.9); details on type of indication, INTERMACS class, SCAI class at admission and causes of death in this group of patients are reported in the Table S4 . Ten out of 12 patients (83%) with ADHF‐CS who were transplanted were alive at the last‐available follow‐up.
Among patients discharged, the rate of 30 day readmission was similar between the AMI‐CS and ADHF‐CS groups (13.3% vs. 6%, P = 0.2).
Discussion
We reported all‐comers data from consecutive patients enrolled in the prospective multicentre Altshock‐2 registry. First, our analysis confirmed the increasing incidence of ADHF‐CS (57%), in line with other recent studies showing a non‐ischaemic aetiology in 40–70% of the overall cardiogenic shock patients. 4 , 7 , 12 , 13 The development of more tailored heart failure treatment is increasing life expectancy of patients with chronic heart failure worldwide 14 but inevitably leads to an increased rate of those experiencing the most dramatic form of acute heat failure within this population. 5 Notably, the in‐hospital mortality of patients with heart failure related CS in our cohort (i.e. 43.8%) was significantly higher than that reported in recent large north‐American registries (ranging between 24% and 35%) 7 , 15 and similar to that of AMI‐CS patients' group. However, our data are in line with a recent European multicentre study on ADHF‐CS. 16 Different heart transplantation allocation system's rules, a more common and earlier adoption of long‐term assistance device strategy as well as different characteristics of included population may justify these trans‐Atlantic discrepancies. For example, despite more than two‐third of heart failure patients in our cohort had a known history of cardiomyopathy, the proportion of those treated with heart failure medications at baseline, particularly the inhibitors of the renin‐angiotensin‐aldosterone system, was lower when compared with the rate previously reported in real‐world heart failure registry. 17 These data confirm that the population enrolled in the registry represented the most severe forms of HF characterized by poor haemodynamic tolerability to these drugs and limited tolerance to acute decompensation events.
Our registry demonstrated several distinctive features of patients admitted for ADHF‐CS as compared with AMI‐CS. Despite their younger age, they had more comorbidities and presented with worse renal and liver function, while a lower proportion of them matched the historical cardiogenic shock cut‐off of hypoperfusion (i.e., lactate level above 2 mmol/L) at presentation. Chronic heart failure entails compensatory adaptive mechanisms to the reduced heart pump function so that the shift from an equilibrium state to the cardiogenic shock syndrome is more subtle and less abrupt than in the AMI‐CS setting. 1 , 18 , 19 This is evidenced by the congestive profile (higher CVP at index evaluation) and worsened biventricular echocardiographic features (both structural parameters and systolic function) of ADHF‐CS as compared with the AMI‐CS group. The elevation of right filling pressures is the foremost causal factor for deterioration of kidney function and contributes to cholestasis and acute liver failure together with the reduced cardiac output in ADHF patients. 20 , 21 Treatments aiming to aggressively and early counteract this haemodynamic derangement appear necessary to improve short‐term survival of these groups of patients.
In contrast to recently published experiences derived mainly from North American countries, 3 , 22 , 23 the use of pulmonary artery catheter was infrequent in our cohort (less than one‐fifth of the patients) in favour of a clinical and echocardiography monitoring. Furthermore, a larger adoption of MCS was registered in our cohort of ADHF‐CS patients: 42% of ADHF‐CS patients were implanted with an IABP when compared with 11.3% of the ADHF‐CS group of the recently published paper by Sinha et al. 7 The chronically elevated systemic vascular resistances of heart failure patients may benefit from treatments aiming to reduce the afterload and improving the ventriculo‐arterial coupling. IABP has demonstrated to promote such mechanisms and enhance haemodynamics in the ADHF‐CS cohort unlike in the AMI‐CS one. 24 , 25 Similarly, more than half of ADHF‐CS group in our cohort received sodium nitroprusside. The high prevalence of dilated left ventricle and severe functional mitral regurgitation at index echocardiography as well as the afore‐mentioned congestive haemodynamic profile at presentation create a window of opportunity for vasodilator therapy even in this unstable setting. Nitroprusside may reduce the afterload and increase the anterograde cardiac output improving end‐organ perfusion. Moreover, it produces reduction of heart filling pressures and pulmonary decongestion that are major prognostic determinants and therapeutic target as previously underlined. 26 However, the limited residual functional reserve of end‐organs to the acute insult and the absence of a removable causal factor lead to longer length of hospital stay, longer inotropic and MCS therapies duration, and a lower percentage of patients stabilized and/or recovering in the ADHF‐CS group. 27 Up to one quarter of those who died in hospital in the heart failure group, did it because of a septic deterioration underscoring the complexity of their management and the multifactorial influence of their clinical course. Moreover, mortality of those treated with LVAD during the index hospitalization for CS was particularly high in our cohort and underlined the limitations of such a strategy in this scenario. 28 Of note, lower survival has been reported in patients who underwent LVAD implantations in unstable conditions, that is, INTERMACS Profile 1–2, as compared with those who were implanted in relatively stable or elective scenarios. 29 Furthermore, difficulties in stabilization or reversal of end‐organ dysfunction, long admission‐induced sarcopenia, and failure in adequate assessment of right ventricular functional reserve might be main determinants of this ominous prognosis.
Ultimately, our data show that patients with ADHF‐CS are very challenging as they have unique features at presentation which may theoretically drive delayed diagnosis (possibly secondary to the chronic heart failure therapy on board), and more biventricular and end‐organ dysfunction. This turns into a significantly high rate of mortality, even with heart replacement therapies, which warrants innovative approaches in this patient population. As a matter of fact, in the light of the reported figures, we envision the need for a timely and potent MCS strategy, which might also provide a reliable background for a safe transition to durable LVAD. We speculate that mortality in LVAD patients was driven by right ventricular failure, insufficient end‐organ resuscitation, and systemic frailty before implant. Moreover, the value of inotropic therapy should be re‐discussed as it is usually required for long periods and therefore might be the driver for secondary complications related to hospitalization and chronic hypoperfusion.
Limitations
Our study has several limitations. First, the small sample size of the present analysis limits the deeper inferential analyses in particular on survival predictor for each aetiological form. Besides, patients were treated according to each centre protocols that may vary and create some bias in data interpretation. Considering the limited number of observations, it was not possible to perform sensitivity analyses according to different enrolling centre, type of admission, and de novo vs. chronic heart failure ADHF‐CS to investigate some significant interaction on investigated endpoints.
However, the present work represents the first analysis derived from our multicentre Italian Altshock‐2 Registry on cardiogenic shock and gives a unique real‐world picture of contemporary management of cardiogenic shock focusing on the poorly investigated ADHF‐CS population.
Conclusions
In the present report from the Altshock‐2 Registry, we confirmed the rising prevalence of ADHF‐CS. The heart failure‐related aetiological entity is characterized by a higher prevalence of end‐organ dysfunction at presentation a longer hospital length of stay, a prolonged inotropic and MCS support, and a similar in‐hospital mortality when compared with those with AMI. Less than half of ADHF‐CS patients survived free from the need for urgent heart replacement therapies during the hospitalization for cardiogenic shock, yet with ominous prognosis among LVAD recipients. This warrants reappraisal of the clinical characteristics of these patients and implementation of new treatment strategies.
Conflict of interest
Nuccia Morici and Alice Sacco receives institutional grant support from Getinge Global US; Alice Sacco receives speaker honoraria from AstraZeneca and Menarini; Navin K. Kapur receives consulting/speaker honoraria and institutional grant support from Abbott Laboratories, Abiomed, Inc, Boston Scientific, Edwards, LivaNova, Getinge, Teleflex. and Zoll. Federico Pappalardo is a consultant for Abiomed. Fabrizio Oliva receives speaker honoraria from AstraZeneca, Viphor, Orion, and Novartis and has been involved in the advisory board of Novartis, AstraZeneca, and Bayer. Marco Marini receives consulting/speaker honoraria from AstraZeneca, Orion, and Abiomed. The other authors have nothing to declare.
Funding
None.
Supporting information
Figure S1. Kaplan‐Meyer analysis for mortality at last available follow‐up since index event according to CS aetiology.
Table S1. In‐hospital mortality according to enrolling center and aetiology. Data refers to active enrolling centres and to the 190 patients of the present analysis (excluding 48 patients with other aetiology of cardiogenic shock than AMI‐CS and ADHF related ones). Data are presented as n/N (relative %).
Table S2. Coronary artery disease characteristics and treatment of AMICS group.
Table S3. Cause of death in the overall cohort and according to CS aetiology. Data are presented as n (% on available).
Table S4. Indication, INTERMACS class at implantation, SCAI class at admission and cause of dead of patients treated with. Data are presented as n/N (%).
Figure S2. A–C. Kaplan‐Meyer survival curves according to transitory MCS (tMCS) utilization during in‐hospital stay in the overall cohort (A), AMI‐CS (B) and ADHF‐CS (C). Log rank test for comparison: p value 0.2 in overall cohort, p value 0.7 for AMI‐CS, p value 0.1 for ADHF‐CS.
Acknowledgements
We thank Dr Dario Brunelli for data management and Francesco Chietera (MD) and Mattia Garofalo (MD) for their contribution in data collection from their centre (Cardiology Unit, IRCCS Azienda Ospedaliero‐Universitaria di Bologna, Bologna, Italy).
Open access funding provided by BIBLIOSAN.
Bertaina, M. , Morici, N. , Frea, S. , Garatti, L. , Briani, M. , Sorini, C. , Villanova, L. , Corrada, E. , Sacco, A. , Moltrasio, M. , Ravera, A. , Tedeschi, M. , Bertoldi, L. , Lettino, M. , Saia, F. , Corsini, A. , Camporotondo, R. , Colombo, C. N. J. , Bertolin, S. , Rota, M. , Oliva, F. , Iannaccone, M. , Valente, S. , Pagnesi, M. , Metra, M. , Sionis, A. , Marini, M. , De Ferrari, G. M. , Kapur, N. K. , Pappalardo, F. , and Tavazzi, G. (2023) Differences between cardiogenic shock related to acute decompensated heart failure and acute myocardial infarction. ESC Heart Failure, 10: 3472–3482. 10.1002/ehf2.14510.
References
- 1. Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:1315–1341. doi: 10.1002/ejhf.1922 [DOI] [PubMed] [Google Scholar]
- 2. Iannaccone M, Albani S, Giannini F, Colangelo S, Boccuzzi GG, Garbo R, et al. Short term outcomes of impella in cardiogenic shock: A review and meta‐analysis of observational studies. Int J Cardiol 2021;324:44–51. doi: 10.1016/j.ijcard.2020.09.044 [DOI] [PubMed] [Google Scholar]
- 3. Bertaina M, Galluzzo A, Rossello X, Sbarra P, Petitti E, Prever SB, et al. Prognostic implications of pulmonary artery catheter monitoring in patients with cardiogenic shock: A systematic review and meta‐analysis of observational studies. J Crit Care 2022;69:154024. doi: 10.1016/j.jcrc.2022.154024 [DOI] [PubMed] [Google Scholar]
- 4. Berg DD, Bohula EA, Van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, et al. Epidemiology of shock in contemporary cardiac intensive care units: Data from the critical care cardiology trials network registry. Circ Cardiovasc Qual Outcomes 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017;19:1242–1254. doi: 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 6. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification expert consensus update: A review and incorporation of validation studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association. J Am Coll Cardiol 2022;79:933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 7. Sinha SS, Rosner CM, Tehrani BN, Maini A, Truesdell AG, Ben LS, et al. Cardiogenic shock from heart failure versus acute myocardial infarction: Clinical characteristics, hospital course, and 1‐year outcomes. Circ Heart Fail 2022;15:e009279. doi: 10.1161/CIRCHEARTFAILURE.121.009279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morici N, Oliva F, Ajello S, Stucchi M, Sacco A, Cipriani MG, et al. Management of cardiogenic shock in acute decompensated chronic heart failure: The ALTSHOCK phase II clinical trial. Am Heart J 2018;204:196–201. doi: 10.1016/j.ahj.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 9. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 10. Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med 2007;4:e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the bleeding academic research consortium. Circulation 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 12. Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: Single center analysis. Catheter Cardiovasc Interv 2020;96:1339–1347. doi: 10.1002/ccd.29319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sörensen NA, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv 2020;96:E213–E219. doi: 10.1002/ccd.28707 [DOI] [PubMed] [Google Scholar]
- 14. Tromp J, Ouwerkerk W, van Veldhuisen DJ, Hillege HL, Richards AM, van der Meer P, et al. A systematic review and network meta‐analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2022;10:73–84. doi: 10.1016/j.jchf.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 15. Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez‐Montfort J, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol 2022;80:185–198. doi: 10.1016/j.jacc.2022.04.049 [DOI] [PubMed] [Google Scholar]
- 16. Schrage B, Sundermeyer J, Beer BN, Bertoldi L, Bernhardt A, Blankenberg S, et al. Use of mechanical circulatory support in patients with non‐ischaemic cardiogenic shock. Eur J Heart Fail 2023;25:562–572. doi: 10.1002/ejhf.2796 [DOI] [PubMed] [Google Scholar]
- 17. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP‐HF registry. J Am Coll Cardiol 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 18. Brener MI, Rosenblum HR, Burkhoff D. Pathophysiology and advanced hemodynamic assessment of cardiogenic shock. Methodist Debakey Cardiovasc J 2021;16:7–15. doi: 10.14797/mdcj-16-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhatt AS, Berg DD, Bohula EA, Alviar CL, Baird‐Zars VM, Barnett CF, et al. De novo vs acute‐on‐chronic presentations of heart failure‐related cardiogenic shock: Insights from the critical care cardiology trials network registry. J Card Fail 2021;27:1073–1081. doi: 10.1016/j.cardfail.2021.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biegus J, Hillege HL, Postmus D, Valente MAE, Bloomfield DM, Cleland JGF, et al. Abnormal liver function tests in acute heart failure: Relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail 2016;18:830–839. doi: 10.1002/ejhf.532 [DOI] [PubMed] [Google Scholar]
- 21. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garan AR, Kanwar M, Thayer KL, Whitehead E, Zweck E, Hernandez‐Montfort J, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in‐hospital mortality. JACC Hear Fail 2020;8:903–913. doi: 10.1016/j.jchf.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 23. Thayer KL, Zweck E, Ayouty M, Garan AR, Hernandez‐Montfort J, Mahr C, et al. Invasive hemodynamic assessment and classification of in‐hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail 2020;13:334–349. doi: 10.1161/CIRCHEARTFAILURE.120.007099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morici N, Marini C, Sacco A, Tavazzi G, Saia F, Palazzini M, et al. Intra‐aortic balloon pump for acute‐on‐chronic heart failure complicated by cardiogenic shock. J Card Fail 2022;28:1202–1216. doi: 10.1016/j.cardfail.2021.11.009 [DOI] [PubMed] [Google Scholar]
- 25. Baldetti L, Pagnesi M, Gramegna M, Belletti A, Beneduce A, Pazzanese V, et al. Intra‐aortic balloon pumping in acute decompensated heart failure with hypoperfusion: From pathophysiology to clinical practice. Circ Heart Fail 2021;14:e008527. doi: 10.1161/CIRCHEARTFAILURE.121.008527 [DOI] [PubMed] [Google Scholar]
- 26. Baldetti L, Sacchi S, Pazzanese V, Calvo F, Gramegna M, Barone G, et al. Longitudinal invasive hemodynamic assessment in patients with acute decompensated heart failure‐related cardiogenic shock: A single‐center experience. Circ Heart Fail 2022;15:E008976. doi: 10.1161/CIRCHEARTFAILURE.121.008976 [DOI] [PubMed] [Google Scholar]
- 27. Hernandez‐Montfort J, Sinha SS, Thayer KL, Whitehead EH, Pahuja M, Garan AR, et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ Heart Fail 2021;14:542–552. doi: 10.1161/CIRCHEARTFAILURE.120.007924 [DOI] [PubMed] [Google Scholar]
- 28. Molina EJ, Shah P, Kiernan MS, Cornwell WK, Copeland H, Takeda K, et al. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg 2021;111:778–792. doi: 10.1016/j.athoracsur.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 29. de By TMMH, Schoenrath F, Veen KM, Mohacsi P, Stein J, Alkhamees KMM, et al. The European registry for patients with mechanical circulatory support of the European Association for Cardio‐Thoracic Surgery: Third report. Eur J Cardiothorac Surg 2022;62: doi: 10.1093/ejcts/ezac032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan‐Meyer analysis for mortality at last available follow‐up since index event according to CS aetiology.
Table S1. In‐hospital mortality according to enrolling center and aetiology. Data refers to active enrolling centres and to the 190 patients of the present analysis (excluding 48 patients with other aetiology of cardiogenic shock than AMI‐CS and ADHF related ones). Data are presented as n/N (relative %).
Table S2. Coronary artery disease characteristics and treatment of AMICS group.
Table S3. Cause of death in the overall cohort and according to CS aetiology. Data are presented as n (% on available).
Table S4. Indication, INTERMACS class at implantation, SCAI class at admission and cause of dead of patients treated with. Data are presented as n/N (%).
Figure S2. A–C. Kaplan‐Meyer survival curves according to transitory MCS (tMCS) utilization during in‐hospital stay in the overall cohort (A), AMI‐CS (B) and ADHF‐CS (C). Log rank test for comparison: p value 0.2 in overall cohort, p value 0.7 for AMI‐CS, p value 0.1 for ADHF‐CS.


