Abstract
Aims
Heart failure (HF) and sleep‐disordered breathing (SDB) frequently coexist. We aimed to compare the prognostic value of different nocturnal hypoxic burden metrics in hospitalized HF patients.
Methods and results
HF patients underwent polygraphy screening for SDB in this prospective cohort. Hypoxic burden metrics assessed using pulse oximetry included time < 90% oxygen saturation (T90), proportion of total recording time < 90% oxygen saturation (TRT90), oxygen desaturation index (ODI), and mean oxygen saturation (meanSO2). The prespecified endpoints were the composite of cardiovascular death or admission for worsening HF. This study included 764 hospitalized HF patients, 16.5% and 36.6% of whom had obstructive and central sleep apnoea, respectively. With a median follow‐up time of 2.2 years, endpoint events occurred in 410 (53.7%) patients. In univariate and multivariate analyses, T90, TRT90, and meanSO2 were substantially associated with the composite outcome, whereas ODI was not. After multivariate Cox model adjustment, patients with 5.0 ≤ T90 ≤ 52.0 min [hazard ratio (HR) 1.32, 95% confidence interval (CI): 1.02–1.71, P = 0.034] or T90 > 52.0 min (HR 1.56, 95% CI: 1.21–2.02, P = 0.001) had a greater risk of the composite outcome than those with T90 < 5.0 min. The TRT90 and T90 results were similar. Compared with meanSO2 > 95%, meanSO2 < 93% (HR 1.47, 95% CI: 1.16–1.88, P = 0.002) was correlated with adverse outcomes.
Conclusions
The hypoxic burden metrics T90, TRT90, and meanSO2, but not ODI, were independent predictors of cardiovascular death or readmission for worsening HF. Indicators of duration and severity, not just the frequency of nocturnal hypoxaemia, should be valued and considered for intervention to improve outcomes in HF patients.
Keywords: Heart failure, Sleep‐disordered breathing, Cardiovascular, Outcome, Sleep apnoea
Introduction
Sleep‐disordered breathing (SDB) influences at least half of heart failure (HF) patients and is accompanied by adverse clinical outcomes. 1 , 2 , 3 , 4 The severity of SDB is usually identified and quantified using the apnoea–hypopnoea index (AHI), which may be a risk factor for postdischarge death in patients with acute HF. 1 Pathophysiological abnormalities in patients with SDB (both obstructive and central), such as sympathetic nervous system activation, systemic inflammation, and intermittent nocturnal hypoxaemia, may accelerate adverse cardiovascular events and aggravate HF. 2 , 5 , 6 The sleep apnoea‐related hypoxic burden also contributes to the development of stroke and HF. 7 , 8
The nocturnal hypoxic burden is often assessed using a finger pulse oximeter. Time < 90% oxygen saturation (T90), an indication of nocturnal hypoxaemia, has been shown to be an independent predictor of all‐cause death in individuals with chronic stable HF. 9 In an older male community‐based cohort, T90 was also shown to be associated with cardiovascular mortality. 10 Furthermore, the obstructive sleep apnoea‐related hypoxic burden may be utilized to forecast cardiovascular death and all‐cause mortality. 11 The nocturnal hypoxic burden, caused by apnoea and hypopnoea, may be a major contributor to the impairment of the cardiovascular system by SDB. 12
There is limited research comparing the prognostic value of different nocturnal hypoxic burden indicators in hospitalized HF patients. It is unclear whether different nocturnal hypoxic burden metrics have different effects on the cardiovascular outcomes of HF patients. We hypothesized that metrics of duration and severity but not frequency of nocturnal hypoxaemia would be independent predictors of the composite outcome in hospitalized HF patients. This study attempted to determine the value of different nocturnal hypoxic burden metrics on the composite of cardiovascular death or readmission for worsening HF.
Methods
Study population and participants
In this prospective cohort study, HF patients who underwent a sleep study while hospitalized at the HF Center at Fuwai Hospital between May 2015 and July 2018 were included. The inclusion criteria were as follows: (i) signs and symptoms of increased fluid load and/or hypoperfusion and the appropriate supporting tests to confirm worsening cardiac function, such as echocardiography, natriuretic peptide levels, electrocardiography, and chest X‐ray; (ii) adults over 18 years old; and (iii) N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) values > 300 pg/mL. The exclusion criteria included heart transplantation during hospitalization, chronic obstructive pulmonary disease, acute myocarditis, in‐hospital death, sleep monitoring < 4 h, and loss to follow‐up. Patients who were currently using or had previously used home oxygen treatment or any other form of positive airway pressure therapy were also excluded. All patients completed an informed consent form, the study was performed in line with the principles of the Helsinki Declaration, and the study protocol was approved by the Fuwai Hospital Ethics Committee.
Sleep studies
Patients were screened with overnight sleep monitoring within 48 h of admission or during hospitalization. All sleep studies were performed in an unattended hospital setting using a multichannel cardiorespiratory monitor ApneaLink Plus (ResMed) for sleep monitoring. During sleep monitoring, the patient was free of hypotension (systolic blood pressure < 90 mmHg) and did not require ventilator‐assisted ventilation. The patients did not inhale oxygen during sleep monitoring, and the decision to administer oxygen therapy during the rest of the time was made according to the patient's symptoms and disease severity. From 10:00 PM to 6:00 AM, the patient's body posture, snoring, chest respiratory motion, pulse oximetry, and nasal airflow pressure were all monitored by the sleep monitor. Night shift nurses regularly checked the status of the sleep monitoring devices on the patients and made the necessary adjustments. ApneaLink Version 10.2 software automatically evaluated the collected data, and then two sleep specialists manually analysed and corrected it. The final statistical analysis only considered data with a valid analysis time of at least 4 h.
Apnoea and hypopnoea episodes were scored in accordance with the American Association of Sleep Medicine guideline recommendations. 13 An apnoea event was defined as a decrease in nasal airway pressure of 90% or greater from baseline levels lasting at least 10 s (Supporting Information, Table S1 ). A hypopnoea event was defined as a nasal airflow pressure reduction of 30% or more from baseline values followed by a 3% or greater drop in oxygen saturation that lasted for at least 10 s. AHI was defined as the number of apnoea and hypopnoea per hour of sleep. The severity of SDB was assessed by the AHI. No or mild SDB was considered an AHI of <5 or <15 events/h. Patients were classified as having central sleep apnoea if they had an AHI ≥ 15 events/h and central apnoea or hypopnoea episodes > 50%; otherwise, they were classified as having obstructive sleep apnoea. We utilized desaturation counts of at least 3%/h during sleep to calculate the oxygen desaturation index (ODI).
Hypoxic burden metrics and classifications
T90, TRT90 (proportion of total recording time < 90% oxygen saturation), ODI, and mean oxygen saturation (meanSO2) were identified as four common metrics of hypoxic burden. The research population was separated into three groups based on the tertiles of T90: T90 < 5.0 min, 5.0 ≤ T90 ≤ 52.0 min, and T90 > 52.0 min. The reference for statistical analysis was T90 < 5.0 min. Other metrics of hypoxic burden were also grouped by tertile, such as T90.
Follow‐up and outcomes
Patients participated in systematic follow‐up and data collection via outpatient visits or telephone calls after discharge until September 2019. The prespecified endpoints were identified as the composite of cardiovascular death or the first unplanned hospital admission for worsening HF. The collection and definition of endpoint events were completed and confirmed by two cardiovascular specialists.
Statistical analysis
Categorical and continuous variables for baseline characteristics are expressed as frequencies with percentages and means with standard deviations (or medians with interquartile ranges for variables with a skewed distribution), respectively. For group comparisons, analysis of variance (ANOVA) and the χ 2 test or Fisher's exact test were utilized. The Kaplan–Meier curve survival analysis and the log‐rank test employed tertile divides of the metrics for the nocturnal hypoxic burden. Both univariate and multivariate Cox models fulfilled the proportional hazards assumption. The multivariate Cox proportional risk regression model included baseline factors considered to be clinically relevant or univariately involved with outcomes. Given the number of available events, the included variables were carefully chosen. Patient characteristics from the baseline table, comorbidities, lab results, clinical assessments, and medications at discharge were all candidate variables. The multivariate Cox model was completed using stepwise forward selection with a significance threshold of 0.10 for exclusion and re‐entry. Statistics were deemed indicative of significance if P < 0.05. R 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria) software was utilized to conduct all statistical analyses.
Results
Patient characteristics
In total, 764 patients were included (Figure 1 ); 36.6% of patients had central sleep apnoea, and 16.5% of patients had obstructive sleep apnoea. The median follow‐up was 2.2 years [95% confidence interval (CI): 2.0–2.4]. During the follow‐up period, 53.7% (410/764) of patients reached the composite of cardiovascular death or hospital admission for worsening HF.
Figure 1.
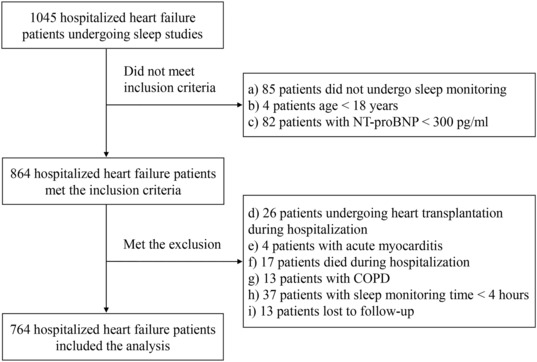
Study flowchart. COPD, chronic obstructive pulmonary disease; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Table 1 shows the characteristics of the patients. The average age was 54.7 ± 14.9 years, with a mean body mass index (BMI) of 24.9 ± 4.5 kg/m2. A total of 27.7% had diabetes, 45.7% had hypertension, more than one‐third had atrial fibrillation and coronary artery disease, and more than half had dyslipidaemia. In 10.7% (82/764) of patients, the oxygen saturation level was never <90%. Patients in the highest T90 tertile had a higher BMI, NT‐proBNP level, and New York Heart Association (NYHA) class, lower left ventricular ejection fraction, and lower kidney function (Table 1 ). Patients with endpoint events had higher T90 and TRT90, lower meanSO2, and lower minimal oxygen saturation but showed no difference in AHI or ODI values compared with those without (Table 2 ).
Table 1.
Clinical characteristics of patients according to T90
| Variable | Total (N = 764) | T90 < 5.0 min (N = 249) | 5.0 ≤ T90 ≤ 52.0 min (N = 260) | T90 > 52.0 min (N = 255) | P value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age (years) | 54.7 (14.9) | 53.0 (15.4) | 54.8 (14.0) | 56.1 (15.1) | 0.064 |
| Male, n (%) | 582 (76.2) | 182 (73.1) | 201 (77.3) | 199 (78.0) | 0.372 |
| Heart rate (b.p.m.) | 78.6 (16.7) | 77.5 (16.4) | 78.0 (16.0) | 80.2 (17.4) | 0.141 |
| BMI (kg/m2) | 24.9 (4.5) | 23.7 (3.8) | 25.0 (4.3) | 26.1 (5.1) | <0.001 |
| ≥28 kg/m2, n (%) | 168 (22.0) | 35 (14.1) | 53 (20.4) | 80 (31.4) | <0.001 |
| Current smoker, n (%) | 153 (20.0) | 41 (16.5) | 58 (22.3) | 54 (21.2) | 0.220 |
| Systolic BP (mmHg) | 117.7 (20.3) | 117.5 (20.0) | 117.6 (19.3) | 117.9 (21.5) | 0.967 |
| Diastolic BP (mmHg) | 72.7 (13.0) | 71.4 (12.5) | 72.7 (12.5) | 73.9 (13.8) | 0.090 |
| NYHA III/IV, n (%) | 645 (84.4) | 200 (80.3) | 216 (83.1) | 229 (89.8) | 0.010 |
| Comorbidities | |||||
| CAD, n (%) | 260 (34.0) | 73 (29.3) | 91 (35.0) | 96 (37.6) | 0.131 |
| Hypertension, n (%) | 349 (45.7) | 105 (42.2) | 105 (40.4) | 139 (54.5) | 0.002 |
| Diabetes, n (%) | 212 (27.7) | 59 (23.7) | 73 (28.1) | 80 (31.4) | 0.155 |
| Atrial fibrillation, n (%) | 270 (35.3) | 87 (34.9) | 88 (33.8) | 95 (37.3) | 0.712 |
| Dyslipidaemia, n (%) | 434 (56.8) | 134 (53.8) | 153 (58.8) | 147 (57.6) | 0.491 |
| Laboratory values | |||||
| Haemoglobin (g/L) | 142.9 (22.0) | 143.2 (20.8) | 143.5 (21.9) | 141.8 (23.3) | 0.647 |
| eGFR (mL/min/1.73 m2) | 71.1 (23.9) | 74.6 (24.3) | 70.4 (23.3) | 68.5 (23.9) | 0.014 |
| <60 mL/min/1.73 m2, n (%) | 244 (31.9) | 62 (24.9) | 89 (34.2) | 93 (36.5) | 0.013 |
| BUN (mg/dL) | 8.3 (4.1) | 7.7 (3.3) | 8.5 (4.5) | 8.7 (4.4) | 0.029 |
| NT‐proBNP (pg/mL) | 2768.0 (1222.3, 6788.5) | 2045.0 (1000.0, 4109.0) | 2999.5 (1148.3, 7214.8) | 4181.0 (1714.0, 8643.0) | <0.001 |
| LAD (mm) | 47.1 (8.5) | 44.7 (8.3) | 47.6 (8.4) | 48.9 (8.2) | <0.001 |
| LVEDD (mm) | 64.6 (12.2) | 62.4 (13.0) | 65.8 (11.4) | 65.5 (12.1) | 0.003 |
| LVESVI (mL/m2) | 83.8 (44.9) | 78.4 (46.6) | 87.7 (41.9) | 85.2 (45.9) | 0.054 |
| LVEDVI (mL/m2) | 124.3 (50.9) | 119.1 (53.4) | 128.0 (47.1) | 125.5 (51.9) | 0.131 |
| LAVI (mL/m2) | 49.4 (20.9) | 46.4 (18.6) | 51.1 (23.0) | 50.7 (20.5) | 0.020 |
| LVEF (%) | 36.2 (13.8) | 38.9 (14.9) | 34.5 (12.6) | 35.3 (13.4) | 0.001 |
| <40%, n (%) | 509 (66.6) | 147 (59.0) | 183 (70.4) | 179 (70.2) | 0.002 |
| 40–49%, n (%) | 104 (13.6) | 34 (13.7) | 41 (15.8) | 29 (11.4) | |
| ≥50%, n (%) | 151 (19.8) | 68 (27.3) | 36 (13.8) | 47 (18.4) | |
| Medication at discharge | |||||
| ACEIs/ARBs, n (%) | 472 (61.8) | 150 (60.2) | 167 (64.2) | 155 (60.8) | 0.601 |
| Beta‐blockers, n (%) | 705 (92.3) | 222 (89.2) | 247 (95.0) | 236 (92.5) | 0.047 |
| MRAs, n (%) | 585 (76.6) | 182 (73.1) | 214 (82.3) | 189 (74.1) | 0.026 |
| Digoxin, n (%) | 432 (56.5) | 123 (49.4) | 156 (60.0) | 153 (60.0) | 0.022 |
| Loop diuretics, n (%) | 723 (94.6) | 228 (91.6) | 254 (97.7) | 241 (94.5) | 0.009 |
| Diuretics, n (%) | 734 (96.1) | 234 (94.0) | 255 (98.1) | 245 (96.1) | 0.059 |
| Statins, n (%) | 347 (45.4) | 113 (45.4) | 122 (46.9) | 112 (43.9) | 0.791 |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LAVI, left atrial volume index; LVEDD, left ventricular end‐diastolic diameter; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index; MRAs, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; T90, time < 90% oxygen saturation.
Table 2.
Sleep variables with and without events
| Variable | Total (N = 764) | Event free (N = 354) | Incident case (N = 410) | P value |
|---|---|---|---|---|
| Apnoea–hypopnoea index, events/h | 15.5 (6.4, 29.3) | 15.2 (6.1, 29.2) | 16.1 (6.6, 29.2) | 0.856 |
| Oxygen desaturation index, events/h | 19.9 (11.4, 32.9) | 19.6 (10.2, 33.0) | 20.4 (12.0, 32.3) | 0.484 |
| T90, min | 19.5 (3.0, 83.0) | 9.0 (2.0, 59.8) | 28.0 (4.0, 94.5) | <0.001 |
| TRT90, % | 5.0 (0.6, 21.0) | 2.4 (0.4, 16.0) | 7.6 (0.9, 23.9) | <0.001 |
| Mean oxygen saturation, % | 94.0 (93.0, 96.0) | 95.0 (93.0, 96.0) | 94.0 (93.0, 96.0) | 0.004 |
| Minimal oxygen saturation, % | 79.0 (74.0, 83.0) | 80.0 (75.0, 83.0) | 79.0 (73.0, 82.0) | 0.003 |
| SDB | 0.477 | |||
| No or mild SDB, n (%) | 358 (46.9) | 166 (46.9) | 192 (46.8) | |
| Obstructive sleep apnoea, n (%) | 126 (16.5) | 64 (18.1) | 62 (15.1) | |
| Central sleep apnoea, n (%) | 280 (36.6) | 124 (35.0) | 156 (38.0) |
SDB, sleep‐disordered breathing; T90, time < 90% oxygen saturation; TRT90, proportion of total recording time < 90% oxygen saturation.
Time < 90% oxygen saturation as a predictor of the composite outcome
As T90 increases, the risk of cardiovascular death or rehospitalization for HF increases. The Kaplan–Meier curves revealed that patients with T90 in the upper tertile had significantly higher event rates for endpoint occurrence than those with T90 < 5 min (Figure 2 ). At 2 years, the composite outcome probabilities of patients with T90 < 5 min, 5.0 ≤ T90 ≤ 52.0 min, and T90 > 52.0 min were 38.6%, 49.2%, and 60.8%, respectively. In the univariate Cox model, T90 (either continuous or categorical) was substantially linked to a risk for the composite outcome. The significant association remained for the 5.0 ≤ T90 ≤ 52.0 min [hazard ratio (HR) 1.32, 95% CI: 1.02–1.71, P = 0.034] and T90 > 52.0 min (HR 1.56, 95% CI: 1.21–2.02, P < 0.001) groups after adjusting for hypertension, age, blood urea nitrogen, left ventricular end‐diastolic diameter, left atrial diameter, NT‐proBNP, loop diuretics, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers (Tables 3 and 4 ). T90 > 5.0 min was independently associated with a greater risk of the composite outcome, with an adjusted HR of 1.44 (95% CI: 1.14–1.80, P = 0.002) using T90 < 5.0 min as a reference (Supporting Information, Figure S1 ). There was no noticeable interaction between T90 and crucial clinical subgroups in our study.
Figure 2.
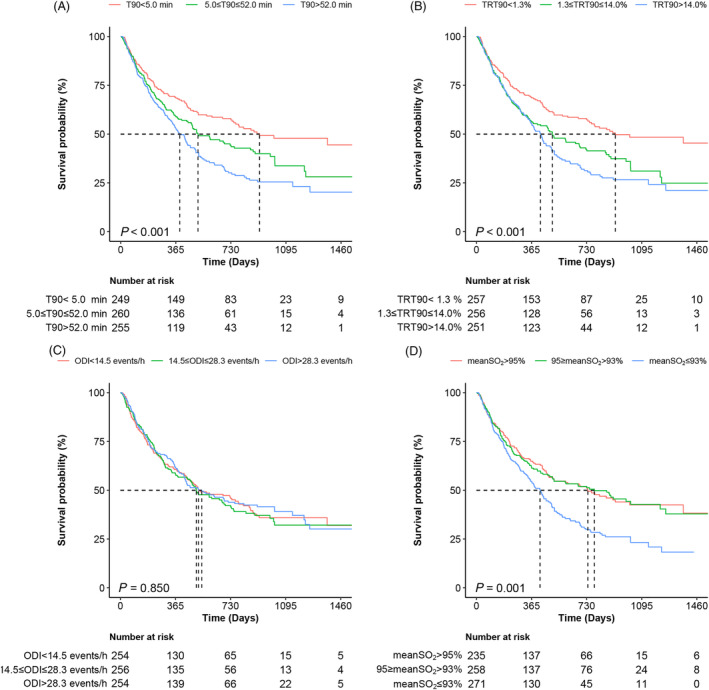
Kaplan–Meier analysis of the survival probability of patients with hypoxic burden metrics. (A) T90, time < 90% oxygen saturation; (B) TRT90, proportion of total recording time < 90% oxygen saturation; (C) ODI, oxygen desaturation index; (D) MeanSO2, mean oxygen saturation.
Table 3.
Association of T90 with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| T90 (<5.0 min) | Reference | — | Reference | — |
| T90 (5.0–52.0 min) | 1.39 (1.08–1.79) | 0.011 | 1.32 (1.02–1.71) | 0.034 |
| T90 (>52.0 min) | 1.80 (1.41–2.30) | <0.001 | 1.56 (1.21–2.02) | 0.001 |
| Age ≥ 60 years | 1.40 (1.15–1.70) | 0.001 | 1.20 (0.98–1.47) | 0.086 |
| BMI ≥ 28 kg/m2 | 0.76 (0.59–0.96) | 0.022 | — | — |
| NYHA III/IV | 1.44 (1.08–1.92) | 0.013 | — | — |
| CAD | 1.21 (0.99–1.48) | 0.068 | — | — |
| Hypertension | 0.79 (0.65–0.96) | 0.017 | 0.77 (0.63–0.95) | 0.014 |
| Atrial fibrillation | 1.37 (1.12–1.67) | 0.002 | — | — |
| BUN, mg/dL | 1.06 (1.04–1.08) | <0.001 | 1.04 (1.01–1.06) | 0.002 |
| eGFR < 60, mL/min/1.73 m2 | 1.48 (1.21–1.81) | <0.001 | — | — |
| LAD, mm | 1.03 (1.02–1.04) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| LVEDD, mm | 1.00 (0.99–1.01) | 0.849 | 0.99 (0.98–1.00) | 0.045 |
| LnNT‐proBNP, pg/mL | 1.43 (1.31–1.56) | <0.001 | 1.24 (1.12–1.36) | <0.001 |
| LVEF | 0.028 | — | ||
| 40–49% | 0.71 (0.52–0.97) | 0.031 | — | — |
| ≥50% | 1.15 (0.91–1.45) | 0.256 | — | — |
| ACEIs/ARBs | 0.52 (0.43–0.64) | <0.001 | 0.66 (0.54–0.82) | <0.001 |
| Beta‐blockers | 0.93 (0.66–1.33) | 0.709 | — | — |
| MRAs | 0.87 (0.69–1.08) | 0.866 | — | — |
| Digoxin | 1.00 (0.82–1.22) | 0.998 | — | — |
| Loop diuretics | 0.89 (0.59–1.35) | 0.588 | 0.62 (0.41–0.95) | 0.028 |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; T90, time < 90% oxygen saturation.
Table 4.
Association of nocturnal hypoxic burden metrics with the composite outcome by Cox regression
| Variable | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| T90 (85 min, per SD) | 1.14 (1.05–1.24) | 0.002 | 1.08 (0.99–1.18) | 0.092 |
| T90 (<5.0 min) | Reference | — | Reference | — |
| T90 (5.0–52.0 min) | 1.39 (1.08–1.79) | 0.011 | 1.32 (1.02–1.71) | 0.034 |
| T90 (>52.0 min) | 1.80 (1.41–2.30) | <0.001 | 1.56 (1.21–2.02) | 0.001 |
| TRT90 (22%, per SD) | 1.18 (1.08–1.29) | <0.001 | 1.11 (1.02–1.22) | 0.018 |
| TRT90 (<1.3%) | Reference | — | Reference | — |
| TRT90 (1.3–14.0%) | 1.50 (1.17–1.92) | 0.001 | 1.38 (1.07–1.78) | 0.013 |
| TRT90 (>14.0%) | 1.75 (1.37–2.24) | <0.001 | 1.51 (1.17–1.95) | 0.002 |
| ODI (15.3 events/h, per SD) | 1.00 (0.91–1.10) | 0.972 | 1.00 (0.91–1.11) | 0.936 |
| ODI (<14.5 events/h) | Reference | — | Reference | — |
| ODI (14.5–28.3 events/h) | 1.04 (0.82–1.31) | 0.767 | 0.99 (0.77–1.26) | 0.905 |
| ODI (>28.3 events/h) | 0.97 (0.76–1.23) | 0.794 | 0.96 (0.75–1.22) | 0.716 |
| MeanSO2 (4.6%, per SD) | 0.89 (0.83–0.96) | 0.001 | 0.90 (0.84–0.96) | 0.002 |
| MeanSO2 (>95%) | Reference | — | Reference | — |
| MeanSO2 (93–95%) | 1.02 (0.79–1.32) | 0.858 | 1.07 (0.82–1.38) | 0.623 |
| MeanSO2 (<93%) | 1.54 (1.22–1.96) | <0.001 | 1.47 (1.16–1.88) | 0.002 |
CI, confidence interval; HR, hazard ratio; MeanSO2, mean oxygen saturation; ODI, oxygen desaturation index; SD, standard deviation; T90, time < 90% oxygen saturation; TRT90, proportion of total recording time < 90% oxygen saturation.
Adjusted for age (categorical), hypertension, blood urea nitrogen, left ventricular end‐diastolic diameter, left atrial diameter, N‐terminal pro‐brain natriuretic peptide, loop diuretics, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers.
Proportion of total recording time < 90% oxygen saturation as a predictor of the composite outcome
Similar to the T90 Kaplan–Meier curve, the composite outcome event rate was considerably higher in the 1.3 ≤ TRT90 ≤ 14% and TRT90 > 14% patient groups than in the TRT90 < 1.3% group. The composite outcome event rates for patients with TRT90 < 1.3%, 1.3 ≤ TRT90 ≤ 14%, and TRT90 > 14% were 38.5%, 51.2%, and 59.4%, respectively, at 2 years. In multivariate Cox models, compared with those with TRT90 < 1.3%, patients with 1.3 ≤ TRT90 ≤ 14% (HR 1.38, 95% CI: 1.07–1.78, P = 0.013) and TRT90 > 14% (HR 1.51, 95% CI: 1.17–1.95, P = 0.002) had an increased risk of the composite outcome event (Table 4 and Supporting Information, Table S2 ). For every 22% (per standard deviation) increase in TRT90, the risk of a composite outcome event increased by 11% (HR 1.11, 95% CI: 1.02–1.22, P = 0.018).
Oxygen desaturation index as a predictor of the composite outcome
The 2 year composite outcome event rates were similar among the different ODI groups, as shown in Figure 2 . In univariate and multivariate Cox models, ODI as a categorical and continuous variable was not significantly associated with the composite outcome event (Table 4 and Supporting Information, Table S3 ). After multivariate adjustment, 14.5 ≤ ODI ≤ 28.3 events/h (HR 0.99, 95% CI: 0.77–1.26, P = 0.905) and ODI > 28.3 events/h (HR 0.96, 95% CI: 0.75–1.22, P = 0.716) were not linked to an elevated risk of composite outcome events compared with ODI < 14.5 events/h (Table 4 ).
Mean oxygen saturation as a predictor of the composite outcome
Figure 2 shows that patients with 93 ≤ meanSO2 ≤ 95% had no difference in composite outcome events at 2 years compared with patients with a meanSO2 > 95%, whereas patients with a meanSO2 < 93% experienced more events. Only a meanSO2 < 93% (HR 1.47, 95% CI: 1.16–1.88, P = 0.002) was correlated with a considerably greater risk of composite outcome events in the multivariate Cox models (Table 4 and Supporting Information, Table S4 ). The risk of the composite endpoint event declined by 10% (HR 0.90, 95% CI: 0.84–0.96, P = 0.002) for every 4.6% (per standard deviation) increase in the meanSO2.
Association of nocturnal hypoxic burden metrics
T90 had various correlations with other sleep monitoring metrics, such as the AHI, ODI, TRT90, and meanSO2. The correlation coefficients of T90 with AHI, ODI, and TRT90 were 0.54, 0.61, and 0.98, respectively. T90 and meanSO2 were negatively correlated (Spearman's correlation: r = −0.8, P < 0.01). The scatterplots of AHI and T90 are shown in Supporting Information, Figure S3 .
Restricted cubic spline curve analysis of the composite outcome
Restrictive cubic splines are shown for different metrics of hypoxic burden (Figure 3 and Supporting Information, Figure S2 ). The splines showed similar trends in T90 and TRT90, with an increase in the composite outcome for patients with increased T90 or TRT90, adjusted for the same variables as in the multivariate Cox model. For T90 and TRT90, 20 min and 5% were the optimal cut‐off thresholds, respectively. With increasing ODI, no change in the composite outcomes was observed. The optimal cut‐off point for the meanSO2 was 94%, as shown in Figure 3 .
Figure 3.
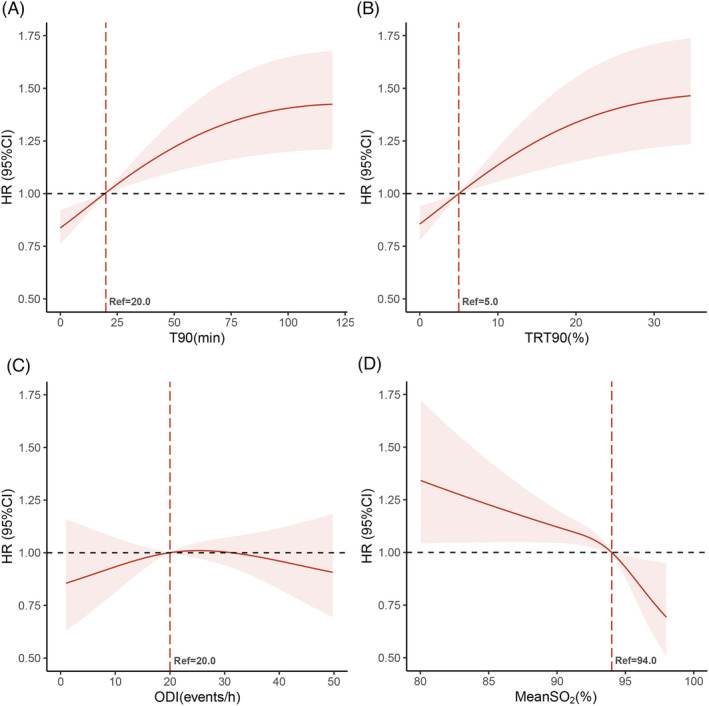
Restricted cubic splines of the hazard ratios (HRs) for the composite outcome among hypoxic burden metrics. (A) T90, time < 90% oxygen saturation; (B) TRT90, proportion of total recording time < 90% oxygen saturation; (C) ODI, oxygen desaturation index; (D) MeanSO2, mean oxygen saturation. Adjusted for age (categorical), hypertension, blood urea nitrogen, left ventricular end‐diastolic diameter, left atrial diameter, N‐terminal pro‐brain natriuretic peptide, loop diuretics, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers. CI, confidence interval.
Discussion
In the current study, we address variations in the prognostic impact of different nocturnal hypoxic burden metrics in patients with HF for the composite of cardiovascular death or rehospitalization for worsening HF. T90 and TRT90 were shown to be substantially and independently linked to the composite outcome but not with ODI. T90 and TRT90 are independent predictors of composite outcome events in patients with HF. Moreover, a meanSO2 < 93% was associated with more composite outcome events than a meanSO2 > 95%. In HF patients, nocturnal hypoxaemia duration and severity, but not frequency, were independent predictors of the composite outcome.
T90 is a reliable predictor of prognosis in HF and SDB patients. Oldenburg et al. observed that the severity of T90 was an independent predictor of prognosis and that T90 was connected to higher all‐cause mortality in patients with stable HF. 9 T90 was also a robust independent predictor of poor outcomes in hospitalized patients with HF in our research. In comparison with the cohort investigated by Oldenburg et al., the current study population was younger, had a lower BMI, and had longer durations of hypoxaemia. This could be attributed to the research population's higher NYHA class, heavier fluid load, and longer circulation time. 14 It is more likely that the association between hypoxaemia and HF is bidirectional, with fluid retention in HF promoting the development of hypoxaemia, which in turn contributes to the progression of HF. Furthermore, in community‐dwelling older men, T90 was an independent predictor of cardiovascular mortality, and its prognostic value was unaffected by either the presence or severity of AHI and SDB. 10 The underprediction of SDB severity by the AHI may be due to the frequency of sleep apnoea not fully reflecting the disease burden and hypoxaemia consequences. 15
TRT90 represents the severity of the hypoxic burden and is associated with adverse cardiovascular outcomes. The SHHS study showed an association between sleep‐related intermittent hypoxaemia and all‐cause mortality in younger men. 16 Similarly, severe blood oxygen desaturation was independently associated with all‐cause mortality in older men in the MrOS study. 17 Combining both cohorts revealed that hypoxic burden was a predictor of cardiovascular mortality in adults older than age 40. 11 Notably, the thresholds and definitions of ‘hypoxic burden’ differ, as do the disease cohorts. More prospective HF cohorts are needed to determine the strength and prognostic value of the association of TRT90 with HF rehospitalization or cardiovascular death events.
The current research found no association between ODI and cardiovascular mortality in hospitalized HF patients, similar to previous findings in chronic HF. 9 Although the ODI is indicative of intermittent hypoxaemia, it does not offer in‐depth or lengthy information on hypoxic events. In contrast, Eiichi Watanabe et al. reported that the proportion of recorded time to 4% desaturation events was an independent prognostic predictive predictor in 112 HF patients with combined central sleep apnoea. 18 Furthermore, Barnabas Gellen et al. found that the number of desaturations < 90%/h was related to adverse outcomes in patients with HF with reduced ejection fraction. 19 The ODI and AHI were moderately correlated with T90, implying that event frequency‐based metrics may not effectively represent the severity of the hypoxic burden.
A lower meanSO2 is associated with a higher risk of adverse composite outcomes. This study showed that hospitalized HF patients with a meanSO2 < 93% had a higher risk of the composite outcome than those with a meanSO2 > 95%. In a longitudinal cohort study of obstructive sleep apnoea, a meanSO2 < 93% and minimal oxygen saturation < 78% were associated with a 2.93‐fold and 2.60‐fold risk of sudden cardiac death, respectively. 20 Multiple oxygen saturation metrics reflecting hypoxic burden are reliable predictors of sudden cardiac death. 20 A 93% ≤ meanSO2 ≤ 95% brought no increased risk of events, suggesting that a certain threshold of hypoxaemia is required to cause adverse cardiovascular outcomes. A meanSO2 < 93% (HR 1.7, 95% CI: 1.3–2.3, P < 0.001) has been linked to the highest risk of death in chronic kidney disease patients. 21
Hypoxic burden metrics due to sleep apnoea and hypopnoea can also be increased by hypoxia–reoxygenation effects, and other mechanisms such as pulmonary venous congestion or pulmonary oedema, prolonged circulation time, and subclinical lung disease may also increase the hypoxic burden. The nocturnal hypoxic burden caused by apnoea and hypopnoea may more precisely indicate the adverse effects of SDB on cardiovascular outcomes and may have risk prediction and therapeutic implications. In one study, brain natriuretic peptide increased by 9.6% (95% CI: 1.5–17.7%, P = 0.02) for every 10% increase in T90 in HF patients with reduced ejection function, but there was no relationship between brain natriuretic peptide level and apnoea or hypopnoea episodes. 22 The current treatment for SDB focuses on reducing nocturnal apnoea and hypopnoea and concomitant desaturation events. The presence of daytime central apnoea or Cheyne–Stokes respiration in patients with HF has been associated with poor prognosis. 23 , 24 This finding indicates that continuous positive airway pressure targeting nocturnal sleep apnoea is insufficient in patients with HF. The use of AHI‐guided positive airway pressure therapy to prevent cardiovascular disease and improve survival remains controversial. 25 , 26 Furthermore, adaptive servo‐ventilation increases cardiovascular mortality in HF patients with predominantly central sleep apnoea. 27
Reducing the nocturnal hypoxic burden is also a sign of the efficacy of SDB treatment. Nocturnal oxygen supplementation reduced the AHI in obstructive sleep apnoea by a mean of 17.9 events/h and significantly improved oxygen saturation and T90, even though there was no change in subjective sleep quality or cognitive performance. 28 In addition, in patients with HF and Cheyne–Stokes breathing, nocturnal oxygen therapy improves not only sympathetic activation but also sleep, 29 cognitive function, and exercise tolerance. 30 In the CHF‐HOT trial, nocturnal low‐flow oxygen improved the AHI, nocturnal hypoxaemia, left ventricular ejection fraction, and quality of life in HF patients with SDB. 31 These findings imply that nocturnal oxygen therapy may be an alternative treatment to positive‐pressure ventilation.
The role of oxygen therapy in acute HF is controversial. 12 , 32 Oxygen therapy does not improve in‐hospital mortality in acute HF patients without hypoxaemia. 33 The use of hyperoxia in patients with HF increases systemic vascular resistance, left ventricular filling pressure, and the impact of cardiac relaxation and cardiac output. 34 , 35 Nocturnal oxygen therapy treats SDB associated with HF, and clinical trial researchers are studying its effect on mortality and cardiovascular outcomes. 12 Moreover, buspirone and transvenous nerve stimulation effectively treat central sleep apnoea and hypoxaemia. 36 , 37 , 38 These novel therapeutic approaches may become new options for the treatment of HF in patients with SDB.
Limitations
In this prospective cohort study, we first evaluated the impact of different nocturnal hypoxic burden indicators on the prognosis of hospitalized patients with HF. Our participants were from the most prestigious cardiovascular hospital in Asia, and most HF patients were tertiary referrals from all over the country. The patient's symptoms were severe and complex, and the findings of our research may not be generalizable to other populations. Second, we did not analyse the composition of the hypoxic burden, and the features of the hypoxic burden may vary among patients with different types of sleep apnoea. We also did not assess the degree of daytime hypoxaemia in HF patients. Third, T90 may be increased due to episodes of hypoxia–reoxygenation, apnoea and hypopnoea events, pulmonary venous congestion, or pulmonary oedema and for longer periods due to other mechanisms of hypoxaemia. Fourth, we did not exclude or record which patients received hypoxaemia‐related therapy following discharge. Finally, due to the limitations of the observational study design, unknown potentially confounding factors may influence the study's results, even after adjusting for demographics, comorbidities, and medications.
Conclusions
In conclusion, we found that different metrics of nocturnal hypoxic burden have different prognostic values in HF patients. The duration and severity but not frequency of nocturnal hypoxaemia can be used to better assess the prognosis of HF patients with SDB. The nocturnal hypoxic burden metrics T90 and TRT90 are independent predictors of the composite of cardiovascular death or readmission for worsening HF in HF patients. Considering the high prevalence of SDB among HF patients, the degree of nocturnal hypoxic burden should be of concern. Screening for SDB and assessing the nocturnal hypoxic burden should be part of the management of patients with HF. Further prospective randomized controlled trials are necessary to determine whether minimizing the nocturnal hypoxic burden improves clinical outcomes in HF patients.
Conflict of interest
None declared.
Funding
This work was supported by the Key Projects in the National Science and Technology Pillar Program of the 13th Five‐Year Plan Period (grant number 2017YFC1308300), Beijing, People's Republic of China; the Key Projects in the National Science and Technology Pillar Program of the 12th Five‐Year Plan Period (grant number 2011BAI11B08), Beijing, People's Republic of China; and the CAMS Innovation Fund for Medical Science (grant number 2020‐I2M‐1‐002).
Supporting information
Table S1: Various definitions and terms related to sleep disordered breathing.
Table S2: Association of TRT90 with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression.
Table S3: Association of ODI with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression.
Table S4: Association of meanSO2 with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression. MeanSO2, mean oxygen saturation. Abbreviations as in Table 3.
Figure S1: Subgroup analysis of T90 and the composite outcome.
Figure S2: Unadjusted restricted cubic splines of the hazard ratios for the composite outcome among hypoxic burden metrics.
Figure S3: Scatterplots of AHI and T90. AHI, apnea‐hypopnea index; T90, time < 90% oxygen saturation.
Acknowledgements
Thanks to the statisticians, cardiologists, and sleep specialists for their valuable advice and help and to all HFCU staff and patients who participated in the study.
Huang, B. , Huang, Y. , Zhai, M. , Zhou, Q. , Ji, S. , Liu, H. , Zhuang, X. , Zhang, Y. , and Zhang, J. (2023) Association of hypoxic burden metrics with cardiovascular outcomes in heart failure and sleep‐disordered breathing. ESC Heart Failure, 10: 3504–3514. 10.1002/ehf2.14526.
Contributor Information
Yuhui Zhang, Email: yuhuizhangjoy@163.com.
Jian Zhang, Email: fwzhangjian62@126.com.
References
- 1. Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, et al. Sleep disordered breathing and post‐discharge mortality in patients with acute heart failure. Eur Heart J 2015;36:1463‐1469. doi: 10.1093/eurheartj/ehu522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered breathing and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol 2021;78:608‐624. doi: 10.1016/j.jacc.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 3. Lévy P, Naughton MT, Tamisier R, Cowie MR, Bradley TD. Sleep apnoea and heart failure. Eur Respir J 2022;59:2101640. doi: 10.1183/13993003.01640-2021 [DOI] [PubMed] [Google Scholar]
- 4. Cowie MR, Gallagher AM. Sleep disordered breathing and heart failure: What does the future hold? JACC Heart Fail 2017;5:715‐723. doi: 10.1016/j.jchf.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 5. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El‐Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021;144:e56‐e67. doi: 10.1161/CIR.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Fan J, Guo R, Hao W, Gong W, Yan Y, et al. Association of OSA with cardiovascular events in women and men with acute coronary syndrome. Eur Respir J 2023;61:2201110. doi: 10.1183/13993003.01110-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azarbarzin A, Sands SA, Taranto‐Montemurro L, Vena D, Sofer T, Kim S‐W, et al. The sleep apnea‐specific hypoxic burden predicts incident heart failure. Chest 2020;158:739‐750. doi: 10.1016/j.chest.2020.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanchard M, Gervès‐Pinquié C, Feuilloy M, Le Vaillant M, Trzepizur W, Meslier N, et al. Hypoxic burden and heart rate variability predict stroke incidence in sleep apnoea. Eur Respir J 2021;57:2004022. doi: 10.1183/13993003.04022-2020 [DOI] [PubMed] [Google Scholar]
- 9. Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J 2016;37:1695‐1703. doi: 10.1093/eurheartj/ehv624 [DOI] [PubMed] [Google Scholar]
- 10. Baumert M, Immanuel SA, Stone KL, Litwack Harrison S, Redline S, Mariani S, et al. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community‐dwelling men. Eur Heart J 2020;41:533‐541. doi: 10.1093/eurheartj/ehy838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azarbarzin A, Sands SA, Stone KL, Taranto‐Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease‐related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 2019;40:1149‐1157. doi: 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeineddine S, Rowley JA, Chowdhuri S. Oxygen therapy in sleep‐disordered breathing. Chest 2021;160:701‐717. doi: 10.1016/j.chest.2021.02.017 [DOI] [PubMed] [Google Scholar]
- 13. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597‐619. doi: 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yumino D, Redolfi S, Ruttanaumpawan P, Su M‐C, Smith S, Newton GE, et al. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010;121:1598‐1605. doi: 10.1161/CIRCULATIONAHA.109.902452 [DOI] [PubMed] [Google Scholar]
- 15. Linz D, Baumert M, Catcheside P, Floras J, Sanders P, Lévy P, et al. Assessment and interpretation of sleep disordered breathing severity in cardiology: Clinical implications and perspectives. Int J Cardiol 2018;271:281‐288. doi: 10.1016/j.ijcard.2018.04.076 [DOI] [PubMed] [Google Scholar]
- 16. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep‐disordered breathing and mortality: A prospective cohort study. PLoS Med 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smagula SF, Stone KL, Redline S, Ancoli‐Israel S, Barrett‐Connor E, Lane NE, et al. Actigraphy‐ and polysomnography‐measured sleep disturbances, inflammation, and mortality among older men. Psychosom Med 2016;78:686‐696. doi: 10.1097/PSY.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe E, Kiyono K, Matsui S, Somers VK, Sano K, Hayano J, et al. Prognostic importance of novel oxygen desaturation metrics in patients with heart failure and central sleep apnea. J Card Fail 2017;23:131‐137. doi: 10.1016/j.cardfail.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gellen B, Canouï‐Poitrine F, Boyer L, Drouot X, Le Thuaut A, Bodez D, et al. Apnea‐hypopnea and desaturations in heart failure with reduced ejection fraction: Are we aiming at the right target? Int J Cardiol 2016;203:1022‐1028. doi: 10.1016/j.ijcard.2015.11.108 [DOI] [PubMed] [Google Scholar]
- 20. Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, et al. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J Am Coll Cardiol 2013;62:610‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jhamb M, Ran X, Abdalla H, Roumelioti M‐E, Hou S, Davis H, et al. Association of sleep apnea with mortality in patients with advanced kidney disease. Clin J Am Soc Nephrol 2020;15:182‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gottlieb JD, Schwartz AR, Marshall J, Ouyang P, Kern L, Shetty V, et al. Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol 2009;54:1706‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Emdin M, Mirizzi G, Giannoni A, Poletti R, Iudice G, Bramanti F, et al. Prognostic significance of central apneas throughout a 24‐hour period in patients with heart failure. J Am Coll Cardiol 2017;70:1351‐1364. [DOI] [PubMed] [Google Scholar]
- 24. Giannoni A, Gentile F, Sciarrone P, Borrelli C, Pasero G, Mirizzi G, et al. Upright Cheyne‐Stokes respiration in patients with heart failure. J Am Coll Cardiol 2020;75:2934‐2946. [DOI] [PubMed] [Google Scholar]
- 25. Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005;353:2025‐2033. doi: 10.1056/NEJMoa051001 [DOI] [PubMed] [Google Scholar]
- 26. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016;375:919‐931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 27. Cowie MR, Woehrle H, Wegscheider K, Angermann C, D'Ortho M‐P, Erdmann E, et al. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095‐1105. doi: 10.1056/NEJMoa1506459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan L, Li T, Zhang Y, He D, Luo L, Lei F, et al. Effect of one night of nocturnal oxygen supplementation on highland patients with OSA: A randomized, crossover trial. Chest 2021;160:690‐700. doi: 10.1016/j.chest.2021.02.046 [DOI] [PubMed] [Google Scholar]
- 29. Staniforth AD, Kinnear WJ, Starling R, Hetmanski DJ, Cowley AJ. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne‐Stokes respiration. Eur Heart J 1998;19:922‐928. doi: 10.1053/euhj.1997.0861 [DOI] [PubMed] [Google Scholar]
- 30. Andreas S, Clemens C, Sandholzer H, Figulla HR, Kreuzer H. Improvement of exercise capacity with treatment of Cheyne‐Stokes respiration in patients with congestive heart failure. J Am Coll Cardiol 1996;27:1486‐1490. [DOI] [PubMed] [Google Scholar]
- 31. Nakao YM, Ueshima K, Yasuno S, Sasayama S. Effects of nocturnal oxygen therapy in patients with chronic heart failure and central sleep apnea: CHF‐HOT study. Heart Vessels 2016;31:165‐172. doi: 10.1007/s00380-014-0592-6 [DOI] [PubMed] [Google Scholar]
- 32. Sepehrvand N, Ezekowitz JA. Oxygen therapy in patients with acute heart failure: Friend or foe? JACC Heart Fail 2016;4:783‐790. doi: 10.1016/j.jchf.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 33. Yu Y, Yao R‐Q, Zhang Y‐F, Wang S‐Y, Xi W, Wang J‐N, et al. Is oxygen therapy beneficial for normoxemic patients with acute heart failure? A propensity score matched study. Mil Med Res 2021;8:38. doi: 10.1186/s40779-021-00330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol 1996;27:353‐357. [DOI] [PubMed] [Google Scholar]
- 35. Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest 2001;120:467‐473. doi: 10.1378/chest.120.2.467 [DOI] [PubMed] [Google Scholar]
- 36. Giannoni A, Borrelli C, Mirizzi G, Richerson GB, Emdin M, Passino C. Benefit of buspirone on chemoreflex and central apnoeas in heart failure: A randomized controlled crossover trial. Eur J Heart Fail 2021;23:312‐320. doi: 10.1002/ejhf.1854 [DOI] [PubMed] [Google Scholar]
- 37. Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, et al. Transvenous neurostimulation for central sleep apnoea: A randomised controlled trial. Lancet 2016;388:974‐982. doi: 10.1016/S0140-6736(16)30961-8 [DOI] [PubMed] [Google Scholar]
- 38. Oldenburg O, Costanzo MR, Germany R, McKane S, Meyer TE, Fox H. Improving nocturnal hypoxemic burden with transvenous phrenic nerve stimulation for the treatment of central sleep apnea. J Cardiovasc Transl Res 2021;14:377‐385. doi: 10.1007/s12265-020-10061-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Various definitions and terms related to sleep disordered breathing.
Table S2: Association of TRT90 with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression.
Table S3: Association of ODI with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression.
Table S4: Association of meanSO2 with the composite of cardiovascular death or worsening heart failure rehospitalization by Cox regression. MeanSO2, mean oxygen saturation. Abbreviations as in Table 3.
Figure S1: Subgroup analysis of T90 and the composite outcome.
Figure S2: Unadjusted restricted cubic splines of the hazard ratios for the composite outcome among hypoxic burden metrics.
Figure S3: Scatterplots of AHI and T90. AHI, apnea‐hypopnea index; T90, time < 90% oxygen saturation.


