Abstract
Aims
This study aimed to assess the effectiveness of adaptive servo‐ventilation (ASV) for lowering hypoxaemic burden components in heart failure with reduced ejection fraction (HFrEF) patients.
Methods and results
Fifty‐six stable HFrEF patients with left ventricular ejection fraction ≤ 40 were randomized to receive either ASV (n = 27; 25 males) or optimal medical management or optimal medical management alone (n = 29; 26 males). Patients underwent overnight polysomnography at baseline and a 12 week follow‐up visit. We quantified hypoxaemic as time spent at <90% oxygen saturation (T90) decomposed into desaturation‐related components (T90desaturation) and non‐specific drifts (T90non‐specific). In the ASV arm, T90 significantly shortened by nearly 60% from 50.1 ± 95.8 min at baseline to 20.5 ± 33.0 min at follow‐up compared with 59.6 ± 88 and 65.4 ± 89.6 min in the control arm (P = 0.009). ASV reduced the apnoea‐related component (T90desaturation) from 37.7 ± 54.5 to 2.1 ± 7.3 min vs. 37.7 ± 54.5 and 40.4 ± 66.4 min in the control arm (P = 0.008). A significant non‐specific T90 component of 19.6 ± 31.8 min persisted during ASV. In adjusted multivariable regression, T90desaturation was significantly associated with the ratio of the forced expiratory volume in the first second to the forced vital capacity of the lungs (β = 0.336, 95% confidence interval 0.080 to 0.593; P = 0.011) and T90non‐specific with left ventricular ejection fraction (β = −0.345, 95% confidence interval −0.616 to −0.073; P = 0.014).
Conclusions
ASV effectively suppresses the sleep apnoea‐related component of hypoxaemic burden in HFrEF patients. A significant hypoxaemic burden not directly attributable to sleep apnoea but related to the severity of heart failure remains and may adversely affect cardiovascular long‐term outcomes.
Keywords: Heart failure with sleep apnoea, Adaptive servo‐ventilation, Randomized controlled trial
Aims
This study aimed to assess the effectiveness of adaptive servo‐ventilation (ASV) for lowering hypoxaemic burden decomposed into desaturation‐related components (T90desaturation) and non‐specific drifts (T90non‐specific) in heart failure with reduced ejection fraction (HFrEF).
Background
In HFrEF patients, obstructive sleep apnoea (OSA) and central sleep apnoea (CSA) are highly prevalent and associated with significant morbidity and mortality. 1 , 2 ASV controls both OSA and CSA, 3 but the SERVE‐HF and ADVENT‐HF (NCT01128816) landmark trials have shown no mortality benefit. 4 Importantly, the nocturnal hypoxaemic burden is associated with cardiovascular mortality in heart failure rather than the frequency of apnoeas and hypopnoeas. 5 , 6 , 7 , 8 , 9 Possible residual hypoxaemic burden on ASV may therefore explain the lack of mortality benefit. 10 Hence, we tested the effects of ASV on desaturation‐related components and non‐specific drifts in T90 in a randomized controlled trial.
Methods
In this non‐prespecified ancillary study of the multicentre, parallel, open‐label auto‐servo‐ventilation in heart failure with sleep apnoea trial (ISRCTN04353156), 11 we analysed the nocturnal hypoxaemic burden in New York Heart Association class II–III heart failure patients with a left ventricular ejection fraction ≤ 40% and stable clinical status who presented with an apnoea/hypopnea index (AHI) ≥ 20 1/h. 12 All patients gave written informed consent to participate in the trial. The protocol was approved by the local ethics boards of each participating centre. Patients were randomly assigned to either the control group, receiving optimal medical management for heart failure, or the ASV group, receiving ASV therapy in addition to optimal medical management. ASV was titrated as described before. 12
Polysomnography was performed and analysed according to standard diagnostic criteria. 12 We characterize the composition of hypoxaemic burden with a custom‐made automated algorithm:
Oxygen desaturation index is the number of desaturations per hour of sleep (in h−1).
T90 is the accumulated sleep time spent below 90% oxygen saturation (in min).
T90desaturation is the time of sleep spent below 90% oxygen saturation associated with acute desaturation patterns of at least 4% (in min).
T90non‐specific is the time of sleep spent below 90% oxygen saturation associated with non‐specific drifts (in min).
We compared the hypoxaemic burden between the treatment and control arms at baseline and 12 weeks of follow‐up using analysis of covariance, where follow‐up measurements were the dependent variables, treatment was a fixed factor, and baseline values were included as covariates.
Results
Of the 72 patients randomized in the original study, 27 entered the ASV arm and 29 entered the control arm. Sixteen patients were excluded due to loss at follow‐up or inadequate polysomnograms (Supporting Information, Figure S1 ). Table 1 summarizes patient characteristics in both arms.
Table 1.
Baseline characteristics of patients in the control and adaptive servo‐ventilation (ASV) arms
| Total population | Control group | ASV group | P‐value | |
|---|---|---|---|---|
| Subject (n) | 56 | 29 | 27 | |
| Age (years) | 64.1 ± 9.8 | 64.5 ± 9.5 | 63.6 ± 10.2 | 0.747 |
| Males (n) | 51 (91%) | 26 (90%) | 25 (93%) | 0.393 |
| BMI (kg/m2) | 30.6 ± 4.8 | 31.7 ± 5.0 | 29.4 ± 4.3 | 0.071 |
| Overweight | 23 (41.7%) | 12 (41%) | 11 (41%) | 0.961 |
| Obese | 28 (50%) | 17 (59%) | 11 (41%) | 0.178 |
| FEV1/FVC ratio | 0.774 ± 0.079 | 0.773 ± 0.082 | 0.776 ± 0.077 | 0.884 |
| Medical history | ||||
| Afib | 16 (28.6%) | 11 (38%) | 5 (19%) | 0.099 |
| MI | 29 (51.8%) | 15 (52%) | 14 (52%) | 0.782 |
| Angina | 10 (17.9%) | 4 (14%) | 6 (21%) | 0.822 |
| HT | 27 (48.2%) | 14 (48%) | 15 (48%) | 0.796 |
| OSA | 30 (53.6%) | 16 (55%) | 14 (52%) | 0.578 |
Afib, atrial fibrillation; BMI, body mass index; FEV1/FVC ratio, the ratio of the forced expiratory volume in the first second to the forced vital capacity of the lungs; HT, hypertension; MI, myocardial infarction, OSA, obstructive sleep apnoea.
P‐values represent the results of the two‐tailed t‐test and the χ 2 test.
ASV reduced the event‐driven measures of OSA and CSA: AHI reduced from 50.5 ± 18.6 to 9.2 ± 8.7 1/h in the ASV arm compared with no change in the control arm (46.2 ± 19 vs. 46.4 ± 23.1 1/h) (P < 0.001). Similarly, the oxygen desaturation index dropped significantly from 15.8 ± 16.4 1/h on average by 79% in the ASV arm compared with steady levels in the control group (23.3 ± 18.4 vs. 23.8 ± 20.3 1/h) (P < 0.001) (Supporting Information, Table S1 ).
In the ASV arm, T90 shortened nearly 60% from 50.1 ± 95.8 min at baseline to 20.5 ± 33.0 min at follow‐up compared with a 10% prolongation from 59.6 ± 88 to 65.4 ± 89.6 min in the control arm (P = 0.009) (Figure 1 ). ASV supressed T90 associated with frank apnoeas and hypopnoeas (T90desaturation) from 19.9 ± 40.4 min at baseline to 2.1 ± 7.3 min at follow‐up compared with 37.7 ± 54.5 and 40.4 ± 66.4 min in the control arm (P = 0.008). In contrast, non‐specific contributions to T90 (T90non‐specific), accounting for 37.7 ± 54.5 and 19.9 ± 40.4 min at baseline in the control and treatment arms, respectively, were not significantly suppressed by ASV (P = 0.249).
Figure 1.
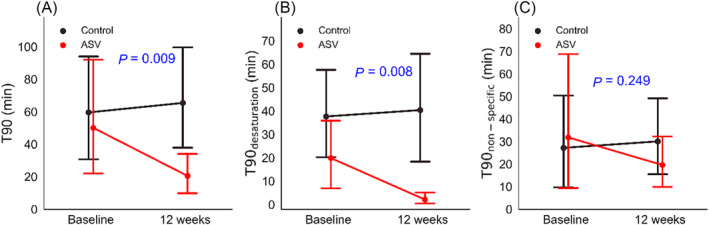
Nocturnal hypoxaemic burden in heart failure patients randomized to adaptive servo‐ventilation (ASV) and standard treatment (control) at baseline and 12 weeks of follow‐up quantified as T90 (A), T90 due to apnoea‐related desaturations (B), and non‐specific drifts in SpO2 (T90non‐specific) (C).
All components of T90 correlated with body mass index (Supporting Information, Table S2 ), T90desaturation was associated with the ratio of the forced expiratory volume in the first second to the forced vital capacity of the lungs (FEV1/FVC ratio), and T90non‐specific was higher in patients with very low left ventricular ejection fraction (Figure 2 ). Multivariable regression (Supporting Information, Table S3 ) corroborated the significant association between left ventricular ejection fraction and T90non‐specific and T90desaturation and the FEV1/FVC ratio.
Figure 2.
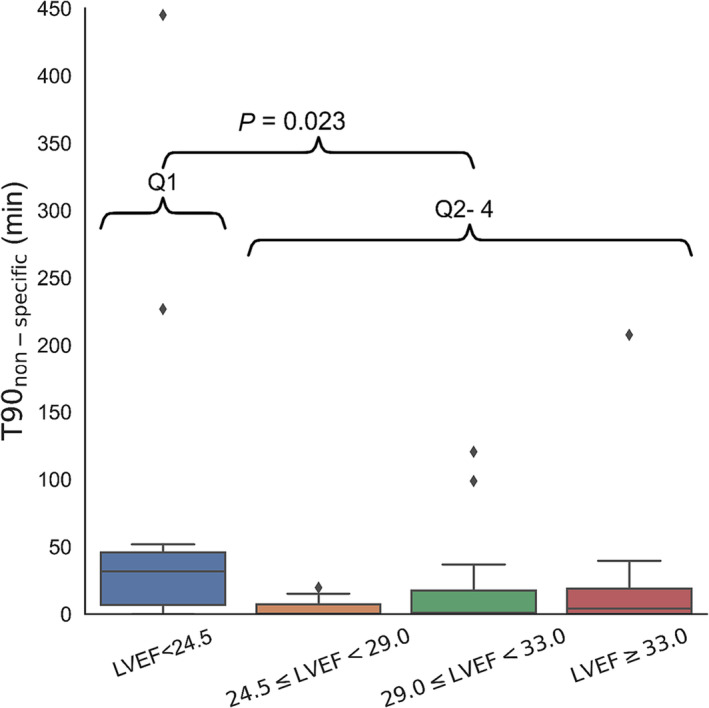
Non‐specific nocturnal hypoxaemic burden across patient quartiles with different severity of left ventricular dysfunction. LVEF, left ventricular ejection fraction.
Conclusions
By decomposing T90 into components that primarily relate to apnoeas and hypopnoeas and non‐specific reductions in oxygen saturation levels, we demonstrate that ASV effectively reduces the OSA‐ and CSA‐related hypoxaemic burden in HFrEF patients, but a significant non‐specific hypoxaemic burden remains.
A previous uncontrolled study of different CSA aetiologies showed that neither ASV nor CPAP suppresses T90non‐specific effectively with a considerable persisting nocturnal hypoxaemic burden. 10 At baseline, T90 varied between 70 and 110 min on average, and ASV reduced that time by ~35–55%. In SERVE‐HF, T90 was approximately 50 min at baseline. ASV treatment only shortened it to 18–20 min, 4 comparable with our data, in which the non‐specific drifts contributed about half of the total T90. Considering a cohort study in chronic HFrEF patients that showed that T90 > 22 min was associated with all‐cause mortality, 5 our analysis may partly explain why ASV may not provide mortality benefits for HF patients. Our data also suggest that patients with very poor left ventricular function tended to have a higher non‐specific hypoxaemic burden (Figure 2 and Supporting Information, Table S3 ) that may be caused by chronic pulmonary congestion/pulmonary oedema. Obesity may also contribute to non‐specific hypoxaemic burden. Body mass index was comparable in both study arms and did not change significantly at follow‐up.
Our study has important implications for treating sleep apnoea in heart failure patients. ASV effectively reduces hypoxaemic burden in HFrEF patients and may even help monitor cardiac decompensation. 13 Transvenous phrenic nerve stimulation constitutes an alternative therapy for CSA, also effectively reducing AHI, heart rate perturbations, and hypoxaemic burden. 14 Decomposing hypoxaemic burden into apnoea‐related and non‐specific components as performed here also demonstrated significant non‐specific hypoxaemic burden persisting during transvenous phrenic nerve stimulation. 15 Thus, reducing the nocturnal hypoxaemic burden by addressing concomitant comorbidities, such as obesity, pulmonary disease, and chronic pulmonary congestion/pulmonary oedema, may constitute important additional treatment targets.
Our ancillary study is limited by its relatively small sample size and retrospective, hypothesis‐generating nature. The original trial was not designed to determine the cause of increased hypoxaemic burden in HFrEF patients. Consequently, assessment of other comorbidities (e.g. pulmonary disease) that may cause hypoxaemic burden was not available. Prospective studies are required to determine if reducing non‐specific hypoxaemic burden improves outcomes in HFrEF patients.
Conflict of interest
None declared.
Funding
This work was supported by grant support from Philips Home Healthcare Solutions (Murrysville, PA, USA). M.A. has received grant support from Philips Respironics, the ResMed Foundation, and the Else‐Kroener Fresenius Foundation, and lecture and consulting fees from the ResMed Foundation and Philips Respironics. The sponsor played no role in the design and conduct of the study, the analysis and interpretation of the data, or the preparation, review, and approval of the manuscript.
Supporting information
Table S1: Patient characteristics in the control arm and adaptive servo‐ventilation (ASV) arm at baseline and after 12 weeks of follow‐up. P‐values represent ANCOVA test results.
Table S2: Spearman correlation analysis of hypoxemic burden on baseline PSG and clinical variables. For convenience, p‐values <0.1 are printed in bold.
Table S3: Multivariable regression of hypoxemic burden measures (T90, T90desaturation and T90non‐specific) with patients' body mass index, apnea‐hypopnea index, FEV1/FVC ratio, and left ventricular ejection fraction.
Figure S1: Flowchart of the auto‐servo ventilation study.
Baumert, M. , Linz, D. , Pfeifer, M. , Tafelmeier, M. , Felfeli, P. , Arzt, M. , and Shahrbabaki, S. S. (2023) Hypoxaemic burden in heart failure patients receiving adaptive servo‐ventilation. ESC Heart Failure, 10: 3725–3728. 10.1002/ehf2.14556.
References
- 1. Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered breathing and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol 2021;78:608‐624. doi: 10.1016/j.jacc.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 2. Arzt M, Oldenburg O, Graml A, Schnepf J, Erdmann E, Teschler H, et al. Prevalence and predictors of sleep‐disordered breathing in chronic heart failure: The SchlaHF‐XT registry. Eur J Heart Fail 2022;9:4100‐4111. doi: 10.1002/ehf2.14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J 2017;49:1600959. doi: 10.1183/13993003.00959-2016 [DOI] [PubMed] [Google Scholar]
- 4. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, et al. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095‐1105. doi: 10.1056/NEJMoa1506459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J 2016;37:1695‐1703. doi: 10.1093/eurheartj/ehv624 [DOI] [PubMed] [Google Scholar]
- 6. Linz D, Baumert M, Catcheside P, Floras J, Sanders P, Levy P, et al. Assessment and interpretation of sleep disordered breathing severity in cardiology: Clinical implications and perspectives. Int J Cardiol 2018;271:281‐288. doi: 10.1016/j.ijcard.2018.04.076 [DOI] [PubMed] [Google Scholar]
- 7. Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep‐disordered breathing and cardiovascular disease: An outcome‐based definition of hypopneas. Am J Respir Crit Care Med 2008;177:1150‐1155. doi: 10.1164/rccm.200712-1884OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azarbarzin A, Sands SA, Stone KL, Taranto‐Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease‐related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 2019;40:1149‐1157. doi: 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baumert M, Immanuel SA, Stone KL, Litwack Harrison S, Redline S, Mariani S, et al. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community‐dwelling men. Eur Heart J 2020;41:533‐541. doi: 10.1093/eurheartj/ehy838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linz D, Malfertheiner MV, Werner N, Lerzer C, Gfullner F, Linz B, et al. Nocturnal hypoxemic burden during positive airway pressure treatment across different central sleep apnea etiologies. Sleep Med 2021;79:62‐70. doi: 10.1016/j.sleep.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 11. Respironics Inc. (USA) . In: Respironics Inc. (USA).
- 12. Arzt M, Schroll S, Series F, Lewis K, Benjamin A, Escourrou P, et al. Auto‐servoventilation in heart failure with sleep apnoea: A randomised controlled trial. Eur Respir J 2013;42:1244‐1254. doi: 10.1183/09031936.00083312 [DOI] [PubMed] [Google Scholar]
- 13. Fox H, Rudolph V, Munt O, Malouf G, Graml A, Bitter T, et al. Early identification of heart failure deterioration through respiratory monitoring with adaptive servo‐ventilation. J Sleep Res 2023;32:e13749. doi: 10.1111/jsr.13749 [DOI] [PubMed] [Google Scholar]
- 14. Oldenburg O, Costanzo MR, Germany R, McKane S, Meyer TE, Fox H. Improving nocturnal hypoxemic burden with transvenous phrenic nerve stimulation for the treatment of central sleep apnea. J Cardiovasc Transl Res 2021;14:377‐385. doi: 10.1007/s12265-020-10061-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baumert M, Immanuel S, McKane S, Linz D. Transvenous phrenic nerve stimulation for the treatment of central sleep apnea reduces episodic hypoxemic burden. Int J Cardiol 2023;378:89‐95. doi: 10.1016/j.ijcard.2023.02.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Patient characteristics in the control arm and adaptive servo‐ventilation (ASV) arm at baseline and after 12 weeks of follow‐up. P‐values represent ANCOVA test results.
Table S2: Spearman correlation analysis of hypoxemic burden on baseline PSG and clinical variables. For convenience, p‐values <0.1 are printed in bold.
Table S3: Multivariable regression of hypoxemic burden measures (T90, T90desaturation and T90non‐specific) with patients' body mass index, apnea‐hypopnea index, FEV1/FVC ratio, and left ventricular ejection fraction.
Figure S1: Flowchart of the auto‐servo ventilation study.


