Abstract
Aims
Heart failure with reduced ejection fraction (HFrEF) is associated with excessive sympathetic and impaired parasympathetic activity. The Barostim Neo™ device is used for electronical baroreflex activation therapy (BAT) to counteract autonomic nervous system dysbalance. Randomized trials have shown that BAT improves walking distance and reduces N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) levels at least in patients with only moderate elevation at baseline. Its impact on the risk of heart failure hospitalization (HFH) and death is not yet established, and experience in clinical routine is limited.
Methods and results
We report on patient characteristics and clinical outcome in a retrospective, non‐randomized single‐centre registry of BAT in HFrEF. Patients in the New York Heart Association (NYHA) Classes III and IV with a left ventricular ejection fraction (LVEF) <35% despite guideline‐directed medical therapy were eligible. Symptom burden, echocardiography, and laboratory testing were assessed at baseline and after 12 months. Clinical events of HFH and death were recorded at routine clinical follow‐up. Data are shown as number (%) or median (inter‐quartile range). Between 2014 and 2020, 30 patients were treated with BAT. Median age was 67 (63–77) years, and 27 patients (90%) were male. Most patients (83%) had previous HFH. Device implantation was successful in all patients. At 12 months, six patients had died and three were alive but did not attend follow‐up. NYHA class was III/IV in 26 (87%)/4 (13%) patients at baseline, improved in 19 patients, and remained unchanged in 5 patients (P < 0.001). LVEF improved from 25.5 (20.0–30.5) % at baseline to 30.0 (25.0–36.0) % at 12 months (P = 0.014). Left ventricular end‐diastolic diameter remained unchanged. A numerical decrease in NT‐proBNP [3165 (880–8085) vs. 1001 (599–3820) pg/mL] was not significant (P = 0.526). Median follow‐up for clinical events was 16 (10–33) months. Mortality at 1 (n = 6, 20%) and 3 years (n = 10, 33%) was as expected by the Meta‐Analysis Global Group in Chronic Heart Failure risk score. Despite BAT, event rate was high in patients with NYHA Class IV, NT‐proBNP levels >1600 pg/mL, or estimated glomerular filtration rate (eGFR) <30 mL/min at baseline. NYHA class and eGFR were independent predictors of mortality.
Conclusions
Patients with HFrEF who are selected for BAT are in a stage of worsening or even advanced heart failure. BAT appears to be safe and improves clinical symptoms and—to a modest degree—left ventricular function. The risk of death remains high in advanced disease stages. Patient selection seems to be crucial, and the impact of BAT in earlier disease stages needs to be established.
Keywords: Chronic heart failure, Device therapy, Baroreflex activation therapy, Barostim Neo™
Introduction
Heart failure (HF) is a chronic and progressive syndrome affecting billions of patients worldwide. Despite recent major advances in medical treatment, a large proportion of patients suffering from HF with reduced ejection fraction (HFrEF) remain symptomatic and thus limited in quality of life even after treatment initialization and stabilization on therapy. Furthermore, in these putatively ‘stable’ patients, the residual risk for recurrent cardiovascular events, that is, HF hospitalization and premature death, has shown to be much higher in absolute rates compared with risk of cardiovascular events in patients, for example, with atherosclerotic cardiovascular disease. 1
The concept of worsening HF (WHF) was introduced to focus on patients with progressive disease indicated by deterioration of cardiac function, increase in biomarkers, and signs and symptoms of cardiac decompensation with need to intensify medical treatment especially with use of intravenous diuretics either in the outpatient setting or during hospitalization. 1 Hospitalization for WHF has shown to be associated with a three‐fold higher risk for mortality with the highest risk within the first months after discharge in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) trial population. 2 Similarly, 25% of patients hospitalized for WHF died or were readmitted within 30 days of discharge in a contemporary US real‐world registry. 3
Therefore, there is need for new therapeutic options beyond established medical therapy to improve symptoms and outcome in patients suffering from HFrEF with persistent limitation and progressive disease.
Whereas cardiac resynchronization therapy (CRT) is established for selected patients with left bundle branch block and wide QRS complex, 4 several device therapies are being evaluated to treat HFrEF via modulation of the autonomic nervous system (ANS) with the intention to restore the balance between sympathetic and parasympathetic nervous system function. 5 Previous studies have shown that HF is associated with excessive sympathetic nervous system activation and concomitant impairment of parasympathetic activity already early in the course of the disease. 6 , 7 , 8 The ANS is regulated by complex interaction of afferent reflexes including baroreceptor, chemoreceptor, muscle metaboreceptor, and mechanoreceptor reflexes and renal nerve reflexes, which are integrated within the central nervous system to regulate cardiac output and vital organ perfusion via efferent sympathetic and vagal signalling including ß‐adrenergic receptor stimulation, neurohumoral activation, inflammation, and nitric oxide synthesis signalling (for detailed review, see Floras and Ponikowski 9 ). The disequilibrium of the ANS in HF correlates to impaired heart rate (HR) variability (HRV), cardiomyocyte dysfunction and apoptosis, neurohumoral activation, impaired nitric oxide signalling, inflammation and susceptibility to arrhythmia, and sudden death. 5
Baroreflex activation therapy (BAT) is administered through a pacemaker‐like device with an extravascular lead that activates the carotid baroreceptor electronically, simulating an increase in blood pressure and provoking a central‐mediated increase in parasympathetic activity. 5 , 9 , 10 BAT was initially designed to treat drug‐resistant arterial hypertension. 11 In HFrEF, it has been proven to reduce N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) levels and improve New York Heart Association (NYHA) class and exercise capacity effectively in a randomized controlled trial, the BeAT‐HF trial. 12 Nevertheless, the impact of BAT on the rate of HF hospitalization and death is unknown and the experience with BAT in HFrEF in clinical routine regarding patient selection, safety, and outcome is sparse.
In a retrospective, monocentric registry, all HFrEF patients treated with BAT at the Heart Centre Dresden were included to gain information on patient selection in a real‐world setting, on safety, and on outcome.
Methods
The BAT ‐ HFrEF registry was designed as a monocentric, retrospective registry to characterize all HFrEF patients implanted with a BAT device (Barostim Neo™, CVRx Inc., MN, USA) at the Heart Centre Dresden between 2014 (when the CE mark for the treatment of HFrEF was conferred) and September 2020. The surgical procedure of device implantation followed a standardized protocol as reported elsewhere. 13 Patients were invited regularly after BAT implantation for routine clinical assessment and control of device functionality in the outpatient HF clinic. This study was in accordance with the Declaration of Helsinki and was approved by the local ethics committee (Technische Universität Dresden, BO‐EK‐204042021).
Study population
Patients were eligible for the implantation of BAT as per clinical standard: chronic HF (CHF) with left ventricular (LV) ejection fraction (LVEF ≤ 35%) and persistent dyspnoea according to NYHA Class III, IV, or II when former IV despite optimized medical treatment including the highest tolerated dose of angiotensin‐converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), or angiotensin receptor/neprilysin inhibitors (ARNIs), beta‐blockers (BBs), and mineralocorticoid receptor antagonists (MRAs) for at least 3 months and clinically stable for at least 4 weeks. Systolic blood pressure had to be at least 100 mmHg. Patients with an indication for CRT or recent CRT implantation within the last 6 months were deemed ineligible. Surgical suitability was given when at least one carotid bifurcation was below the level of the mandible and free from atherosclerotic plaques reducing the inner luminal diameter of the distal common carotid artery or the internal carotid artery by more than 50%. Decision for BAT implantation was made by the institutional multidisciplinary heart failure team in consent of the informed patient.
Data collection
Data on patient characteristics at baseline including demographics, symptoms, medical treatment, comorbidities, imaging findings, and laboratory values were compiled from clinical records. Periprocedural parameters of the BAT device implantation were taken from surgery reports and discharge letters. Follow‐up information was gathered during routine clinical care 1 month after BAT implantation and at 3, 6, 12, 24, 36, 48, and 60 months thereafter. Ultra‐short‐term HRV was calculated as standard deviation of all RR intervals (SDNN) and the root mean square of successive RR interval differences (RMSSD) from resting electrocardiograms (ECGs) before BAT implantation and at follow‐up as described previously. 14 Statistical analysis was performed with all available data up to September 2021, at least 12 months after BAT implantation in every patient.
Statistical analysis
All data were analysed using SPSS Version 27 (IBM Corporation, Chicago, IL, USA). Continuous variables are depicted as median and inter‐quartile range. Categorical variables are given as numbers and percentages. Normal distribution was tested by applying the Shapiro–Wilk test. Differences in continuous variables between baseline and 12 month follow‐up were analysed by paired Student's t‐test or non‐parametric Wilcoxon signed‐rank test, as appropriate. Categorical variables were tested by applying Fisher's exact test. Survival time analysis or event‐free time analysis regarding HF hospitalizations was evaluated with the Kaplan–Meier method by applying the log‐rank test for group comparisons. A stepwise multivariate binary logistic Cox regression analysis with survival at 3 years was used to identify predictors of survival. A P value of <0.05 was considered statistically significant.
Results
We report on patient characteristics and clinical results after 12 months of treatment. Due to small numbers, open‐label design, and missing control group, the results are descriptive. We also performed an outcome analysis including total individual follow‐up time.
Patient characteristics at baseline
Between the years 2014 and 2020, 30 patients with chronic HFrEF were treated with BAT. Table 1 shows the patients' characteristics. Median age in our cohort was 67 (63–77) years, 27 patients (90%) were male, all patients were Caucasian, and median body mass index (BMI) was 29 (26–35) kg/m2. CHF was diagnosed 5 (3–10) years before initiation of BAT. The vast majority of patients (83%) were previously hospitalized due to decompensated HF. Nearly half of the patients (47%) were hospitalized for this reason at least three times. Median LV end‐diastolic diameter (LVEDD) was enlarged with 63 (58–69) mm, and LVEF was severely reduced with 25 (20–31) %. Secondary mitral regurgitation of at least moderate severity was found in four patients (13%) and tricuspid regurgitation in nine patients (30%). History of atrial fibrillation or flutter was documented in 57% of patients. An implantable cardioverter defibrillator (ICD) was implanted in 73% of patients. One third of patients were treated with a CRT device and classified as CRT non‐responder. Most patients (87%) reported dyspnoea according to NYHA Class III, whereas four patients suffered from dyspnoea with any physical activity or intermittently at rest according to NYHA Class IV. Accordingly, NT‐proBNP levels were found to be highly elevated with 3165 (880–8085) pg/mL in median. The median estimated glomerular filtration rate (eGFR) was 55 mL/min. Twenty per cent of patients had a baseline eGFR < 30 mL/min indicating end‐organ damage. Typical comorbidities as obesity (40%), diabetes mellitus (53%), and arterial hypertension (67%) were seen frequently.
Table 1.
Baseline demographic characteristics and treatment
| Variables (n = 30) | Median (IQR)/n (%) |
|---|---|
| Age (years) | 67 (63–77) |
| Male gender (n) | 27 (90) |
| Caucasian race (n) | 30 (100) |
| BMI (kg/m2) | 29 (26–35) |
| Obesity (BMI > 30 kg/m2) (n) | 12 (40) |
| Ischaemic heart disease (n) | 18 (60) |
| LVEF (%) | 25 (20–31) |
| LVEDD (mm) | 63 (58–69) |
| NYHA Class II/III/IV (n) | 0/26/4 (0/87/13) |
| Duration of CHF (years) | 5 (3–10) |
| Heart failure hospitalization (n) | 25 (83) |
| ≥3 heart failure hospitalizations (n) | 14 (47) |
| NT‐proBNP (pg/mL) | 3165 (880–8085) |
| NT‐proBNP > 1600 pg/mL (n) | 18 (64) a |
| Systolic blood pressure | 128 (113–136) |
| Diastolic blood pressure | 72 (66–81) |
| Mitral valve insufficiency ≥ moderate (n) | 4 (13) |
| Tricuspid valve insufficiency ≥ moderate (n) | 9 (30) |
| Atrial fibrillation/flutter (n) | 17 (57) |
| Diabetes mellitus (n) | 16 (53) |
| Arterial hypertension (n) | 20 (67) |
| COPD (n) | 4 (13) |
| History of malignant disease (n) | 5 (17) |
| Creatinine (μmol/L) | 120 (95–165) |
| eGFR (mL/min/kg) | 55 (34–70) |
| Cardiac devices | |
| ICD (n) | 22 (73) |
| CRT (n) | 10 (33) |
| Diuretics (n) | 28 (93) |
| Ivabradine (n) | 4 (13) |
| Digitoxin (n) | 1 (3) |
BMI, body mass index; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IQR, inter‐quartile range; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association.
Two values missing.
Medical treatment of HF included ACEi or ARB in 43% of patients, and 50% of the patients received an ARNI. BBs were used in 97% of patients and MRAs in 63% of patients. Patients treated with a particular drug class reached in mean of 61% (ACEi/ARB), 67% (ARNI), 61% (BB), and 54% (MRA) of target dose, respectively. At baseline, sodium–glucose cotransporter 2 inhibitors (SGLT2is) were given only to a minority of 17% of patients (Table 2 ).
Table 2.
Treatment with heart failure medications of prognostic relevance at baseline and 12 month follow‐up
| Class of drug | Baseline, n (%) | % target dose | 12 months, n (%) | % target dose |
|---|---|---|---|---|
| Beta‐blocker (n) | 29 (97) | 61 | 18 (86) | 64 |
| ACEi or ARB (n) | 13 (43) | 61 | 6 (29) | 58 |
| ARNI (n) | 15 (50) | 67 | 15 (71) | 63 |
| MRA (n) | 19 (63) | 54 | 13 (62) | 50 |
| SGLT2i (n) | 5 (17) | n.a. | 9 (43) | n.a. |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor/neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Data were obtained in 30 patients at baseline and 21 patients at follow‐up.
Safety
Complications associated with device implantation were rare: device pocket bleeding and red blood cell transfusion occurred in two patients and transient nerve injury in two patients. A surgical revision of the pocket site was necessary in one patient due to haematoma. Both patients with transient nerve injury reported ipsilateral paraesthesia or hypoesthesia of the tongue, and one of them suffered from dysphagia. All complaints were resolved within the first 3 months. Periprocedural strokes did not occur. There were no device infections in the periprocedural time nor during the long‐term follow‐up.
Clinical outcomes
The success rate of Barostim Neo™ implantation was 100%, and the system was activated in all patients. After 12 months of BAT, six patients had died (20%). Three patients were alive but did not attend follow‐up visits so that further information on clinical status was not available.
In the remaining patients (70%), blood pressure and HR remained stable (Figure 1 ). BAT did not hinder intensification of medical therapy with initiation of SGLT2i in four patients and ARNI in five patients during follow‐up. Median dosage of ACEi/ARB, ARNI, BB, and MRA remained stable during follow‐up (Table 2 ).
Figure 1.
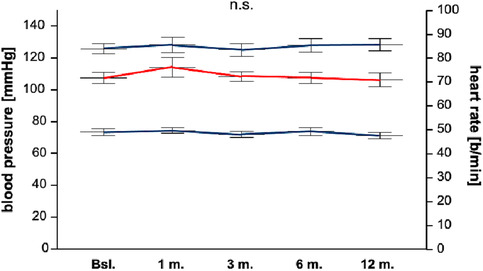
Systolic blood pressure, diastolic blood pressure, and heart rate. The median systolic blood pressure at baseline was 128 (113–136) and 130 (122–141) mmHg after 12 months. The median diastolic blood pressure at baseline was 72 (66–81) and 70 (65–76) mmHg after 12 months. The median heart rate at baseline was 72 (66–79) and 70 (65–76) b.p.m. after 12 months. There were no significant differences between baseline and 12 months.
NYHA functional class significantly improved between baseline and 12 month follow‐up (P < 0.001) (Figure 2 ). This was accompanied by a numerical decrease in NT‐proBNP [3165 (880–8085) vs. 1001 (599–3820) pg/mL]. However, this change did not reach statistical significance (P = 0.526). A numerical, statistically non‐significant reduction in NT‐proBNP was seen in both subgroups of patients with an initial NT‐proBNP level below or above 1600 pg/mL (P = 0.196 and P = 0.314) (Figure 3 ).
Figure 2.
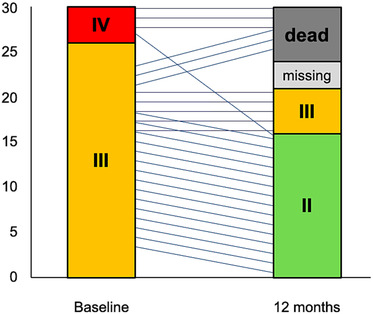
New York Heart Association (NYHA) functional class is depicted at baseline and after 12 months, showing a significant improvement to a lower NYHA class at follow‐up. Six patients were dead at the 12 month follow‐up, and three values are missing due to loss of follow‐up.
Figure 3.
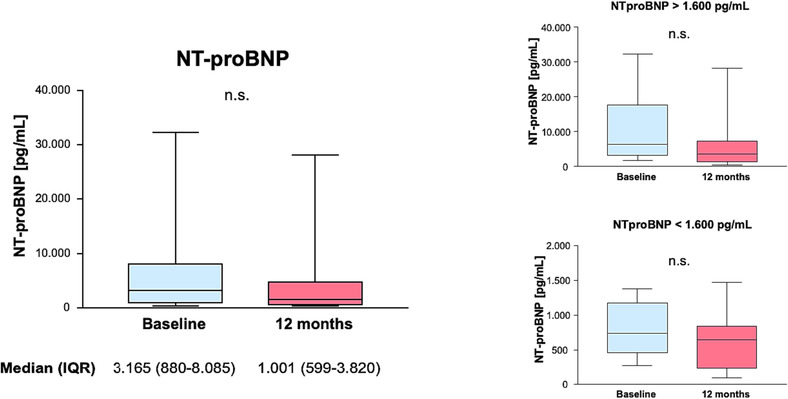
N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP). There was no significant change in NT‐proBNP between baseline and after 12 months, neither in total nor after separating patients into two groups using an NT‐proBNP of 1600 pg/mL as arbitrary cut‐off. IQR, inter‐quartile range.
The LVEF improved from 25.5 (20.0–30.5) % at baseline to 30.0 (25.0–36.0) % at 12 months (P = 0.014), whereas LVEDD remained unchanged (P = 0.284) (Figure 4 ). Further echocardiographic findings are depicted in Table 3 . Left atrial diameter and volume index were found to be enlarged at baseline and declined after 12 months of BAT in trend. Right ventricular systolic pressure (RVSP) estimated by the maximum tricuspid regurgitation velocity was elevated at baseline and numerically lower at follow‐up. However, this was not statistically significant and potentially biased by underreporting in patients with normal RVSP and those without tricuspid regurgitation.
Figure 4.
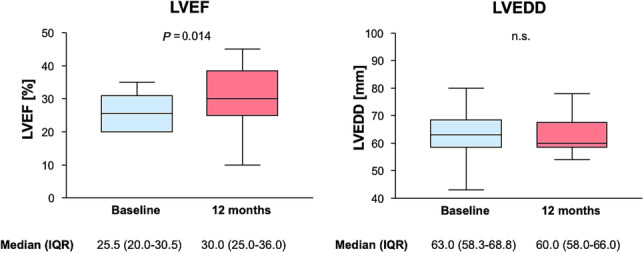
Left ventricular ejection fraction (LVEF) and left ventricular end‐diastolic diameter (LVEDD). LVEF showed a slight but statistically significant improvement from baseline to 12 months. LVEDD showed no significant improvement after 12 months. IQR, inter‐quartile range.
Table 3.
Echocardiographic assessment at baseline and 12 month follow‐up
| Baseline | 12 months | P value | |
|---|---|---|---|
| LA diameter (mm) | 47 (44–52) | 47 (41–50) | 0.021 |
| LAVI (mL/m2) | 48 (39–58) | 37 (30–56) | 0.099 |
| LVEDD (mm) | 63 (58–69) | 60 (58–66) | 0.284 |
| LVESD (mm) | 56 (49–64) | 53 (45–64) | 0.244 |
| LVEF (%) | 26 (20–30) | 30 (25–36) | 0.014 |
| IVS thickness (mm) | 12 (10–13) | 12 (10–13) | 0.623 |
| RV diameter (mm) | 33 (27–42) | 34 (25–42) | 0.431 |
| RVSP (mmHg) | 54 (38–56) | 42 (29–56) | 0.441 |
| TAPSE (mm) | 13 (11–19) | 14 (12–18) | 0.832 |
IVS, interventricular septum; LA, left atrium in parasternal long‐axis view; LAVI, left atrial volume index; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; RV, right ventricular; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Ultra‐short‐term HRV was assessed in 18 patients without atrial fibrillation or atrial pacemaker stimulation at baseline and after follow‐up of 12 months. There was no significant change in SDNN [18.8 (11.3–29.5) vs. 14.5 (10.4–30.3) ms; P = 0.586] or RMSSD [17.8 (12.8–38.0) vs. 19.0 (13.8–36.4) ms; P = 0.943].
Median follow‐up time for clinical events defined as death or HF hospitalization was 16 (10–33) months. During this time, a total of 10 patients died [2 HF, 3 not related to HF (sepsis, renal failure, and malignancy), and 5 unexplained]. This accounts for a number of deaths of 16.8 per 100 patient years. Mortality at 1 and 3 years was 20% and 33.3%, which was as expected when risk of all‐cause death was estimated using the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score with 20.6% mortality at 1 year and 42.5% at 3 years (Figure 5 ). 15
Figure 5.
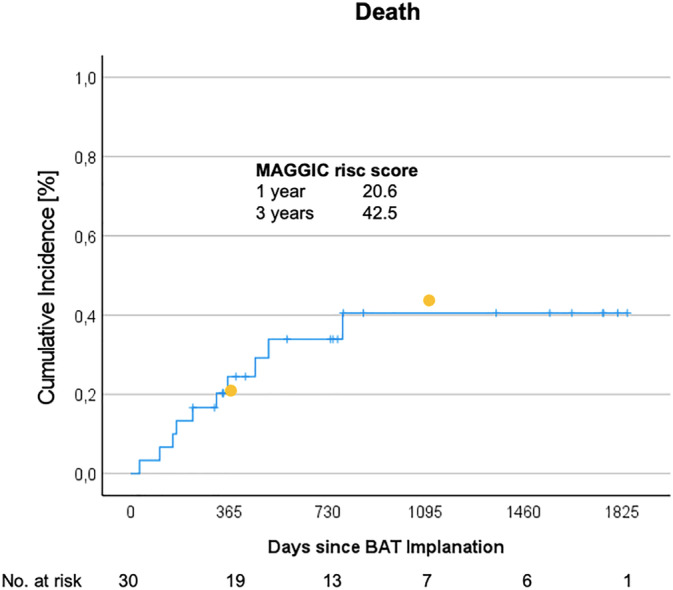
Mortality. Cumulative mortality after baroreflex activation therapy (BAT) implantation is depicted. Mortality was 20% after 1 year and 44% after 3 years. Yellow dots indicate mortality as predicted by the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score at 1 year (20.6%) and 3 years (42.5%).
A total of 14 patients were hospitalized due to HF during follow‐up. One of those patients was hospitalized twice for this reason, resulting in an event rate of 15. The number of first and recurrent HF hospitalizations was 28.5 per 100 patient years.
Advanced HF symptoms defined as NYHA Class IV, high NT‐proBNP levels >1600 pg/mL, and severely impaired renal function with an eGFR < 30 mL/min at baseline significantly predicted the risk of death or HF hospitalization (Figure 6 ). In a multivariate analysis of survival at 3 years, NYHA Class IV and an NT‐proBNP above median (3165 pg/mL) remained statistically significant independent predictors of mortality (Table 4 ).
Figure 6.
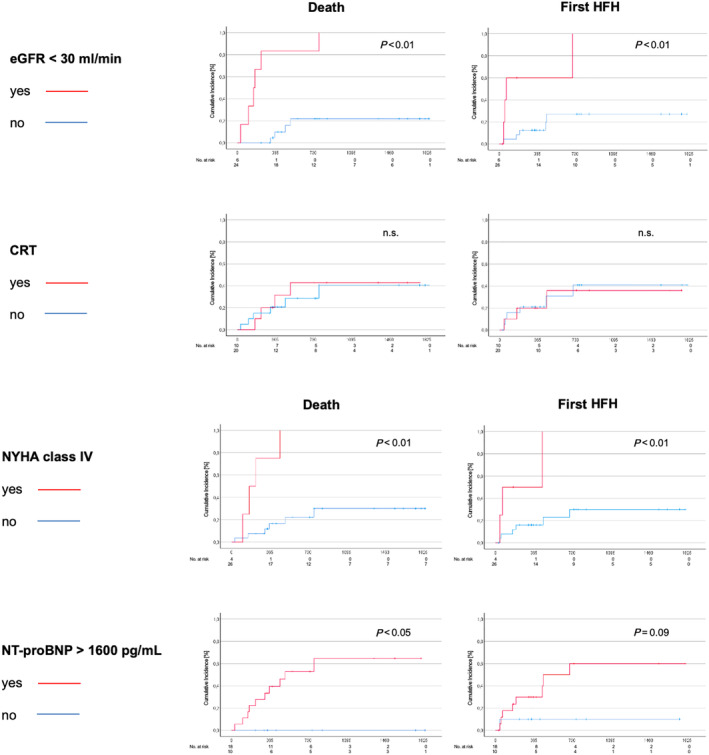
Predictors of outcome. New York Heart Association (NYHA) Class IV, estimated glomerular filtration rate (eGFR) <30 mL/min, and N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) >1600 pg/mL were statistically significant predictors for worse outcome regarding death or heart failure hospitalization (HFH). Whether patients had a cardiac resynchronization therapy (CRT) device or not was not an outcome predictor.
Table 4.
Predictors of death at 3 years
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| eGFR < 30 mL/min | 12.70 (3.49; 46.15) | <0.001 | 2.85 (0.48; 16.88) | 0.105 |
| NYHA Class IV | 8.81 (2.17; 35.71) | 0.002 | 7.02 (1.60; 30.71) | 0.010 |
| NT‐proBNP > median 3165 pg/mL | 12.47 (1.56; 99.68) | 0.017 | 11.43 (1.41; 92.68) | 0.023 |
| CRT | 1.11 (0.31; 3.95) | 0.869 | ||
Stepwise multivariate binary logistic Cox regression analysis with survival at 3 years as depending variable. Median NT‐proBNP level was used for categorization due to missing events in patients with NT‐proBNP < 1600 pg/mL.
CI, confidence interval; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HR, hazard ratio; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association.
Regarding patients with or without cardiac resynchronization therapy at baseline, there were no differences in change of LVEF [5.3 (0.0–5.0) vs. 5.5 (−1.5 to 10.0); P = 0.837] or NT‐proBNP [350 (−3309 to 2249) vs. −391 (−1035 to 245); P = 0.659] between baseline and 12 months. CRT did not impact the risk for HF hospitalization or death (Figure 6 and Table 4 ).
Discussion
Main findings
To the best of our knowledge, this is the largest observational registry aiming to characterize patients with CHF treated with BAT in routine clinical practice and to analyse safety and clinical outcome during intermediate‐term follow‐up. The data demonstrate that (i) patients who are selected for BAT are in a stage of worsening or even advanced HF; (ii) the risk of HF hospitalizations and death remains extremely high at least in those patients who present in NYHA Class IV and have high NT‐proBNP levels or severe renal comorbidity; (iii) BAT in HFrEF appears safe, at least in a setting of a specialized unit with standardized procedures of patient selection, device implantation, and long‐term follow‐up; and (iv) BAT improves clinical symptoms and—to a modest degree—LV function.
Patient population
Patients included in this registry were in a state of worsening or even advanced HFrEF and frequently suffered from comorbidities such as diabetes, impaired renal function, atrial fibrillation, or obesity. Compared with the randomized trials of BAT in HFrEF, the HOPE4HF and BeAT‐HF trials, patients in our registry were slightly older and had a slightly worse renal function, significantly higher NT‐proBNP values, and a higher rate of prior HF hospitalizations. 12 , 16 Patients in this registry had more severe symptoms regarding NYHA class and higher NT‐proBNP levels along with worse LVEF and renal function indicating secondary organ failure as compared with patients in the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial, which tested the effect of vericiguat compared with placebo in a dedicated HFrEF population of patients with worsening symptoms and recent event of HF decompensation in spite of already initialized medical treatment. 17 All of these characteristics are related to outcome in terms of HF hospitalization and death and are used for the prognostication of survival. 15
Autonomic function/heart rate variability
Heart rate and beat‐to‐beat HRV are regulated by the ANS. Reduced HRV as determined by SDNN carried a significant risk in cardiac mortality in patients after myocardial infarction with incremental risk when LVEF was below 35%. 18 Whereas BAT was shown to improve RMSSD among other time‐ and frequency‐domain measures but not SDNN in patients with arterial hypertension, the impact of BAT on HRV in HFrEF has not been studied so far. 11 , 19 In our cohort, there was no change in HR with BAT on top of background BB therapy and no change in HRV as determined by the time‐domain measures of SDNN or RMSSD over time. Either this might be related to technical issues because the method of ultra‐short‐term HRV calculated from 10 s ECG recordings is less well established compared with short‐term and longer term recordings of 10 min or 24 h. 14 Moreover, patients in our cohort having high NT‐proBNP levels might be in a stage of HF too advanced to be susceptible for ANS modulation by BAT. 19 , 20 Furthermore, HRV per se might be suboptimal to monitor the effects of BAT on ANS in HF. However, there is no reference standard measure of ANS activity clinically available (for review, see Patel et al. 5 ). Nevertheless, in a recent study in 425 patients with HFrEF on optimal medical and device therapy, abnormal baroreceptor and chemoreceptor sensitivity strongly predicted lower HRV and exercise tolerance as well as impaired risk of adequate ICD shocks and cardiac death. 21 These findings underscore the need to establish reliable measures of ANS activity to guide proper patient selection in an individualized therapeutic approach to treat HF. 19
Clinical benefit
Patient characterization is of major impact to contextualize clinical outcome in our patient cohort. The risk of death in the treatment arm of the EMPEROR‐Reduced trial, a patient group that was treated according to current guidelines but with less high‐risk characteristics, was 10.1 per 100 patient years. 22 In contrast, rate of death was 16.9 in the placebo arm of the VICTORIA trial 17 and 16.8 per 100 patient years in our registry population. In addition, the number of deaths after 1 and 3 years in our population was as expected according to the MAGGIC risk score. On the other hand, the number of first and recurrent HF hospitalizations in our cohort was lower with 28.5 compared with 42.4 per 100 patient years in the VICTORIA trial. These findings might indicate that BAT does not have major impact on mortality but reduces the risk of HF hospitalizations. The BeAT‐HF trial (NCT02627196), even though in a non‐blinded fashion, was designed to answer the important question if BAT is able to reduce morbidity and mortality in HFrEF. Results of this trial are expected to be published this year.
A lower than expected number of HF hospitalizations are in line with the improvement in LVEF and NYHA class seen in our cohort. Data on echocardiographic parameters in BAT are sparse. In the HOPE4HF trial, a non‐significant trend of 2.5 ± 1.7% LVEF between‐group difference (P = 0.15) in favour of BAT compared with medical therapy was shown. 16 A small‐sized prospective registry comparing 10 patients with BAT and 30 patients with medical therapy only demonstrated a 10% increase in LVEF already 3 months after BAT. However, groups were not balanced at baseline with significantly lower LVEF in the BAT group. 23 Whereas BAT might therefore have an impact on cardiac contractility, cardiac structure as measured by LVEDD seems to be unaffected at least in median‐term follow‐up. A dedicated mechanistic study from Grossman et al., which was already published in 1991, demonstrated a close relationship between total peripheral resistance and echocardiographically determined LV filling patterns. As compared with patients with normal ventricular filling, those with impaired LV filling had a 4.5 times higher increase in norepinephrine levels in response to isometric exercise and more than two times higher total peripheral resistance but a five times lower increase in cardiac output despite an equivalent increase in mean arterial blood pressure. 24 In other words, high peripheral resistance and sympathetic overdrive are associated with impaired LV function and inability to generate an adequate cardiac output in response to exercise. Peripheral vasoconstriction and resistance are thought to be a main consequence of ANS imbalance in HF. 5 , 9 Thus, clinical findings from the HOPE4HF trial are of importance, showing a significant increase in systolic blood pressure and pulse pressure as a result of BAT in HF, which might be caused by improved stroke volume due to reduced vascular resistance. 16 A raise in cardiac contractility as a result of reduced afterload is also in line with a reduction in LV filling pressure and—as it is seen in our patients—with a reduced left atrial volume overload. However, dedicated echocardiographic assessment is necessary to understand the impact of BAT on cardiac structure and function. There is a BAT post‐market multicentre study in Germany designed to assess cardiac function and remodelling in HFrEF patients, the BiRD‐HF trial, using core lab analysis of echocardiographic parameters. This registry attempts to enrol 110 patients and is still ongoing.
The improvement in NYHA classification in our cohort is of clinical importance and consistent with the findings of the HOPE4HF and BeAT‐HF trials as well as a meta‐analysis of these trials. 20 This clinical benefit was shown to be independent of age, gender, or presence of comorbidities such as atrial fibrillation in these randomized trials and went along with a significant improvement in walking distance in a 6 min hall walk test and quality‐of‐life assessment. 20 The reduction in symptom burden seems to be given on top of an optimized medical therapy including ARNI as indicated by the registry by Guckel et al. 23
In contrast to other trials and registries, we did not detect any statistically significant changes in NT‐proBNP after BAT in our patient cohort. Two assumptions may account: first, the sample size is just too small and the variability of NT‐proBNP is too high to reach statistical significance despite a numerical reduction of −68% in NT‐proBNP. Second, there is no reduction in NT‐proBNP because our patient cohort is already too sick to benefit from BAT. The BeAT‐HF trial data, in contrast to previous trials, revealed that patients initially randomized to BAT vs. control (Cohort A) did not have any benefit regarding NT‐proBNP. Post hoc analyses identified the eligibility criterion of NT‐proBNP > 1600 pg/mL in patients without previous HF hospitalization, which was used to enrich the study population with patients at high risk for morbid and mortal events, as a marker for more advanced HF and a marker for a patient group, which might have a lower treatment response. In patients with baseline NT‐proBNP levels <1600 pg/mL, a significant reduction in NT‐proBNP with BAT could be confirmed. In a complex restructure of the study population and in agreement with the Food Drug Administration (FDA), final analysis focused on patients with baseline NT‐proBNP < 1600 pg/mL (Cohorts B and C). 12 The FDA finally approved Barostim Neo™ in 2019 for the use in patients with HFrEF and NT‐proBNP < 1600 pg/mL. Several trials suggest that patients with lower NT‐proBNP levels might have greater response to HF therapies. The treatment effect of vericiguat vs. placebo on the primary composite endpoint of cardiovascular death or HF hospitalization in the VICTORIA trial was greatest in patients with NT‐proBNP levels below 4000 pg/mL but not obvious anymore in those with levels >8000 pg/mL. 25 The LCZ696 in Advanced Heart Failure (LIFE) trial taught us that striking achievements of ARNI in patients predominantly in NYHA Class II in the PARADIGM‐HF study cannot necessarily be extrapolated to patients with advanced HF. 26 , 27 This double‐blind randomized clinical trial compared sacubitril/valsartan with valsartan in patients in NYHA Class IV. After 24 weeks, there were no significant differences between groups regarding NT‐proBNP and a composite of clinical outcome parameters. 26 We could not detect a difference in treatment response depending on baseline NT‐proBNP with an arbitrary cut‐off of 1600 pg/mL or when split by median (data not shown). Nevertheless, most of our patients were in NYHA Class III and some even in Class IV with a median NT‐proBNP level >3100 pg/mL, so that it could be argued that our patients' NT‐proBNP and mortality did not improve because they were already too sick and would only benefit from a mechanical assist device or heart transplant. This is supported by the finding that NYHA Class IV, NT‐proBNP levels >1600 pg/mL, and secondary organ dysfunction with eGFR < 30 mL/min significantly predicted HF hospitalizations and death with eGFR < 30 mL/min and NYHA Class IV as independent predictors of survival at 3 years. Whether patients in a markedly earlier disease stage and NYHA Class II situation represent a patient group with particular potential to benefit from BAT is unclear. To our knowledge, there are no studies at this point in time investigating Barostim Neo™ in patients in NYHA Class II and/or the full LVEF range for HFrEF with an LVEF ≤ 40% as defined by current guidelines 4 rather the restriction to use in NYHA III and LVEF < 35% by CE mark and FDA approval. If an NT‐proBNP cut‐off of 1600 pg/mL is optimal to predict treatment response needs to be established. 20 Our data indicate that patient selection is crucial to utilize the therapeutic potential of BAT in HFrEF.
A subanalysis of the HOPE4HF study indicated that patients without CRT have more pronounced treatment effects with BAT compared with those with CRT. 28 Patients with CRT therefore were excluded from study participation in the BeAT‐HF trial, and subsequently, the FDA approved Barostim Neo™ only in patients without CRT. One third of the patients in our cohort were treated with CRT for at least 6 months before BAT. Our data do not indicate any difference in treatment effect on NT‐proBNP, LVEF, or clinical events in patients with or without CRT. This is in line with findings from Guckel et al. In this registry, 90% of BAT patients were CRT non‐responder. 23 Further studies are necessary to define the efficacy of BAT in CRT patients.
Safety
As already shown in the setting of clinical trials, complication rates of Barostim Neo™ implantation were also low in routine clinical care. Bleeding and nerve injuries occurred, all of which resolved completely. No device had to be extracted because of infection or for other reasons. Theoretically, there is no risk of device endocarditis, because the lead is located strictly extravascular.
Blood pressure and HR remained stable during BAT in our cohort, which serves as an important safety marker, because HFrEF patients often suffer from symptomatic hypotension that limits HF therapy (Figure 1 ). Our patients received adequate guideline‐directed HF medication (Table 2 ). Almost all patients were treated with either ARNI or ACEi/ARB in addition to a BB, and 60% of patients were on triple therapy in combination with an MRA. Regarding drug classes used and percentage of target doses achieved, patients in our cohort were treated more intense compared with those in the recent registries or clinical trials. 17 , 22 , 29 BAT did not result in down‐titration of medical therapy and did not hinder medical treatment intensification. It is relevant to point out that only five patients were treated with SGLT2i at baseline. However, results of outcome trials with SGLT2i in CHF were pending when most of the patients in this registry were scheduled for BAT. 22 , 30 Treatment with an SGLT2i was initiated in four patients at follow‐up.
Limitations
All conclusions drawn from the data obtained from our registry have to be taken with outmost caution due to its monocentric, retrospective, non‐randomized, and non‐blinded design and its small sample size, which makes our analysis prone to bias. This study is neither designed nor powered to assess the impact of BAT on morality or morbidity in HFrEF. In addition, our study lacks a control group. This means that the observed changes over time could be unrelated or not entirely due to BAT. Intensification of medical HF treatment in some patients during follow‐up might have impacted the results. Moreover, our patient collective does not represent a general HF population due to the selection process in a highly specialized centre with an overrepresentation of patients in a more advanced stage of the disease. Women are fairly underrepresented with 10% of the study population. This was also a limitation of previous randomized trials and cohorts. Therefore, further research is necessary to evaluate the effectiveness of BAT in women. Besides HRV, no other measures of ANS activity such as baroreceptor or chemoreceptor sensitivity have been obtained to monitor the effect of BAT on ANS and to identify potential non‐responders.
Conclusions
In a real‐world situation of BAT in WHF, the Barostim Neo™ system has shown to be safe and effective in reduction of symptom burden and in a modest improvement of LV contractility. The subsequent number of HF hospitalizations in median‐term follow‐up was lower than expected, whereas BAT seems not to impact mortality. In patients with more advanced HF, the risk for HF hospitalization and death remains extremely high despite BAT. So treatment with the Barostim Neo™ system seems to be futile or at least of questionable effectiveness in this subgroup. Further studies are essential to demonstrate the effects of BAT on outcome and to identify most susceptible patient groups.
Conflict of interest
A.L. reports grants from Novartis; personal fees from Abbott, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Edwards Lifesciences, Medtronic, Novartis, and Pfizer; and other fees from Claret Medical, Picardia, and Transverse Medical outside the submitted work. N.M. has received personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Bayer, Abbott, Abiomed, and Boston Scientific outside the submitted work. M.K. has received personal fees from Boston, CVRx, Medtronic, Philips, and Zoll outside the submitted work. E.B.W. reports grants from Boehringer Ingelheim and personal fees from Amarin, Amgen, AstraZeneca, Daiichi Sankyo, Bayer, Boehringer Ingelheim, CVRx, and Novartis outside the submitted work. All remaining authors do not report any possible conflicts of interest connected to the submitted work.
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Blanco, C. , Madej, T. , Mangner, N. , Hommel, J. , Grimm, S. , Knaut, M. , Linke, A. , and Winzer, E. B. (2023) Baroreflex activation therapy in patients with heart failure with reduced ejection fraction: a single‐centre experience. ESC Heart Failure, 10: 3373–3384. 10.1002/ehf2.14508.
References
- 1. Greene SJ, Bauersachs J, Brugts JJ, Ezekowitz JA, Lam CSP, Lund LH, et al. Worsening heart failure: Nomenclature, epidemiology, and future directions: JACC review topic of the week. J Am Coll Cardiol 2023;81:413–424. doi: 10.1016/j.jacc.2022.11.023 [DOI] [PubMed] [Google Scholar]
- 2. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906 [DOI] [PubMed] [Google Scholar]
- 3. Greene SJ, Triana TS, Ionescu‐Ittu R, Shi S, Guerin A, DeSouza MM, et al. Patients hospitalized for de novo versus worsening chronic heart failure in the United States. J Am Coll Cardiol 2021;77:1023–1025. doi: 10.1016/j.jacc.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 5. Patel HC, Bauersachs J, Bohm M, Borggrefe M, Brutsaert D, Coats AJS, et al. The autonomic nervous system as a therapeutic target in heart failure: A scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2017;19:1361–1378. doi: 10.1016/j.jacc.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 6. van Bilsen M, Amorim DS, Dargie HJ, Heer K, Brown M, Jenner D, et al. Is there autonomic impairment in congestive (dilated) cardiomyopathy? Lancet 1981;1:525–527. doi: 10.1016/s0140-6736(81)92863-4 [DOI] [PubMed] [Google Scholar]
- 7. Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, et al. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 1995;92:3206–3211. doi: 10.1161/01.CIR.92.11.3206 [DOI] [PubMed] [Google Scholar]
- 8. Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 1971;285:877–883. doi: 10.1056/NEJM197110142851602 [DOI] [PubMed] [Google Scholar]
- 9. Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 2015;36:1974–82b. doi: 10.1093/eurheartj/ehv087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byku M, Mann DL. Neuromodulation of the failing heart: Lost in translation? JACC Basic Transl Sci 2016;1:95–106. doi: 10.1016/j.jacbts.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, et al. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug‐resistant arterial hypertension. Hypertension 2009;54:530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023 [DOI] [PubMed] [Google Scholar]
- 12. Zile MR, Lindenfeld J, Weaver FA, Zannad F, Galle E, Rogers T, et al. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol 2020;76:1–13. doi: 10.1016/j.jacc.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 13. Knaut M, Madej T. Implantation von Systemen zur Barorezeptorstimulation. In: Ennker J, Falk V, Photiadis J, Starck C, Weymann A, eds. Referenz Herzchirurgie. Thieme; 2023:945–951. [Google Scholar]
- 14. Munoz ML, van Roon A, Riese H, Thio C, Oostenbroek E, Westrik I, et al. Validity of (ultra‐)short recordings for heart rate variability measurements. PLoSOne 2015;10:e0138921. doi: 10.1371/journal.pone.0138921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 16. Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail 2015;3:487–496. doi: 10.1016/j.jchf.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 17. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 18. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ, ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators . Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478–484. doi: 10.1016/S0140-6736(97)11144-8 [DOI] [PubMed] [Google Scholar]
- 19. Gentile F, Passino C, Emdin M, Giannoni A. Baroreflex activation therapy in heart failure: Targeting the right patient. Eur J Heart Fail 2022;24:1674–1676. doi: 10.1002/ejhf.2627 [DOI] [PubMed] [Google Scholar]
- 20. Coats AJS, Abraham WT, Zile MR, Lindenfeld JA, Weaver FA, Fudim M, et al. Baroreflex activation therapy with the Barostim™ device in patients with heart failure with reduced ejection fraction: A patient level meta‐analysis of randomized controlled trials. Eur J Heart Fail 2022;24:1665–1673. doi: 10.1002/ejhf.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giannoni A, Gentile F, Buoncristiani F, Borrelli C, Sciarrone P, Spiesshoefer J, et al. Chemoreflex and baroreflex sensitivity hold a strong prognostic value in chronic heart failure. JACC Heart Fail 2022;10:662–676. doi: 10.1016/j.jchf.2022.02.006 [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 23. Guckel D, Eitz T, El HM, Braun M, Khalaph M, Imnadze G, et al. Baroreflex activation therapy in advanced heart failure therapy: Insights from a real‐world scenario. ESC Heart Fail 2023;10:284–294. doi: 10.1002/ehf2.14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grossman E, Oren S, Messerli FH. Left ventricular filling and stress response pattern in essential hypertension. Am J Med 1991;91:502–506. doi: 10.1016/0002-9343(91)90186-2 [DOI] [PubMed] [Google Scholar]
- 25. Ezekowitz JA, O'Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, et al. N‐terminal pro‐B‐type natriuretic peptide and clinical outcomes: Vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail 2020;8:931–939. doi: 10.1016/j.jchf.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 26. Mann DL, Givertz MM, Vader JM, Starling RC, Shah P, McNulty SE, et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA Cardiol 2022;7:17–25. doi: 10.1001/jamacardio.2021.4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 28. Zile MR, Abraham WT, Weaver FA, Butter C, Ducharme A, Halbach M, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction: Safety and efficacy in patients with and without cardiac resynchronization therapy. Eur J Heart Fail 2015;17:1066–1074. doi: 10.1002/ejhf.299 [DOI] [PubMed] [Google Scholar]
- 29. Zeymer U, Clark AL, Barrios V, Damy T, Drozdz J, Fonseca C, et al. Utilization of sacubitril/valsartan in patients with heart failure with reduced ejection fraction: Real‐world data from the ARIADNE registry. Eur Heart J Qual Care Clin Outcomes 2022;8:469–477. doi: 10.1093/ehjqcco/qcab019 [DOI] [PubMed] [Google Scholar]
- 30. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]


