Abstract
Aims
A high red blood cell distribution width (RDW) at admission or discharge is associated with a worse prognosis in hospitalized patients with heart failure (HF), and the prognostic value of the in‐hospital change in RDW (∆RDW) remains debatable.
Methods and results
We included 5514 patients with critical illness and HF from the MIMIC‐IV database. The ΔRDW was calculated by the RDW at discharge minus that at admission. Clinical outcomes included all‐cause mortality at 90 day, 180 day, and 1 year after discharge. The median age of the patients was 73.91 years, and 46.37% were women. Kaplan–Meier curve and Cox regression analyses were used to examine the association between the ΔRDW and all‐cause mortality at different time points. A multivariable Cox proportional hazard model showed that the ΔRDW (per 1% increase) was independently associated with all‐cause mortality at 90 day, 180 day, and 1 year after adjusting for confounding factors (hazard ratio [HR] = 1.17, 95% confidence interval [CI] = 1.13–1.21, P < 0.001; HR = 1.17, 95% CI = 1.14–1.20, P < 0.001; and HR = 1.18, 95% CI = 1.15–1.20, P < 0.001, respectively). Restricted cubic splines showed a non‐linear relationship between the ΔRDW and the risk of clinical outcomes. High ΔRDW was associated with a high risk of mortality at different time points. A subgroup analysis showed that this positive association remained consistent in pre‐specified subgroups.
Conclusions
Our study suggests that an increased RDW during hospitalization is independently associated with short‐ or long‐term all‐cause mortality in critical‐ill patients with HF.
Keywords: Critical care, Heart failure, Prognosis, Red blood cell
Introduction
The red blood cell distribution width (RDW) reflects variation in the dimension of red blood cells and is widely used as a diagnostic tool for anaemia. 1 Several studies have shown that an increased RDW is related to an adverse outcome and is a novel risk factor for risk stratification in patients with cardiovascular diseases. 2 , 3 , 4 , 5 , 6 , 7
Heart failure (HF) is one of the most prevalent cardiovascular diseases with an increasing mortality rate. In patients with HF, accurate risk stratification for a poor prognosis is crucial. Previous studies have shown that an increased RDW is associated with poor short‐ and long‐term outcomes in acute or chronic HF. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 An increased RDW indicates the deregulation of erythrocyte homeostasis and abnormal survival of red blood cells. This situation may lead to a variety of underlying pathophysiological disorders, such as oxidative stress, inflammation, and a poor nutritional status. 21 Baseline RDW has been incorporated in recent HF prognostic models and might be a promising treatsment target for patients with HF.
Although a high RDW at admission or discharge is associated with a worse prognosis in hospitalized patients with HF, the prognostic importance of an in‐hospital change in RDW (∆RDW) remains debatable. 22 Several studies have shown that a persistent increase in the RDW during hospitalization is associated with adverse short‐or long‐term outcomes in patients with acute or chronic HF. 23 , 24 , 25 , 26 , 27 , 28 However, the RDW value may be different from that of general inpatients or outpatients with HF who are critically ill. A recent study showed that the baseline RDW was associated with in‐hospital and short‐term mortality in critically ill patients with HF. 9 However, the in‐hospital ∆RDW and the mortality rate in the short or long term in critically ill patients with HF is unclear. Therefore, we aimed to perform a retrospective study to examine whether the in‐hospital ∆RDW is independently associated with mortality at different time points in patients who are critically ill with HF.
Methods
Study design and data source
This study was a retrospective analysis using data from the open‐accessed Medical Information Mart for Intensive Care database (MIMIC‐IV), which is an updated version of MIMIC‐III. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected by an institutional review board approval. The Institutional Review Boards of the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center gave their approval for this project. The medical records, laboratory results, medical therapies, demographic information, and International Classification of Diseases (ICD) disease codes of patients who were admitted to the intensive care unit at Beth Israel Deaconess Medical Center between 2008 and 2019 are contained in the MIMIC‐IV database. The patients' identity information was concealed to protect their privacy.
Inclusion and exclusion criteria
Patients with HF who were first admitted to the intensive care unit were identified on the basis of the ICD‐9 and ICD‐10 codes. HF may be not registered as the principal diagnosis. Therefore, we excluded records with HF in positions not in the first five diagnosis based on the ICD sequence. Patients with in‐hospital death or no RDW records at admission or discharge were excluded. Additionally, patients with a length of hospitalization shorter than 3 days, those with malignant cancer or haematological disease, those with red blood cells transfusion during hospitalization, or with missing data (<10% of the total sample size) were also excluded (Figure S1 ).
Study variables
The RDW at admission was recorded as the first RDW measured within 24 h after admission. The RDW at discharge was recorded as the last measurement within 24 h before discharge. The in‐hospital ΔRDW was calculated by the RDW at discharge minus the RDW at admission.
Other variables included demographic characteristics (age, sex, and race), body mass index (BMI), length of hospitalization, co‐morbidities, physical examination results, laboratory test results, medication, and treatment. Co‐morbidities included essential hypertension, type 2 diabetes mellitus, atrial fibrillation, myocardial infarction, cerebrovascular disease, peripheral vascular disease, pulmonary disease, liver disease, renal disease, and the Charlson co‐morbidity index. A physical examination included heart rate, systolic and diastolic blood pressure, and mean arterial pressure. Laboratory tests included haemoglobin values, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, white blood cell count, platelet count, and concentrations of potassium, sodium, chloride, glucose, creatinine, blood urea nitrogen, and N terminal pro‐brain natriuretic peptide (NT‐proBNP). Medication and treatment included vasoactive agents (dobutamine, dopamine, epinephrine, milrinone, nesiritide, nitrate, nitroprusside sodium, norepinephrine, and vasopressin), angiotensin‐converting enzyme inhibitors/angiotensin receptor antagonists, beta‐blockers, mineralocorticoid receptor antagonists, calcium channel blockers, diuretics, digoxin, ventilation, dialysis, an intra‐aortic balloon pump, and extracorporeal membrane oxygenation. In addition, Sequential Organ Failure Assessment (SOFA) and the Simplified Acute Physiology Score II (SAPSII) were recorded.
The clinical outcome comprised all‐cause 90 day (short term) mortality, 180 day (medium term) mortality, and 1 year (long term) mortality.
Statistical analysis
The baseline characteristics of our study population were compared after grouping by tertiles of the ΔRDW. All continuous variables are shown as the mean and standard deviation or median and interquartile range and were compared with one‐way analysis of variance or Kruskal–Wallis one‐way analysis of variance according to the distribution of data. Categorical variables, which are expressed as counts and percentages, were compared with the χ2 test. P values for trend were calculated by the Mantel–Haenszel test. Kaplan–Meier curves for the outcomes were compared using the log‐rank test according to ΔRDW tertiles.
A Cox regression analysis was conducted to evaluate the association between the ΔRDW tertiles and short‐, medium‐, and long‐term mortality. In each outcome, adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were computed with the ΔRDW as a continuous variable. Three multivariable models were constructed. Model 1 was adjusted for age, sex, race, BMI, and the length of hospitalization. Model 2 was adjusted for the same variables as those in Model 1 plus hypertension, type 2 diabetes mellitus, atrial fibrillation, myocardial infarction, the Charlson co‐morbidity index, mean arterial pressure, heart rate, haemoglobin, white blood cells, platelets, and creatinine. Model 3 was adjusted for the same variables as those in Model 2 plus NT‐proBNP, SOFA, SAPSII, and RDW at admission. Regarding the variables of BMI and NT‐proBNP with missing values of >20% of the total sample size, dummy variables were used to indicate missing covariate values.
To further validate the predictive value of the ΔRDW for mortality, we calculated Harrell's C‐index before and after adding ΔRDW to Model 3. We then performed the reclassification analysis to evaluate the improvement between the model containing the ΔRDW in addition to Model 3 and original Model 3. Continuous net reclassification improvement and integrated discrimination improvement were used in the reclassification analysis.
The relationship between the ΔRDW and the adjusted HR of each outcome is shown using four‐knot restricted cubic splines. These splines were used to determine the reference point of the ΔRDW and investigate whether there was a non‐linear relationship between the ΔRDW and mortality. A threshold analysis was further performed to examine if the association between the ΔRDW and mortality was non‐linear. To further minimize the confounding bias, we also divided the study population into two groups according to the reference point of the ΔRDW and performed propensity matched score analysis (calliper was 0.05, ratio was 1:1) to balance the baseline characteristics.
Subgroup analyses for the association between the ΔRDW as a continuous variable (per 1%) and outcomes were performed in patients with different sexes, ages, baseline mean arterial pressure, haemoglobin concentrations, RDW at admission, and SOFA scores, and those with or without essential hypertension, type 2 diabetes mellitus, atrial fibrillation, or myocardial infarction. We further investigated the possibility of interactions by incorporating interaction terms into the adjusted models. A sensitivity analysis was performed by reanalysing when removing the missing data of BMI and NT‐proBNP. Statistical software comprising Stata 17.0 (Stata Corporation, College Station, TX, USA), Free statistics software version 1.7.1 (Clinicalscientists Inc, Beijing, China), EmpowerStats version 4.1 (X&Y solutions Inc, Boston, USA), and package R 4.2.2 (Vienna, Austria) were used for all statistical analyses. Two‐tailed P values <0.05 were considered statistically significant.
Results
Baseline characteristics and outcome
We finally included 5514 patients according to the inclusion and exclusion criteria (Figure S1 ). The median age was 73.91 years, 46.37% of the patients were women, 48.11% had a history of hypertension, and 27.78% had a history of type 2 diabetes mellitus. Table 1 shows the baseline characteristics and mortality rate of our study population according to the tertiles of the ΔRDW. Across the tertiles, the median age, proportions of female sex and White race, length of hospitalization, common co‐morbidities (hypertension, type 2 diabetes mellitus, myocardial infarction, atrial fibrillation, cerebrovascular disease, peripheral vascular disease, pulmonary disease, and renal disease), the Charlson co‐morbidity index, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, RDW at discharge, platelet count, use of vasoactive agents, angiotensin‐converting enzyme inhibitors or angiotensin receptor antagonists, beta‐blockers, mineralocorticoid receptor antagonists, calcium channel blockers, diuretics, digoxin, the proportion of dialysis, the mean SOFA score, and the median SAPSII were progressively higher with increasing tertiles. The baseline heart rate, diastolic blood pressure, mean arterial pressure, white blood cell count, RDW at admission, and use of an intra‐aortic balloon pump were progressively lower with increasing tertiles (all P for trend <0.05, Table 1 ). However, there were no significant differences in BMI, the proportion of liver disease, systolic blood pressure, concentrations of potassium, sodium, chloride, glucose, creatinine, blood urea nitrogen or NT‐proBNP, the proportion of ventilation, or the proportion of extracorporeal membrane oxygenation across the ΔRDW tertiles (all P for trend >0.05, Table 1 ).
Table 1.
Baseline characteristics and mortality of the study population according to ΔRDW tertiles
| Items | Total | 1st tertile | 2nd tertile | 3rd tertile | P for trend |
|---|---|---|---|---|---|
| ΔRDW | −11.7% to 0.19% | 0.2% to 1.49% | 1.5 to 16.3% | ||
| N | 5514 | 1835 | 1768 | 1911 | |
| Age median (IQR), year | 73.91 (63.67–83.20) | 71.00 (60.59–81.37) | 75.03 (64.39–83.53) | 75.50 (65.72–84.29) | <0.001 |
| Female, n (%) | 2557 (46.37) | 789 (43.00) | 821 (46.44) | 947 (49.56) | <0.001 |
| Race, n (%) | <0.001 | ||||
| White | 3652 (66.23) | 1164 (63.43) | 1183 (66.91) | 1305 (68.29) | |
| Black | 532 (9.65) | 168 (9.16) | 150 (8.48) | 214 (11.20) | |
| Others | 1330 (24.12) | 503 (27.41) | 435 (24.60) | 392 (20.51) | |
| BMI, median (IQR), kg/m2 (n = 3277) | 29.93 (25.91–35.03) | 30.34 (26.11–35.08) | 29.97 (26.18–35.09) | 29.53 (25.52–34.85) | 0.464 |
| Length of hospitalization, median (IQR), days | 7.82 (5.29–12.11) | 7.09 (4.98–10.85) | 7.55 (5.21–11.78) | 8.79 (5.91–13.85) | <0.001 |
| Co‐morbidities, n (%) | |||||
| Hypertension | 2653 (48.11) | 795 (43.32) | 812 (45.93) | 1046 (54.74) | <0.001 |
| Type 2 diabetes mellitus | 1532 (27.78) | 468 (25.50) | 440 (24.89) | 624 (32.65) | <0.001 |
| Atrial fibrillation | 1795 (32.55) | 549 (29.92) | 498 (28.17) | 748 (39.14) | <0.001 |
| Myocardial Infarction | 2002 (36.31) | 612 (33.35) | 623 (35.24) | 767 (40.14) | <0.001 |
| Cerebrovascular disease | 324 (5.88) | 85 (4.63) | 94 (5.32) | 145 (7.59) | <0.001 |
| Peripheral vascular disease | 855 (15.51) | 235 (12.81) | 247 (13.97) | 373 (19.52) | <0.001 |
| Pulmonary disease | 944 (17.12) | 285 (15.53) | 257 (14.54) | 402 (21.04) | <0.001 |
| Liver disease | 156 (2.83) | 56 (3.05) | 37 (2.09) | 63 (3.30) | 0.633 |
| Renal disease | 516 (9.36) | 153 (8.34) | 128 (7.24) | 235 (12.30) | <0.001 |
| Charlson co‐morbidity index, mean ± SD | 6.45 ± 2.30 | 6.16 ± 2.35 | 6.37 ± 2.31 | 6.79 ± 2.21 | <0.001 |
| Physical examination | |||||
| Heart rate, median (IQR), b.p.m. | 83 (73–96) | 84 (73–97) | 83 (73–95) | 82 (72–95) | 0.015 |
| Systolic blood pressure, mean ± SD, mmHg | 123.39 ± 24.75 | 124.07 ± 24.34 | 123.41 ± 24.99 | 122.72 ± 24.91 | 0.095 |
| Diastolic blood pressure, mean ± SD, mmHg | 65.80 ± 17.27 | 67.12 ± 17.40 | 65.70 ± 17.46 | 64.64 ± 16.90 | <0.001 |
| Mean arterial pressure, mean ± SD, mmHg | 82.03 ± 18.10 | 83.17 ± 18.26 | 81.77 ± 17.73 | 81.20 ± 18.25 | <0.001 |
| Laboratory test | |||||
| Haemoglobin, median (IQR), g/dL | 12.5 (11.1–13.8) | 12.4 (10.7–13.8) | 12.6 (11.3–13.9) | 12.5 (11.2–13.7) | 0.081 |
| Haematocrit, median (IQR), % | 37.7 (33.7–41.3) | 37.4 (33–41.3) | 38.1 (34.1–41.5) | 37.7 (34–41.1) | 0.227 |
| MCV median (IQR), fL | 91 (87–95) | 91 (86–95) | 91 (87–95) | 91 (87–95) | 0.024 |
| MCH median (IQR), pg | 30.1 (28.6–31.5) | 30 (28.4–31.5) | 30.2 (28.8–31.5) | 30.2 (28.6–31.6) | 0.002 |
| MCHC, median (IQR), g/dL | 33.1 (32.1–34) | 33 (31.9–34) | 33.1 (32.2–34.1) | 33.1 (32.1–34) | 0.011 |
| RDW at admission, mean±SD, % | 14.53 ± 1.73 | 15.16 ± 2.06 | 14.17 ± 1.44 | 14.25 ± 1.43 | <0.001 |
| RDW at discharge, mean ± SD, % | 15.70 ± 2.46 | 14.34 ± 1.63 | 14.89 ± 1.50 | 17.76 ± 2.50 | <0.001 |
| White blood cell, median (IQR), K/μL | 8.2 (6.5–10.8) | 8.3 (6.6–11) | 8.3 (6.6–10.9) | 8.1 (6.4–10.6) | 0.004 |
| Platelet, median (IQR), K/μL | 226 (180–284) | 224 (177–282) | 225 (179–280) | 230 (182–289) | 0.019 |
| Potassium, mean ± SD mmol/L | 4.30 ± 0.69 | 4.30 ± 0.71 | 4.28 ± 0.67 | 4.32 ± 0.69 | 0.422 |
| Sodium, median (IQR), mmol/L | 139 (137–141) | 139 (137–141) | 139 (137–141) | 139 (137–141) | 0.514 |
| Chloride, median (IQR), mmol/L | 102 (99–104) | 102 (98–105) | 102 (99–104) | 102 (99–104) | 0.429 |
| Glucose, median (IQR), mg/dL | 118 (98–157) | 118 (99–156) | 119.5 (97–157) | 117 (97–161) | 0.100 |
| Creatinine, median (IQR), mg/dL | 1.1 (0.9–1.4) | 1.1 (0.8–1.4) | 1.1 (0.8–1.4) | 1.1 (0.9–1.5) | 0.329 |
| Blood urea nitrogen, median (IQR), mg/dL | 22 (16–31) | 21 (15–32) | 22 (16–30) | 22 (16–32) | 0.508 |
| NT‐proBNP, median (IQR), pg/mL (n = 3622) | 2578.5 (933–6724) | 2439 (848–6053) | 2511 (864–6576) | 2737.5 (1041.5–7428.5) | 0.218 |
| Medications, n (%) | |||||
| Vasoactive agents | 2631 (47.71) | 800 (43.60) | 813 (45.98) | 1018 (53.27) | <0.001 |
| ACEI/ARB | 3869 (70.17) | 1257 (68.50) | 1220 (69.00) | 1392 (72.84) | 0.004 |
| Beta‐blockers | 4952 (89.81) | 1605 (87.47) | 1593 (90.10) | 1754 (91.78) | <0.001 |
| Mineralocorticoid receptor antagonist | 1072 (19.44) | 332 (18.09) | 274 (15.50) | 466 (24.39) | <0.001 |
| Calcium channel blockers | 1947 (35.31) | 582 (31.72) | 604 (34.16) | 761 (39.82) | <0.001 |
| Diuretics | 5165 (93.67) | 1668 (90.90) | 1653 (93.50) | 1844 (96.49) | <0.001 |
| Digoxin | 844 (15.31) | 259 (14.11) | 239 (13.52) | 346 (18.11) | <0.001 |
| Treatment, n (%) | |||||
| Ventilation | 4731 (85.80) | 1552 (84.58) | 1552 (87.78) | 1627 (85.14) | 0.647 |
| Dialysis | 367 (6.66) | 105 (5.72) | 95 (5.37) | 167 (8.74) | <0.001 |
| IABP | 146 (2.65) | 61 (3.32) | 45 (2.55) | 40 (2.09) | 0.019 |
| ECMO | 14 (0.25) | 3 (0.16) | 3 (0.17) | 8 (0.42) | 0.119 |
| SOFA, mean ± SD | 3.50 ± 2.48 | 3.38 ± 2.51 | 3.49 ± 2.40 | 3.63 ± 2.51 | 0.002 |
| SAPSII, median (IQR) | 35 (29–42) | 34 (27–41) | 35 (29–42) | 37 (30–44) | <0.001 |
| Mortality, n (%) | |||||
| 90 days | 528 (9.58) | 115 (6.27) | 152 (8.60) | 261 (13.66) | <0.001 |
| 180 days | 739 (13.40) | 156 (8.50) | 209 (11.82) | 374 (19.57) | <0.001 |
| 1 year | 1069 (19.39) | 234 (12.75) | 301 (17.02) | 534 (27.94) | <0.001 |
ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin receptor antagonists; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aotic ballon pump; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; NT‐proBNP, N terminal pro‐brain natriuretic peptide; RDW, red cell distribution width; SAPS, Simplified Acute Physiology Scores; SOFA, Sequential Organ Failure Assessment.
Among the 5514 patients, the 90 day, 180 day, and 1 year mortality rates were 9.58%, 13.40%, and 19.39%, respectively (Table 1 ). Across the ΔRDW tertiles, the 90 day, 180 day and 1 year mortality rates were progressively higher with increasing tertiles (all P for trend <0.05, Table 1 ).
Change in red blood cell distribution width and mortality
According to Kaplan–Meier curves (Figure 1 ), patients in the third tertile had a higher risk of 90 day, 180 day, and 1 year mortality than those in the first and second tertiles (log‐rank P < 0.001, Figure 1 A–C ).
Figure 1.
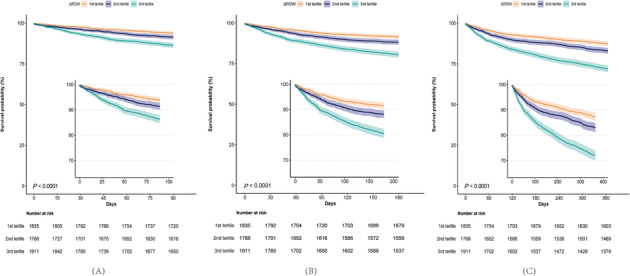
Kaplan–Meier curves for outcomes by ΔRDW tertiles. RDW, red cell distribution width.
The univariable and multivariable Cox proportional hazard models and HRs for 90 day, 180 day, and 1 year mortality are shown in Table 2 . After adjusting for the confounding factors of age, sex, race, BMI, length of hospitalization; hypertension, type 2 diabetes mellitus, atrial fibrillation, myocardial infarction, Charlson co‐morbidity index, mean arterial pressure, heart rate, haemoglobin, white blood cell count, platelet count, creatinine, NT‐proBNP, SOFA, SAPSII, and RDW at admission, patients in the third tertile of the ΔRDW still had an independent high risk of 90 day mortality (HR = 2.56, 95% CI = 2.02–3.24, P < 0.001), 180 day mortality (HR = 2.70, 95% CI = 2.21–3.30, P < 0.001), and 1 year mortality (HR = 2.67, 95% CI = 2.26–3.14, P < 0.001). The ΔRDW as a continuous variable (per 1% increase) was independently associated with a high risk of all‐cause mortality (HR = 1.17, 95% CI = 1.13–1.21, P < 0.001 for 90 day mortality; HR = 1.17, 95% CI = 1.14–1.20, P < 0.001 for 180 day mortality; and HR = 1.18, 95% CI = 1.15–1.20, P < 0.001 for 1 year mortality).
Table 2.
Association between ΔRDW value and risk of mortality
| Outcome | Univariable model | P for trend | Model 1 | P for trend | Model 2 | P for trend | Model 3 | P for trend |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| 90 day mortality | ||||||||
| ΔRDW tertiles | ||||||||
| 1st tertile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 2nd tertile | 1.34 (1.04–1.71) | 0.022 | 1.18 (0.92–1.52) | 0.187 | 1.20 (0.94–1.54) | 0.150 | 1.47 (1.14–1.91) | 0.003 |
| 3rd tertile | 2.30 (1.85–2.88) | <0.001 | 1.89 (1.51–2.36) | <0.001 | 2.08 (1.66–2.61) | <0.001 | 2.56 (2.02–3.24) | <0.001 |
| ΔRDW, % | 1.13 (1.09–1.16) | <0.001 | 1.10 (1.07–1.14) | <0.001 | 1.14 (1.10–1.18) | <0.001 | 1.17 (1.13–1.21) | <0.001 |
| 180 day mortality | ||||||||
| ΔRDW tertiles | ||||||||
| 1st tertile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 2nd tertile | 1.38 (1.12–1.70) | 0.003 | 1.22 (0.99–1.51) | 0.060 | 1.24 (1.01–1.54) | 0.044 | 1.53 (1.23–1.91) | <0.001 |
| 3rd tertile | 2.47 (2.05–2.98) | <0.001 | 2.05 (1.70–2.48) | <0.001 | 2.19 (1.81–2.66) | <0.001 | 2.70 (2.21–3.30) | <0.001 |
| ΔRDW, % | 1.14 (1.11–1.16) | <0.001 | 1.11 (1.08–1.14) | <0.001 | 1.14 (1.11–1.17) | <0.001 | 1.17 (1.14–1.20) | <0.001 |
| 1 year mortality | ||||||||
| ΔRDW tertiles | ||||||||
| 1st tertile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 2nd tertile | 1.34 (1.13–1.60) | 0.001 | 1.21 (1.01–1.43) | 0.034 | 1.23 (1.03–1.46) | 0.020 | 1.51 (1.26–1.81) | <0.001 |
| 3rd tertile | 2.42 (2.07–2.82) | <0.001 | 2.06 (1.76–2.41) | <0.001 | 2.19 (1.87–2.56) | <0.001 | 2.67 (2.26–3.14) | <0.001 |
| ΔRDW, % | 1.14 (1.12–1.16) | <0.001 | 1.12 (1.10–1.15) | <0.001 | 1.15 (1.12–1.18) | <0.001 | 1.18 (1.15–1.20) | <0.001 |
Model 1 include age, gender, race, BMI, and length of hospitalization. Model 2 include model 1 plus hypertension, type 2 diabetes mellitus, atrial fibrillation, myocardial infarction, Charlson co‐morbidity index, mean arterial pressure, heart rate, haemoglobin, white blood cell, platelet, and creatinine. Model 3 include model 2 plus NT‐proBNP, SOFA, SAPSII, and RDW at admission.
BMI, body mass index; CI, confidential interval; HR, hazard ratio; NT‐proBNP, N terminal pro‐brain natriuretic peptide; RDW, red cell distribution width; SAPS, Simplified Acute Physiology Scores; SOFA, sequential organ failure assessment.
Table 3 shows that a model containing the ΔRDW and all variables in Model 3 had a higher Harrell's C‐index than that in the original Model 3 for predicting the mortality at different time points (Table 3 ). In the reclassification analysis, the addition of a ΔRDW to Model 3 showed a continuous net reclassification improvement of 20.2% (95% CI = 13.6–25.8, P < 0.001) and integrated discrimination improvement of 1.4% (95% CI = 0.7–2.4, P < 0.001) for 90 day mortality. The continuous net reclassification improvement and integrated discrimination improvement for the model remained positive for 180 day and 1 year mortality (Table 4 ). Our results suggested that including the ΔRDW in an established predictive model enhanced the prediction performance for all‐cause mortality.
Table 3.
Comparison between ΔRDW in addition to Model 3 and original Model 3 for predicting mortality
| Outcomes | Model 3 | ΔRDW+ Model 3 | P value | |
|---|---|---|---|---|
| 90 day mortality | ||||
| Harrell's C‐index (95% CI) | 0.741 (0.720–0.762) | 0.761 (0.742–0.781) | <0.001 | |
| 180 day mortality | ||||
| Harrell's C‐index (95% CI) | 0.724 (0.706–0.741) | 0.748 (0.731–0.765) | <0.001 | |
| 1 year mortality | ||||
| Harrell's C‐index (95% CI) | 0.711 (0.696–0.726) | 0.739 (0.725–0.753) | <0.001 | |
Model 3 adjusted for age, gender, race, BMI, length of hospitalization; hypertension, type 2 diabetes mellitus, atrial fibrillation, myocardial infarction, Charlson co‐morbidity index, mean arterial pressure, heart rate, haemoglobin, white blood cell, platelet, creatinine, NT‐proBNP, SOFA, SAPSII, and RDW at admission.
BMI, body mass index; CI, confidential interval; HR, hazard ratio; NT‐proBNP, N terminal pro‐brain natriuretic peptide; RDW, red cell distribution width; SAPS, Simplified Acute Physiology Scores; SOFA, Sequential Organ Failure Assessment.
Table 4.
Reclassification analysis for a model containing ΔRDW in addition to Model 3 and original Model 3 for predicting mortality
| Outcomes | Continuous NRI (%, 95% CI) | P value | IDI (%, 95% CI) | P value |
|---|---|---|---|---|
| 90 day mortality | 20.2 (13.6–25.8) | <0.001 | 1.4 (0.7–2.4) | <0.001 |
| 180 day mortality | 19.7 (15.1–24.6) | <0.001 | 1.6 (0.9–2.5) | <0.001 |
| 1 year mortality | 23.4 (18.4–27.6) | <0.001 | 2.4 (1.6–3.4) | <0.001 |
Model 3 adjusted for age, gender, race BMI, length of hospitalization; hypertension, type 2 diabetes mellitus, atrial fibrillation, myocardial infarction, Charlson co‐morbidity index, mean arterial pressure, heart rate, haemoglobin, white blood cell, platelet, creatinine, NT‐proBNP, SOFA, SAPSII, and RDW at admission.
BMI, body mass index; CI, confidential interval; HR, hazard ratio; IDI, integrated discrimination improvement; NT‐proBNP, N terminal pro‐brain natriuretic peptide; NRI, net reclassification improvement; RDW, red cell distribution width; SAPS, Simplified Acute Physiology Scores; SOFA, Sequential Organ Failure Assessment.
Restricted cubic splines (Figure 2 ) showed a non‐linear relationship between the ΔRDW and the adjusted HRs for all outcomes (all P for non‐linearity <0.001). A high ΔRDW was associated with a high risk of 90 day (Figure 2 A ), 180 day (Figure 2 B ), and 1 year mortality (Figure 2 C ). The splines showed that a ΔRDW = 0.7% was the reference point. When we used a model of two‐piecewise linear regression, the corresponding thresholds for the ΔRDW at 6.65%, 6.19%, and 4.7% were identified for 90 day, 180 day, and 1 year mortality, respectively (Table S1 ). The ΔRDW in the majority of patients was below the threshold, and the all‐cause mortality risk increased with a change in the ΔRDW (adjusted HR = 1.22, 95% CI = 1.16–1.28 for 90 day mortality; HR = 1.25, 95% CI = 1.20–1.30 for 180 day mortality; and HR = 1.27, 95% CI = 1.22–1.33 for 1 year mortality). However, in the minority of patients above the threshold, the mortality risk decreased with an increase in the ΔRDW for 90 day mortality (adjusted HR = 0.59, 95% CI = 0.41–0.86). Additionally, the association between the ΔRDW and the risk of 180 day and 1 year mortality was not significant above the threshold (both P > 0.05).
Figure 2.

Relationship between the ΔRDW and the risk of mortality shown by restricted cubic splines. RDW, red cell distribution width.
To further verify the association between the ΔRDW and mortality, we conducted a propensity‐matched analysis according to the reference point of the ΔRDW (0.7%). Finally, 3818 patients were included after matching, and the baseline characteristics except the length of hospitalization were well balanced (Table S2 ). Kaplan–Meier curves for the matched data (Figure S2 ) showed that patients with a ΔRDW ≥0.7% had a higher risk of 90 day, 180 day, and 1 year mortality than patients with a ΔRDW <0.7% (all log‐rank P < 0.001, Figure S2 A–C). Patients with a ΔRDW ≥0.7% still had an independent high risk of 90 day (HR = 2.17, 95% CI = 1.72–2.74, P < 0.001), 180 day (HR = 2.13, 95% CI = 1.76–2.58, P < 0.001), and 1 year mortality (HR = 2.03, 95% CI = 1.74–2.38, P < 0.001) in the matched data after adjusting for confounding factors in line with the unmatched data. The ΔRDW as a continuous variable was an independent risk factor for each outcome (Table S3 ).
Subgroup analysis
Subgroup analyses (Figure 3 ) showed that the association between the ΔRDW as a continuous variable (per 1% increase) and mortality at 90 days, 180 days, and 1 year remained consistent among patients in subgroups with different sexes, ages, baseline mean arterial pressure, haemoglobin values, RDW at admission, and SOFA scores, and in patients with or without hypertension, diabetes mellitus, atrial fibrillation, or myocardial infarction. There was no interaction between the ΔRDW and the risk of mortality in these subgroups (all P for interaction >0.05, Figure 3 ).
Figure 3.

Risk of mortality for a 1% increase in the ΔRDW in pre‐specified subgroups. CI, confidence interval; HR, hazard ratio; RDW, red cell distribution width.
Sensitivity analysis
After excluding the patients with missing values for BMI or NT‐proBNP (n = 3493), the results remained positive as in the original study population in the sensitivity analysis (Table S4 ). In 2021 patients without missing data, a high ΔRDW tertile or a ΔRDW as a continuous variable (per 1%) was independently associated with a higher risk of mortality at different time points (all P < 0.05).
Association between red blood cell distribution width at discharge and risk of mortality
To investigate the predictive value of RDW at discharge, we further include the RDW at discharge instead of ΔRDW as a continuous variable in the multivariable Cox proportional hazard model (Model 3). Our results also showed that RDW at discharge as a continuous variable was also positive associated with high risk of mortality at each time points (Table S5 ). Restricted cubic splines (Figure S3 ) also showed a non‐linear relationship between the RDW at discharge and the adjusted HRs for all outcomes (all P for non‐linearity <0.001). The splines showed that RDW = 15.2% was the reference point. A higher RDW at discharge was associated with higher risk of mortality at each time point.
To compare the predictive value of RDW at admission, at discharge, and ΔRDW on the all‐cause mortality, we carried out the ROC curve analysis for all outcomes. Our results showed that RDW at discharge had a better predictive value for mortality at each time points than RDW at admission or ΔRDW (all P < 0.001, Table S6 and Figure S4 ).
Independent factors associated with high change in red blood cell distribution width
A further analysis showed that patients with an older age, female sex, Black race, a prolonged length of hospitalization, hypertension, atrial fibrillation, peripheral vascular disease, pulmonary disease, renal disease, a higher Charlson co‐morbidity index, lower mean arterial pressure, mean corpuscular haemoglobin, RDW at admission, a higher platelet count, and a higher SAPSII were associated with a higher ΔRDW (Table S7 ).
Discussion
In this study, we found that a higher ΔRDW during hospitalization was associated with a higher risk of all‐cause mortality at different time points and improved the risk stratification for mortality in critically ill patients with HF. Our results suggested that an in‐hospital ΔRDW independently predicted a poor outcome in critically ill patients with HF. A dynamic ΔRDW during hospitalization could provide useful information to identify the risk of adverse events and guide the optimal treatment for patients with HF.
Several previous studies have shown that an elevated RDW during hospitalization is associated with adverse events in patients with acute HF. 23 , 24 , 25 , 27 , 28 However, recently, Xanthopoulos et al. reported that the in‐hospital ΔRDW was not associated with a poor prognosis in patients with HF, 29 which indicates that the predictive value of a ΔRDW for mortality remains uncertain. Because of the small sample size or short‐term follow‐up in previous studies, we performed a retrospective cohort analysis from the MIMIC‐IV database and finally included 5792 critically ill patients with HF. We investigated the association between the ΔRDW and mortality at different time points. The large sample, multiple regression, and propensity score matching analysis ensured a high credibility of our results, which are superior to those in the previous studies. We also showed the independent predictive value of the ΔRDW and all‐cause mortality at different time points in critically ill patients with HF, which is consistent with previous studies. 23 , 24 , 25 , 27 , 28
Recently, Zhang et al. showed that a higher RDW at baseline was an independent predictor for in‐hospital and short‐term all‐cause mortality in critically ill patients with HF. 9 However, the dynamic ΔRDW in critically ill patients with HF is unknown. In our study, we focused on the in‐hospital ΔRDW and short‐ and long‐term mortality and showed the predictive value of a dynamic ΔRDW for adverse outcomes. Our finding emphasized that an in‐hospital ΔRDW was associated with a poor prognosis and could improve the risk stratification in mortality in patients with HF. This conclusion is in accordance with a previous finding that a high RDW was a prognostic marker for mortality in patients with HF. 30 The potential mechanisms of how the RDW is related to adverse outcomes include abnormalities of inflammation and metabolism, iron deficiency, 16 , 31 anaemia, and malnutrition, 21 which are common and related to adverse events in HF. In addition, echocardiographic findings in acute HF have shown that a higher RDW is associated with a higher elevated left ventricular filling pressure and poorer left ventricular deformation. 32 , 33 , 34 In patients with chronic HF, studies have reported that a higher RDW is associated with an impaired exercise tolerance and poor stem cell mobilization. 35 , 36 , 37 These changes in patients with HF would also contribute to a poor prognosis.
Our subgroup analysis showed that the predictive value of a high ΔRDW for mortality was consistent in pre‐specified subgroups. This finding indicated that an increased ΔRDW was associated with a high risk of all‐cause mortality, regardless of the presence of anaemia, and common co‐morbidities such as diabetes mellitus and coronary heart disease. This finding is in accordance with previous studies. 12 , 38 , 39 , 40 , 41 However, because HF is a complex syndrome caused by various heart diseases, the predictive value of the RDW for the prognosis could be different. Zhang et al. reported that the RDW was a prognostic indicator for patients with HF caused by coronary heart disease and dilated cardiomyopathy, but not valvular heart disease. 42 Moreover, several studies have shown that a high RDW is associated with an increased risk of mortality in patients with HF and a preserved ejection fraction, but not with a reduced ejection fraction. 43 , 44 Therefore, because of the complexity of HF syndrome, the predictive value of RDW for adverse events will be affected by the cause or co‐morbidities of HF. Although there was no interaction between the ΔRDW and prespecified subgroups on mortality in our study, the ΔRDW value was associated with several factors such as co‐morbidities of HF. Therefore, the prognostic value of the ΔRDW requires further investigation in HF with various heart diseases in future studies.
Our study also showed that the established model for mortality was improved after including the variable of ΔRDW, which suggested that the ΔRDW could improve the risk stratification in critically ill patients with HF. This finding is consistent with that in previous studies on baseline RDW. 9 Several studies showed that the RDW in combination with NT‐proBNP improved the prognostic value in a traditional model in patients with acute or chronic HF. 12 , 15 , 45 In addition, many studies have shown that the baseline RDW is an independent predictive marker for all‐cause mortality in patients with acute or chronic HF. 8 , 13 , 14 , 46 Moreover, a high RDW was also associated with increased all‐cause mortality, even in patients with advanced HF who underwent cardiac resynchronization therapy, 47 , 48 implantation of a left ventricular assist device, 49 , 50 or heart transplantation. 51 In our study, the ΔRDW improved the risk stratification for short‐ and long‐term mortality in the established model including baseline RDW and NT‐proBNP. This finding suggested that a ΔRDW is a novel prognosticator in critically ill patients with HF.
To ensure the robustness of our results, we used a multivariable regression analysis to adjust for confounding factors and propensity score matching to balance the baseline variable, which could improve the credibility of our results. Restricted cubic splines showed that the relationship between the ΔRDW and the risk of all‐cause mortality was non‐linear. Additionally, the threshold effect analysis showed that a small part (<10%) of the study population did not show consistent results with the main results of our study. The reclassification analysis showed that the inclusion of the ΔRDW enhanced the predictive ability for mortality in the established model. In summary, our study showed the independent prognostic value of the ΔRDW during hospitalization for short‐ and long‐term all‐cause mortality. Physicians could regard an increased RDW during hospitalization as a novel marker to improve the risk stratification of adverse events in critically ill patients with HF.
Several studies showed that red blood cell transfusion would increase the RDW value in critically ill patients and affect the predictive value of RDW on mortality. 52 , 53 When considering this confounding factor, we excluded the patients who underwent red blood cell transfusion during hospitalization or patients with haematological diseases (leukaemia, aplastic anaemia, or polycythemia vera) in our analysis. Thus, the dynamic change of RDW may only influenced by the change of the condition in critically ill patients with HF during hospitalization.
In our study, we also examine the predictive value of RDW at discharge on the risk of mortality in different time points. Our results showed that RDW at discharge was also an independent predictor for the all‐cause mortality, which was inconsistent with previous studies. 17 , 28 This finding suggested that the dynamic change of RDW could improve the risk stratification for mortality than baseline RDW. The potential mechanism might be the therapeutic effect during hospitalization on the change of RDW. Critically ill HF patients with high RDW at baseline would be improved during treatment while the RDW might be decreased. Thus, continuous elevated RDW would have better predictive value on mortality in HF patients. In addition, RDW at discharge would be better than ΔRDW during hospitalization on predicting the adverse events, and this finding suggested that the updated RDW would be more accurate in the risk stratification than the dynamic change of RDW. The possible mechanism might be that the updated RDW value could reflect the improved condition of the patients at discharge better than the dynamic changes during hospitalization.
There are several limitations to our study. First, our study was a retrospective cohort analysis of the MIMIC database, and some bias of this study design is possible. Prospective cohort studies with a large sample should be carried out in the future to investigate the association between the ΔRDW and adverse events. Second, data on the left ventricular ejection fraction were not available in the database. Therefore, we failed to analyse the association between the ΔRDW and mortality in patients with HF and different phenotypes. Third, the variables of iron, ferritin, vitamin B12, and folic acid were not available in the database. A change in haematopoietic material during hospitalization should be taken into consideration in future studies. Fourth, although we used dummy variables in the multivariable analysis and removed missing data in the sensitivity analysis, the effect of the missing data for BMI and NT‐proBNP cannot be neglected. Finally, our study focused on the in‐hospital change and outcomes in HF. The longitudinal change of the RDW after discharge requires further study. Although several studies showed that serial increases in the RDW were associated with a poor long‐term prognosis, 26 , 54 the longitudinal ΔRDW at a longer follow‐up period and its relation to the risk of mortality in HF are unclear.
Conclusion
An increased RDW during hospitalization is independently associated with all‐cause mortality in critically ill patients with HF. An in‐hospital ΔRDW could be a novel marker for risk stratification for mortality in patients with HF.
Conflict of interest
None declared.
Funding
This study was funded by Tianjin Key Medical Discipline(Specialty) Construction Project (TJYXZDXK‐055B) and the Natural Science Foundation of Tianjin City, China (S20ZDB477).
Supporting information
Figure S1. Flow chart of the study population.
Figure S2. Kaplan–Meier curves for mortality in matched patients (A) 90‐day mortality (B) 180‐day mortality (C) 1‐year mortality.
Figure S3. Relationship between RDW at discharge and risk of mortality presented by restricted cubic splines (A) 90‐day mortality (B) 180‐day mortality (C) 1‐year mortality.
Figure S4. Comparison of the AUC of ROC curve of RDW at admission, ∆RDW and RDW at discharge for the prediction value of mortality (A) 90‐day mortality (B) 180‐day mortality (C) 1‐year mortality.
Table S1. Threshold effect analysis of ΔRDW on mortality.
Table S2. Baseline characteristics of study population before and after matching according the reference point of ΔRDW (0.7%).
Table S3. Association between ΔRDW and risk of mortality before and after matching.
Table S4. Association between ΔRDW and mortality in patients without missing data (n = 2021).
Table S5. Association between RDW at discharge and risk of mortality.
Table S6. Comparison of the AUC of ROC curve of RDW at admission, ΔRDW and RDW at discharge for the prediction value of mortality.
Table S7. Associations between baseline characteristics and high ΔRDW(ΔRDW≥0.7).
Acknowledgements
The authors thank Medlive Inc. for scientific editing of this manuscript and review services.
Zhang, Q. , Zhou, B. , Li, X. , and Cong, H. (2023) In‐hospital changes in the red blood cell distribution width and mortality in critically ill patients with heart failure. ESC Heart Failure, 10: 3287–3298. 10.1002/ehf2.14513.
References
- 1. Bessman JD, Gilmer PR Jr, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol 1983;80:322‐326. doi: 10.1093/ajcp/80.3.322 [DOI] [PubMed] [Google Scholar]
- 2. Fava C, Cattazzo F, Hu ZD, Lippi G, Montagnana M. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: Useful or hype? Ann Transl Med 2019;7:581. doi: 10.21037/atm.2019.09.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pernow J, Mahdi A, Yang J, Zhou Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc Res 2019;115:1596‐1605. doi: 10.1093/cvr/cvz156 [DOI] [PubMed] [Google Scholar]
- 4. Salvagno GL, Sanchis‐Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86‐105. doi: 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 5. Li N, Zhou H, Tang Q. Red blood cell distribution width: A novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers 2017;2017:7089493. doi: 10.1155/2017/7089493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uyarel H, Isik T, Ayhan E, Ergelen M. Red cell distrubition width (RDW): A novel risk factor for cardiovascular disease. Int J Cardiol 2012;154:351‐352. doi: 10.1016/j.ijcard.2011.10.126 [DOI] [PubMed] [Google Scholar]
- 7. Talarico M, Manicardi M, Vitolo M, Malavasi VL, Valenti AC, Sgreccia D, et al. Red cell distribution width and patient outcome in cardiovascular disease: A “real‐world” analysis. J Cardiovasc Dev Dis 2021;8:120. doi: 10.3390/jcdd8100120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim M, Lee CJ, Kang HJ, Son NH, Bae S, Seo J, et al. Red cell distribution width as a prognosticator in patients with heart failure. ESC Heart Fail 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Wang Y, Chen N, Liu Y, Xiao J, Lin Z, et al. Red cell distribution width is associated with short‐term mortality in critically ill patients with heart failure. ESC Heart Fail 2022;9:3210‐3220. doi: 10.1002/ehf2.14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al‐Najjar Y, Goode KM, Zhang J, Cleland JG, Clark AL. Red cell distribution width: An inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail 2009;11:1155‐1162. doi: 10.1093/eurjhf/hfp147 [DOI] [PubMed] [Google Scholar]
- 11. Jackson CE, Dalzell JR, Bezlyak V, Tsorlalis IK, Myles RC, Spooner R, et al. Red cell distribution width has incremental prognostic value to B‐type natriuretic peptide in acute heart failure. Eur J Heart Fail 2009;11:1152‐1154. doi: 10.1093/eurjhf/hfp157 [DOI] [PubMed] [Google Scholar]
- 12. van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL Jr. Red blood cell distribution width and 1‐year mortality in acute heart failure. Eur J Heart Fail 2010;12:129‐136. doi: 10.1093/eurjhf/hfp179 [DOI] [PubMed] [Google Scholar]
- 13. Zhu X, Cheang I, Xu F, Gao R, Liao S, Yao W, et al. Long‐term prognostic value of inflammatory biomarkers for patients with acute heart failure: Construction of an inflammatory prognostic scoring system. Front Immunol 2022;13:1005697. doi: 10.3389/fimmu.2022.1005697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenei ZM, Förhécz Z, Gombos T, Pozsonyi Z, Jánoskuti L, Prohászka Z. Red cell distribution width as predictive marker in CHF: Testing of model performance by reclassification methods. Int J Cardiol 2014;174:783‐785. doi: 10.1016/j.ijcard.2014.04.107 [DOI] [PubMed] [Google Scholar]
- 15. Su JL, Zhang SG, Gao RJ, Han QF, Wang LH, Zhou YH, et al. Red cell distribution width is a predictor of mortality in patients with chronic heart failure. Int J Cardiol 2016;212:79‐81. doi: 10.1016/j.ijcard.2016.03.064 [DOI] [PubMed] [Google Scholar]
- 16. Hullin R, Barras N, Abdurashidova T, Monney P, Regamey J. Red cell distribution width and prognosis in acute heart failure: Ready for prime time! Intern Emerg Med 2019;14:195‐197. doi: 10.1007/s11739-018-1995-7 [DOI] [PubMed] [Google Scholar]
- 17. Melchio R, Rinaldi G, Testa E, Giraudo A, Serraino C, Bracco C, et al. Red cell distribution width predicts mid‐term prognosis in patients hospitalized with acute heart failure: The RDW in acute heart failure (RE‐AHF) study. Intern Emerg Med 2019;14:239‐247. doi: 10.1007/s11739-018-1958-z [DOI] [PubMed] [Google Scholar]
- 18. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al.; CHARM Investigators . Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM program and the Duke databank. J Am Coll Cardiol 2007;50:40‐47. doi: 10.1016/j.jacc.2007.02.067 [DOI] [PubMed] [Google Scholar]
- 19. Salvatori M, Formiga F, Moreno‐Gonzalez R, Chivite D, Migone De Amicis M, Cappellini MD, et al. Red blood cell distribution width as a prognostic factor of mortality in elderly patients firstly hospitalized due to heart failure. Kardiol Pol 2019;77:632‐638. doi: 10.33963/KP.14818 [DOI] [PubMed] [Google Scholar]
- 20. Wolowiec L, Rogowicz D, Banach J, Buszko K, Surowiec A, Blazejewski J, et al. Prognostic significance of red cell distribution width and other red cell parameters in patients with chronic heart failure during two years of follow‐up. Kardiol Pol 2016;74:657‐664. doi: 10.5603/KP.a2016.0004 [DOI] [PubMed] [Google Scholar]
- 21. Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: Prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659‐666. doi: 10.1016/j.ahj.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 22. Xanthopoulos A, Giamouzis G, Dimos A, Skoularigki E, Starling RC, Skoularigis J, et al. Red blood cell distribution width in heart failure: Pathophysiology, prognostic role, controversies and dilemmas. J Clin Med 2022;11:1951. doi: 10.3390/jcm11071951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makhoul BF, Khourieh A, Kaplan M, Bahouth F, Aronson D, Azzam ZS. Relation between changes in red cell distribution width and clinical outcomes in acute decompensated heart failure. Int J Cardiol 2013;167:1412‐1416. doi: 10.1016/j.ijcard.2012.04.065 [DOI] [PubMed] [Google Scholar]
- 24. Turcato G, Zorzi E, Prati D, Ricci G, Bonora A, Zannoni M, et al. Early in‐hospital variation of red blood cell distribution width predicts mortality in patients with acute heart failure. Int J Cardiol 2017;243:306‐310. doi: 10.1016/j.ijcard.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 25. Muhlestein JB, Lappe DL, Anderson JL, Muhlestein JB, Budge D, May HT, et al. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30‐day outcomes. Int J Lab Hematol 2016;38:328‐337. doi: 10.1111/ijlh.12490 [DOI] [PubMed] [Google Scholar]
- 26. Cauthen CA, Tong W, Jain A, Tang WH. Progressive rise in red cell distribution width is associated with disease progression in ambulatory patients with chronic heart failure. J Card Fail 2012;18:146‐152. doi: 10.1016/j.cardfail.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Watanabe H, et al. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. J Cardiol 2016;67:268‐273. doi: 10.1016/j.jjcc.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 28. Ferreira JP, Girerd N, Arrigo M, Medeiros PB, Ricardo MB, Almeida T, et al. Enlarging red blood cell distribution width during hospitalization identifies a very high‐risk subset of acutely decompensated heart failure patients and adds valuable prognostic information on top of Hemoconcentration. Medicine (Baltimore) 2016;95:e3307. doi: 10.1097/MD.0000000000003307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xanthopoulos A, Papamichalis M, Zajichek A, Milinovich A, Kattan MW, Skoularigis J, et al. In‐hospital red blood cell distribution width change in patients with heart failure. Eur J Heart Fail 2019;21:1659‐1661. doi: 10.1002/ejhf.1546 [DOI] [PubMed] [Google Scholar]
- 30. Turcato G, Cervellin G, Bonora A, Prati D, Zorzi E, Ricci G, et al. Red blood cell distribution width improves reclassification of patients admitted to the emergency department with acute decompensated heart failure. J Med Biochem 2018;37:299‐306. doi: 10.1515/jomb-2017-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tseliou E, Terrovitis JV, Kaldara EE, Ntalianis AS, Repasos E, Katsaros L, et al. Red blood cell distribution width is a significant prognostic marker in advanced heart failure, independent of hemoglobin levels. Hellenic J Cardiol 2014;55:457‐461. [PubMed] [Google Scholar]
- 32. Oh J, Kang SM, Hong N, Choi JW, Lee SH, Park S, et al. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J Card Fail 2009;15:517‐522. doi: 10.1016/j.cardfail.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 33. Senthong V, Hudec T, Neale S, Wu Y, Hazen SL, Tang WH. Relation of red cell distribution width to left ventricular end‐diastolic pressure and mortality in patients with and without heart failure. Am J Cardiol 2017;119:1421‐1427. doi: 10.1016/j.amjcard.2017.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eroglu E, Kilicgedik A, Kahveci G, Bakal RB, Kirma C. Red cell distribution width and its relationship with global longitudinal strain in patients with heart failure with reduced ejection fraction: A study using two‐dimensional speckle tracking echocardiography. Kardiol Pol 2018;76:580‐585. doi: 10.5603/KP.a2017.0256 [DOI] [PubMed] [Google Scholar]
- 35. van Craenenbroeck EM, Pelle AJ, Beckers PJ, Possemiers NM, Ramakers C, Vrints CJ, et al. Red cell distribution width as a marker of impaired exercise tolerance in patients with chronic heart failure. Eur J Heart Fail 2012;14:54‐60. doi: 10.1093/eurjhf/hfr136 [DOI] [PubMed] [Google Scholar]
- 36. Poglajen G, Sever M, Cernelc P, Haddad F, Vrtovec B. Increased red cell distribution width is associated with poor stem cell mobilization in patients with advanced chronic heart failure. Biomarkers 2015;20:365‐370. doi: 10.3109/1354750X.2015.1094137 [DOI] [PubMed] [Google Scholar]
- 37. Hong SJ, Youn JC, Oh J, Hong N, Lee HS, Park S, et al. Red cell distribution width as an independent predictor of exercise intolerance and ventilatory inefficiency in patients with chronic heart failure. Yonsei Med J 2014;55:635‐643. doi: 10.3349/ymj.2014.55.3.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pascual‐Figal DA, Bonaque JC, Redondo B, Caro C, Manzano‐Fernandez S, Sanchez‐Mas J, et al. Red blood cell distribution width predicts long‐term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail 2009;11:840‐846. doi: 10.1093/eurjhf/hfp109 [DOI] [PubMed] [Google Scholar]
- 39. Dai Y, Konishi H, Takagi A, Miyauchi K, Daida H. Red cell distribution width predicts short‐ and long‐term outcomes of acute congestive heart failure more effectively than hemoglobin. Exp Ther Med 2014;8:600‐606. doi: 10.3892/etm.2014.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katsiadas N, Xanthopoulos A, Giamouzis G, Skoularigkis S, Skopeliti N, Moustaferi E, et al. The effect of SGLT ‐ 2i administration on red blood cell distribution width in patients with heart failure and type 2 diabetes mellitus: A randomized study. Front Cardiovasc Med 2022;9:984092. doi: 10.3389/fcvm.2022.984092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siedlecki L, Szygula‐Jurkiewicz B, Szczurek W, Pyka L, Niedziela J, Gasior M. Mortality risk factors in patients with advanced heart failure and diabetes mellitus. Kardiol Pol 2019;77:604‐609. doi: 10.33963/KP.14813 [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Wang Y, Kang JS, Yu JX, Yin SJ, Cong XF, et al. Differences in the predictive value of red cell distribution width for the mortality of patients with heart failure due to various heart diseases. J Geriatr Cardiol 2015;12:647‐654. doi: 10.11909/j.issn.1671-5411.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sotiropoulos K, Yerly P, Monney P, Garnier A, Regamey J, Hugli O, et al. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Fail 2016;3:198‐204. doi: 10.1002/ehf2.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imai R, Uemura Y, Okumura T, Takemoto K, Uchikawa T, Koyasu M, et al. Impact of red blood cell distribution width on non‐cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J Cardiol 2017;70:591‐597. doi: 10.1016/j.jjcc.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 45. Liang L, Huang L, Zhao X, Zhao L, Tian P, Huang B, et al. Prognostic value of RDW alone and in combination with NT‐proBNP in patients with heart failure. Clin Cardiol 2022;45:802‐813. doi: 10.1002/clc.23850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xanthopoulos A, Giamouzis G, Tryposkiadis K, Paraskevopoulou E, Paraskevopoulou P, Karagiannis G, et al. A simple score for early risk stratification in acute heart failure. Int J Cardiol 2017;230:248‐254. doi: 10.1016/j.ijcard.2016.12.131 [DOI] [PubMed] [Google Scholar]
- 47. Topaz G, Haim M, Kusniec J, Kazum S, Goldenberg G, Golovchiner G, et al. Association between red cell distribution width and mortality after cardiac resynchronization therapy. Isr Med Assoc J 2015;17:505‐509. [PubMed] [Google Scholar]
- 48. Boros AM, Perge P, Jenei Z, Karady J, Zima E, Molnar L, et al. Measurement of the red blood cell distribution width improves the risk prediction in cardiac resynchronization therapy. Dis Markers 2016;2016:7304538. doi: 10.1155/2016/7304538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller PE, Houston BA, Schneider AL, Bush AL, Whitman GJ, Stevens GR, et al. Associations of preimplant red blood cell distribution width with clinical outcomes among individuals with left ventricular assist devices. ASAIO J 2016;62:677‐683. doi: 10.1097/MAT.0000000000000431 [DOI] [PubMed] [Google Scholar]
- 50. Truby LK, Sridharan L, Flores RJ, Garan AR, Jennings D, Yuzefpolskaya M, et al. Red cell distribution width predicts 90 day mortality in continuous‐flow left ventricular assist device patients. ASAIO J 2019;65:233‐240. doi: 10.1097/MAT.0000000000000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szygula‐Jurkiewicz B, Szczurek W, Skrzypek M, Nadziakiewicz P, Siedlecki L, Zakliczynski M, et al. Red blood cell distribution width in end‐stage heart failure patients is independently associated with all‐cause mortality after orthotopic heart transplantation. Transplant Proc 2018;50:2095‐2099. doi: 10.1016/j.transproceed.2018.02.141 [DOI] [PubMed] [Google Scholar]
- 52. Spadaro S, Taccone FS, Fogagnolo A, Franchi F, Scolletta S, Ragazzi R, et al. The effects of blood transfusion on red blood cell distribution width in critically ill patients: A pilot study. Transfusion 2018;58:1863‐1869. doi: 10.1111/trf.14759 [DOI] [PubMed] [Google Scholar]
- 53. Mahmoodpoor A, Gamari AA, Sanaie S, Dolati S, Yusefi B, Nader ND. Post‐transfusion changes in red cell distribution width predicts survival in critically ill patients. J Clin Anesth 2021;73:110335. doi: 10.1016/j.jclinane.2021.110335 [DOI] [PubMed] [Google Scholar]
- 54. Oh J, Kang SM, Won H, Hong N, Kim SY, Park S, et al. Prognostic value of change in red cell distribution width 1 month after discharge in acute decompensated heart failure patients. Circ J 2012;76:109‐116. doi: 10.1253/circj.cj-11-0664 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the study population.
Figure S2. Kaplan–Meier curves for mortality in matched patients (A) 90‐day mortality (B) 180‐day mortality (C) 1‐year mortality.
Figure S3. Relationship between RDW at discharge and risk of mortality presented by restricted cubic splines (A) 90‐day mortality (B) 180‐day mortality (C) 1‐year mortality.
Figure S4. Comparison of the AUC of ROC curve of RDW at admission, ∆RDW and RDW at discharge for the prediction value of mortality (A) 90‐day mortality (B) 180‐day mortality (C) 1‐year mortality.
Table S1. Threshold effect analysis of ΔRDW on mortality.
Table S2. Baseline characteristics of study population before and after matching according the reference point of ΔRDW (0.7%).
Table S3. Association between ΔRDW and risk of mortality before and after matching.
Table S4. Association between ΔRDW and mortality in patients without missing data (n = 2021).
Table S5. Association between RDW at discharge and risk of mortality.
Table S6. Comparison of the AUC of ROC curve of RDW at admission, ΔRDW and RDW at discharge for the prediction value of mortality.
Table S7. Associations between baseline characteristics and high ΔRDW(ΔRDW≥0.7).


