This randomized clinical trial aims to determine if a personalized, multidomain risk-reduction intervention improves cognition and dementia risk profile among adults 70 years and older.
Key Points
Question
Can a personalized, multidomain risk-reduction intervention improve cognition and dementia risk factor profile among older adults?
Findings
In this 2-year randomized clinical trial of 172 adults aged 70 to 89 years who were at high risk for dementia, randomization to the personalized, multidomain intervention group led to modest improvements in cognitive composite score, dementia risk factors, and quality of life compared with a health education control group.
Meaning
Modifiable risk-reduction strategies should be considered for older adults at risk for dementia.
Abstract
Importance
Modifiable risk factors are hypothesized to account for 30% to 40% of dementia; yet, few trials have demonstrated that risk-reduction interventions, especially multidomain, are efficacious.
Objective
To determine if a personalized, multidomain risk reduction intervention improves cognition and dementia risk profile among older adults.
Design, Setting, and Participants
The Systematic Multi-Domain Alzheimer Risk Reduction Trial was a randomized clinical trial with a 2-year personalized, risk-reduction intervention. A total of 172 adults at elevated risk for dementia (age 70-89 years and with ≥2 of 8 targeted risk factors) were recruited from primary care clinics associated with Kaiser Permanente Washington. Data were collected from August 2018 to August 2022 and analyzed from October 2022 to September 2023.
Intervention
Participants were randomly assigned to the intervention (personalized risk-reduction goals with health coaching and nurse visits) or to a health education control.
Main Outcomes and Measures
The primary outcome was change in a composite modified Neuropsychological Test Battery; preplanned secondary outcomes were change in risk factors and quality of life (QOL). Outcomes were assessed at baseline and 6, 12, 18, and 24 months. Linear mixed models were used to compare, by intention to treat, average treatment effects (ATEs) from baseline over follow-up. The intervention and outcomes were initially in person but then, due to onset of the COVID-19 pandemic, were remote.
Results
The 172 total participants had a mean (SD) age of 75.7 (4.8) years, and 108 (62.8%) were women. After 2 years, compared with the 90 participants in the control group, the 82 participants assigned to intervention demonstrated larger improvements in the composite cognitive score (ATE of SD, 0.14; 95% CI, 0.03-0.25; P = .02; a 74% improvement compared with the change in the control group), better composite risk factor score (ATE of SD, 0.11; 95% CI, 0.01-0.20; P = .03), and improved QOL (ATE, 0.81 points; 95% CI, −0.21 to 1.84; P = .12). There were no between-group differences in serious adverse events (24 in the intervention group and 23 in the control group; P = .59), but the intervention group had greater treatment-related adverse events such as musculoskeletal pain (14 in the intervention group vs 0 in the control group; P < .001).
Conclusions and Relevance
In this randomized clinical trial, a 2-year, personalized, multidomain intervention led to modest improvements in cognition, dementia risk factors, and QOL. Modifiable risk-reduction strategies should be considered for older adults at risk for dementia.
Trial Registration
ClinicalTrials.gov Identifier: NCT03683394
Introduction
Alzheimer disease and related dementias (ADRD) are highly prevalent, costly, and some of the most feared illnesses of older adults. Yet, few effective preventions or treatments exist,1,2 and recently approved amyloid-reduction drugs are expensive, have fairly strict eligibility, and require extensive monitoring for adverse effects.3,4,5,6 The urgent unmet need for prevention and alternative therapies has prompted interest in risk-reduction strategies, given that up to 40% of ADRD risk might be modifiable by targeting lifestyle, medical, and behavioral risk factors.7,8
Several, but not all, trials have supported the role for interventions such as cardiovascular risk factor reduction, especially aggressive systolic blood pressure control, increased physical or cognitive activity, and other single-domain behavioral interventions for ADRD prevention.9,10,11,12 In addition, some multidomain trials, primarily those conducted in Europe, have reported that interventions targeting multiple risk factors simultaneously can slow cognitive decline and reduce cognitive impairment,13,14 although results have been mixed.15,16 Studies are needed to clarify the effects of different intervention approaches, including a personalized approach, in which risk factors are targeted based on a participant’s risk profile, preferences, and priorities for risk reduction. Herein, we present results from the Systematic Multi-Domain Alzheimer Risk Reduction Trial (SMARRT), a pilot randomized clinical trial (RCT) testing a personalized, multidomain AD risk reduction intervention in an integrated US health care delivery system.
Methods
Study Design and Participants
A detailed SMARRT protocol was published previously17 and can be found in Supplement 1. Participants were members of Kaiser Permanente Washington (KPWA), an integrated health care delivery system in the Seattle area. Eligible participants were KPWA members aged 70 to 89 years with at least 2 dementia risk factors targeted by the intervention: (1) physical inactivity, (2) uncontrolled hypertension, (3) poor sleep, (4) taking a prescription medication that may adversely affect cognition, (5) high depressive symptoms, (6) uncontrolled diabetes, (7) social isolation, and (8) currently smoking (as defined in the eTable in Supplement 2).
Initial eligibility was determined through analyzing electronic health records (EHRs) algorithmically, using diagnosis codes, medication dispensings, blood pressure values, and laboratory test results. Individuals who gave KPWA permission to be contacted for research and who had at least 1 risk factor identified from their EHR were eligible for further screening. To obtain a diverse sample, Hispanic individuals and individuals who were a race other than White were oversampled in the EHRs. Eligible individuals then completed a telephone screening to determine interest and further eligibility, including additional assessment and confirmation of the 8 risk factors. We excluded members who had fewer than 2 risk factors, resided in a skilled nursing facility, were receiving hospice care, had dementia (determined from the EHR or score <25 on a telephone screening of the Cognitive Abilities Screening Instrument [CASI]),18 or had certain chronic medical and mental health conditions that would limit trial participation. Comorbidities were summarized with the Elixhauser Comorbidity Index.19,20
All study procedures were approved by institutional review boards at KPWA and the University of California, San Francisco. All study participants provided written informed consent. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Randomization and Blinding
After the baseline visit, participants were randomized in a 1:1 ratio to intervention or control using randomly permuted blocks of size 2, stratified by clinic, age (70-79 years compared with 80-89 years), and race and ethnicity (non-Hispanic White compared with races and ethnicities other than White [American Indian, Asian, Black, Hispanic, or other, which included Native Hawaiian or Other Pacific Islander owing to small sample size and self-reported other or unknown race and “prefer not to answer”]). Race and ethnicity were collected as reported in EHRs. After randomization, a study coordinator outlined study next steps, which differed based on group assignment. The KPWA study physician (E.B.L.), study coordinator (L.E.F.), and some members of the intervention team were not blinded (B.H.B., D.E.R., S.D.), but the remaining study personnel, including the outcomes assessors, were blinded to group assignment (K.Y., E.V., D.E.B., C.B.P.).
Intervention
The intervention used a personalized, patient-centered approach delivered through a health coach and nurse with contacts in person (prior to the COVID-19 pandemic) or by telephone (for those who preferred telephone prior to the pandemic and for all participants after onset of the pandemic). Health coaching sessions were offered every 4 to 6 weeks to set goals related to risk factors chosen by participants,17 with more frequent visits in the first 3 months, then every 6 weeks for the final 15 months of the intervention. At the first intervention visit, the health coaches used a visual aid to facilitate conversation about the modifiable risk factors, which was individualized for each participant. A red section listed risk factors that were suboptimal for that participant, and a green section reinforced behaviors the participant was already engaged in. Then the health coaches helped the participants set specific goals for suboptimally controlled risk factors that they were interested in working on (ie, sign up for a class, walk a certain number of steps daily). Goals were reviewed at each coaching visit. Once a goal was reached, a new goal was set either for that risk factor or another risk factor as the participant preferred. Time per coaching session was greater in the first 3 months (approximately 45 minutes), then reduced to 20 minutes for the remainder of the calls. Health coaches had master’s degrees in social work or psychology and received training in motivational interviewing techniques (eg, eliciting values, reinforcing change). A standardized coaching manual and resources for each risk factor were provided. Participants were given information on selected risk factors, self-monitoring tools, and resources (eg, fitness tracker, sleep workbook, brain games), depending on preference. The risk factor targets included the 8 factors from inclusion criteria, but participants could also work on other nontargeted health goals, such as diet and cognitive activity.
Participants with uncontrolled diabetes, uncontrolled hypertension, or risky medication use received quarterly contacts from the intervention nurse. We used a treat-to-target approach based on KPWA clinical guidelines and brief electronic communication with the primary care team as needed. For those targeting hypertension, a portable blood pressure monitor was provided. Risky medications were identified from 2015 Beers criteria21 for medications that can impair cognition (eg, anticholinergics, sedative-hypnotics). When a risky medication was identified, the study nurse outlined possible cognitive risks and encouraged the participant to discuss alternatives with their primary care clinician.
Health Education Control Group
Participants randomized to the health education (HE) group received mailed educational materials (typically 1-2 pages) every 3 months. The materials, from sources such as the Alzheimer’s Association and those provided as part of routine care at KPWA, included information on ADRD and dementia risk reduction, including risk factors targeted in the SMARRT intervention.
Outcomes
Most outcomes were assessed every 6 months (baseline and at 6, 12, 18, and 24 months) in both study groups. All participants had an in-person baseline visit; however, due to the COVID-19 pandemic, which occurred early in the trial, the protocol was modified to include telephone outcome assessment. Since study participants were without dementia, we chose a cognitive outcome that would be sensitive to small cognitive changes over time and was previously used in multidomain ADRD prevention trials. The primary outcome was a composite z score based on the modified Neuropsychological Test Battery,13,22,23,24 composed of the CASI telephone test (global cognition), revised Wechsler Memory Scale Logical Memory test (verbal memory) and Digit Span test (attention), and the Category Fluency and Phonemic Fluency tests (language).
One secondary outcome was a composite z score for risk factors (measured using different methods than for study eligibility) based on the following: (1) the Rapid Assessment of Physical Activity for older adults (range, 1-7, with higher score indicating more activity)25; (2) a waist-worn accelerometer (ActiGraph) to measure steps per day averaged over 7 days (assessed at baseline and 24 months in a subset); (3) blood pressure measures from routine clinical visits (from EHRs) averaged for each 6 months for participants with hypertension; (4) the Pittsburgh Sleep Quality Index (range, 0-21, with lower score indicating better sleep)26; (5) the use of potentially harmful prescription medications (≥2 medication fills in the same class on the 2015 Beers Criteria in the past 6 months); (6) the Center for Epidemiologic Studies Depression Scale (range, 0-60, with lower score indicating fewer symptoms)27; (7) hemoglobin A1c values from EHRs for participants with diabetes averaged over 12 months; (8) the Patient-Reported Outcomes Measurement Information System (PROMIS) satisfaction with social activities short form28 (range, 7-35, with higher score indicating greater social satisfaction); and (9) self-reported smoking. Both composite z scores were calculated as the average of all available scale results, each standardized to have a mean of 0 and SD of 1 before averaging. The other secondary outcome was quality of life (QOL), based on physical, mental, and social health; pain; and fatigue, using the PROMIS Global Health measure.29
Adverse events (AEs) and serious AEs were assessed for both groups by questionnaire every 3 months. Participants completed a standardized questionnaire at regular study assessment visits (6, 12, 18, and 24 months) and via a mailed form in between study visits (3, 9, 15, and 21 months). All events were rated by the study physician (E.B.L.) to determine if they were treatment related. At the 24-month visit, all participants were invited to answer questions about study satisfaction (on a 4-point Likert scale with 1 indicating not satisfied; 2, a little satisfied; 3, satisfied; and 4, very satisfied).
Statistical Analysis
The comparability of the intervention group and HE control group was assessed using t test or χ2 test, as appropriate. In our intention-to-treat approach, all available outcomes were included, without regard to adherence or study completion. For continuous outcomes assessed at baseline and 6, 12, 18, and 24 months, including the primary and both secondary outcomes, treatment effects were estimated using linear mixed models for the changes from baseline to each follow-up assessment, with average treatment effects (ATEs) estimated by the average of the 4-visit–specific between-group differences in mean change from baseline. The linear mixed models included random intercepts to account for the within-participant correlation of the repeated measures. For continuous outcomes measured only at baseline and 24 months, linear models for changes from baseline were used. For binary outcomes, generalized estimating equations logistic models with robust standard errors clustered on participants were used.30
In a sensitivity analysis, adjustment was made for sex, race and ethnicity, education status, the unweighted baseline Elixhauser score (to adjust for group differences postrandomization), and a visit-specific indicator whether the follow-up visit was by telephone (to adjust for the onset of the COVID-19 pandemic and remote assessment). As a second sensitivity analysis, we repeated the models with participants who completed the trial. Data management was performed in SAS, version 9.4 (SAS Institute Inc), and analyses were implemented using Stata, version 17.2 (StataCorp). All statistical tests were 2-sided and statistical significance was determined at P < .05.
Results
Patient Population
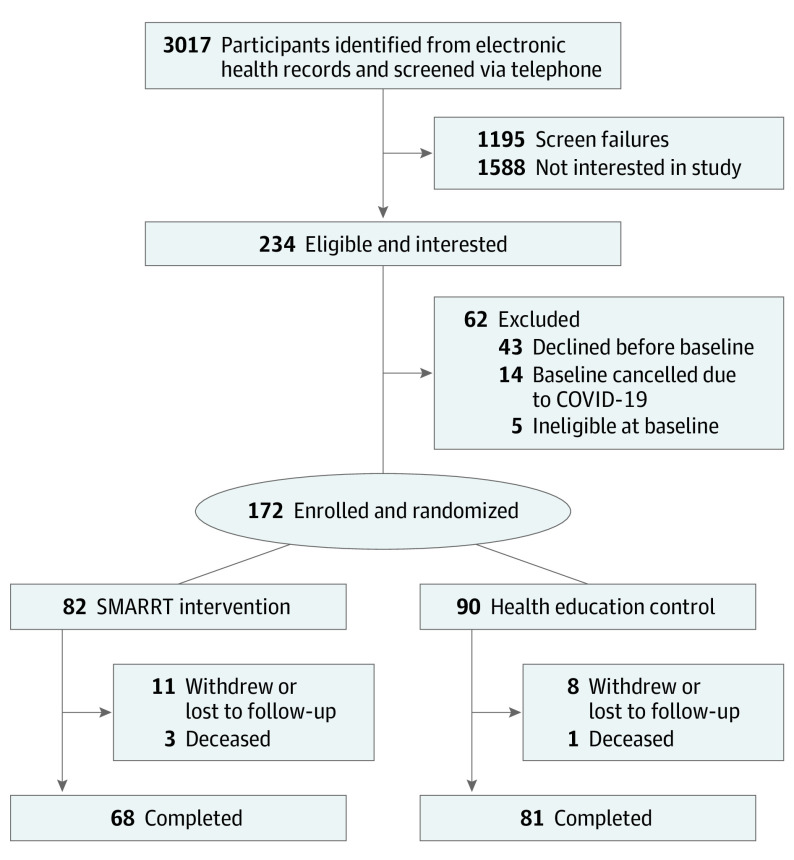
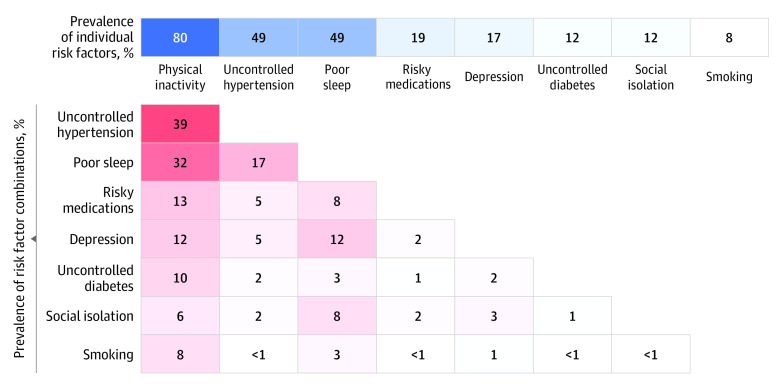
The study recruitment flow and inclusion are depicted in Figure 1. Between August 2018 and March 2020, 3017 individuals were screened by telephone, with 1195 ineligible (mostly due to having only 1 risk factor), 1158 uninterested in participating (primarily due to time), and 234 eligible and interested. Due to the COVID-19 pandemic shutdown, enrollment was curtailed at 172 participants (mean [SD] age, 75.7 [4.8] years; 108 [62.8%] female). Compared with the 90 participants randomized to the HE control, the 82 assigned to the intervention were more likely to be female and had a slightly higher comorbidity score but did not differ on other characteristics, including screening risk factors (Table 1). Mean (SD) baseline telephone CASI scores were also similar (control: 29.8 [1.8]; intervention: 29.6 [2.1]; P = .48). Baseline risk factors assessed via questionnaires/self-report as well as other cognitive function tests were similar across treatment groups (Table 2). The mean (SD) number of risk factors was 2.5 (0.7), with low physical activity, uncontrolled hypertension, and poor sleep being the most prevalent, with many co-occurring in various patterns (Figure 2).
Figure 1. Recruitment Flow for the Systematic Multi-Domain Alzheimer Risk Reduction Trial (SMARRT).
Table 1. Participant Demographics and Baseline Risk.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| Control group (n = 90) | Intervention group (n = 82) | ||
| Age, mean (SD), y | 75.6 (4.7) | 75.8 (4.9) | .82 |
| Sex | |||
| Female | 51 (56.7) | 57 (69.5) | .08 |
| Male | 39 (43.3) | 25 (30.5) | |
| Racea | |||
| American Indian | 4 (4.4) | 2 (2.4) | .72 |
| Asian | 5 (5.6) | 3 (3.7) | |
| Black | 9 (6.7) | 9 (11.0) | |
| White | 76 (84.4) | 64 (78.0) | |
| Otherb | 4 (4.4) | 4 (4.9) | |
| Hispanic ethnicitya | 5 (6.5) | 2(3.1) | .46 |
| Education, mean (SD), y | 16.4 (2.4) | 16.0 (2.8) | .34 |
| Elixhauser Comorbidity Index, mean (SD) | 2.3 (1.7) | 2.8 (1.9) | .06 |
| Screening risk factors | |||
| Physical inactivity | 73 (81.1) | 64 (78.0) | .71 |
| Uncontrolled hypertension | 43 (47.8) | 41 (50.0) | .88 |
| Poor sleep | 43 (48.3) | 40 (48.8) | >.99 |
| Risky medications | 18 (20.0) | 15 (18.3) | .85 |
| Depression | 15 (16.9) | 15 (18.3) | .84 |
| Uncontrolled diabetes | 8 (8.9) | 12 (14.6) | .34 |
| Social isolation | 8 (9.0) | 12 (14.6) | .34 |
| Smoking | 6 (6.7) | 7 (9.8) | .77 |
| No. of risk factors, mean (SD) | 2.4 (0.6) | 2.5 (0.7) | .17 |
The race and ethnicity variables were taken from electronic health records.
The other category includes Native Hawaiian or Other Pacific Islander (very small sample size) and self-reported other or unknown race and “prefer not to answer.”
Table 2. Values for Outcome Measures at Baseline by Treatment Group.
| Variable | Mean (SD) | P value | |
|---|---|---|---|
| Control group (n = 90) | Intervention group (n = 82) | ||
| Cognitive outcomes | |||
| CASI telephone screening | 29.8 (1.8) | 29.6 (2.1) | .48 |
| Wechsler Memory Scale Logical Memory test | 39.6 (14.8) | 38.2 (14.3) | .53 |
| Category Fluency test | 21.4 (5.8) | 22.6 (5.8) | .19 |
| Phonemic Fluency test | 39.2 (12.1) | 40.2 (12.4) | .61 |
| Wechsler Memory Scale Digit Span test | 6.0 (2.0) | 6.0 (2.0) | .90 |
| Risk factor outcomes | |||
| Physical activity (RAPA for older adults) | 6.1 (2.3) | 6.4 (2.4) | .36 |
| Systolic blood pressurea | 140.2 (10.1) | 138.9 (11.4) | .59 |
| Diastolic blood pressurea | 75.7 (8.5) | 73.4 (7.1) | .19 |
| Sleep quality (PSQI) | 9.3 (3.3) | 9.3 (3.4) | .89 |
| Risky medications, No. (%)b | 18 (20.0) | 15 (18.3) | .85 |
| Depression (CES-D score) | 10.4 (8.4) | 10.9 (8.8) | .73 |
| Diabetes control (hemoglobin A1c)c | 8.9 (1.3) | 8.5(1.1) | .51 |
| Social satisfaction (PROMIS) | 25.6 (6.7) | 25.7 (6.2) | .96 |
| Smoking, No. (%) | 6 (6.7) | 7 (8.8) | .77 |
| Quality of life | |||
| PROMIS Global Health | 34.0 (5.8) | 33.9 (5.9) | .92 |
Abbreviations: CASI, Cognitive Abilities Screening Instrument; CES-D, Center for Epidemiological Studies Depression Scale; PROMIS, Patient-Reported Outcomes Measurement Information System; PSQI, Pittsburgh Sleep Quality Index; RAPA, Rapid Assessment of Physical Activity.
Average systolic and diastolic blood pressure in the past 6 months.
Two or more fills of medications in the same class in the past 6 months that are deemed risky for cognition (generally anticholinergics and sedative-hypnotics), identified from the 2015 Beers Criteria.21
Average hemoglobin A1c in the past 12 months in participants with diagnosed diabetes.
Figure 2. Prevalence and Combination of Risk Factors for the 172 Participants in the Systematic Multi-Domain Alzheimer Risk Reduction Trial.
Darker shading indicates higher frequency of individual risk factors (blue) or risk factor combinations (red).
Intervention
Among participants assigned to the intervention group, the mean (SD) number of risk factors chosen to work on over the trial was 3.5 (1.4). The most common risk factors worked on were physical activity (n = 78 [95.1%]), hypertension (n = 55 [67.1%]), sleep (n = 43 [52.4%]), depressive symptoms (n = 37 [45.1%]), and social engagement (n = 34 [41.5%]). The remaining risk factors (risky medications, diabetes, and smoking) were chosen by fewer than 25% of participants. Over the 2 years, the mean (SD) number of health coaching contacts was 18.8 (5.5; range, 1-28). Participants chose to use the following resources: fitness tracker devices for physical activity (n = 42), home blood pressure monitoring kits (n = 24), and sleep workbooks (n = 22). The cost of the supplies averaged less than $80 per participant.
Outcomes
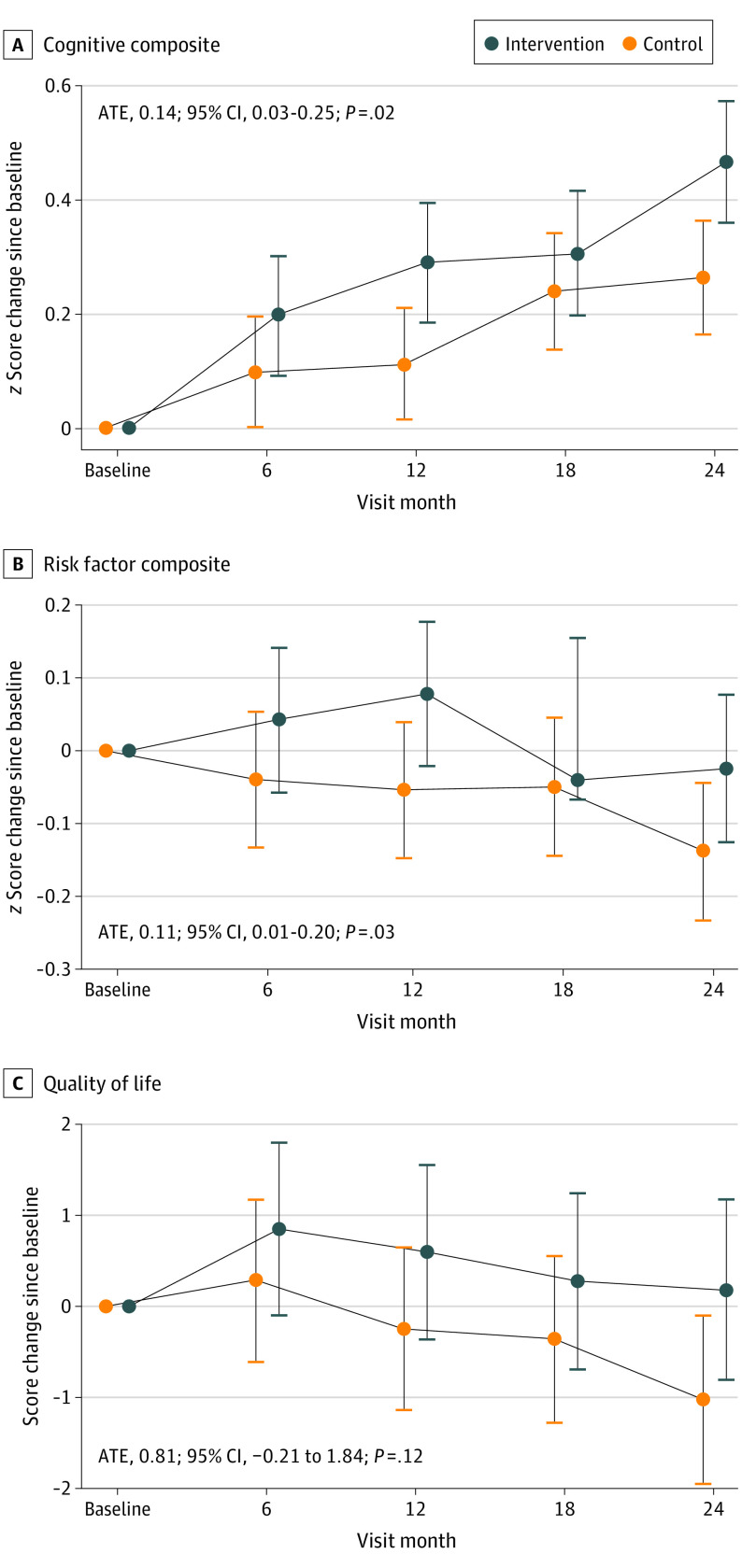
Over the 2 years, 19 participants (11.0%) withdrew or were lost to follow-up (11 in the intervention group and 8 in the control group; P = .34), there were 4 deaths unrelated to the study (3 in the intervention group and 1 in the control group; P = .27), and 149 participants completed the trial. In the intention-to-treat analysis of the composite cognitive primary outcome, the intervention group improved more than the control group (ATE of SD, 0.14; 95% CI, 0.03-0.25; P = .02; Figure 3) (within-group change for intervention: SD, 0.32; 95% CI, 0.24-0.40; within-group change for control: SD, 0.18; 95% CI, 0.11-0.26). This translates to a 74% greater increase in cognition among the intervention group compared with the control group. A similar pattern was observed for most of the individual cognitive domains (eFigure 1 in Supplement 2). In an exploratory analysis, incidence of a low CASI score (<27; consistent with cognitive impairment)18 or diagnosis of mild cognitive impairment (MCI) or dementia was examined. Over the trial, 8 participants in the control group and 5 in the intervention group met this criteria (age- and sex-adjusted odds ratio, 0.68; 95% CI, 0.19-2.19).
Figure 3. Primary and Secondary Outcomes.
Primary (A) and secondary (B and C) outcomes in the Systematic Multi-Domain Alzheimer Risk Reduction Trial by treatment group. ATE indicates average treatment effect. Error bars represent 95% CIs.
Both secondary outcomes also improved in the intervention group compared with the control group, including the composite risk factor score (ATE of SD, 0.11; 95% CI, 0.01-0.20; P = .03; Figure 3) (within-group change for intervention: SD, 0.03; 95% CI, −0.04 to 0.10; within-group change for control: SD, −0.07; 95% CI, −0.14 to 0.01) and the PROMIS QOL score (ATE, 0.81 points; 95% CI, −0.21 to 1.84 points; P = .12; Figure 3) (within-group change for intervention: 11.46 points; 95% CI, 8.35-14.58 points; within-group change for control: 10.65 points; 95% CI, 7.52-13.79 points). The increase in risk factor score translates to a roughly 145% improvement in risk factor profile in the intervention group compared with changes in the control group. For the QOL score, the intervention group improved 8% compared with the control group.
Changes in individual risk factor outcomes by treatment group were also examined, and nearly all were consistent with greater improvement in the intervention group compared with control, with trend level or statistically significant improvements in sleep, depressive symptoms, diabetes control, and social satisfaction (eFigure 2 in Supplement 2). Analyses adjusted for sex, race and ethnicity, education status, comorbidity score, and telephone assessment for all comparisons were almost identical to the unadjusted analyses (eFigure 3 in Supplement 2), and analyses restricted to those completing the intervention demonstrated similar results to the primary analysis for all outcomes.
Study Satisfaction
At the end of the trial, 138 participants (92.6% of completers) answered the study satisfaction survey. Overall satisfaction was high (mean [SD], 3.6 [0.6]); compared with the control group, intervention participants were slightly more satisfied with the study (intervention: mean [SD], 3.7 [0.5]; control: mean [SD], 3.5 [0.7]). All participants were satisfied with the study’s ability to improve their health (mean [SD], 3.1 [0.9]); however, intervention participants had slightly higher satisfaction (intervention: mean [SD], 3.4 [0.7]; control: mean [SD], 2.9 [1.0]).
AEs and Serious AEs
All serious AEs (death, serious illness, hospitalizations) were judged to be unrelated to treatment and were similar by group (24 in the intervention group and 23 in the control group; P = .59). The intervention group reported 14 AEs possibly related to treatment; there were no treatment-related AEs in the control group. The related AEs included 9 musculoskeletal complaints (eg, calf cramp, joint pain, muscle pull), 2 incidents related to anxiety, 2 elevated blood pressure readings at in-person study visits, and 1 skin rash from an activity tracker.
Discussion
In this 2-year, personalized, multidomain pilot RCT for ADRD risk reduction, we found that older adults without dementia and with at least 2 dementia risk factors randomized to the SMARRT intervention demonstrated improvements in composite measures of cognition, dementia risk factors, and QOL compared with those randomized to the HE control. Furthermore, most participants expressed high levels of satisfaction with the intervention. The trial represents a promising strategy that could be examined in larger future trials to determine whether these 2-year gains translate into reduced risk or delayed onset of ADRD.
Several multidomain risk-reduction trials demonstrated a positive effect on cognition, including a recent RCT with 119 participants (MCI or subjective memory complaints), which demonstrated, compared with an education control, greater composite cognition gains and more favorable risk factor profiles after an 8-week multidomain trial (physical activity, Mediterranean diet, and cognitive activity).14 Longer-duration multidomain RCTs that spanned 1 to 2 years have had conflicting results. The 2-year FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) trial demonstrated slightly greater cognitive improvement (as measured by the modified Neuropsychological Test Battery) in the intervention group (cardiovascular risk management, physical activity, cognitive activity, and diet) compared with the control group.13 However, other multidomain RCTs, including preDIVA (Prevention of Dementia by Intensive Vascular Care) and MAPT (Multidomain Alzheimer Prevention Trial), have not found cognitive benefits.15,16 Disparate results may be partly explained by heterogeneity in participants, interventions, and cognitive outcomes. A recent Cochrane meta-analysis (focusing on 8 multidomain trials) concluded that there was little evidence for dementia prevention (only 2 trials included this outcome) but evidence of a small, statistically significant improvement in cognition with an ATE of 0.03 SD on a composite cognitive score.31 The present observed ATE of 0.14 on the composite cognitive outcome is similar to a Cohen d and would be consistent with a small clinical effect.
Like other primary prevention trials in cognitively normal older adults, cognitive scores for both groups in SMARRT improved over time, presumably from practice effects or trial participation. The observed effect size for the intervention, while modest, is greater than most prior multidomain trials or the meta-analysis.31,32 A personalized approach may be more effective in an at-risk population where dementia risk factors and motivations to work on risk reduction vary substantially.33 Prior to SMARRT, we surveyed almost 600 KPWA members, and most respondents were concerned about ADRD, wanted to know their personal risk factors, and were highly motivated to make lifestyle changes to lower dementia risk.34
Given the recent fast pace of ADRD drug discovery, new disease-modifying agents are now an exciting reality.3,4,5,6 Several drugs have recently or will be approved for early AD and MCI (not primary prevention), will require fairly intensive monitoring for AEs (such as amyloid-related imaging abnormalities),35,36 and will be costly. It is challenging to compare efficacy between risk-reduction strategies for primary prevention and pharmacological treatments for AD. Nonetheless, given their low cost, excellent safety profile, and benefit on health and QOL overall, risk-reduction strategies may be used in combination with medications or offered as primary prevention for those at risk for ADRD. Hopefully, in the future, treatment of ADRD will be like cardiovascular disease management, with a combination of risk reduction and specific drugs targeted for disease mechanisms.
Strengths and Limitations
SMARRT has several strengths, especially being, to our knowledge, the first personalized multidomain intervention for ADRD prevention in which participants chose to work on individual risk factors and identified goals and ways to achieve behavioral change. However, there are some limitations, including that the trial was conducted at 1 site and within an integrated health care system, which differs from how many US patients receive health care. Elements of the intervention could generalize to other integrated health care systems, such as the Veterans Affairs health system, but also to other health care systems, using EHRs to identify risk factors and other eligibility criteria and provide the opportunity for remote health coaching and outcome assessment.
As this was a pilot, the trial was not powered to detect small treatment effects on individual risk factors, specific cognitive domains, or incident MCI or dementia. Inherent in the study design was greater interaction with the SMARRT intervention group. Although both groups had 5 outcome visits, the intervention group had greater contact with health and nurse coaches, so it is possible that some of the intervention group’s observed benefits could be due to social contact in addition to changes in risk factors. We focused on risk factors that could be primarily ascertained by EHR data and then verified using standardized questionnaires and, thus, did not include all previously studied ADRD risk factors.
The trial was initiated prior to the COVID-19 pandemic, but the shutdown shortened enrollment and necessitated administering the intervention and measuring outcomes remotely. It is likely that the pandemic negatively affected the targeted dementia risk factors, particularly social isolation, physical activity, and depression,37,38 but this exogenous shock, starting near the time of the 6-month visit, is expected to have affected both treatment groups, avoiding bias in the ATE estimate. We hypothesize that effect sizes for the primary and secondary outcomes may have been greater if the pandemic had not occurred. While the transition from in-person to telephone assessments has been shown to be valid for cognitive tests,39 the in-person cognitive battery administered at baseline included more sensitive tests and assessed more cognitive domains. Furthermore, while we oversampled KPWA clinics with greater diversity to increase enrollment of Hispanic participants and participants who were a race other than White, we only achieved 20% diversity. While this reflects the population of older adults in the Seattle area, it fell short of our study goal of 30%. A higher proportion of racial and ethnic minority individuals were unable to commit to the requirements of the study due to time commitments and transportation difficulties.
Conclusions
In summary, this pilot RCT with a multidomain, personalized intervention demonstrated benefits for cognition, ADRD risk factors, and QOL among older adults without dementia. Given the low rate of AEs and benefits to overall health, especially cardiovascular, in addition to brain health, even the modest benefit observed with this intervention could have great public health effect. The results support offering modifiable risk-reduction strategies to older adults at risk for dementia and further investigation of this approach in a larger multisite trial.
Trial Protocol
eTable. Instruments and EHR Definitions Used for Risk Factor Screening and Enrollment Eligibility
eFigure 1. Individual Cognitive Test Outcomes: Treatment Effect over 24 Months for Cognitive Tests in the SMARRT Study
eFigure 2. Individual Risk Factor Outcomes: Treatment Effect over 24 Months for Individual Risk Factor Outcomes in the SMARRT Study
eFigure 3. Primary and secondary outcomes in the Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT) by treatment group, models adjusted for sex, race/ethnicity, education, comorbidity score and phone assessment
Data Sharing Statement
References
- 1.Mehegan L, Rainville C. 2021 AARP survey on the perceptions related to a dementia diagnosis: adults age 40-plus. AARP . June 22, 2021. Accessed October 20, 2023. https://www.aarp.org/content/dam/aarp/research/surveys_statistics/health/2021/dementia-diagnosis-perceptions.doi.10.26419-2Fres.00471.001.pdf
- 2.2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 3.Cummings J, Lee G, Nahed P, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y). 2022;8(1):e12295. doi: 10.1002/trc2.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Bokhoven P, de Wilde A, Vermunt L, et al. The Alzheimer’s disease drug development landscape. Alzheimers Res Ther. 2021;13(1):186. doi: 10.1186/s13195-021-00927-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 6.Sims JR, Zimmer JA, Evans CD, et al. ; TRAILBLAZER-ALZ 2 Investigators . Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JD, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553-561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebok GW, Ball K, Guey LT, et al. ; ACTIVE Study Group . Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24. doi: 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downey A, Stroud C, Landis S, Leshner AI, eds. Preventing Cognitive Decline and Dementia: A Way Forward. National Academies Press; 2017. [PubMed] [Google Scholar]
- 12.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027-1037. doi: 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- 13.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 14.McMaster M, Kim S, Clare L, et al. Lifestyle risk factors and cognitive outcomes from the multidomain dementia risk reduction randomized controlled trial, Body Brain Life for Cognitive Decline (BBL-CD). J Am Geriatr Soc. 2020;68(11):2629-2637. doi: 10.1111/jgs.16762 [DOI] [PubMed] [Google Scholar]
- 15.Andrieu S, Guyonnet S, Coley N, et al. ; MAPT Study Group . Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377-389. doi: 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
- 16.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388(10046):797-805. doi: 10.1016/S0140-6736(16)30950-3 [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Barnes DE, Rosenberg D, et al. Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT): study protocol. J Alzheimers Dis. 2019;70(s1):S207-S220. doi: 10.3233/JAD-180634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45-58. doi: 10.1017/S1041610294001602 [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 21.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 22.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657-665. doi: 10.1016/j.jalz.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64(9):1323-1329. doi: 10.1001/archneur.64.9.1323 [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, Aguirre E, Barber JA, et al. APPLE-Tree (Active Prevention in People at Risk of Dementia: Lifestyle, Behaviour Change and Technology to Reduce Cognitive and Functional Decline) programme: protocol. Int J Geriatr Psychiatry. 2020;35(8):811-819. doi: 10.1002/gps.5249 [DOI] [PubMed] [Google Scholar]
- 25.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 28.Hahn EA, Beaumont JL, Pilkonis PA, et al. The PROMIS satisfaction with social participation measures demonstrated responsiveness in diverse clinical populations. J Clin Epidemiol. 2016;73:135-141. doi: 10.1016/j.jclinepi.2015.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873-880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. Springer-Verlag; 2012. doi: 10.1007/978-1-4614-1353-0 [DOI] [Google Scholar]
- 31.Hafdi M, Hoevenaar-Blom MP, Richard E. Multi-domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst Rev. 2021;11(11):CD013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coley N, Giulioli C, Aisen PS, Vellas B, Andrieu S. Randomised controlled trials for the prevention of cognitive decline or dementia: a systematic review. Ageing Res Rev. 2022;82:101777. doi: 10.1016/j.arr.2022.101777 [DOI] [PubMed] [Google Scholar]
- 33.How to sustain brain healthy behaviors: applying lessons of public health and science to drive change. Global Council on Brain Health . 2022. Accessed October 20, 2023. https://www.aarp.org/content/dam/aarp/health/brain_health/2022-03/gcbh-behavior-change-report-english.doi.10.26419-2Fpia.00106.001.pdf.
- 34.Marcum ZA, Rosenberg D, Barnes DE, Yaffe K, Larson EB. Engaging patients to design the Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT) intervention: findings from a web-based survey. J Alzheimers Dis Rep. 2020;4(1):255-260. doi: 10.3233/ADR-200210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delrieu J, Bateman RJ, Touchon J, Sabbagh M, Cummings J. The future of AD clinical trials with the advent of anti-amyloid therapies: an CTAD Task Force report. J Prev Alzheimers Dis. 2022;9(3):393-399. doi: 10.14283/jpad.2022.48 [DOI] [PubMed] [Google Scholar]
- 36.Withington CG, Turner RS. Amyloid-related imaging abnormalities with anti-amyloid antibodies for the treatment of dementia due to Alzheimer’s disease. Front Neurol. 2022;13:862369. doi: 10.3389/fneur.2022.862369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson CD, Roscoe H, Green E, et al. Impact of COVID-19 policies on perceptions of loneliness in people aged 75 years and over in the cognitive function and aging study (CFAS II). J Am Geriatr Soc. 2023;71(2):463-473. doi: 10.1111/jgs.18099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beydoun HA, Beydoun MA, Gautam RS, et al. COVID-19 pandemic impact on trajectories in cardiometabolic health, physical activity, and functioning among adults from the 2006-2020 Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2022;77(7):1371-1379. doi: 10.1093/gerona/glac028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JR, Gibbons LE, Crane PK, et al. Shifting of cognitive assessments between face-to-face and telephone administration: measurement considerations. J Gerontol B Psychol Sci Soc Sci. 2023;78(2):191-200. doi: 10.1093/geronb/gbac135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Instruments and EHR Definitions Used for Risk Factor Screening and Enrollment Eligibility
eFigure 1. Individual Cognitive Test Outcomes: Treatment Effect over 24 Months for Cognitive Tests in the SMARRT Study
eFigure 2. Individual Risk Factor Outcomes: Treatment Effect over 24 Months for Individual Risk Factor Outcomes in the SMARRT Study
eFigure 3. Primary and secondary outcomes in the Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT) by treatment group, models adjusted for sex, race/ethnicity, education, comorbidity score and phone assessment
Data Sharing Statement