Abstract
Background:
Data on platinum sensitivity of low-grade serous ovarian carcinoma (LGSOC) in the upfront setting is lacking, and there is limited and contradictory information on chemotherapy responses in recurrent disease.
Methods:
Patients with LGSOC seen at a comprehensive cancer center from 1/1/1998–9/30/2021 were identified from institutional databases. Response to neoadjuvant chemotherapy (NACT) or adjuvant platinum-based chemotherapy and to second- to fifth-line regimens were retrospectively characterized by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Wilcoxon rank sum and two-tailed Fisher exact tests were employed.
Results:
Of 50 patients, 12 received platinum doublets for suboptimal residual disease and 11 as NACT. Of 12 patients with suboptimal residual disease, 7 (58%) achieved objective responses (5 partial responses [PRs] and 2 complete responses); of the 11 patients who underwent NACT, 1 (9%) achieved a PR (P=.027). The 15 remaining patients had stable disease on first-line platinum chemotherapy. Of 44 patients who recurred, 20 had RECIST-evaluable responses to second-line and 27 to third-line chemotherapy. Objective response rates to platinum-based chemotherapy were 22% (2 of 9) in the second line and 10% (1 of 10) in the third. In second and third lines, highest response rates were observed with non-platinum chemotherapy with bevacizumab, at 100% (2 of 2) and 30% (3 of 10), respectively.
Conclusions:
Primary platinum-based chemotherapy has moderate activity in LGSOC and minimal activity in the recurrent setting, suggesting standard definitions of platinum sensitivity may not apply in LGSOC. In the second and third lines, non-platinum chemotherapy/bevacizumab elicited the highest response rates.
Keywords: Low-grade serous ovarian cancer, neoadjuvant, KRAS, cytoreduction surgical procedures, chemotherapy
Precis:
Front-line platinum-based chemotherapy has moderate activity in low-grade serous ovarian carcinoma (LGSOC), and minimal activity in the recurrent setting, suggesting standard definitions of platinum sensitivity may not apply in LGSOC. In the second and third lines, non-platinum chemotherapy/bevacizumab elicited the highest response rates.
Introduction
Low-grade serous ovarian cancers (LGSOCs), which account for 5%−10% of epithelial ovarian cancers, are characterized by low mutational burden, low frequency of TP53 mutations, and no clear germline association.1–4 Clinically, LGSOC has a bimodal age of onset distribution (20–30 and 50–60 years), is less platinum-responsive than high-grade serous ovarian carcinoma (HGSOC), and typically exhibits indolent behavior.5–7 Patients with advanced LGSOC have a median survival of approximately 10 years and often receive multiple lines of therapy throughout their disease course.
Although the tumor biology and clinical behavior of LGSOC differ from HGSOC, a similar treatment paradigm is commonly used; patients with recurrent disease are classified as either platinum sensitive or platinum resistant, which is used to inform subsequent therapy choices. In patients with LGSOC who recur >6 months after completion of platinum therapy (ie, those with platinum-sensitive disease), retreatment with platinum-based doublets with or without bevacizumab is a preferred regimen.8 However, response rates to platinum-based chemotherapies in the upfront and recurrent settings are underreported. Similarly, data on response rates to other cytotoxic regimens, such as single-agent non-platinum agents with or without bevacizumab, are also limited.9 Thus, many patients may be enduring unnecessary treatment side effects in the face of unknown benefits.
In this study, we sought to assess response of LGSOC to platinum-based therapy in the primary setting using a cohort of patients who presented to a single comprehensive cancer center at time of initial diagnosis. We also sought to determine LGSOC response to platinum and other non-platinum cytotoxic agents in the recurrent setting. We investigated these questions using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 to retrospectively assess response rate.
Methods
This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSK; IRB #15–200). First, patients with LGSOC (including grade 1 serous ovarian cancer, as specified in older pathology reports) who presented at time of diagnosis from 1/1/1998 to 9/30/2021 were identified from an institutional database. Cases with co-existing or subsequent high-grade carcinoma were excluded. Next, two groups of patients were identified as study populations of interest. The first group included patients with evaluable responses to first-line platinum chemotherapy; these patients received platinum-based chemotherapy as neoadjuvant chemotherapy (NACT) or after suboptimal primary debulking surgery (>1 cm of residual tumor in maximum diameter left after upfront surgery). The second group included patients who had recurred and had radiographically evaluable disease and treatment in the second- to fifth-line settings. In all cases, low-grade serous histology was confirmed by subspecialist gynecologic pathologists at our institution.
Clinicopathologic variables of interest were collected from the electronic medical record and included the following: body mass index (BMI); volume of residual disease at time of initial cytoreduction, defined as complete gross resection (CGR; no visible/macroscopic residual tumor), optimal residual disease (cytoreduction to ≤1 cm residual disease), or suboptimal residual disease (cytoreduction to >1 cm residual disease) as described by the operative report; stage at diagnosis (per the 2014 International Federation of Gynaecology and Obstetrics [FIGO] staging system); and chemotherapeutic regimens. Platinum sensitivity was defined as tumor recurrence ≥6 months from date of last primary platinum-based chemotherapy; platinum resistance was defined as tumor recurrence <6 months from date of last primary platinum-based chemotherapy or progression on platinum-based chemotherapy. Massively parallel sequencing was performed in the Clinical Laboratory Improvement Amendments (CLIA)-certified MSK Molecular Diagnostics Service Laboratory on a subset of LGSOC samples and matched normal blood using the US Food and Drug Administration–cleared MSK–Integrated Mutation Profiling of Actionable Cancer Targets (IMPACT) assay targeting 341–505 cancer-related genes. In addition, the presence of KRAS mutations in exons 2 and 3, which includes KRAS G12, G13, and q61 hotspots, were also collected.10
All patients received their treatment at a single comprehensive cancer center and had digitized computed tomography (CT) images available for review. Image review from all time points and RECIST scoring, beginning with diagnosis and continuing until time of progression, were conducted by a study radiologist (JG) who specializes in clinical trials and RECIST evaluation of treatment responses. This radiologist was blinded to chemotherapeutic regimen. Responses to first-line and recurrent therapy were retrospectively classified by RECIST v1.1. Patients who did not have RECIST-evaluable CT scans were excluded. Response rate was determined based on best response to line of treatment, with best overall response defined as complete response (CR), partial response (PR), stable disease (SD), or progression of disease (POD).11 Responses to endocrine treatment or targeted treatments, such as MEK inhibitors, were outside the scope of this study.12,13
Associations between categorical clinicopathologic variables and continuous clinical outcomes were compared using the Wilcoxon rank sum test, and comparisons of categorical variables and categorical clinical outcomes were performed using a two-tailed Fisher exact test. A P value <.05 was considered significant. All statistical analyses were performed using R version 4.2.1 (https://cran.r-project.org/).
Results
Clinicopathologic demographics
Demographics and details of the cohort are presented in Table 1 and Figure 1. One hundred twenty-four patients presented to MSK with LGSOC in the study timeframe. Thirty-eight patients were excluded due to a component of high-grade carcinoma on histology and an additional 36 patients were excluded as they did not have RECIST-evaluable CT imaging available. The final cohort of 50 patients included in the study were diagnosed with advanced-stage disease. The median age at diagnosis was 53 years (interquartile range [IQR]: 42–64 years). Among 30 patients who received tumor sequencing, 6 (20%) had a KRAS mutation. Forty-eight (96%) of 50 patients underwent cytoreduction (primary or interval debulking surgery). Of 38 patients who underwent primary cytoreduction, 22 (58%) achieved CGR, 9 (24%) had an optimal cytoreduction, and 6 (16%) had a suboptimal cytoreduction. The extent of residual tumor was not specified in the operative record of 1 patient (3%). At time of primary cytoreduction, in addition to standard surgical staging, 17 (45%) of 38 patients had bowel resections, 15 (39%) of 38 patients had upper abdominal procedures, including diaphragm resections, splenectomies, and distal pancreatectomies, and 0 (0%) patients had major urologic procedures (eg, ureteral resection). Of 11 patients who underwent NACT, median number of cycles was 5 (IQR: 3–6 cycles). Of 10 patients who underwent surgery after NACT, 4 (40%) achieved CGR, 5 (50%) had optimal cytoreduction, and 1 (10%) had suboptimal cytoreduction. At time of interval cytoreduction, in addition to standard surgical staging, 7 (70%) of 10 patients had bowel resections, 6 (60%) of 10 patients had upper abdominal procedures, including diaphragm resections, splenectomies, and distal pancreatectomies, and 2 (20%) of 10 patients had major urologic procedures, both with ureteral resections with reimplantation.
Table 1:
Patient characteristics
| Characteristic | No. of patients (%) N = 50 |
|---|---|
| Median age at diagnosis, years (IQR) | 53 (42, 64) |
| Race | |
| White | 39 (78%) |
| Black or African American | 3 (6%) |
| Asian | 6 (12%) |
| Unknown | 2 (4 %) |
| Median BMI at diagnosis, kg/m2 (IQR) | 25 (22, 29) |
| Stage at diagnosis | |
| III | 39 (78%) |
| IV | 11 (22%) |
| KRAS profiling | |
| KRAS wild type | 24 (80%) |
| KRAS mutation | 6 (20%) |
| Unknown | 20 |
| Postoperative residual tumor | |
| Complete gross resection | 26 (55%) |
| Optimal (≤1 cm) | 14 (30%) |
| Suboptimal (>1 cm) | 7 (15%) |
| No surgery/unknown | 3 |
| Surgical procedure performed beyond standard staging * | |
| Bowel resections | 24 (50%) |
| Upper abdominal procedures | 21 (44%) |
| Major urologic procedures | 2 (4%) |
IQR, interquartile range; BMI, body mass index
Denominator is patients who underwent surgery, n=48
Figure 1a:
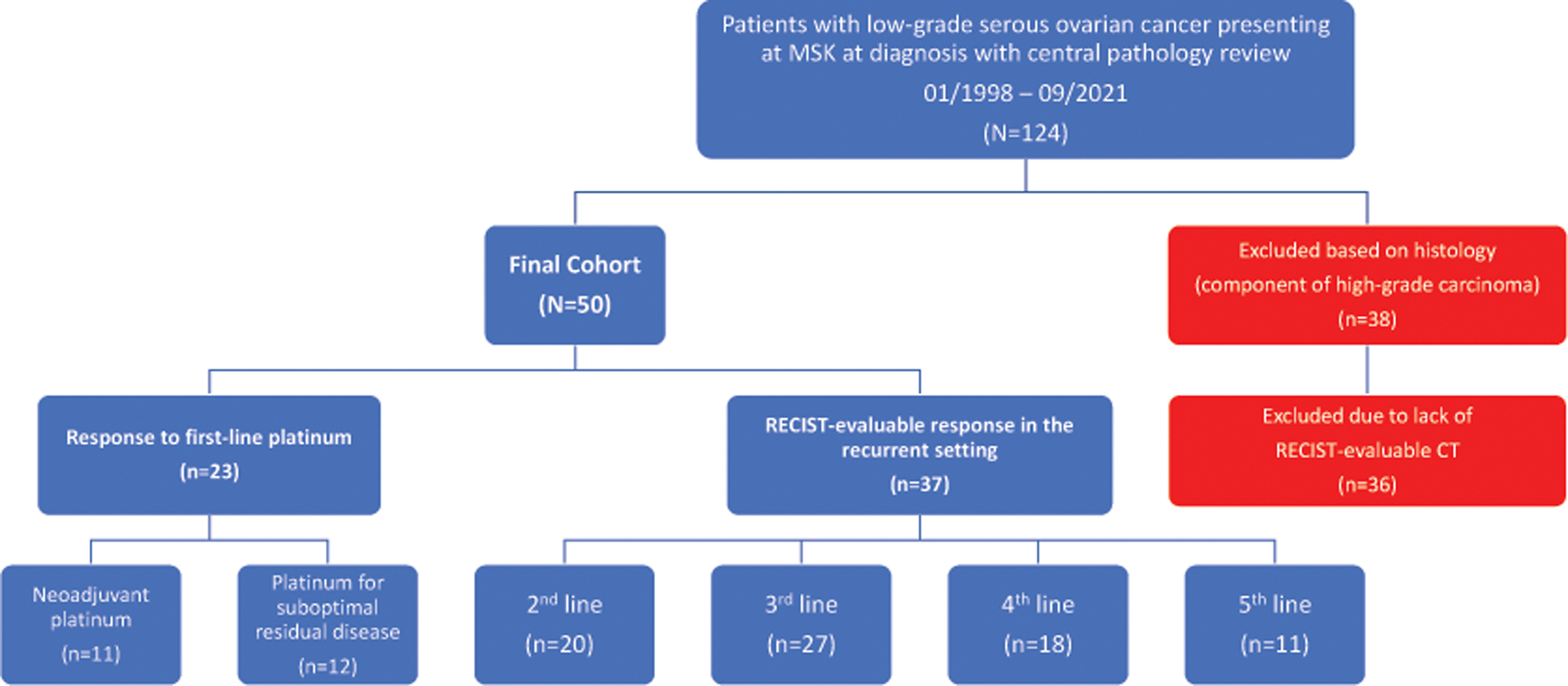
Characteristics of study population
MSK, Memorial Sloan Kettering Cancer Center; RECIST, Response Evaluation Criteria in Solid Tumors; CT, computed tomography
Response to first-line platinum therapy
Response to first-line platinum therapy was assessed in patients who had undergone NACT (n=11) or a suboptimal primary cytoreductive surgery as described in operative or imaging reports prior to initiation of postoperative chemotherapy (n=12; Figure 2). Of these 23 patients, 19 (83%) received a regimen of carboplatin and paclitaxel every 3 weeks, 2 (9%) received intravenous/intraperitoneal cisplatin-paclitaxel, and 2 (9%) received bevacizumab in addition to carboplatin and paclitaxel.
Figure 2:
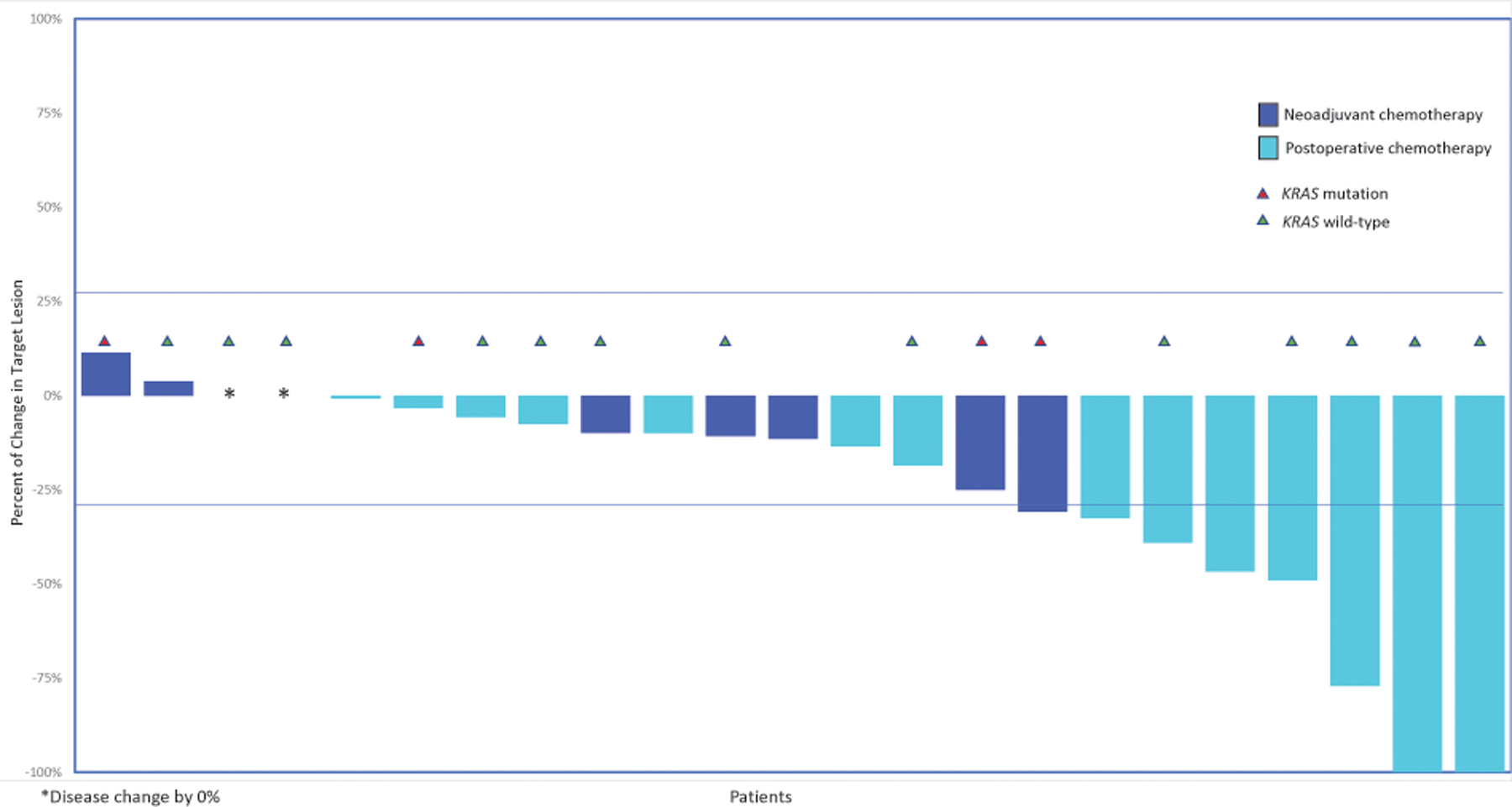
Response to first-line platinum therapy (patients receiving NACT [n=11] or postoperative chemotherapy for suboptimal residual disease [n=12])
*Disease change by 0%
NACT, neoadjuvant chemotherapy
Reduction of initial high disease burden to increase chances of resectability was the rationale for administering NACT for all patients who received this treatment. No patients achieved a CR, 1 (9%) achieved a PR, 10 (91%) had SD, and none had POD. Among the 12 patients who received platinum-based therapy following a suboptimal primary cytoreduction, 2 (17%) achieved a CR, 5 (42%) achieved a PR, 5 (42%) had SD, and none had POD. The overall response rate (ORR) was 9% for the NACT group and 58% for patients who received chemotherapy following suboptimal primary cytoreduction (P=.027). The median percent change in volume of target lesions at the time of best response was −7.5% (IQR: −11.1%, −2.1%) versus −25.5% (IQR: −47.3%, −7.5%), respectively. Sixteen of the 23 patients who received NACT or adjuvant chemotherapy for measurable suboptimal residual disease had tumor sequencing data, of whom 4 (25%) had a KRAS mutation. All 4 patients with KRAS-mutated LGSOC had SD to first-line platinum therapy. Among the remaining 12 patients with KRAS wild-type LGSOC, 7 (58%) had SD and 5 (42%) responded to first-line platinum chemotherapy.
Response in the recurrent setting
Of the entire cohort of 50 patients, 37 patients had available imaging for RECIST v1.1 evaluation of treatment response: 20 patients in the second line; 27 in the third line; 18 in the fourth line; and 11 in the fifth line. At time of first recurrence, 37 patients were considered platinum sensitive: 14 (38%) patients who were considered platinum sensitive had received hormonal maintenance therapy, while 23 (62%) patients who were considered platinum sensitive had not received hormonal maintenance therapy. Five patients (25%) responded to second-line treatment with any cytotoxic agent, while 12 (60%) had SD and 3 (15%) had POD (Figure 3). Two (22%) of 9 patients responded to platinum-based therapy, while 3 (27%) of 11 responded to non-platinum-based chemotherapy. Nine patients who received second-line treatment had tumor molecular profiling; 2 (67%) of 3 patients with a KRAS mutation responded to second-line treatment versus 0 (0%) of 6 patients with KRAS wild-type LGSOC (P=.083).
Figure 3:
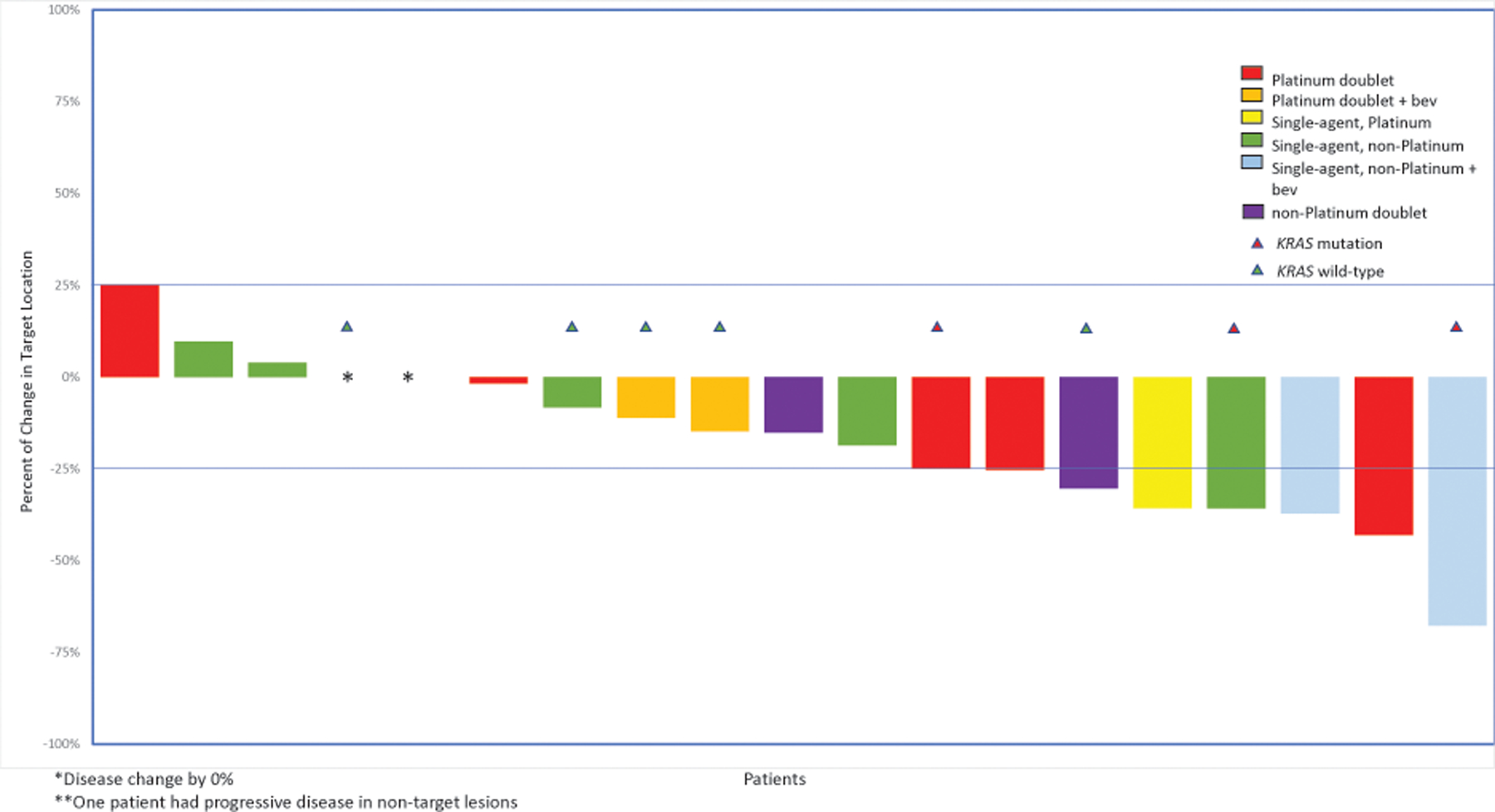
Response to second-line cytotoxic treatment (n=20 patients)
*Disease change by 0%
**One patient had progressive disease in non-target lesions
Bev, bevacizumab
Among 37 patients considered platinum sensitive at time of first recurrence, 13 (35%) received a second-line cytotoxic agent and had a RECIST-evaluable scan. Of 7 patients considered platinum-sensitive who had undergone retreatment with platinum in the second line, 2 (29%) achieved a PR, 4 (57%) had SD, and 1 (14%) had POD. Of 6 patients with platinum-sensitive disease who received non-platinum-based chemotherapy in the second line, 1 (17%) achieved a PR, 4 (67%) had SD, and 1 (17%) had PD.
In the third line, 27 patients received treatment and were assessable: the ORR was 22% with any cytotoxic chemotherapy (n=6; Figure 4). Patients with LGSOC responded more often to single-agent non-platinum regimens with bevacizumab in the third line (n=3/10, 30%) or to single-agent bevacizumab (n=2/3, 67%). Response rates to platinum-based chemotherapies were 10% (n=1/10). For subsequent treatment lines, the ORR was 11% (2/18) in the fourth line and 0% (0/11) in the fifth line (Figures 5 and Supplementary Figure 1). The most common responses in the fourth line were to single-agent non-platinum regimens with bevacizumab (n=1/4, 25%).
Figure 4:
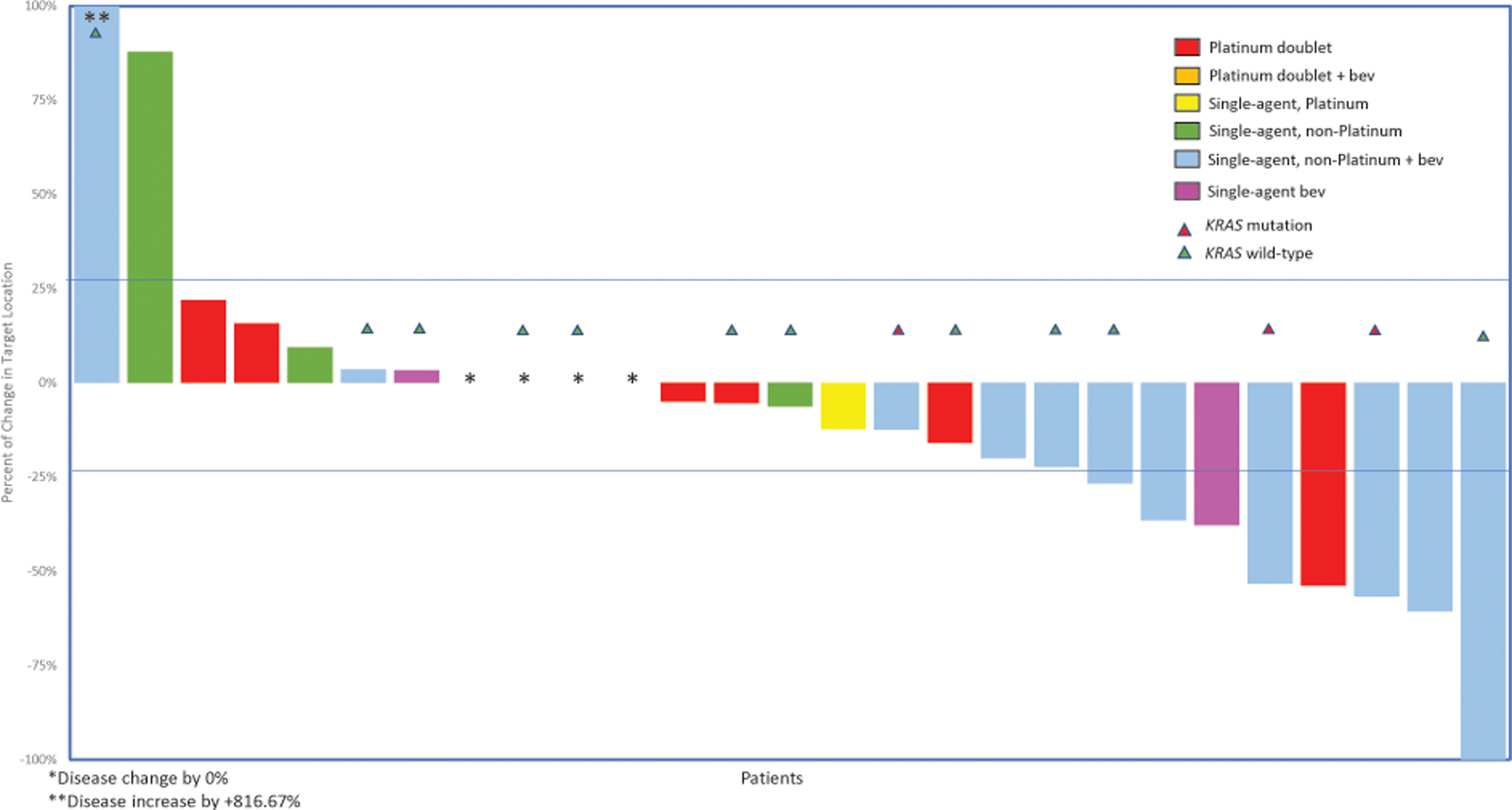
Response to third-line cytotoxic treatment (n=26 patients)
*Disease change by 0%
**Disease increase by +816.67%
Bev, bevacizumab
Figure 5:
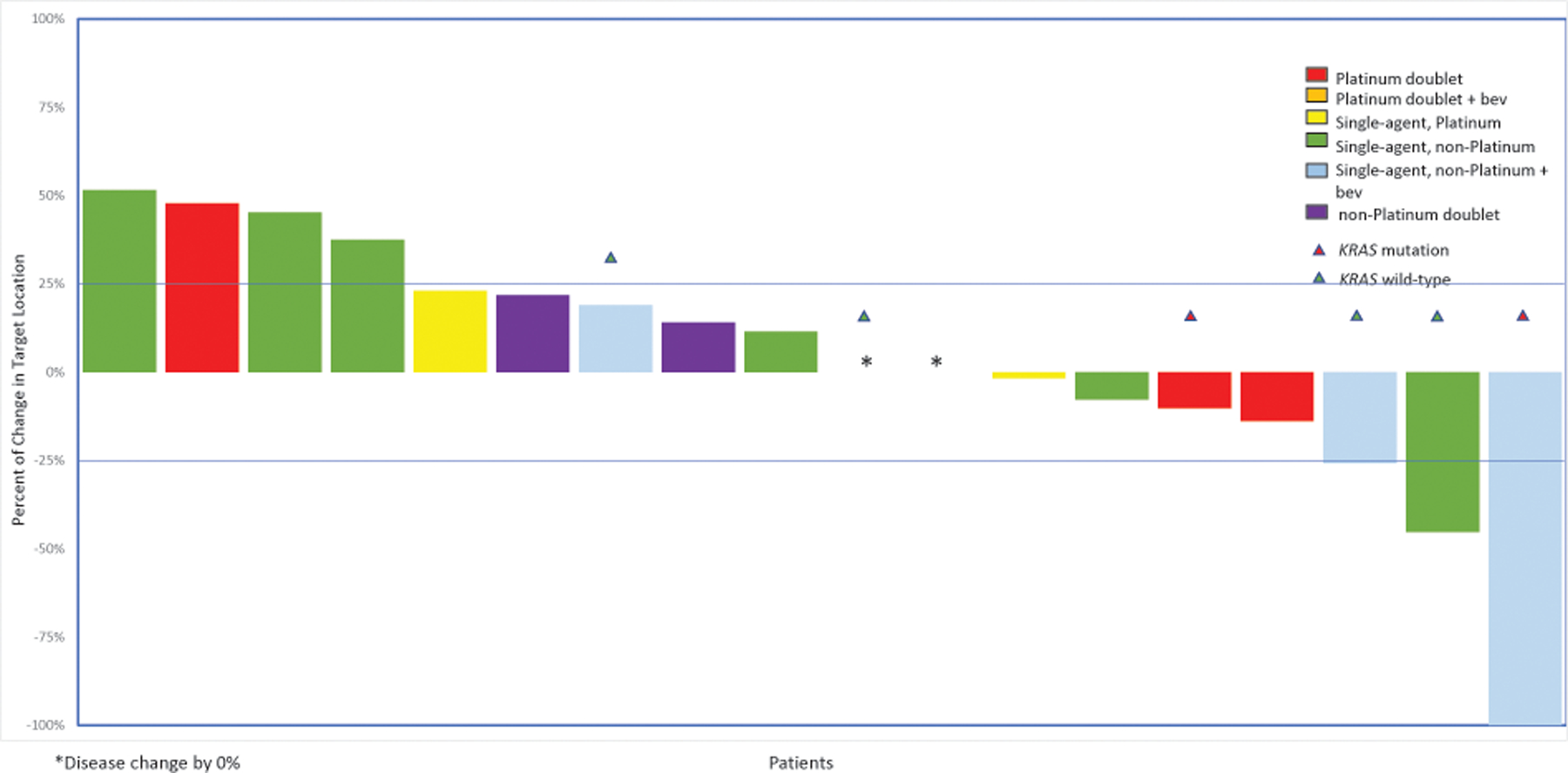
Response to fourth-line cytotoxic treatment (n=18 patients)
*Disease change by 0%
Bev, bevacizumab
Discussion
Here, we report an ORR of 35% to first-line platinum therapy among women with LGSOC who underwent chemotherapy as part of NACT or after suboptimal primary cytoreduction. In the NACT setting, the ORR was low at 9%; however, CGR was achieved in 40% and optimal cytoreduction in 50% of patients at interval debulking surgery despite high initial burden of disease. Response rates to first-line platinum in the postoperative setting were 58%. Among patients with recurrent disease, we found that 25% of patients responded to cytotoxic chemotherapy in the second-line setting, with a response rate of 22% to platinum-based chemotherapy and 27% to non-platinum-based chemotherapy. Overall, the highest response rates in the second through fourth lines were demonstrated with single-agent non-platinum regimens with bevacizumab, ranging from 25% in the fourth line (1 of 4 patients) to 100% in the second line (2 of 2 patients). This finding is in line with prior studies demonstrating the clinical benefit of bevacizumab in the management of LGSOC, with previously reported overall response rates of 48%−55% of bevacizumab-containing regimens.9,14
While earlier studies have reported response rates of 4%−11% to platinum in the neoadjuvant setting, these data are limited by their inclusion of patients having received historical regimens, such as cyclophosphamide.15,16 An analysis of patients with LGSOC who received platinum-based regimens for suboptimal residual disease in prospective randomized Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) trials reported a response rate of 23%.6 While we report a response rate of 9% with modern regimens of NACT, we also found a higher ORR (58%) in patients who received first-line platinum for >1 cm of residual disease on pretreatment imaging. There are several possible explanations for the observed differences. First, most low-grade serous tumors have lower mitotic activity and typically slower disease growth compared to high-grade serous carcinoma.17 Since chemotherapy is more effective in actively proliferating cells, the duration of treatment in patients who received NACT may not have been adequate to illicit the degree of tumor shrinkage observed in patients who received all cycles of chemotherapy postoperatively. A second possible explanation is that partial tumor debulking, leading to lower volume of disease, is associated with improved response to chemotherapy due to improvement in drug penetration into smaller residual tumors (ie, removal of pharmacologic sanctuaries), removal of less chemosensitive tumor cells in the plateau phase of cell growth, resection of chemoresistant clones, and that small tumors may respond to fewer cycles of chemotherapy, thus decreasing the likelihood of acquiring chemoresistance. A third possible explanation is that resolution of inflammatory and other changes in the postoperative setting may have erroneously contributed to the “tumor shrinkage” detected on imaging.
We argue, however, that the 58% ORR observed in patients who received postoperative chemotherapy highlights the importance of carefully considering the option of postoperative chemotherapy in patients with LGSOC, especially in those with >1 cm of residual disease. Over the past several years, chemotherapy in the primary adjuvant setting for patients with LGSOC has been increasingly omitted at select institutions, as evidenced by its inclusion as a therapeutic option in National Comprehensive Cancer Network guidelines (2B recommendation); however, this shift may be premature in the absence of prospective data to firmly support this change.8 An ongoing phase III study (NCT04095364) randomizing patients with newly diagnosed advanced LGSOC who have undergone primary debulking surgery to treatment with 6 cycles of platinum/taxane chemotherapy followed by letrozole versus letrozole alone is exploring the potential impacts of this change.
While the data reported here present an important benchmark in the clinical understanding of upfront therapy for patients with LGSOC, perhaps the most relevant application of these data are in the recurrent setting. In recurrent LGSOC, response rates of 2%−5% to cytotoxic chemotherapies have been reported; however, these data are limited by the lack of standardized radiographic evaluation of disease response and by referral biases.18 It has been difficult to adequately design prospective trials given these limitations. For example, the MILO/ENGOT-ov11 trial compared the difference in progression-free survival for patients with LGSOC and measurable disease who were randomized 2:1 to the MEK inhibitor bimetinib versus physician’s choice of chemotherapy (pegylated liposomal doxorubicin, paclitaxel, or topotecan). Ultimately, this study closed early after crossing a prespecified futility boundary. Most notably, the ORR to physician’s choice of chemotherapy was 13%, which was greater than anticipated based on previously reported, single-institution retrospective case series.12 Here, we report a 25% ORR to second-line cytotoxic chemotherapy, which adds data to the existing literature on expected response rates to chemotherapy in this setting.
Beyond trial design, it is vitally important to understand expected response rates to available treatments for recurrent LGSOC, as this information can inform patient counseling and provider decision making. While MEK inhibitors have emerged as second-line treatment options, ≥3 grade toxicities are frequent. For example, in the MILO/ENGOT-ov11 study, in which the ORR to a MEK inhibitor was 16%, 76% of patients randomized to the MEK inhibitor experienced a grade 3 or 4 adverse event. In comparison, the rate of ≥3 grade toxicities with physician’s choice of chemotherapy reported in the MILO/ENGOT-ov11 study was 44% and the ORR was 13%.12 While MEK inhibitors are a valuable addition to the armamentarium of therapies used to treat LGSOC, chemotherapy remains a standard option. The population analyzed in this study predates the routine use of MEK inhibitors in the LGSOC population and allows for an analysis of the impact of chemotherapy in the recurrent setting.
We found that while the majority of patients with LGSOC met the traditional definition of platinum sensitivity (presumably due to the indolent tumor growth rate associated with this disease), few patients responded to platinum retreatment. Of 13 patients with evaluable disease treated at time of first recurrence, the 7 patients who received a platinum therapy (2 PR, 4 SD, 1 POD) responded similarly to the 6 patients who received a non-platinum therapy (1 PR, 4 SD, 1 POD). This finding suggests that providers should not rely on the traditional paradigm for treatment of recurrent ovarian cancer, which recommends retreatment with platinum in patients recurring more than 6 months after platinum-based chemotherapy. Instead, retreatment with platinum should be weighed carefully, given the potential equivalent response rates and lesser toxicity with other cytotoxic regimens.
Although our sample size was limited with only 6 of 30 patients demonstrating a KRAS mutation, we found no difference in response rates to primary platinum chemotherapy among women with KRAS-mutated LGSOC compared to KRAS wild-type LGSOC. While prior studies have suggested that having a KRAS mutation serves as a favorable prognostic factor and increases sensitivity to platinum chemotherapies, further studies with larger cohorts are needed to fully elucidate the impact of KRAS mutations on response to therapy.1,13,19,20
This study has several limitations. We sought to minimize referral bias by only including patients who presented to our institution for initial care, and therefore, the patient population was small. Similarly, while RECIST reads were performed as an objective assessment of tumor response, these assessments were performed outside a clinical trial, and thus, the interval between imaging studies was non-standardized. In addition, due to the retrospective nature of this study, confirmatory scans were not performed for confirmation of RECIST response. Further studies with larger cohorts of patients should be conducted to confirm the optimal treatment in patients with LGSOC, particularly in the recurrent setting.
The data reported above further reinforce that LGSOC is a distinct entity that merits a disease-specific treatment paradigm. Primary surgical debulking should be the goal in all patients newly diagnosed with LGSOC. While platinum-based chemotherapy provides objective responses in the adjuvant setting, the traditional clinical definition of platinum sensitivity may not predict responses to platinum-based chemotherapy in the recurrent setting.
Supplementary Material
Figure 1b:
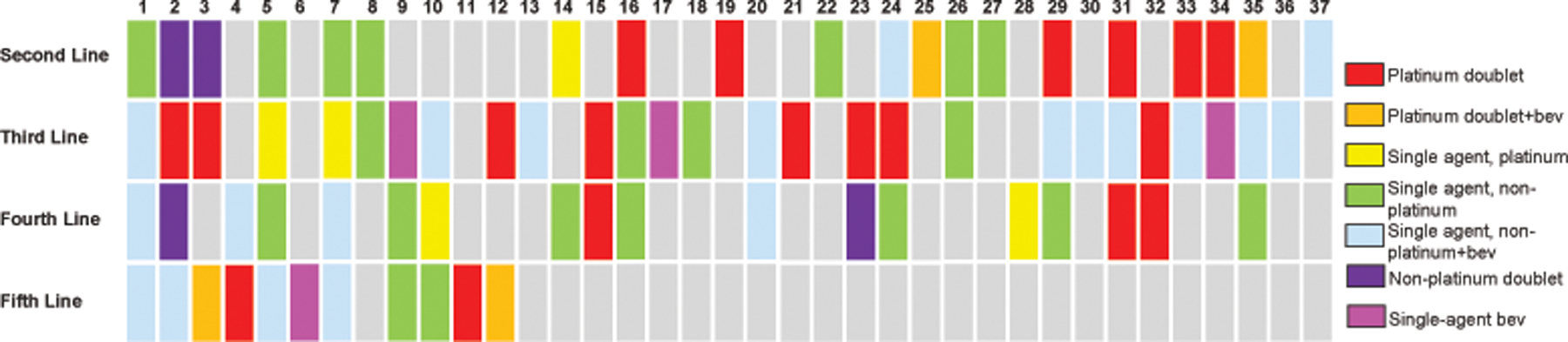
Patients treated for recurrent disease
Patients are represented by columns and lines of treatment by rows. Regimens are color-coded according to the legend. Bev, bevacizumab.
Funding:
This work was supported by the National Cancer Institute at the National Institutes of Health (P30CA008748).
Footnotes
Conflict of Interest Disclosure: Outside of the submitted work, AI reports consulting fees from Mylan. RG reports honoraria from GSK, AstraZeneca, Natera, Springworks, Corcept, MJH, and PER. DC reports personal fees from Apyx Medical, Verthermia Inc., Biom ‘Up, and AstraZeneca, as well as recent or current stock/options ownership of Apyx Medical, Verthemia, Intuitive Surgical, Inc., TransEnterix, Inc., Doximity, Moderna, and BioNTech SE. CA has received research grants from Abbvie, Clovis, Genentech, and Astra Zeneca and served on advisory boards for Abbvie, AstraZeneca/Merck, Eisai/Merck, Mersana Therapeutics, Repare Therapeutics, and Roche/Genentech. MHC serves on the advisory board for Roche and Verastem Oncology. All other authors have no potential conflicts of interest to disclose.
Ethics Approval Statement: This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSK; IRB #15–200).
Data availability statement:
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Manning-Geist B, Gordhandas S, Liu YL, et al. MAPK pathway genetic alterations are associated with prolonged overall survival in low-grade serous ovarian carcinoma. Clin Cancer Res Apr 20 2022;doi: 10.1158/1078-0432.CCR-21-4183 [DOI] [PMC free article] [PubMed]
- 2.Vineyard MA, Daniels MS, Urbauer DL, et al. Is low-grade serous ovarian cancer part of the tumor spectrum of hereditary breast and ovarian cancer? Gynecol Oncol Feb 2011;120(2):229–32. doi: 10.1016/j.ygyno.2010.10.033 [DOI] [PubMed] [Google Scholar]
- 3.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene Feb 3 2005;24(6):1053–65. doi: 10.1038/sj.onc.1208298 [DOI] [PubMed] [Google Scholar]
- 4.Norquist BM, Harrell MI, Brady MF, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol Apr 2016;2(4):482–90. doi: 10.1001/jamaoncol.2015.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol Apr 2008;198(4):459 e1–8; discussion 459 e8–9. doi: 10.1016/j.ajog.2008.01.035 [DOI] [PubMed] [Google Scholar]
- 6.Grabowski JP, Harter P, Heitz F, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol Mar 2016;140(3):457–62. doi: 10.1016/j.ygyno.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 7.Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol Aug 2006;108(2):361–8. doi: 10.1097/01.AOG.0000227787.24587.d1 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw Feb 2 2021;19(2):191–226. doi: 10.6004/jnccn.2021.0007 [DOI] [PubMed] [Google Scholar]
- 9.Grisham RN, Iyer G, Sala E, et al. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer Jul 2014;24(6):1010–4. doi: 10.1097/IGC.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn May 2015;17(3):251–64. doi: 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Jan 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Monk BJ, Grisham RN, Banerjee S, et al. MILO/ENGOT-ov11: Binimetinib Versus Physician’s Choice Chemotherapy in Recurrent or Persistent Low-Grade Serous Carcinomas of the Ovary, Fallopian Tube, or Primary Peritoneum. J Clin Oncol Nov 10 2020;38(32):3753–3762. doi: 10.1200/JCO.20.01164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet Feb 5 2022;399(10324):541–553. doi: 10.1016/S0140-6736(21)02175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton HJ, Fleming ND, Sun CC, Bhosale P, Schmeler KM, Gershenson DM. Activity of bevacizumab-containing regimens in recurrent low-grade serous ovarian or peritoneal cancer: A single institution experience. Gynecol Oncol Apr 2017;145(1):37–40. doi: 10.1016/j.ygyno.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol Mar 2008;108(3):510–4. doi: 10.1016/j.ygyno.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 16.Cobb LP, Sun CC, Iyer R, et al. The role of neoadjuvant chemotherapy in the management of low-grade serous carcinoma of the ovary and peritoneum: Further evidence of relative chemoresistance. Gynecol Oncol Sep 2020;158(3):653–658. doi: 10.1016/j.ygyno.2020.06.498 [DOI] [PubMed] [Google Scholar]
- 17.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol Apr 2004;28(4):496–504. doi: 10.1097/00000478-200404000-00009 [DOI] [PubMed] [Google Scholar]
- 18.Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol Jul 2009;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 19.Gershenson DM, Sun CC, Wong KK. Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br J Cancer Nov 3 2015;113(9):1254–8. doi: 10.1038/bjc.2015.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grisham RN, Vergote I, Banerjee SN, et al. Molecular results and potential biomarkers identified from MILO/ENGOT-ov11 phase 3 study of binimetinib versus physicians choice of chemotherapy (PCC) in recurrent low-grade serous ovarian cancer (LGSOC). J Clin Oncol May 2021;39(no. 15 suppl):5519. doi: 10.1200/JCO.2021.39.15_suppl.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


