Abstract
Theranostic materials research is experiencing rapid growth driven by the interest in integrating both therapeutic and diagnostic modalities. These materials offer the unique capability to not only provide treatment but also track the progression of a disease. However, to create an ideal theranostic biomaterial without compromising drug encapsulation, diagnostic imaging must be optimized for improved sensitivity and spatial localization. Herein, we create a protein-engineered fluorinated coiled-coil fiber, Q2TFL, capable of improved sensitivity to 19F magnetic resonance spectroscopy (MRS) detection. Leveraging residue-specific noncanonical amino acid incorporation of trifluoroleucine (TFL) into the coiled-coil, Q2, which self-assembles into nanofibers, we generate Q2TFL. We demonstrate that fluorination results in a greater increase in thermostability and 19F magnetic resonance detection compared to the nonfluorinated parent, Q2. Q2TFL also exhibits linear ratiometric 19F MRS thermoresponsiveness, allowing it to act as a temperature probe. Furthermore, we explore the ability of Q2TFL to encapsulate the anti-inflammatory small molecule, curcumin (CCM), and its impact on the coiled-coil structure. Q2TFL also provides hyposignal contrast in 1H MRI, echogenic signal with high-frequency ultrasound and sensitive detection by 19F MRS in vivo illustrating fluorination of coiled-coils for supramolecular assembly and their use with 1H MRI, 19F MRS and high frequency ultrasound as multimodal theranostic agents.
Keywords: protein fibers, 19F MRS, theranostic, drug encapsulation, imaging, biomaterial
Introduction
Theranostic agents are a growing field in biomedicine that help to overcome limitations in biomaterials providing therapy and diagnosis of diseases.1 These materials help to monitor the development of disease after therapeutic treatment as well as provide a simultaneous diagnosis and treatment of a disease.1 Currently, theranostics largely focus on synthetic approaches while using inorganic materials such as quantum dots or radiolabeling to confer diagnostic properties.1−3 Quantum dots suffer from stability and aggregation, which greatly reduces their diagnostic sensitivity and limit their ability to effectively penetrate tissues with their signal.4 The practical application of radiolabeling can be challenging due to the short half-lives of radioactive isotopes, which impose logistic constraints. The resulting limited time window necessitates the use of efficient synthesis methods to ensure timely labeling. However, it also raises concerns about potential prolonged radiation exposure during the labeling process.5 Theranostics are also challenged by combining drug delivery techniques that possess targeting moieties with high specificity, thus reducing therapeutic efficacy and signal sensitivity.6 To create an ideal theranostic biomaterial, without compromising drug encapsulation, diagnostic imaging must be optimized for improved detection.7 One such method to improve this specificity is the incorporation of fluorine into biomaterials.8
Since fluorine is largely absent from organisms, yet exists in 100% natural abundance, it is useful as a contrast agent due to its specific signal in 19F MRS.9 In light of this, many 19F MRS materials have been developed for biomedical applications10−14 such as MRI cell tracking15,16 and tumor imaging17,18 as well as monitoring tumor cell hypoxia19 and proliferation.20 These agents are often synthetically derived to create fluorine-based polymers17,21−23 or nanoemulsions.24−26
With recent advancements in synthetic and chemical biology, protein engineered theranostic agents have been developed27 where fluorinated proteins can be produced through methods such as noncanonical amino acid (NCAA) incorporation28,29 or solid-phase peptide synthesis (SPPS).30 We have previously developed a protein-based 19F MRS-traceable micelle by residue-specific NCAA incorporation of trifluoroleucine (TFL) into a thermoresponsive assembled protein (TRAP), resulting in F-TRAP.31 Whereas previous fluorinated proteins suffer from unfavorable relaxation properties necessary to directly visualize 19F protein nuclei in MRI, the supramolecular micelle assembly of F-TRAP provides the opportunity for fluorine amplification due to ordering and structural constriction.31
Conversely, we have also shown the ability to use protein fibers for theranostic imaging by biorthogonal azide–alkyne cycloaddition of a designed coiled-coil protein with azidohomoalanine (AHA), QAHA, to an alkyne-bearing iron oxide templating peptide, CMms6.32 The hybrid QAHA-X-CMms6 bearing the templated ultrasmall superparamagnetic iron oxide (USPIO) biomaterial is capable of doxorubicin encapsulation and exhibits sensitive T2*-weighted MRI darkening in part due to the multitude of USPIOs spaced along a single protein fiber assembly.32 The QAHA also establishes our fibers to be capable of concentrated and sustained release.32 While Q highlights the benefits of using a self-assembling fiber to confine many MR-sensitive USPIOs and provides unique T2*-darkening, it suffers from the addition of several postpurification synthesis steps. In contrast, biosynthetic fluorination by NCAA incorporation of TFL is achieved in a single step.
Given the strong decrease in 19F T2 relaxation times as a result of F-TRAP micelle ordering and constriction as well as the evidence of the USPIO agent ordering along hybrid QAHA-X-CMms6 fibers, we similarly propose that a 19F nuclei dense coiled-coil fiber may prove to be a sensitive 19F MRS theranostic agent. Fibrous biomaterials also benefit from the ability to form scaffolds for cell growth, tissue function33,34 as well as retain composition and localization for drug delivery.35 While we have previously studied the impact of TFL incorporation into Q,36 we have not studied the candidacy of a coiled-coil protein fiber for 19F MRS.
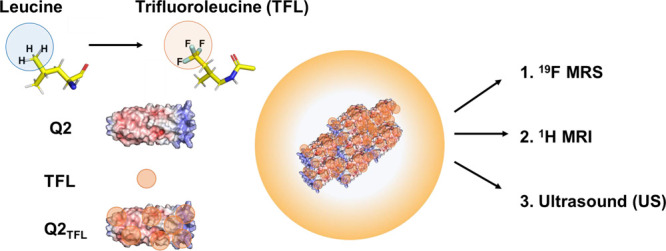
Herein, we develop a protein-based fluorinated self-assembling fiber, Q2TFL as a theranostic agent capable of 19F MRS. We demonstrate that Q2TFL has increased sensitivity for 19F MRS, and increased thermostability compared to previous constructs, and can encapsulate the hydrophobic small molecule, curcumin (CCM), which provides further stabilization. Furthermore, we show that Q2TFL may be used in vivo as a visible fiber assembly via 1H MRI and high-frequency ultrasound as well as a sensitive biomaterial using 19F MRS. Interestingly, we show that Q2TFL possesses a ratiometric 19F MRS signal proportional to its protein structure and environmental temperature indicating its potential as a multifunctional in vivo probe.
Materials and Methods
Materials
Electrically competent LAM1000 E. coli cells37 were gifted from David Tirrell at California Institute of Technology. Bacto-tryptone, sodium chloride (NaCl), yeast extract, tryptic soy agar, ampicillin sodium salt, sodium phosphate dibasic anhydrous (Na2HPO4), sodium hydroxide (NaOH), dextrose monohydrate (d-glucose), magnesium sulfate (MgSO4), calcium chloride (CaCl2), manganese chloride tetrahydrate (MnCl2·4H2O), cobaltous chloride hexahydrate (CoCl2·6H2O), isopropyl β-D-1-thiogalactopyranoside (IPTG), Pierce bicinchoninic acid (BCA) assay kit, Pierce snakeskin dialysis tubing 3.5 K molecular weight cutoff (MWCO), sodium dodecyl sulfate (SDS), Nunc ninety-six well plates, BD Clay Adams glass microscopy slides, Pierce C18 tips, and 5,5,5-trifluoroleucine were acquired from Thermo Fisher Scientific. The 20 naturally occurring amino acids, dimethyl sulfoxide (DMSO), nickel(III) chloride hexahydrate (NiCl2·6H2O), sodium molybdate dihydrate (Na2MoO4·2H2O), iron(III) chloride (FeCl3), iron(II) chloride tetrahydrate (FeCl2·4H2O), thiamine hydrochloride (vitamin B), thioflavin T (ThT), curcumin (CCM), trifluoroacetic acid (TFA), ProteoMass peptide and protein MALDI-MS calibration kit containing sinnapinic acid, D2O, and copper(II) sulfate pentahydrate (CuSO4·5H2O) were purchased from Sigma-Aldrich. Hydrochloric acid (HCl), Coomassie Brilliant Blue G-250 were purchased from VWR. HiTrap FF 5 mL columns for protein purification were purchased from Cytiva Life Sciences. Macrosep and Microsep Advance Centrifugal Devices 3K MWCO and 0.2 μm syringe filters were purchased from PALL. Acrylamide/bis solution (30%) 29:1, and natural polypeptide sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) standard were purchased from Bio-Rad. Copper(II) chloride anhydrous (CuCl2), sodium selenite (Na2SeO3), and imidazole were purchased from Acros Organics. Formvar/carbon-coated copper grids (FCF400-Cu) and 1% uranyl acetate for transmission electron microscopy were purchased from Electron Microscopy Sciences. Borosilicate glass capillaries (0.2 × 2 × 75 mm) were purchased from VitroCom.
Expression and Purification
Q2TFL and QTFL proteins were expressed as described previously.36 While and pQE30/Q38 was used from our prior studies, pQE60/Q239 plasmid was cloned and purchased from Genscript and Integrated DNA Technologies, respectively. Q and Q2 were expressed in leucine auxotrophic LAM1000 E. coli cells in supplemented M9 minimal media. Prior to induction, expression media was allowed to grow to an optical density at 600 nm (OD600) of 0.8–1.0 before pelleting at 5000 × g at 4 °C for 30 min in an Avanti J-25 centrifuge (Beckman Coulter). Cells were washed a total of three times by resuspending in 0.9% NaCl previously stored at 4 °C overnight, centrifuging to repellet the cells in between washes. Following the final wash and centrifugation cycle, the cell pellet was resuspended in M9 media supplemented instead with 19 amino acids (minus leucine) and containing all other media chemicals. The expression culture was then incubated for 15 min at 37 °C and 350 rpm allowing for recovery while starving of leucine before addition of 555 μg/mL of TFL and 200 μg/mL of IPTG to induce expression. After incubation at 37 °C and 350 rpm for 3 h, cells were harvested by centrifugation at 5000 × g at 4 °C for 30 min in an Avanti J-25 centrifuge (Beckman Coulter) and stored at −20 °C until purification. 12% SDS-PAGE was used to confirm expression of QTFL and Q2TFL. Protein was purified using affinity chromatography on a cobalt-charged HiTrap IMAC FF 5 mL column with Buffer A (50 mM Tris-HCl, 500 mM NaCl, pH 8.0). Protein was eluted using a gradient of Buffer B (50 mM Tris-HCl, 500 mM NaCl, 500 mM imidazole, pH 8.0) possessing an imidazole concentration range from 10–500 mM. Pure fractions were then dialyzed in six consecutive 5 L volumes of Buffer A and concentrated to approximately 2 mM using 3 kDa Macrosep centrifugal filters (Pall). Protein purity was confirmed by 12% SDS-PAGE and concentration determined by BCA assay.
Assessment of Trifluoroleucine Incorporation
Trifluoroleucine (TFL) was assessed by matrix-assisted laser desorption/ionization- time-of-flight mass spectrometry (MALDI-TOF MS) using a Bruker UltrafleXtreme MALDI-TOF/TOF. Protein was diluted 1:50 in water before being mixed in equal parts diluted sample to sinnapinic acid matrix. Protein sample was spotted onto a Bruker MTP 384 steel target plate and vacuum-dried in a desiccator. Using the same protocol, Sigma-Aldrich peptide standards were also spotted onto the target plate. The spectra were then deconvoluted to Gaussian functions in PeakFit software to its maximum goodness of fit by R2 value using one peak to represent full incorporation, and ≥1 peak to represent masses less than full incorporation. The relative percent area of the incorporated Gaussian peak was used to determine the incorporation based on n number of peaks deconvoluted and if the Gaussian fit peak of the expected TFL peak was less than the expected m/z, the % difference was incorporated into the assessment (eq 1).
 |
1 |
Circular Dichroism Spectroscopy
The secondary structures of Q2TFL and QTFL were assessed using a Jasco J-815 circular dischroism (CD) spectrometer with a PTC-423S single position Peltier temperature control system. Wavelength scans were performed from 195 to 250 at 1 nm step sizes by diluting the protein into water (at approximately 10 μM) in order to minimize the effects of sodium chloride. Temperature scans were performed from 20 to 85 °C in water and in phosphate buffer (50 mM Na2HPO4, pH 8.0) and in phosphate buffer in the presence of saturated CCM (as determined by binding data) at 1 °C/min as done previously.40 The mean residue ellipticity (MRE) was calculated as described in previous studies.41
Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
Secondary structure of Q2TFL and QTFL protein was confirmed using attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy with a Nicolet 6700 Fourier Transform Infrared Spectrometer equipped with a diamond ATR accessory and a mercury cadmium telluride (MCT)-A detector. Spectra were collected for 5 μL of 1 mM protein from 4000 to 400 cm–1 with 4.0 cm–1 increments. Sample spectra were normalized using buffer background and analyzed from 1700 to 1600 cm–1 corresponding to the amide I region. Peaks were deconvoluted using Gaussian functions in PeakFit software until the goodness of fit reached r2 ≥ 0.99.42,43
Curcumin Binding
CCM was bound to Q2TFL as described previously.40 Briefly, increasing ratios of CCM:Q2TFL were made at final volumes of 1 mL with final concentrations of Q2TFL at 15 μM and a final concentration of 1% v/v DMSO. Samples were loaded onto a 96-well black plate and excited at 420 nm, and emission was read at 520 nm using a BioTek Synergy H1 microplate reader at room temperature (RT). Normalized relative fluorescence intensities were calculated and analyzed in Graphpad Prism (GraphPad Software). Binding affinity was calculated using the specific binding kinetics equation.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) images were taken with an FEI Talos L120C transmission electron microscope. Samples were diluted to 50 μM and 3 μL was spotted on Formvar/carbon-coated copper grids followed by a 5 μL wash with water and 3 μL staining with 1% v/v uranyl acetate solution each with incubation times of 1 min. Between steps, filter paper was used to wick the grids. Following imaging, fibrils were sized in ImageJ software (Version 1.52q).44
Confocal Microscopy
Q2TFL was diluted to 50 μM and saturated with 40 μM curcumin (solubilized in DMSO) as determined by the binding affinity in drug-binding experiments. The final concentration of samples for confocal microscopy possessed 1% v/v DMSO. Five μL of sample was deposited onto a microslide and covered with a 22 × 22 mm #1 microscope cover glass. Images were taken with a Leica TCS SP8 X laser confocal microscope using a dry 10x objective at RT. Samples were excited at 460 nm and images were taken with a 470–550 nm detection window.
19F Nuclear Magnetic Resonance
19F detection was
studied using a Bruker AVIII-500 (11.7 T) nuclear
magnetic resonance (NMR) instrument equipped with a broadband BB(F)O
CryoProbe. One-pulse sequence was used to acquire the 19F signal with a spectral width 113,636.4 Hz corresponding to 241.5
ppm, 0.577 s acquisition time, and 256 scans. 1D 19F NMR
spectra of QTFL and Q2TFL in 10% v/v D2O were collected in the approximate range of 0.25–2.0 mM based
on concentrations measured by BCA assay following dilution in 10%
v/v D2O spiked buffer (50 mM Tris, 500 mM HCl, pH = 8.0).
90% TFA/10% D2O was acquired with the same sequence for
comparison. Topspin 3.2 software was used to visualize spectra and
quantify the signal-to-noise ratio (SNR) using the Bruker SINO command
by calculating the ratio of the peak amplitude (signal) to the standard
deviation of the noise level in the spectrum. To facilitate a comparison
of SNR signals between Q2TFL and F-TRAP,31 we estimated the gain in SNR for F-TRAP when measured at
9.4 T and translated into an 11.7 T magnetic field strength. This
estimation was made under the assumption of identical experimental
conditions and negligible differences in relaxation times, utilizing
a simplified version of the relationship between SNR and magnetic
field strength, Bo, as 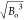 .45,46
.45,46
T1 and T2 relaxation times of the fluorine nuclei in Q2TFL were examined using the inversion recovery and Carr–Purcell–Meiboom–Gill pulse sequences, respectively. The T1 measurement was performed with variable inversion times (TI) of 0.001, 0.05, 0.1, 0.25, 0.8, 1.5, 3.0, and 5.0 s and a 4 s repetition time (TR), averaged over 200 scans. The T2 measurement was conducted using variable echo times (TE) of 0.002, 0.02, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1, 1.4, 1.6, 1.8, 2.5, 5, 10, and 20 ms with a 4 s TR, averaged over 512 scans. T1 and T2 relaxation times were calculated based on a monoexponential fitting analysis using Graphpad Prism software.
Phantom and In Vivo Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) and spectroscopy (MRS) were performed on a Biospec 70/30 micro-MRI system (Bruker – Billerica MA, USA) equipped with zero helium boil-off 300 mm horizontal bore 7-T (7-T) superconducting magnet (300 MHz) based on ultrashield refrigerated magnet technology (USR). The magnet is interfaced to an actively shielded gradient coil insert (Bruker BGA-12S-HP; OD = 198 mm, ID = 114 mm, 660 mT/m gradient strength, 130 μs rise time) and powered by a high-performance gradient amplifier (IECO, Helsinki – Finland) operating at 300A/500 V. This installation is controlled by an Avance-3HD console operated under Paravision 6.1 and TopSpin 3.1. The MR imaging and spectroscopy setup utilized in this study involved the in-house design of two distinct radiofrequency (rf) resonators for scanning a mouse body. The first coil was a volume transmit-receive linear birdcage rf coil with 16 rungs, possessing an outer diameter (OD) of 72 mm, an inner diameter (ID) of 42 mm, and a length (L) of 64 mm (Figure S1a). This rf coil was tuned to 300.16 MHz, corresponding to the 1H proton Larmor frequency. It served the purpose of transmitting and receiving signals during the imaging process, providing radio frequency coverage for the mouse body. A rectangular flexible rf coil was also designed to enable specific detection of the fluorine (19F) nuclei (282 MHz) at 7 T. This flexible surface coil was fabricated by attaching adhesive flat copper tape circuitry affixed to a sheet transparency film. The coil had dimensions of L = 10 mm and a width (W) of 30 mm (Figure S1b). The coil incorporated four distributed fixed ceramic capacitors (Kyocera Co Ltd., Kyoto, JP), which facilitated electrically balanced tuning to a frequency near 282 MHz, corresponding to the 19F Larmor frequency.
The flexible rf coil was skillfully wrapped into the inner part of the cylindrical birdcage rf coil and positioned to optimize inductive coupling and radiofrequency (rf) coverage (Figure S1c). This configuration enabled the achievement of dual resonance for both 1H and 19F nuclei by utilizing the single port of the volume birdcage coil. The single port was connected to a tune/match box, which served as an interface between the volume coil and the spectrometer and also enabled the fine-tuning readjustment of either the proton or fluorine resonances (Figure S1d). The utilization of this single port dual-resonance setup via mutual inductive coupling facilitated the acquisition of imaging and spectroscopy data for both proton (1H) and fluorine (19F) signals, allowing for comprehensive analysis and investigation in the study. A set of 19F magnetic resonance spectra were acquired for the calibration and characterization of the custom-designed RF coil setup and overall sensitivity. The acquisition parameters included a TR= 5 s to enable full magnetization recovery, a number of averages (Nav) = 1, and 4096 sample points for the acquisition with a spectral width (SW) = 85.227 kHz corresponding to 301.6 ppm resulting in spectral resolution of 21 Hz/pt.
A set of water phantoms doped with copper sulfate (CuSO4: 1 g/L, 4.01 M) and NMR tubes filled individually with 100 μL of 100% water, 13 mM trifluoroacetic acid (TFA, 100%) and 1 mM Q2TFL were used for characterizing the 1H/19F rf coil set coverage, sensitivity, and performance. After conducting rf power and shim calibrations using the 1H signal, serial dilutions of TFA NMR tubes were utilized as a reference to evaluate the limit of detection (LOD) for the 19F signal in our experimental setup. The LOD was established by determining the concentration that achieved a SNR above 3 standard deviations of the noise floor.47 The 19F signal optimization of Q2TFL was subsequently carried out. To achieve a constant scan time of 4 min, TR was incrementally increased from 50 to 1000 ms by adjusting the number of averages. The objective of this optimization was to determine the best combination of TR and Nav to acquire Q2TFL spectra with maximum sensitivity under 4 min. Additionally, the same optimization process was repeated at a reduced acquisition time of 1 min to evaluate the impact of improved temporal resolution on SNR. The SNR values were calculated using the “sino” command in Bruker Topspin software. Specifically, the 19F signal interval was defined between −50 ppm and −100 ppm, while the background noise region was selected within the 0 ppm −50 ppm chemical shift range. The spectra were set to a line broadening (LB) value of 30 for display purposes only. By following this experimental protocol, the calibration of the rf power, shim adjustments, and optimization of TR were achieved, ensuring accurate NMR spectral acquisition and analysis for the Q2TFL samples. Specifically, an optimization between length of the scan time and TR was found prior to in vivo experiments to maximize SNR of Q2TFL in 19F MRS. Here, scan times were used at 1 min for dynamic studies and 4 min for static studies, where the number of averages was varied at different TR.
In our in vivo1H MRI/19F MRS mouse experiments, we opted to utilize isoflurane as the preferred anesthetic agent. Isoflurane stands as the predominant choice in small animal studies due to its advantages, including ease of administration, rapid onset, and swift offset of action. These attributes collectively contribute to a streamlined and highly predictable experimental workflow.
Our decision to employ isoflurane was influenced by the specific focus of our study, which involves the measurement of fluorine signals. Concerns regarding the potential interference from isoflurane-derived fluorine background signals prompted careful consideration. It is imperative to acknowledge that isoflurane may introduce unwanted signals that could overlap with other peaks, such as those arising from Q2TFL.
By contrast, for previous investigations involving the F-TRAP biomaterial,31 we chose to utilize ketamine-xylazine (KX) – a regulated and controlled injectable anesthetic devoid of any 19F background signal. However, the use of KX anesthesia carries its own set of limitations, including an irreversible and relatively extended duration of action, which can result in prolonged recovery times. These limitations have the potential to significantly impact the experimental timeline and data collection significantly.
Therefore, our preference for isoflurane over KX anesthesia was grounded in the pursuit of a smoother and more predictable experimental workflow. Isoflurane provides greater control and reversibility in terms of adjusting the depth of anesthesia during the experimental sessions. This level of control is crucial not only during the initial testing and characterization phases, where precise timing is often challenging to predict, but also in the context of long-term disease studies, where maintaining a stable physiological state remains paramount.
Following isoflurane induction, the lower body of the mouse was centered within the rf coil and positioned at the isocenter of the magnet to ensure comprehensive anatomical coverage, with the knee closely fitting the rectangular surface coil. To provide anatomical context, a 1H MRI scan was performed using a 3D gradient echo (3D-GE) Flash sequence. The scan parameters were set as follows: TR= 40 ms, echo time (TE) = 2.1 ms, flip angle (FA) = 30°, matrix size (Mx) = 256 × 128 × 128, field of view (FOV) = 51.2 × 25.6 × 25.6 mm, NAV = 2. The acquisition time for this scan was less than 22 min, resulting in an isotropic image resolution of 200 μm. The primary objective of this scan was to accurately visualize the intra-articular location of Q2TFL injected at 1 mM (50 μL) within the mouse.
For 19F scans, the rf coil resonance was readjusted to fine-tune/match the 282 MHz Larmor frequency and to perform phantom MRI settings utilized as a reference, with an acquisition time of 10 min (TR = 100 ms, NAV = 6000). This setup ensured consistent imaging conditions for the 19F scans, enabling an accurate comparison and analysis of the Q2TFL biomaterial.
To assess and compare the 19F MRS sensitivity of the current Q2TFL biomaterial with that of a previously studied TFL-incorporated construct called F-TRAP,31 we adjusted the scan time to 6 min. 40 s (TR= 100 ms, NAV = 4,000). This modification allowed us to evaluate the overall performance of both the experimental rf coil setup and biomaterials. Importantly, these adjustments were implemented while maintaining the MRI settings optimized for Q2TFL and utilizing the same coil setup employed throughout this study for both in vitro and in vivo. Consequently, the following parameters were employed: 4096 points and SW = 85.227 kHz, resulting in a spectral resolution of 21 Hz per data point.
Ultrasound Guided Injection
The image-guided intra-articular injection of the Q2TFL was performed using a Vevo 3100 high-frequency ultrasound (US) system (Visualsonics/Fujifilm, Toronto ON, CA). The system was equipped with an adjustable rail system designed for small animal handling, precise positioning, and optimization. This setup allowed for noninvasive in vivo imaging under accurate physiological conditions, which included a temperature-controlled heated stage, gas anesthesia, and a syringe injection system for simultaneous compound administration.
A 50 MHz high-frequency US transducer (MX700 D) was utilized, providing an axial resolution of 30 μm and enabling real-time imaging at a rate of up to 300 frames per second. To ensure optimal imaging conditions, mice were positioned in a dorsal recumbent posture on the US heated stage. The hind limbs were flexed and externally rotated approximately 45° while a surgical tape was applied to immobilize the limbs and facilitate access to the knee joint.
Prior to the injection, a sterile US gel was applied over the joint area to enhance visualization and guidance during the injection process. The US transducer was positioned parallel to the femur, allowing for clear visualization of the patellar ligament, which appeared as a dark band in the ultrasound image.
For the injection itself, a needle was carefully inserted laterally into the patellar tendon within the joint capsule. The Q2TFL (1 mM, 50 μL) solution was slowly infused through the needle, while the intra-articular release was continuously monitored using ultrasound imaging.
By employing this technique, the image-guided intra-articular injection of the Q2TFL was successfully performed, ensuring reproducible targeting and delivery of the compound within the joint space while allowing for real-time monitoring of the injection process.
Statistical Analysis
GraphPad Prism (GraphPad Software) was employed for statistical analysis using a student’s t-test.
Results and Discussion
Rationale and Protein Synthesis
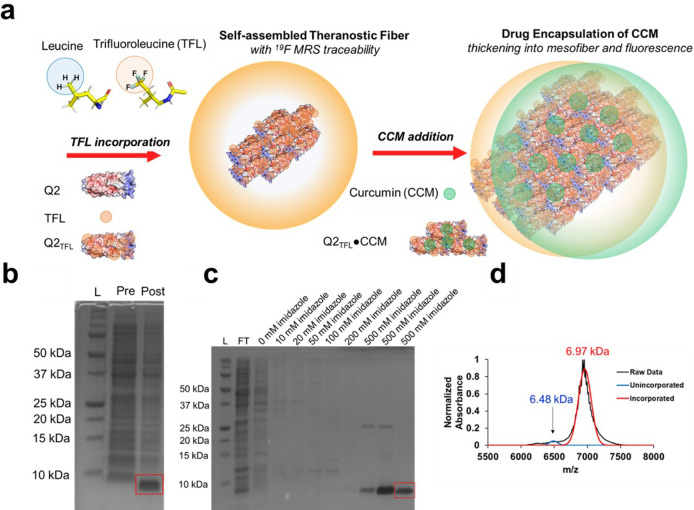
Q2TFL was designed for greater thermostability possessing 9 leucines, compared to 7 in QTFL, which was confirmed by a lower Rosetta Score39,40 with the aim of creating a fluorinated fiber capable of curcumin (CCM) encapsulation (Figure 1a). Q2TFL was generated by residue-specific noncanonical amino acid incorporation of trifluoroleucine (TFL) using leucine auxotrophic LAM1000 E. coli cells.37,48 Protein expression (Figure 1b) and purification (Figure 1c) were assessed by 12% SDS-PAGE gels showing protein bands at a molecular weight of 6.97 kDa for Q2TFL. Percent of TFL incorporation was assessed using MALDI-TOF based on the molecular weight of Q2 (6.48 kDa) (Table S1). Q2TFL showed an expected increase in molecular weight upon incorporation of TFL of 0.49 kDa based on the difference in molecular weight of TFL (185.14 Da) and leucine (131.17 Da) and the number of leucines. Using best-fit Gaussian peaks based on the expected molecular weight of incorporated and unincorporated proteins, Q2TFL was determined to have an average incorporation of 95.0 ± 2.3% (Figure 1d, Figure S4, and Table S2) with this value near the expected range based on previous incorporation levels for TFL in coiled-coils from previous studies, which ranged from 90.7% – 95.1%.36 As a control, QTFL was biosynthesized, purified and confirmed for TFL incorporation as previously described (Figures S2 and S5 and Tables S1 and S3).
Figure 1.
(a) Scheme of TFL incorporation and CCM encapsulation to generate Q2TFL and Q2TFL•CCM (b) of Q2TFL protein (6.97 kDa) after expression. L: Ladder, Pre: Preinduction with IPTG, Post: Postinduction with IPTG. (c) Q2TFL protein after purification. L: Ladder, FT: Flow-through, following are increasing concentrations of imidazole. (d) Representative MALDI-TOF spectra showing incorporation of TFL into Q2 by the size increase to 6.97 kDa.
Fluorinated Coiled-Coil Structure
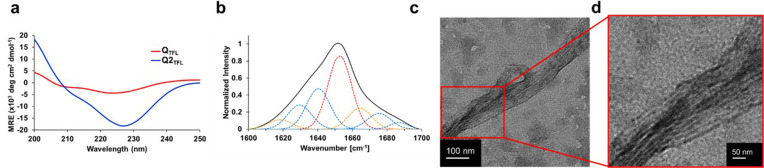
The secondary structure of Q2TFL was assessed by using CD spectroscopy. Q2TFL exhibited a characteristic α-helical spectrum with a double minimum of −100 deg cm2 dmol–1 and −15 000 deg cm2 dmol–1 at 208 and 222 nm, respectively (Figure 2a, Table S4). Additionally, Q2TFL possessed a 222/208 ratio of 150. The large magnitude of the 222/208 ratio suggests that α-helices were found in proximity of other α-helices reflecting the coiled-coil and fibrous nature of Q2TFL.49−51 To further explore the impact of the higher TFL content in Q2TFL, we compared the data with the previously fluorinated fiber, QTFL.36 The parent QTFL exhibited a double minimum of −500 deg cm2 dmol–1 and −4,300 deg·cm2·dmol–1 at 208 and 222 nm, respectively and a 222/208 ratio of 8.6 (Figure 2a, Table S4). Strikingly, Q2TFL demonstrated a much stronger coiled-coil structure and α-helical characteristic minimum at 222 nm, in agreement with previous studies of fluorination on coiled-coil structure.37,52−54
Figure 2.
(a) CD wavelength scan of QTFL and Q2TFL in water performed at 20 °C from 195 to 250 nm. Spectra are the average of three independent trials. (b) Representative ATR-FTIR spectra of Q2TFL. Overall spectra by deconvolution are in black and individual peak deconvolutions are in dotted red lines (α-helix), blue lines (β-sheet), and orange lines (random coil/turns). (c) Transmission electron micrograph of the Q2TFL protein. (d) Higher resolution micrograph of Q2TFL protein highlighting striations composing the fiber.
In addition, the Q2TFL secondary structure in its native buffer conditions was assessed using ATR-FTIR of the samples at 2 mM (Figure 2b). In agreement with CD results, Q2TFL revealed a helical content of 38.4% after deconvolution (Table S5), which was 13.6% higher than the parent QTFL (Figure S5, Table S5), indicating the positive effect of additional TFLs on the coiled-coil structure.
Supramolecular Assembly and Microstructure
Given the nature of the Q proteins to undergo supramolecular assembly at low temperatures,39 Q2TFL was incubated at 4 °C after concentration to 2 mM in 50 mM Tris, 500 mM NaCl, pH 8.0 buffer. Q2TFL underwent supramolecular assembly into nanofibers. Lower resolution micrographs showed Q2TFL fiber morphology to appear similar to those found for QTFL36 containing large diameter and sheet-like structures (Figure 2c, Figure S6). Higher resolution micrograph images revealed a fibrous structure composed of striations measuring 3.6 ± 0.8 nm in size (n = 20) (Figure 2d, Figure S6), approximately the diameter of a single coiled-coil domain and in agreement with the 3.5 ± 0.5 nm protofibril diameters measured in Q previously and suggesting a similar end-to-end stacking mechanism.55
Overall, fiber assemblies are measured to be 215.8 ± 38.6 nm (n = 20) in size by TEM (Figure 2c, S6). The large standard error is explained by the presence of large fiber aggregates as large as 840 nm in diameter. As a result, we view the median diameter, 181.7 nm, as a better representation of a typical fiber diameter. Whereas we have previously strongly associated nanofibril diameter size with the electrostatic potential of protofibril termini,40,55 the increase in diameter of Q2TFL fibrils suggests the size can also be modulated by hydrophobicity, namely by fluorinating or modifying the number of hydrophobic residues lining the coiled-coil pore. To this extent, this agrees with phenomena associated with fiber thickening upon the introduction of hydrophobic small molecule curcumin (CCM) in our fibers. Strong interaction of CCM in the hydrophobic pore and in between fibers causes hydrophobic residues to be further buried and increases the exposure of nonpolar residue groups and thus increases protein surface activity.55−57 We similarly associate the introduction of hydrophobic residues with increased hydrophobic residue packing and surface activity, which in turn increases protofibril interaction, resulting in a population of larger fiber diameters.
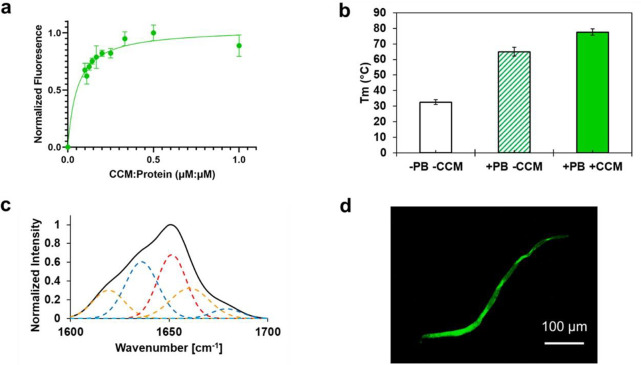
Q2TFL thermostability was measured by CD temperature scans from 20 to 85 °C (Figure S7). In only water, in the absence of salts or buffers, Q2TFL exhibited a melting temperature of 32.6 ± 1.6 °C (Figure 3a). However, under physiologically relevant buffer conditions such as the phosphate buffer used in this study, Q2TFL possessed a melting temperature of 65.0 ± 2.9 °C. This range spans physiological temperature where Q2TFL meets the criteria of an ionic strength-responsive protein biomaterial for controlled drug release.58 In previous studies,36 it was observed that QTFL exhibited an increase in melting temperature, rising from 39 to 52 °C. This substantial enhancement in thermostability aligns with previous research indicating that fluorinated coiled-coils tend to improve stability.37,52−54 Notably, a higher content of TFL resulted in a more significant increase in stability, highlighting the relationship between the TFL concentration and improved stability.
Figure 3.
(a) Spectroscopic fluorescence of Q2TFL at different curcumin:protein molar ratios. Fluorescence was measured by excitation at 450 nm and emission at 520 nm. Error bars represent the standard deviation and are the result of three independent trials. (b) Melting temperature of Q2TFL in the presence of phosphate buffer (PB) with and without CCM. Melting temperature is measured by CD and error bars are the result of three independent trials. (c) Representative ATR-FTIR spectra of Q2TFL•CCM. Overall spectra by deconvolution are shown in black and individual peak deconvolutions in dotted red lines (α-helix), blue lines (β-sheet), and orange lines (random coil/turns). (d) Fluorescent confocal micrograph of Q2TFL•CCM showing fiber thickening to the mesoscale.
Curcumin Binding
Coiled-coil proteins have traditionally been studied for their hydrophobic small molecule-binding ability due to the presence of a hydrophobic pore.32,36,40,59 In particular, curcumin (CCM)36,40 has been used as a model candidate drug due to its therapeutic use as an antiproliferative, antibacterial, and anti-inflammatory agent.60−62 We assess the ability of Q2TFL to bind CCM (Figure 3b) and the impact of encapsulation on Q2TFL structure and stability, where Q2TFL exhibits a Kd of 0.06 μM/μM [CCM:protein] (0.03–0.09 μM/μM @ 95% CI), which translates to an 8:1 ratio of monomer to CCM, a significant increase compared to Q2. We use 2Kd or a ratio of 0.12 to mark saturation of CCM binding and where a negligible increase in fluorescence is seen.40 Moreover, CCM-bound Q2TFL (Q2TFL·CCM) exhibits a 12.6 °C increase in melting temperature to 77.6 ± 2.0 °C (Figure 3b) via CD, which is consistent with previously reported increases upon CCM binding.40
ATR-FTIR was used to decipher the secondary structure of Q2TFL (Figure 2b) and compared to Q2TFL·CCM (Figure 3c). After deconvolution of the spectra, Q2TFL exhibited 42.4 ± 8.6% α-helical content, 38.4 ± 14.0% β-sheet content, and 19.2 ± 9.7% random coil content (Table S5). Upon binding to CCM, noted by broadening of the ATR-FTIR spectra, Q2TFL·CCM possesses 30.8 ± 6.9% α-helical content, 33.0 ± 13.7% β-sheet content, 33.3 ± 8.3% random coil content (Table S5) exhibiting a 14.1% loss in ordered structure–a behavior consistent with previous fiber·CCM binding studies.40
We have previously established a linear model40 correlating the increase in thermostability upon binding CCM, and the loss of ordered structure as measured by ATR-FTIR.40 Based on this model, a 14.5% loss of structure is predicted, which translates to an error of just 0.4% from our measured structure loss of 14.1%, within the root mean squared error (RMSE) of the model,40 which is calculated here to be 0.9%. These results both validate the linear model and strengthen our conclusion that Q2TFL possesses similar binding behavior to nonfluorinated fibers previously studied. While CCM-binding imposes a negative impact on the ordered structure of the coiled-coil, a loss of secondary structure measured by ATR-FTIR has been associated with a positive interaction of CCM in the hydrophobic pore, which helps stabilize the protein and increase thermostability.40 Thus, the fluorination of Q2TFL results in a strengthened interaction with CCM.
Binding of CCM causes fiber thickening of Q2TFL (Figure 3d, Figure S8), consistent with our recent analysis of supramolecular coiled-coil fibers.40 Fiber-thickening by CCM has been established for collagen activity56,57 as well as all coiled-coil fibers designed in our lab so far40,55 and is explained by increased solvation of polar groups and burying of the hydrophobic residues leading to increased surface activity.56,57 The average fiber diameter of 10.8 ± 5.4 μm is similar to the median fiber diameter predicted by our recently established relationship between the nanofiber and CCM-thickened fiber diameters. While this relationship was assessed for nonfluorinated CCM fibers, the predicted fiber diameter is 12.9 μm based on a 181.7 nm Q2TFL fiber diameter translating to an error of 2.1 μm, just outside our model’s root mean squared error (RMSE) of 0.8 μm (Figure S9). These results suggest that Q2TFL supramolecular fiber assembly upon CCM-binding remains similar to our previous nonfluorinated constructs.
19F Nuclear Magnetic Resonance
To determine the potential for Q2TFL as a noninvasive 19F MR dynamic probe, initial 19F NMR was performed on a 500 MHz NMR spectrometer. QTFL and Q2TFL exhibited triplet NMR peaks (Figure S10a, b) consistent with the triple fluorinated residue motif. Due to the presence of peak overlap in the spectrum of Figure S10a, b, the accurate identification and distinction of individual peaks becomes challenging. Specifically, peak 3, characterized by the largest chemical shift, overlaps with peak 2, making it difficult to reliably detect and distinguish them. We attribute this overlap and reduced clarity to protein conformational heterogeneity, which can result in line broadening. This overlap hinders the clear resolution of the individual contributions of these peaks, potentially complicating their proper identification and quantification. Despite the challenges posed by the peak overlap, we were able to characterize the overall T1 and T2 relaxation times of Q2TFL in its 19F NMR spectrum. Q2TFL demonstrated a 19F T1 relaxation time of 329 ms and a T2 relaxation time of 120 μs in its 19F NMR spectrum at 25 °C and 5.6 mg/mL. In comparison, previous findings from our group reported that F-TRAP at a concentration of 1 mg/mL and 22 °C, exhibited 19F T1 of 393 ms and a T2 of 1.2 ms31 suggesting the increased rigidity of Q2TFL.
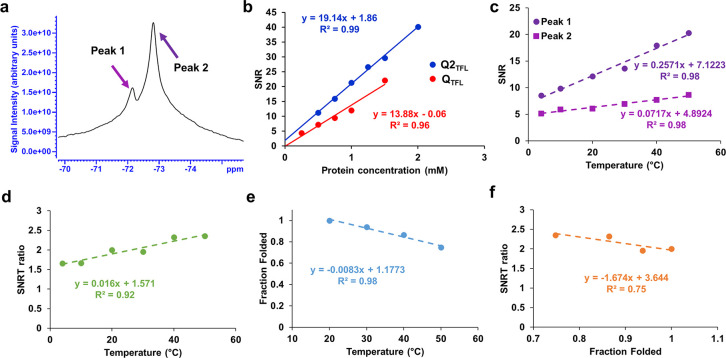
Q2TFL and QTFL were measured at molar concentrations 0.25–2.0 mM (Table S6) and the signal-to-noise ratio (SNR) was measured for each spectrum using 50–100 ppm to represent all signals that appeared in the spectra and 0–50 ppm, where no signal appeared, to represent the noise. TFA exhibited a chemical shift of −76.1 ppm (Figure S10a), consistent with reported values.63 Q2TFL displayed a chemical shift of −72.8 ppm (Figure 4a, Figure S10b), whereas parent QTFL exhibited a chemical shift of −72.6 (Figure S10c). Additionally, Q2TFL demonstrated a SNR dependence on the 19F molar concentration of 19.14 mM–1, while QTFL showed a relationship of 13.88 mM–1 to SNR (Figure 4b, Figure S11). Notably, the SNR efficiency with respect to the 19F molar concentration of Q2TFL was 1.38 times greater than that of QTFL, which is consistent with the expected increase based on the theoretical 9/7 TFL ratio of Q2TFL/QTFL.
Figure 4.
(a) NMR spectrum at 500 MHz (11.7-T) of Q2TFL at 1.5 mM shows two peaks (magenta and purple arrows), (b) SNR of Q2TFL and QTFL as a function of protein concentration. (c) Temperature dependence of SNR from independent peaks. (d) Linear correlation of temperature with SNRT ratio showing the ability to predict temperature from 19F MRS. (e) Linear correlation of temperature with average (n = 3) fraction folded of Q2TFL as assessed by CD. (f) Linear correlation of average fraction folded (n = 3) as assessed by CD with SNRT ratio showing ability to predict relative structure from 19F MRS.
In comparison, our previous fluorinated
construct, F-TRAP, exhibited
an SNR efficiency with respect to 19F molar concentration
of ∼13.6 mM–1 at a magnetic field strength
of 400 MHz.31 To account for the difference
in magnetic field strength B0, we conducted
an estimation of SNR performance at 500 MHz, based on its well-established
dependence on the static magnetic field strength, 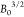 .45,64 Using this relationship,
our projection indicates that F-TRAP could achieve an SNR performance
of ∼19.0 mM–1 at 500 MHz. This estimation
suggests that Q2TFL, as an improved protein-engineered
drug delivery agent, generates a stronger 19F MR signal
at equal molar concentrations compared to our previously detectable 19F MR biomaterial, F-TRAP.31 Furthermore,
Q2TFL possesses 9 TFL per monomer with a monomeric molecular
weight of 6.97 kDa, whereas F-TRAP has 11 TFL per monomer with a monomeric
molecular weight of 16.74 kDa. This translates to an SNR slope of
2.74 mg/mL–1 for Q2TFL, which is 2.4
times higher than the 1.14 mg/mL–1 SNR slope for
F-TRAP. These results suggest that Q2TFL is significantly
more powerful by mass.
.45,64 Using this relationship,
our projection indicates that F-TRAP could achieve an SNR performance
of ∼19.0 mM–1 at 500 MHz. This estimation
suggests that Q2TFL, as an improved protein-engineered
drug delivery agent, generates a stronger 19F MR signal
at equal molar concentrations compared to our previously detectable 19F MR biomaterial, F-TRAP.31 Furthermore,
Q2TFL possesses 9 TFL per monomer with a monomeric molecular
weight of 6.97 kDa, whereas F-TRAP has 11 TFL per monomer with a monomeric
molecular weight of 16.74 kDa. This translates to an SNR slope of
2.74 mg/mL–1 for Q2TFL, which is 2.4
times higher than the 1.14 mg/mL–1 SNR slope for
F-TRAP. These results suggest that Q2TFL is significantly
more powerful by mass.
Finally, Q2TFL was assessed for temperature dependence by altering the environmental temperature in NMR. Q2TFL exhibited an increase in SNR with an increase in temperature, dominated by peak 2 at all temperatures. SNR of each peak was assessed individually by acquiring 1 ppm breadths around peaks 1 and 2 resulting in independent temperature coefficients (Figure 4c). At constant concentration, the ratio of these slopes was used to determine an independent SNR-temperature coefficient for Q2TFL dubbed the SNRT ratio. As expected, linear temperature dependence was retained with the SNRT ratio (Figure 4d), illustrating the intuitive capability to predict temperature using the ratio of peaks 1 and peak 2. This suggested that it could serve as a valuable tool for temperature monitoring. Furthermore, Q2TFL possessed a linear correlation at in vivo relevant temperatures with an R2 = 0.98 (Figure 4e). Thus, the SNRT ratio was correlated with the fraction folded assessed by CD at in vivo relevant temperatures with an R2 = 0.75 (Figure 4f), indicating a strong linear relationship and demonstrating the ability to predict relative structure from overall SNRT ratio alone. These preliminary results show promising potential for the applications of SNRT. Further investigations can explore its use as a valuable tool for in vivo monitoring of QTFL structure and temperature, particularly in areas such as hyperthermia and drug release control. However, conducting in vivo experiments and exploring these applications require additional research and careful considerations. This study serves as an initial step toward these possibilities.
Phantom Magnetic Resonance Imaging
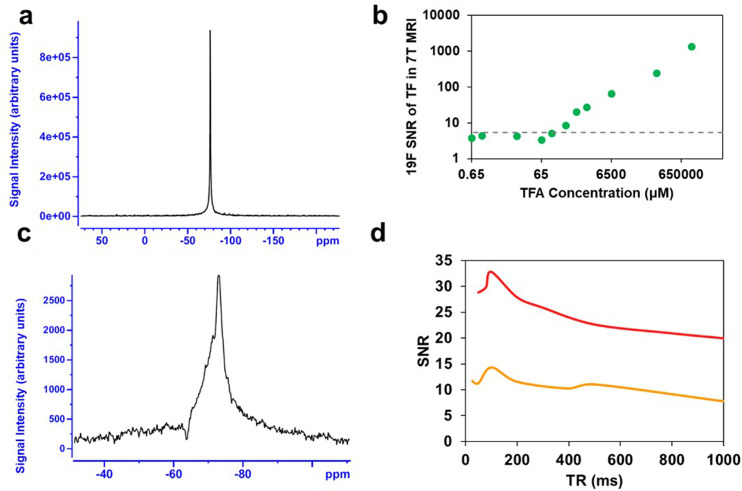
Q2TFL’s potential as a traceable drug delivery agent was assessed through in vivo experiments conducted with our customized rf coil specifically designed for the 7-T Bruker 7030 Biospec μ-MRI system. This single-port dual-resonance (1H/19F) rf coil was tailored to provide optimal coverage of body extremities, such as the knees, during the experiments. The MRI sequence parameters were first optimized by phantom imaging of 100 μL of 100% TFA (13 mM) and 1 mM Q2TFL samples. The limit of detection (LOD) (Figure 5a) was assessed for 19F using TFA (Figure 5b) with a spectral resolution of 41.6 Hz/pt. The threshold for the LOD calculation was achieved at a SNR of 5.3, which is equal to three standard deviations above the baseline noise level. This threshold was reached at 130 μM TFA. Based on the relative SNR of Q2TFL, this suggests that the LOD would be reached by ∼100 μM Q2TFL using the 1.46 Q2TFL:TFA (mM:mM) SNR ratio as determined by NMR.
Figure 5.
(a). Representative in vitro TFA spectra (100%, 13 mM) were acquired using our experimental setup and custom RF coil on a 7-T animal MRI scanner (300 MHz) employing a single pulse sequence. (b) Corresponding 19F SNRs at 7-T MRI are presented for serial dilutions of 100% TFA (green), progressively diluted until reaching the limit of detection (LOD) threshold indicated by dashed lines. (c) Representative 19F MR scan (scan time = 4 min, TR = 80 ms). (d) SNR of Q2TFL obtained from 19F MRS using a 7-T MRI scanner, with scans acquired under both 4 min (red) and 1 min (orange) scan times, while varying the TR.
Prior to in vivo studies, we aimed to optimize the SNR of Q2TFL in 19F MRS by finding a balance between the length of the scan time and the repetition time (TR). Here, we varied TR using a shorter scan time (1 min) and a longer scan time (4 min). To do so, the number of averages was adjusted to maintain a consistent scan time across different TR values (Figure 5c, Table S7). Here, we empirically optimize the performance of our pulse sequence parameters while adhering to a fixed imaging time frame, where 4 min scans were used for static studies and 1 min for dynamic studies (which aided us in identifying potential overlap with fluorinated anesthetics, such as isoflurane). Notably, Q2TFL exhibited the highest signal performance at TR between 80 and 100 ms. Longer scan time of 4 min (Figure 5d) showed a significant improvement in SNR, while shorter 1 min scan times yielded an expected ∼2× reduction in SNR. Nevertheless, the sensitivity of the 1 min scan remained above the LOD, allowing for the acquisition of Q2TFL spectra with improved temporal resolution for traceability purposes. Overall, these studies allowed us to determine a suitable balance between scanning parameters: TR and number of averages.
In Vivo Magnetic Resonance Imaging
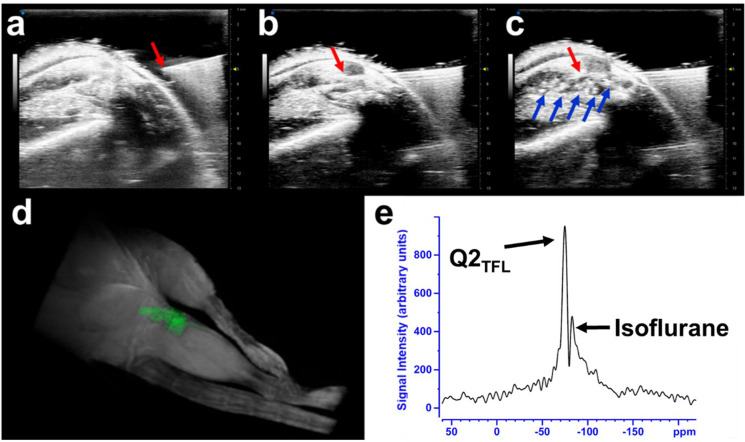
4-to-6-week-old C57Bl6 mice were used to demonstrate the in vivo1H MRI and 19F MRS traceability of Q2TFL. Mice were intra-articularly injected with a 50 μL volume of 1 mM Q2TFL protein, guided by ultrasound (Figure 6a–c). Consistent with our recent work using a coiled-coil fusion protein to target disease prevention in osteoarthritis,65 we use the knee joint as model for localized injection, where here we focus on imaging modalities of Q2TFL. Throughout the imaging experiments, the Q2TFL fibers appeared immobilized using both high frequency ultrasound and MRI. Notably, Q2TFL revealed high frequency echogenic properties as shown using a phantom setup (Figure S12) and in vivo experiments (Figure 6a–c).
Figure 6.
Ultrasound guided injection imaging: (a) Sagittal view of the hindlimb right before the needle insertion which is adequately tilted at 45° to expose to joint and ease the infusion. (b) The needle insertion within the hindlimb knee joint. (c) Successful injection of Q2TFL into the hindlimb knee joint appearing as an echogenic signal using high frequency ultrasound. Red arrow indicates the syringe tip, and blue arrows indicate the presence of Q2TFL. (d) 3D rendering of 1H MRI imaging of the mouse hindlegs where Q2TFL fibers (highlighted in green) appeared as a hypointense signal in the 3D MRI data sets in the injected hindleg. (e) 19F MR spectroscopy performed in vivo after injection of Q2TFL using 10 min scan (TR = 100 ms, NAV = 6000).
Three-dimensional gradient echo (3D-GE) imaging of mouse hindlimbs was conducted under 200-μm isotropic resolution (Figure 6d). The images revealed the presence of Q2TFL in the injected joint, observed as a hypo-signal on MRI due to its short T2 transverse relaxation time (Figure S13). T2-shortening of Q2TFL could be attributed to the semisolid fibers, which provide rigidity and result in dipolar interactions within the protein.66 Additionally, the high protein concentration creates a hydrophobic environment, restricting water mobility and further contributing to the observed hypo-signal.67,68
In vivo19F MR spectroscopy showed a chemical shift of −72.8 ppm (Figure 6e) corresponding to Q2TFL with a SNR of 20.6. Interestingly, the spectra also revealed a neighboring peak with a chemical shift of −78.0 ppm, which we attribute to the use of isoflurane as an inhaled anesthetic during in vivo mouse imaging. This was verified by turning off isoflurane while performing a series of 1 min scans over time. As respiration increased due to clearance of the anesthetic, the SNR of the peak at −78.0 ppm gradually decreased, while the Q2TFL SNR remained stable (Figure S14). This observation provides further evidence supporting the identification of the peak at −86.7 ppm as a result of the isoflurane presence in the spectra.
Finally, a comparative analysis was conducted to assess the relative SNR of Q2TFLin vivo compared to the previous fluorinated construct, F-TRAP.31In vivo19F MRS was performed using the same sequence timing as used for F-TRAP, while ensuring optimized conditions for Q2TFL pulse sequence parameters (TE, TR, NAV) and MRI coil. The scan consisted of 4000 averages with a TR of 100 ms, resulting in a total scan time of 6 min and 40 s. The obtained SNR for Q2TFL was 11.45 using 7.0 mg/mL (corresponding to 1 mM Q2TFL and 9 mM 19F) (Figure S15). The results demonstrated a substantial improvement in the SNR of Q2TFL, in terms of both weight (2.0×) and mM yield of 19F (2.5x), which can be attributed to a higher 19F-protein ratio and monomer density in the fiber morphology, leading to stronger 19F packing. The relatively short T2 of Q2TFL indicates that signal enhancement may be further improved via sequence optimization. Nevertheless, the notable SNR already observed in the 19F MRS holds promise for the application of Q2TFL as an imaging agent.
We have demonstrated Q2TFL to possess bimodal mapping through echogenicity for high-frequency ultrasound visualization (Figure 6, Figure S12), and T2-darkening MRI contrast relative the surround tissue (Figure 6 and Figure S15) while also being traceable by 19F MRS. To the best of our knowledge, this is the first protein fiber to have the capability of a multimodal imaging agent. Furthermore, Q2TFL exhibited utility as a probe for both environmental temperature and protein structure analysis. In addition to its encapsulation ability, which increased thermostability and thickness, these attributes represent a foundation for the future development of biomaterials that possess novel theranostic behavior.
Conclusions
Q2TFL forms fibers on the nano- to mesoscale and generates a larger increase in thermostability and SNR compared to our previously fluorinated fiber construct, QTFL, at the same concentration, demonstrating its ability for 19F magnetic resonance detection. Furthermore, Q2TFL’s therapeutic potential in the form of drug delivery has been demonstrated by its ability to encapsulate CCM. We further explore its ability to thicken and thermostabilize upon CCM binding, as well as its stimuli-responsiveness to ionic strength. Processing of TFL triplet behavior in Q2TFL potentially allows for additional function as a temperature probe and monitoring of the relative protein structure of the agent. Finally, we demonstrate the ability of Q2TFL to provide multimodal contrast in both 1H MRI and high frequency ultrasound with sensitive traceability by 19F MRS in vivo. The results here provide important criteria toward fluorination of coiled-coils for supramolecular assembly and design toward 19F MRS theranostic agents. These results provide a foundation for future in vivo investigations in this area and to explore the potential applications of Q2TFLin vivo.
Acknowledgments
A special thanks to Dr. Michael Meleties for his help and edits during the drafting of this manuscript. The authors thank Dr. Chin Lin and Dr. Joel Tang at the NYU Chemistry Department Shared Instrumentation Facility for his guidance with 19F NMR and subsequent analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsanm.3c04357.
Figures of experimental MR setup designed in-house, QTFL expression and purification, representative QTFL ATR-FTIR spectra, MALDI-TOF spectra for Q2TFL and QTFL calculations, NMR spectra of QTFL and TFA, representative TEM images of Q2TFL, representative fluorescent confocal micrographs of CCM-bound Q2TFL, model of CCM-bound fiber diameters compared to native fiber diameters and relative placement of Q2TFL, in vivo19F MR spectra of Q2TFL at 6 min 40 s scan time, Q2TFL phantom echogenicity, Q2TFL spectroscopy over time after turning off fluorinated anesthetic, and Tables for secondary structure compositions by CD and ATR-FTIR deconvolution, TFL incorporation calculation results for independent trials of QTFL and Q2TFL, SNR values for Q2TFL and QTFL by NMR, and SNR values for Q2TFL at varying TR (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by NSF-DMREF under Award Number DMR 1728858. ATR-FTIR experiments were performed at the NYU Chemistry Department Shared Instrument Facility. The facility is supported by the National Science Foundation (NSF) Chemistry Research Instrumentation and Facilities Program (CHE-0840277) and Materials Research Science and Engineering Center (MRSEC) Program (DMR-1420073). Part of this work was performed at the NYU Grossman School Medicine Preclinical Imaging Laboratory, a shared resource partially supported by the NIH/SIG 1S10OD018337–01, the Laura and Isaac Perlmutter Cancer Center Support Grant, NIH/NCI 5P30CA016087, and the NIBIB Biomedical Technology Resource Center Grant NIH P41 EB017183. This work was partially supported by the NYU Shifrin Myers Breast Cancer Discovery Fund (SMBCDF).
The authors declare no competing financial interest.
This paper was published ASAP on November 6, 2023. The Table of Contents, Figures 3 and 4 were replaced, and the corrected version was reposted on November 8, 2023.
Supplementary Material
References
- Jeyamogan S.; Khan N. A.; Siddiqui R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Archives of Medical Research 2021, 52 (2), 131–142. 10.1016/j.arcmed.2020.10.016. [DOI] [PubMed] [Google Scholar]
- Ballinger J. R. Theranostic radiopharmaceuticals: established agents in current use. Br J. Radiol 2018, 91 (1091), 20170969. 10.1259/bjr.20170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodei L.; Herrmann K.; Schöder H.; Scott A. M.; Lewis J. S. Radiotheranostics in oncology: current challenges and emerging opportunities. Nature Reviews Clinical Oncology 2022, 19 (8), 534–550. 10.1038/s41571-022-00652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdellatif A. A. H.; Younis M. A.; Alsharidah M.; Al Rugaie O.; Tawfeek H. M. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomedicine 2022, 17, 1951–1970. 10.2147/IJN.S357980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W.; Zhang J.; Wang Y.; Jiao C.; Song Z.; Ma Y.; Ding Y.; Zhang Z.; He X.. Radiolabeling of Nanomaterials: Advantages and Challenges. Frontiers in Toxicology 2021, 3, 10.3389/ftox.2021.753316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Zhou F.-L.; Davies G.-L.; Williams G. R. Theranostics for MRI-guided therapy: Recent developments. VIEW 2022, 3 (3), 20200134. 10.1002/VIW.20200134. [DOI] [Google Scholar]
- Eggeling C. Advances in bioimaging—challenges and potentials. J. Phys. D: Appl. Phys. 2018, 51 (4), 040201. 10.1088/1361-6463/aaa259. [DOI] [Google Scholar]
- Monkovic J. M.; Gibson H.; Sun J. W.; Montclare J. K. Fluorinated Protein and Peptide Materials for Biomedical Applications. Pharmaceuticals 2022, 15 (10), 1201. 10.3390/ph15101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E. N. G.; Suzuki Y. Using 19F NMR to Probe Biological Interactions of Proteins and Peptides. ACS Chem. Biol. 2014, 9 (6), 1242–1250. 10.1021/cb500111u. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello J.; Barnett B. P.; Bottomley P. A.; Bulte J. W. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 2011, 24 (2), 114–129. 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hequet E.; Henoumont C.; Muller R. N.; Laurent S. Fluorinated MRI contrast agents and their versatile applications in the biomedical field. Future Med. Chem. 2019, 11 (10), 1157–1175. 10.4155/fmc-2018-0463. [DOI] [PubMed] [Google Scholar]
- Makela A. V.; Foster P. J. Preclinical (19)F MRI cell tracking at 3 T. Magma 2019, 32 (1), 123–132. 10.1007/s10334-018-0715-7. [DOI] [PubMed] [Google Scholar]
- Wang C.; Adams S. R.; Ahrens E. T. Emergent Fluorous Molecules and Their Uses in Molecular Imaging. Acc. Chem. Res. 2021, 54 (15), 3060–3070. 10.1021/acs.accounts.1c00278. [DOI] [PubMed] [Google Scholar]
- Chen J.; Lanza G. M.; Wickline S. A. Quantitative magnetic resonance fluorine imaging: today and tomorrow. WIREs Nanomedicine and Nanobiotechnology 2010, 2 (4), 431–440. 10.1002/wnan.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjic J. M.; Ahrens E. T. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip Rev. Nanomed Nanobiotechnol 2009, 1 (5), 492–501. 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte J. W. M. Detecting Different Cell Populations Using Multispectral 19F MRI. Radiology 2019, 291 (2), 358–359. 10.1148/radiol.2019190377. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Moonshi S. S.; Han Y.; Puttick S.; Peng H.; Magoling B. J. A.; Reid J. C.; Bernardi S.; Searles D. J.; Král P.; Whittaker A. K. PFPE-Based Polymeric 19F MRI Agents: A New Class of Contrast Agents with Outstanding Sensitivity. Macromolecules 2017, 50 (15), 5953–5963. 10.1021/acs.macromol.7b01285. [DOI] [Google Scholar]
- Guo C.; Xu S.; Arshad A.; Wang L. A pH-responsive nanoprobe for turn-on 19F-magnetic resonance imaging. Chem. Commun. 2018, 54 (70), 9853–9856. 10.1039/C8CC06129G. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Hallac R. R.; Lopez R.; Denney R.; MacDonough M. T.; Li L.; Liu L.; Graves E. E.; Trawick M. L.; Pinney K. G.; Mason R. P. Evaluation of tumor ischemia in response to an indole-based vascular disrupting agent using BLI and (19)F MRI. Am. J. Nucl. Med. Mol. Imaging 2015, 5 (2), 143–153. [PMC free article] [PubMed] [Google Scholar]
- Ko I. O.; Jung K. H.; Kim M. H.; Kang K. J.; Lee K. C.; Kim K. M.; Noh I.; Lee Y. J.; Lim S. M.; Kim J. Y.; Park J. A. Preliminary (19)F-MRS Study of Tumor Cell Proliferation with 3′-deoxy-3′-fluorothymidine and Its Metabolite (FLT-MP). Contrast Media Mol. Imaging 2017, 2017, 3981358. 10.1155/2017/3981358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.; Guo W.; Yang G.; Song H.; Wang Y.; Wang C.; Kong D.; Wang W. Fluorine Meets Amine: Reducing Microenvironment-Induced Amino-Activatable Nanoprobes for (19)F-Magnetic Resonance Imaging of Biothiols. ACS Appl. Mater. Interfaces 2018, 10 (22), 18532–18542. 10.1021/acsami.8b03764. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhang H.; Guo C.; Hu G.; Wang L. Multiresponsive Nanoprobes for Turn-On Fluorescence/(19)F MRI Dual-Modal Imaging. Anal. Chem. 2020, 92 (17), 11739–11746. 10.1021/acs.analchem.0c01786. [DOI] [PubMed] [Google Scholar]
- Moonshi S. S.; Zhang C.; Peng H.; Puttick S.; Rose S.; Fisk N. M.; Bhakoo K.; Stringer B. W.; Qiao G. G.; Gurr P. A.; Whittaker A. K. A unique (19)F MRI agent for the tracking of non phagocytic cells in vivo. Nanoscale 2018, 10 (17), 8226–8239. 10.1039/C8NR00703A. [DOI] [PubMed] [Google Scholar]
- Shin S. H.; Park E. J.; Min C.; Choi S. I.; Jeon S.; Kim Y. H.; Kim D. Tracking Perfluorocarbon Nanoemulsion Delivery by (19)F MRI for Precise High Intensity Focused Ultrasound Tumor Ablation. Theranostics 2017, 7 (3), 562–572. 10.7150/thno.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela A. V.; Foster P. J. Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magn Reson Med. 2018, 80 (3), 1138–1147. 10.1002/mrm.27081. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Oeh J.; Hitz A.; Hedehus M.; Eastham-Anderson J.; Peale F. V. Jr; Hamilton P.; O’Brien T.; Sampath D.; Carano R. A. D. Monitoring and Targeting Anti-VEGF Induced Hypoxia within the Viable Tumor by (19)F-MRI and Multispectral Analysis. Neoplasia 2017, 19 (11), 950–959. 10.1016/j.neo.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Y.; Miao D.; Zhou M.; Wang L.; Zhou H.; Su G.. Bio-Inspired Protein-Based Nanoformulations for Cancer Theranostics. Frontiers in Pharmacology 2018, 9, 10.3389/fphar.2018.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee C. T.; Arntson K. E.; Urick A. K.; Mishra N. K.; Hawk L. M. L.; Wisniewski A. J.; Pomerantz W. C. K. Protein-observed 19F-NMR for fragment screening, affinity quantification and druggability assessment. Nat. Protoc. 2016, 11 (8), 1414–1427. 10.1038/nprot.2016.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramini J. M.; Hamilton K.; Ma L.-C.; Swapna G. V. T.; Leonard P. G.; Ladbury J. E.; Krug R. M.; Montelione G. T. (19)F NMR reveals multiple conformations at the dimer interface of the nonstructural protein 1 effector domain from influenza A virus. Structure (London, England: 1993) 2014, 22 (4), 515–525. 10.1016/j.str.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirberger S. E.; Maltseva S. D.; Manulik J. C.; Einstein S. A.; Weegman B. P.; Garwood M.; Pomerantz W. C. K. Synthesis of Intrinsically Disordered Fluorinated Peptides for Modular Design of High-Signal 19F MRI Agents. Angew. Chem., Int. Ed. 2017, 56 (23), 6440–6444. 10.1002/anie.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L. K.; Frezzo J. A.; Katyal P.; Hoang D. M.; Ben Youss Gironda Z.; Xu C.; Xie X.; Delgado-Fukushima E.; Wadghiri Y. Z.; Montclare J. K. Protein Engineered Nanoscale Micelles for Dynamic 19F Magnetic Resonance for Therapeutic Drug Delivery. ACS Nano 2019, 13 (3), 2969–2985. 10.1021/acsnano.8b07481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L. K.; Britton D.; Jihad T.; Punia K.; Xie X.; Delgado-Fukushima E.; Liu C. F.; Mishkit O.; Liu C.; Hu C.; Meleties M.; Renfrew P. D.; Bonneau R.; Wadghiri Y. Z.; Montclare J. K. Engineered protein-iron oxide hybrid biomaterial for MRI-traceable drug encapsulation. Molecular Systems Design & Engineering 2022, 7 (8), 915–932. 10.1039/D2ME00002D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal P.; Meleties M.; Montclare J. K. Self-Assembled Protein- and Peptide-Based Nanomaterials. ACS Biomater Sci. Eng. 2019, 5 (9), 4132–4147. 10.1021/acsbiomaterials.9b00408. [DOI] [PubMed] [Google Scholar]
- Scheibel T. Protein fibers as performance proteins: new technologies and applications. Curr. Opin. Biotechnol. 2005, 16 (4), 427–433. 10.1016/j.copbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tiwari R.; Tiwari G.; Lahiri A.; R V.; Rai A. K. Localized Delivery of Drugs through Medical Textiles for Treatment of Burns: A Perspective Approach. Adv. Pharm. Bull. 2021, 11 (2), 248–260. 10.34172/apb.2021.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More H. T.; Zhang K. S.; Srivastava N.; Frezzo J. A.; Montclare J. K. Influence of fluorination on protein-engineered coiled-coil fibers. Biomacromolecules 2015, 16 (4), 1210–1217. 10.1021/bm5019062. [DOI] [PubMed] [Google Scholar]
- Montclare J. K.; Son S.; Clark G. A.; Kumar K.; Tirrell D. A. Biosynthesis and Stability of Coiled-Coil Peptides Containing (2S,4R)-5,5,5-Trifluoroleucine and (2S,4S)-5,5,5-Trifluoroleucine. ChemBioChem. 2009, 10 (1), 84–86. 10.1002/cbic.200800164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L. K.; Meleties M.; Katyal P.; Xie X.; Delgado-Fukushima E.; Jihad T.; Liu C.-F.; O’Neill S.; Tu R. S.; Renfrew P. D.; et al. Thermoresponsive Protein-Engineered Coiled-coil Hydrogel for Sustained Small Molecule Release. Biomacromolecues 2019, 20, 3340–3351. 10.1021/acs.biomac.9b00107. [DOI] [PubMed] [Google Scholar]
- Britton D.; Meleties M.; Liu C.; Jia S.; Mahmoudinobar F.; Renfrew P. D.; Bonneau R.; Montclare J. K. Tuning a coiled-coil hydrogel via computational design of supramolecular fiber assembly. Molecular Systems Design & Engineering 2023, 8 (2), 217–226. 10.1039/D2ME00153E. [DOI] [Google Scholar]
- Britton D.; Monkovic J.; Jia S.; Liu C.; Mahmoudinobar F.; Meleties M.; Renfrew P. D.; Bonneau R.; Montclare J. K. Supramolecular Assembly and Small-Molecule Binding by Protein-Engineered Coiled-Coil Fibers. Biomacromolecules 2022, 23 (11), 4851–4859. 10.1021/acs.biomac.2c01031. [DOI] [PubMed] [Google Scholar]
- Gunasekar S. K.; Asnani M.; Limbad C.; Haghpanah J. S.; Hom W.; Barra H.; Nanda S.; Lu M.; Montclare J. K. N-Terminal Aliphatic Residues Dictate the Structure, Stability, Assembly, and Small Molecule Binding of the Coiled-Coil Region of Cartilage Oligomeric Matrix Protein. Biochemistry 2009, 48 (36), 8559–8567. 10.1021/bi900534r. [DOI] [PubMed] [Google Scholar]
- Wang P.; Bohr W.; Otto M.; Danzer K. M.; Mizaikoff B. Quantifying amyloid fibrils in protein mixtures via infrared attenuated-total-reflection spectroscopy. Anal. Bioanal. Chem. 2015, 407 (14), 4015–4021. 10.1007/s00216-015-8623-4. [DOI] [PubMed] [Google Scholar]
- Hu X.; Kaplan D.; Cebe P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39 (18), 6161–6170. 10.1021/ma0610109. [DOI] [Google Scholar]
- Schneider C. A.; Rasband W. S.; Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9 (7), 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult D. I.; Lauterbur P. C. The sensitivity of the zeugmatographic experiment involving human samples. Journal of Magnetic Resonance (1969) 1979, 34 (2), 425–433. 10.1016/0022-2364(79)90019-2. [DOI] [Google Scholar]
- Liang Z.-P.; Lauterbur P. C.. Principles of Magnetic Resonance Imaging: A Signal Processing Perspective; Wiley-IEEE Press, 1999. [Google Scholar]
- Shrivastava A.; Gupta V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of Young Scientists 2011, 2, 21–25. 10.4103/2229-5186.79345. [DOI] [Google Scholar]
- Montclare J. K.; Tirrell D. A. Evolving proteins of novel composition. Angew. Chem., Int. Ed. Engl. 2006, 45 (27), 4518–4521. 10.1002/anie.200600088. [DOI] [PubMed] [Google Scholar]
- Lau S. Y.; Taneja A. K.; Hodges R. S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 1984, 259 (21), 13253–13261. 10.1016/S0021-9258(18)90686-1. [DOI] [PubMed] [Google Scholar]
- Kwok S. C.; Hodges R. S. Stabilizing and destabilizing clusters in the hydrophobic core of long two-stranded alpha-helical coiled-coils. J. Biol. Chem. 2004, 279 (20), 21576–21588. 10.1074/jbc.M401074200. [DOI] [PubMed] [Google Scholar]
- Shepherd N. E.; Hoang H. N.; Abbenante G.; Fairlie D. P. Left- and right-handed alpha-helical turns in homo- and hetero-chiral helical scaffolds. J. Am. Chem. Soc. 2009, 131 (43), 15877–15886. 10.1021/ja9065283. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Ghirlanda G.; Vaidehi N.; Kua J.; Mainz D. T.; Goddard I. W.; DeGrado W. F.; Tirrell D. A. Stabilization of coiled-coil peptide domains by introduction of trifluoroleucine. Biochemistry 2001, 40 (9), 2790–2796. 10.1021/bi0022588. [DOI] [PubMed] [Google Scholar]
- Bilgiçer B.; Fichera A.; Kumar K. A Coiled Coil with a Fluorous Core. J. Am. Chem. Soc. 2001, 123 (19), 4393–4399. 10.1021/ja002961j. [DOI] [PubMed] [Google Scholar]
- Buer B. C.; de la Salud-Bea R.; Al Hashimi H. M.; Marsh E. N. G. Engineering Protein Stability and Specificity Using Fluorous Amino Acids: The Importance of Packing Effects. Biochemistry 2009, 48 (45), 10810–10817. 10.1021/bi901481k. [DOI] [PubMed] [Google Scholar]
- Hume J.; Sun J.; Jacquet R.; Renfrew P. D.; Martin J. A.; Bonneau R.; Gilchrist M. L.; Montclare J. K. Engineered Coiled-Coil Protein Microfibers. Biomacromolecules 2014, 15 (10), 3503–3510. 10.1021/bm5004948. [DOI] [PubMed] [Google Scholar]
- Nishad Fathima N.; Saranya Devi R.; Rekha K. B.; Dhathathreyan A. Collagen-curcumin interaction — A physico-chemical study. Journal of Chemical Sciences 2009, 121 (4), 509–514. 10.1007/s12039-009-0061-4. [DOI] [Google Scholar]
- Fathima N. N.; Dhathathreyan A.; Ramasami T. Directed 2-dimensional organisation of collagen: Role of cross-linking and denaturing agents. Journal of Chemical Sciences 2010, 122 (6), 881–889. 10.1007/s12039-010-0076-x. [DOI] [Google Scholar]
- García M. C.14 - Ionic-strength-responsive polymers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf A. S. H., Abu-Thabit N. Y., Eds.; Woodhead Publishing, 2019; pp 393–409. 10.1016/B978-0-08-101995-5.00014-3 [DOI] [Google Scholar]
- Yin L.; Agustinus A. S.; Yuvienco C.; Minashima T.; Schnabel N. L.; Kirsch T.; Montclare J. K. Engineered Coiled-Coil Protein for Delivery of Inverse Agonist for Osteoarthritis. Biomacromolecules 2018, 19 (5), 1614–1624. 10.1021/acs.biomac.8b00158. [DOI] [PubMed] [Google Scholar]
- Rai D.; Singh J. K.; Roy N.; Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410 (1), 147–155. 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- Zsila F.; Molnár P.; Deli J.; Lockwood S. F. Circular dichroism and absorption spectroscopic data reveal binding of the natural cis-carotenoid bixin to human alpha1-acid glycoprotein. Bioorganic chemistry 2005, 33 (4), 298–309. 10.1016/j.bioorg.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Yallapu M. M.; Jaggi M.; Chauhan S. C. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discovery Today 2012, 17 (1), 71–80. 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A.A.; Glen M.J. 19F-13C, 1H-13C Multi-Bond and Single-Bond 2D Correlations and Determinations of Coupling-Constant Signs. Journal of Magnetic Resonance, Series A 1994, 107, 158–166. 10.1006/jmra.1994.1063. [DOI] [Google Scholar]
- Edelstein W. A.; Glover G. H.; Hardy C. J.; Redington R. W. The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med. 1986, 3 (4), 604–618. 10.1002/mrm.1910030413. [DOI] [PubMed] [Google Scholar]
- Katyal P.; Hettinghouse A.; Meleties M.; Hasan S.; Chen C.; Cui M.; Sun G.; Menon R.; Lin B.; Regatte R.; Montclare J. K.; Liu C. J. Injectable recombinant block polymer gel for sustained delivery of therapeutic protein in post traumatic osteoarthritis. Biomaterials 2022, 281, 121370. 10.1016/j.biomaterials.2022.121370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y.; Huo X.; Yin Z. Protein-protein interactions at high concentrations: Effects of ArgHCl and NaCl on the stability, viscosity and aggregation mechanisms of protein solution. Int. J. Pharm. 2021, 601, 120535. 10.1016/j.ijpharm.2021.120535. [DOI] [PubMed] [Google Scholar]
- Kumar C. S.; Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv Rev. 2011, 63 (9), 789–808. 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger M.; Pruessmann K. P. Short-T2MRI: Principles and recent advances. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 237–270. 10.1016/j.pnmrs.2019.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.