Abstract
Objective
The purpose of this study was to develop and externally validate multivariable prediction models for future pain intensity outcomes to inform targeted interventions for patients with neck or low back pain in primary care settings.
Methods
Model development data were obtained from a group of 679 adults with neck or low back pain who consulted a participating United Kingdom general practice. Predictors included self-report items regarding pain severity and impact from the STarT MSK Tool. Pain intensity at 2 and 6 months was modeled separately for continuous and dichotomized outcomes using linear and logistic regression, respectively. External validation of all models was conducted in a separate group of 586 patients recruited from a similar population with patients’ predictor information collected both at point of consultation and 2 to 4 weeks later using self-report questionnaires. Calibration and discrimination of the models were assessed separately using STarT MSK Tool data from both time points to assess differences in predictive performance.
Results
Pain intensity and patients reporting their condition would last a long time contributed most to predictions of future pain intensity conditional on other variables. On external validation, models were reasonably well calibrated on average when using tool measurements taken 2 to 4 weeks after consultation (calibration slope = 0.848 [95% CI = 0.767 to 0.928] for 2-month pain intensity score), but performance was poor using point-of-consultation tool data (calibration slope for 2-month pain intensity score of 0.650 [95% CI = 0.549 to 0.750]).
Conclusion
Model predictive accuracy was good when predictors were measured 2 to 4 weeks after primary care consultation, but poor when measured at the point of consultation. Future research will explore whether additional, nonmodifiable predictors improve point-of-consultation predictive performance.
Impact
External validation demonstrated that these individualized prediction models were not sufficiently accurate to recommend their use in clinical practice. Further research is required to improve performance through inclusion of additional nonmodifiable risk factors.
Keywords: Back Pain, External Validation, Neck Pain, Prediction Model Development, Targeted Interventions
Introduction
Despite substantial research focused on improving patient outcomes in those with neck and/or low back pain (NLBP), the impact of these conditions persists and they are among the top 10 reasons for overall disease burden in terms of disability-adjusted-life-years.1 Although most NLBP episodes are not long-lasting, the proportion of people for whom these symptoms develop into disabling problems is growing.2 The task of improving first contact treatment for such a large public health problem is an international priority,3 particularly among low-income and middle-income countries where the disease burden is rising fastest.4
This research builds on epidemiological studies, which have consistently highlighted that the transition from acute NLBP episodes into persistent NLBP can be predicted.5,6 In addition, the use of risk stratification tools to discriminate between risk subgroups to better match initial treatment7,8 has demonstrated advantages to first contact treatment decision-making3,9 and is, therefore, recommended by international guidelines.10–12
A key next step is not only to stratify individuals into subgroups based on prognostic information, but to also develop and validate individual patient prediction models and to produce communication aids for consultations, including clear visualizations of predictions. Accurately predicting an individual’s future pain intensity scores may allow for the development of clinician decision support tools that enable more tailored, individualized clinical care. Existing tools, such as the Keele STarT Back risk stratification tool,8 determine risk subgroups but do not predict an individual patient’s future pain intensity outcomes, which could be used to shape patient and clinician expectations and lead to more personalized health care.
Existing prediction tools for patients with NLBP have been developed using data collected through self-report questionnaires or interviews, often after first presentation in primary care, rather than during the consultation where most of these tools are intended to be used.7,13,14 It is important to understand to what extent predictions based on such data align with predictions based on information collected by clinicians during a routine consultation.
This research forms part of a larger body of work, developing digital health technology for first contact consultations to support clinical decision-making for patients with NLBP based on individual outcome predictions, as part of a Horizon 2020 European research program (http://backup-project.eu/).15 This incorporates the Keele STarT MSK Tool (www.keele.ac.uk/startmsk), which predicts poor outcomes in patients in primary care settings who are consulting due to musculoskeletal pain,16 alongside a set of recommended risk-matched treatment options developed through consensus.9,17,18
In the present study, we used predictor items that were agreed to be clinically relevant during the forming of the Keele STarT MSK Tool to develop new models to predict an individual’s future pain intensity. We report on the development, internal validation, and external validation of prognostic models to predict 2- and 6- month pain intensity score, which was also modeled when dichotomized as low/moderate-high pain intensity. We explored the predictive performance of these models in external data, using predictor information collected at 2 distinct time points: during the consultation and through patient self-report questionnaires collected 2 to 4 weeks afterwards.
Methods
Source of Data
For this study, secondary analysis of data from 2 existing datasets, the Keele Aches and Pains Study (KAPS)16 and the STarT MSK Pilot Trial (STarT MSK-pilot),9 was combined for model development and internal validation, while external validation of the prediction models was conducted in patients from a third existing dataset: the STarT MSK Main Trial (STarT MSK-MT).18 Eligible patients were defined in the same way in all 3 datasets: those aged 18 and over, consulting at a participating general practice with musculoskeletal pain. The present study included only the subset of patients who consulted with NLBP. Further details of the datasets used in these analyses are included in Supplementary Appendix I.
Outcome Definitions
The outcome was future pain intensity score, which was measured at 2- and 6-month follow-up, through participants’ self-reported response to the question “How intense was your pain, on average, over the last 2 weeks? [Responses on a 0-10 scale, where 0 is ‘no pain’ and 10 is ‘worst pain ever’].” This score was modeled continuously and separately as a binary outcome, dichotomized as 0 to 4 (low pain intensity) versus 5 to 10 (moderate-high pain intensity). A cut-off of 5 on a 0–10 numerical rating scale to indicate at least moderate pain intensity has been reported previously in the literature19–21 and was considered clinically meaningful by the physical therapists in the research team (ie, was considered to be the most appropriate cut-point to group patients into those with good pain intensity outcomes [score of 0 to 4] and those with poor pain intensity outcomes [5 or more]).
Predictors
The 10 items from the Keele STarT MSK Tool were considered as predictors in all models (Tab. 1). These were pain intensity (on a scale from 0 to 10), pain self-efficacy, pain impact, walking short distances only, pain elsewhere, thinking their condition will last a long time, other important health problems, emotional well-being, fear of pain-related movement, and pain duration.16 No statistical selection was conducted, as these predictor variables had all been considered clinically important during the development of the Keele STarT MSK Tool.16 We included the additional predictor of primary pain site (back or neck pain) as this was identified through discussion with the wider research team as being potentially clinically important for both accurate prediction and face validity.
Table 1.
Baseline Predictor Responsesa
| Model Development | External Validation | ||||
|---|---|---|---|---|---|
| STarT MSK-Pilot | KAPS | Total | POC | 2–4 Wk | |
| n = 214 | n = 465 | n = 679 | n = 275 | n = 586 | |
| Age (at consultation, y), mean (SD) | 58.2 (15.9) | 54.6 (17) | 55.7 (16.7) | 56.6 (15.5) | 58.5 (16.0) |
| Sex, female | 134 (62.6) | 267 (57.4) | 401 (59.1) | 167 (60.7) | 353 (60.2) |
| Primary pain site, neck | 59 (27.6) | 57 (12.3) | 116 (17.1) | 61 (22.2) | 129 (22.0) |
| Pain duration | |||||
| <3 mo | 67 (31.3) | 116 (25.0) | 183 (27.0) | 63 (22.9) | 140 (23.9) |
| 3–6 mo | 33 (15.4) | 67 (14.4) | 100 (14.7) | 41 (14.9) | 91 (15.5) |
| 7–12 mo | 29 (13.6) | 33 (7.1) | 62 (9.1) | 33 (12.0) | 70 (12.0) |
| Over 1 y | 85 (39.7) | 240 (51.6) | 325 (47.9) | 134 (48.7) | 278 (47.4) |
| Comorbidities (self-reported) | |||||
| Diabetes | 19 (8.9) | 41 (8.8) | 60 (8.8) | 23 (8.4) | 59 (10.0) |
| Respiratory problem | 37 (17.3) | 67 (14.4) | 104 (15.3) | 48 (17.5) | 102 (17.4) |
| Heart problem | 65 (30.4) | 118 (25.4) | 183 (27.0) | 75 (27.3) | 171 (29.2) |
| Chronic fatigue | 14 (6.5) | 11 (2.4) | 25 (3.7) | 16 (5.8) | 43 (7.3) |
| Anxiety/depression | 48 (22.4) | 97 (20.9) | 145 (21.4) | 61 (22.2) | 147 (25.1) |
| Other | 46 (21.5) | 121 (26.0) | 167 (24.6) | 71 (25.8) | 132 (22.5) |
| EQ5D—Usual activities | |||||
| No problem | 44 (20.6) | 138 (29.7) | 182 (26.8) | 40 (14.6) | 96 (16.4) |
| Slight problem | 80 (37.4) | 139 (29.9) | 219 (32.3) | 95 (34.6) | 206 (35.2) |
| Moderate problem | 52 (24.3) | 106 (22.8) | 158 (23.3) | 86 (31.3) | 183 (31.2) |
| Severe problem | 28 (13.1) | 60 (12.9) | 88 (13.0) | 38 (13.8) | 70 (12.0) |
| Unable/extreme problem | 10 (4.7) | 15 (3.2) | 25 (3.7) | 12 (4.6) | 23 (3.9) |
| Help to read instructions | |||||
| Never | 162 (75.7) | 354 (76.1) | 516 (76.0) | 221 (80.4) | 476 (82.2) |
| Rarely | 33 (15.4) | 48 (10.3) | 81 (11.9) | 22 (8.0) | 51 (8.7) |
| Sometimes | 9 (4.2) | 36 (7.7) | 45 (6.6) | 19 (6.9) | 32 (5.5) |
| Often | 2 (0.9) | 14 (3.0) | 16 (2.4) | 9 (3.3) | 19 (3.2) |
| Always | 3 (1.4) | 13 (2.8) | 16 (2.4) | 1 (0.4) | 2 (0.3) |
| NDI—Baseline score, mean (SD) | 16.1 (8) | 17.5 (8.7) | 16.6 (8.7) | ||
| NDI %—Baseline score, mean (SD) | 32.2 (16) | 35.1 (17.4) | 33.2 (17.4) | ||
| RMDQ—Baseline score, median (IQR) | 9 (5–13) | 10 (5–15) | 9 (5–14) | ||
| STarT MSK Tool score (baseline), median (IQR) | 6 (4–7) | 7 (4–9) | 7 (4–9) | 7 (5–8) | 7 (5–9) |
| STarT MSK Tool score subgroup (baseline) | |||||
| High risk | 29 (13.6) | 118 (25.4) | 147 (21.6) | 95 (34.6) | 140 (23.9) |
| Medium risk | 105 (49.1) | 176 (37.9) | 281 (41.4) | 120 (43.6) | 273 (46.6) |
| Low risk | 63 (29.4) | 125 (26.9) | 188 (27.7) | 40 (14.6) | 101 (17.4) |
| 1) Pain intensity, median (IQR) On average, how intense was your pain [where 0 is “no pain” and 10 is “pain as bad as it could be”]? | 7 (5–8) | 7 (4–8) | 7 (5–8) | 7 (6–8) | 7 (5–8) |
| 2) Pain self-efficacy Do you often feel unsure about how to manage your pain condition? | 144 (67.3) | 225 (48.4) | 369 (54.3) | 163 (59.3) | 279 (47.6) |
| 3) Pain impact Over the last 2 wk, have you been bothered a lot by your pain? | 113 (52.8) | 330 (71.0) | 443 (65.2) | 228 (82.9) | 462 (78.8) |
| 4) Walking short distances only Have you only been able to walk short distances because of your pain? | 117 (54.7) | 248 (53.3) | 365 (53.8) | 143 (52.0) | 344 (58.7) |
| 5) Pain elsewhere Have you had troublesome joint or muscle pain in more than 1 part of your body? | 147 (68.7) | 304 (65.4) | 451 (66.4) | 126 (45.8) | 398 (67.9) |
| 6) Thinking their condition will last a long time Do you think your condition will last a long time? | 154 (72.0) | 315 (67.7) | 469 (69.1) | 211 (76.7) | 477 (81.4) |
| 7) Other important health problems Do you have other important health problems? | 81 (37.9) | 183 (39.4) | 264 (38.9) | 88 (32.0) | 242 (41.3) |
| 8) Emotional well-being Has pain made you feel down or depressed in the last 2 wk? | 132 (61.7) | 284 (61.1) | 416 (61.3) | 170 (61.8) | 388 (66.2) |
| 9) Fear of pain-related movement Do you feel it is unsafe for a person with a condition like yours to be physically active? | 56 (26.2) | 133 (28.6) | 189 (27.8) | 144 (52.4) | 322 (55.0) |
| 10) Pain duration Have you had your current pain problem for 6 months or more? | 114 (53.3) | 219 (47.1) | 333 (49.0) | 121 (44.0) | 345 (58.9) |
a Baseline characteristics and responses to the Keele STarT MSK tool items (“As you answer these questions, think about how you have been over the last two weeks:”) in model development and external validation data sets. Values are n (%) unless otherwise stated. EQ5D = EuroQol 5 Dimension; IQR = interquartile range; KAPS = Keele Aches and Pains Study; NDI = Neck Disability Index; POC = point of consultation; RMDQ = Roland Morris Disability Questionnaire; SD = standard deviation.
Predictor information was collected through a postal questionnaire sent to patients within a few days of their general practice consultation (KAPS, STarT MSK-pilot, and STarT MSK-MT). For STarT MSK-MT (the external validation data), predictor information was also collected at the time of general practice consultation in patients in the intervention arm, and these data were used for additional validation analyses.
Further detail on all candidate predictors is given in Supplementary Appendix IV (Tab. S1).
Statistical Analysis
Sample Size
The sample size for all analyses was fixed due to the size of the available datasets. We compared the available number of participants for each analysis (Fig. 1) to sample size recommendations for developing22,23 and externally validating24–26 clinical prediction models. Further details on the sample size calculations are included in Supplementary Appendix II.
Figure 1.
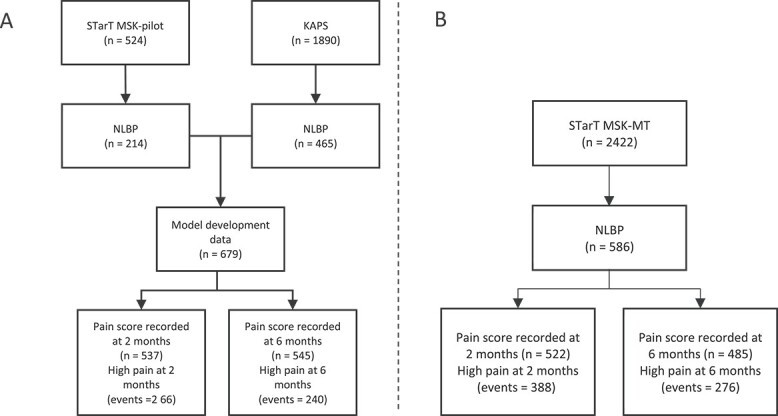
Patient flow summary at model development (A) and at external validation (B). KAPS = Keele Aches and Pains Study; NLBP = neck and/or low back pain.
Based on the anticipated inclusion of 11 predefined predictor parameters (1 continuous predictor, modeled linearly, and 10 binary predictors), we required 311 participants for the development of models for continuous pain intensity score, and at least 824 participants (with 412 “moderate-high pain intensity” events) for binary pain intensity outcomes. Thus, our available data exceeded the requirements for the continuous pain intensity score model but was not enough for the binary pain intensity outcome.
For precise estimation of model performance, we required 892 and 1946 participants to externally validate the continuous and binary outcome models, respectively; thus, estimates of predictive performance are subject to some uncertainty.
Missing Data
Multiple imputation by chained equations was used to account for missing data in both predictor and outcome measurements, under the assumption that data were missing at random.27,28 Multiple imputation was performed separately for each dataset to allow for the clustering of individuals within that dataset, with the number of imputations chosen to exceed the percentage of incomplete cases.28 Preliminary checks for associations between missingness and predictor values were conducted to check for obvious violations of the missing at random assumption. Results of analyses were pooled across imputations using Rubin rules where appropriate.27
Model Development
Continuous pain intensity score outcomes were modeled using random-effects linear regression, while binary outcomes of moderate-high pain intensity were modeled using random-effects logistic regression.29 Outcomes were modeled using multilevel mixed-effects models to account for heterogeneity across the 2 model development datasets, resulting in average model intercepts across the KAPS and STarT MSK-pilot datasets.30 Models were fitted using restricted maximum likelihood (REML), with an unstructured variance–covariance for the random effects on the intercept term.31 Continuous predictors were modeled linearly on their continuous scale, and all predictors were forced into all models.32,33
Internal Validation
Predictive performance of the developed models was assessed through calibration for the continuous outcome models, and through calibration and discrimination for the binary outcome models.34 Calibration was assessed using the calibration slope, calibration in the- large (CITL), and the ratio of observed to expected cases (O/E, for binary outcome models only). Discrimination was assessed through the C statistic. The proportion of variance in the outcome explained by the predictors in each model was determined using the adjusted 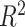 (or pseudo
(or pseudo 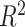 for binary outcomes, using Nagelkerke and Cox-Snell approaches).
for binary outcomes, using Nagelkerke and Cox-Snell approaches).
Internal validation was conducted simultaneously for all models, using bootstrapping with 1000 samples to provide optimism-adjusted estimates of predictive performance.27,29,35
The optimism-adjusted calibration slope was used as an estimate of the uniform shrinkage factor for each model, with regression coefficients multiplied by this shrinkage factor to correct for overfitting.29,35,36
External Validation
Model equations for the 4 prediction models were applied for the participants in the STarT MSK-MT data to calculate the prediction values from each model. Predictive performance measures were calculated, as described for the internal validation, including measures of calibration (calibration slope, CITL, O/E ratio) and discrimination (C statistic), and measures of overall model fit (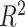 or Nagelkerke pseudo
or Nagelkerke pseudo 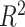 for binary outcomes). Model performance was also assessed within subgroups to check consistency in performance across age ranges, sex, treatment group (matched treatment or usual care), and pain durations prior to presentation.
for binary outcomes). Model performance was also assessed within subgroups to check consistency in performance across age ranges, sex, treatment group (matched treatment or usual care), and pain durations prior to presentation.
Predictors in the STarT MSK-MT data were recorded for each patient at 2 time points. Data on predictors were available for each participant when assessed (i) within the general practice consultation, and (ii) after the consultation using a self-reported questionnaire, which was returned by post around 2 to 4 weeks after the consultation.
The timing of risk predictors collected 2 to 4 weeks after consultation was more similar to the recording of the predictor variables in the model development data, while predictor variable collection at the point of consultation better reflects the models’ intended future use. We therefore tested model performance for both data collection time points to assess the validity of our assumption that the developed models could be used in practice at point of consultation.
Extended statistical methods are given in Supplementary Appendix III. All analyses were performed using Stata MP Version 16 (StataCorp). This paper adheres to the TRIPOD checklist for the transparent reporting of multivariable prediction models, see Supplementary Appendix VI.37
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Study Population
Development Data
Across the 2 model development datasets, 679 patients with NLBP were available for inclusion in the analysis (Fig. 1A). Patients predominantly presented with back pain (83%), with a much smaller group presenting with neck pain (17%), and had a median baseline pain intensity score of 7 (interquartile range = 5–8) out of 10. Patient demographics across the 2 datasets are given in Table 1.
Table 1 also shows a summary of predictor responses. When summarized across both model development datasets, most patients (n = 451, 66.4%) had troublesome musculoskeletal pain in more than 1 part of their bodies, with 69.1% (n = 469) thinking their condition would last a long time. A further 27.8% (n = 189) participants were reported a “fear of pain-related movement.”
Few predictors’ variables showed substantial differences (larger than 10%) in distribution between the STarT MSK-pilot and KAPS datasets, as can be seen in Table 1. Notable differences in predictor variable distributions included those for pain self-efficacy (“unsure about how to manage [their] pain condition”; STarT MSK-pilot 67.3%, KAPS 48.4%); and for pain impact (“bothered a lot by [their] pain” in the preceding 2 weeks; STarT MSK-pilot 52.8%, KAPS 71.0%).
External Validation Data
The STarT MSK-MT data included 586 patients with NLBP for the external validation of the above models (Fig. 1B). Patients predominantly presented with back pain (78%) and had a median baseline pain intensity score of 7 (Interquartile range [IQR] = 6–8) out of 10 reported at point of consultation, and a median of 7 (IQR = 5–8) when reporting in the data collected 2 to 4 weeks after consultation.
The majority of patients reported experiencing moderate-high pain intensity at 2 months, with a prevalence of moderate-high pain intensity at 57.7% (slightly higher than the 49.5% prevalence seen in the development data). This dropped to 47.1% at 6 months follow-up (44.0% for the development data). Baseline pain intensity scores on a scale from 0 to 10 were reported consistently across the data collected 2 to 4 weeks after consultation (median = 7; IQR = 5–8) and at the point of consultation (median = 7; IQR = 6–8), with a slightly narrower spread of scores recorded at point of consultation.
Predictors regarding pain impact and pain self-efficacy were both reported as present in a higher proportion of patients when collected at point of consultation, while the remaining predictor items showed a higher prevalence when recorded 2 to 4 weeks after consultation. The largest difference was seen for the “pain elsewhere” item, with 68% answering “yes” 2 to 4 weeks after consultation compared to only 46% answering “yes” at point of consultation.
Model Development and Internal Validation
The final models for predicting pain intensity scores in patients with NLBP, after optimism adjustment, are presented in Table 2, along with the shrinkage factor estimated via bootstrapping. Detailed results from the internal validation can be seen in Supplementary Appendix IV (Tab. S2 and Fig. S1).
Table 2.
Prognostic Modelsa
| Coefficients for Continuous Outcome, Pain Score (β) | Odds Ratios for Binary Outcome, High Pain | |||
|---|---|---|---|---|
| 2 mo | 6 mo | 2 mo | 6 mo | |
| Pain intensity | 0.236 | 0.269 | 1.23 | 1.26 |
| Pain self-efficacy | 0.526 | 0.212 | 1.38 | 1.20 |
| Pain impact | 0.859 | 0.632 | 2.33 | 1.40 |
| Walking short distances only | 0.447 | 0.934 | 1.57 | 2.37 |
| Pain elsewhere | 0.49 | 0.278 | 1.51 | 1.33 |
| Thinking their condition will last a long time | 1.22 | 1.673 | 2.51 | 3.61 |
| Other important health problems | 0.783 | 0.578 | 1.73 | 1.38 |
| Emotional well-being | −0.032 | −0.002 | 0.91 | 1.28 |
| Fear of pain-related movement | 0.041 | −0.434 | 1.07 | 0.63 |
| Pain duration | 1.029 | 1.129 | 2.82 | 2.19 |
| Primary pain site | −0.171 | 0.515 | 0.69 | 1.02 |
| Intercept | −0.304 | −1.153 | −4.010 | −4.324 |
| Var (intercept) | 0.237 | 0.132 | 0.030 | 0.041 |
| Shrinkage factor | 0.975 | 0.982 | 0.930 | 0.938 |
a Prognostic models after optimism adjustment. Numbers are intercepts (α) and coefficients (β) and for continuous outcome models, intercepts (α), and odds ratios (exp[β]) and for binary outcome models. Uniform shrinkage factors for each model were obtained through bootstrapping with 1000 replications.
Conditional on other variables in the model, baseline pain intensity and thinking their condition would last a long time were the strongest predictors of pain intensity at both follow-up time points, with higher baseline pain intensity and expecting the condition to last a long time both being associated with higher pain intensity scores at follow-up. Episode duration (whether the patient had experienced pain for longer than 6 months at the time of their general practice consultation) was also an important predictor, associated with higher pain intensity at both 2 and 6 months. Figure 2 gives a demonstration of how the models for pain intensity could be used to calculate predictions for pain intensity score and the probability of moderate-high pain intensity for individual patients at 6 months.
Figure 2.
Demonstration of prediction calculation. LP = linear predictor; NLBP = neck and/or low back pain.
External Validation
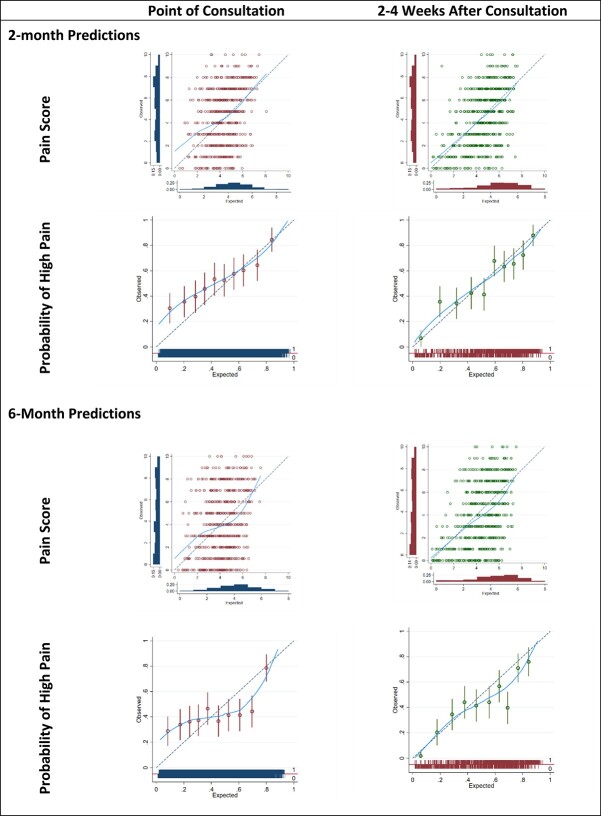
Details of the model performance on external validation are given in Table 3, while calibration plots for all models can be seen in Figure 3.
Table 3.
External Validation, Predictive Performance a
| Time | Outcome | Measure | Point of Consultation | 2–4 Wk After Consultation |
|---|---|---|---|---|
| 2 mo | Pain score | Calibration slope | 0.650 (0.549 to 0.750) | 0.848 (0.767 to 0.928) |
| CITL | 0.649 (0.506 to 0.792) | 0.378 (0.249 to 0.507) | ||
| R 2 median (IQR) | 11.1% (10.0% to 12.1%) | 25.3% (24.9% to 26.0%) | ||
| High pain | Calibration slope | 0.436 (0.338 to 0.535) | 0.657 (0.556 to 0.758) | |
| CITL | 1.081 (0.951 to 1.21) | 0.798 (0.665 to 0.931) | ||
| O/E | 1.599 (1.552 to 1.647) | 1.369 (1.329 to 1.41) | ||
| C statistic | 0.649 (0.618 to 0.679) | 0.726 (0.696 to 0.753) | ||
| Pseudo R2 median (IQR) | 12.1% (10.5% to 13.3%) | 27.1% (26.1% to 27.9%) | ||
| 6 mo | Pain score | Calibration slope | 0.593 (0.499 to 0.688) | 0.735 (0.656 to 0.815) |
| CITL | −0.93 (−1.088 to −0.773) | −1.262 (−1.408 to −1.116) | ||
| R 2 median (IQR) | 10.4% (9.4% to 11.4%) | 20.8% (20.2% to 21.3%) | ||
| High pain | Calibration slope | 0.526 (0.417 to 0.635) | 0.71 (0.598 to 0.823) | |
| CITL | 0.028 (−0.101 to 0.157) | −0.307 (−0.438 to −0.176) | ||
| O/E | 1.015 (0.984 to 1.046) | 0.88 (0.854 to 0.908) | ||
| C statistic | 0.663 (0.632 to 0.693) | 0.721 (0.692 to 0.749) | ||
| Pseudo R2 median (IQR) | 8.7% (7.6% to 10.2%) | 22.1% (21.1% to 23.1%) | ||
a Predictive performance of models for pain on external validation in the STarT MSK Main Trial data. CITL = calibration in the large; IQR = interquartile range; O/E = observed/expected ratio.
Figure 3.
Model calibration in external validation data.
Predictions for continuous pain intensity score generated using point-of-consultation data were poorly calibrated when compared to observed values, with calibration slopes of 0.65 (0.55–0.75) and 0.59 (0.50–0.69) at 2 and 6 months respectively. However, the predictions generated in data from 2 to 4 weeks after consultation showed better calibration at both time points, with calibration slopes of 0.85 (0.77–0.93) and 0.74 (0.66–0.82).
Estimates of CITL suggest that the 2-month pain intensity score model systematically underpredicted patients 2-month pain intensity scores by an average of 0.65 pain intensity points in the point-of-consultation predictions (95% CI = 0.51 to 0.79), and by an average of 0.38 pain intensity points (95% CI = 0.25 to 0.51) in predictions generated from predictor responses 2 to 4 weeks after consultation. The pain intensity score model at 6 months systematically overpredicted patients’ pain intensity scores for both sources of predictor data, with predicted pain intensity scores being an average of 0.93 points too high in the point-of-consultation data (95% CI = 0.77 to 1.09), and 1.20 points too high in the data from 2 to 4 weeks after consultation (95% CI = 1.12 to 1.41).
The model to predict pain intensity score at 2 months performed better than the 6-month model by all predictive performance measures, in both sources of predictor variables at point of consultation and 2 to 4 weeks after consultation, as can be seen in Table 3.
Calibration performance was poor for the models predicting the binary outcome of moderate-high pain intensity. In the point-of-consultation data, the calibration slope was 0.44 (95% CI = 0.34 to 0.54) for predicting high pain intensity at 2 months and 0.53 (95% CI = 0.42 to 0.64) at 6 months, indicating predictions were too high in those at low risk of high pain intensity and were too low in those at high risk of moderate-high pain. As with the continuous outcome pain intensity models, calibration slopes indicated better calibration performance for predictions generated using predictors collected 2 to 4 weeks after consultation.
Discrimination performance was consistent for models predicting moderate-high pain intensity at both time points. C statistics of 0.65 (0.62–0.68) and 0.66 (0.63–0.69) at 2 and 6 months, respectively, suggest that around 65% and 66% of concordant pairs were correctly identified by the models for these outcome time points, based on predictors recorded at point of consultation. Again, using predictor values from 2 to 4 weeks after consultation to generate predictions gave better discriminative performance, with 73% of concordant pairs correctly identified at 2 months and 72% identified at 6 months.
Analyses to assess model performance across different subgroups (shown in Suppl. Appendix V) suggest that model performance was reasonably consistent across age ranges, sex, treatment group, and pain duration prior to presentation.
Discussion
We have developed and externally validated new individualized prediction models for pain intensity outcomes at 2- and 6-month follow-up in patients consulting with NLBP in general practice, based on the Keele STarT MSK Tool items.
The findings from our external validation demonstrated that models applied in the predictor data collected 2 to 4 weeks after the consultation achieved better predictive performance than data collected at the point of consultation. For example, in terms of calibration performance, the model predicting pain intensity score at 2 months had a calibration slope of 0.848 (0.767–0.928) in data from 2 to 4 weeks after consultation, but only 0.650 (0.549–0.750) at point of consultation. The discriminative ability of the models for predicting 6-month binary outcomes was more consistent between validations in data from 2 to 4 weeks after consultation and point of consultation, with a reasonable C statistic of 0.66 for moderate–high pain intensity when using data collected at point of consultation compared with 0.72 for predictions based on data from 2 to 4 weeks after consultation. The continuous outcome models showed a systematic underprediction of pain intensity scores at 2 month, and systematic overprediction at 6 months. When predictions were generated using data collected at point of consultation, this overprediction was by around 0.9 points on the 0–10 pain intensity scale, which is not trivial.
The models presented here were based on known predictors for outcomes in patients with NLBP from the team’s previous risk stratification work.16 The choice of candidate predictors was therefore limited to our previously validated Keele STarT MSK Tool items: A decision that proved in hindsight to be a limitation to exploring options for greater accuracy in our predictions of pain intensity outcomes. In understanding reasons for the disappointing performance of these prediction models, it is important to recognize that the Keele STarT MSK Tool was designed for risk-stratification to inform clinicians about risk-matched treatment options. The Keele STarT MSK Tool, therefore, contains mainly items considered to be treatment modifiable risk factors (with pain duration being the only nonmodifiable item). Other known non-modifiable risk factors that were not considered during the model development process presented here include factors such as employment status and other socioeconomic indicators, previous surgery, comorbidities, and previous pain episodes.38 The next step to improve model performance for individualized predictions will be to explore whether adding such nonmodifiable factors to our models improves their predictive accuracy.
Comparison With Other Studies
Other prediction models currently exist to predict various outcomes in those with NLBP, such as time to recovery for patients with acute low back pain,13 global perceived effect for patients with persistent neck pain,39 or disability in patients undergoing surgery for lumbar degeneration.40 However, as far as we are aware, this is the first time that models have specifically been developed to predict an individual patient’s levels of pain intensity at future time points. Prediction models in the field which have previously been updated and externally validated13,41 often performed suboptimally in external samples. It is not uncommon for risk prediction models to need updating depending on their specific purpose or clinical population, as we have found here.
Several studies have previously shown baseline pain intensity to be a reliable predictor of future pain intensity among patients with low back pain, suggesting that initial pain levels could be a useful indicator of long-term pain outcomes and poor recovery.42–44 Our findings are also consistent with recent data from Brazil showing that the Keele STarT Back Tool is more predictive of outcomes when collected a few weeks after a physical therapist consultation than at the consultation itself.28 Our results further agree with a study in United Kingdom and Dutch primary care which suggests that additional assessment of pain intensity after 4 to 6 weeks results in better predictions than when using baseline pain intensity alone.45 However, although predictor–outcome associations are known to be weaker for time-varying predictors (such as pain intensity) when measured at consultation than when measured 2 to 4 weeks after consultation, it is still recommended for such predictors to be measured at the time of intended model use to improve the applicability of the model in practice.46
In patients with neck pain, expectations and previous clinical course of symptoms were found to predict global perceived effect.41 In patients with low back pain of short duration (<4 weeks) and a pain intensity ≥2/10, the duration of the current episode, pain intensity, and depression was found to predict time to recovery from pain.14 Despite some differences in populations, we also found that pain intensity, episode duration, and thinking their condition will last a long time to predict future pain intensity (conditional on other model variables). Thus, some predictive factors seem consistent across the literature.
Our findings of the limited predictive performance of low mood, however, contrast with the results of an umbrella review of systematic reviews in this area.38 Differences in the predictive ability of mood may be related to differences in the populations used for analysis, or due to other variables in our models (such as bothersomeness or pain intensity) assimilating the prognostic impact of mood.
It should be noted that all variables included in our models were self-report measures, with no variables arising from clinical examination, existing electronic medical record data, or imaging results. This decision was made due to a lack of a standardized clinical examination for patients consulting with NLBP, and the expectation that using self-report items could overcome the wide variation in general practitioner clinical examinations. Furthermore, general practitioners rarely have imaging results on which to base a treatment decision. Indeed, previous work suggests that clinical examination and MRI scan results add little to outcome predictions in patients with low back pain over-and-above predictors such as younger age, attitudes and beliefs regarding pain, or depression.47,48
Strengths and Limitations
We acknowledge that to achieve a comprehensive understanding of a patient’s health status over time, a variety of outcomes are needed. Therefore, a key limitation here is that our new prognostic model focusses solely on predicting pain intensity. Although pain intensity is undoubtedly an essential aspect of measuring a patient’s pain experience, it does not reflect the complexity of pain and its wider impact on an individual’s physical, emotional, and social well-being. We are, therefore, planning to similarly publish prognostic models for a broader range of outcomes, including physical function (restriction in usual activities) and time off work. Collectively, these models will provide useful information that could inform treatment decisions and guide patient care.
A barrier to implementation in practice is the complexity for clinicians to calculate outcome predictions for individual patients, which may require a prebuilt calculator. Such a calculator has been incorporated into the Back-UP first contact WebApp dashboard,15 however, reflecting on the relatively low Cstatistic seen within the external validation at the point of consultation; however, further research is needed to improve the discriminative performance of these models before we could recommend use in clinical practice.
Evaluating the performance of predictions at the point of consultation is a clear strength to our validation, with predictive performance assessment at the point where the models are intended to be used in practice. However, small sample sizes for this external validation resulted in some uncertainty around performance estimates in this population, where all models performed less well than when used in data from 2 to 4 weeks after consultation. Although further external validation in a larger dataset would reduce our uncertainty in predictive performance estimates, the current validation gives a good indication that for these models to perform well at point of consultation, where they would be used in practice, it is likely that updating (for example, to incorporate nonmodifiable risk factors) or recalibration would be needed.
A strength of the external validation is in the representativeness of the sample used. An anonymized medical record audit of all patients with MSK conditions in primary care settings suggested that there was no evidence of selection bias in baseline pain intensity or risk severity in the participants within the STarT MSK MT population. Therefore, we are confident that the sample used was representative of patients consulting to primary care in the United Kingdom.
It was not possible to produce separate prediction models for patients with neck pain and lower back pain, due to the limited number of patients available with the neck as their primary pain site. Rather than neglecting to include these patients with neck pain in our analyses, we instead combined the patient populations and introduced “primary pain site” as a predictor in the model. For this reason, predictor effects are likely to be highly weighted toward the effects experienced by patients with low back pain as their primary pain site, and thus future validation in separate neck pain and low back pain populations would be required to assess the extent to which our conclusions (and models) can be applied to a population with neck pain only.
Implications
Predictions of binary pain intensity outcomes, as included here, provide clinicians and patients with a simple assessment of expected pain intensity at future time points. These offer results that are easy to interpret and can contribute quickly to decision making in clinical settings. However, binary pain intensity outcomes do not provide detailed information about the magnitude or severity of pain, which may limit their usefulness for monitoring changes in pain intensity over time. In contrast, pain intensity predictions on the continuous scale, which this study also presents, give a clear indication of the change in pain intensity score over time, allowing identification of clinically meaningful, smaller changes in expected pain intensity. Analyzing pain intensity outcomes on the continuous scale maximizes the available information for detecting predictor–outcome associations and allows these models to have greater flexibility to be used in different contexts or locations, where a different dichotomy of pain intensity score might be preferred.
The initial individualized prediction models developed within this study were incorporated into an online demonstrator, the Back-UP First Contact web app (http://backup-project.eu/?p=767).15 For the first time, clinicians were able to see individualized patient predictions on hypothetical patients and give their feedback to the research team about the usability of individual predictions and visualizations to inform treatment decision-making. A future paper will report on the acceptability of the prediction visualizations to both clinicians and patients. The findings of this study, however, suggest that, at present, the prediction models we have are not yet adequate for clinical use for prediction purposes at the point of consultation. Further research is therefore required to improve the prediction models.
Conclusion
We have developed and externally validated models to predict pain intensity outcomes for individual patients consulting in primary care with NLBP. The variables included within the risk prediction models were limited to the existing Keele STarT MSK Tool items. External validation demonstrated that these individualized prediction models, particularly when evaluated at the point of consultation, were not sufficiently accurate to recommend their use in clinical practice. Further research is therefore required to improve the prediction models through inclusion of additional nonmodifiable risk factors.
Supplementary Material
Acknowledgments
The authors thank the patients who gave their time to participate in the 3 original studies generating the data for this study, as well as the participating general practices.
Contributor Information
Lucinda Archer, School of Medicine, Keele University, Keele, Staffordshire, UK; Institute for Applied Health Research, University of Birmingham, Birmingham, UK; National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre, UK.
Kym I E Snell, School of Medicine, Keele University, Keele, Staffordshire, UK; Institute for Applied Health Research, University of Birmingham, Birmingham, UK; National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre, UK.
Siobhán Stynes, School of Medicine, Keele University, Keele, Staffordshire, UK; Midlands Partnership Foundation NHS Trust, North Staffordshire Musculoskeletal Interface Service, Haywood Hospital, Staffordshire, UK.
Iben Axén, Unit of Intervention and Implementation Research for Worker Health, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, Stockholm, Sweden.
Kate M Dunn, School of Medicine, Keele University, Keele, Staffordshire, UK.
Nadine E Foster, School of Medicine, Keele University, Keele, Staffordshire, UK; Surgical Treatment and Rehabilitation Service (STARS) Education and Research Alliance, The University of Queensland and Metro North Hospital and Health Service, Queensland, Australia.
Gwenllian Wynne-Jones, School of Medicine, Keele University, Keele, Staffordshire, UK.
Daniëlle A van der Windt, School of Medicine, Keele University, Keele, Staffordshire, UK.
Jonathan C Hill, School of Medicine, Keele University, Keele, Staffordshire, UK.
Author Contributions
Lucinda Archer (Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Kym I.E. Snell (Methodology, Supervision, Writing—original draft, Writing—review & editing), Siobhán Stynes (Conceptualization, Data curation, Funding acquisition, Project administration, Writing—original draft, Writing—review & editing), Iben Axén (Conceptualization, Funding acquisition, Writing—original draft, Writing—review & editing), Kate M. Dunn (Conceptualization, Data curation, Funding acquisition, Writing—original draft, Writing—review & editing), Nadine E. Foster (Conceptualization, Data curation, Funding acquisition, Writing—original draft, Writing—review & editing), Gwenllian Wynne-Jones (Conceptualization, Funding acquisition, Writing—original draft, Writing—review & editing), Danielle A. van der Windt (Conceptualization, Funding acquisition, Writing—original draft, Writing—review & editing), and Jonathan C. Hill (Conceptualization, Data curation, Funding acquisition, Project administration, Writing—original draft, Writing—review & editing)
Funding
This study presents work conducted as part of a project funded by the European Horizon 2020 Research and Innovation Program (Grant Agreement No. 777090). This study uses data collected as part of independent research funded by the National Institute for Health Research under its Programme Grants for Applied Research scheme (STarT MSK program RP-PG-1211-20010) as well as by Centre of Excellence funding from Versus Arthritis (Grant No. 20202). L. Archer and K. Snell were supported by funding from the Evidence Synthesis Working Group, which was funded by the National Institute for Health and Care Research School for Primary Care Research (Project No. 390) and by funding from the National Institute for Health and Care Research Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. K. Snell was funded by the National Institute for Health and Care Research School for Primary Care Research (NIHR SPCR Launching Fellowship). N. Foster was a National Institute for Health and Care Research senior investigator and supported through a National Institute for Health and Care Research Research Professorship (NIHR-RP-011-015).
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest. The views expressed in this study are those of the authors and not necessarily those of the European Union, the National Health Service, the National Institute for Health and Care Research, the funding bodies, or the Department of Health and Social Care. This study reflects only the views of the authors, and the European Commission is not liable for any use that may be made of its contents. The information in this paper is provided “as is,” without warranty of any kind, and we accept no liability for loss or damage suffered by any person using this information. Portions of this study were presented at the 41st International Society for Clinical Biostatistics, August 23–27, 2020, Krakow, Poland, and the 18th International Association for the Study of Pain World Congress, August 4–8, 2020, Amsterdam, the Netherlands.
References
- 1. Vos T, Abajobir A, Abate K, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1211–1259. 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchbinder R, Underwood M, Hartvigsen J, Maher CG. The lancet series call to action to reduce low value care for low back pain: an update. Pain. 2020;161:S57–S64. 10.1097/j.pain.0000000000001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster N, Mullis R, Hill J, et al. Effect of stratified care for low Back pain in family practice (IMPaCT Back): a prospective population-based sequential comparison. Ann Fam Med. 2014;12:102–111. 10.1370/afm.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the global burden of disease study 2017. Ann Transl Med. 2020;8:299. 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4:e2037371. 10.1001/jamanetworkopen.2020.37371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Traeger AC, Henschke N, Hübscher M, et al. Estimating the risk of chronic pain: development and validation of a prognostic model (PICKUP) for patients with acute low Back pain. PLoS Med. 2016;13:e1002019. 10.1371/journal.pmed.1002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill J, Whitehurst D, Lewis M, et al. Comparison of stratified primary care management for low Back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378:1560–1571. 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill J, Dunn K, Lewis M, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–641. 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 9. Hill J, Garvin S, Chen Y, et al. Stratified primary care versus non-stratified care for musculoskeletal pain: findings from the STarT MSK feasibility and pilot cluster randomized controlled trial. BMC Fam Pract. 2020;21:30. 10.1186/s12875-019-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence . Low Back Pain and Sciatica in Over 16s: Assessment and Management (NG59). NICE; 2016. Accessed October 13, 2023. https://www.nice.org.uk/guidance/ng59. [PubMed] [Google Scholar]
- 11. Jonckheer P, Desomer A, Depreitere B, et al. Low Back Pain and Radicular Pain: Development of a Clinical Pathway. Health Services Research (HSR). Brussels: Belgian Health Care Knowledge Centre (KCE); 2017. [Google Scholar]
- 12. Bailly F, Trouvin AP, Bercier S, et al. Clinical guidelines and care pathway for management of low back pain with or without radicular pain. Joint Bone Spine. 2021;88:105227. 10.1016/j.jbspin.2021.105227. [DOI] [PubMed] [Google Scholar]
- 13. Silva T, Macaskill P, Kongsted A, Mills K, Maher C, Hancock M. Predicting pain recovery in patients with acute low back pain: updating and validation of a clinical prediction model. Eur J Pain. 2019;23:341–353. 10.1002/ejp.1308. [DOI] [PubMed] [Google Scholar]
- 14. Silva T, Macaskill P, Mills K, et al. Predicting recovery in patients with acute low back pain: a clinical prediction model. Eur J Pain. 2017;21:716–726. 10.1002/ejp.976. [DOI] [PubMed] [Google Scholar]
- 15. Back-UP . Personalised Prognostic Models to Improve Well-being and Return to Work After Neck and Low Back Pain. 2020. Accessed September 14, 2021. http://backup-project.eu/.
- 16. Dunn K, Campbell P, Lewis M, et al. Refinement and validation of a tool for stratifying patients with musculoskeletal pain. Eur J Pain. 2021;25:2081–2093. 10.1002/ejp.1821. [DOI] [PubMed] [Google Scholar]
- 17. Hill J, Garvin S, Chen Y, et al. Computer-based stratified primary Care for Musculoskeletal Consultations Compared with usual care: study protocol for the STarT MSK cluster randomized controlled trial. JMIR Res Protoc. 2020;9:e17939. 10.2196/17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hill JC, Garvin S, Bromley K, et al. Risk-based stratified primary care for common musculoskeletal pain presentations (STarT MSK): a cluster-randomised, controlled trial. Lancet Rheumatol. 2022;4:E591–E602. 10.2139/ssrn.3925482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aun C, Lam Y, Collect B. Evaluation of the use of visual analogue scale in Chinese patients. Pain. 1986;25:215–221. 10.1016/0304-3959(86)90095-3. [DOI] [PubMed] [Google Scholar]
- 20. Visual Analogue Scale. Physiopedia; 2021. Accessed September 14, 2021. https://www.physio-pedia.com/Visual_Analogue_Scale. [Google Scholar]
- 21. Von Korff M, Ormel J, Keefe F, Dworkin S. Grading the severity of chronic pain. Pain. 1992;50:133–149. 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 22. Riley RD, Snell KIE, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: part I—continuous outcomes. Stat Med. 2019;38:1262–1275. 10.1002/sim.7993. [DOI] [PubMed] [Google Scholar]
- 23. Riley RD, Snell KI, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: PART II—binary and time-to-event outcomes. Stat Med. 2019;38:1276–1296. 10.1002/sim.7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Archer L, Snell KI, Ensor J, Hudda MT, Collins GS, Riley RD. Minimum sample size for external validation of a clinical prediction model with a continuous outcome. Stat Med. 2020;40:133–146. 10.1002/sim.8766. [DOI] [PubMed] [Google Scholar]
- 25. Snell KI, Archer L, Ensor J, et al. External validation of clinical prediction models: simulation-based sample size calculations were more reliable than rules-of-thumb. J Clin Epidemiol. 2021;135:79–89. 10.1016/j.jclinepi.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riley RD, Debray TPA, Collins GS, et al. Minimum sample size for external validation of a clinical prediction model with a binary outcome. Stat Med. 2021;40:4230–4251. 10.1002/sim.9025. [DOI] [PubMed] [Google Scholar]
- 27. Vergouwe Y, Royston P, Moons KGM, Altman DG. Development and validation of a prediction model with missing predictor data: a practical approach. J Clin Epidemiol. 2010;63:205–214. 10.1016/j.jclinepi.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 28. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 29. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2009. [Google Scholar]
- 30. Debray TPA, Moons KGM, Ahmed I, Koffijberg H, Riley RD. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat Med. 2013;32:3158–3180. 10.1002/sim.5732. [DOI] [PubMed] [Google Scholar]
- 31. Debray TP, Damen JA, Riley RD, et al. A framework for meta-analysis of prediction model studies with binary and time-to-event outcomes. Stat Methods Med Res. 2018;28:2768–2786. 10.1177/0962280218785504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 33. Royston P, Moons KGM, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 34. Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 35. Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 36. Van Houwelingen J, Le Cessie S. Predictive value of statistical models. Stat Med. 1990;9:1303–1325. 10.1002/sim.4780091109. [DOI] [PubMed] [Google Scholar]
- 37. Collins GS, Reitsma JB, Altman DG, Moons KG. For the members of the Tg. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol. 2014;13:1. 10.1186/s12916-014-0241-z. [DOI] [PubMed] [Google Scholar]
- 38. Burgess R, Mansell G, Bishop A, Lewis M, Hill J. Predictors of functional outcome in musculoskeletal healthcare: an umbrella review. Eur J Pain. 2019;24:51–70. 10.1002/ejp.1483. [DOI] [PubMed] [Google Scholar]
- 39. Schellingerhout J, Heymans M, Verhagen A, Lewis M, Vet H, Koes B. Prognosis of patients with nonspecific neck pain: development and external validation of a prediction rule for persistence of complaints. Spine. 2010;35:E827–E835. 10.1097/BRS.0b013e3181d85ad5. [DOI] [PubMed] [Google Scholar]
- 40. McGirt MJ, Sivaganesan A, Asher AL, Devin CJ. Prediction model for outcome after low-back surgery: individualized likelihood of complication, hospital readmission, return to work, and 12-month improvement in functional disability. Neurosurg Focus. 2015;39:E13. 10.3171/2015.8.FOCUS15338. [DOI] [PubMed] [Google Scholar]
- 41. Myhrvold BL, Kongsted A, Irgens P, Robinson HS, Thoresen M, Vøllestad NK. Broad external validation and update of a prediction model for persistent neck pain after 12 weeks. Spine. 2019;44:E1298–E1310. 10.1097/BRS.0000000000003144. [DOI] [PubMed] [Google Scholar]
- 42. Campbell P, Foster NE, Thomas E, Dunn KM. Prognostic indicators of low back pain in primary care: five-year prospective study. J Pain. 2013;14:873–883. 10.1016/j.jpain.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costa LCM, Maher CG, McAuley JH, et al. Prognosis for patients with chronic low back pain: inception cohort study. BMJ. 2009;339:b3829. 10.1136/bmj.b3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henschke N, Maher CG, Refshauge KM, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008;337:a171. 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mansell G, Jordan KP, Peat GM, et al. Brief pain re-assessment provided more accurate prognosis than baseline information for low-back or shoulder pain. BMC Musculoskelet Disord. 2017;18:139. 10.1186/s12891-017-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whittle R, Royle KL, Jordan KP, Riley RD, Mallen CD, Peat G. Prognosis research ideally should measure time-varying predictors at their intended moment of use. Diagn Progn Res. 2017;1:1. 10.1186/s41512-016-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schepper EI, Koes BW, Oei EH, Bierma-Zeinstra SM, Luijsterburg PA. The added prognostic value of MRI findings for recovery in patients with low back pain in primary care: a 1-year follow-up cohort study. Eur Spine J. 2016;25:1234–1241. 10.1007/s00586-016-4423-6. [DOI] [PubMed] [Google Scholar]
- 48. Jarvik JG, Hollingworth W, Heagerty PJ, Haynor DR, Boyko EJ, Deyo RA. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine (Phila Pa 1976). 2005;30:1541–1548. 10.1097/01.brs.0000167536.60002.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.