Abstract
The adsorption energy of a molecule onto the surface of a material underpins a wide array of applications, spanning heterogeneous catalysis, gas storage, and many more. It is the key quantity where experimental measurements and theoretical calculations meet, with agreement being necessary for reliable predictions of chemical reaction rates and mechanisms. The prototypical molecule–surface system is CO adsorbed on MgO, but despite intense scrutiny from theory and experiment, there is still no consensus on its adsorption energy. In particular, the large cost of accurate many-body methods makes reaching converged theoretical estimates difficult, generating a wide range of values. In this work, we address this challenge, leveraging the latest advances in diffusion Monte Carlo (DMC) and coupled cluster with single, double, and perturbative triple excitations [CCSD(T)] to obtain accurate predictions for CO on MgO. These reliable theoretical estimates allow us to evaluate the inconsistencies in published temperature-programed desorption experiments, revealing that they arise from variations in employed pre-exponential factors. Utilizing this insight, we derive new experimental estimates of the (electronic) adsorption energy with a (more) precise pre-exponential factor. As a culmination of all of this effort, we are able to reach a consensus between multiple theoretical calculations and multiple experiments for the first time. In addition, we show that our recently developed cluster-based CCSD(T) approach provides a low-cost route toward achieving accurate adsorption energies. This sets the stage for affordable and reliable theoretical predictions of chemical reactions on surfaces to guide the realization of new catalysts and gas storage materials.
Introduction
The adsorption energy (Eads) of a molecule on the surface of a material is a quantity of fundamental importance. For example, adsorption (or desorption) forms the primary rate-limiting step of many critical reactions in heterogeneous catalysis,1,2 with overall reaction rates determined by their Eads.3,4 It is also used to determine the selectivity of a surface for binding a particular molecule, relevant for the storage and sequestration of gases pertinent to energy applications.5 These properties depend sensitively on the value of Eads, making it vitally important to obtain this quantity accurately with either theoretical calculations or experimental measurements.
Touted as the ‘hydrogen molecule of surface science’,6 the CO adsorption energy onto the MgO (001) surface has served as the quintessential test for both theory and experiment.7−14 It is highly representative of many important processes (e.g., CO oxidation15 and N2 reduction16 in surface catalysis, as well as CO217 adsorption in gas storage), and the weak van der Waals (vdW) dispersion interactions that govern the Eads make it a stringent test. As such, a method incapable of obtaining the Eads of CO on MgO accurately cannot be trusted to reliably predict molecule–surface interactions for more complex surface phenomena. In this context, an Eads prediction is typically considered reliable if it reaches “chemical accuracy” of 43 meV (1 kcal/mol).18 This level of precision on Eads (together with smaller temperature contributions) is essential for the dependable estimation of crucial thermodynamic properties, including chemical reaction rates.19
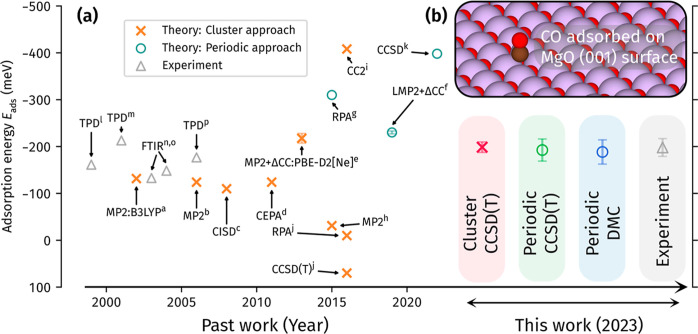
Unfortunately, obtaining an accurate Eads is highly challenging for both theory and experiment. Despite a large body of experimental and theoretical investigations (Figure 1a), the Eads of CO on MgO is still under debate. Even nominally accurate many-body theoretical methods (Figure 1a) can produce a range of nearly 500 meV (11 kcal/mol) on Eads, encompassing predictions going from weak physisorption to moderate chemisorption. At room temperature, this range can lead to over 8 orders of magnitude change in reaction rate predictions. Experimental measurements have covered a similar range in the past,11−14 while recent estimates (Figure 1a) have settled to between −133 and −208 meV, this range is still too large. Crucially, it has not been possible to establish agreement on the CO on MgO Eads between multiple theoretical approaches and multiple experiments at the same time (see Section S1.2 of the Supporting Information).
Figure 1.
(a) Adsorption energy Eads of CO on MgO from previous experimental and theoretical investigations. For the past theory work, we focus on many-body wave function studies employing either a cluster or periodic approach. The past experimental work involves either the Fourier transform infrared (FTIR) or the TPD technique, which we discuss in Section S9 of the Supporting Information. The CO on MgO system is visualized in the top panel of (b), and in its bottom panel, we give accurate estimates to Eads from this work utilizing CCSD(T) with a cluster approach, CCSD(T) with a periodic approach, and DMC with a periodic approach. A best estimate of the experimental value has also been made by reanalyzing the previous experimental work with an improved pre-exponential factor (discussed in the text). Error bars have been determined for all estimates made from this work. References for past simulation work are as follows: (a) Ugliengo and Damin.20 (b) Herschend et al.21 (c) Qin et al.22 (d) Staemmler.23 (e) Boese and Sauer.24 (f) Alessio et al.25 (g) Bajdich et al.26 (h) Li et al.27 (i) Heuser and Höfener.28 (j) Mazheika and Levchenko.29 (k) Mitra et al.30 References for past experiments are as follows: (l) Wichtendahl et al.31 (m) Dohnálek et al.32 (n) Spoto et al.33 (o) Spoto et al.34 (p) Sterrer et al.35
Modeling the weak vdW interactions that govern the binding of CO on MgO requires a rigorous treatment of its electronic structure. This raises questions over common electronic structure methods, such as density functional theory (DFT) or second-order Møller–Plesset perturbation theory (MP2). The former does not naturally incorporate vdW dispersion in its standard approximations (although approaches36−38 are available), while the latter lacks higher-order dispersion effects.39 For modeling these interactions in small molecules, the methods of choice are quantum diffusion Monte Carlo (DMC)40 and coupled cluster with single, double, and perturbative triple particle-hole excitation operators [CCSD(T)].41 While both DMC42−46 and CCSD(T)47−51 have been successfully used for several surface adsorption (and even dissociation/reaction52,53) problems, there remain open questions on their reliability for extended systems (i.e., surfaces and large molecules). For example, recent work54 has indicated significant differences in the interaction energies between large and complex molecules (many analogous to molecule–surface interactions). These unresolved questions prompt a fresh review of the CO on MgO system to clarify the origin of its discrepancies among theoretical techniques.
Applying DMC or CCSD(T) to surface problems is highly challenging because of the steep scaling of their computational complexity with the number of atoms. With these methods, surfaces can be modeled either as a finite cluster or a repeating supercell slab, termed cluster and periodic approaches, respectively. To date, neither DMC nor CCSD(T) has been applied to examine CO adsorption on MgO with a periodic approach. While CCSD(T) with a cluster approach, termed cluster CCSD(T) hereafter, has been previously performed, it is difficult to converge. For example, the aforementioned 500 meV range arises from cluster CC-based Eads estimates that are not adequately converged. Here, the challenge lies in simultaneously converging both the surface model (size) and the electronic structure settings. The former requires large system sizes (both cluster and periodic) to reach the bulk (infinite size) limit and a dilute CO coverage, while the latter requires large basis sets and the inclusion of correlation from electrons in subvalence metal shells. These requirements all contribute to a significant computational burden that can become intractable.
In this work, we reach a consensus for the CO on MgO Eads, achieving agreement between theory and experiment. For theory, we leverage the latest advances in periodic DMC, periodic CCSD(T), and cluster CCSD(T) to produce three high-quality estimates of the Eads. With this, we establish an agreement between all three theoretical techniques to subchemical accuracy. This has allowed us to evaluate and understand the inconsistencies in previous theoretical calculations and experimental measurements. For example, we establish that the discrepancies among previous temperature-programed desorption (TPD) experiments arise predominantly from the use of different pre-exponential factors. Subsequently, we derive new Eads values for these TPD experiments with a more accurate pre-exponential factor (while removing thermal and zero-point contributions). This effort has made it possible for this study to become the first to establish a consensus between a variety of theoretical techniques and multiple experimental measurements. These estimates from both theory and experiment place the CO on MgO system squarely in the physisorption regime, all lying within the −199 ± 11 meV range set by our best Eads estimate from the cluster CCSD(T) technique. Crucially, we demonstrate that our employed cluster CCSD(T) technique, combining the recently developed SKZCAM protocol55 with reduced-scaling CCSD(T), can achieve its high accuracy at a low cost comparable to (hybrid) DFT. This opens the door for its use as a routine benchmark tool56−58 as well as within high-throughput frameworks for predicting new and improved catalyst59 and gas storage materials.60
Methods
Before assessing the final Eads obtained for the three theoretical techniques [cluster CCSD(T), periodic CCSD(T), and periodic DMC], we will discuss how we have been able to reach such high-quality estimates in this section. Each theoretical technique approaches the final Eads differently based on the choice of the electronic structure method [CCSD(T) or DMC] and surface model (periodic or cluster). For example, CCSD(T)61 tackles the many-electron Schrödinger equation via an expansion of electronic configurations (using particle-hole excitation operators) from a reference wave function, while DMC62 achieves this via an imaginary time projection to the ground state from a trial wave function. Accordingly, these two electronic structure methods depend on different factors, such as the basis-set size for CCSD(T) and the time step for DMC as described in Section S5 of the Supporting Information. In fact, to reach sufficient accuracy, this even affects how we go about computing the Eads, which we discuss first below. Thereafter, we will also describe how the separate surface models reach the bulk limit and dilute coverage regimes.
Computing Adsorption Energy
The primary quantity of interest in this work is the adsorption energy, which physically represents the energy released when a CO molecule in the gas-phase adsorbs onto a pristine MgO surface and can be defined as
| 1 |
where E[CO + MgO], E[MgO], and E[CO] are the energies of the CO on MgO (CO + MgO), pristine MgO, and gas-phase CO systems, respectively. In practice, we actually compute the interaction energy, where we have two definitions depending on the theoretical technique
| 2 |
The first definition
is similar to Eads but calculates the
energy of the separate  and
and 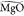 systems with
structures frozen from the
CO + MgO system (as indicated by
systems with
structures frozen from the
CO + MgO system (as indicated by 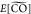 and
and 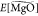 , respectively). Computing Eint (over Eads directly) allows
for basis-set superposition error (BSSE) corrections63 to be applied to cluster CCSD(T) calculations. For periodic
DMC and periodic CCSD(T), we use the second definition of Eint, where the
, respectively). Computing Eint (over Eads directly) allows
for basis-set superposition error (BSSE) corrections63 to be applied to cluster CCSD(T) calculations. For periodic
DMC and periodic CCSD(T), we use the second definition of Eint, where the 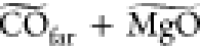 system corresponds to the frozen
system corresponds to the frozen 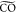 displaced >5
Å away from the frozen
surface, both taken from the CO + MgO system. It differs from the
(formal) first definition of Eint by less
than 5 meV (Section S5.2 of the Supporting Information) and was used to mitigate finite-size errors44 for both calculations, while also enabling larger timesteps
to make DMC more economical.
displaced >5
Å away from the frozen
surface, both taken from the CO + MgO system. It differs from the
(formal) first definition of Eint by less
than 5 meV (Section S5.2 of the Supporting Information) and was used to mitigate finite-size errors44 for both calculations, while also enabling larger timesteps
to make DMC more economical.
Reaching the final Eads from Eint then requires the addition of a Δgeom term; it represents the energy required to relax the separate frozen CO and MgO geometries back into their equilibrium geometries. As obtaining forces (and thus equilibrium geometries) is challenging for both CCSD(T) and DMC, the CO, MgO, and CO + MgO structures as well as Δgeom, a small term, were approximated at the DFT level. Specifically, we chose the revPBE-D4 exchange–correlation functional64 (and dispersion treatment65) due to its reasonable Eads and geometrical parameters compared to CCSD(T) and experiment (see Section S3 of the Supporting Information). As discussed in Section S4 of the Supporting Information, the errors arising from the use of revPBE-D4 geometries have been conservatively estimated by assessing its effect on an ensemble of high-quality DFT functionals along Jacob’s ladder.36,66
Periodic Approaches
Assuming converged electronic structure methods (Section S5 of the Supporting Information), we must ensure that the surface models used (see Figure 2) have converged to the bulk limit and dilute CO coverage regimes. Periodic approaches can achieve this in a straightforward fashion via the supercell approach (Figure 2a) by increasing the surface supercell size and number of slab layers. As shown in Section S5.4 of the Supporting Information, we find that a four-layer (4L) (4 × 4) supercell of the MgO (001) surface is sufficient to converge Eads to less than 1 meV at the DFT level. We performed periodic CCSD(T) with the Cc4s code50,67−69 and periodic DMC with CASINO.70 Even with the latest advances, direct calculation (at converged settings) on the 4L (4 × 4) supercell can be computationally expensive for both CCSD(T) and DMC, although the more favorable system size scaling of DMC can enable such systems to be tackled.52 Instead, we have computed Eint on a 2L supercell cleaved from the original 4L supercell and, in the vein of Pople’s model chemistry,71 approximated the remaining (much) smaller contributions with computationally economical methods, as elaborated in Section S6 of the Supporting Information.
Figure 2.
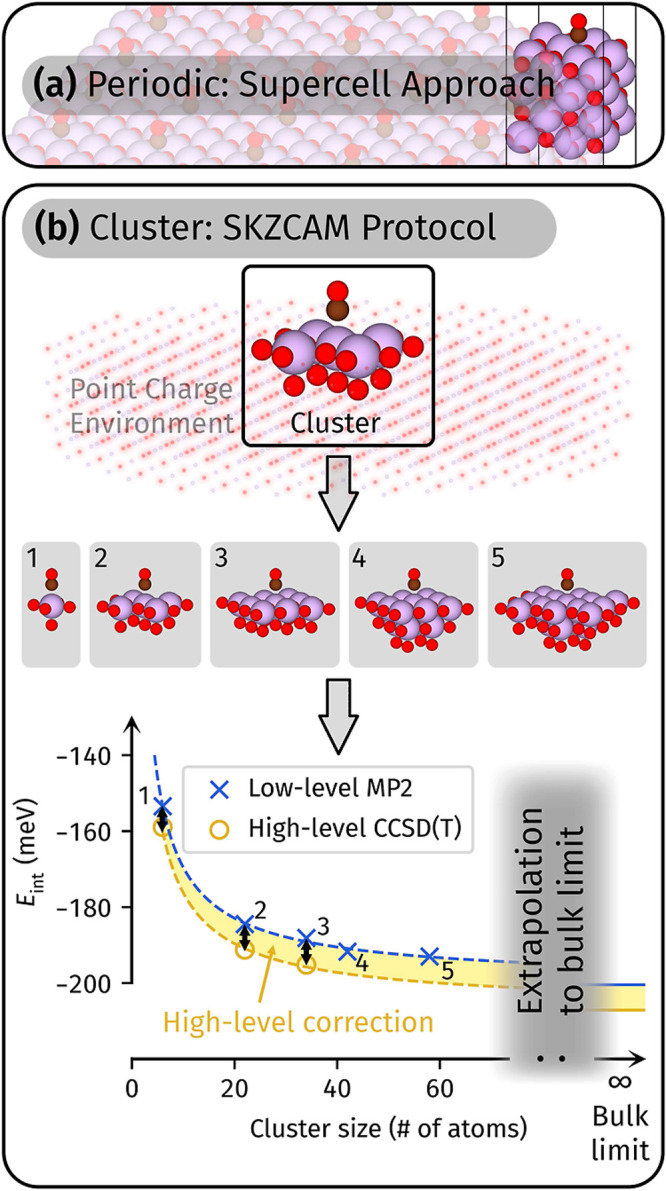
Schematic of the (a) periodic supercell approach and (b) cluster approach used in this study to compute the adsorption energy of CO on MgO. We performed both DMC and CCSD(T) with the periodic supercell approach. The cluster approach in (b) is based on our recently developed SKZCAM protocol,55 which generates a series of quantum clusters of increasing size under an electrostatic embedding framework (see top panel). We have computed the interaction energy Eint for the first three (five) clusters at the LNO-CCSD(T) (MP2) level, which are numbered in the bottom panel of (b). The MP2 bulk limit was estimated by extrapolating this series of clusters. A subsequent high-level correction to the CCSD(T) level was estimated from a subset of these clusters via the LNO-CCSD(T) approach.
Cluster Approaches
Cluster approaches work by placing a finite cluster within appropriate embedding environments. They naturally provide dilute coverage estimates, but convergence toward the bulk limit is challenging. As discussed in our previous study,55 the convergence of a finite cluster depends on interdependent factors such as (1) embedding approach (e.g., mechanical;6 electrostatic,72 incremental73 or quantum74−78); (2) shape; (3) size; and (4) charge of the cluster. The use of steep scaling methods such as CCSD(T) severely limits the cluster size that can be reached. In this study, we use the local natural orbital (LNO) scheme79−82 [LNO-CCSD(T)] in Mrcc(83) to further extend the feasible system sizes while maintaining high accuracy (Section S7 of the Supporting Information). The challenge is then to keep the (quantum) cluster small enough to make well-converged LNO-CCSD(T) computations routinely affordable while also reaching the bulk limit.
Our recently proposed SKZCAM protocol is particularly suited for tackling this challenge. It is based upon the electrostatic embedding approach72,84−86 (top panel of Figure 2b) and provides the design rubrics to generate a series of quantum clusters of systematically increasing size (middle panel of Figure 2b). We have shown previously55 and here (bottom panel of Figure 2b) that these clusters converge smoothly and rapidly to the bulk limit. Although initially devised for calculating oxygen vacancy formation energies, it has been extended to encompass adsorption on metal-oxide surfaces as part of this study. We take advantage of the smooth convergence with cluster size in the SKZCAM protocol to extrapolate (see Section S7.1) a small number of clusters to the bulk limit. This extrapolation (inspired by the form of the Jost correction87 and empirical dispersion corrections37) is expected to naturally incorporate any missing long-range polarization and dispersion effects. As shown in the bottom panel of Figure 2b and Table S10 of the Supporting Information, only the first five clusters are required to converge to within 5 meV.
While the largest cluster size (∼60 atoms) is amenable at the MP2 level, it is intractable with canonical CCSD(T). Fortunately, convergence to the bulk limit of CCSD(T) can be accelerated by evaluating a LNO-CCSD(T) level correction to the bulk limit MP2 for a series of smaller clusters in the fashion of the ΔCC correction from Boese et al.24 This correction is highly accurate because another quality of the SKZCAM protocol is the good cancellation of finite-size errors between many-body methods such as MP2 and CCSD(T) across its clusters. Specifically, we find deviations of only 3 meV in this correction across the first three clusters of the SKZCAM protocol. Note that this correction is different for every new molecule–surface system. The resulting computations in this protocol require only a few days on a single computer node, easily accessible in commodity computer clusters.
Results
Agreement between Many-Body Methods
As discussed in the Methods, the final Eads we obtain for each of the three techniques is actually composed of several terms, where besides Δgeom, Eint itself consists of several contributions. As shown in Section S6 of the Supporting Information, each of these terms has been carefully converged, with conservative error bars estimated for the most important terms. With this effort, we come to a final Eads estimate (in meV) of −199 ± 11 for cluster CCSD(T), −193 ± 24 for periodic CCSD(T), and −188 ± 26 for periodic DMC (summarized in Table 1). This agreement is better than chemical accuracy; in fact, we reach subchemical accuracy with a maximum deviation of 11 meV (1 kJ/mol) across the three theoretical techniques, smaller than their error bars. These Eads values place the adsorption behavior of CO on MgO squarely in the physisorption regime, right in the middle of the aforementioned large 500 meV range across previous theoretical calculations (Figure 1). To give some perspective, the H2O monomer, known to chemisorb on MgO, has an Eads in the −480 to –550 meV25,42 range, close to some previous theoretical estimates for CO on MgO.
Table 1. Comparison of the Final Eads Estimates between the Cluster CCSD(T), Periodic CCSD(T), and Periodic DMC Techniques as Well as Our Best Estimate from Experimenta.
| technique | Eads (meV) | cost (kCPUh) | max RAM (GB) |
|---|---|---|---|
| cluster CCSD(T) | –199 ± 11 | ∼20 | ∼20 |
| periodic CCSD(T) | –193 ± 24 | ∼200 | ∼3000 |
| periodic DMC | –188 ± 26 | ∼1000 | negligible |
| experiment | –198 ± 19 | N/A | N/A |
Estimates on the computational cost in 1000 CPU core hours (kCPUh) and maximum RAM usage in gigabytes (GB) are also given. No RAM usage has been given for DMC because it uses a negligible amount relative to CCSD(T).
Reaching agreement for the CO on MgO Eads across fundamentally distinct electronic structure methods [DMC and CCSD(T)] and surface models (cluster and periodic) that have been systematically converged gives us confidence in using these estimates to evaluate past theoretical and experimental literature. In particular, the low cost of the cluster CCSD(T) approach (elaborated in the Discussion) allows for effects of electronic structure settings, such as basis-set size, frozen core size, and cluster size, on the Eads to be studied. For example, in Section S8 of the Supporting Information, we show that inadequate basis-set size, large frozen core size (i.e., only including valence electrons in the many-body correlation treatment), and small cluster size all lead to weaker binding (i.e., less negative Eads). On the basis of this convergence analysis, we have been able to attribute many of the underestimated literature values to inadequate convergence of these properties. Similarly, we show that the studies that overestimate the binding strength largely result as they do not correct for BSSE, which becomes particularly strong for small basis sets. The advances in accuracy of the techniques in this study point toward an agreement with only the work from Sauer’s group, first computed by Boese and Sauer24 and then by Alessio et al.,25 reaffirming the reliability of their High-level:Low-level approach.24,25,88−91
Re-evaluating Previous Experimental Measurements
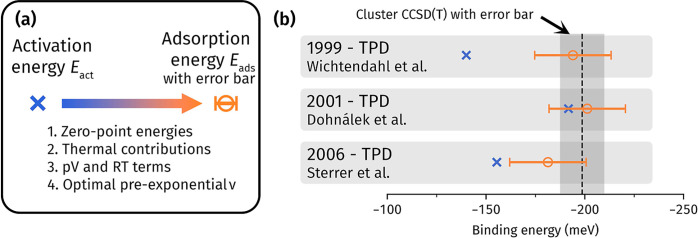
Our reliable theoretical estimates now give us the opportunity to evaluate the discrepancies between past experiments. These previous experiments, of which there are many, have spanned a broad 300 meV range (see Section S2 of the Supporting Information). As discussed before6,9,92 and in Section S9 of the Supporting Information, some of these measurements are not reliable, and we focus only on the recent (three) TPD experiments. In their original TPD measurements, (Arrhenius) activation energies (Eact) of −140, −192, and −155 meV were measured by Wichtendahl et al.,31 Dohnálek et al.,32 and Sterrer et al.,35 respectively. Notably, there is still a deviation24 of 52 meV (>1 kcal/mol) that is too large.
To compare these TPD experiments against our theoretical calculations, the original Eact values must be converted into Eads. The importance of this conversion has only been noted in a handful of recent CO on MgO studies.10,24 Typically, it involves removing thermal and zero-point contributions, as well as pV and RT terms (i.e., effects 1, 2, and 3, but not 4, in Figure 3a). It is common to compute these terms accurately using DFT (as performed in Section S9.2 of the Supporting Information). However, this only constitutes a constant shift of −19 meV for each TPD experiment and does not solve the large noted deviation.24 For example, with only these effects accounted for, Wichtendahl et al., Dohnálek et al., and Sterrer et al. predict Eads of −156, −208, and −172 meV, respectively (see Section S2 of the Supporting Information and Figure 1a).
Figure 3.
(a) Effects considered during conversion of the Arrhenius activation energies Eact in TPD experiments to adsorption energies Eads suitable for comparison to theory. (b) Resulting Eads converted from Eact match to within error bars with our cluster CCSD(T) estimate for all three TPD experiments (from Wichtendahl et al.,31 Dohnálek et al.,32 and Sterrer et al.35).
Our theoretical estimates (between −188 and −199 meV) are in the middle of the above Eads range, with Wichtendahl et al. and Sterrer et al. underestimating while Dohnálek et al. overestimating. This differing behavior points toward the pre-exponential factor (ν) being the culprit. For example, ν is not typically known and commonly assigned to log(ν) = 13 (e.g., by Wichtendahl et al. and Sterrer et al.), while Dohnálek et al. have estimated (with large ±2 error bars) it to be log(ν) = 15. Since these original experiments, ν has received considerable attention,38,93,94 and, importantly, an estimate of log(ν) = 13.8 ± 1.6 has been given by Campbell and Sellers,95 agreeing with a theoretical estimate [log(ν) = 14.2] from Nygren and Pettersson.9 Thus, there is now the prospect of making corrections38 toward a better ν value (effect 4 in Figure 3a). In Figure 3b, we have made these ν corrections to Eact, combining it with the aforementioned thermal contributions and using a newer analysis95 of the TPD curve from the original study by Wichtendahl et al. The resulting experimental Eads range falls to within 20 meV (i.e., better than chemical accuracy), and all three experiments now agree with our theoretical estimates, where Wichtendahl et al., Dohnálek et al., and Sterrer et al. predict Eads of −194, −201 and −181 meV, respectively, with ±19 meV error bars arising from uncertainty in ν.
Discussion
The achieved agreement is a testament to the algorithmic and methodological developments made in the past decades on all three theoretical techniques to enable such high accuracy at a tractable computational cost. As discussed previously, the accuracy and reliability of the cluster CCSD(T) Eads value have been made possible with the SKZCAM protocol combined with the recent advances in local approximations to CCSD(T) [e.g., LNO-CCSD(T),79−82,96 DLPNO-CCSD(T),97−101 PNO-LCCSD(T),102−104 etc.]. While canonical CCSD(T) could only be performed for the smallest quantum clusters of up to 1–2 dozen atoms,82 LNO-CCSD(T) can tackle molecules involving hundreds of atoms54,81 and ionic crystal clusters of around 100 atoms.55 For periodic DMC, the introduction of ccECP pseudopotentials105,106 gives confidence in calculations involving elements beyond the first row, while the ZSGMA107,108 algorithm and determinant localization approximation (DLA)109 enable larger timesteps for the same accuracy. While DMC has had a long history spanning several decades,42,46,110−112 the periodic CCSD(T) technique has only come into maturation in recent years113−116 and besides the significant algorithmic improvements,69,117 it is particularly the recent developments in finite-size corrections50,53,67 that have enabled chemical accuracy to be reached for CO on MgO and indeed other surface adsorption problems.118,119
We compare the computational costs of the three techniques
in Table 1. While a
one-to-one
comparison cannot be made because the calculations were performed
on different computing architectures, it is clear that cluster CCSD(T)
is cheaper by 1 or 2 orders of magnitude compared to either of the
periodic techniques. In fact, this cost is comparable to periodic
hybrid DFT calculations, which takes ∼1k CPU-hours to compute.
From previous work, we have found that the (cluster-based) SKZCAM
protocol, combined with the reduced scaling and efficient implementation
of LNO-CCSD(T), can actually become
cheaper than periodic hybrid DFT for more complex surfaces such as
TiO2. The cluster CCSD(T) calculations require a small
amount of memory (∼20 GB on a single node), amenable on standard
computing hardware (typically containing >128 GB on a single node).
On the other hand, periodic CCSD(T) can require ∼3000 GB of
RAM distributed across high-memory nodes. It should be noted that
while periodic DMC  has been more expensive than periodic CCSD(T)
has been more expensive than periodic CCSD(T)  for the CO on MgO system studied here,
its better scaling with system size, excellent parallelization (across
computer nodes), and low memory requirements should enable it to be
more efficient for larger surfaces and molecules.
for the CO on MgO system studied here,
its better scaling with system size, excellent parallelization (across
computer nodes), and low memory requirements should enable it to be
more efficient for larger surfaces and molecules.
The true Eads value for each technique (i.e., when both electronic structure settings and the surface model are converged) is anticipated to lie within its respective error range in Table 1. Out of the three theoretical techniques, the cluster CCSD(T) calculation has the lowest error bars, and this is achieved by design, thanks to the SKZCAM protocol. For example, finite-size errors from the MP2 extrapolation to the bulk limit can be estimated by including more clusters into the formula and likewise the high-level correction up to CCSD(T), as discussed in Section S7 of the Supporting Information. For this reason, we consider the Eads estimate of −199 ± 11 meV by the cluster CCSD(T) technique to be the best estimate. We chose not to combine all three theoretical techniques into one best estimate because their errors have distinct origins and behaviors. For example, the periodic CCSD(T) error bars are systematic, arising from an incomplete basis set, while the errors are stochastic for periodic DMC.
It will form the topic of future work whether the high accuracy and low cost of this cluster approach will persist for other molecule–surface systems. Tackling surfaces with metallic120,121 or covalent122 character will require the use of alternative embedding approaches. In particular, for (transition) metals, new effective core potentials123 and developments in applying CC-based theories to metals124,125 should now enable high-accuracy and low-cost cluster approaches to be developed for these systems. We expect that the SKZCAM protocol’s core principle—extrapolating bulk properties from a small series of well-constructed clusters—will persist for these covalent122 and metallic121 systems as well. Future periodic CCSD(T) calculations of metal surfaces will also become more economical and feasible. Thanks to recent developments,117 the previous requirement for numerous twist averages in metals, to address independent-particle finite-size errors, has been streamlined to a single special twist angle, promising a substantial reduction in costs by 1 or 2 orders of magnitude.
The accuracy of the three theoretical techniques has come to such high precision that it is now possible to benchmark the accuracy of experiments. In particular, it has demonstrated the necessity of utilizing accurate pre-exponential factors in TPD experiments to reach reliable agreement. This means that while agreement has been achieved previously for theoretical calculations and specific experiments, these must be viewed with skepticism. Out of the re-evaluated Eads values in Figure 3b, we expect the reanalyzed Eads estimates from the TPD experiments by Wichtendahl et al. and Dohnálek et al. to provide a more accurate estimate than Sterrer et al. as they involve lower CO surface coverages (see Section S9.3 of the Supporting Information). As such, we take the average of the two to come to the best experimental estimate of –198 ± 19 meV, which we use in Figure 1b and Table 1. Our cluster CCSD(T) estimate of −199 ± 11 meV demonstrates near-exact agreement to this experimental estimate, and its smaller error bars underscore its status as the best estimate of the CO on MgO Eads out of all theoretical calculations and experimental measurements.
Conclusions
In summary, we have resolved the value of the adsorption energy (Eads) for CO on MgO to −199 ± 11 meV, achieving consensus between three independent theoretical calculations [cluster CCSD(T), periodic CCSD(T), and periodic DMC] and three separate TPD experiments. It establishes both DMC and CCSD(T) as methods that have matured sufficiently to benchmark surface phenomena. For example, we used reliable theoretical estimates to assess and understand the discrepancies in the previous literature (both theory and experiment). In particular, we demonstrate that the differences between previous experimental TPD measurements and our theoretical estimates arise from differing pre-exponential factors. A subsequent re-evaluation with a more precise pre-exponential factor has now allowed for the agreement to be achieved, highlighting the importance of considering this factor in future work. Furthermore, we show that the cluster CCSD(T) technique, made possible with the SKZCAM protocol and the reduced scaling LNO-CCSD(T) method, demonstrates high accuracy at low cost; requiring only a few days on a single computer node.
While agreement between theory and experiment has been achieved before for specific surfaces,6,49 the SKZCAM protocol55 used here promises the ability to generalize this accuracy to other surfaces and properties systematically, amenable for automated high-throughput calculations. Combined with its accuracy and low cost, these properties of the SKZCAM protocol open the door toward studying the interaction of many molecules and surfaces simultaneously at reference quality with the cluster CCSD(T) technique. With this, we can create large benchmark databases suitable for assessing the quality of DFT functionals—currently sorely lacking for metal-oxide surfaces.93 Furthermore, we can now go beyond adsorption to study catalytic reaction steps on technologically relevant surfaces. Here, the combination of theoretical calculations and experimental measurements, now capable of reaching a consensus, will enable the unveiling of precise mechanistic insights126,127 into these surface reaction phenomena.
Acknowledgments
The authors are grateful for resources provided by the Cambridge Service for Data Driven Discovery (CSD3) operated by the University of Cambridge Research Computing Service (www.csd3.cam.ac.uk), provided by Dell EMC and Intel using Tier-2 funding from the Engineering and Physical Sciences Research Council (capital grant EP/P020259/1), and DiRAC funding from the Science and Technology Facilities Council (www.dirac.ac.uk); the Cirrus UK National Tier-2 HPC Service at EPCC (http://www.cirrus.ac.uk) funded by the University of Edinburgh and EPSRC (EP/P020267/1); and computational support from the UK national high-performance computing service, ARCHER2, for which access was obtained via the UKCP consortium and funded by EPSRC grant ref EP/X035891/1. B.X.S. acknowledges support from the EPSRC Doctoral Training Partnership (EP/T517847/1). V.K. acknowledges support from the Ernest Oppenheimer Early Career Fellowship and the Sydney Harvey Junior Research Fellowship, Churchill College, University of Cambridge. P.R.N. is grateful for financial support from the ERC grant no. 101076972, “aCCuracy”, the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, and the National Research, Development, and Innovation Office (NKFIH, grant nos. KKP126451 and FK142489). A.M. acknowledges support from the European Union under the “n-AQUA” European Research Council project (Grant no. 101071937).
Supporting Information Available
The input and output files associated with this study and all analysis can be found on GitHub at github.com/benshi97/Data_CO_on_MgO or viewed (and analyzed) online on Colab. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c09616.
Discussion of past CO on MgO estimates, estimating the uncertainty of the revPBE-D4 geometry, details on computational techniques and convergence, final Eads estimates and computational costs, ,and re-evaluation of previous experimental Eads estimates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ertl G. Elementary Steps in Heterogeneous Catalysis. Angew. Chem., Int. Ed. 1990, 29, 1219–1227. 10.1002/anie.199012191. [DOI] [Google Scholar]
- Liu X.; Jiao Y.; Zheng Y.; Jaroniec M.; Qiao S.-Z. Building Up a Picture of the Electrocatalytic Nitrogen Reduction Activity of Transition Metal Single-Atom Catalysts. J. Am. Chem. Soc. 2019, 141, 9664–9672. 10.1021/jacs.9b03811. [DOI] [PubMed] [Google Scholar]
- Gao W.; Chen Y.; Li B.; Liu S.-P.; Liu X.; Jiang Q. Determining the Adsorption Energies of Small Molecules with the Intrinsic Properties of Adsorbates and Substrates. Nat. Commun. 2020, 11, 1196. 10.1038/s41467-020-14969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Ye S.; Hu W.; Sharman E.; Liu R.; Liu Y.; Luo Y.; Jiang J. Electric Dipole Descriptor for Machine Learning Prediction of Catalyst Surface–Molecular Adsorbate Interactions. J. Am. Chem. Soc. 2020, 142, 7737–7743. 10.1021/jacs.0c01825. [DOI] [PubMed] [Google Scholar]
- Morris R. E.; Wheatley P. S. Gas Storage in Nanoporous Materials. Angew. Chem., Int. Ed. 2008, 47, 4966–4981. 10.1002/anie.200703934. [DOI] [PubMed] [Google Scholar]
- Sauer J. Ab Initio Calculations for Molecule–Surface Interactions with Chemical Accuracy. Acc. Chem. Res. 2019, 52, 3502–3510. 10.1021/acs.accounts.9b00506. [DOI] [PubMed] [Google Scholar]
- Neyman K. M.; Rösch N. CO Bonding and Vibrational Modes on a Perfect MgO(001) Surface: LCGTO-LDF Model Cluster Investigation. Chem. Phys. 1992, 168, 267–280. 10.1016/0301-0104(92)87161-2. [DOI] [Google Scholar]
- Mejías J. A.; Márquez A. M.; Fernández Sanz J.; Fernández-García M.; Ricart J. M.; Sousa C.; Illas F. On Modelling the Interaction of CO on the MgO(100) Surface. Surf. Sci. 1995, 327, 59–73. 10.1016/0039-6028(94)00831-0. [DOI] [Google Scholar]
- Nygren M. A.; Pettersson L. G. M. Comparing Ab Initio Computed Energetics with Thermal Experiments in Surface Science: CO/MgO(001). J. Chem. Phys. 1996, 105, 9339–9348. 10.1063/1.472724. [DOI] [Google Scholar]
- Valero R.; Gomes J. R. B.; Truhlar D. G.; Illas F. Good Performance of the M06 Family of Hybrid Meta Generalized Gradient Approximation Density Functionals on a Difficult Case: CO Adsorption on MgO(001). J. Chem. Phys. 2008, 129, 124710. 10.1063/1.2982923. [DOI] [PubMed] [Google Scholar]
- Furuyama S.; Fujii H.; Kawamura M.; Morimoto T. Physisorption of Nitric Oxide, Carbon Monoxide, Nitrogen, and Oxygen by Magnesium Oxide Powder. J. Phys. Chem. 1978, 82, 1028–1032. 10.1021/j100498a014. [DOI] [Google Scholar]
- Paukshtis E. A.; Soltanov R. I.; Yurchenko E. N. Determination of the Strength of Aprotic Acidic Centers on Catalyst Surfaces from the IR Spectra of Adsorbed Carbon Monoxide. React. Kinet. Catal. Lett. 1981, 16, 93–96. 10.1007/BF02077036. [DOI] [Google Scholar]
- Henry C. R.; Chapon C.; Duriez C. Precursor State in the Chemisorption of CO on Supported Palladium Clusters. J. Chem. Phys. 1991, 95, 700–705. 10.1063/1.461419. [DOI] [Google Scholar]
- Jian-Wei H.; Estrada C. A.; Corneille J. S.; Ming-Cheng W.; Wayne Goodman D. CO Adsorption on Ultrathin MgO Films Grown on a Mo(100) Surface: An IRAS Study. Surf. Sci. 1992, 261, 164–170. 10.1016/0039-6028(92)90228-X. [DOI] [Google Scholar]
- Sarma B. B.; Plessow P. N.; Agostini G.; Concepción P.; Pfänder N.; Kang L.; Wang F. R.; Studt F.; Prieto G. Metal-Specific Reactivity in Single-Atom Catalysts: CO Oxidation on 4d and 5d Transition Metals Atomically Dispersed on MgO. J. Am. Chem. Soc. 2020, 142, 14890–14902. 10.1021/jacs.0c03627. [DOI] [PubMed] [Google Scholar]
- Du H.-L.; Chatti M.; Hodgetts R. Y.; Cherepanov P. V.; Nguyen C. K.; Matuszek K.; MacFarlane D. R.; Simonov A. N. Electroreduction of Nitrogen with Almost 100% Current-to-Ammonia Efficiency. Nature 2022, 609, 722–727. 10.1038/s41586-022-05108-y. [DOI] [PubMed] [Google Scholar]
- Mutch G. A.; Shulda S.; McCue A. J.; Menart M. J.; Ciobanu C. V.; Ngo C.; Anderson J. A.; Richards R. M.; Vega-Maza D. Carbon Capture by Metal Oxides: Unleashing the Potential of the (111) Facet. J. Am. Chem. Soc. 2018, 140, 4736–4742. 10.1021/jacs.8b01845. [DOI] [PubMed] [Google Scholar]
- Neese F.; Atanasov M.; Bistoni G.; Maganas D.; Ye S. Chemistry and Quantum Mechanics in 2019: Give Us Insight and Numbers. J. Am. Chem. Soc. 2019, 141, 2814–2824. 10.1021/jacs.8b13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. W. J.; Xu L.; Mavrikakis M. Computational Methods in Heterogeneous Catalysis. Chem. Rev. 2021, 121, 1007–1048. 10.1021/acs.chemrev.0c01060. [DOI] [PubMed] [Google Scholar]
- Ugliengo P.; Damin A. Are Dispersive Forces Relevant for CO Adsorption on the MgO(001) Surface?. Chem. Phys. Lett. 2002, 366, 683–690. 10.1016/S0009-2614(02)01657-3. [DOI] [Google Scholar]
- Herschend B.; Baudin M.; Hermansson K. Influence of Substrate Dynamics on CO–MgO(001) Bonding–Using Molecular Dynamics Snapshots in Quantum-Chemical Calculations. J. Phys. Chem. B 2006, 110, 5473–5479. 10.1021/jp0538262. [DOI] [PubMed] [Google Scholar]
- Qin C. CI Study of CO Adsorption on MgO(100). Chem. Phys. Lett. 2008, 460, 457–460. 10.1016/j.cplett.2008.06.046. [DOI] [Google Scholar]
- Staemmler V. Method of Local Increments for the Calculation of Adsorption Energies of Atoms and Small Molecules on Solid Surfaces. 2. CO/MgO(001). J. Phys. Chem. A 2011, 115, 7153–7160. 10.1021/jp200047d. [DOI] [PubMed] [Google Scholar]
- Boese A. D.; Sauer J. Accurate Adsorption Energies of Small Molecules on Oxide Surfaces: CO–MgO(001). Phys. Chem. Chem. Phys. 2013, 15, 16481–16493. 10.1039/c3cp52321g. [DOI] [PubMed] [Google Scholar]
- Alessio M.; Usvyat D.; Sauer J. Chemically Accurate Adsorption Energies: CO and H2O on the MgO(001) Surface. J. Chem. Theory Comput. 2019, 15, 1329–1344. 10.1021/acs.jctc.8b01122. [DOI] [PubMed] [Google Scholar]
- Bajdich M.; Nørskov J. K.; Vojvodic A. Surface Energetics of Alkaline-Earth Metal Oxides: Trends in Stability and Adsorption of Small Molecules. Phys. Rev. B 2015, 91, 155401. 10.1103/PhysRevB.91.155401. [DOI] [Google Scholar]
- Li W.; Chen C.; Zhao D.; Li S. LSQC: Low Scaling Quantum Chemistry Program. Int. J. Quantum Chem. 2015, 115, 641–646. 10.1002/qua.24831. [DOI] [Google Scholar]
- Heuser J.; Höfener S. Wave-Function Frozen-Density Embedding: Approximate Analytical Nuclear Ground-State Gradients. J. Comput. Chem. 2016, 37, 1092–1101. 10.1002/jcc.24301. [DOI] [PubMed] [Google Scholar]
- Mazheika A.; Levchenko S. V. Ni Substitutional Defects in Bulk and at the (001) Surface of MgO from First-Principles Calculations. J. Phys. Chem. C 2016, 120, 26934–26944. 10.1021/acs.jpcc.6b09505. [DOI] [Google Scholar]
- Mitra A.; Hermes M. R.; Cho M.; Agarawal V.; Gagliardi L. Periodic Density Matrix Embedding for CO Adsorption on the MgO(001) Surface. J. Phys. Chem. Lett. 2022, 13, 7483–7489. 10.1021/acs.jpclett.2c01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichtendahl R.; Rodriguez-Rodrigo M.; Härtel U.; Kuhlenbeck H.; Freund H.-J. Thermodesorption of CO and NO from Vacuum-Cleaved NiO(100) and MgO(100). Phys. Status Solidi A 1999, 173, 93–100. . [DOI] [Google Scholar]
- Dohnálek Z.; Kimmel G. A.; Joyce S. A.; Ayotte P.; Smith R. S.; Kay B. D. Physisorption of CO on the MgO(100) Surface. J. Phys. Chem. B 2001, 105, 3747–3751. 10.1021/jp003174b. [DOI] [Google Scholar]
- Spoto G.; Gribov E.; Damin A.; Ricchiardi G.; Zecchina A. The IR Spectra of Mg52+(CO) Complexes on the (001) Surfaces of Polycrystalline and Single Crystal MgO. Surf. Sci. 2003, 540, L605–L610. 10.1016/S0039-6028(03)00830-6. [DOI] [Google Scholar]
- Spoto G.; Gribov E. N.; Ricchiardi G.; Damin A.; Scarano D.; Bordiga S.; Lamberti C.; Zecchina A. Carbon Monoxide MgO from Dispersed Solids to Single Crystals: A Review and New Advances. Prog. Surf. Sci. 2004, 76, 71–146. 10.1016/j.progsurf.2004.05.014. [DOI] [Google Scholar]
- Sterrer M.; Risse T.; Freund H.-J. CO Adsorption on the Surface of MgO(001) Thin Films. Appl. Catal., A 2006, 307, 58–61. 10.1016/j.apcata.2006.03.007. [DOI] [Google Scholar]
- Klimeš J.; Michaelides A. Perspective: Advances and Challenges in Treating van Der Waals Dispersion Forces in Density Functional Theory. J. Chem. Phys. 2012, 137, 120901. 10.1063/1.4754130. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Hansen A.; Brandenburg J. G.; Bannwarth C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105–5154. 10.1021/acs.chemrev.5b00533. [DOI] [PubMed] [Google Scholar]
- Rangarajan S.; Mavrikakis M. A Comparative Analysis of Different van Der Waals Treatments for Molecular Adsorption on the Basal Plane of 2H-MoS2. Surf. Sci. 2023, 729, 122226. 10.1016/j.susc.2022.122226. [DOI] [Google Scholar]
- Al-Hamdani Y. S.; Tkatchenko A. Understanding Non-Covalent Interactions in Larger Molecular Complexes from First Principles. J. Chem. Phys. 2019, 150, 010901. 10.1063/1.5075487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W. M. C.; Mitas L.; Needs R. J.; Rajagopal G. Quantum Monte Carlo Simulations of Solids. Rev. Mod. Phys. 2001, 73, 33–83. 10.1103/RevModPhys.73.33. [DOI] [Google Scholar]
- Raghavachari K.; Trucks G. W.; Pople J. A.; Head-Gordon M. A Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 1989, 157, 479–483. 10.1016/S0009-2614(89)87395-6. [DOI] [Google Scholar]
- Karalti O.; Alfè D.; Gillan M. J.; Jordan K. D. Adsorption of a Water Molecule on the MgO(100) Surface as Described by Cluster and Slab Models. Phys. Chem. Chem. Phys. 2012, 14, 7846–7853. 10.1039/c2cp00015f. [DOI] [PubMed] [Google Scholar]
- Mostaani E.; Drummond N. D.; Fal’ko V. I. Quantum Monte Carlo Calculation of the Binding Energy of Bilayer Graphene. Phys. Rev. Lett. 2015, 115, 115501. 10.1103/PhysRevLett.115.115501. [DOI] [PubMed] [Google Scholar]
- Zen A.; Roch L. M.; Cox S. J.; Hu X. L.; Sorella S.; Alfè D.; Michaelides A. Toward Accurate Adsorption Energetics on Clay Surfaces. J. Phys. Chem. C 2016, 120, 26402–26413. 10.1021/acs.jpcc.6b09559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hamdani Y. S.; Rossi M.; Alfè D.; Tsatsoulis T.; Ramberger B.; Brandenburg J. G.; Zen A.; Kresse G.; Grüneis A.; Tkatchenko A.; Michaelides A. Properties of the Water to Boron Nitride Interaction: From Zero to Two Dimensions with Benchmark Accuracy. J. Chem. Phys. 2017, 147, 044710. 10.1063/1.4985878. [DOI] [PubMed] [Google Scholar]
- Hsing C.-R.; Chang C.-M.; Cheng C.; Wei C.-M. Quantum Monte Carlo Studies of CO Adsorption on Transition Metal Surfaces. J. Phys. Chem. C 2019, 123, 15659–15664. 10.1021/acs.jpcc.9b03780. [DOI] [Google Scholar]
- Grajciar L.; Bludský O.; Nachtigall P. Water Adsorption on Coordinatively Unsaturated Sites in CuBTC MOF. J. Phys. Chem. Lett. 2010, 1, 3354–3359. 10.1021/jz101378z. [DOI] [PubMed] [Google Scholar]
- Voloshina E.; Usvyat D.; Schütz M.; Dedkov Y.; Paulus B. On the Physisorption of Water on Graphene: A CCSD(T) Study. Phys. Chem. Chem. Phys. 2011, 13, 12041–12047. 10.1039/c1cp20609e. [DOI] [PubMed] [Google Scholar]
- Kubas A.; Berger D.; Oberhofer H.; Maganas D.; Reuter K.; Neese F. Surface Adsorption Energetics Studied with “Gold Standard” Wave-Function-Based Ab Initio Methods: Small-Molecule Binding to TiO2(110). J. Phys. Chem. Lett. 2016, 7, 4207–4212. 10.1021/acs.jpclett.6b01845. [DOI] [PubMed] [Google Scholar]
- Gruber T.; Liao K.; Tsatsoulis T.; Hummel F.; Grüneis A. Applying the Coupled-Cluster Ansatz to Solids and Surfaces in the Thermodynamic Limit. Phys. Rev. X 2018, 8, 021043. 10.1103/PhysRevX.8.021043. [DOI] [Google Scholar]
- Lau B. T. G.; Knizia G.; Berkelbach T. C. Regional Embedding Enables High-Level Quantum Chemistry for Surface Science. J. Phys. Chem. Lett. 2021, 12, 1104–1109. 10.1021/acs.jpclett.0c03274. [DOI] [PubMed] [Google Scholar]
- Doblhoff-Dier K.; Meyer J.; Hoggan P. E.; Kroes G.-J. Quantum Monte Carlo Calculations on a Benchmark Molecule–Metal Surface Reaction: H2 + Cu(111). J. Chem. Theory Comput. 2017, 13, 3208–3219. 10.1021/acs.jctc.7b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsoulis T.; Sakong S.; Groß A.; Grüneis A. Reaction Energetics of Hydrogen on Si(100) Surface: A Periodic Many-Electron Theory Study. J. Chem. Phys. 2018, 149, 244105. 10.1063/1.5055706. [DOI] [PubMed] [Google Scholar]
- Al-Hamdani Y. S.; Nagy P. R.; Zen A.; Barton D.; Kállay M.; Brandenburg J. G.; Tkatchenko A. Interactions between Large Molecules Pose a Puzzle for Reference Quantum Mechanical Methods. Nat. Commun. 2021, 12, 3927. 10.1038/s41467-021-24119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B. X.; Kapil V.; Zen A.; Chen J.; Alavi A.; Michaelides A. General Embedded Cluster Protocol for Accurate Modeling of Oxygen Vacancies in Metal-Oxides. J. Chem. Phys. 2022, 156, 124704. 10.1063/5.0087031. [DOI] [PubMed] [Google Scholar]
- Dittmer A.; Izsák R.; Neese F.; Maganas D. Accurate Band Gap Predictions of Semiconductors in the Framework of the Similarity Transformed Equation of Motion Coupled Cluster Theory. Inorg. Chem. 2019, 58, 9303–9315. 10.1021/acs.inorgchem.9b00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H. R.; Zhai H.; Umrigar C. J.; Chan G. K.-L. The Chromium Dimer: Closing a Chapter of Quantum Chemistry. J. Am. Chem. Soc. 2022, 144, 15932–15937. 10.1021/jacs.2c06357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A.; Laude G.; Khire S. S.; Gurav N. D.; Qu C.; Conte R.; Yu Q.; Li S.; Houston P. L.; Gadre S. R.; Richardson J. O.; Evangelista F. A.; Bowman J. M. Ring-Polymer Instanton Tunneling Splittings of Tropolone and Isotopomers Using a Δ-Machine Learned CCSD(T) Potential: Theory and Experiment Shake Hands. J. Am. Chem. Soc. 2023, 145, 9655–9664. 10.1021/jacs.3c00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt A. F.; Henle J. J.; Rose B. T.; Wang Y.; Darrow W. T.; Denmark S. E. Prediction of Higher-Selectivity Catalysts by Computer-Driven Workflow and Machine Learning. Science 2019, 363, eaau5631 10.1126/science.aau5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer C. E.; Leaf M.; Lee C. Y.; Farha O. K.; Hauser B. G.; Hupp J. T.; Snurr R. Q. Large-Scale Screening of Hypothetical Metal–Organic Frameworks. Nat. Chem. 2012, 4, 83–89. 10.1038/nchem.1192. [DOI] [PubMed] [Google Scholar]
- Bartlett R. J.; Musiał M. Coupled-Cluster Theory in Quantum Chemistry. Rev. Mod. Phys. 2007, 79, 291–352. 10.1103/RevModPhys.79.291. [DOI] [Google Scholar]
- Foulkes W. M. C.; Mitas L.; Needs R. J.; Rajagopal G. Quantum Monte Carlo Simulations of Solids. Rev. Mod. Phys. 2001, 73, 33–83. 10.1103/RevModPhys.73.33. [DOI] [Google Scholar]
- Boys S.; Bernardi F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. 10.1080/00268977000101561. [DOI] [Google Scholar]
- Hammer B.; Hansen L. B.; Nørskov J. K. Improved Adsorption Energetics within Density-Functional Theory Using Revised Perdew-Burke-Ernzerhof Functionals. Phys. Rev. B 1999, 59, 7413–7421. 10.1103/PhysRevB.59.7413. [DOI] [Google Scholar]
- Caldeweyher E.; Mewes J.-M.; Ehlert S.; Grimme S. Extension and Evaluation of the D4 London-dispersion Model for Periodic Systems. Phys. Chem. Chem. Phys. 2020, 22, 8499–8512. 10.1039/D0CP00502A. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Schmidt K. Jacob’s Ladder of Density Functional Approximations for the Exchange-Correlation Energy. AIP Conf. Proc. 2001, 577, 1–20. 10.1063/1.1390175. [DOI] [Google Scholar]
- Liao K.; Grüneis A. Communication: Finite Size Correction in Periodic Coupled Cluster Theory Calculations of Solids. J. Chem. Phys. 2016, 145, 141102. 10.1063/1.4964307. [DOI] [PubMed] [Google Scholar]
- Hummel F.; Tsatsoulis T.; Grüneis A. Low Rank Factorization of the Coulomb Integrals for Periodic Coupled Cluster Theory. J. Chem. Phys. 2017, 146, 124105. 10.1063/1.4977994. [DOI] [PubMed] [Google Scholar]
- Irmler A.; Gallo A.; Grüneis A. Focal-Point Approach with Pair-Specific Cusp Correction for Coupled-Cluster Theory. J. Chem. Phys. 2021, 154, 234103. 10.1063/5.0050054. [DOI] [PubMed] [Google Scholar]
- Needs R. J.; Towler M. D.; Drummond N. D.; López Ríos P.; Trail J. R. Variational and Diffusion Quantum Monte Carlo Calculations with the CASINO Code. J. Chem. Phys. 2020, 152, 154106. 10.1063/1.5144288. [DOI] [PubMed] [Google Scholar]
- Pople J. A. Quantum Chemical Models (Nobel Lecture). Angew. Chem., Int. Ed. 1999, 38, 1894–1902. . [DOI] [PubMed] [Google Scholar]
- Bramley G. A.; Beynon O. T.; Stishenko P. V.; Logsdail A. J. The Application of QM/MM Simulations in Heterogeneous Catalysis. Phys. Chem. Chem. Phys. 2023, 25, 6562–6585. 10.1039/d2cp04537k. [DOI] [PubMed] [Google Scholar]
- Voloshina E.; Paulus B. On the Application of the Incremental Scheme to Ionic Solids: Test of Different Embeddings. Theor. Chem. Acc. 2005, 114, 259–264. 10.1007/s00214-005-0689-1. [DOI] [Google Scholar]
- Manby F. R.; Stella M.; Goodpaster J. D.; Miller T. F. I. A Simple, Exact Density-Functional-Theory Embedding Scheme. J. Chem. Theory Comput. 2012, 8, 2564–2568. 10.1021/ct300544e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hégely B.; Nagy P. R.; Ferenczy G. G.; Kállay M. Exact density functional and wave function embedding schemes based on orbital localization. J. Chem. Phys. 2016, 145, 064107. 10.1063/1.4960177. [DOI] [Google Scholar]
- Libisch F.; Huang C.; Carter E. A. Embedded Correlated Wavefunction Schemes: Theory and Applications. Acc. Chem. Res. 2014, 47, 2768–2775. 10.1021/ar500086h. [DOI] [PubMed] [Google Scholar]
- Pham H. Q.; Hermes M. R.; Gagliardi L. Periodic Electronic Structure Calculations with the Density Matrix Embedding Theory. J. Chem. Theory Comput. 2020, 16, 130–140. 10.1021/acs.jctc.9b00939. [DOI] [PubMed] [Google Scholar]
- Schäfer T.; Libisch F.; Kresse G.; Grüneis A. Local Embedding of Coupled Cluster Theory into the Random Phase Approximation Using Plane Waves. J. Chem. Phys. 2021, 154, 011101. 10.1063/5.0036363. [DOI] [PubMed] [Google Scholar]
- Nagy P. R.; Kállay M. Optimization of the Linear-Scaling Local Natural Orbital CCSD(T) Method: Redundancy-Free Triples Correction Using Laplace Transform. J. Chem. Phys. 2017, 146, 214106. 10.1063/1.4984322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P. R.; Samu G.; Kállay M. Optimization of the Linear-Scaling Local Natural Orbital CCSD(T) Method: Improved Algorithm and Benchmark Applications. J. Chem. Theory Comput. 2018, 14, 4193–4215. 10.1021/acs.jctc.8b00442. [DOI] [PubMed] [Google Scholar]
- Nagy P. R.; Kállay M. Approaching the Basis Set Limit of CCSD(T) Energies for Large Molecules with Local Natural Orbital Coupled-Cluster Methods. J. Chem. Theory Comput. 2019, 15, 5275–5298. 10.1021/acs.jctc.9b00511. [DOI] [PubMed] [Google Scholar]
- Gyevi-Nagy L.; Kállay M.; Nagy P. R. Integral-Direct and Parallel Implementation of the CCSD(T) Method: Algorithmic Developments and Large-Scale Applications. J. Chem. Theory Comput. 2020, 16, 366–384. 10.1021/acs.jctc.9b00957. [DOI] [PubMed] [Google Scholar]
- Kállay M.; et al. The MRCC Program System: Accurate Quantum Chemistry from Water to Proteins. J. Chem. Phys. 2020, 152, 074107. 10.1063/1.5142048. [DOI] [PubMed] [Google Scholar]
- Scanlon D. O.; Dunnill C. W.; Buckeridge J.; Shevlin S. A.; Logsdail A. J.; Woodley S. M.; Catlow C. R. A.; Powell M. J.; Palgrave R. G.; Parkin I. P.; Watson G. W.; Keal T. W.; Sherwood P.; Walsh A.; Sokol A. A. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. 10.1038/nmat3697. [DOI] [PubMed] [Google Scholar]
- Buckeridge J.; Butler K. T.; Catlow C. R. A.; Logsdail A. J.; Scanlon D. O.; Shevlin S. A.; Woodley S. M.; Sokol A. A.; Walsh A. Polymorph Engineering of TiO2: Demonstrating How Absolute Reference Potentials Are Determined by Local Coordination. Chem. Mater. 2015, 27, 3844–3851. 10.1021/acs.chemmater.5b00230. [DOI] [Google Scholar]
- Zhang X.; Zhu L.; Hou Q.; Guan J.; Lu Y.; Keal T. W.; Buckeridge J.; Catlow C. R. A.; Sokol A. A. Toward a Consistent Prediction of Defect Chemistry in CeO2. Chem. Mater. 2023, 35, 207–227. 10.1021/acs.chemmater.2c03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol A. A.; Bromley S. T.; French S. A.; Catlow C. R. A.; Sherwood P. Hybrid QM/MM Embedding Approach for the Treatment of Localized Surface States in Ionic Materials. Int. J. Quantum Chem. 2004, 99, 695–712. 10.1002/qua.20032. [DOI] [Google Scholar]
- Tosoni S.; Sauer J. Accurate Quantum Chemical Energies for the Interaction of Hydrocarbons with Oxide Surfaces: CH4/MgO(001). Phys. Chem. Chem. Phys. 2010, 12, 14330–14340. 10.1039/c0cp01261k. [DOI] [PubMed] [Google Scholar]
- Boese A. D.; Sauer J. Accurate Adsorption Energies for Small Molecules on Oxide Surfaces: CH4/MgO(001) and C2H6/MgO(001). J. Comput. Chem. 2016, 37, 2374–2385. 10.1002/jcc.24462. [DOI] [PubMed] [Google Scholar]
- Alessio M.; Bischoff F. A.; Sauer J. Chemically Accurate Adsorption Energies for Methane and Ethane Monolayers on the MgO(001) Surface. Phys. Chem. Chem. Phys. 2018, 20, 9760–9769. 10.1039/C7CP08083B. [DOI] [PubMed] [Google Scholar]
- Rehak F. R.; Piccini G.; Alessio M.; Sauer J. Including Dispersion in Density Functional Theory for Adsorption on Flat Oxide Surfaces, in Metal–Organic Frameworks and in Acidic Zeolites. Phys. Chem. Chem. Phys. 2020, 22, 7577–7585. 10.1039/D0CP00394H. [DOI] [PubMed] [Google Scholar]
- Pacchioni G. Quantum Chemistry of Oxide Surfaces: From CO Chemisorption to the Identification of the Structure and Nature of Point Defects on MgO. Surf. Rev. Lett. 2000, 07, 277–306. 10.1142/S0218625X00000336. [DOI] [Google Scholar]
- Campbell C. T.; Sellers J. R. V. Enthalpies and Entropies of Adsorption on Well-Defined Oxide Surfaces: Experimental Measurements. Chem. Rev. 2013, 113, 4106–4135. 10.1021/cr300329s. [DOI] [PubMed] [Google Scholar]
- Schmid M.; Parkinson G. S.; Diebold U. Analysis of Temperature-Programmed Desorption via Equilibrium Thermodynamics. ACS Phys. Chem. Au 2023, 3, 44–62. 10.1021/acsphyschemau.2c00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. T.; Sellers J. R. V. The Entropies of Adsorbed Molecules. J. Am. Chem. Soc. 2012, 134, 18109–18115. 10.1021/ja3080117. [DOI] [PubMed] [Google Scholar]
- Nagy P. R.; Samu G.; Kállay M. An Integral-Direct Linear-Scaling Second-Order Møller–Plesset Approach. J. Chem. Theory Comput. 2016, 12, 4897–4914. 10.1021/acs.jctc.6b00732. [DOI] [PubMed] [Google Scholar]
- Riplinger C.; Neese F. An Efficient and near Linear Scaling Pair Natural Orbital Based Local Coupled Cluster Method. J. Chem. Phys. 2013, 138, 034106. 10.1063/1.4773581. [DOI] [PubMed] [Google Scholar]
- Riplinger C.; Sandhoefer B.; Hansen A.; Neese F. Natural Triple Excitations in Local Coupled Cluster Calculations with Pair Natural Orbitals. J. Chem. Phys. 2013, 139, 134101. 10.1063/1.4821834. [DOI] [PubMed] [Google Scholar]
- Riplinger C.; Pinski P.; Becker U.; Valeev E. F.; Neese F. Sparse Maps—A Systematic Infrastructure for Reduced-Scaling Electronic Structure Methods. II. Linear Scaling Domain Based Pair Natural Orbital Coupled Cluster Theory. J. Chem. Phys. 2016, 144, 024109. 10.1063/1.4939030. [DOI] [PubMed] [Google Scholar]
- Bistoni G.; Riplinger C.; Minenkov Y.; Cavallo L.; Auer A. A.; Neese F. Treating Subvalence Correlation Effects in Domain Based Pair Natural Orbital Coupled Cluster Calculations: An Out-of-the-Box Approach. J. Chem. Theory Comput. 2017, 13, 3220–3227. 10.1021/acs.jctc.7b00352. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Riplinger C.; Becker U.; Liakos D. G.; Minenkov Y.; Cavallo L.; Neese F. Communication: An Improved Linear Scaling Perturbative Triples Correction for the Domain Based Local Pair-Natural Orbital Based Singles and Doubles Coupled Cluster Method [DLPNO-CCSD(T)]. J. Chem. Phys. 2018, 148, 011101. 10.1063/1.5011798. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Schwilk M.; Köppl C.; Werner H.-J. Scalable Electron Correlation Methods. 4. Parallel Explicitly Correlated Local Coupled Cluster with Pair Natural Orbitals (PNO-LCCSD-F12). J. Chem. Theory Comput. 2017, 13, 4871–4896. 10.1021/acs.jctc.7b00799. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Werner H.-J. Scalable Electron Correlation Methods. 5. Parallel Perturbative Triples Correction for Explicitly Correlated Local Coupled Cluster with Pair Natural Orbitals. J. Chem. Theory Comput. 2018, 14, 198–215. 10.1021/acs.jctc.7b01141. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Werner H.-J. Explicitly Correlated Local Coupled-Cluster Methods Using Pair Natural Orbitals. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2018, 8, e1371 10.1002/wcms.1371. [DOI] [Google Scholar]
- Bennett M. C.; Melton C. A.; Annaberdiyev A.; Wang G.; Shulenburger L.; Mitas L. A New Generation of Effective Core Potentials for Correlated Calculations. J. Chem. Phys. 2017, 147, 224106. 10.1063/1.4995643. [DOI] [PubMed] [Google Scholar]
- Bennett M. C.; Wang G.; Annaberdiyev A.; Melton C. A.; Shulenburger L.; Mitas L. A New Generation of Effective Core Potentials from Correlated Calculations: 2nd Row Elements. J. Chem. Phys. 2018, 149, 104108. 10.1063/1.5038135. [DOI] [PubMed] [Google Scholar]
- Zen A.; Sorella S.; Gillan M. J.; Michaelides A.; Alfè D. Boosting the Accuracy and Speed of Quantum Monte Carlo: Size Consistency and Time Step. Phys. Rev. B 2016, 93, 241118. 10.1103/PhysRevB.93.241118. [DOI] [Google Scholar]
- Zen A.; Brandenburg J. G.; Klimeš J.; Tkatchenko A.; Alfè D.; Michaelides A. Fast and Accurate Quantum Monte Carlo for Molecular Crystals. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 1724–1729. 10.1073/pnas.1715434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen A.; Brandenburg J. G.; Michaelides A.; Alfè D. A New Scheme for Fixed Node Diffusion Quantum Monte Carlo with Pseudopotentials: Improving Reproducibility and Reducing the Trial-Wave-Function Bias. J. Chem. Phys. 2019, 151, 134105. 10.1063/1.5119729. [DOI] [PubMed] [Google Scholar]
- Rajagopal G.; Needs R. J.; Kenny S.; Foulkes W. M. C.; James A. Quantum Monte Carlo Calculations for Solids Using Special k Points Methods. Phys. Rev. Lett. 1994, 73, 1959–1962. 10.1103/PhysRevLett.73.1959. [DOI] [PubMed] [Google Scholar]
- Alfè D.; Gillan M. J.; Towler M. D.; Needs R. J. Diamond and β-Tin Structures of Si Studied with Quantum Monte Carlo Calculations. Phys. Rev. B 2004, 70, 214102. 10.1103/PhysRevB.70.214102. [DOI] [Google Scholar]
- Alfè D.; Gillan M. J. The Energetics of Oxide Surfaces by Quantum Monte Carlo. J. Phys.: Condens. Matter 2006, 18, L435–L440. 10.1088/0953-8984/18/35/L01. [DOI] [PubMed] [Google Scholar]
- McClain J.; Sun Q.; Chan G. K.-L.; Berkelbach T. C. Gaussian-Based Coupled-Cluster Theory for the Ground-State and Band Structure of Solids. J. Chem. Theory Comput. 2017, 13, 1209–1218. 10.1021/acs.jctc.7b00049. [DOI] [PubMed] [Google Scholar]
- Zhang I. Y.; Grüneis A. Coupled Cluster Theory in Materials Science. Front. Mater. 2019, 6, 123. 10.3389/fmats.2019.00123. [DOI] [Google Scholar]
- Sun Q.; Zhang X.; Banerjee S.; Bao P.; Barbry M.; Blunt N. S.; Bogdanov N. A.; Booth G. H.; Chen J.; Cui Z. H.; et al. Recent Developments in the PySCF Program Package. J. Chem. Phys. 2020, 153, 024109. 10.1063/5.0006074. [DOI] [PubMed] [Google Scholar]
- Neufeld V. A.; Ye H.-Z.; Berkelbach T. C. Ground-State Properties of Metallic Solids from Ab Initio Coupled-Cluster Theory. J. Phys. Chem. Lett. 2022, 13, 7497–7503. 10.1021/acs.jpclett.2c01828. [DOI] [PubMed] [Google Scholar]
- Mihm T. N.; Schäfer T.; Ramadugu S. K.; Weiler L.; Grüneis A.; Shepherd J. J. A Shortcut to the Thermodynamic Limit for Quantum Many-Body Calculations of Metals. Nat. Comput. Sci. 2021, 1, 801–808. 10.1038/s43588-021-00165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg J. G.; Zen A.; Fitzner M.; Ramberger B.; Kresse G.; Tsatsoulis T.; Grüneis A.; Michaelides A.; Alfè D. Physisorption of Water on Graphene: Subchemical Accuracy from Many-Body Electronic Structure Methods. J. Phys. Chem. Lett. 2019, 10, 358–368. 10.1021/acs.jpclett.8b03679. [DOI] [PubMed] [Google Scholar]
- Schäfer T.; Gallo A.; Irmler A.; Hummel F.; Grüneis A. Surface Science Using Coupled Cluster Theory via Local Wannier Functions and In-RPA-embedding: The Case of Water on Graphitic Carbon Nitride. J. Chem. Phys. 2021, 155, 244103. 10.1063/5.0074936. [DOI] [PubMed] [Google Scholar]
- Libisch F.; Huang C.; Carter E. A. Embedded Correlated Wavefunction Schemes: Theory and Applications. Acc. Chem. Res. 2014, 47, 2768–2775. 10.1021/ar500086h. [DOI] [PubMed] [Google Scholar]
- Araujo R. B.; Rodrigues G. L. S.; dos Santos E. C.; Pettersson L. G. M. Adsorption Energies on Transition Metal Surfaces: Towards an Accurate and Balanced Description. Nat. Commun. 2022, 13, 6853. 10.1038/s41467-022-34507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert C.; Piras A.; Gryn’ova G. CO2 on Graphene: Benchmarking Computational Approaches to Noncovalent Interactions. ACS Omega 2023, 8, 35768–35778. 10.1021/acsomega.3c03251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaberdiyev A.; Wang G.; Melton C. A.; Bennett M. C.; Shulenburger L.; Mitas L. A New Generation of Effective Core Potentials from Correlated Calculations: 3d Transition Metal Series. J. Chem. Phys. 2018, 149, 134108. 10.1063/1.5040472. [DOI] [PubMed] [Google Scholar]
- Masios N.; Irmler A.; Schäfer T.; Grüneis A.. Averting the Infrared Catastrophe in the Gold Standard of Quantum Chemistry. 2023, arXiv:2303.16957. arXiv preprint. [DOI] [PubMed] [Google Scholar]
- Neufeld V. A.; Berkelbach T. C.. Highly Accurate Electronic Structure of Metallic Solids from Coupled-Cluster Theory with Nonperturbative Triple Excitations. 2023, arXiv:2303.11270. arXiv preprint. [DOI] [PubMed] [Google Scholar]
- Du Y.; Deskins N. A.; Zhang Z.; Dohnálek Z.; Dupuis M.; Lyubinetsky I. Imaging Consecutive Steps of O2 Reaction with Hydroxylated TiO2(110): Identification of HO2 and Terminal OH Intermediates. J. Phys. Chem. C 2009, 113, 666–671. 10.1021/jp807030n. [DOI] [Google Scholar]
- Zhao Q.; Martirez J. M. P.; Carter E. A. Electrochemical Hydrogenation of CO on Cu(100): Insights from Accurate Multiconfigurational Wavefunction Methods. J. Phys. Chem. Lett. 2022, 13, 10282–10290. 10.1021/acs.jpclett.2c02444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.