Abstract
Electrical storm (ES) is a life-threatening state of electrical instability characterized by 3 or more episodes of sustained ventricular arrhythmia (VA) within 24 hours. Most cases of ES arise in the setting of underlying structural heart disease which provides an arrhythmogenic substrate, often provoked by excessive sympathetic activation or other aggravating factors. Identification of the underlying cardiac substrate and reversible triggers is needed, in addition to diligent interrogation and programming of the implantable cardioverter-defibrillator that is often present. Medical management includes membrane-active anti-arrhythmic drugs, beta-adrenergic blockade, sedation, and often hemodynamic support for hypotension from cardiac decompensation. The intensity of these interventions should be matched to the severity of the ES syndrome and risk of recurrent VA and adverse outcomes. A stepped-care algorithm can be used, involving escalating treatments for higher-risk presentations or recurrent VA. Many patients with ES are considered for catheter ablation and are at elevated risk of hemodynamic compromise, which may justify the use of temporary mechanical circulatory support in selected patients. A multidisciplinary collaborative approach to the management of ES is essential. Outcomes after ES are poor, including frequent recurrences of VA and deaths due to progressive heart failure and other cardiac causes. Evaluation for heart transplantation or palliative care is often appropriate, even for patients who survive the initial episode. In this State-of-the-Art review, all aspects of the evaluation and management of ES are described to enable providers to provide comprehensive care for these patients with critical illness caused by recurrent VA.
Introduction
Electrical storm (ES) is a life-threatening state of cardiac electrical instability characterized by repetitive clusters of sustained ventricular arrhythmias (VA) over a short period.(1,2) The majority of these VA are monomorphic (MM) ventricular tachycardia (VT) but may include polymorphic VT (PMVT) and ventricular fibrillation (VF). Although VA may self-terminate, medical intervention or external defibrillation is usually required in the absence of a functioning implantable cardioverter-defibrillator (ICD). A standard clinical definition of ES is three or more sustained VA episodes (including appropriate ICD shocks) separated by at least 5 minutes over 24 hours.(1,2)
Using this standard definition, ES occurs in up to 28% of patients receiving an ICD for secondary prevention.(3–6) For those receiving an ICD for primary prevention, the incidence of ES is lower, reported at 2.3% per year over an average follow-up of 21 months.(7) The multicenter OBSERVational registry On long-term outcome of ICD patients (OBSERVO-ICD) demonstrated an overall incidence of ES of 4.7% over a median of 39 months, being higher for secondary prevention patients than primary prevention patients (10.5% versus 3.9%).(8) Defining the prevalence of ES in patients without an ICD is challenging, as many experience sudden cardiac death (SCD) at home.
Most patients with ES (77–94%) have underlying structural heart disease, with the majority having advanced cardiomyopathy (ischemic or nonischemic).(9) A minority of ES occurs in patients with macroscopically normal but molecularly abnormal hearts (e.g., channelopathies).(8–10) Most patients with ES have a pre-existing ICD due to underlying cardiomyopathy as an ICD indication and the ability of an ICD to abort SCD during ES resulting in survival to hospital admission.(9) Lower left ventricular ejection fraction (LVEF), older age, prolonged QRS duration, lack of appropriate guideline-directed medical therapy (GDMT), chronic kidney disease, and previous VA episodes (especially MMVT as the presenting rhythm) are reported ES risk factors.(5–7,9,11)
ES is a risk factor for cardiovascular mortality and generally portends a poor outcome.(3,4,7–10,12) It is essential to distinguish ES from a single or limited number of isolated episodes of VA, which have a more favorable prognosis.(12) Nonetheless, the occurrence of VA in patients with ICDs carries high short-term and long-term mortality from both arrhythmic and non-arrhythmic causes.(3,4,8,13,14)
Overall, ES presents with a wide spectrum of illness severity and clinical manifestations resulting from a complex interplay of anatomic, metabolic, autonomic, and epigenetic factors. ES represents a medical emergency, foreshadows an increased risk of death, and requires a multimodality therapeutic approach typically necessitating cardiac intensive care unit (CICU) admission. This State-of-the-Art review describes the pathophysiology, diagnostic/prognostic assessment, medical management, and interventional management of ES.
Pathophysiology of ES
Mechanisms of Ventricular Arrhythmias and Electrical Storm
Development of ES usually requires both an arrhythmic substrate and a proarrhythmic trigger (Figure 1). Most ES develops on a backdrop of either structural heart disease or pathogenic ion channel defects (channelopathies) (Table 1). Structural heart disease can cause arrhythmogenic remodeling with the development of myocardial scar that can form the basis of reentry, or by impaired expression and function of ion channels and alterations in calcium handling. The risk for ES increases when structural myocardial disease and myocardial conduction/repolarization derangements coincide.
Figure 1:
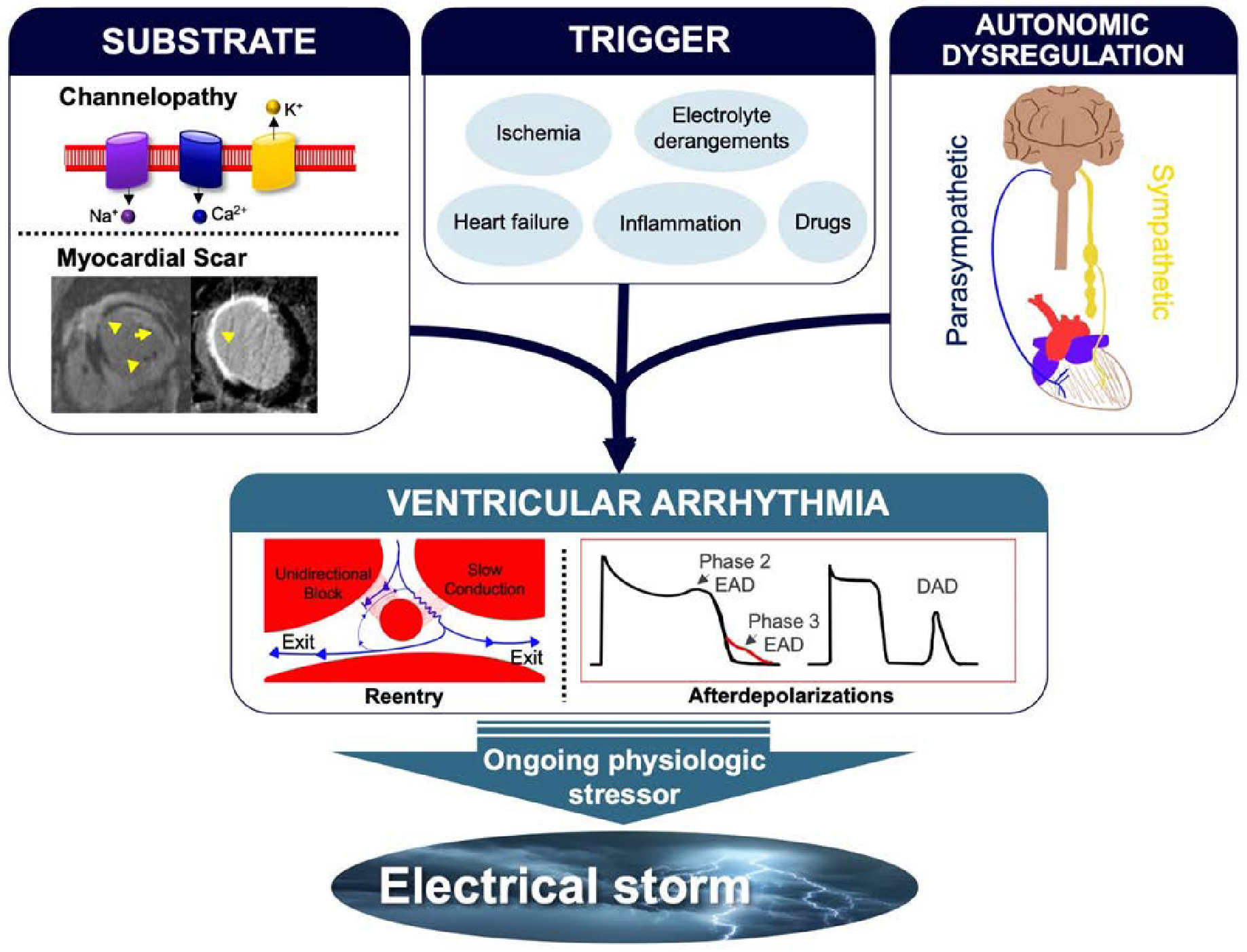
Mechanisms of arrhythmogenesis in ES. Triggers such as myocardial ischemia, inflammation, or hemodynamic decompensation, as well as drug and electrolyte effects, often with accompanying autonomic nervous system imbalance, can lead to sustained VA due to reentry and/or after depolarizations in those with vulnerable anatomic or electrical substrates (e.g., myocardial scar). Perpetuation of the inciting trigger and the resulting sympathetic nervous system response leads to recurrent VA and ES.
Table 1:
| Substrate | Triggers for ES | Disease-targeted ES therapy |
|---|---|---|
| STRUCTURAL HEART DISEASE | ||
| Ischemic cardiomyopathy | Active ischemia Sympathetic tone Decompensated heart failure |
Revascularization if indicated Catheter ablation |
| Nonischemic cardiomyopathy | Sympathetic tone Decompensated heart failure |
Hemodynamic support Consider catheter ablation |
| Arrhythmogenic cardiomyopathy | Sympathetic tone | Catheter ablation |
| Cardiac sarcoidosis | Active granulomatous disease | Immune suppression if active inflammation Catheter ablation |
| Chagas disease | Inflammation | Autonomic modulation Catheter Ablation |
| Viral myocarditis | Inflammation | Hemodynamic support Consider catheter ablation |
| Giant cell myocarditis | Inflammation | Immune suppressive therapy Hemodynamic support |
| CONDUCTION DEFECTS (CHANNELOPATHIES) | ||
| Congenital long QT syndrome | QT-prolonging agents Sympathetic tone |
Avoid QT-prolonging agents Beta-blockers Atrial pacing Autonomic modulation |
| Acquired long QT syndrome | QT-prolonging agents Bradycardia |
Avoid QT-prolonging agents IV magnesium Atrial pacing Lidocaine |
| CPVT | Sympathetic tone ICD shocks |
Beta-blockers Flecainide Autonomic modulation |
| Brugada syndrome | Parasympathetic tone Fever Excessive alcohol intake |
Avoid sodium channel blockers Avoid provoking drugs/conditions Isoproterenol or quinidine Consider catheter ablation |
| Early repolarization syndrome or idiopathic VF | Parasympathetic tone | Isoproterenol or quinidine Consider catheter ablation for PVC triggers |
| Short QT syndrome | Parasympathetic tone | Isoproterenol or quinidine |
| Idiopathic or short-coupled VF | Parasympathetic tone | IV verapamil Isoproterenol or quinidine Consider catheter ablation for PVC triggers |
| Idiopathic (outflow tract) VT | Sympathetic tone | Beta-blockers or verapamil |
The most common mechanism for VA during ES is macro-reentry caused by slow conduction through surviving tissue channels in scar resulting in MMVT.(1,15) Additionally, functional reentry occurs via heterogeneously impaired excitability and decreased repolarization reserve, which may be augmented by myocardial stretch and elevated sympathetic tone during decompensated heart failure (HF).(1,15,16) Reentry requires an area of anatomic or functional conduction block, an electrical pathway with unidirectional block, and a pathway with slow or heterogeneous conduction. This substrate for reentry is present in patients with myocardial scar from a prior myocardial infarction (MI), interstitial fibrosis from dilated nonischemic cardiomyopathy (NICM), focal inflammation, or infiltrative cardiomyopathy.(15)
Less commonly, ES is secondary to triggered activity from early afterdepolarization (EAD) or delayed afterdepolarization (DAD). EADs occur during systolic phase 2 or 3 of the action potential, primarily driven by a reduction in repolarization reserve by the L-type Ca current (Ica,L) and Na-Ca exchange current (INCX).(1) EADs are the primary mechanisms for PMVT and torsades de pointes (TdP) in congenital or acquired long QT syndrome.(1) DADs occur during diastolic phase 4 of the action potential when repolarization is complete but before the next action potential occurs.(1) DADs are secondary to increased intracellular calcium concentrations from sarcoplasmic reticulum calcium release and can occur in myocardial ischemia, digoxin or catecholamine toxicity, or catecholaminergic PMVT.(1,16)
Reentry and triggered activity are not mutually exclusive for ventricular arrhythmogenesis, and patients typically have an underlying myocardial substrate allowing reentry with VA initiated acutely by triggered activity. Underlying electrolyte alterations, acid-base imbalance, abnormal metabolism, and drug toxicity are often inciting factors.(1,2) Sympathetic activation can decrease the VA threshold by increasing afterdepolarizations and can cause dispersion of action potential duration (heterogeneity of repolarization) in myocardial tissue allowing for higher susceptibility to VA.(16) In diseased myocardium, afterdepolarization resulting in a short-coupled premature ventricular contraction (PVC) often initiates reentry VT or VF from conduction block in one limb of the reentry circuit and slow conduction in the other limb.
Substrates for electrical storm
Structural heart disease
Patients with chronic infarcts and ischemic cardiomyopathy (ICM) most frequently present with MMVT caused by reentry through subendocardial scar (Figure 2).(1) Less commonly, PVCs arising from Purkinje fibers in the scar border zone cause PMVT and VF.(1) Acute myocardial ischemia produces myocardial tissue repolarization heterogeneity and depolarization of the ischemic tissue leading to reentry and PMVT or VF, and reperfusion itself can be arrhythmogenic.(1)
Figure 2:
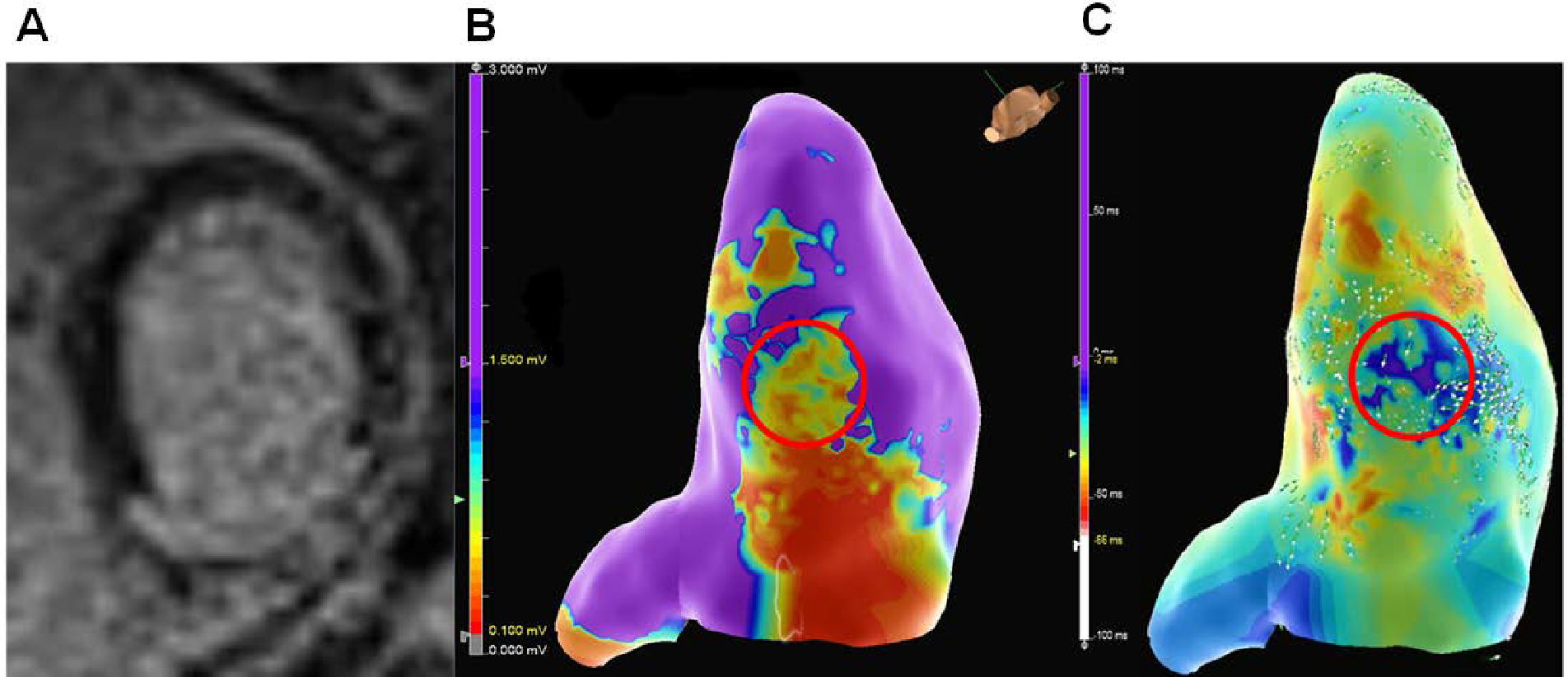
A patient with an ICM presenting with ES secondary to inferior MI, resulting in inferior left ventricular scar as shown by (A) late gadolinium enhancement on cardiovascular magnetic resonance imaging. (B) The imaging correlated with areas of low voltage (red circle) on electroanatomical mapping during VT ablation in the inferior left ventricle. (C) Activation mapping in sinus rhythm demonstrated areas of late activation and slow conduction (red circle) that corresponded to the area of scar.
Patients with NICM, particularly inherited cardiomyopathy syndromes, may have distinct scar regions which facilitate reentrant VT or trigger VF.(15) Certain NICM substrates have a higher propensity for VA and ES, including arrhythmogenic (right ventricular) cardiomyopathy, cardiac sarcoidosis, and Chagas cardiomyopathy.(17–20) In these diseases, acute myocardial injury/inflammation and chronic myocardial scarring can be proarrhythmic, causing reentrant MMVT or PVC-mediated VF. Acute (fulminant) myocarditis, including idiopathic, viral, or giant-cell myocarditis, can provoke ES. In one analysis, nearly half of patients requiring venoarterial (VA) extracorporeal membrane oxygenator (ECMO) support for fulminant giant-cell myocarditis presented with ES.(21,22) In cardiac sarcoidosis and acute fulminant myocarditis (particularly giant cell), immunosuppression is essential, recognizing that initiation of immunosuppression can trigger VA.(1,18,21,22)
Conduction defects (Channelopathies)
Inherited or acquired alterations in ion channels and transporters causing impaired depolarization and repolarization can provoke VA despite a structurally normal heart.(23,24) Ion channel mutations and medications can impair repolarizing currents or augment depolarizing currents, prolonging the QT interval and predisposing to bradycardia-dependent TdP due to triggered EADs.(24) Early repolarization syndromes (especially an early repolarization pattern in the inferior/lateral leads) can trigger VF, but the overall ES incidence in this population remains relatively low.(23) Idiopathic VF can be initiated by a short-coupled triggering PVC (often with the PVC arising from structures dense with Purkinje fibers like the moderator band and papillary muscle), and this population appears to have a higher risk of ES at presentation and recurrent ES than others with idiopathic VF.(25,26) Idiopathic MMVT in patients with structurally normal hearts, including outflow tract VT and fascicular VT, is typically considered benign but may rarely result in ES.(1) Specific antiarrhythmic drugs (AADs) or other therapies to use or avoid in the setting of VA have been identified for many of these conditions (Table 1).(24)
Diagnostic assessment and risk stratification
Arrhythmia assessment
The initial diagnostic evaluation for ES patients focuses on evaluating the arrhythmia, identifying triggers, understanding the cardiac substrate, assessing the hemodynamic state, and performing risk stratification (Figure 3). Information regarding the frequency and duration of VA episodes should be sought from ICD interrogation and cardiac telemetry when available, and initiation of continuous (ideally 12-lead) electrocardiographic monitoring is invaluable. Higher-risk VA features that may justify a more aggressive initial strategy include VF/PMVT, faster ventricular rate, more frequent or incessant VA episodes, a tendency to degenerate to VF, failure of ICD therapies, and VA triggered by short-coupled PVCs. The patient’s history of VA and prior therapies including AADs or catheter ablation will help predict the likelihood of treatment success. Patients naïve to AADs may have a more favorable response to medical therapy, while those presenting with ES despite long-term AAD therapy are more likely to require catheter ablation. Patients presenting with drug-refractory VA despite prior ablation attempts are less likely to respond to conventional treatment strategies (e.g., repeat catheter ablation), and may require heart transplantation or left ventricular assist device (LVAD) implantation.
Figure 3:
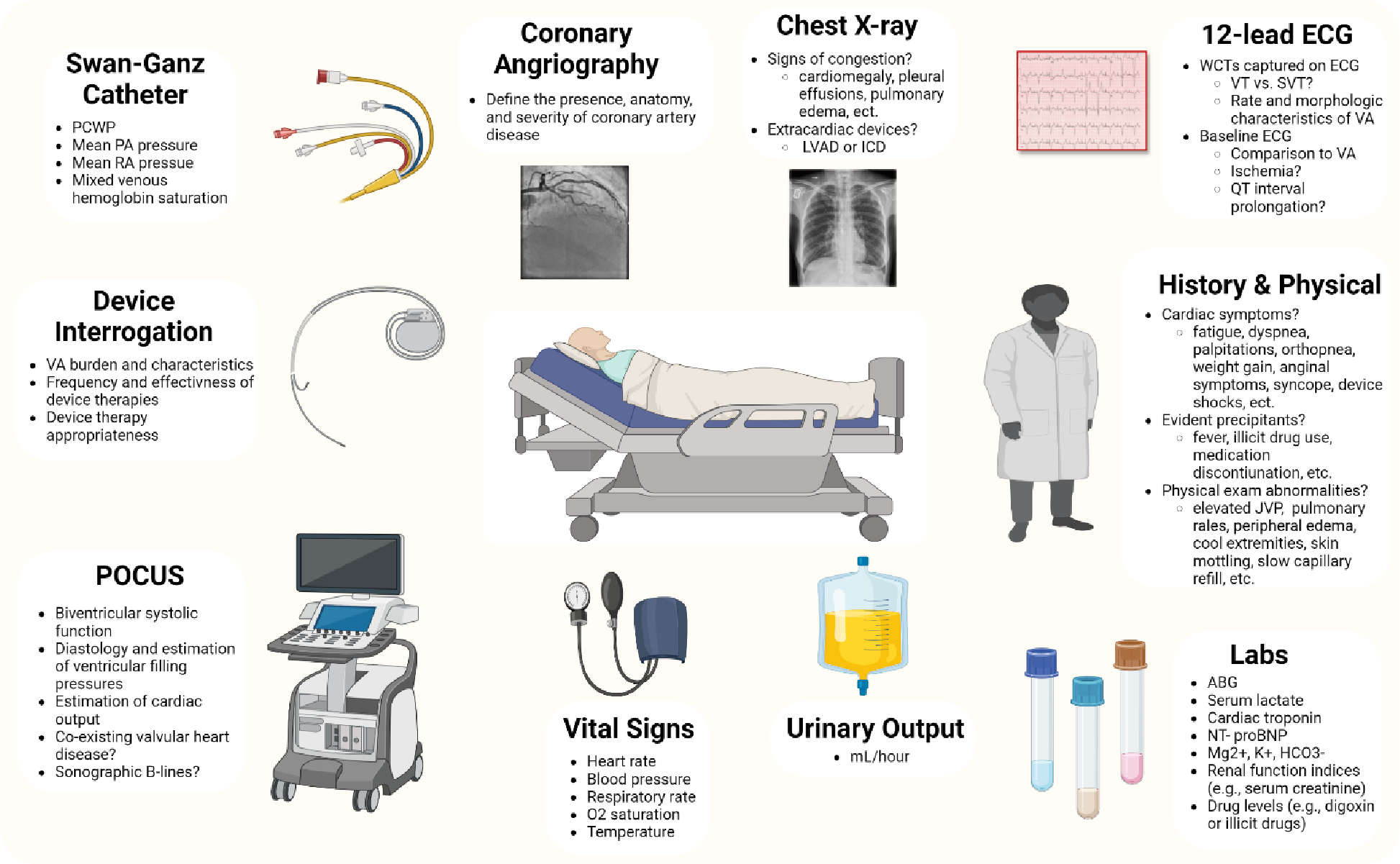
Diagnostic and clinical assessment and risk stratification for patients with electrical storm.
Identification of triggers
A triggering mechanism (Central Illustration) is found in only a minority of ES patients.(3,11) Potential triggers include myocardial ischemia, worsening HF or volume overload causing myocardial stretch, concomitant acute illness with fever, recent medication usage or changes causing drug toxicity or QT prolongation, imbalances in sympathetic and parasympathetic activity, non-cardiac organ failure, thyrotoxicosis, and electrolyte derangements (particularly hypo- or hyperkalemia and hypomagnesemia).(2,3,11) Identification and discontinuation of offending proarrhythmic drugs is important.(2) Elevated sympathetic tone and adrenergic excess are proarrhythmic and often drive ES.(16,27) Initial laboratory evaluation includes electrolytes, lactate, kidney/liver/thyroid function, and cardiac biomarkers.(2) A 12-lead electrocardiogram should be obtained during both the native rhythm and VT if possible. It is essential to exclude myocardial ischemia, particularly for patients with established coronary artery disease (CAD) or ICM, recognizing that nonischemic processes may increase serum cardiac troponin levels. A coronary angiogram is often indicated to identify obstructive CAD that could produce myocardial ischemia, even for patients without clear evidence of acute MI. Computed tomography coronary angiography can be considered in selected stable patients when the clinical suspicion is low.
Central Illustration:
Global approach to the evaluation and management of ES including diagnostic assessment, risk stratification, medical therapy, and catheter ablation.
Hemodynamic and cardiac substrate assessment
Clinicians must recognize that ES often portends hemodynamic destabilization, which can be exacerbated by standard medical therapies for ES. Myocardial stunning can result from repetitive ICD shocks, molecular and cellular changes, and global cardiac hypoperfusion from VA itself, resulting in a vicious cascade of progressive hemodynamic and electrical instability.(28) Identifying coexisting decompensated HF or cardiogenic shock (CS) is crucial during ES evaluation, as these patients are less likely to tolerate recurrent VA or standard ES therapies. Initial clinical evaluation should include assessment of perfusion and volume overload (Figure 4). Echocardiography can assess the underlying cardiac substrate and hemodynamic profile by identifying ventricular systolic dysfunction and noninvasively estimating biventricular filling pressures and cardiac output, which may be supplemented by invasive hemodynamic measurements from an arterial, central venous, or pulmonary artery catheter. New, worsening, or severe ventricular dysfunction should raise suspicion for progressive cardiomyopathy as the key ES driver, and evidence of advanced HF should be investigated.(29) Cardiac magnetic resonance imaging or positron emission tomography can be diagnostic when arrhythmogenic cardiomyopathy, cardiac sarcoidosis, acute myocarditis, or Chagas cardiomyopathy are suspected or when planning catheter ablation.(18) ES in the setting of acute (fulminant) myocarditis is a potential indication for endomyocardial biopsy.(22)
Figure 4:
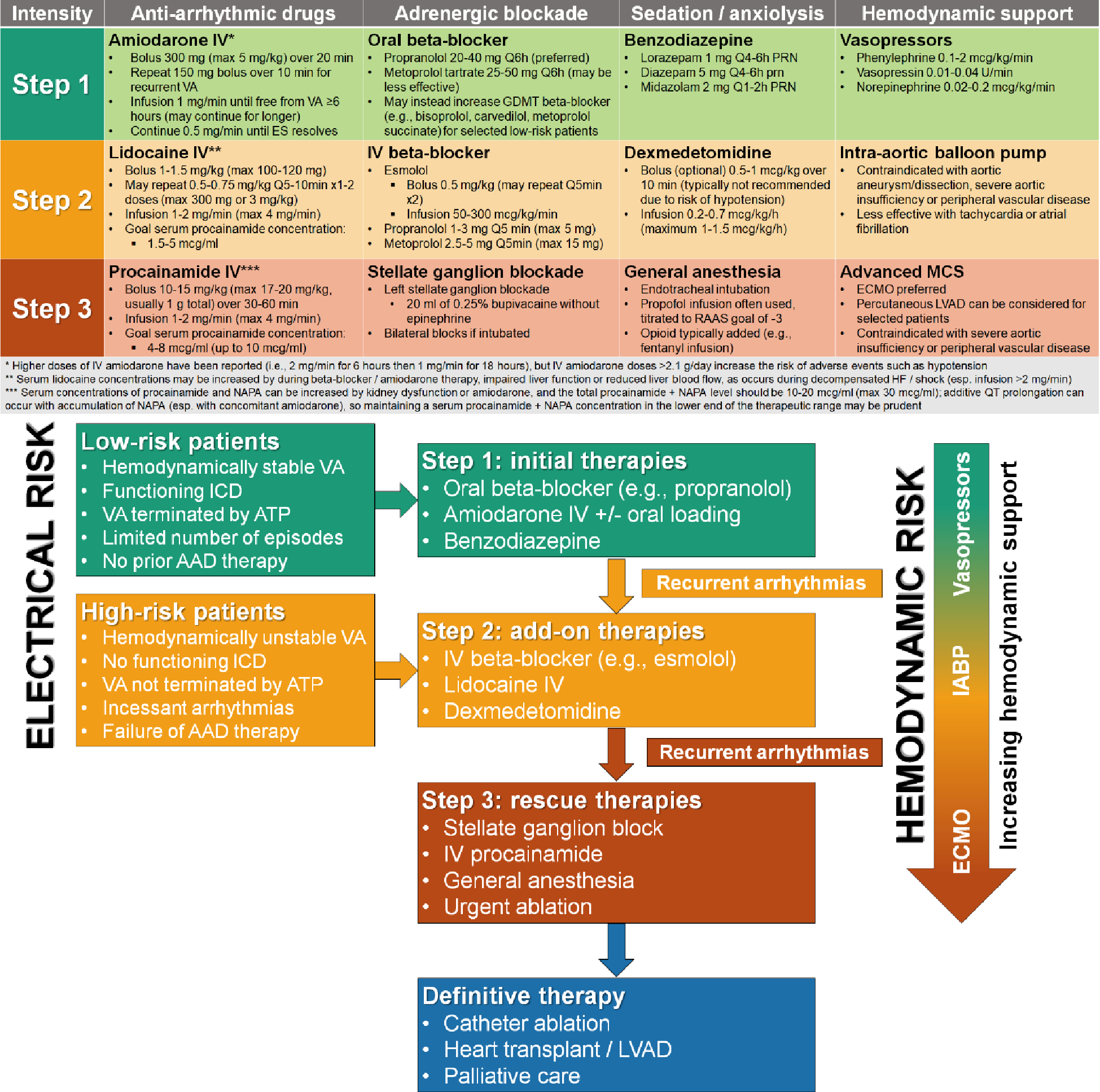
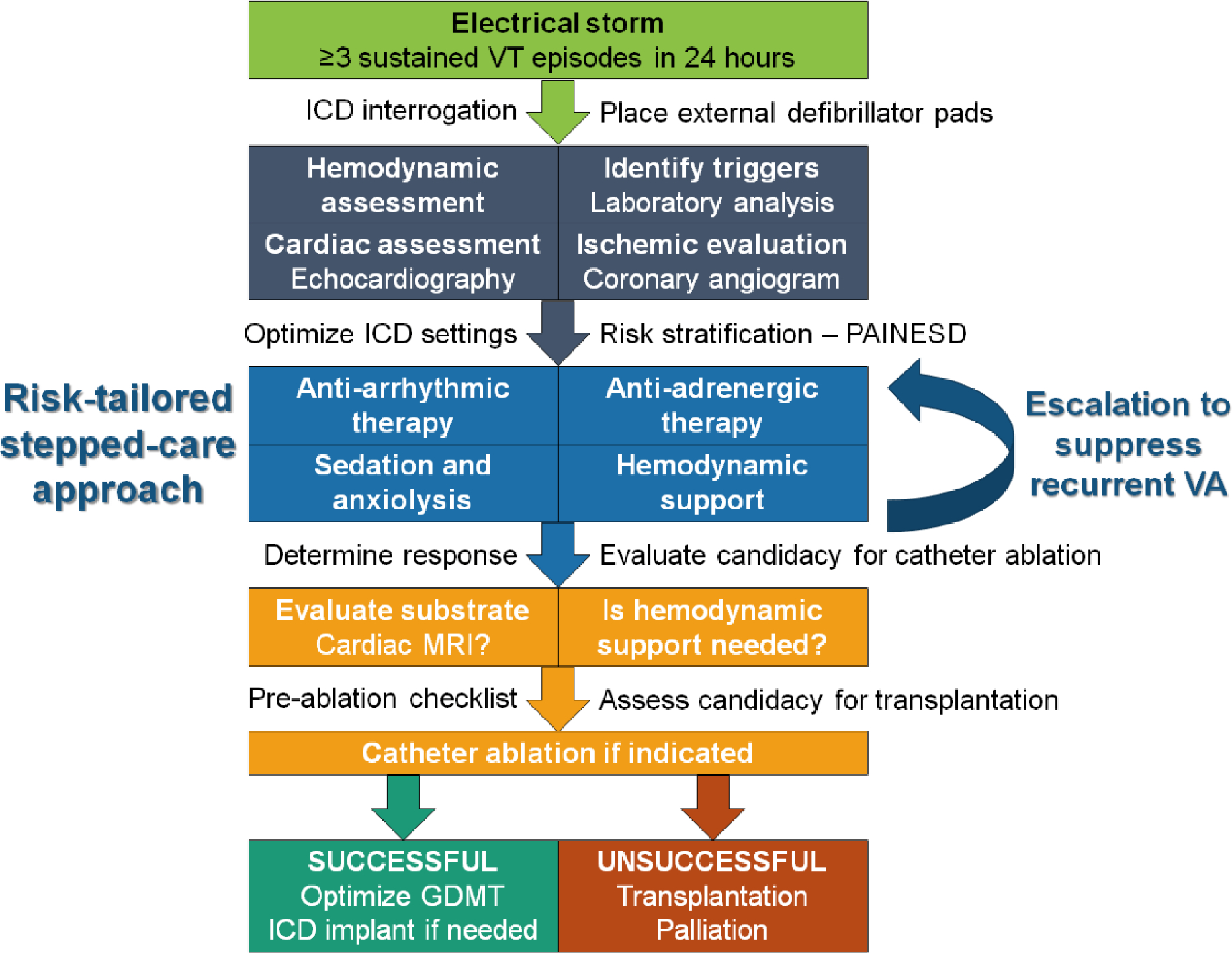
Stepped-care algorithm for rational escalation of medical therapy in ES. Higher-risk presentations may justify starting at step 2 and increasing to the next step is warranted in case of recurrent VA.
Left ventricular assist device patients
Over one-third of LVAD recipients develop VA and this portends a worse prognosis.(30,31) In a single-center observational study, ES occurred in 10% of 730 patients after LVAD and was associated with high 1-year mortality; risk factors for ES after LVAD included prior VA, prior VT ablation, AAD use, and perioperative MCS.(31) Patients requiring an LVAD inherently possess an arrhythmogenic substrate from ventricular scar and remodeling due to advanced cardiomyopathy, which is not necessarily ameliorated by the LVAD. Furthermore, apical scarring from the LVAD cannula and hemodynamic perturbations such as hypovolemia triggering suction events represent other proarrhythmic mechanisms among LVAD patients.(32) Although many LVAD patients tolerate VA relatively well hemodynamically, VA episodes can lead to right ventricular (RV) dysfunction and inadequate left ventricular preload, which can impair device flow and produce low-output sequelae, thrombus formation, or suction events.(30–32)
Implantable cardioverter defibrillator management during ES
Importance of ICD in patients with ES
The ICD is an indispensable treatment option for patients at risk of SCD, substantially mitigating the risk of arrhythmic death during ES.(1,2) Patients with ICDs account for most of those with ES, making appropriate management of the ICD during ES crucial to patient care.(3,5,6,8,10,11,13) In ICD patients, ES can trigger multiple ICD therapies including anti-tachycardia pacing (ATP) or ICD shocks depending on the device programming and the VA rate. ICD shocks can further exacerbate ES by provoking pain and emotional distress (including anxiety, depression, phantom shocks, and post-traumatic stress disorder), which further stimulate sympathetic drive and increase the risk of subsequent VA episodes potentially leading to a vicious cycle of VA and recurrent ICD therapies.(2,16,33) An ICD that can successfully terminate VT using ATP is an important protective factor during ES, and patients without an ICD or whose ICD is ineffective for terminating VA (or requires multiple shocks to succeed) are at higher risk and require a more aggressive initial approach to therapy.
Immediate management
While every effort should be made to interrogate the ICD urgently, the immediate goal of treatment is to avoid or minimize repetitive, ineffective, or inappropriate shocks (including those for hemodynamically tolerated or nonsustained VA). This can be achieved acutely by ICD reprogramming or applying a magnet over the ICD, which suspends VA detection and therapies while maintaining the pacing function.(2) If the clinical VT is below the ICD detection rate, ATP therapies (or an ICD shock) can be manually administered through the device to terminate the VT as appropriate. It is advisable to place external defibrillator pads on all patients (including those with a functioning ICD) for external cardioversion if ICD therapies do not terminate the VT, recognizing that most ICDs have an automatic maximal shock limit for each VA episode and repetitive ICD shocks can decrease battery life.
ICD interrogation
The goals of ICD interrogation are to quantify the frequency of VA and ICD therapies, determine if the ICD therapies were appropriate, assess the VT morphology, identify the mechanism of initiation, recognize any failure of appropriate therapies to abort VA episodes, and allow reprogramming to optimize detection and treatment of VA episodes. If the VT rate is below the ICD therapy zones, the rate cut-offs can be adjusted. Contemporary ICDs allow for programming different therapies into 2 or more zones based on rate, allowing ICD therapies to be tailored to the observed VA.
Appropriate versus inappropriate therapies
It is crucial to determine if the ICD therapies are appropriate (i.e., for sustained VA) or inappropriate. Inappropriate ICD therapies can be for arrhythmias such as nonsustained VT or supraventricular tachycardia (e.g., atrial fibrillation with rapid ventricular rate), or due to artifactual signals such as T wave oversensing or lead noise (e.g., lead fracture).(34) Programming strategies using SVT discriminators can significantly decrease the incidence of inappropriate ICD shocks.(34)
ICD reprogramming to prevent ICD shocks
If the VT is well tolerated without significant symptoms or hemodynamic compromise, the ICD can be reprogrammed by increasing the detection time or programming an ATP-only zone. ATP can be programmed to facilitate shock-free termination of VT and is effective in approximately three-quarters of MMVT episodes.(35) ATP can be potentially optimized to improve MMVT termination by:
Increasing the number of cycles of ATP therapies
Increasing the number of bursts per ATP cycle
Decreasing the ATP cycle length (i.e., a lower percentage of VT cycle length)
Decreasing the ATP coupling interval with every subsequent burst (“scan” programming)
Progressively decreasing the R-R interval during an individual ATP delivery (“ramp” programming)
Device proarrhythmia
While an ICD can effectively treat VA, it can also be proarrhythmic; the same can be true of cardiac resynchronization therapy (CRT).(36,37) There is an inverse relationship between the aggressiveness and safety of ATP. More aggressive ATP therapies (shorter coupling intervals, ramp ATP, more ATP attempts, more bursts per attempt) can be more effective at VT termination but may risk accelerating a well-tolerated VT or degenerate VT into VF. Randomized studies have not shown a consistent difference between the proarrhythmic effects of burst versus ramp ATP therapies.(38) Acceleration of VT is more likely with shorter or variable cycle lengths of VT and less likely in the presence of AADs.(36) Low energy ICD shocks can potentially lead to VT acceleration or degeneration to VF, making it important to program back-up high-energy ICD shocks following ATP or low-energy ICD shocks.(36) For patients with ES occurring shortly after upgrade to CRT, turning off the LV lead or changing to backup VVI pacing mode may be appropriate.(37)
Device implantation or upgrade
ES survivors without an ICD should generally receive a secondary-prevention ICD during hospitalization, with an upgrade to CRT if indicated.(1,2,37) Placing an ICD during ES can provoke multiple ICD shocks, so this is typically considered only at the time of hospital discharge. ICD implantation is contraindicated for incessant VA or when the patient has advanced HF, unless they are being bridged to transplant or LVAD.(1,2) Complete deactivation of ICD therapies is justified for patients pursuing a palliative care approach.
Acute medical management
General principles
ES spans a spectrum of acuity and associated risk, necessitating a flexible management strategy tailored to the severity of the presentation. The core medical management elements of ES include 1) membrane active AADs, 2) adrenergic blockade, 3) sedation, and 4) hemodynamic support, with individual treatments ranging in potential efficacy, invasiveness, and risk of complications (Figure 4). Recommendations for the management of ES from the recent ESC guidelines are summarized in Table 2.(2)
Table 2:
ESC Guideline recommendations for electrical storm.2
| Class I recommendations |
| Mild to moderate sedation is recommended in patients with electrical storm to alleviate psychological distress and reduce sympathetic tone (LOE: C) |
| Antiarrhythmic therapy with beta-blockers (non-selective preferred) in combination with intravenous amiodarone is recommended in patients with structural heart disease and electrical storm unless contraindicated (LOE: B) |
| Intravenous magnesium with supplementation of potassium is recommended in patients with TdP (LOE: C) |
| Isoproterenol or transvenous pacing to increase heart rate is recommended in patients with acquired long QT syndrome and recurrent TdP despite correction of precipitating conditions and magnesium (LOE: C) |
| Catheter ablation is recommended in patients presenting with incessant VT or electrical storm due to MMVT refractory to AADs (LOE: B) |
| Class IIa recommendations |
| Deep sedation/intubation should be considered in patients with an intractable electrical storm refractory to drug treatment (LOE: C) |
| Catheter ablation should be considered in patients with recurrent episodes of PMVT/VF triggered by a similar PVC, non-responsive to medical treatment or coronary revascularization (LOE: C) |
| Class IIb recommendations |
| Quinidine may be considered in patients with CAD and electrical storm due to recurrent PMVT when other AAD therapy fails (LOE: C) |
| Autonomic modulation may be considered in patients with electrical storm refractory to drug treatment and in whom catheter ablation is ineffective or not possible (LOE: C) |
| Institution of mechanical circulatory support may be considered in the management of drug-refractory electrical storm and cardiogenic shock (LOE: C) |
Abbreviations: AAD, antiarrhythmic drug; CAD, coronary artery disease; LOE, level of evidence; MMVT, monomorphic ventricular tachycardia; PMVT, polymorphic ventricular tachycardia; PVC, premature ventricular contraction; TdP, torsades des pointes; VF, ventricular fibrillation; VT, ventricular tachycardia.
The most effective intervention for acute termination of VA is synchronized electrical cardioversion (for MMVT) or defibrillation (for PMVT or VF), either externally or using an existing ICD (with or without first attempting ATP).(1,2) Immediate electrical cardioversion or defibrillation is always preferred for patients with hemodynamically unstable VA and is appropriate for hemodynamically stable VT when the risk of sedation is low.(1,2)
Membrane-active anti-arrhythmic drugs
Membrane-active AADs targeting cardiomyocyte ion channels have a central role in the management of ES to achieve chemical cardioversion, to facilitate the success of electrical cardioversion or ATP, or to reduce the risk of VA recurrence after cardioversion.(1,2) AADs used for ES include class I AADs (i.e., lidocaine and procainamide) that terminate VT by reducing electrical excitability and slowing conduction and class III AADs (i.e., sotalol and amiodarone) that terminate VT by prolonging the refractory period and inhibiting reentry.(1,2)
Intravenous (IV) AADs indicated for acute termination of VT include amiodarone, lidocaine, procainamide, and sotalol, each having important strengths and limitations.(1,2) Based on small head-to-head comparison studies in relatively hemodynamically stable patients, the acute efficacy for VT termination appears to be greatest for procainamide, intermediate for amiodarone and sotalol, and lowest for lidocaine.(1,39–45) Due to its higher acute efficacy, procainamide carries a class IIa recommendation for acute termination of VT (particularly hemodynamically stable VT), compared with a class IIb recommendation for amiodarone or sotalol.(1,2) However, procainamide is contraindicated in severe structural heart disease, decompensated HF, acute MI, and advanced kidney disease, all of which are common in ES populations. Therefore, IV amiodarone is generally preferred in patients with ES, particularly for facilitating ATP or electrical cardioversion and for preventing recurrent VT.(2) After acute termination of VT, it is logical to continue an AAD that was effective for chemical cardioversion in either IV or oral form to prevent recurrence. AADs can cause dose- and infusion rate-dependent hypotension via vasodilation from alpha-1 blockade (procainamide and amiodarone), negative inotropy via beta-1 blockade (sotalol and amiodarone) and other mechanisms (procainamide and lidocaine); the incidence of hypotension appears lowest with lidocaine.(39–45)
To prevent recurrent VT, IV and oral AADs can be utilized separately or together. Amiodarone IV is recommended as the first line AAD in patients with ES based on its greater efficacy for preventing recurrent VT, including efficacy for suppressing VT that occurs despite other AADs.(2,39–41,46) The anti-arrhythmic efficacy and receptor-binding profile of amiodarone differs with IV and oral administration, and oral loading with accumulation of an active metabolite increases its efficacy.(47) When chronic amiodarone is appropriate for preventing recurrent VT, IV amiodarone is typically continued during initial oral amiodarone loading (e.g., 800–1600 mg/day up to a total of 10–20 g) until the patient has been free from VA for ≥48 hours.(1,2,47) Sotalol can occasionally be substituted when amiodarone is not desired due to concerns about long-term toxicity (e.g., younger patients who are naïve to AADs).(2,46) To avoid proarrhythmia, sotalol and procainamide should only be considered when the baseline QT interval is not prolonged, serum potassium and magnesium are normal, kidney function is not severely impaired, and the patient is not concomitantly receiving QT prolonging drugs (e.g., amiodarone).
When amiodarone is ineffective as monotherapy or for higher-risk presentations, lidocaine is often added as a second line AAD to suppress VA during further amiodarone loading; lidocaine is more effective in ischemic myocardium. Lidocaine is well tolerated and can be safely combined with QT-prolonging drugs, but can accumulate during decompensated HF or CS, as is common in ES. Procainamide is usually a third line AAD in ES due to its potential toxicity, risk of accumulation of the active QT-prolonging metabolite N-acetylprocainamide (NAPA) with kidney dysfunction, and lack of an oral equivalent.(44) Due to similar ion channel effects (including additive QT prolongation), amiodarone is typically discontinued when procainamide is added; however, adding procainamide at a low dose to ongoing amiodarone can be considered in selected patients with close monitoring. Serum drug concentration monitoring is necessary for patients receiving lidocaine or procainamide, along with QTc monitoring for patients receiving QT-prolonging AADs.
When transitioning off IV lidocaine, oral mexiletine is often added to amiodarone. Ranolazine is an anti-ischemic drug possessing anti-arrhythmic effects that appears effective as add-on therapy for refractory VA in case series; however, ranolazine was not effective for prevention of ICD shocks in a randomized trial, so the role of ranolazine in ES remains uncertain.(1,48,49) Intravenous magnesium sulfate is recommended for TdP, even when the serum magnesium level is normal. For bradycardia-dependent TdP, increasing the heart rate via transvenous pacing or isoproterenol is indicated.(1,2) Quinidine blocks the transient outward potassium current (ITO) and may be effective for suppressing VA in Brugada syndrome and other inherited arrhythmia syndromes, as well as for selected patients with VA that are refractory to other AADs.(1,2,24)
Adrenergic blockade
Recurrent VT in ES is often promoted by stimulation of cardiac beta-adrenergic receptors, and beta-adrenergic blockade is a crucial component of ES management (especially in the setting of acute myocardial ischemia).(1,2,16,27) Sotalol and amiodarone (particularly IV amiodarone) have beta-blocking properties, but adding another beta-blocker can enhance their efficacy.(39,40,46) Adding or up-titrating a GDMT beta-blocker (e.g. metoprolol succinate, bisoprolol, carvedilol) can be considered, although the alpha-1 blockade produced by carvedilol often causes dose-limiting hypotension.(1,50,51) While the beta-1 blocker metoprolol tartrate can be initiated and rapidly titrated for patients who are beta-blocker naïve, nonselective beta-1/2 blockers (e.g., propranolol) are preferred during ES.(1,2) Propranolol should be considered for patients with ES, either as initial therapy or when a beta-1 blocker is ineffective.(2,52,53) Propranolol (160 mg/day) displayed better efficacy than metoprolol tartrate (200 mg/day) in preventing recurrent VT during ES in a randomized controlled trial of 60 ICD patients receiving IV amiodarone, including higher freedom from VA at 24 hours (47% versus 10%).(52) Theoretical advantages of propranolol over metoprolol include blockade of both beta-1 and beta-2 adrenergic receptors, strong central nervous system penetration which may reduce sympathetic outflow, more comprehensive beta-receptor inhibition via inverse agonism, and mild sodium channel blockade at very high doses.(53) When rapid initiation of beta-blockade is needed, both metoprolol and propranolol are available in IV formulations, recognizing that these IV formulations are very potent (particularly propranolol). Alternatively, esmolol and landiolol are ultrashort-acting IV beta-1 blockers that can be added to oral beta-blockers as a second-tier therapy in ES, having the advantage of rapid onset and easy uptitration with quick offset in case of hypotension.(2,27,54) The cardiac antiarrhythmic effects of esmolol have faster onset and offset than the vascular hypotensive effects, which may result in delayed hypotension.(54)
When beta-blockers are ineffective or not tolerated, ablation of cardiac sympathetic innervation via percutaneous cervical sympathetic (stellate) ganglion blockade is potentially beneficial.(2,27,55,56) In 30 patients with medically-refractory ES who underwent stellate ganglion blockade, 60% were free from VA after 24 hours.(56) With appropriate training, stellate ganglion blockade can be performed quickly and easily at bedside by providers with expertise in ultrasound-guided jugular venous access.(55) Most of the cardiac sympathetic innervation comes from the left stellate ganglion, so left-sided stellate ganglion blockade is performed first, with bilateral stellate ganglion blockade reserved for intubated patients due to the potential risk of phrenic nerve paresis that could compromise respiration.(55) Surgical cardiac sympathetic denervation may be considered for refractory ES in patients who respond favorably to stellate ganglion blockade.(1)
Sedation
Sedation during ES is an extension of adrenergic blockade that reduces central sympathetic outflow.(2) The heightened risk of further VT triggered by anxiety and post-traumatic stress can be mitigated by appropriate sedation/anxiolysis.(2,33) A first-line sedation strategy includes oral or IV benzodiazepines for anxiolysis and to induce amnesia surrounding cardioversion or ICD shocks. Dexmedetomidine is a second-line sedative medication that exerts specific anti-adrenergic effects by reducing central sympathetic outflow via alpha-2 receptor activation, potentially resulting in a reduced risk of tachyarrhythmias.(57) Low dose dexmedetomidine can be used in spontaneously breathing patients without causing respiratory depression. For severe or refractory ES, endotracheal intubation with general anesthesia is justified, but can worsen hemodynamic instability.(2,58,59) This approach may prevent recurrent VT and mitigate the traumatic experience of repeated defibrillations. When ES results in recurrent cardiac arrest, this also ensures a secured airway. If general anesthesia is necessary, initiating dexmedetomidine prior to attempts at awakening reduces the risk of rebound sympathetic activation.
Hemodynamic support
Medical treatments for ES can trigger or aggravate hemodynamic instability via vasodilatory, negative inotropic, and negative chronotropic effects. This often causes hypotension, and susceptible patients with advanced HF may develop a low-output HF state or overt CS. GDMT often must be held or reduced in the acute phase of ES due to dose-limiting hypotension from beta-blockade and AAD therapy. Fluid resuscitation should be performed cautiously, as ES patients are often volume overloaded and this may promote recurrent VA. Most vasopressors and inotropes have proarrhythmic effects mediated by direct activation of beta-adrenergic receptors or augmentation of their downstream second messenger systems.(60) Inotropes and vasopressors should be avoided or used at the minimum dose that restores organ perfusion. Pure vasoconstrictors such as phenylephrine or vasopressin can reverse drug-induced peripheral vasodilation, and may be anti-arrhythmic by promoting central sympathetic withdrawal via the baroreflex.(60) Pure vasoconstrictors reduce cardiac output and should be avoided in low-output states or CS (particularly when the serum lactate is elevated). Norepinephrine has better hemodynamic efficacy and can be substituted despite a slight risk of proarrhythmia.(60)
When vasopressor and inotropic therapy are either ineffective for restoring hemodynamic stability or result in proarrhythmia, temporary mechanical circulatory support (MCS) should be considered.(61) An intra-aortic balloon pump (IABP) can provide mild augmentation of arterial pressure and cardiac output with a limited risk of complications, but provides minimal support during VT.(61) While the IABP has not been shown to improve outcomes in patients with CS, patients with low-output HF due to chronic cardiomyopathy may have a favorable hemodynamic response.(61) Percutaneous VADs (pVADs) such as the Impella (Abiomed, Danvers, MA) or TandemHeart (Livanova, London, UK) provide more robust hemodynamic support than the IABP but produce a higher risk of complications.(61) While these devices can provide hemodynamic support during VT, this can be limited by RV dysfunction. Each support modality has advantages and disadvantages related to use during ES or catheter ablation (Table 3). One important limitation of the transvalvular Impella pump is the need for the device to remain in the left ventricle, which itself can be proarrhythmic (particularly during a suction event due to low preload). When higher-level hemodynamic support is needed in ES (with or without CS), VA ECMO can entirely replace the native cardiac output even during VF and cardiac arrest.(62) Among patients receiving VA ECMO, those with ES generally have better outcomes due to their reversible etiology and limited end-organ failure. Any decision regarding the use of temporary MCS, particularly VA ECMO, should occur in the context of the reversibility of the patient’s hemodynamic compromise, treatability of their ES, and candidacy for advanced HF therapies.
Table 3:
MCS support modalities and their role in catheter ablation for ES.
| Modality | Function | Advantages | Disadvantages |
|---|---|---|---|
|
| |||
| IABP | Inflation in diastole increases coronary perfusion; deflation during systole reduces afterload | Easy to place and widely available Lower risk of complications Unloads LV by reducing afterload |
Primarily effective in sinus rhythm Ineffective at higher heart rates or with non-sinus rhythms Contraindicated in patients with AI Modest hemodynamic support (increase in cardiac output & MAP) |
| Impella | A continuous-flow pump placed across the AV provides LV unloading and augments cardiac output | Relatively easy to place and widely available Significant increase in cardiac output: 3 L/min (CP) or 5 L/min (5.5) No reliance on sinus rhythm Directly unloads LV |
Requires surgical cut down (5.5) Contraindicated in AS or mechanical AV (must cross aortic valve) Crowded LVOT limits retro-aortic approach to ablation Can cause ventricular ectopy & EMI Does not provide RV support without use of a second device |
| TandemHeart | Arterial bypass system with transseptal LA access and external pump | Significant reduction in cardiac preload and workload Full cardiac output support Addition of oxygenation circuit is possible No reliance on sinus rhythm |
Not widely available Requires transseptal access (leaving a residual ASD) Large arterial and venous sheaths with risk of vascular complications Risk of LA thrombus and hemolysis Does not provide RV support without use of a second device |
| ECMO | Portable complete cardiopulmonary bypass | Full biventricular cardiac and pulmonary support No reliance on sinus rhythm Can be placed at bedside without fluoroscopy Can use either transseptal or retrograde aortic approach |
Large arterial and venous sheaths with risk of vascular complications Thromboembolic and bleeding risks Standard configurations increase left ventricular afterload |
AI, aortic insufficiency; AS, aortic stenosis; ASD, atrial septal defect; AV, aortic valve; DBP, diastolic blood pressure; ECMO, extracorporeal membrane oxygenation; EMI, electromagnetic interference; IABP, intraaortic balloon pump; LA, left atrium; LV, left ventricle; LVOT, left ventricular outflow tract; MAP, mean arterial pressure; RV, right ventricular.
Stepped-care algorithm
A stepped-care approach to ES management can match the initial intensity of the four central management components to the acuity and risk of the presentation and then escalate proportionately in case of recurrent VA (Figure 4). This ensures that the strength of intervention (and potential risk of complications) matches the clinical situation. In this paradigm, a low-risk ES patient (e.g., hemodynamically stable MMVT, functioning ICD) starts at step 1 for each of the medical management components, with standard initial therapies including IV amiodarone monotherapy plus an oral beta-blocker and an oral benzodiazepine.(2) A higher-risk ES patient (e.g., no ICD, hemodynamically unstable VA) starts at step 2 with standard add-on therapies, including a second AAD (typically lidocaine), esmolol, and dexmedetomidine, in addition to step 1 initial therapies. Patients with recurrent VA escalate to the next higher step, and rescue therapies that can be added include stellate ganglion block, third line AADs (e.g., procainamide), general anesthesia, and escalating degrees of hemodynamic support. The threshold for escalation should be lower for low-risk interventions (e.g., dexmedetomidine or stellate ganglion block) versus high-risk interventions (e.g., general anesthesia or VA ECMO). It remains unclear whether all ES patients should initially receive combination AAD therapy, propranolol, esmolol, and/or stellate ganglion block as a first-line approach.
The role of catheter ablation in ventricular arrhythmias and electrical storm
Consideration of catheter ablation is recommended for patients with sustained VA refractory to medical therapy, making ablation an important long-term strategy for preventing ES recurrence.(1,2,63) Randomized trials, consisting primarily of stable patients with ICM and MMVT, demonstrate a benefit of catheter ablation over medical therapy for prevention of VT recurrence and ICD shocks, without a clear survival benefit; catheter ablation can also be effective in NICM.(64–67) In the Ventricular Tachycardia Ablation versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease (VANISH) trial, ablation was superior to escalation of AADs (increased amiodarone dosing +/− mexiletine), but the composite incidence of death, ES and appropriate ICD shocks remained frequent (59.1% versus 68.5%).(66,68) Approximately one-fourth of patients in the VANISH trial presented with ES, and this subgroup appeared to derive similar benefit from catheter ablation over intensification of AAD therapy.(69) Catheter ablation carries a class I indication in patients with ICM and ES with failure or intolerance of AADs and a class IIa indication for patients with refractory VA and NICM.(1,2,63)
Observational studies report improved outcomes after catheter ablation compared with medical therapy in ES cohorts; however, these are limited by small size and potential selection bias.(70) In a large modern multicenter series of catheter ablation in ES, elimination of clinical VT was demonstrated in 87%, and 64% had complete non-inducibility of VT at the end of the procedure.(71) Recurrence of VT and one-year mortality after catheter ablation were higher in those with ES, highlighting the severe underlying illness in this population and substantial risks even among survivors.(71) Elimination of clinical VT and post-procedural non-inducibility after catheter ablation predict lower VT recurrence, lower ES recurrence, and higher survival rates.(70–73) Risk factors for VT recurrence after ablation include lower LVEF, previous ablation, and presence of an ICD.(12)
Patient selection and pre-procedural evaluation
In ES patients with structural heart disease and MMVT without reversible causes, catheter ablation during the index hospitalization should be strongly considered, and early catheter ablation can acutely stabilize incessant VA. Important pre-procedural considerations include the following (Figure 5):
Figure 5:
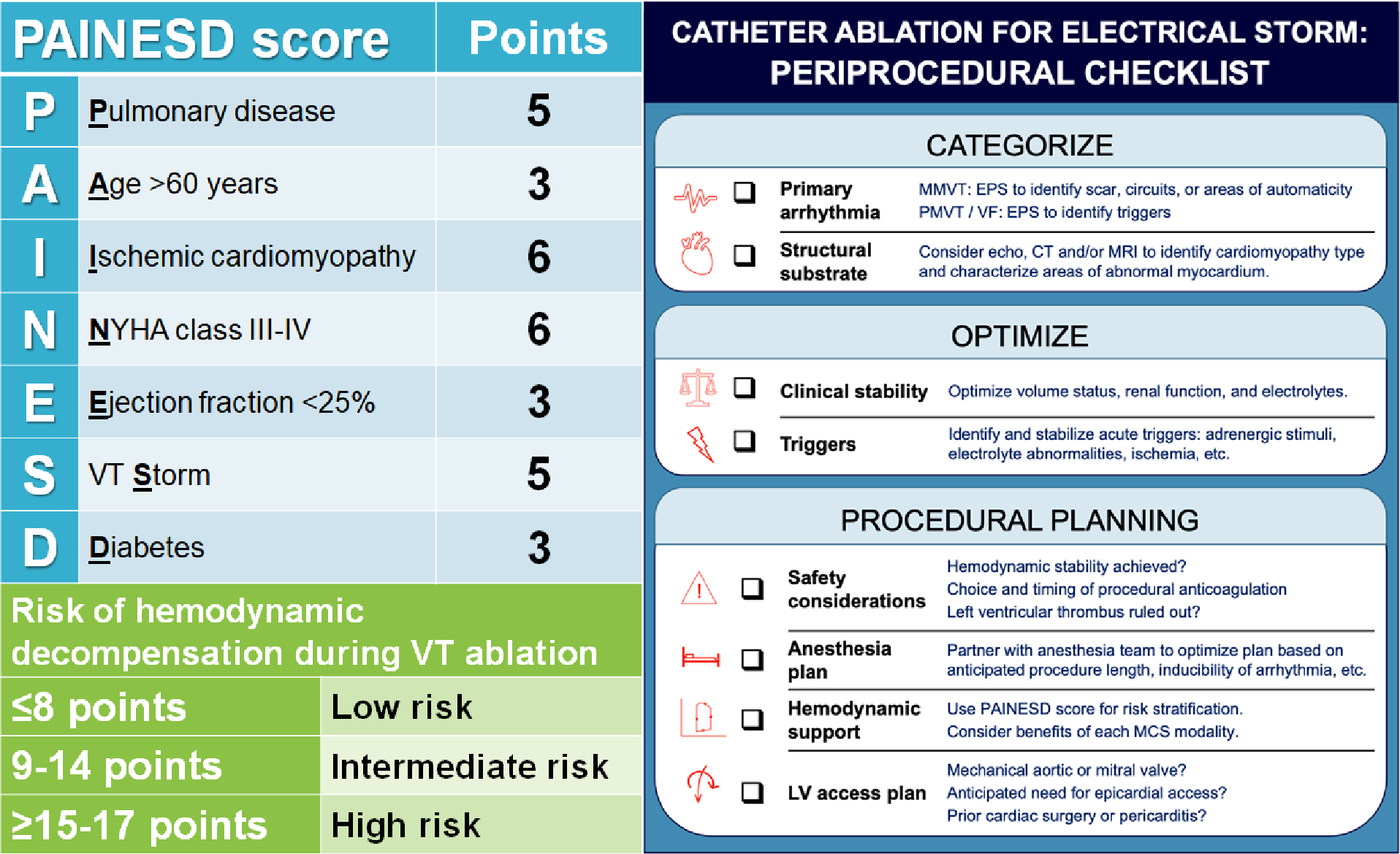
Risk stratification using the PAINESD score (left) and procedural checklist (right) for planning prior to catheter ablation.
1). Hemodynamic stabilization.
Catheter ablation involves moderate sedation or general anesthesia and repeated induction of VT for mapping purposes, which can lead to hemodynamic decompensation even with previously tolerated VT. Pre-procedure, patients should receive acute stabilization with vasoactive medications and/or MCS, as well as risk stratification for intraprocedural hemodynamic decompensation.(74)
2). Addressing triggers.
In patients with identified triggers, initial efforts should focus on correcting these factors.(2) In such patients, catheter ablation may still be beneficial for identification and elimination of the underlying VT substrate; however, mapping and assessment of procedural success will be more straightforward without residual triggers.
3). Substrate.
VT circuits are often easier to localize in ICM than NICM.(75) Though observational studies in VT ablation suggest higher procedural failure, VT recurrence, and mortality in NICM compared to ICM, smaller studies focused on ES report similar short- and intermediate-term outcomes after ablation in ICM and NICM populations.(73,76) Successful catheter ablation has been reported after ES in arrhythmogenic cardiomyopathy, cardiac sarcoidosis and Chagas disease; epicardial ablation is often required in these patients.(18–20,75,77) Patients with cardiac sarcoidosis historically have higher VA recurrence and worse long-term outcomes than other patients with NICM.(77) Successful catheter ablation has been reported targeting RVOT substrate in Brugada syndrome and VF-triggering PVCs in early repolarization syndrome and idiopathic VF.(23,25,75,78)
Catheter Ablation Strategies in ES
Mapping and ablation for VT
VT ablation in structural heart disease is performed using a combination of two primary strategies. Activation mapping involves definition of the full activation circuit during VT, often with the aid of entrainment mapping, followed by ablation targeting the critical isthmus.(15,75,78) Substrate modification includes delineation of scar borders and all potential critical channels through scar while in sinus rhythm, followed by ablation aimed at eliminating identified channels and abnormal signals within the scar.(15) The major limitation of activation mapping is the need to induce and maintain VT for the duration of mapping, leading to potential hemodynamic compromise. Limitations of substrate modification include a longer procedure, less certainty regarding ablation targets, and potential for proarrhythmic effect if incomplete ablation is performed within heterogenous scar tissue. Substrate modification in addition to activation-based ablation was shown to be superior to activation mapping alone for preventing VT recurrence, though data are limited comparing these approaches in ES.(79)
Mapping and ablation for VF
VF is not characterized by a consistent reentrant circuit, but VF driver activity at scar border zones may be mapped and targeted for ablation.(15,78) Additionally, triggering PVCs from the outflow tract, papillary muscles, and/or Purkinje system may be reproducibly identified and targeted for ablation with success rates exceeding 80%.(15,72,75,78) Catheter ablation of PVC triggers in patients with PMVT or VF refractory to medical therapy or coronary revascularization carries a Class IIa indication.(2)
Measures of procedural success
Benchmarks for procedural success in VT ablation include termination of VT during ablation, non-inducibility of VA, elimination of all abnormal signals within scar, or elimination of the triggering PVCs.(63–66) A crucial challenge is the presence of multiple clinical or inducible VT morphologies, as often occurs in advanced cardiomyopathy. Up to 35% of the ES population may be too unstable to perform post-procedural programmed stimulation, and recurrence is high in those with residual inducibility of VT.(71)
Mechanical circulatory support for catheter ablation in ES
Peri-ablation acute hemodynamic decompensation is associated with higher likelihood of procedural failure, more VT recurrence, and increased in-hospital and long-term mortality.(74,80,81) Interventions to minimize harm related to the catheter ablation procedure include pre-procedural optimization of hemodynamic status and end-organ function, avoidance of general anesthesia if possible, careful attention to intraprocedural hemodynamic status and fluid balance, and choosing substrate modification over VT induction in higher-risk patients. MCS can be valuable for providing hemodynamic support during procedural sedation and while mapping unstable VTs, particularly in patients with prior failed substrate-based ablation and/or extensive scar with anticipated long duration of ablation. However, the potential for complications necessitates careful patient selection.(62,82)
MCS during catheter ablation is used more frequently for ES patients, and in-hospital and short-term mortality remains high among ES patients requiring MCS.(62,71,80,82) Use of MCS improves hemodynamic stability during catheter ablation, but has not been shown to reduce VT recurrence or improve long-term outcomes, with similar findings in ES cohorts.(70,80,82) Small propensity matched analyses showed lower risk of hemodynamic decompensation, higher likelihood of post-procedure non-inducibility, and lower mortality with up-front MCS compared to rescue or no MCS, although outcomes are mixed.(74,80,82)
The PAINESD score (Figure 5) is a risk-stratification tool developed to predict acute hemodynamic decompensation during VT ablation and post-procedural mortality; ES is one of the risk factors.(81–83) A PAINESD score of ≥17 (≥15 when general anesthesia is excluded as a risk factor) is associated with higher hemodynamic risk and greater need for MCS, and has been proposed as a criterion that can be used to select patients for pre-emptive MCS during ablation.(81–83) When using MCS to support ablation, pre-emptive initiation prior to the procedure should be considered to avoid acute hemodynamic decompensation and the need for bailout MCS.(62,74,82,83)
Advanced heart failure evaluation
The development of VA and ES may be a symptom of worsening cardiomyopathy heralding the transition to advanced HF.(3,4,13,14,29,72) Patients with advanced HF and ES should be considered for advanced HF therapies, such as heart transplantation or durable LVAD.(29,51) The decision to list a patient for heart transplantation or proceed with LVAD depends on the patient’s probability of recovery, suitability for transplant, degree of hemodynamic compromise and anticipated waiting time for transplantation. Decision-making is challenging in the setting of ES, when a patient may not have the severe ventricular dysfunction typical of advanced HF and might have myocardial recovery if VA are suppressed. In most cases, heart transplantation is preferred for advanced HF patients with refractory VA, and LVAD is reserved for those patients who are not considered favorable candidates for transplantation or who are too unstable to survive until transplantation.
ES is rarely the sole reason for heart transplantation, with refractory VA historically accounting for fewer than 1% of adult heart transplants according to United Network for Organ Sharing (UNOS).(84) In October 2018, UNOS approved a new heart allocation system which prioritizes patients with refractory VA.(85) Under the revised UNOS allocation, patients with MCS and life-threatening VA are prioritized as a Status 1 (the highest priority) and those with life-threatening VA without MCS can be listed as Status 2. This may facilitate urgent heart transplantation for patients with ES and truly refractory VA.
Patient selection for LVAD in the setting of refractory VA requires a thoughtful approach, as the arrhythmogenic myocardial substrate can persist after LVAD implantation and VA after LVAD can predispose to RV dysfunction and adverse outcomes.(29–32) Patients with a history of VA before LVAD implantation are at the highest risk of recurrent VA following LVAD implantation, but otherwise may have comparable 1-year survival when stratified by INTERMACS profile.(32,86) Ablation at the time of LVAD implantation can be considered for selected patients.(87)
Given the high short-term risk of death and poor long-term outcomes among patients who survive ES, timely palliative care consultation during hospitalization is important to establish overall goals of care. Among the sizeable group of ES patients with advanced HF, comparatively few will receive heart transplant or LVAD, and hospice care may be an appropriate option. Ongoing palliative care follow-up after discharge can be beneficial in patients with advanced HF, and should be considered for ES survivors with HF.(51)
Management of patients after recovery from ES
Antiarrhythmic drugs
Oral amiodarone is recommended for long-term management in patients with ES due to MMVT or repeated ICD discharges with a low VA burden, following ICD optimization.(2) Higher doses of amiodarone may be needed chronically to suppress VT, recognizing the greater toxicity and potential excess risk of non-cardiac death in patients with more severe HF.(14,47) If amiodarone is not desirable or tolerated, then guidelines recommend alternate AAD therapy according to underlying disease and cardiac function.(2) Addition of mexiletine and/or ranolazine to oral amiodarone can be considered, although efficacy evidence is limited and ablation is generally superior.(48,49,68,88) AADs are usually continued in patients with a successful ablation due to the substantial risk of recurrent VA. Patients with an unsuccessful ablation or those who are not candidates for ablation often receive combination AAD therapy including amiodarone, but rates of recurrent VA are high (exceeding 40%).(49,66,68,71,74,80) Cardiac stereotactic body radiotherapy may be an alternative for highly selected patients with refractory VA who fail or are not candidates for repeat catheter ablation.(89) Recurrent ES is common, occurring in up to one-third to one-half of ES patients and being more common in those with lower LVEF, older age and not receiving angiotensin-converting enzyme (ACE) inhibitors.(3,6,71–73)
Guideline-directed medical therapy
Optimization of GDMT is an important step after recovery for ES patients with underlying cardiomyopathy.(51) Reinstitution of GDMT prior to hospital discharge is essential given the elevated risk of death due to HF in patients with VA and the recognition of inadequate GDMT as a risk factor for ES.(4–6,13,14,51,72) While ACE inhibitors, angiotensin receptor blockers, and angiotensin-neprilysin inhibitors do not have a definite effect on VA, aldosterone antagonists (and perhaps SGLT2 inhibitors) do appear to reduce the risk of SCD and it is essential to initiate all indicated GDMT classes in patients with cardiomyopathy.(51) Digoxin may be proarrhythmic and is often discontinued. A crucial unanswered question is whether patients with reduced LVEF who receive propranolol during ES should be switched to a GDMT beta-blocker, recognizing that propranolol has not been studied for HF with reduced LVEF.(2,51) It seems prudent to ensure that a patient remains free from VA before transitioning between beta-blockers.
Follow-up
ES survivors remain at substantial risk of VA, HF, and other adverse events, justifying close multidisciplinary follow-up after hospital discharge.(9,10,72,73) Frequent contact with a cardiologist, particularly a heart rhythm specialist, is warranted. Remote ICD monitoring can alert clinicians to recurrent VA, including asymptomatic nonsustained or ICD-treated events. Diligent monitoring for noncardiac toxicities is necessary for patients receiving chronic amiodarone.(47) Crucially, most ES survivors should be considered for follow-up with an advanced HF expert, particularly if they may be a candidate for advanced HF therapies.
Conclusion
ES is a true heart rhythm emergency with a high risk of morbidity and mortality necessitating CICU admission. ES management requires an integrated multidisciplinary team, including providers with expertise in critical care cardiology, heart rhythm, and advanced HF. ES typically develops in patients with cardiomyopathy and may be a manifestation of cardiac deterioration reflecting a transition to advanced HF that can require heart transplantation or LVAD. Most ES patients have a pre-existing ICD for primary or secondary prevention, and diligent ICD programming is beneficial. Suppression of arrhythmias in ES patients integrates membrane active AADs (typically amiodarone), adrenergic blockade, and sedation/anxiolysis tailored to the severity of the clinical presentation in a stepped-care paradigm. Early use of propranolol and stellate ganglion blockade may be beneficial. Many patients require catheter ablation to resolve ES and reduce the risk of further VA. Hemodynamic compromise is common, potentially requiring hemodynamic support before, during, and after catheter ablation. Optimization of GDMT during hospitalization is essential along with close multidisciplinary follow-up, as many ES survivors will develop complications from progressive HF. Collaborative multicenter clinical trials are needed to define best practices for ES patients, recognizing the wide spectrum of severity that can manifest.
Source of funding:
No extramural funding was directly involved in the conduct of this research. PAN receives research funding from National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI, R21AG 62580-1, R01HL 131535-4, R01HL 143070-2] the National Institute on Aging [NIA, R01AG 062436-1]), Agency for Healthcare Research and Quality (AHRQ, R01HS 25402-3), Food and Drug Administration (FDA, FD 06292), and the American Heart Association (18SFRN34230146, AHA). AHK is supported by NIH T32 HL007111 and the Department of Cardiovascular Medicine at Mayo Clinic in Rochester, MN. JET is supported by the National Heart, Lung and Blood Institute, the American Heart Association, and the Indiana Clinical & Translational Sciences Institute.
Footnotes
Disclosure: MAS receives research support from the National Institutes of Health Clinical Center intramural research funds. PAN and Mayo Clinic have filed patents related to the application of AI to the ECG for diagnosis and risk stratification and have licensed several A-ECG algorithms to Anumana. PAN and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. PAN is a study investigator in an ablation trial sponsored by Medtronic. PAN has served on an expert advisory panel for OptumLabs. The other authors have no relevant disclosures or conflicts of interest.
Sponsored by the American College of Cardiology Critical Care Cardiology and Electrophysiology Sections
References
- 1.Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 2.Zeppenfeld K, Tfelt-Hansen J, de Riva M et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 3.Bansch D, Bocker D, Brunn J, Weber M, Breithardt G, Block M. Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol 2000;36:566–73. [DOI] [PubMed] [Google Scholar]
- 4.Exner DV, Pinski SL, Wyse DG et al. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation 2001;103:2066–71. [DOI] [PubMed] [Google Scholar]
- 5.Arya A, Haghjoo M, Dehghani MR et al. Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator. Am J Cardiol 2006;97:389–92. [DOI] [PubMed] [Google Scholar]
- 6.Streitner F, Kuschyk J, Veltmann C et al. Predictors of electrical storm recurrences in patients with implantable cardioverter-defibrillators. Europace 2011;13:668–74. [DOI] [PubMed] [Google Scholar]
- 7.Sesselberg HW, Moss AJ, McNitt S et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm 2007;4:1395–402. [DOI] [PubMed] [Google Scholar]
- 8.Guerra F, Palmisano P, Dell’Era G et al. Implantable cardioverter-defibrillator programming and electrical storm: Results of the OBSERVational registry On long-term outcome of ICD patients (OBSERVO-ICD). Heart Rhythm 2016;13:1987–92. [DOI] [PubMed] [Google Scholar]
- 9.Guerra F, Shkoza M, Scappini L, Flori M, Capucci A. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace 2014;16:347–53. [DOI] [PubMed] [Google Scholar]
- 10.Noda T, Kurita T, Nitta T et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol 2018;255:85–91. [DOI] [PubMed] [Google Scholar]
- 11.Brigadeau F, Kouakam C, Klug D et al. Clinical predictors and prognostic significance of electrical storm in patients with implantable cardioverter defibrillators. Eur Heart J 2006;27:700–7. [DOI] [PubMed] [Google Scholar]
- 12.Vergara P, Tzou WS, Tung R et al. Predictive Score for Identifying Survival and Recurrence Risk Profiles in Patients Undergoing Ventricular Tachycardia Ablation: The I-VT Score. Circ Arrhythm Electrophysiol 2018;11:e006730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole JE, Johnson GW, Hellkamp AS et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer DL, Prutkin JM, Hellkamp AS et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation 2009;120:2170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dukkipati SR, Koruth JS, Choudry S, Miller MA, Whang W, Reddy VY. Catheter Ablation of Ventricular Tachycardia in Structural Heart Disease: Indications, Strategies, and Outcomes-Part II. J Am Coll Cardiol 2017;70:2924–2941. [DOI] [PubMed] [Google Scholar]
- 16.Cauti FM, Rossi P, Sommer P. The sympathetic nervous system and ventricular arrhythmias: an inseparable union. Eur Heart J 2021;42:3588–3590. [DOI] [PubMed] [Google Scholar]
- 17.Zhai L, Hu Y, Li X et al. Incidence, Predictors and Clinical Impact of Ventricular Electrical Storm in Arrhythmogenic Cardiomyopathy Patients with an Implantable Cardioverter-Defibrillator: A Single-Center Report with Medium-Term Follow-Up. Int J Gen Med 2021;14:10055–10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birnie DH, Sauer WH, Bogun F et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305–23. [DOI] [PubMed] [Google Scholar]
- 19.Romero J, Velasco A, Pisani CF et al. Advanced Therapies for Ventricular Arrhythmias in Patients With Chagasic Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;77:1225–1242. [DOI] [PubMed] [Google Scholar]
- 20.Laredo M, Oliveira Da Silva L, Extramiana F et al. Catheter ablation of electrical storm in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2020;17:41–48. [DOI] [PubMed] [Google Scholar]
- 21.Montero S, Aissaoui N, Tadie JM et al. Fulminant giant-cell myocarditis on mechanical circulatory support: Management and outcomes of a French multicentre cohort. Int J Cardiol 2018;253:105–112. [DOI] [PubMed] [Google Scholar]
- 22.Kociol RD, Cooper LT, Fang JC et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation 2020;141:e69–e92. [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Wilde AA, Horie M et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 24.Napolitano C, Bloise R, Monteforte N, Priori SG. Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation. Circulation 2012;125:2027–34. [DOI] [PubMed] [Google Scholar]
- 25.Cheniti G, Vlachos K, Meo M et al. Mapping and Ablation of Idiopathic Ventricular Fibrillation. Front Cardiovasc Med 2018;5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg C, Davies B, Mellor G et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: a report from the CASPER registry. Eur Heart J 2021;42:2827–2838. [DOI] [PubMed] [Google Scholar]
- 27.Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm : sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 2000;102:742–7. [DOI] [PubMed] [Google Scholar]
- 28.Jentzer JC, Chonde MD, Dezfulian C. Myocardial Dysfunction and Shock after Cardiac Arrest. Biomed Research International 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of Ventricular Arrhythmias in Patients With Advanced Heart Failure. J Am Coll Cardiol 2017;69:1842–1860. [DOI] [PubMed] [Google Scholar]
- 30.Gordon JS, Maynes EJ, Choi JH et al. Ventricular arrhythmias following continuous-flow left ventricular assist device implantation: A systematic review. Artif Organs 2020;44:E313–E325. [DOI] [PubMed] [Google Scholar]
- 31.Rehorn MR, Black-Maier E, Loungani R et al. Electrical storm in patients with left ventricular assist devices: Risk factors, incidence, and impact on survival. Heart Rhythm 2021;18:1263–1271. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A, Amin M, Boilson BA, Killu AM, Madhavan M. Ventricular Arrhythmias in Patients With Left Ventricular Assist Device (LVAD). Curr Treat Options Cardiovasc Med 2019;21:75. [DOI] [PubMed] [Google Scholar]
- 33.Sears SF, Hauf JD, Kirian K, Hazelton G, Conti JB. Posttraumatic stress and the implantable cardioverter-defibrillator patient: what the electrophysiologist needs to know. Circ Arrhythm Electrophysiol 2011;4:242–50. [DOI] [PubMed] [Google Scholar]
- 34.Cheng A, Auricchio A, Schloss EJ et al. SVT discrimination algorithms significantly reduce the rate of inappropriate therapy in the setting of modern-day delayed high-rate detection programming. J Cardiovasc Electrophysiol 2019;30:2877–2884. [DOI] [PubMed] [Google Scholar]
- 35.Cheng A, Joung B, Brown ML et al. Characteristics of ventricular tachyarrhythmias and their susceptibility to antitachycardia pacing termination in patients with ischemic and nonischemic cardiomyopathy: A patient-level meta-analysis of three large clinical trials. J Cardiovasc Electrophysiol 2020;31:2720–2726. [DOI] [PubMed] [Google Scholar]
- 36.Pinski SL, Fahy GJ. The proarrhythmic potential of implantable cardioverter-defibrillators. Circulation 1995;92:1651–64. [DOI] [PubMed] [Google Scholar]
- 37.Deif B, Ballantyne B, Almehmadi F et al. Cardiac resynchronization is pro-arrhythmic in the absence of reverse ventricular remodelling: a systematic review and meta-analysis. Cardiovasc Res 2018;114:1435–1444. [DOI] [PubMed] [Google Scholar]
- 38.Calkins H, el-Atassi R, Kalbfleisch S, Langberg J, Morady F. Comparison of fixed burst versus decremental burst pacing for termination of ventricular tachycardia. Pacing Clin Electrophysiol 1993;16:26–32. [DOI] [PubMed] [Google Scholar]
- 39.Kowey PR, Levine JH, Herre JM et al. Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. The Intravenous Amiodarone Multicenter Investigators Group. Circulation 1995;92:3255–63. [DOI] [PubMed] [Google Scholar]
- 40.Scheinman MM, Levine JH, Cannom DS et al. Dose-ranging study of intravenous amiodarone in patients with life-threatening ventricular tachyarrhythmias. The Intravenous Amiodarone Multicenter Investigators Group. Circulation 1995;92:3264–72. [DOI] [PubMed] [Google Scholar]
- 41.Levine JH, Massumi A, Scheinman MM et al. Intravenous amiodarone for recurrent sustained hypotensive ventricular tachyarrhythmias. Intravenous Amiodarone Multicenter Trial Group. J Am Coll Cardiol 1996;27:67–75. [DOI] [PubMed] [Google Scholar]
- 42.Gorgels AP, van den Dool A, Hofs A et al. Comparison of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Am J Cardiol 1996;78:43–6. [DOI] [PubMed] [Google Scholar]
- 43.Somberg JC, Bailin SJ, Haffajee CI et al. Intravenous lidocaine versus intravenous amiodarone (in a new aqueous formulation) for incessant ventricular tachycardia. Am J Cardiol 2002;90:853–9. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz M, Martin A, Arribas F et al. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J 2017;38:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho DS, Zecchin RP, Richards DA, Uther JB, Ross DL. Double-blind trial of lignocaine versus sotalol for acute termination of spontaneous sustained ventricular tachycardia. Lancet 1994;344:18–23. [DOI] [PubMed] [Google Scholar]
- 46.Connolly SJ, Dorian P, Roberts RS et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA 2006;295:165–71. [DOI] [PubMed] [Google Scholar]
- 47.Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA 2007;298:1312–22. [DOI] [PubMed] [Google Scholar]
- 48.Gupta T, Khera S, Kolte D, Aronow WS, Iwai S. Antiarrhythmic properties of ranolazine: A review of the current evidence. Int J Cardiol 2015;187:66–74. [DOI] [PubMed] [Google Scholar]
- 49.Zareba W, Daubert JP, Beck CA et al. Ranolazine in High-Risk Patients With Implanted Cardioverter-Defibrillators: The RAID Trial. J Am Coll Cardiol 2018;72:636–645. [DOI] [PubMed] [Google Scholar]
- 50.Ruwald AC, Gislason GH, Vinther M et al. Importance of beta-blocker dose in prevention of ventricular tachyarrhythmias, heart failure hospitalizations, and death in primary prevention implantable cardioverter-defibrillator recipients: a Danish nationwide cohort study. Europace 2018;20:f217–f224. [DOI] [PubMed] [Google Scholar]
- 51.Heidenreich PA, Bozkurt B, Aguilar D et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263–e421. [DOI] [PubMed] [Google Scholar]
- 52.Chatzidou S, Kontogiannis C, Tsilimigras DI et al. Propranolol Versus Metoprolol for Treatment of Electrical Storm in Patients With Implantable Cardioverter-Defibrillator. J Am Coll Cardiol 2018;71:1897–1906. [DOI] [PubMed] [Google Scholar]
- 53.Chen PS, Doytchinova A. Why Is Propranolol Better Than Metoprolol in Acute Treatment of Electrical Storm? J Am Coll Cardiol 2018;71:1907–1909. [DOI] [PubMed] [Google Scholar]
- 54.Garnock-Jones KP. Esmolol: a review of its use in the short-term treatment of tachyarrhythmias and the short-term control of tachycardia and hypertension. Drugs 2012;72:109–32. [DOI] [PubMed] [Google Scholar]
- 55.Wittwer ED, Radosevich MA, Ritter M, Cha YM. Stellate Ganglion Blockade for Refractory Ventricular Arrhythmias: Implications of Ultrasound-Guided Technique and Review of the Evidence. J Cardiothorac Vasc Anesth 2020;34:2245–2252. [DOI] [PubMed] [Google Scholar]
- 56.Tian Y, Wittwer ED, Kapa S et al. Effective Use of Percutaneous Stellate Ganglion Blockade in Patients With Electrical Storm. Circ Arrhythm Electrophysiol 2019;12:e007118. [DOI] [PubMed] [Google Scholar]
- 57.Zhong Q, Kumar A, Deshmukh A, Bennett C. Dexmedetomidine Reduces Incidences of Ventricular Arrhythmias in Adult Patients: A Meta-Analysis. Cardiol Res Pract 2022;2022:5158362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bundgaard JS, Jacobsen PK, Grand J et al. Deep sedation as temporary bridge to definitive treatment of ventricular arrhythmia storm. Eur Heart J Acute Cardiovasc Care 2020;9:657–664. [DOI] [PubMed] [Google Scholar]
- 59.Martins RP, Urien JM, Barbarot N et al. Effectiveness of Deep Sedation for Patients With Intractable Electrical Storm Refractory to Antiarrhythmic Drugs. Circulation 2020;142:1599–1601. [DOI] [PubMed] [Google Scholar]
- 60.Jentzer JC, Hollenberg SM. Vasopressor and Inotrope Therapy in Cardiac Critical Care. J Intensive Care Med 2021;36:843–856. [DOI] [PubMed] [Google Scholar]
- 61.Rihal CS, Naidu SS, Givertz MM et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 2015;65:e7–e26. [DOI] [PubMed] [Google Scholar]
- 62.Vallabhajosyula S, Vallabhajosyula S, Vaidya VR et al. Venoarterial Extracorporeal Membrane Oxygenation Support for Ventricular Tachycardia Ablation: A Systematic Review. ASAIO J 2020;66:980–985. [DOI] [PubMed] [Google Scholar]
- 63.Cronin EM, Bogun FM, Maury P et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020;17:e2–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reddy VY, Reynolds MR, Neuzil P et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuck KH, Schaumann A, Eckardt L et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40. [DOI] [PubMed] [Google Scholar]
- 66.Sapp JL, Wells GA, Parkash R et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111–21. [DOI] [PubMed] [Google Scholar]
- 67.Tung R, Xue Y, Chen M et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent With Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation 2022;145:1839–1849. [DOI] [PubMed] [Google Scholar]
- 68.Deyell MW, Steinberg C, Doucette S et al. Mexiletine or catheter ablation after amiodarone failure in the VANISH trial. J Cardiovasc Electrophysiol 2018;29:603–608. [DOI] [PubMed] [Google Scholar]
- 69.Deyell MW, Doucette S, Parkash R et al. Ventricular tachycardia characteristics and outcomes with catheter ablation vs. antiarrhythmic therapy: insights from the VANISH trial. Europace 2022;24:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jimenez Candil J, Castro JC, Hernandez J et al. Timing of Ablation and Prognosis of Patients With Electrical Storm and Scar-Related Left Ventricular Dysfunction. Am J Cardiol 2020;136:87–93. [DOI] [PubMed] [Google Scholar]
- 71.Vergara P, Tung R, Vaseghi M et al. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm 2018;15:48–55. [DOI] [PubMed] [Google Scholar]
- 72.Nayyar S, Ganesan AN, Brooks AG, Sullivan T, Roberts-Thomson KC, Sanders P. Venturing into ventricular arrhythmia storm: a systematic review and meta-analysis. Eur Heart J 2013;34:560–71. [DOI] [PubMed] [Google Scholar]
- 73.Muser D, Liang JJ, Pathak RK et al. Long-Term Outcomes of Catheter Ablation of Electrical Storm in Nonischemic Dilated Cardiomyopathy Compared With Ischemic Cardiomyopathy. JACC Clin Electrophysiol 2017;3:767–778. [DOI] [PubMed] [Google Scholar]
- 74.Muser D, Liang JJ, Castro SA et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: A propensity-score matched analysis. Heart Rhythm 2018;15:1500–1506. [DOI] [PubMed] [Google Scholar]
- 75.Bhaskaran A, Tung R, Stevenson WG, Kumar S. Catheter Ablation of VT in Non-Ischaemic Cardiomyopathies: Endocardial, Epicardial and Intramural Approaches. Heart Lung Circ 2019;28:84–101. [DOI] [PubMed] [Google Scholar]
- 76.Anderson RD, Ariyarathna N, Lee G et al. Catheter ablation versus medical therapy for treatment of ventricular tachycardia associated with structural heart disease: Systematic review and meta-analysis of randomized controlled trials and comparison with observational studies. Heart Rhythm 2019;16:1484–1491. [DOI] [PubMed] [Google Scholar]
- 77.Siontis KC, Santangeli P, Muser D et al. Outcomes Associated With Catheter Ablation of Ventricular Tachycardia in Patients With Cardiac Sarcoidosis. JAMA Cardiol 2022;7:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dukkipati SR, Choudry S, Koruth JS, Miller MA, Whang W, Reddy VY. Catheter Ablation of Ventricular Tachycardia in Structurally Normal Hearts: Indications, Strategies, and Outcomes-Part I. J Am Coll Cardiol 2017;70:2909–2923. [DOI] [PubMed] [Google Scholar]
- 79.Di Biase L, Burkhardt JD, Lakkireddy D et al. Ablation of Stable VTs Versus Substrate Ablation in Ischemic Cardiomyopathy: The VISTA Randomized Multicenter Trial. J Am Coll Cardiol 2015;66:2872–2882. [DOI] [PubMed] [Google Scholar]
- 80.Turagam MK, Vuddanda V, Atkins D et al. Hemodynamic Support in Ventricular Tachycardia Ablation: An International VT Ablation Center Collaborative Group Study. JACC Clin Electrophysiol 2017;3:1534–1543. [DOI] [PubMed] [Google Scholar]
- 81.Santangeli P, Frankel DS, Tung R et al. Early Mortality After Catheter Ablation of Ventricular Tachycardia in Patients With Structural Heart Disease. J Am Coll Cardiol 2017;69:2105–2115. [DOI] [PubMed] [Google Scholar]
- 82.Mariani S, Napp LC, Lo Coco V et al. Mechanical circulatory support for life-threatening arrhythmia: A systematic review. Int J Cardiol 2020;308:42–49. [DOI] [PubMed] [Google Scholar]
- 83.Pothineni NVK, Enriquez A, Kumareswaran R et al. Outcomes of a PAINESD score-guided multidisciplinary management approach for patients with ventricular tachycardia storm and advanced heart failure: A pilot study. Heart Rhythm 2022. [DOI] [PubMed] [Google Scholar]
- 84.Olivari MT, Windle JR. Cardiac transplantation in patients with refractory ventricular arrhythmias. J Heart Lung Transplant 2000;19:S38–42. [DOI] [PubMed] [Google Scholar]
- 85.Kilic A, Mathier MA, Hickey GW et al. Evolving Trends in Adult Heart Transplant With the 2018 Heart Allocation Policy Change. JAMA Cardiol 2021;6:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cowger J, Shah P, Stulak J et al. INTERMACS profiles and modifiers: Heterogeneity of patient classification and the impact of modifiers on predicting patient outcome. J Heart Lung Transplant 2016;35:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tankut S, Gosev I, Yoruk A et al. Intraoperative Ventricular Tachycardia Ablation During Left Ventricular Assist Device Implantation in High-Risk Heart Failure Patients. Circ Arrhythm Electrophysiol 2022;15:e010660. [DOI] [PubMed] [Google Scholar]
- 88.Gao D, Van Herendael H, Alshengeiti L et al. Mexiletine as an adjunctive therapy to amiodarone reduces the frequency of ventricular tachyarrhythmia events in patients with an implantable defibrillator. J Cardiovasc Pharmacol 2013;62:199–204. [DOI] [PubMed] [Google Scholar]
- 89.Krug D, Blanck O, Andratschke N et al. Recommendations regarding cardiac stereotactic body radiotherapy for treatment refractory ventricular tachycardia. Heart Rhythm 2021;18:2137–2145. [DOI] [PubMed] [Google Scholar]


