Abstract
BACKGROUND AND AIMS:
The 13C-methacetin breath test (MBT) is a noninvasive, quantitative hepatic metabolic function test. The aim of this prospective, multicenter study was to determine the utility of initial and serial 13C-MBT in predicting 21-day outcomes in adults with acute liver failure (ALF) and non-acetaminophen acute liver injury (ALI).
APPROACH AND RESULTS:
The 13C-MBT BreathID device (Exalenz Biosciences, Ltd.) provided the percent dose recovery (PDR) for a duration of 60 minutes after administration of 13C-methacetin solution as the change in exhaled 13CO2/12CO2 compared with pre-ingestion ratio on study days 1, 2, 3, 5, and 7. Results were correlated with 21-day transplant-free survival and other prognostic indices. A total of 280 subjects were screened for enrollment between May 2016 and August 2019. Median age of the 62 enrolled patients with adequate data was 43 years, 79% were Caucasian, 76% had ALF with the remaining 24% having ALI. The mean PDR peak on day 1 or day 2 was significantly lower in nonsurvivors compared with transplant-free survivors (2.3%/hour vs. 9.1%/hour; P < 0.0001). In addition, serial PDR peaks were consistently lower in nonsurvivors versus survivors (P < 0.0001). The area under the receiver operating characteristic curve (AUROC) of the 13C-MBT in the combined cohort was 0.88 (95% CI: 0.79-0.97) and higher than that provided by King’s College (AUROC = 0.70) and Model for End-Stage Liver Disease scores (AUROC = 0.83). The 13C-MBT was well tolerated with only two gastrointestinal adverse events reported.
CONCLUSIONS:
The 13C-MBT is a promising tool to estimate the likelihood of hepatic recovery in patients with ALF and ALI. Use of the PDR peak data from the 13C-MBT point-of-care test may assist with medical decision making and help avoid unnecessary transplantation in critically ill patients with ALF and ALI. (Hepatology 2021;74:961-972).
Acute liver failure (ALF) is a rare but potentially devastating syndrome defined by the sudden onset of coagulopathy (i.e., an international normalized ratio [INR] of ≥ 1.5) and hepatic encephalopathy in a patient without known pre-existing liver disease.(1) Although a multitude of disease processes can lead to severe acute liver injury (ALI) and ALF, there are only about 3,000 cases of ALF each year in the United States.(2) Recent studies have demonstrated the importance of ALF etiology in distinguishing favorable short-term prognosis with a 50%-70% transplant free survival (TFS) (hepatitis A, acetaminophen [APAP] overdose, and ischemia) from conditions with an unfavorable prognosis (idiosyncratic DILI, autoimmune, hepatitis B), with a 10%-30% TFS.(2,3) Although emergency liver transplantation (LT) is associated with excellent 1-year survival, the ongoing shortage of donor organs preclude many patients with ALF from undergoing life-saving LT. Conversely, reliable identification of patients destined to recover with their native liver is essential to avoid unnecessary LT. Therefore, an accurate and reliable bedside test to identify patients with ALF who will recover with supportive care from those who will die without LT remains a critical unmet need. Although many studies have attempted to identify features of ALF that predict outcome, all suffer from relatively poor diagnostic accuracy.(3)
The Acute Liver Failure Study Group (ALFSG) is a consortium of North American academic medical centers that has prospectively studied the etiologies and outcomes of adults with ALF since 1998.(4) In addition, a prospective registry of adults with severe ALI, defined by an INR ≥ 2.0, serum alanine aminotransferase (ALT) ≥ 10 × upper limit of normal (ULN), and bilirubin ≥ 3 mg/dL, was established in 2010.(5) Over the years, the ALFSG has conducted clinical trials in an effort to improve TFS in both patients with both ALF and ALI.(6,7) The 13C-methacetin breath test (13C-MBT) is a semi-quantitative, noninvasive hepatic metabolic function test that assays the biotransformation of 75 mg of 13C-labeled methacetin by hepatic cytochrome-P450 1A2 (CYP1A2) to 13CO2 and APAP.(8) The 13C-MBT has been shown to be more accurate than the Model for End-Stage Liver Disease (MELD) score in predicting decompensation and survival in patients with cirrhosis undergoing LT evaluation.(9) The 13C-MBT may also be useful in patients with severe acute liver disease, but the number of patients studied has been limited.(10) The aim of the current study is to demonstrate the potential utility of the initial and serial 13C-MBT measurements as prognostic tools in adult subjects with ALF and severe non-APAP ALI who were followed until death, LT, or discharge from the hospital 21 days after enrollment.
Materials and Methods
OBJECTIVE
The predefined primary study outcome was used to assess the relationship between the day 1 (initial) percent dose recovery (PDR) rate, known as PDR peak, which reflects the maximum rate of metabolism of 13C-methacetin measured as the change in 13CO2/12CO2 ratio after ingestion of a 75-mg dose of 13C-methacetin normalized using the patient’s height and weight, and TFS at day 21 in eligible adult patients with ALF and non-APAP ALI. Our research hypothesis was that the initial PDR peak values would be significantly higher in patients who survived compared with those who died or underwent LT by day 21. The secondary outcomes in this study were to determine the optimal cutoff point for the PDR peak that best distinguishes between outcomes (TFS vs. death/LT). Other exploratory secondary outcomes included the change in 13C-MBT measurements over a maximum of 7 days from enrollment, to assess the relationship between single time points of 13C-MBT measurements and outcome. Finally, we set out to compare the PDR peak as a prognostic tool for predicting day 21 TFS in conjunction with other clinical parameters such as etiology, laboratory measurements, and other published prognostic indices.
STUDY POPULATION
The study protocol was approved by local institutional review boards (IRBs) of participating sites. An Investigational Device Exemption (IDE) application with the US Food and Drug Administration was approved (IDE #G150226) in November 2015. Written, informed consent to participate was obtained from the patient or a legally authorized representative before enrollment per local regulations. The study was initiated in May 2016 in patients with ALF between the ages of 18 and 70 years with any degree of encephalopathy and an INR ≥ 1.5 caused by an illness of <24-week duration. Initially, exclusion criteria included consumption of a multitude of medications thought to potentially interfere with the metabolism of 13C-methacetin (see Supporting Table S1).(9) After further consideration of these exclusions, the eligibility criteria were modified in March 2018, to increase the upper age range to 80 years and allow for use of famotidine or acyclovir within 48 hours of enrollment as well as use of statins in the 30 days before enrollment. Eligibility criteria were also widened to include patients with severe non-APAP ALI, as these patients have been shown to have a 41% risk of progressing to ALF or dying within 21 days, with a similar proportion dying or undergoing LT as those with ALF.(5) In contrast, patients with APAP-ALI remained excluded due to very low rates of progression to ALF (4%). These changes in eligibility criteria were approved by all IRBs and regulatory authorities.
STUDY DESIGN
Testing involved the administration of a ready-to-use solution of a 75-mg dose of 13C-methacetin mixed into 150 mL of water followed by retrieval of expired 13CO2 and 12CO2 over a period of 1 hour after substrate administration.(9) A maximum of five tests were administered on study days 1, 2, 3, 5, and 7. Subjects were required to have been fasting from solid food for a minimum of 6 hours, and a nasoenteric tube feeding had to be held for a minimum of 4 hours, before substrate administration. In addition, other medications could not be given in the hour before test substrate administration. The solution of 13C-methacetin was swallowed by nonintubated patients who were upright and cooperative; substrate was otherwise administered via nasoenteric tube to other participants. Exhaled breath was collected at the bedside for a total of 75 minutes, including up to 15 minutes for baseline determination and 60 minutes of 13CO2/12CO2 ratio collection following administration of 13C-methacetin using a nasal cannula for conscious patients or a ventilator hose adaptor for the endotracheal tube of intubated patients connected to the BreathID MCS device (Exalenz Biosciences Ltd., Modiin, Israel). During the breath collection period, all other intensive care unit (ICU) care including continuous renal replacement, intravenous medications, and other treatments were continued. Investigators were blinded to the MBT results to avoid bias in patient management. Calculation of the PDR peak, cumulative PDR at 20 minutes (cPDR20), and other MBT parameters were performed at the data coordinating center, independent of the sponsor and site investigators. The investigational substrate was stored locally at room temperature.
Previous studies have demonstrated that the intertest coefficient of variation of the 13C-MBT in 53 healthy volunteers and patients with liver disease is 13.2% (95% CI: 11.1%-15.2%) for the PDR peak, and the coefficient of variation is 23.9% (95% CI: 20.3%-27.4%) for the cPDR20.(10,11)
SAFETY ASSESSMENTS
All serious adverse events (SAEs) were collected from day 1 through day 21 of the study and graded per standard protocol; relatedness to the test device was determined by the local investigator. An independent medical safety monitor reviewed each SAE in real time for expectedness and relatedness. All nonserious adverse events were collected from day 1 to day 7. An independent Data and Safety Monitoring Board reviewed aggregate data throughout the study period. Because the primary product of the demethylation of methacetin is acetaminophen, serum APAP-cysteine adducts testing was performed in the first 20 study participants before and after each 13C-MBT test administration. Serum APAP-cysteine adducts were measured in the research lab of Dr. Laura James at the University of Arkansas using a previously described HPLC method.(12) The detection of a serum APAP-cysteine adduct in non-APAP-induced patients with ALI/ALF was considered significant if a value of >1.0 nmol/L was detected. In subjects with presumed APAP hepatotoxicity, an increase of serum APAP-cysteine adduct levels above their initial value was assessed in conjunction with serum liver biochemistries. An independent group of experienced clinical investigators reviewed the adduct data as they became available, to ensure that the small dose of APAP per test administration would not result in additional liver injury. After reviewing the adduct test results from 20 consecutively evaluable patients in whom no values >1.0 nmol/L were observed, the independent group concluded that there were no safety concerns, and further testing for APAP-cysteine adducts was halted.
SAMPLE SIZE
Up to 200 evaluable patients meeting the eligibility criteria were to be consecutively enrolled at the 11 sites of the ALFSG participating in this study. An evaluable patient is one who completed one or more 13C-MBT tests measured for a minimum of 30 minutes after ingestion of 13C-methacetin.
STATISTICAL ANALYSIS
A futility analysis was planned after 100 consecutive enrollments to consider stopping the study early if there were no signal differences in the initial PDR peak distribution between those who reached 21-day TFS and those who died or underwent LT by day 21. The final analysis was based on a comparison of the initial mean PDR peak between the TFS and non-TFS outcome groups using a two-sided significance level of 0.10. Planned exploratory analyses for hypothesis generation included subgroup analyses, examination of the cumulative cPDR20, and the comparison of the initial PDR peak to the currently available ALFSG Prognostic Index for predicting 21-day TFS. All statistical analyses are two-sided tests and were conducted using SAS V9.3 or higher (SAS Institute, Inc., Cary, NC).
Results
STUDY POPULATION
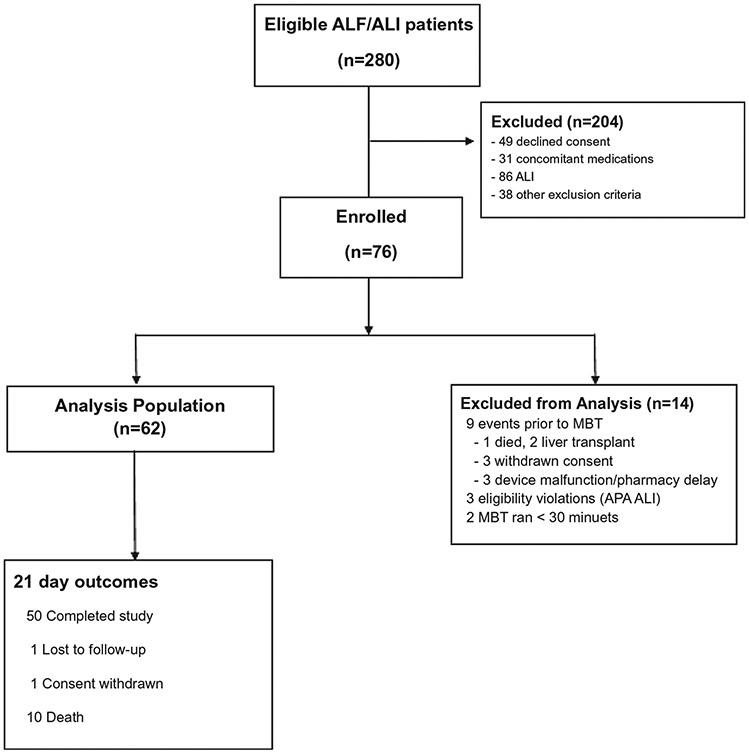
Between May 2016 and August 2019, 280 patients were screened for eligibility and 76 were enrolled into the study (Fig. 1). A total of 9 subjects never received an 13C-MBT due to death (1), LT (2), withdrawal of consent (3), and device malfunction/pharmacy delay.(3) In addition, 3 subjects had an eligibility violation of APAP-ALI, and 2 subjects had nonevaluable data (<30 minutes duration of the 13C-MBT), leaving a total of 62 subjects with adequate 13C-MBT data for analysis.
FIG. 1.
Overview of eligible and enrolled patients. A total of 280 patients with ALF and ALI were screened for the study and 76 were enrolled. The analysis population included 62 patients with available 13C-MBT results and 21-day outcome, of whom 56 had a day 1 or day 2 13C-MBT test result for efficacy analysis.
Demographics of the study population are depicted in Table 1. The median age of the 62 subjects was 43 years (range: 18 to 73), 61% were female, 79% were Caucasian, 76% had ALF due to a multitude of etiologies, and 24% had non-APAP ALI. At enrollment into the study, 42% had grade 3 or 4 hepatic encephalopathy with a median bilirubin of 7.2 mg/dL (range: 0.6 to 36) and a median INR of 2.7 (range: 1.4 to 10.7). In addition, 34% of the study population were intubated and 21% were receiving renal replacement therapy.
TABLE 1.
Baseline Characteristics of the patient population
| Overall Group | ALF Group | ALI Group | |
|---|---|---|---|
| Parameter | n = 62 | n = 47 | n = 15 |
| Median age (years) | 43 (18, 73) | 40 (18, 73) | 50 (25, 66) |
| Female (%) | 61% | 70% | 33% |
| Race/ethnicity (%) | |||
| White | 79% | 79% | 80% |
| Black | 18% | 19% | 13% |
| Asian | 3% | 2% | 7% |
| Hispanic | 7% | 6% | 7% |
| Etiology (%) | |||
| APAP | 42% | 55% | 0% |
| Hepatitis A | 11% | 2% | 40% |
| DILI | 11% | 9% | 20% |
| Hepatitis B | 8% | 6% | 13% |
| Autoimmune | 5% | 4% | 7% |
| Shock* | 5% | 4% | 7% |
| Indeterminate | 5% | 4% | 7% |
| Pregnancy | 2% | 2% | 0% |
| Wilson disease | 2% | 0% | 7% |
| Other virus | 2% | 2% | 0% |
| Other | 8% | 11% | 0% |
| Median entry labs | |||
| ALT (U/L) | 2,738 (141, 15,817) | 2,860 (141, 15,817) | 1,702(578, 8631) |
| Bilirubin (mg/dL) | 7.1 (0.6, 36.0) | 5.5 (0.6, 36.0) | 12.4 (4.3, 21.5) |
| INR | 2.7 (1.4, 10.7) | 2.9 (1.4, 10.7) | 2.4 (2.0, 5.0) |
| Creatinine (mg/dL) | 0.9 (0.4, 7.7) | 1.2 (0.4, 7.7) | 0.7 (0.5, 5.9) |
| MELD | 30.0 (14, 47) | 31.0 (14, 47) | 26.0 (20, 39) |
| Complications at entry | |||
| Renal replacement | 21% | 28% | 0% |
| Intubated | 34% | 45% | 0% |
| Vasopressors | 18% | 21% | 7% |
| NAC (%) | 90% | 96% | 73% |
| Grade ½ HE | 34% | 45% | 0% |
| Grade ¾ HE | 42% | 55% | 0% |
| Ever listed for LT | 32% | 34% | 27% |
| First 21-day outcome | |||
| LT† | 21% | 23% | 13% |
| Death | 15% | 20% | 0% |
| TFS | 61% | 55% | 80% |
| Unknown‡ | 3% | 2% | 7% |
Shock was an allowable etiology if not severe at study entry (i.e., on no pressors [n = 2] or one pressor [n = 1]). Data are presented as median (range).
One subject died following transplant; data reported as median (range).
Two unknown 21-day outcomes due to consent withdrawal and lost to follow-up.
Abbreviations: HE, hepatic encephalopathy; NAC, N-acetylcysteine.
PDR PEAK AND CLINICAL OUTCOME
The primary outcome assessment was the comparison of the day 1 PDR peak between those that had 21-day TFS versus those who died or underwent LT before day 21. A total of 37 (59.6%) subjects had the initial 13C-MBT conducted on day 1 and an additional 21 subjects had the initial test on day 2, while the 4 remaining subjects had the first study after day 2. The reasons for a delay in testing included the need for other clinical procedures and the subject not having fasted for the required time period. Of the 58 who had 13C-MBT data on day 1 or 2, 2 had missing outcome data, which is how we came to 56 evaluable patients in Table 2.
TABLE 2.
Maximal PDR Peak and cPDR20 by 21-Day Outcomes
| Outcome | ||
|---|---|---|
| 21-Day TFS† | 21-Day Nonsurvivor† |
|
| Primary outcome* | n = 23 | n = 12 |
| Day 1 mean PDR peak (%/hour [SD]) | 10.2 (7.8) | 1.9 (0.6) |
| Difference in means (90% CI) | 8.3 (4.4, 12.1) | |
| P value | <0.0001 | |
| Secondary outcomes* | n = 35 | n = 21 |
| Day 1 or 2 mean PDR peak (%/hour [SD]) | 9.1 (6.8) | 2.3 (0.9) |
| Difference in means (90% CI) | 6.8 (4.8, 8.8) | |
| P value | <0.0001 | |
| Day 1 or 2 mean cPDR20 (%/hour [SD]) | 1.4 (1.3) | 0.2 (.2) |
| Difference in means (90% CI) | 1.2 (0.8, 1.6) | |
| P value | <0.0001 | |
Two participants missing outcome due to consent withdrawal and lost to follow-up.
Data reported as mean (SD).
P value is based on a two-sided Student t test at an alpha level of 0.10.
As per Table 2, the mean PDR peak on day 1 was significantly lower in 21-day nonsurvivors compared with transplant-free survivors (1.9%/hour [SD: 0.6] vs. 10.2%/hour [SD: 7.8]; P < 0.0001). Expanding the PDR peak values to include either day 1 or day 2 showed similar results (9.1%/hour [SD: 6.8] vs. 2.3%/ hour [SD: 0.9]; P < 0.0001). When we evaluated the 21 patients with APAP overdose, the day 1 or 2 PDR peak was significantly higher in the 21-day TFS group compared with the nonsurvivors (7.9%/hour vs. 2.6%/hour; P = 0.001), as well as in the 35 patients with non-APAP ALI/ ALF (10.1%/hour vs. 2.2 %/ hour; P = 0.0003). When the cPDR20 was compared between the two outcome groups, similar differences in the mean values were observed (data not shown).
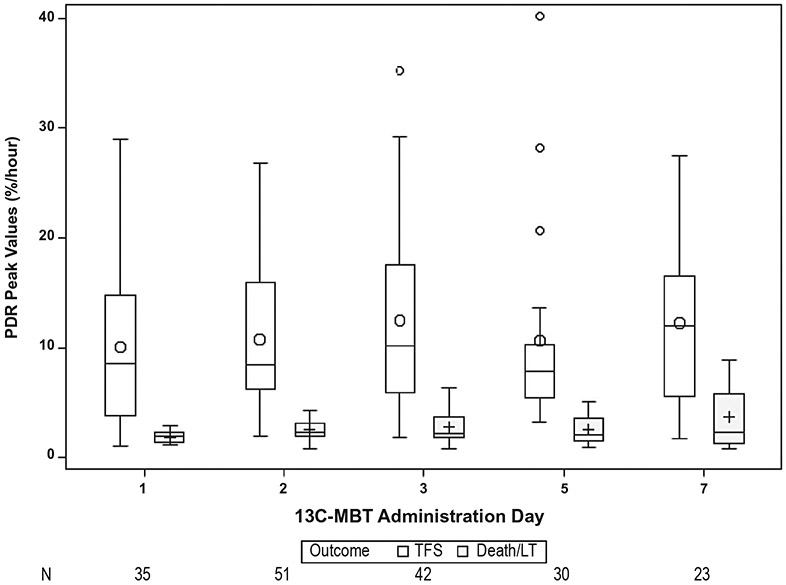
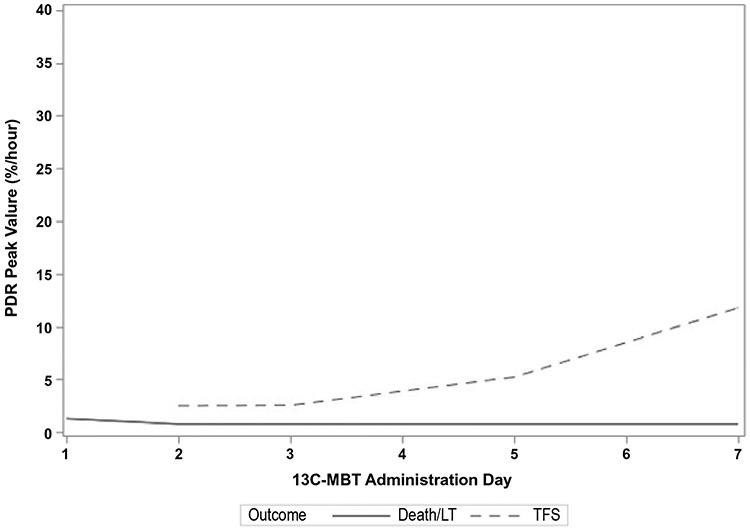
Assessment of serial PDR peak values revealed that the PDR peaks were consistently higher in transplant-free survivors versus nonsurvivors (Fig. 2). This difference remained significant over time (P < 0.001). An example of the serial PDR peak values of an idiosyncratic DILI patient who survived and another DILI patient who required LT can be seen in Fig. 3.
FIG. 2.
PDR peak by outcome and 13C-MBT administration day. The PDR peak values were significantly lower in the nonsurvivors compared with the transplant-free survivors (P < 0.001, repeated-measures analysis). Circles indicate means; boxes indicate interquartile ranges; horizontal line within the boxes indicate medians; bars indicate 1.5 times the interquartile range; and plus signs indicate outliers.
FIG. 3.
Serial 13C-MBT results in a DILI patient with ALF patient who survived and a DILI patient with ALI who required LT. (A) A 52-year-old female with presumed DILI due to tizanidine and ropinirole was enrolled with a MELD score of 26 and a total bilirubin of 9.3 mg/dL, INR of 2.8, and grade 3 hepatic encephalopathy. Her ALFSG prognostic score was 7.7% likelihood of 21-day TFS. She received intravenous N-acetylcysteine on day 1 and was on famotidine starting on day 2, but never on pressors nor intubated. Over time her clinical status improved and by day 7 her hepatic encephalopathy grade was 1 and she was discharged to go home at day 14. Her 13C-MBT results improved from day 2 through day 7. (B) A 53-year-old female with DILI due to clindamycin was enrolled with ALI and no encephalopathy. During follow-up, she rapidly deteriorated and was listed for LT. Her initial MELD score was 30. She was never on pressors but was intubated on day 6. She eventually underwent LT on study day 7 and was discharged to go home on day 20. Her 13C-MBT results demonstrated a very low PDR peak that never improved.
TFS IN THE COMBINED COHORT OF PATIENTS WITH ALI AND ALF
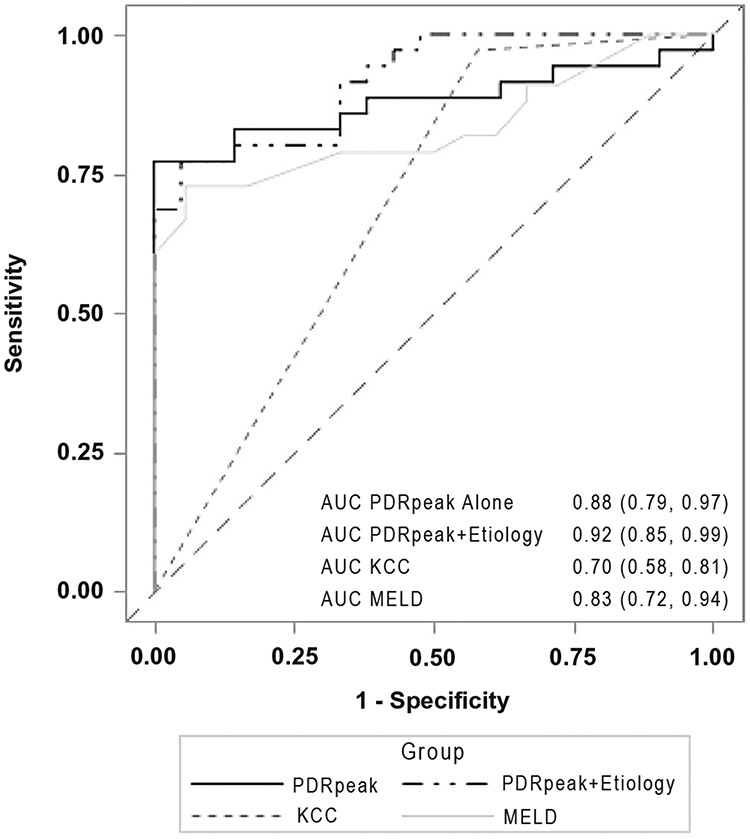
In the 56 patients with ALF and ALI with evaluable day 1 or 2 PDR peak values, the area under the receiver operator characteristics curve (AUROC) was 0.88 (95% CI: 0.79, 0.97). The addition of favorable etiology, defined as hepatitis A, APAP overdose, ischemic injury not requiring extensive pressors, or pregnancy- related ALF, to the 13C-MBT results further improved the AUROC to 0.92 (95% CI: 0.85, 0.99). In contrast, the AUROC of the King’s College Criteria (KCC) was 0.70 (95% CI: 0.58, 0.81), and the AUROC for the MELD score was 0.83 (95% CI: 0.72, 0.94) (Fig. 4). Additional models that compared APAP to non-APAP etiologies and intubated to nonintubated patients or inclusion of admission lab values failed to provide any incremental predictive capabilities (data not shown).
FIG. 4.
Twenty-one-day TFS in the combined ALF/ALI cohort. Among the 56 patients with ALF and ALI with day 1 or 2 measures, the AUROC for the 13C-MBT PDR peak was 0.88, which improved to 0.92 with the inclusion of the etiology of ALI/ALF. The AUROC for 21-day survival was 0.79 for the KCC and 0.83 for the day 1 MELD score.
TFS MODELS IN PATIENTS WITH ALF
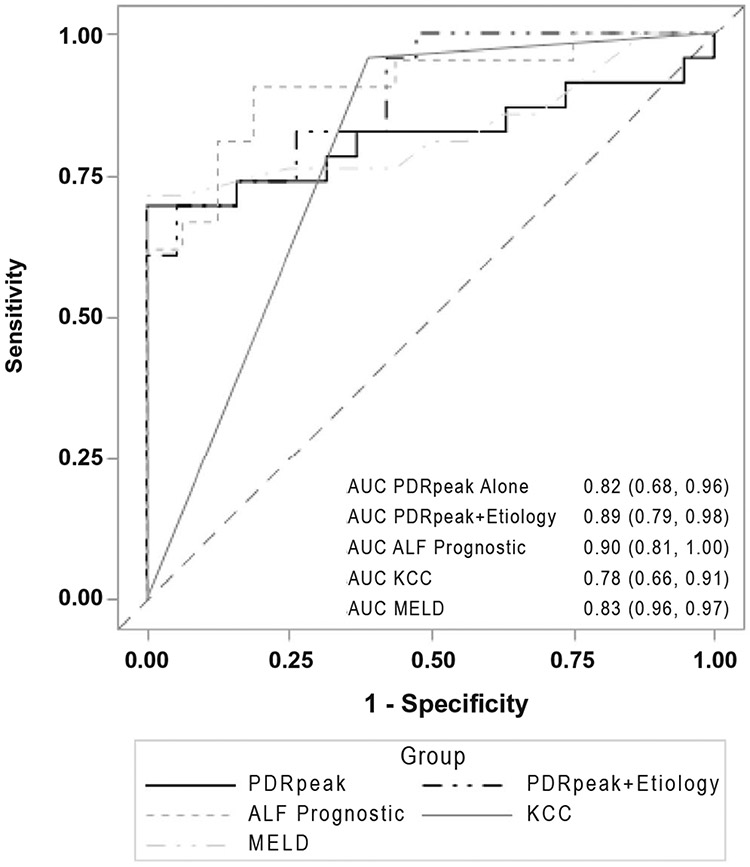
In the 42 patients with ALF only, with evaluable day 1 or 2 PDR peak values, the mean PDR peak was significantly higher in TFS versus non-TFS (8.5%/ hour vs. 2.4%/hour; P = 0.0005), and the AUROC was 0.82 (95% CI: 0.68-0.96). The addition of a favorable etiology of ALF to the PDR peak value from the 13C- MBT improved the AUROC to 0.89 (95% CI: 0.79, 0.98). In contrast, the AUROC of the ALFSG prognostic index was 0.90 (95% CI: 0.81, 1.0), the AUROC for KCC alone was 0.78 (95% CI: 0.66, 0.91), and the AUROC for MELD alone was 0.83 (95% CI: 0.69, 0.97) (Fig. 5).
FIG. 5.
Twenty-one-day TFS in the patients with ALF alone. Among the 42 patients with ALF with available data, the AUROC for the 13C-MBT PDR peak was 0.82, which improved to 0.89 with inclusion of ALF etiology and to 0.93 when combined with the ALFSG prognostic index. The AUROC was 0.78 for KCC and 0.83 for MELD scores.
SAFETY ASSESSMENTS
Review of the serum APAP-cysteine adduct results obtained in the first 20 evaluable patients demonstrated no evidence of inadvertent APAP hepatotoxicity or accumulation of APAP-cysteine adducts in either non-APAP or APAP-related patients.(12) There were a total of 28 SAEs reported from 25 subjects, none of which were deemed related to the investigational product. In addition, four potentially related, nonserious adverse events with a reported severity of mild to moderate were noted, including nausea, vomiting, and two events of emesis that occurred in nonintubated patients during test administration.
Discussion
The aim of our study was to determine the safety and accuracy of a bedside, noninvasive hepatic metabolic test to provide real-time prognostic data for clinical decision making in critically ill patients with ALF or severe non-APAP ALI. The initial 13C-MBT results performed quite well in predicting 21-day TFS in our patient population (Fig. 2). In addition, serial assessment of 13C-MBT results demonstrated that subjects who died or underwent LT within 21 days had persistently lower values compared to patients with TFS, indicating that this test may be a useful dynamic measure of global hepatic function. Interestingly, the single subject with a persistently low 13C-MBT PDR peak, who did not die or undergo LT, had Wilson disease and recovered within 21 days. We presume that the low 13C-MBT results in this patient may have been due to unsuspected advanced fibrosis or cirrhosis that is present in most subjects with Wilson disease who present with ALF.(13)
Acetaminophen is minimally absorbed from the stomach but rapidly absorbed by the small intestine through passive diffusion.(14,15) Following absorption, 13C-methacetin undergoes extensive first-pass clearance in the liver through metabolism of hepatic CYP1A2 into APAP and 13CO2. The BreathID device is based on continuous measurement of 13CO2 and 12CO2 concentrations by molecular correlation spectroscopy, which can detect variations less than 1:1,000 in the 13CO2/12CO2 ratio. Use of the 13C-MBT in critically ill patients requires the study staff to bring the portable equipment into the patient’s room. In 31% of the 185 tests initiated, 13C-methacetin was given via nasoenteric tube. One potential concern of using an oral substrate-based breath test in intubated patients receiving sedation is the potential for gastroparesis, which could delay absorption of the substrate and affect test results. In the current study, the 13C-MBT PDR peak reliably identified those more likely to survive, whether they were intubated (22 patients, 10.5%/ hour vs. 2.7%/hour; P = 0.03) or not intubated at the time of test administration (34 patients, 8.6%/hour vs. 1.9%/hour; P < 0.001). An unrelated study previously demonstrated that intubated medical ICU patients receiving vasopressors and opiates frequently had evidence of impaired gastric emptying.(16) Another study of intubated trauma patients with intracranial hemorrhage reported that the median gastric emptying time was 13.9 hours compared with 3.0 hours in healthy volunteers.(17) Furthermore, small bowel motility was also severely impaired in these ICU patients.(17) Finally, a single dose of narcotics in healthy volunteers has been shown to significantly delay the emptying of acetaminophen from the stomach.(14,15) With these data, we were concerned that many of our patients with ALI and ALF may have had functional gastroparesis, leading to a reduction in 13C-methacetin absorption. Although none of the enrolled patients had a history of known gastroparesis, there were three instances of gastrointestinal adverse events. Two of these events occurred during the 13C-methacetin administration, suggesting that some of these critically ill patients may have had functional gastroparesis. However, individual patients with low 13C-MBT test results generally remained low during serial assessments over subsequent days. We also did not find an association between the use of mechanical ventilation (P = 0.34) or vasopressor administration (P = 0.09) at study admission with 13C-MBT results, nor with the route of substrate administration through the oral versus naso-enteric/orogastric route (P = 0.37). Therefore, we presume that the 13C-MBT provides a robust index of hepatic function in critically ill patients with ALF and non-APAP ALI being cared for in the ICU. Other groups have reported on the use of intravenous administration of 13C-methacetin with periodic collection of exhaled 13C02 in patients with cirrhosis undergoing surgery and patients with ALF.(18,19) However, most of these data are in small, retrospective cohorts, and the kinetics of 13C-methacetin hepatic metabolism are very different when given intravenously compared to orally. In addition, a larger dose of methacetin is required for intravenous administration and needs to be dosed by patient body weight, leading to additional complexity and safety concerns as well as challenges with manufacturing sterile 13C-methacetin for human use.(20) Therefore, due to its rapid absorption in the fasted state from the small intestine, extensive hepatic extraction and first-pass metabolism in the liver, and simplicity of fixed dosing, we believe that oral administration of 13C-methacetin may lead to a more accurate and reproducible measure of global hepatic function.(21)
The accurate determination of prognosis in ALF has been an elusive goal, with many investigators using regression analyses of clinical features to identify which patient will die without a liver transplant.(3,22) Such prognostic indices have generally suffered from poor accuracy. The 13C-MBT, however, does not rely on static clinical features but rather actual metabolic capacity of the liver. Other groups have identified that serial arterial lactate levels as a modification to the KCC can also be of value in identifying patients with APAP overdose with a poor prognosis.(23-25) In addition, the 13C-MBT not only reflects CYP450 activity and therefore the severity of hepatocyte injury/necrosis, but also hepatocyte regeneration, as shown by the recovery of activity over days after admission. The clinical importance of serial 13C-MBT measurements deserves emphasis, as an increase in PDR peak over time provides objective evidence of recovery, and suggests waiting another day for a final decision regarding LT (Fig. 3). The present study, therefore, represents the first attempt to predict the outcome of ALF and non-APAP ALI using a truly dynamic liver function assessment tool.
Other prognostic models in ALF using widely available clinical and laboratory data include the KCC and the MELD score. A recent meta-analysis of these indices showed that the KCC performed better than the MELD score in patients with APAP hepatotoxicity.(25,26) In contrast, the MELD score had a superior prognostic value for nonsurvival in non-APAP patients. The ALFSG Prognostic Index was recently shown to have superior performance to both the King’s College and MELD score in a large derivation and validation cohort of North American patients with ALF.(3) This index includes five variables including the etiology of ALF (favorable vs. unfavorable as defined previously), use of vasopressors, encephalopathy grade, total bilirubin, and INR level. In the current study, the 13C-MBT plus etiology had comparable AUROC to the ALFSG Prognostic Index whether one grouped etiology as APAP versus non-APAP or favorable versus unfavorable. Furthermore, the dynamic nature of the 13C- MBT makes it more attractive for medical decision making. The combination of the 13C-MBT PDR peak and the ALFSG prognostic index variables showed a minimal improvement in prediction of 21-day TFS performance characteristics in our limited cohort. This is primarily due to the high AUROC that each measure had alone. To validate its utility, testing of the 13C-MBT in additional large cohorts of patients with ALI and ALF will be needed.
There were several important lessons learned from this study, including the challenges of enrolling patients into a clinical trial who have with a rare, sudden onset disease. As per Fig. 1, informed consent was not able to be obtained from 16% of the screened patients. Other studies of critically ill patient populations have demonstrated a consent failure rate of 1 in 3 to 1 in 4, particularly when a therapeutic intervention is not being offered, as in our case. In addition, several patients were initially excluded due to use of concomitant medications like acyclovir, famotidine and statins, which could have theoretically affected the 13C-MBT results through competitive inhibition of CYP1A2. However, after further review with the manufacturer and regulatory authorities, we were able to remove this exclusion criteria to allow more patients to be enrolled. In addition, we increased the upper age of enrollment to 80 and allowed for enrollment of non-APAP patients with ALI who have a limited 21-day TFS, to further help reach our recruitment goals. Other challenges with this study included device malfunction, which prohibited the collection of 13C-MBT data in 5 of the 76 enrolled participants. In order for the BreathID device to operate, the equipment must come to the ambient temperature of the room. In addition, it was at times challenging to complete the 13C-MBT in critically ill patients who were receiving enteral feedings. However, reliable data using the T-shaped ventilator hose adaptor could be obtained from intubated patients. Therefore, use of the 13C- MBT appears to be feasible in a population with ALI and ALF. Finally, the testing for serum APAP-cysteine adducts demonstrated that there was no evidence of new or worsening acetaminophen exposure with the small, 75-mg test dose of 13C-methacetin needed to conduct the study.(27) Other studies have also demonstrated no adverse effects from the small dose of methacetin used.(9,11) Finally, there were no device-related SAEs reported, although, as mentioned, 4 patients did experience transient nausea and emesis, which may have been related to underlying gastroparesis.
Limitations of the current study include the modest sample size that did not meet our intended recruitment goal of 200 patients. This arose from the presence of multiple exclusion criteria in many patients who were receiving various concomitant contraindicated medications. In addition, it can be difficult to establish a good rapport with family members of patients with ALF and ALI due to the sudden nature of the illness and looming concerns regarding the need for urgent LT. During the study, we were able to eliminate some of the medication exclusion criteria, but early on this factor proved to be problematic. Overall, there was a clear difference in breath-test results between the TFS and those needing urgent LT and dying. The observation that a given trajectory over time associates with their outcome suggests that additional studies of serial 13C-MBT are needed.
In conclusion, we demonstrated that the 13C-MBT is a feasible and promising means of risk-stratifying critically ill patients with ALF and non-APAP ALI for adverse outcomes. Further studies with broader inclusion criteria are needed to further develop the use of this simple, noninvasive bedside technology that can provide instantaneous objective information to clinicians and hopefully reduce the need for unnecessary transplants in patients with ALF and non-APAP ALI.
Supplementary Material
Acknowledgment:
The authors thank Dr. Laura James of the University of Arkansas for performing the serum APAP-cysteine adducts in the first 20 patients enrolled. The authors also thank the officers of Exalenz Biosciences, Ltd., for their assistance with conducting the trial, including Gil Guggenheim, Avraham Hershkowitz, Ofer Schlesinger, and James McLaughlin. The investigators would like to thank the following individuals for their willingness to serve on the Safety Review Committee ( Jorge Rakela, M.D., Ray Chung, M.D., and Willis Maddrey, M.D.), the Data and Safety Monitoring Board (Michael Lucey, M.D. [chair], Karla Ballman, Ph.D., and Patrick Northup, M.D.), and the independent Medical Safety Monitor, Dean Karvellas, M.D.
Abbreviations:
- ALF
acute liver failure
- ALFSG
Acute Liver Failure Study Group
- ALI
acute liver injury
- ALT
alanine aminotransferase
- APAP
acetaminophen
- AUROC
area under the receiver operating characteristic curve
- cPDR20
cumulative percent dose recovery of methacetin at 20 minutes
- CYP1A2
cytochrome P-450 IA2
- HE
hepatic encephalopathy
- ICU
intensive care unit
- INR
international normalized ratio of the prothrombin time
- KCC
King’s College Criteria
- LT
liver transplantation
- MBT
Methacetin breath test
- MELD
Model for End-Stage Liver Disease
- NAC
N-acetylcysteine
- PDR
percent dose recovery
- SAE
serious adverse event
- TFS
transplant-free survival
- ULN
upper limit of normal
Footnotes
Potential conflict of interest: Dr. Lee consults for Pfizer, Genentech, SeaGen, Karuna, Forma, and Cortexyme. He received grants from Merck, Bristol-Myers Squibb, Intercept, Gilead, Novo Nordisk, Cumberland, Aurora, and Conatus. Dr. Fontana consults for Sanofi. He received grants from Bristol-Myers Squibb, Gilead, and AbbVie. Dr. Stravitz received grants from Exalenz. Dr. Hanje is on the speakers bureau for Salix and Intercept. Dr. Hameed advises and received grants from Gilead. He advises Surrozen, Mallinckrodt, and Pleiogenix. He received grants from Intercept, Conatus, Genfit, and Salix/Valeant. Dr. Ganger advises Alexion and is on the speakers bureau for Gilead. Dr. Olson advises Mallinckrodt.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.31783/suppinfo.
REFERENCES
- 1).Stravitz RT, Kramer AH, Davern T, Shaikh AOS, Caldwell SH, Mehta RL, et al. ; Acute Liver Failure Study Group. Intensive care of patients with acute liver failure: recommendations of the US Acute Liver Failure Study Group. Crit Care Med 2007;35:2498–2508. [DOI] [PubMed] [Google Scholar]
- 2).Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in adults with acute liver failure between 1993 and 2013: an observational cohort study. Ann Int Med 2016;164:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Koch DG, Tillman H, Durkalski V, Lee WM, Reuben A. Development of a model to predict transplant free survival of patients with acute liver failure. Clin Gastroenterol Hepatol 2016;14:1199–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SHB, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Int Med 2002;137:947–954. [DOI] [PubMed] [Google Scholar]
- 5).Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, et al. The natural history of severe acute liver injury. Am J Gastroenterol 2017;112:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Stravitz RT, Gottfried M, Durkalski V, Fontana RJ, Hanje AJ, Koch D, et al. Safety, tolerability and pharmacokinetics of l-ornithine phenylacetate in patients with acute liver injury/failure and hyperammonemia. Hepatology 2018;67:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Ilan Y. Review article: the assessment of liver function using breath tests. Aliment Pharmacol Ther 2007;26:1293–1302. [DOI] [PubMed] [Google Scholar]
- 9).Stravitz RT, Reuben A, Mizrahi M, Lalazar G, Brown K, Gordon SC, et al. Use of the methacetin breath test to classify the risk of cirrhotic complications and mortality in patients evaluated/listed for liver transplantation. J Hepatol 2015;63:1345–1351. [DOI] [PubMed] [Google Scholar]
- 10).Lalazar G, Pappo O, Heathcovici T, Hadjad T, Shubi M, Ohana H, et al. A continuous 13C methacetin breath test for noninvasive assessment of intrahepatic inflammation and fibrosis in patients with chronic HCV infection and normal ALT. J V Hepatitis 2008;15:716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Lalazar G, Adar T, Ilan Y. Point-of-care continuous 13C-methacetin breath test improves decision making in acute liver disease: results of a pilot clinical trial. World J Gastroenterol 2009;15:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Roberts DW, Lee WM, Hinson JA, Bai S, Swearingen CJ, Stravitz RT, et al. An immunoassay to rapidly measure acetaminophen protein adducts accurately identifies patients with acute liver injury or failure. Clin Gastroenterol Hepatol 2017;15:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Camarata M, Gottfried M, Rule JA, Ala A, Lee WM, Schilsky SRT, et al. Outcomes of acute liver injury in adults due to Wilson disease: is survival without transplant possible? Liver Transpl 2020;26:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Raffa RB, Pergolizzi JV, Taylor R, Decker JF, Patrick JT. Acetaminophen oral absorption and clinical influences. Pain Pract 2014;14:668–677. [DOI] [PubMed] [Google Scholar]
- 15).Srinivas NR. Acetaminophen absorption kinetics in altered gastric emptying: establishing a relevant pharmacokinetic surrogate using published data. J Pain Pall Care Pharmacother 2015;29:115–119. [DOI] [PubMed] [Google Scholar]
- 16).Tarling MM, Toner CC, Withington PS, Baxter MK, Whelpton R, Goldhill DR. A model of gastric emptying using paracetamol absorption in intensive care patients. Int Care Med 1997;23:256–260. [DOI] [PubMed] [Google Scholar]
- 17).Rauch S, Krueger K, Turan A, You J, Roewer N, Sessler DI. Use of wireless motility capsule to determine gastric emptying and small intestinal transit times in critically ill trauma patients. J Crit Care 2012;27:534–539. [DOI] [PubMed] [Google Scholar]
- 18).Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg 2009;250:119–125. [DOI] [PubMed] [Google Scholar]
- 19).Lock JF, Kotobi AN, Malinowski M, Schulz A, Jara M, Neuhaus P, et al. Predicting the prognosis in acute liver failure: results from a retrospective, pilot study using the LiMAx test. Ann Hepatol 2013;12:388–394. [PubMed] [Google Scholar]
- 20).Tugtekin I, Radermacher P, Wachter U, Barth E, Weidenbach H, Adler G, et al. Comparison between the oral and IV 13-C phenylalanine breath test for the assessment of liver function. Isot Environ Health Stud 1999;35:147–156. [DOI] [PubMed] [Google Scholar]
- 21).Candelli M, Cazzato IA, Eista EC, Pignataro G, Gasbarrini A. 13C-methacetin breath test and oxygen supply. Aliment Pharm Ther 2003;18:1176. [DOI] [PubMed] [Google Scholar]
- 22).McPhail MJW, Farne H, Senvar N, Wendon JA, Bernal W. Ability of King’s College criteria and model for end stage liver disease scores to predict mortality of patients with acute liver failure: a meta-analysis. Clin Gastroenterol Hepatol 2016;14:516–525. [DOI] [PubMed] [Google Scholar]
- 23).Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet 2002;359:558–563. [DOI] [PubMed] [Google Scholar]
- 24).Macquillan GC, Seyam MS, Nightingale P, Neuberger JM, Murphy N. Blood lactate but not serum phosphate levels can predict patient outcome in fulminant hepatic failure. Liver Transpl 2005;11:1073–1079. [DOI] [PubMed] [Google Scholar]
- 25).Craig DGN, Ford AC, Hayes PC, Simpson KY. Systematic review: prognostic tests of paracetamol-induced acute liver failure. Aliment Pharmacol Ther 2010;31:1064–1076. [DOI] [PubMed] [Google Scholar]
- 26).EASL Clinical Practice Guidelines. EASL Clinical practice Guidelines on the management of acute /fulminant liver failure. J Hepatol 2017;66:1047–1081. [DOI] [PubMed] [Google Scholar]
- 27).Stravitz RT, James L, Wendon J, Pop OT, Audimoolam VK, Lee WM. Does methacetin use in breath testing increase acetaminophen hepatotoxicity in the setting of acute liver failure? Hepatology 2015;62:179A.25914217 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.