Abstract
Diuretic resistance implies a failure to increase fluid and sodium (Na+) output sufficiently to relieve volume overload, edema or congestion despite escalating doses of a loop diuretic to a ceiling level [80 mg of furosemide once or twice daily or greater in those with reduced glomerular filtration rate (GFR) or heart failure (HF)]. It is a major cause of recurrent hospitalizations in patients with chronic heart failure (CHF) and predicts death but is difficult to diagnose unequivocally. Pharmacokinetic mechanisms include the low and variable bioavailability of furosemide and the short duration of all loop diuretics that provides time for the kidneys to restore diuretic-induced Na+ losses between doses. Pathophysiological mechanisms of diuretic resistance include an inappropriately high daily salt intake that exceeds the acute diuretic-induced salt loss, hyponatremia or hypokalemic, hypochloremic metabolic alkalosis and reflex activation of the renal nerves. Nephron mechanisms include tubular tolerance that can develop even during the time that the renal tubules are exposed to a single dose of diuretic, or enhanced reabsorption in the proximal tubule that limits delivery to the loop, or an adaptive increase in reabsorption in the downstream distal tubule and collecting ducts that offsets ongoing blockade of Na+ reabsorption in the loop of Henle. These provide rationales for novel strategies including the concurrent use of diuretics that block these nephron segments and even sequential nephron blockade with multiple diuretics and aquaretics combined in severely diuretic resistant patients with heart failure.
Keywords: Furosemide, torsemide, edema, diuretic combinations
Introduction definition and clinical impact:
We review the pathophysiology of diuretic resistance and its implications for improving the management of patients with chronic heart failure (CHF). Diuretic resistance is a failure to increase fluid and sodium (Na+) output sufficiently to relieve volume overload, edema or congestion despite a full dose of a loop diuretic. More quantitative definitions include a failure of oral furosemide (160 mg twice daily or equivalent) to increase Na+ excretion by at least 90 mmol over 3 days. 1 Alternatively, a spot urine sample obtained 1 – 2 hours after a loop diuretic can be used to predicts Na+ output. An Na+ output < 50 mmol is generally insufficient to induce a negative Na+ balance with loop diuretics and therefore predicts diuretic resistance. This was validated prospectively in 50 patients 2:
Na+ output (mmol) = eGFR (BSA/1.73) (SCr/UCr) * 60 min * 2.5 hr * (UNa/1000 ml) where BSA is body surface area, Cr is urine or serum creatinine and UNa is urine sodium concentration 2.
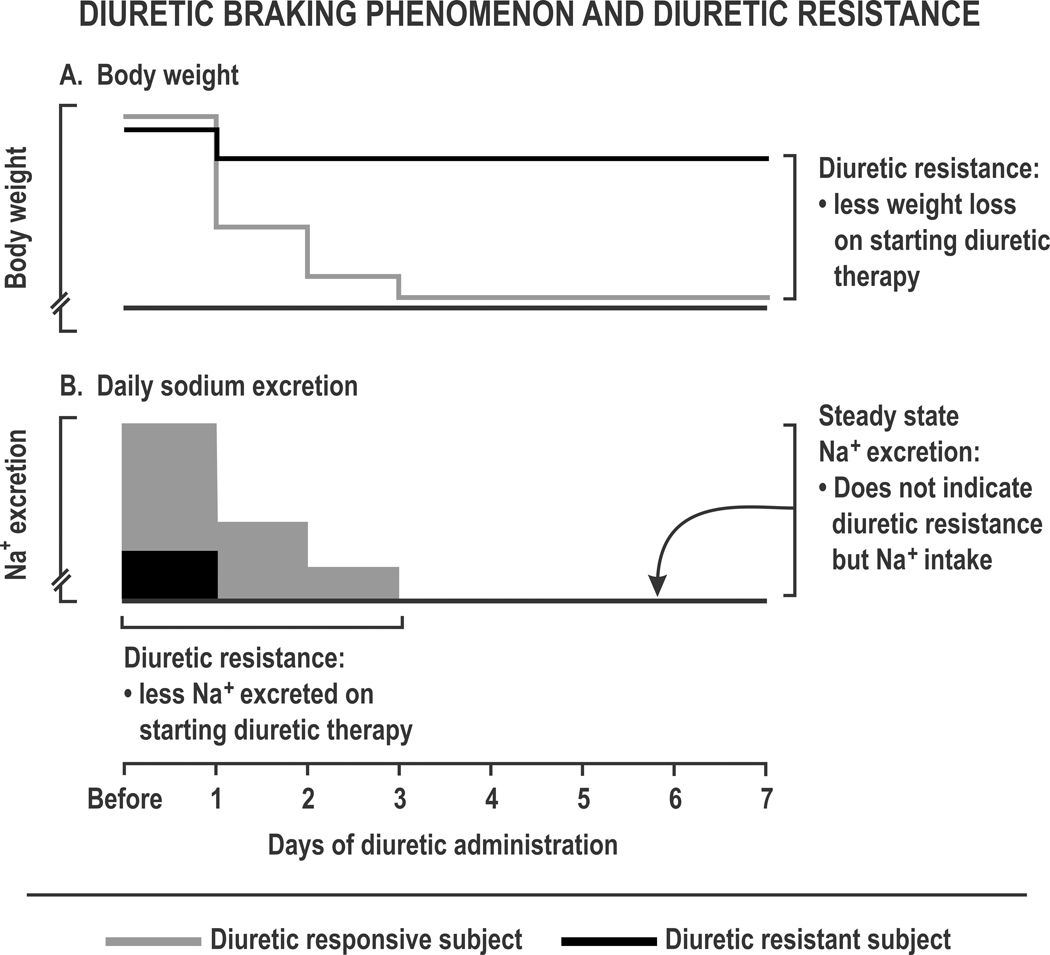
A poor diuretic response predicts subsequent death, readmission or renal complications from CHF 3. Recognition of diuretic resistance is hampered by imprecise metrics. Intravenous diuretics for patients hospitalized with decompensated HF can reduce body weight by 11 Kg yet signs of hypervolemia and congestion persist in > 50% and blood volume, that predicts mortality, 4 remains ~ 30% expanded 5. Thus interstitial fluid, including peripheral and pulmonary edema, is depleted selectively but blood volume is well defended. Indeed, > 85% of fluid removed by diuretics is from extravascular sites that include peripheral and pulmonary edema. Steady state measurements of daily Na+ excretion can indicate daily Na+ intake, but cannot diagnose diuretic resistance (Figure 1). Presently, a practical and quantitative definition of diuretic resistance remains elusive.
Figure 1. Diuretic braking phenomenon and diuretic resistance:
Schematic representation of a diuretic responsive (grey lines and grey boxes) or resistant subject (dark lines and solid boxes), showing body weight (Panel A) and daily sodium excretion (Panel B) during loop diuretic administration.
Loop diuretics and dosage:
Furosemide diuresis normally lasts about 4 hours. Bumetanide is somewhat shorter and torsemide somewhat longer. An approximate dose conversion ratio is 1:20:40:50 for bumetanide: torsemide: furosemide: ethacrynic acid. The normal ceiling daily dose of furosemide above which little further natriuresis occurs is 80 mg once or twice daily, increasing to 160 and 240 mg in patients with chronic kidney disease (CKD) stages 3 and 4 or nephrotic syndrome or 80 – 160 mg in patients with cirrhosis or HF with preserved glomerular filtration rate (GFR). Very high doses of circa 500 mg of furosemide may be required in patients with end stage renal disease (ESRD) 6. The higher furosemide doses required for patients with CKD are a consequence of many factors including a decreased diuretic delivery to the kidney because of decreased renal blood flow (RBF), an increased volume of distribution of the protein-bound diuretic because of hypoalbuminemia, a decreased proximal tubule secretion of the diuretic by the organic anion transporters (OATs) because of competition by urate and other organic anions that are retained in the plasma in patients with CKD and a decreased filtered load of Na+ because of a decreased GFR 7. However, the response to the diuretic delivered to the loop of Henle is well maintained in CKD. In practice, the dose of loop diuretics should generally be increased in proportion to the reduction in eGFR. Patients with CHF may have an impaired absorption of loop diuretics and an impaired tubular response mandating higher doses often given twice daily 8.
Special problems with furosemide include a low and variable bioavailability of 10 – 80% that is impaired further in the elderly and those with HF or CKD 9. In theory, this should be addressed by iv dosing or by substituting torsemide or bumetanide that have higher and more consistent bioavailabilities 10. However, iv infusions are not strikingly superior to oral or bolus iv dosing (see later below) and variations in the gene expressions for the OATs and other genes adds to torsemide variability 11, 12.
Individual diuretic responsiveness:
The natriuretic response depends on salt intake 13, diuretic dose, renal function and right atrial pressure 14. Whereas a low eGFR in patients with HF predicts a poor outcome, a worsening eGFR during hospitalization for acute heart failure (AHF) in the DOSE study paradoxically predicted a better outcome 15. This may reflect a successful hemodynamic response to renin-angiotensin-aldosterone system (RAAS) blockade that itself reduces the GFR but is beneficial for long term renal function 2.
General mechanisms of diuretic resistance and tubular responsiveness:
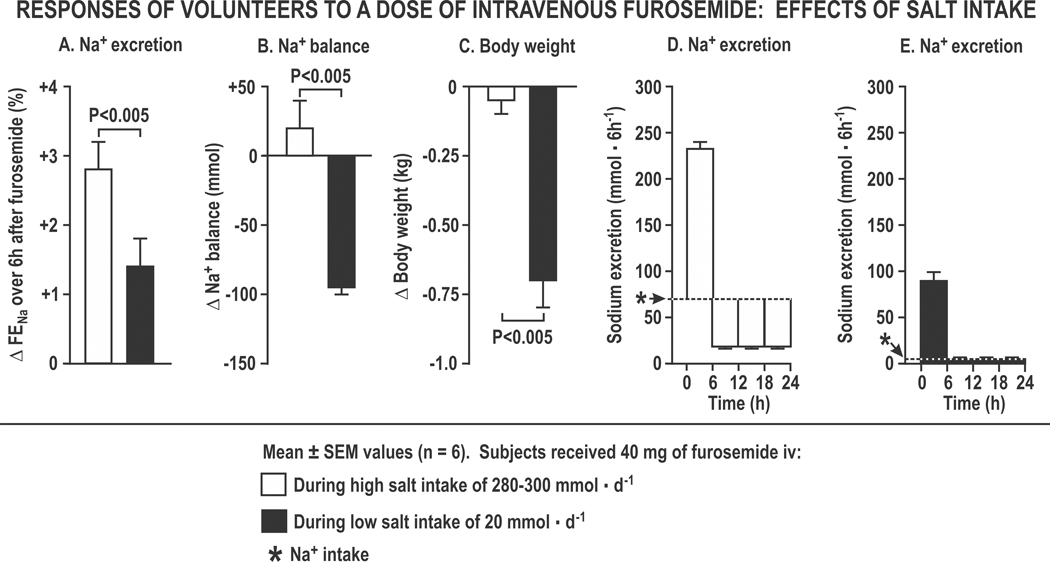
Losses of body weight and Na+ are normally attenuated progressively during diuretic therapy (“braking phenomenon”; Figure 1). The initial 6-hourly natriuresis is enhanced during high salt intake (Figure 2A), yet salt restriction is required to reduce Na+ balance (Figure 2B) or body weight (Figure 2C) 13 because, during a high Na+ intake, the acute Na+ loss is restored by the kidneys after the diuresis by reduced Na+ excretion below intake, resulting in an unchanged Na+ balance (Figure 2D). However, during a low Na+ intake, even a very low level of post-diuretic Na+ excretion cannot restore Na+ balance because the acute natriuresis exceeds the dietary Na+ intake (Figure 2E). Thus, for diuretic resistant patients, the daily Na+ intake should be less than the acute Na+ loss with the diuretic to ensure a negative Na+ balance. This value is 120 – 150 mmol in normal subjects but is reduced in those with CHF to about 50 – 100 mmol 16. However, whereas salt restriction increases diuretic-induced negative salt balance 13, it also increases renin, angiotensin II (Ang II), aldosterone and renal K+ losses 17, 18 and may increase the risk of death in patients with HF 19. Restriction of daily Na+ intake to 80–120 mmol (2 – 3 grams) is a reasonable target for patients with modest CHF and diuretic resistance 6, 20.
Figure 2. The effects of salt intake on the responses of volunteers to furosemide:
Mean (± sem) changes over 6 hours (Panel A) or over 24 hours (Panels B to E) after 40 mg of furosemide over three days during a daily sodium intake of 280 mmol (open boxes) or 20 mmol (closed boxes) depicting changes in fractional excretion of sodium for 6 hours after the first dose of furosemide (Panel A), daily sodium balance (Panel B), changes in daily body weight over three days (Panel C) and the patterns of 6 hourly sodium excretion (Panels D and E).
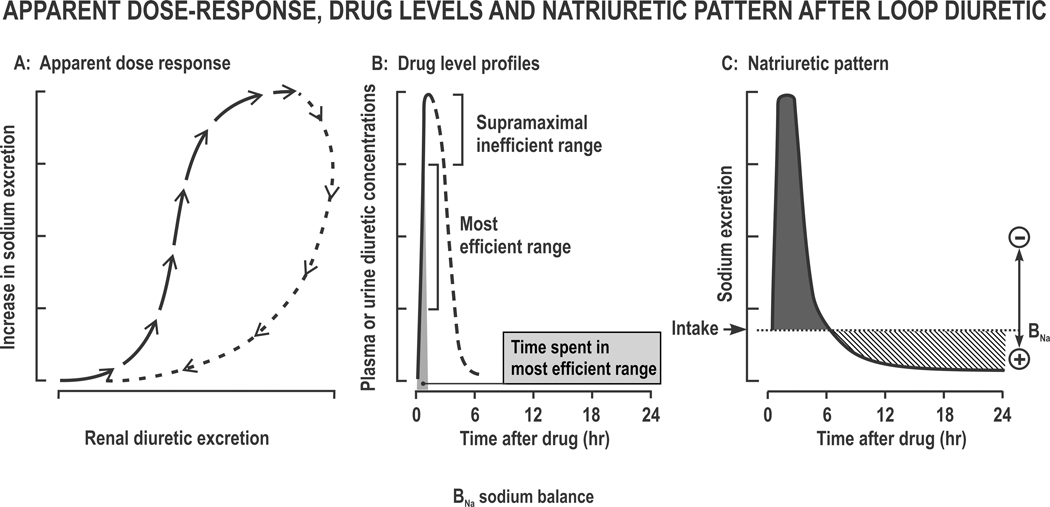
After proximal tubule (PT) secretion, diuretics are confined to the tubular lumen. Thus, their rate of excretion approximates to their rate of delivery to their active site on the tubular lumen of the loop of Henle (LH). A plot of increased Na+ excretion against diuretic excretion thereby provides an “apparent dose-response” relationship that approximates to tubular diuretic responsiveness 21. However, this relationship has marked hysteresis. Thus, the early points that fall on the ascent of the curve lead to much greater increases in Na+ excretion than the later points that fall on the descent of the curve. This finding implies the development of within-dose diuretic tolerance 22 (Figure 3A). The initial few points on the steep part of the apparent “dose response curve” represent highly efficient natriuresis but thereafter several points are effectively “supra-maximal”, while later points fall on the truly inefficient descending curve (Figure 3B). Thus, a more prolonged delivery by an extended release diuretic formulation can enhance diuretic efficiency 22.
Figure 3. Apparent Dose-Response, diuretic plasma or urine levels and natriuretic pattern after a loop diuretic:
The apparent dose-response relationship at time points after a single dose (Panel A), the profile of diuretics with the most efficient levels corresponding to the middle 50% of the rising phase (Panel B) and the natriuretic pattern of brief sodium loss (negative balance) followed by Na+ retention (positive balance) (Panel C).
Post diuretic sodium retention:
Although post-diuretic Na+ retention follows a period of volume loss and activation of the RAAS and sympathetic nervous system (SNS) 17, 18, 23, it is not normally modified by blockade of the RAAS or the SNS 23, 24. Indeed, 94% of an iv test load of 100 mmol NaCl given to volunteers was excreted over two days but, when given after bumetanide accompanied by an infusion of Na+, Cl− and fluid sufficient to prevent any volume depletion, only 9% was excreted 25. Thus, post diuretic renal Na+ retention entails a “memory effect” of the pharmacological action of the diuretic on the renal tubules, rather than a response to volume loss.
Renal hemodynamics:
A reduction in the RBF limits the delivery of diuretic to the kidneys while a reduction in the GFR limits the tubular Na+ delivery. Thus, reductions in RBF or GFR can contribute to diuretic resistance 26.
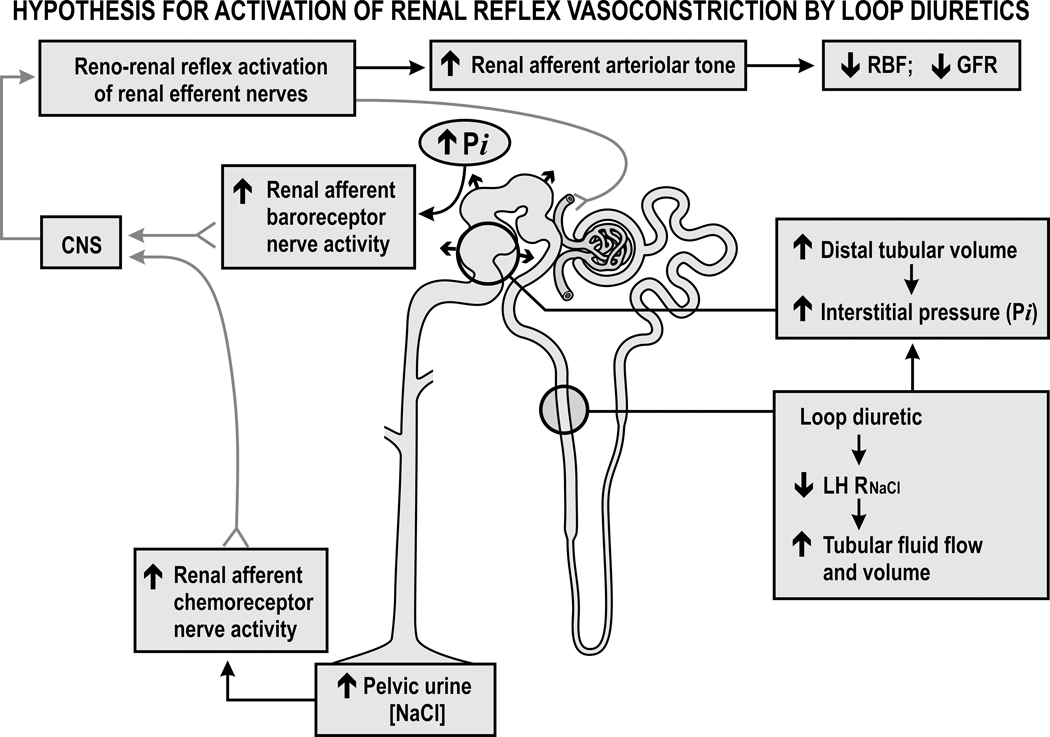
Loop diuretics inhibit reabsorption by the tubular macula densa segment at the end of the LH that normally initiates afferent arteriolar vasoconstriction by the tubuloglomerular feedback (TGF) response. Thus TGF blockade by loop diuretics should increase the RBF and GFR 27. Indeed, furosemide increases the GFR in dogs 28. However, it normally reduces the GFR and the RBF in rats 29, mice 30 and humans 31. The GFR falls by ~23% after furosemide 13, 18, 23 or torsemide 22 in normal subjects or those with HF 26, 32. The fall in GFR in rats is independent of volume depletion or activation of the RAAS 33 but entails reflex renal vasoconstriction 33, 34. Inhibition of tubular fluid reabsorption increases the tubular fluid volume 35 and this increases the intrarenal pressures since the kidney is encapsulated 36, 37. This increase in renal turgor will directly restrict the RBF 38 but also will raise the renal interstitial pressure (Pi) that activates the interstitial baroreceptors. In parallel, the diuretic will increase the urinary NaCl concentration that will activate the renal pelvic chemoreceptors. The ensuing increase in renal afferent nerve discharge should initiate a reno-renal reflex to increase renal efferent nerve activity and reduce the GFR and RBF 39 (Figure 4). Indeed, the fall in GFR with furosemide in rats is mitigated by renal nerve deafferentation 34, 40. Renal afferent nerve activity is increased in rats with HF and enhances tubular Na+ reabsorption, renin release and renal vasoconstriction 41. While radiofrequency renal nerve ablation for patients with HF reduces parameters of volume overload and symptoms modestly 42–44, any effects on diuretic resistance have yet to be studied.
Figure 4. Hypothesis for activation of renal reflex vasoconstriction by loop diuretics:
Diuretics reduce the reabsorption of sodium chloride in the loop of Henle (LH RNaCl), distend the distal tubules and increase the renal interstitial pressure (Pi) that activates renal afferent baroreceptor nerves but also increase the pelvic urine sodium chloride concentration that activates renal afferent chemoreceptor nerves. The increased afferent nerve discharge activates a central nervous system (CNS) reno-renal reflex that increases the renal efferent nerve activity and the afferent arteriolar tone that reduces the renal blood flow and glomerular filtration rate.
An aggressive diuresis in patients with diuretic-resistant CHF can increase the serum creatinine concentration (Scr). This is often ascribed to the concurrent use of RASS inhibitors and taken as a sign of renal injury requiring a reduction in diuretic dosage or a change in therapy. However, whereas infusion of furosemide into euvolemic rats reduces their GFR and increases their renal vascular resistance the concurrent administration of losartan does not modify the fall in GFR and reduces the renal vasoconstriction and filtration fraction 33. Thus, a fall in GFR in this setting is not a manifestation of renal ischemia or damage. Moreover, an angiotensin converting enzyme inhibitor (ACEI) increases furosemide natriuresis modestly in normal subjects thereby reducing the work required for Na+ reabsorption 45. A study of patients with acute heart failure, most of whom were taking an RASS inhibitor, reported that aggressive diuresis with an average daily intravenous dose of 150 mg of furosemide reduced the eGFR only modestly. Moreover worsening renal function was not associated with any increase in the excretion of markers of renal tubular injury. Indeed, an increase in the excretion of neutrophil gelatinase-associated lipocalin, N-acetyl-β-D-glucosaminidase and kidney injury molecule 1 were associated paradoxically with an improved 180-day patient survival 46. These data indicate that the effects of an RASS inhibitor on the renal hemodynamic response to a loop diuretic may entail some further reduction in GFR, but likely a maintained or improved RBF and sometimes an increase in Na+ excretion. The outcome of an improved RBF would be an improved renal O2 delivery while the outcome of a reduced GFR and increased Na+ excretion would be a reduced renal O2 usage because of a reduced work load by the tubules. This favorable O2 balance should not induce renal ischemia or enhance renal tubular injury. Thus, the modest reduction in eGFR during intravenous loop diuretics during therapy with an RASS inhibitor likely represents a favorable renal hemodynamic response rather than renal tubular injury, and therefore should not normally be labeled “acute kidney injury”. It follows that a modest initial rise in Scr of < 20 – 30% should not normally trigger withdrawal of the RASS inhibitor therapy, but does require careful monitoring.
Hyponatremia and hypokalemic, hypochloremic, metabolic alkalosis:
Loop diuretics and heart failure both provoke non-osmolar release of arginine vasopressin (AVP) 47. The increase in AVP in HF may enhance both vasopressin type 1a receptor (V1aR)-mediated vasoconstriction and V2R-mediated free water retention. Together, these may contribute to cardiac strain, worsening congestion and hyponatremia 48. The increase in plasma levels of AVP predict bad outcomes in CHF 49 while the increase in AVP with loop diuretics predicts poor diuresis, hyponatremia and bad outcomes 50. Tolvaptan blocks V2Rs and induces free water diuresis that corrects hyponatremia, but can further increase AVP whose activation of VIaRs further enhances peripheral resistance and renal vasoconstriction 51. Conivaptan blocks V2Rs and V1aRs and may therefore have a further benefit, but its interaction with diuretics requires study 52.
Mild metabolic alkalosis reduces the natriuretic response to bumetanide by ~20%, likely because of increased presentation of HCO3−, without Cl−, to the sodium, potassium, 2 chloride (NKCC2) co-transporter in the LH that has an absolute requirement for Cl− for reabsorption with Na+ and K+ 53. Diuretic responses in hypochloremic patients are improved after correction of hypochloremia with lysine chloride 54. Potassium depletion in rats halves the natriuretic response to furosemide 55. Thus hypokalemic, hypochloremic metabolic alkalosis should be corrected by concurrent use of a collecting duct (CD) diuretic (e.g. amiloride or triamterene), a mineralocorticosteroid receptor antagonists (MRA; e.g. spironolactone or eplerenone) or oral KCl.
Hypoalbuminemia, albuminuria and albumin infusion:
Since loop diuretics are bound to albumen, hypoproteinemia increases their volume of distribution and reduces their renal delivery 56. Mixing iv furosemide in a syringe with human serum albumin (HSA) improved urine output modestly in one study of patients with the nephrotic syndrome 56. However, furosemide should dissociate from circulating albumin in the circulation almost instantly and these studies were not confirmed 57. Proteinuria per se might impair the diuretic response by binding of the secreted diuretics to filtered albumin in tubular fluid. However, the co-administration of furosemide with sulfisoxazole that displaces bound furosemide did not modify the responses of proteinuric subjects to a loop diuretic 58.
Finally, infusions of “salt poor” HSA might increase the RBF and the delivery of the diuretic to the kidneys. However, an HSA infusion that increased the plasma volume by 23% actually reduced the Na+ excretion and the GFR 59. Moreover, although patients with the nephrotic syndrome infused with salt poor HSA had a modest increase in furosemide natriuresis, this was balanced by increased Na+ intake from the “salt poor” albumin, resulting in unchanged Na+ balance 60. Infusion of albumin can cause hypertension, respiratory distress, HF 61 and nephropathy 62. Thus there is no clear place for albumin infusions to enhance diuretic responsiveness but rather these studies highlight many associated problems.
Diuretic combinations:
Diuretic therapy can upregulate Na+ transport in the PT that limits Na+ delivery to the LH, or upregulates Na+ transport in downstream nephron segments that limits natriuresis (Figure 5). These findings provide rationales for combining diuretics with actions on different nephron segments.
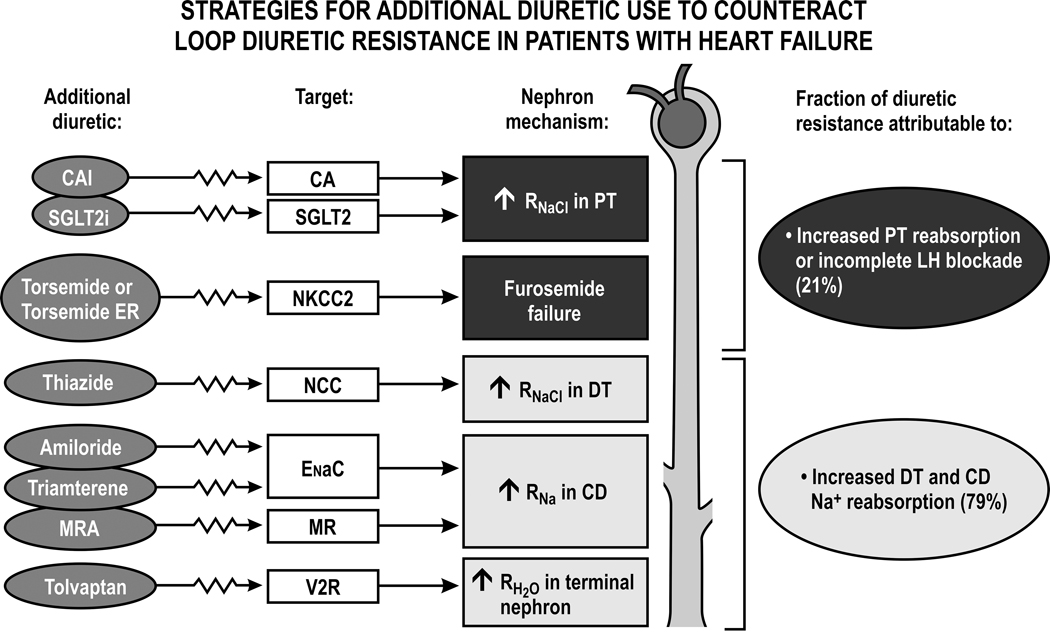
Figure 5. Strategies for the use of additional diuretics to counteract loop diuretic resistance in patients with heart failure:
Schematic representation of nephron sites contributing to loop diuretic resistance and the major classes of diuretic drug to correct these. CAIs, carbonic anhydrase inhibitors; CA, carbonic anhydrase; SGLT2i’s, sodium glucose linked transport 2 inhibitors; torsemide ER, torsemide extended release; NKCC2, sodium, potassium, 2 chloride transporter; NCC, sodium, chloride cotransporter; ENaC, epithelial sodium channel; V2 vasopressin type 2 receptor; MRA’s, mineralocorticosteroid receptor antagonists; RNaCl, reabsorption of sodium chloride; PT, proximal tubule; LH, Loop of Henle; DT, distal tubule; CD, collecting duct. Also shown is a summary from studies of patients with diuretic resistant heart failure that apportioned the contribution to diuretic resistance between proximal effects that limited diuretic and sodium delivery to the LH and distal effects of increased RNaCl in the DT and CD. After Rao, V et al, J Am Soc Nephrol 28:3414–3424, 2017, with permission.
Loop diuretic plus proximal tubule (PT) diuretic:
Blockade of PT reabsorption by acetazolamide (Diamox) does not normally augment furosemide natriuresis 63, 64 likely because it increases the LH delivery of Na+ with HCO3−, whereas reabsorption by the NKCC2 transporter in the loop of Henle is limited by Cl− delivery 53. Although acetazolamide increased diuretic responsiveness in one experimental study, the authors related this to downregulation of Cl−/HCO3− exchange by pendrin in the CD 65.
Sodium glucose linked transporter type 2 inhibitors (SGLT2i’s) reduce PT reabsorption and synergize with loop diuretics to enhance natriuresis 66 as discussed 67. Normal volunteers studied at a daily Na+ intake of 110 mmol were randomized to dapagliflozen or bumetanide for one week and crossed over. Natriuresis with bumetanide was increased significantly by 36% when tested after one week of dapagliflozin and natriuresis with dapagliflozin was increased significantly by 190% when tested after one week of bumetanide. The augmented loop diuretic response after adaptation to dapagliflozin likely occurred because the SGLT2i reduced PT reabsorption of Na+ 67 and thereby upregulated the downstream reabsorption of Na+ in the loop of Henle that is the target for loop diuretics 68. The augmented SGLT2i response after adaptation to bumetanide likely occurred because the loop diuretic released renin that generated Ang II that upregulated the expression of SGLT2 in the PT 69 that is the target for SGLT2i’s. In another study, patients with CHF and type 2 diabetes mellitus were randomized for two weeks to empagliflozin vs placebo. Empagliflozin increased the Na+ excretion with loop diuretics without enhancing the K+ excretion 70 and reduced the plasma volume over two weeks. This occurred without activation of the RAAS or SNS and without a decline in the GFR or the appearance of tubular biomarkers of nephron damage 70. Thus, SGLT2i’s may become important adjuncts to loop diuretic therapy for patient with CHF even in the absence of diabetes mellitus. Indeed, the recent DAPA-HF trial in patients with HF but without diabetes mellitus reported a significant reduction in CVD events and mortality in those randomized to dapagliflozin 71. These results provide a rationale for the use of an SGLT2i to manage diuretic resistance in a wider range of patients with CHF, but this requires further evaluation.
Loop diuretic plus early distal tubule (DT) diuretic:
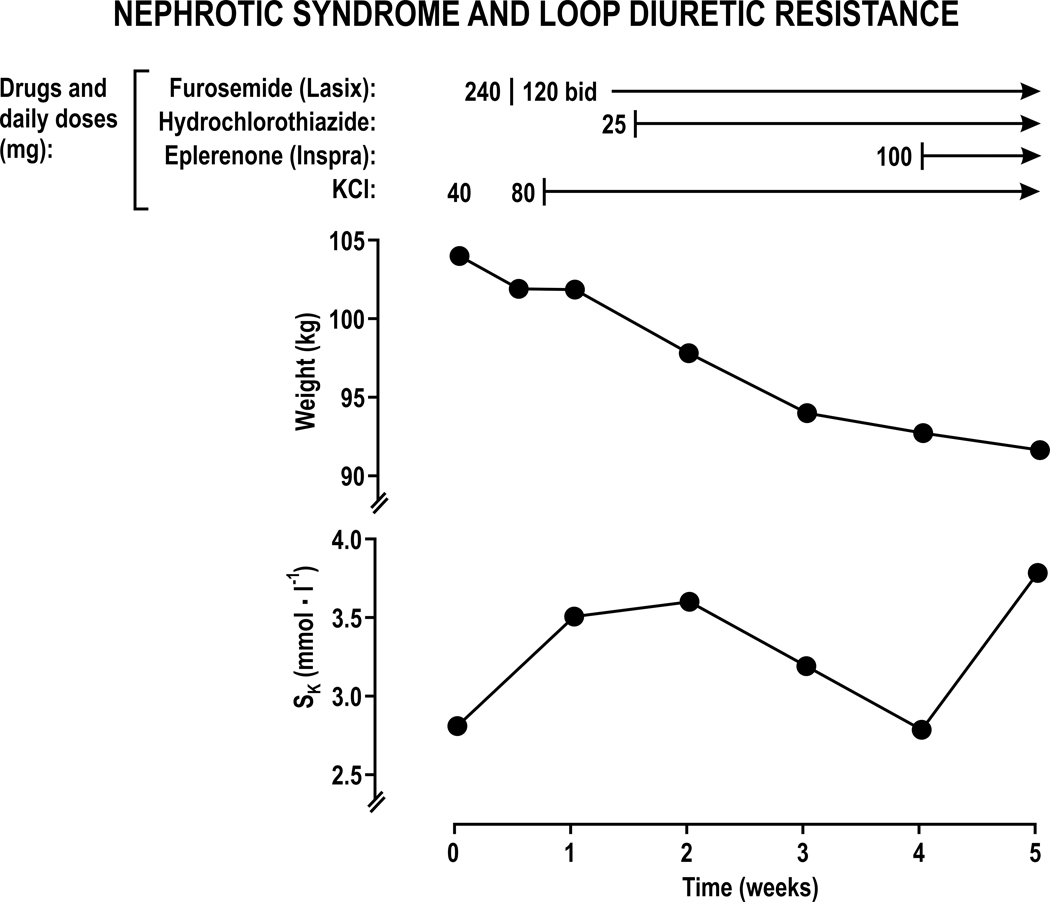
Rats given furosemide develop massive hypertrophy of the DT 72, CD 73, and intercalated cells 74 and a 3-fold increase in the Na+ reabsorption by the distal tubule 75. Therefore, it is rational for patients resistant to loop diuretics to add a thiazide that blocks DT reabsorption 76–78. Frequently, metolazone is selected since it is available as an intravenous or oral preparation. Metolazone has a thiazide-like action and an additional effect to reduce PT reabsorption. An observational study reported that the use of metolazone with loop diuretics for patients with AHF was associated with frequent electrolyte abnormalities and worsening renal function whereas high-dose furosemide therapy was associated with better outcomes 79. Thus, uptitration of loop diuretics is a preferred initial step in the management of patients with diuretic resistant AHF. Although combined therapy with a loop diuretic and a thiazide can be helpful in patients with CHF 80 or edema 81, 82, this strategy increases the frequency of hypokalemia, hypochloremia, hypomagnesemia, alkalosis and azotemia and requires careful management. The benefits and complications of combined loop and thiazide diuretic therapy are illustrated in the clinical account of a patient with the nephrotic syndrome and resistance to 240 mg daily of furosemide (Figure 6). Dividing the daily furosemide dose in two had little effect. The addition of 80 mmol of KCl daily improved the hypokalemia modestly. However, the addition of only 25 mg of hydrochlorothiazide daily while maintaining the loop diuretic led to a 10 Kg loss of body weight, but hypokalemia returned despite the large dose of oral KCl. The further addition of 100 mg of eplerenone daily to block the mineralocoricostroid receptor (MR) restored a normal serum potassium and led to a small further weight loss.
Figure 6. Response of a patient with furosemide-resistant edema from the nephrotic syndrome to treatment with diuretics and potassium chloride.
Loop diuretic plus collecting duct (CD) diuretic:
Amiloride and triamterene block the epithelial sodium channel (ENaC) in the connecting tubule and CD. They can correct hypokalemic metabolic alkalosis since they secondarily inhibit the secretion of K+ and H+. Urine from proteinuric patients contains sufficient proteases such as plasmin to hydrolyze the luminal peptide loops of ENaC thereby opening the Na+ channel and promoting Na+ reabsorption 83, 84. This can be blocked by a CD diuretic. Indeed, CD diuretics enhance furosemide diuresis in children with minimal change glomerulonephritis 85, patients with proteinuria and CHF 86 and in other proteinuric conditions 87. However, randomized clinical trials are presently lacking and CD diuretics can cause dangerous hyperkalemia.
Loop diuretic plus mineralocorticoid receptor antagonists (MRAs):
The ATHENA-HF trial reported that the addition of 100 mg of spironolactone daily to standard diuretic therapy for patients with worsening HF failed to improve outcomes 88. This might be a consequence of prescribing spironolactone for only four days that may be insufficient to accumulate its active metabolite canrenone.
Loop diuretics plus PT and DT diuretic, MRA and aquaretic:
Diuretic resistance entails upregulation of the downstream Na+ transporters, thereby leading to impaired natriuresis on the one hand and to enhanced tubular K+ and H+ secretion in the distal nephron thereby leading to hypokalemic metabolic alkalosis on the other. This provides a rationale for segmental nephron blockade with multiple diuretic agents given concurrently to enhance diuretic responses but reduce electrolyte disturbances. Patients with acute HF and severe diuretic resistance treated with furosemide, metolazone and spironolactone plus, in some, tolvaptan and acetazolamide and supplemental KCl had a daily loss of 3 – 4 l of fluid yet remarkably their serum electrolytes and creatinine were unchanged 89. This novel strategy of segmental nephron blockade merits further prospective evaluation.
Dose escalation or intravenous infusions of loop diuretics:
Stepwise increases in the daily doses of iv furosemide from 125 to 250 to 500 mg failed to increase Na+ or fluid output of patients with refractory CHF 90. Doubling the dose of furosemide in the DOSE study did not affect the global assessment of symptoms in patients with severe HF 91. However, in patients with less severe AHF, each doubling of the dose of a loop diuretic increased daily Na+ excretion, albeit modestly, by 21 mmol 92. Thus, while dose escalation and twice daily administration of loop diuretics is an appropriate initial therapy for most patients, increasing the daily dose above the ceiling does not usually relieve diuretic resistance. Many patients become resistant and require additional measures 6, 20.
Another approach is to change from oral to iv loop diuretic administration. Infusions of furosemide, compared to intermittent doses, in patients with HF increase urine output in some trials but the effects are generally modest 93, often unaccompanied by symptomatic improvement 4 and unconfirmed in other trials 91, 94, 95. This may relate to the rapid development of tubular tolerance 22 (Figure 3A).
Long acting formulations:
Recently, 20 mg of an extended release (ER) formulation of torsemide, compared to 20 mg of an immediate release formulation, was reported to double the Na+ and fluid losses without increasing K+ excretion 22. This was related to a longer time on the efficient ascending phase of the natriuresis/torsemide excretion relationship (Figure 3A), a limited time for post-diuretic Na+ retention (Figure 3C) and a better maintained GFR. Torsemide ER produced a negative Na+ balance in subjects receiving ~ 300 mmol of Na+ daily that, if confirmed, might lessen the need for restriction of salt intake.
Perspectives:
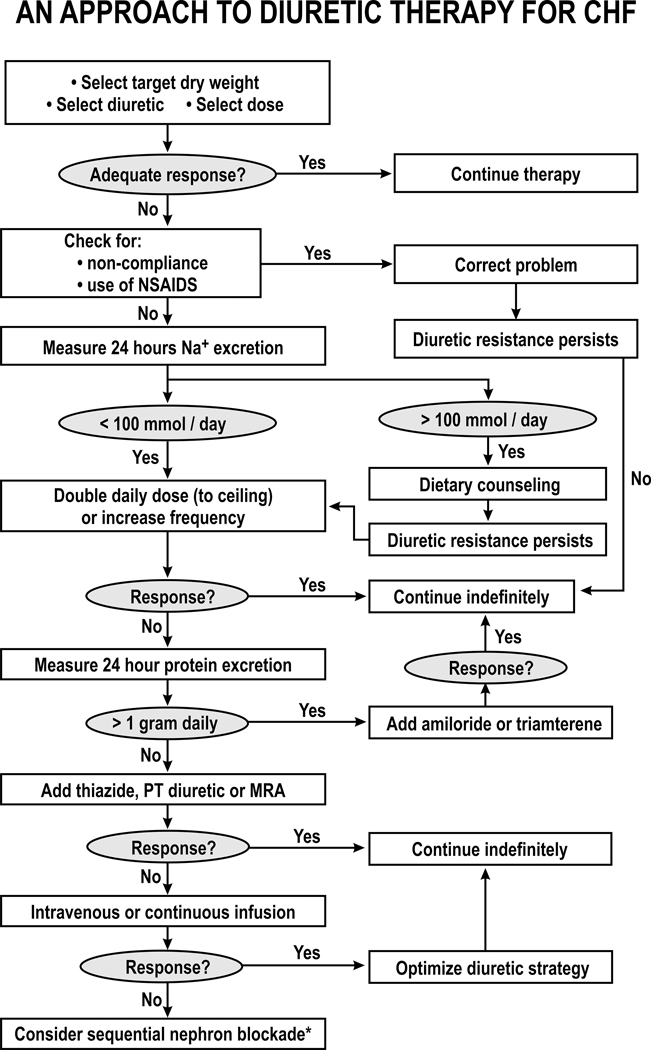
Resistance to diuretics is a frequent, but a sometimes preventable or reversible, cause of hospitalization for congestion and worsening symptoms. Unfortunately, clinical signs and symptoms are often unreliable to detect diuretic resistance. The development of new diuretics, strategies or combinations are important to overcome diuretic resistance. Many factors can contribute to diuretic resistance that provide rationales for the use of specific interventions. As recently presented 6 these strategies are shown in Figure 7. However, they have not been rigorously tested in clinical trials. Therefore, this should be used as a guide for consideration of appropriate treatment rather than a rigorous algorithm.
Figure 7. Diagrammatic representation of an approach to the management of diuretic resistance in patients with heart failure:
DCT, distal convoluted tubule diuretic; NSAIDs, non-steroidal anti-inflammatory agents; PT, proximal tubule; MRA, mineralocorticosteroid antagonist *, experimental therapy.
Sources of Funding:
Work in CSW’s laboratory is supported by the Walters Family Chair of Cardiovascular Research and from gifts to the Georgetown University Hypertension Center and the Gildenhorn/Speisman Family Fund and the Smith-Kogod Foundation.
Footnotes
Disclosures:
CSW is an academic advisor to Sarfez Inc that evaluated 22 and is developing an extended release formulation of torsemide that is supported by an SBIR grant from the NIDDK of the NIH (DK098856). He was the unpaid PI of a trial of dapagliflozin/bumetanide interaction that was funded by Bristol Myers Squibb and Astra Zeneca and was published 66
References
- 1.ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nature reviews. Cardiology. 2015;12:184–192 [DOI] [PubMed] [Google Scholar]
- 2.Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A, Chen M, Wilson FP, Darlington A, Bellumkonda L, Jacoby D, Tang WH, Parikh CR. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circulation. Heart failure. 2016;9:e002370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiernan MS, Stevens SR, Tang WHW, Butler J, Anstrom KJ, Birati EY, Grodin JL, Gupta D, Margulies KB, LaRue S, Davila-Roman VG, Hernandez AF, de Las Fuentes L. Determinants of diuretic responsiveness and associated outcomes during acute heart failure hospitalization: An analysis from the nhlbi heart failure network clinical trials. Journal of cardiac failure. 2018;24:428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strobeck JE, Feldschuh J, Miller WL. Heart failure outcomes with volume-guided management. JACC. Heart failure. 2018;6:940–948 [DOI] [PubMed] [Google Scholar]
- 5.Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: Role for blood volume quantitation. JACC. Heart failure. 2014;2:298–305 [DOI] [PubMed] [Google Scholar]
- 6.Hoorn EJ, Wilcox CS, Ellison DH. Chapter 50, diuretics. Brenner and rector’s the kidney eleventh edition. Elsevier; 2020:1708–1740.
- 7.Wilcox CS. New insights into diuretic use in patients with chronic renal disease. Journal of the American Society of Nephrology : JASN. 2002;13:798–805 [DOI] [PubMed] [Google Scholar]
- 8.Brater DC. Pharmacokinetics of loop diuretics in congestive heart failure. British heart journal. 1994;72:S40–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Furosemide absorption altered in decompensated congestive heart failure. Annals of internal medicine. 1985;102:314–318 [DOI] [PubMed] [Google Scholar]
- 10.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clinical pharmacology and therapeutics. 1995;57:601–609 [DOI] [PubMed] [Google Scholar]
- 11.Vormfelde SV, Schirmer M, Hagos Y, Toliat MR, Engelhardt S, Meineke I, Burckhardt G, Nürnberg P, Brockmöller J. Torsemide renal clearance and genetic variation in luminal and basolateral organic anion transporters. British journal of clinical pharmacology. 2006;62:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthaei J, Brockmöller J, Tzvetkov MV, Sehrt D, Sachse-Seeboth C, Hjelmborg JB, Möller S, Halekoh U, Hofmann U, Schwab M, Kerb R. Heritability of metoprolol and torsemide pharmacokinetics. Clinical pharmacology and therapeutics. 2015;98:611–621 [DOI] [PubMed] [Google Scholar]
- 13.Wilcox CS, Mitch WE, Kelly RA, Skorecki K, Meyer TW, Friedman PA, Souney PF. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. The Journal of laboratory and clinical medicine. 1983;102:450–458 [PubMed] [Google Scholar]
- 14.Aronson D, Burger AJ. Diuretic response: Clinical and hemodynamic predictors and relation to clinical outcome. Journal of cardiac failure. 2016;22:193–200 [DOI] [PubMed] [Google Scholar]
- 15.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: Insights from the dose trial. Journal of cardiac failure. 2016;22:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brater DC. Diuretic therapy in congestive heart failure. Congestive heart failure (Greenwich, Conn.). 2000;6:197–201 [PubMed] [Google Scholar]
- 17.Wilcox CS, Mitch WE, Kelly RA, Friedman PA, Souney PF, Rayment CM, Meyer TW, Skorecki KL. Factors affecting potassium balance during frusemide administration. Clinical science (London, England : 1979). 1984;67:195–203 [DOI] [PubMed] [Google Scholar]
- 18.Kelly RA, Wilcox CS, Mitch WE, Meyer TW, Souney PF, Rayment CM, Friedman PA, Swartz SL. Response of the kidney to furosemide. Ii. Effect of captopril on sodium balance. Kidney international. 1983;24:233–239 [DOI] [PubMed] [Google Scholar]
- 19.Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin MF, Richardson D, Powell LH. Impact of dietary sodium restriction on heart failure outcomes. JACC. Heart failure. 2016;4:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoorn EJ, Ellison DH. Diuretic resistance. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brater DC. Use of diuretics in cirrhosis and nephrotic syndrome. Seminars in nephrology. 1999;19:575–580 [PubMed] [Google Scholar]
- 22.Shah S, Pitt B, Brater DC, Feig PU, Shen W, Khwaja FS, Wilcox CS. Sodium and fluid excretion with torsemide in healthy subjects is limited by the short duration of diuretic action. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed]
- 23.Wilcox CS, Guzman NJ, Mitch WE, Kelly RA, Maroni BJ, Souney PF, Rayment CM, Braun L, Colucci R, Loon NR. Na+, k+, and bp homeostasis in man during furosemide: Effects of prazosin and captopril. Kidney international. 1987;31:135–141 [DOI] [PubMed] [Google Scholar]
- 24.Osborn JL, Holdaas H, Thames MD, DiBona GF. Renal adrenoceptor mediation of antinatriuretic and renin secretion responses to low frequency renal nerve stimulation in the dog. Circulation research. 1983;53:298–305 [DOI] [PubMed] [Google Scholar]
- 25.Almeshari K, Ahlstrom NG, Capraro FE, Wilcox CS. A volume-independent component to postdiuretic sodium retention in humans. Journal of the American Society of Nephrology : JASN. 1993;3:1878–1883 [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. American journal of physiology. Renal physiology. 2016;310:F958–971 [DOI] [PubMed] [Google Scholar]
- 27.Bell T, Araujo M, Luo Z, Tomlinson J, Leiper J, Welch WJ, Wilcox CS. Regulation of fluid reabsorption in rat or mouse proximal renal tubules by asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase 1. American journal of physiology. Renal physiology. 2018;315:F74–F78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods LL, DeYoung DR, Smith BE. Regulation of renal hemodynamics after protein feeding: Effects of loop diuretics. The American journal of physiology. 1991;261:F815–823 [DOI] [PubMed] [Google Scholar]
- 29.Ghys A, Denef J, Delarge J, Georges A. Renal effects of the high ceiling diuretic torasemide in rats and dogs. Arzneimittel-Forschung. 1985;35:1527–1531 [PubMed] [Google Scholar]
- 30.Nusing RM, Treude A, Weissenberger C, Jensen B, Bek M, Wagner C, Narumiya S, Seyberth HW. Dominant role of prostaglandin e2 ep4 receptor in furosemide-induced salt-losing tubulopathy: A model for hyperprostaglandin e syndrome/antenatal bartter syndrome. Journal of the American Society of Nephrology : JASN. 2005;16:2354–2362 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Zhang Y, Yang X, Wang X, Zhang J, Fang J, Jiang X. Hemodynamic effects of furosemide on renal perfusion as evaluated by asl-mri. Academic radiology. 2012;19:1194–1200 [DOI] [PubMed] [Google Scholar]
- 32.Feola M, Lombardo E, Taglieri C, Vallauri P, Piccolo S, Valle R. Effects of levosimendan/furosemide infusion on plasma brain natriuretic peptide, echocardiographic parameters and cardiac output in end-stage heart failure patients. Medical science monitor : international medical journal of experimental and clinical research. 2011;17:Pi7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araujo M, Solis G, Welch WJ, Wilcox CS. Renal nerve deafferentation attenuates the fall in gfr during intravenous infusion of furosemide in anesthetized rats. Kidney & blood pressure research. 2020;45:70–83 [DOI] [PubMed] [Google Scholar]
- 34.Petersen JS. Interactions between furosemide and the renal sympathetic nerves. Pharmacology & toxicology. 1999;84 Suppl 1:1–47 [DOI] [PubMed] [Google Scholar]
- 35.Simeoni M, Boyde A, Shirley DG, Capasso G, Unwin RJ. Application of red laser video-rate scanning confocal microscopy to in vivo assessment of tubular function in the rat: Selective action of diuretics on tubular diameter. Experimental physiology. 2004;89:181–185 [DOI] [PubMed] [Google Scholar]
- 36.Gilmer GG, Deshpande VG, Chou CL, Knepper M. Flow resistance along the rat renal tubule. American journal of physiology. Renal physiology. 2018;315:F1398-f1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araujo M, Welch WJ, Zhou X, Sullivan K, Walsh S, Pasternak A, Wilcox CS. Inhibition of romk blocks macula densa tubuloglomerular feedback yet causes renal vasoconstriction in anesthetized rats. American journal of physiology. Renal physiology. 2017;312:F1120-f1127 [DOI] [PubMed] [Google Scholar]
- 38.Oppermann M, Hansen PB, Castrop H, Schnermann J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. American journal of physiology. Renal physiology. 2007;293:F279–287 [DOI] [PubMed] [Google Scholar]
- 39.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;308:R79–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araujo M, Solis G., Welch WJ., Wilcox CS. Renal nerve deafferentation attenuates the fall in gfr during intravenous infusion of furosemide into anesthetized rats. Kidney & blood pressure research. 2019;45:70–83 [DOI] [PubMed] [Google Scholar]
- 41.Zheng H, Liu X, Katsurada K, Patel KP. Renal denervation improves sodium excretion in rats with chronic heart failure: Effects on expression of renal enac and aqp2. American journal of physiology. Heart and circulatory physiology. 2019, DOI: 10.1152/ajpheart.00299.2019 [DOI] [PMC free article] [PubMed]
- 42.Chen W, Ling Z, Xu Y, Liu Z, Su L, Du H, Xiao P, Lan X, Shan Q, Yin Y. Preliminary effects of renal denervation with saline irrigated catheter on cardiac systolic function in patients with heart failure: A prospective, randomized, controlled, pilot study. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2017;89:E153–e161 [DOI] [PubMed] [Google Scholar]
- 43.Gao JQ, Yang W, Liu ZJ. Percutaneous renal artery denervation in patients with chronic systolic heart failure: A randomized controlled trial. Cardiology journal. 2018 [DOI] [PMC free article] [PubMed]
- 44.Tahir E, Koops A, Warncke ML, Starekova J, Neumann JT, Waldeyer C, Avanesov M, Lund GK, Fischer R, Adam G, Blankenberg S, Wenzel UO, Brunner FJ. Effect of renal denervation procedure on left ventricular mass, myocardial strain and diastolic function by cmr on a 12-month follow-up. Japanese journal of radiology. 2019;37:642–650 [DOI] [PubMed] [Google Scholar]
- 45.Motwani JG, Struthers AD. Captopril augments both basal and frusemide-induced natriuresis in normal man by suppression of circulating angiotensin ii. British journal of clinical pharmacology. 1992;34:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG, Testani JM. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation. 2018;137:2016–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldsmith SR, Francis GS, Cowley AW Jr., Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. Journal of the American College of Cardiology. 1983;1:1385–1390 [DOI] [PubMed] [Google Scholar]
- 48.Goldsmith SR. The role of vasopressin in congestive heart failure. Cleveland Clinic journal of medicine. 2006;73 Suppl 3:S19–23 [DOI] [PubMed] [Google Scholar]
- 49.Lanfear DE, Sabbah HN, Goldsmith SR, Greene SJ, Ambrosy AP, Fought AJ, Kwasny MJ, Swedberg K, Yancy CW, Konstam MA, Maggioni AP, Zannad F, Gheorghiade M. Association of arginine vasopressin levels with outcomes and the effect of v2 blockade in patients hospitalized for heart failure with reduced ejection fraction: Insights from the everest trial. Circulation. Heart failure. 2013;6:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitada S, Kikuchi S, Sonoda H, Yoshida A, Ohte N. Elevation of arginine vasopressin levels following loop diuretic therapy as a prognostic indicator in heart failure. The Journal of international medical research. 2016;44:1430–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo X, Jin Q, Wu Y. Tolvaptan add-on therapy in patients with acute heart failure: A systematic review and meta-analysis. Pharmacology research & perspectives. 2020;8:e00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghali JK, Farah JO, Daifallah S, Zabalawi HA, Zmily HD. Conivaptan and its role in the treatment of hyponatremia. Drug design, development and therapy. 2009;3:253–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loon NR, Wilcox CS. Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clinical science (London, England : 1979). 1998;94:287–292 [DOI] [PubMed] [Google Scholar]
- 54.Hanberg JS, Rao V, Ter Maaten JM, Laur O, Brisco MA, Perry Wilson F, Grodin JL, Assefa M, Samuel Broughton J, Planavsky NJ, Ahmad T, Bellumkonda L, Tang WH, Parikh CR, Testani JM. Hypochloremia and diuretic resistance in heart failure: Mechanistic insights. Circulation. Heart failure. 2016;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hropot M, Klaus E, Unwin R, Giebisch G. Diminished diuretic and natriuretic response to furosemide in potassium-depleted rats. Renal physiology and biochemistry. 1994;17:10–20 [DOI] [PubMed] [Google Scholar]
- 56.Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, Nagase S, Morino Y. Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney international. 1987;32:198–203 [DOI] [PubMed] [Google Scholar]
- 57.Chalasani N, Gorski JC, Horlander JC Sr, Craven R, Hoen H, Maya J, Brater DC. Effects of albumin/furosemide mixtures on responses to furosemide in hypoalbuminemic patients. Journal of the American Society of Nephrology : JASN. 2001;12:1010–1016 [DOI] [PubMed] [Google Scholar]
- 58.Agarwal R, Gorski JC, Sundblad K, Brater DC. Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. Journal of the American Society of Nephrology : JASN. 2000;11:1100–1105 [DOI] [PubMed] [Google Scholar]
- 59.Boer WH, Koomans HA, Dorhout Mees EJ. Renal haemodynamics and sodium handling after hyperoncotic albumin infusion in sodium-restricted normal man. European journal of clinical investigation. 1987;17:442–447 [DOI] [PubMed] [Google Scholar]
- 60.Koomans HA, Geers AB, vd Meiracker AH, Roos JC, Boer P, Dorhout Mees EJ. Effects of plasma volume expansion on renal salt handling in patients with the nephrotic syndrome. American journal of nephrology. 1984;4:227–234 [DOI] [PubMed] [Google Scholar]
- 61.Haws RM, Baum M. Efficacy of albumin and diuretic therapy in children with nephrotic syndrome. Pediatrics. 1993;91:1142–1146 [PubMed] [Google Scholar]
- 62.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, Hou FF. Albumin overload activates intrarenal renin-angiotensin system through protein kinase c and nadph oxidase-dependent pathway. Journal of hypertension. 2011;29:1411–1421 [DOI] [PubMed] [Google Scholar]
- 63.Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, Tang WHW, Droogne W, Mullens W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. European journal of heart failure. 2019;21:1415–1422 [DOI] [PubMed] [Google Scholar]
- 64.Fallahzadeh MA, Dormanesh B, Fallahzadeh MK, Roozbeh J, Fallahzadeh MH, Sagheb MM. Acetazolamide and hydrochlorothiazide followed by furosemide versus furosemide and hydrochlorothiazide followed by furosemide for the treatment of adults with nephrotic edema: A randomized trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69:420–427 [DOI] [PubMed] [Google Scholar]
- 65.Cil O, Haggie PM, Phuan PW, Tan JA, Verkman AS. Small-molecule inhibitors of pendrin potentiate the diuretic action of furosemide. Journal of the American Society of Nephrology : JASN. 2016;27:3706–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. Journal of the American Heart Association. 2018;7:e007046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilcox CS. Antihypertensive and renal mechanisms of sglt2 (sodium-glucose linked transporter 2) inhibitors. Hypertension (Dallas, Tex. : 1979). 2020;75:894–901 [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez-Vicente A, Saez F, Monzon CM, Asirwatham J, Garvin JL. Thick ascending limb sodium transport in the pathogenesis of hypertension. Physiological reviews. 2019;99:235–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reyes-Pardo H, Bautista R, Vargas-Robles H, Rios A, Sánchez D, Escalante B. Role of sodium/glucose cotransporter inhibition on a rat model of angiotensin ii-dependent kidney damage. BMC nephrology. 2019;20:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in heart failure: Diuretic and cardio-renal effects. Circulation. 2020; doi: 10.1161/CIRCULATIONAHA.120.045691 [DOI] [PMC free article] [PubMed]
- 71.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. The New England journal of medicine. 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 72.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. The American journal of physiology. 1985;248:F374–381 [DOI] [PubMed] [Google Scholar]
- 73.Kaissling B, Stanton BA. Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. The American journal of physiology. 1988;255:F1256–1268 [DOI] [PubMed] [Google Scholar]
- 74.Kim J, Welch WJ, Cannon JK, Tisher CC, Madsen KM. Immunocytochemical response of type a and type b intercalated cells to increased sodium chloride delivery. The American journal of physiology. 1992;262:F288–302 [DOI] [PubMed] [Google Scholar]
- 75.Ellison DH, Velázquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. The Journal of clinical investigation. 1989;83:113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco-Bacik MA, Wilson FP, Jacoby D, Chen M, Tang WHW, Cherney DZI, Ellison DH, Testani JM. Compensatory distal reabsorption drives diuretic resistance in human heart failure. Journal of the American Society of Nephrology : JASN. 2017;28:3414–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loon NR, Wilcox CS, Unwin RJ. Mechanism of impaired natriuretic response to furosemide during prolonged therapy. Kidney international. 1989;36:682–689 [DOI] [PubMed] [Google Scholar]
- 78.Kissling KT, Pickworth KK. Comparison of the effects of combination diuretic therapy with oral hydrochlorothiazide or intravenous chlorothiazide in patients receiving intravenous furosemide therapy for the treatment of heart failure. Pharmacotherapy. 2014;34:882–887 [DOI] [PubMed] [Google Scholar]
- 79.Brisco-Bacik MA, Ter Maaten JM, Houser SR, Vedage NA, Rao V, Ahmad T, Wilson FP, Testani JM. Outcomes associated with a strategy of adjuvant metolazone or high-dose loop diuretics in acute decompensated heart failure: A propensity analysis. Journal of the American Heart Association. 2018;7:e009149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dormans TP, Gerlag PG. Combination of high-dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure. European heart journal. 1996;17:1867–1874 [DOI] [PubMed] [Google Scholar]
- 81.Tanaka M, Oida E, Nomura K, Nogaki F, Fukatsu A, Uemura K, Yashiro M, Kimura T, Muso E, Ono T. The na+-excreting efficacy of indapamide in combination with furosemide in massive edema. Clinical and experimental nephrology. 2005;9:122–126 [DOI] [PubMed] [Google Scholar]
- 82.Knauf H, Mutschler E. Sequential nephron blockade breaks resistance to diuretics in edematous states. Journal of cardiovascular pharmacology. 1997;29:367–372 [DOI] [PubMed] [Google Scholar]
- 83.Zheng H, Liu X, Sharma NM, Li Y, Pliquett RU, Patel KP. Urinary proteolytic activation of renal epithelial na+ channels in chronic heart failure. Hypertension (Dallas, Tex. : 1979). 2016;67:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. Journal of the American Society of Nephrology : JASN. 2009;20:299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guigonis V. Abstract 135a. Journal of the American Society of Nephrology. 2001
- 86.Allman S, Norris RJ. An open, parallel group study comparing a frusemide/amiloride diuretic and a diuretic containing cyclopenthiazide with sustained release potassium in the treatment of congestive cardiac failure--a multicentre general practice study. The Journal of international medical research. 1990;18 Suppl 2:17b–23b [PubMed] [Google Scholar]
- 87.Svenningsen P, Andersen H, Nielsen LH, Jensen BL. Urinary serine proteases and activation of enac in kidney--implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Archiv : European journal of physiology. 2015;467:531–542 [DOI] [PubMed] [Google Scholar]
- 88.Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne-Nickens P, Hernandez AF, Braunwald E. Efficacy and safety of spironolactone in acute heart failure: The athena-hf randomized clinical trial. JAMA cardiology. 2017;2:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goyfman M, Zamudio P, Jang K, Chee J, Miranda C, Butler J, Wadhwa NK. Combined aquaretic and diuretic therapy in acute heart failure. International journal of nephrology and renovascular disease. 2017;10:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paterna S, Di Gaudio F, La Rocca V, Balistreri F, Greco M, Torres D, Lupo U, Rizzo G, di Pasquale P, Indelicato S, Cuttitta F, Butler J, Parrinello G. Hypertonic saline in conjunction with high-dose furosemide improves dose-response curves in worsening refractory congestive heart failure. Advances in therapy. 2015;32:971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364:797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M, Sam Broughton J, D’Ambrosi J, Wilson Tang WH, Damman K, Voors AA, Ellison DH, Testani JM. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. European journal of heart failure. 2017;19:1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuriyama A, Urushidani S. Continuous versus intermittent administration of furosemide in acute decompensated heart failure: A systematic review and meta-analysis. Heart failure reviews. 2019;24:31–39 [DOI] [PubMed] [Google Scholar]
- 94.Wu MY, Chang NC, Su CL, Hsu YH, Chen TW, Lin YF, Wu CH, Tam KW. Loop diuretic strategies in patients with acute decompensated heart failure: A meta-analysis of randomized controlled trials. Journal of critical care. 2014;29:2–9 [DOI] [PubMed] [Google Scholar]
- 95.Palazzuoli A, Pellegrini M, Franci B, Beltrami M, Ruocco G, Gonnelli S, Angelini GD, Nuti R. Short and long-term effects of continuous versus intermittent loop diuretics treatment in acute heart failure with renal dysfunction. Internal and emergency medicine. 2015;10:41–49 [DOI] [PubMed] [Google Scholar]