Abstract
Introduction
HIV-1 and Mtb are characterized by immune activation and unbalances production of cytokines, but the expression of IL33 in HIV/TB coinfection remain understudied. This study aimed to evaluate the level of IL-33 in plasma of HIV and M. tuberculosis (HIV/TB) coinfected patients compared to patients with respective mono infections in Yaoundé.
Methods
a cross-sectional study was conducted among patients attending the pneumology service and HIV treatment center of the Yaoundé Jamot Hospital. Plasma samples of 157 HIV/TB coinfected patients (n =26, 50% males and 50% females, mean age 39), HIV-1 monoinfected patients (n = 41, 41% males and 59% females, mean age 35), TB monoinfected patients (n = 48, 56% males and 44% females, mean age 37) and healthy controls (n = 42, 29% males and 71% females, mean age 32) were examined by enzyme-linked immunoassay (ELISA) to detect the levels of IL-33 cytokine.
Results
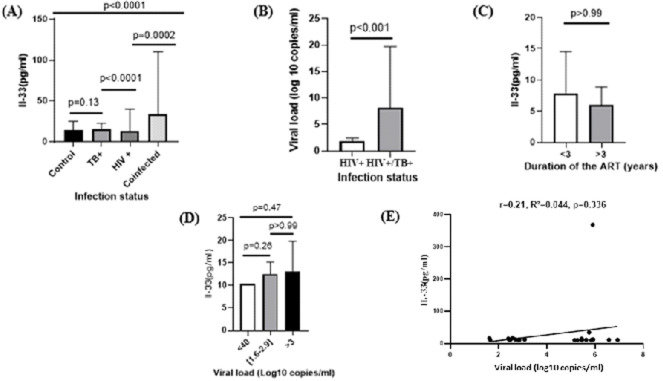
plasma level of IL-33 were higher in HIV/TB coinfected (33.1±30.9 pg/ml) and TB monoinfected individuals (15.1±2.9 pg/ml) compared to healthy controls (14.0±3.4 pg/ml) and could not be detected in most of the HIV-1 monoinfected individuals (12.6±8.7 pg/ml). Interestingly, the increased plasma level of IL-33 in HIV/TB coinfected patients showed a statistically significant difference between healthy controls (33.1±30.9 pg/ml vs 14.0±3.4 pg/ml, P<0.0001) and HIV-1 monoinfected patients (33.1±30.9 pg/ml vs 12.6±8.7 pg/ml, P=0.0002). We further found that IL-33 was higher in patients with high viral load group (40.6±59.7 pg/ml vs 12.6±1.8 pg/ml), P= 0.47) whereas patients under highly active antiretroviral therapy (HAART) showed decreased level of IL-33 concentration as the number of years under ART increased. Our data showed a positive association between plasma IL-33 and viral load in the context of HIV/TB coinfection in our study population with a positive Pearson coefficient of r=0.21.
Conclusion
this study indicates that plasma level of IL-33 differs among HIV/TB coinfected patients and respective monoinfections patients. The increased level of plasma IL-33 reveals that IL-33 measurement in HIV-1 monoinfected patients may represent an early predictor of development of tuberculosis.
Keywords: Interleukin-33, HIV, tuberculosis, coinfection, Cameroon, Africa
INTRODUCTION
Tuberculosis is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb), which is transmitted via aerosols from close contacts with tuberculosis patients. HIV infection has emerged as the principal cause of human immunodepression exposing people living with HIV to opportunistic diseases such as Mtb. The geographical overlapping of the two diseases had increased the number of cases of HIV/TB co-infection causing an immense and continuous burden on public health systems, mostly in Low and Middle Income Countries (LMIC) [1]. In fact, people living with HIV are about 30 times more likely to develop active TB, due to compromised immunity [2]. The Joint United Nations Programme on HIV/AIDS (UNAIDS) has reported in 2016 that one million people died of opportunistic infections out of the 36,7 million people living with HIV [3]. Cameroon is classified among the most affected countries by HIV/AIDS in Central Africa. The prevalence rate is estimated at 4.3% and women and children are the most vulnerable [4]. In Cameroon, it is approximatively 39% of all the tuberculosis cases that were positive to an infection with HIV in 2014 [5]. Currently, it is well known that both HIV and tuberculosis (TB) have a profound effect on the immune system and are characterized by a dysregulation of the normal balance of cytokines and the functioning of the cytokine network. The imbalance of cytokine secretion in HIV infection affects the function of the immune system and the course of the disease, increasing or suppressing viral replication [6]. Numerous data indicate that, despite effective antiretroviral therapy (ART), there is evidence of persistent viral production and immune activation in HIV-infected individuals [7-9]. IL-33 exerts multiple effects on immune cells, and is considered as a key player in the control of viral, bacterial and even parasitic infections. IL-33 is a member of the Il-1 superfamily of cytokines and perform its role as an alarm signal in response to cellular damage induced by infection or injury to alert immune cells expressing the receptor [10]. The nature of the antiviral immune response orchestrated by IL-33 depends on the site of infection, the duration and the cytokine environment [11]. A series of studies followed various parameters in HIV/TB coinfection as compared to HIV monoinfection to conclude that Mtb and HIV act in synergy, accelerating the decline of immunological functions and leading to subsequent death if untreated [12-14]. Therefore, the analysis of cytokines expressed during a HIV/TB coinfection may be important for predicting the course of the disease. While some studies have already been focused on the evaluation of the expression profile of IL-33 in hepatic disease during schistosomiasis [15], hepatitis, malaria [16], HIV infected patients [17] and others on tuberculosis [18], very few studies have been carried out on the expression of IL-33 in HIV/TB coinfection compared to each of the mono infections [19-20]. The goal of this work was to study the expression of IL-33 cytokine in the context of HIV/TB coinfection and to compare to patients with HIV-1 and TB monoinfections.
METHODS
Study design and settings: the study was a cross-sectional study that was carried out at the Jamot Hospital of Yaoundé, located in the Yaoundé I subdivision, Mfoundi Division, in the Center Region of Cameroon. This health facility is a reference center for treatment and care for both HIV and TB-infected patients in-country [21].
Study population: the study population was mainly composed of patients from different population pools at the Jamot Hospital of Yaoundé who voluntarily agreed to participate in the study. These included HIV/TB coinfected patients (n = 26) and TB monoinfected patients (n = 48) who were recruited in the pneumology service of the hospital, HIV-1 monoinfected patients (n = 41) recruited at the HIV treatment service of the hospital and healthy controls (n= 42) from the general population were recruited for the study at the blood bank of the same hospital. Healthy controls repeatedly tested negative for HIV-1 and had no history of TB or exposure to the disease within the past 6 months.
Data collection: informed sessions were done in the presence of patients to clearly explain the objective and the methodology of our study. After obtaining the informed consent, a standardized questionnaire was administered to collect demographic and clinical data. Patients with HIV/TB coinfection were naïve for ART and anti-tuberculosis therapy, HIV-positive patients were under first-line ART regimen (tenofovir disoproxil fumarate (TDF) + lamivudine (3TC) (or emtricitabine, FTC) + efavirenz (EFV) 600 mg), TB- positive patients were under first-line drugs (isoniazid, rifampicin). TB diagnoses were based on clinical symptoms, sputum microscopy and TB-Lamp (Tuberculosis-Loop-Mediated Isothermal Amplification) method. The patients were diagnosed as HIV-positive using Rapid Diagnostic Tests (RDT) following the in-country HIV national algorithm.
Laboratory analysis: sputum samples were stained for acid-fast bacilli and were graded by light microscopy. Cultures were examined weekly until positive for visible colonies or for a maximum of 8 weeks. Plasma was isolated according to the standard procedure. The whole blood was collected in a vacutainer with EDTA and centrifuged at 1000 rpm for 15-20 min with cooling. The plasma was collected, aliquoted, and stored at -80°C at the National Public Health Laboratory until further analysis. The plasma level of IL-33 was measured using ELISA Kit from BioLegend, Inc. USA (catalog number 435907). The test was performed on undiluted plasma following the manufacturer recommendations. The cytokine concentration was determined using a standard curve obtained with the standards provided by the manufacturer (sensitivity 4.14 pg/mL), and the results were expressed in pg/mL.
Statistical analysis: the nonparametric T-test was used to compare two independent groups. The Kruskal-Wallis test was used to compare more than two independent groups. The correlation was evaluated using Spearman´s rank correlation test. The data were analyzed using GraphPad Prism v9.5.0 (GraphPad Software, Boston, MA, USA). Values of p < 0.05 were considered statistically significant.
Ethical consideration: all individuals were over 18 years old and gave written informed consent for participation in the study. According to the General Data Protection Regulation (GDPR) requirements, all participants were deidentified and anonymized by assigning them unique codes expressed as an identifier. All clinical samples, data, and study results were stored in an anonymized form. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee for the Research in Human Health of the School of Health Sciences (approval No.2020/01028/CEIRSH/ESS/MIM).
RESULTS
Characteristics of the study population
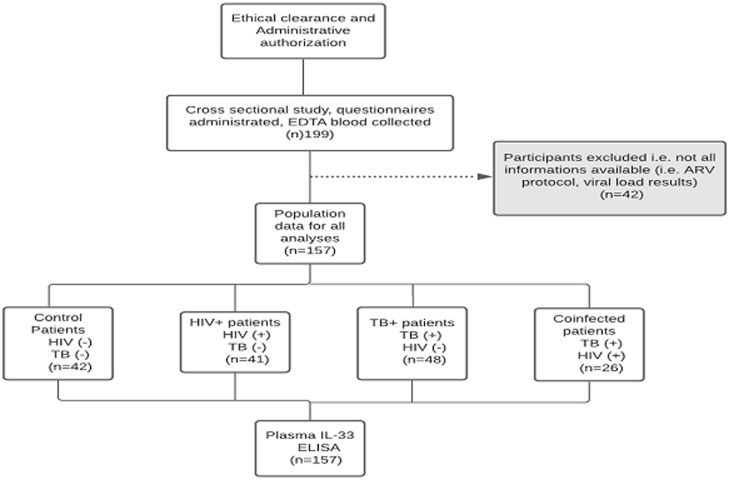
The participants and samples used for the present study were defined as indicated (Figure 1). Samples of participants missing any test result were excluded i.e., missing samples of blood or sputum, ART regimen, viral load results. A total of 157 patients were registered and divided into four different clusters of healthy control, HIV-1 monoinfected, TB monoinfected and HIV/TB coinfected patients. Out of 157 participants enrolled in the study, 26 patients were HIV/TB coinfected patients, 41 HIV-1 monoinfected patients, 48 TB monoinfected patients and 42 healthy controls. The participants' distribution by age, gender and viral load for HIV-positive infected participants are presented below (Table 1). The groups did not differ in demographic indicators. Females predominated (56%) compared to the male gender (43.9%) and the HIV/TB coinfected group had the highest mean age 38.7± 8.6 years. The median viral load in HIV/TB coinfected patients was high (4.22 log10 copies/mL) as compared to that in patients with HIV alone (2.9 log10 copies/mL).
Figure 1.
flow diagram describing the strategy of enrolment and examination of 157 patients from Yaoundé Jamot Hospital (YJH), Cameroon
Table 1.
characteristics of the study population
| Study groups N (%) | ||||
|---|---|---|---|---|
| CONTROL 42(26.7) | HIV (+) 41 (26.1) | TB (+) 48 (30.5) | HIV/TB 26(16.6) | |
| Age | ||||
| Mode [Range] | 34[22-52] | 31[24-59] | 29[23-61] | 39[25-57] |
| Mean (SD) | 31.7 (7.7) | 35.4 (8.2) | 36.7 (11.3) | 38.7 (8.6) |
| Gender | ||||
| Male/Female | 12/30 | 17/24 | 27/21 | 13/13 |
| Viral load (log10 copies/mL), IQR | - | 2.9 (1.7-3.4) | - | 4.2 (2.6-5.8) |
Plasma levels of IL-33 in patients with HIV/TB coinfection compared to HIV and TB monoinfections
To assess whether plasma levels of IL-33 were different from one group to another, plasma IL-33 concentrations were represented for all participants according to their infection status as reported in Figure 2, A. All four patient´s groups differed in the levels of IL-33 expression, with the major differences observed between the patients with TB monoinfection and HIV/TB coinfection. IL-33 secretion in the group of patients with HIV/TB coinfection who were naïve to anti-tuberculosis and antiretroviral therapy was increased by 2.6 times, and 2.4 times compared to the group of patients with HIV alone (33.1 ± 30.9 pg/ml vs 12.6 ± 8.7 pg/ml, p=0.002) and controls (33.1 ± 30.9 pg/ml vs 14.0 ± 3.4 pg/ml, p<0.0001) respectively (Figure 2, A). Comparison of viral load between HIV/TB coinfected participants versus HIV monoinfected participants presented in (Figure 2, B) showed a very high viral load in HIV/TB co-infected participants compared to the other group (8.24 ± 11.4 log10 copies/mL vs 1.7 ± 0.7 log10 copies/mL, p<0.001). Furthermore, we studied the association of levels of IL-33 with disease progression. We divided patients into three subgroups according to their viral loads: low viral load (<40 log10 copies/mL), medium viral load (1.6-2.9 log10 copies/mL) and high viral load (>3 log10 copies/mL). The results showed that IL-33 was higher in patients with high viral load group (69.1 ± 25.3 pg/ml) as compared to those with medium viral load (12.6 ± 1.8 pg/ml ) and low viral load ( 40.6 ± 59.8 pg/ml) (Figure 2, D). Interestingly, although the differences were not statistically significant, there was a tendency for plasma IL-33 levels to decrease as the number of years under ART therapy increased (3.7 ± 6.7 pg/ml vs 3.6 ± 2.8 pg/ml, p>0.99) (Figure 2, C). This was convincingly substantiated by the observation that plasma IL-33 levels increased with viral load in HIV/TB coinfected individuals clearly establishing the positive association between plasma IL-33 and viral load in the context of HIV/TB coinfection in our study population (r=0.21, p=0.34; Figure 2, E).
Figure 2.
plasma levels of IL-33 in patients with HIV/TB coinfection, HIV monoinfection, TB monoinfection, and healthy controls. A) comparative graph of plasma level of IL-33 in HIV/TB coinfection, HIV monoinfection, TB monoinfection individuals cataloged according to their infectious status. B) HIV viral load of all enrolled participants with HIV and HIV/TB coinfection. C) plasma level of IL-33 according to antiretroviral treatment duration in HIV-monoinfected individuals. D) plasma level of IL-33 according to the viral load in HIV/TB coinfected participants. E) correlation between IL-33 and viral load in HIV/TB coinfected participants
DISCUSSION
A full understanding of the pathogenesis of HIV/TB coinfection has yet to be achieved. In the present study, to gain better understanding of the role of IL-33 cytokine in immunopathogenesis of HIV/TB coinfection, we aimed to determine whether HIV/TB coinfected patients compared to patients with respective mono infections are associated with alterations in IL-33. The results of our study showed that plasma levels of IL-33 were elevated in HIV/TB coinfected and TB monoinfected patients compared to healthy controls and could not be detected in most of the HIV-1 monoinfected individuals.
Both HIV-1 infection, even when successfully treated, and Mtb infection are characterized by chronic inflammation. Inflammation is a nonspecific response to pathogens that involves multiple cells, tissues, and organs. While acute inflammation is stopped, chronic inflammation persists, leading to a chronic inflammatory disease characterized by an overproduction of proinflammatory cytokines and recruitment of inflammatory cells (neutrophils and monocytes) to the affected area(s) [20]. Thus, Mtb and HIV-1 exploit different scenarios of immune hyperactivation/chronic inflammation. In this study, we have addressed their overlap in drug-naïve patients in the context of HIV/TB coinfection, HIV-1 infected patients under ART and Mtb under first-line drugs in a resource-limited setting. To the best of our knowledge, no data have clearly explained the expression of IL-33 plasma levels in the context of HIV/TB infection in Africa.
The first main finding of this study was that HIV/TB coinfected and TB monoinfected patients showed an up-regulated concentrations of IL-33. IL-33 is a cytokine of the IL-1 family with pro-inflammatory and anti-inflammatory effects in response to infection [22]. IL-33 attracted attention because, aside from its traditional role as an “alarmin,” it is known to be involved in innate and adaptive immune responses by enhancing natural killer, Th2, CD4 and CD8 T-cell functions which play a central role in the protective immunity against Mtb and HIV infection [19]. In our study, HIV/TB coinfection was characterized by high plasma levels of IL-33, which were increased 2.6-fold and 2.4 fold compared to the levels in patients with HIV-1 and TB monoinfections indicating the additive effects of the two pathogens on IL-33 production. In this way, HIV-induced production of IL-33, protective for HIV-1 patients under highly active antiretroviral therapy, appeared to be detrimental for naïve HIV-1/TB coinfected patients, contributing to HIV-1 persistence and tissue damage in the context of HIV/TB coinfection. Previous studies on the alterations of IL-33 in HIV and TB -infected patients showed inconsistent conclusions [11,17,23-25]. We found that the serum level of Il-33 was slightly higher in the TB monoinfection patients compared to healthy controls, although no statistically significant difference was observed between the two groups, which is consistent with previous research [25]. Perhaps this was due to normal variation above the level of IL-33, which could become significant with increasing the sample size. It should also be considered that the control group included in this study had no history of infection or inflammation, which in turn affects IL-33 levels. It was also found that the expression of IL-33 levels was not increased in HIV infected patients compared to the healthy controls, which is contrary with previous research [24]. The elevation of IL-33 has been observed in a number of types of cancers [26,27], in COVID-19 infection [28] and in chronic hepatitis B infection [29]. Our study was consistent with Miyagaki et al. and Mehraj et al. studies, which didn't find the elevated expression of IL-33 in HIV infected individuals. However, our finding was not consistent with Wu et al. study which found elevated expression of IL-33 in HIV infected individuals. All these studies used ELISA kit to detect the expression levels of IL-33, so the methodology may not be the cause of the different results. We postulated that the differences in clinical characteristics of patients may lead to the different findings in these studies. For example, our study as well as Miyagaki et al. and Mehraj et al. studies enrolled HIV infected patients under ART, while the HIV infected patients enrolled in Wu et al. study were naïve HIV infected individuals. This can be explained by the fact that plasma obtained from HIV-infected individuals under ART display constitutive production of biologically active IL-1α and IL-1β proteins, which are suppressed during the course of antiretroviral therapy [22,23].
We then studied IL-33 expression level with respect to disease progression in HIV infected individuals. Although IL-33 can enhance immune response function, we observed that the increased IL-33 level in naïve HIV/TB coinfected individuals correlates positively with viral load which was consistent with previous research [17]. We postulated that the function of IL-33 in naïve HIV/TB coinfection may be masked due to the complex immune regulation. Additionally, HIV-1 patients under highly active antiretroviral therapy (HAART) showed decreased level of IL-33 concentration as the number of years under ART increased. This is in accordance with the data of other researchers who received similar results [24]. A previous study has shown that mucosal barrier tissues such as gastrointestinal tract store large amounts of IL-33 that may be released upon tissue injury [30]. In HIV infection, gut damage occurs early after infection [31], which can cause the elevation of IL-33 and sST2. Most likely that gut tissue damage in early HIV infection are linked to the elevation of IL-33 observed in naïve HIV/TB coinfected individuals in our study.
This study has a major strength that must be highlighted. Since 2013, the public health policies in Cameroon recommend ART administration as soon as possible after the diagnosis of HIV positivity. Therefore, the HIV+ individuals evaluated in this study present clinical conditions related to infection and clinical progression which are now rare and very difficult to obtain in new studies involving HIV+ populations. As potential limitations of this study, we have to consider (i) the sample size, (ii) we did not exclude parasitic diseases that can also increase or reduce the production of our chosen cytokine, (iii) we did not have the opportunity for a long term follow-up of patients, (iv) the evaluation of a single cytokine instead of a combination of several cytokines profiles. This type of data is useful to indicate general patterns of immune activity and Th1/Th2/Th17 balances. Future studies will be conducted on a wider group of patients and will include a detailed characterization of the Th1, Th2 and Th17 cytokines in plasma of patients to attribute the observed profiles of cytokine expression as well as to correlate it with the clinical course of both infections.
CONCLUSION
We have shown that naïve HIV/TB coinfected patients are characterized by increased plasma levels of IL-33, which is crucial for the control of both infections. The increased levels of plasma IL-33 indicate that IL-33 measurement in HIV-1 monoinfected patients can be a potential surrogate marker of immune activation in the context of HIV-TB coinfection in a resource-limited setting. This data broadens the understanding of HIV pathogenesis and provides critical information for HIV intervention.
What is known about this topic
Both HIV and tuberculosis (TB) have a profound effect on the immune system and are characterized by a dysregulation of the normal balance of cytokines and the functioning of the cytokine network;
Elevation of IL-33 has been observed in a number of types of cancers, in COVID-19 infection, in hepatic disease during schistosomiasis, in malaria and in chronic hepatitis B infection;
Previous studies on the alterations of IL-33 in HIV and TB -infected patients showed inconsistent conclusions.
What this study adds
This study shows the upregulation of IL-33 cytokine in plasma in the context of HIV/TB coinfection compared to patients with respective mono infections;
The increased level of IL-33 in HIV/TB coinfected individuals correlates positively with the viral load;
The measurement of IL-33 in HIV-1 monoinfected patients may represent an early predictor of development of tuberculosis.
Acknowledgments
The authors would like to thank all the patients who participated in this study and are grateful to the Jamot Hospital of Yaoundé where preliminary analyses were performed and for their assistance in providing research authorizations.
Footnotes
Cite this article: René Ghislain Essomba et al. Plasma IL-33 levels and immune activation in HIV-TB coinfection: a cross-sectional study in Yaoundé, Cameroon. Pan African Medical Journal. 2023;46(13).
Competing interests
The authors declare no competing interests.
Authors' contributions
Conceptualization: René Ghislain Essomba and François Xavier Mbopi-Kéou; formal analysis and investigation: René Ghislain Essomba, Rostand Munkam Mbe, Marie Paule Ngogang, Severin Donald Kamdem, Valentina Josiane Ngo Bitoungui, Justin Komguep Nono and Claire Bitchong Ekono; data curation and methodology: René Ghislain Essomba, Severin Donald Kamdem, Pulchérie Thérèse Ateba, Philippe Salomon Nguwoh, Nassif Seni, Valentina Josiane Ngo Bitoungui, Myriam Sylvie Ambomo; writing the original draft preparation: René Ghislain Essomba, Nassif Seni, Marie Claire Okomo Assoumou; resources, supervision and funding acquisition: René Ghislain Essomba, Nassif Seni, Marie Claire Okomo Assoumou, Marie Paule Ngogang, Claire Bitchong Ekono; writing-review and editing: René Ghislain Essomba, Marie Paule Ngogang, Justin Komguep Nono, François Xavier Mbopi-Kéou. All the authors have read and approved the final manuscript.
References
- 1.Akwafuo S, Abah T, Oppong J. Evaluation of the Burden and Intervention Strategies of TB-HIV Co-Infection in West Africa. J Infect Dis Epidemiol. 2020 Jul 17;6 [Google Scholar]
- 2.Zumla A, Malon P, Henderson J, Grange J. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000 May;76(895):259–68. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouanfack OSD, Kouanfack C, Billong SC, Cumber SN, Nkfusai CN, Bede F, et al. Epidemiology of Opportunistic Infections in HIV Infected Patients on Treatment in Accredited HIV Treatment Centers in Cameroon. Int J Matern Child Health AIDS. 2019;8(2):163–72. doi: 10.21106/ijma.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNICEF UNICEF Cameroon Country Programme. Strategy Note HIV/AIDS Programme. 2018;2020:2020. [Google Scholar]
- 5.Noeske J, Nana Yakam A, Abena Foe J-L. Epidemiology of tuberculosis in Cameroon as mirrored in notification data, 2006-2014. Int J Tuberc Lung Dis. 2016 Nov;20(11):1489–1494. doi: 10.5588/ijtld.16.0252. [DOI] [PubMed] [Google Scholar]
- 6.Osuji FN, Onyenekwe CC, Ahaneku JE, Ukibe NR. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. J Biomed Sci. 2018 Dec 3;25(1):88. doi: 10.1186/s12929-018-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs JL, Halvas EK, Tosiano MA, Mellors JW. Persistent HIV-1 Viremia on Antiretroviral Therapy: Measurement and Mechanisms. Front Microbiol. 2019;10:2383. doi: 10.3389/fmicb.2019.02383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoenigl M, Kessler HH, Gianella S. Editorial: HIV-Associated Immune Activation and Persistent Inflammation. Front Immunol. 2019;10:285. doi: 10.3389/fimmu.2019.02858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalfamo M, Le Saout C, Lane HC. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev. 2012;23(4-5):207–14. doi: 10.1016/j.cytogfr.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cayrol C, Girard JP. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev. 2018 Jan;281(1):154–68. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 11.Mehraj V, Ponte R, Routy JP. The Dynamic Role of the IL-33/ST2 Axis in Chronic Viral-infections: Alarming and Adjuvanting the Immune Response. EBioMedicine. 2016 Jul 1;9:37–44. doi: 10.1016/j.ebiom.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012 Feb;8(2):e1002464. doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018 Feb;16(2):80–90. doi: 10.1038/nrmicro.2017.128. [DOI] [PubMed] [Google Scholar]
- 14.Devi P, Khan A, Chattopadhyay P, Mehta P, Sahni S, Sharma S, et al. Co-infections as Modulators of Disease Outcome: Minor Players or Major Players? Front Microbiol. 2021 Jul 6;12:664386. doi: 10.3389/fmicb.2021.664386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamdem SD, Konhawa F, Kuemkon EM, Meyo Kamguia L, Tchanana GK, Nche F, et al. Negative Association of Interleukin-33 Plasma Levels and Schistosomiasis Infection in a Site of Polyparasitism in Rural Cameroon. Front Immunol. 2019;10:2827. doi: 10.3389/fimmu.2019.02827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayimba E, Hegewald J, Ségbéna AY, Gantin RG, Lechner CJ, Agosssou A, et al. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol. 2011 Nov;166(2):218–26. doi: 10.1111/j.1365-2249.2011.04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Li Y, Song CB, Chen YL, Fu YJ, Jiang YJ, et al. Increased Expression of sST2 in Early HIV Infected Patients Attenuated the IL-33 Induced T Cell Responses. Front Immunol. 2018;17 doi: 10.3389/fimmu.2018.02850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karbalaei M, Ghazvini K, Keikha M. IL-33/ST2 Axis as a Well-Known Endogenous Defense against Tuberculosis. Rev Clin Med. 2020 Sep 1;7(3):127–33. [Google Scholar]
- 19.Devalraju KP, Neela VSK, Ramaseri SS, Chaudhury A, Van A, Krovvidi SS, et al. IL-17 and IL-22 production in HIV+ individuals with latent and active tuberculosis. BMC Infect Dis. 2018 Jul 11;18(1):321. doi: 10.1186/s12879-018-3236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosik M, Belikova MG, Ryzhov K, Avdoshina D, Sobkin A, Zverev V, et al. Unique Profile of Proinflammatory Cytokines in Plasma of Drug-Naïve Individuals with Advanced HIV/TB Co-Infection. Viruses. 2023;1330 doi: 10.3390/v15061330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pefura Yone EW, Kengne AP, Kuaban C. Incidence, time and determinants of tuberculosis treatment default in Yaounde, Cameroon: a retrospective hospital register-based cohort study. BMJ Open. 2011 Nov 24;1(2):e000289. doi: 10.1136/bmjopen-2011-000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011 Aug 26;8(1):22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyagaki T, Sugaya M, Yokobayashi H, Kato T, Ohmatsu H, Fujita H, et al. High levels of soluble ST2 and low levels of IL-33 in sera of patients with HIV infection. J Invest Dermatol. 2011 Mar;131(3):794–6. doi: 10.1038/jid.2010.366. [DOI] [PubMed] [Google Scholar]
- 24.He B, Zheng L, Zhou H, He Y, Chen Z, Xiao S, et al. Dynamic observation of IL-33 and its receptors in HIV patients who received HAART. Cell Mol Biol Noisy--Gd Fr. 2017 Mar 31;63(3):73–7. doi: 10.14715/cmb/2017.63.3.14. [DOI] [PubMed] [Google Scholar]
- 25.Youssefi M, Teimouri G, Zahedi Avval F, Ghazvini K, Keikha M. Evaluation of Interleukin 33 and ST2 serum concentration in active tuberculosis patients. Rev Clin Med. 2021;8(3):106–10. [Google Scholar]
- 26.Ngongang P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Dig Dis Sci. 2011 Dec;56(12):3596–601. doi: 10.1007/s10620-011-1760-5. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Shen JX, Hu JL, Huang WH, Zhang GJ. Significance of interleukin-33 and its related cytokines in patients with breast cancers. Front Immunol. 2014;5:141. doi: 10.3389/fimmu.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markovic SS, Jovanovic M, Gajovic N, Jurisevic M, Arsenijevic N, Jovanovic M, et al. IL 33 Correlates With COVID-19 Severity, Radiographic and Clinical Finding. Front Med. 2021 Nov 30;8:749569. doi: 10.3389/fmed.2021.749569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu J, et al. Serum IL-33 Levels Are Associated with Liver Damage in Patients with Chronic Hepatitis B. J Interferon Cytokine Res. 2012 Jun;32(6):248–53. doi: 10.1089/jir.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015 May 14;125(20):3183–92. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponte R, Mehraj V, Ghali P, Couëdel-Courteille A, Cheynier R, Routy JP. Reversing Gut Damage in HIV Infection: Using Non-Human Primate Models to Instruct Clinical Research. EBioMedicine. 2016 Feb;4:40–9. doi: 10.1016/j.ebiom.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]