Abstract
Vascular function is dynamically regulated and dependent on a bevy of cell types and factors that work in concert across the vasculature. The vasoactive eicosanoid, 20-Hydroxyeicosatetraenoic acid (20-HETE) is a key player in this system influencing the sensitivity of the vasculature to constrictor stimuli, regulating endothelial function, and influencing the renin angiotensin system (RAS), as well as being a driver of vascular remodeling independent of blood pressure elevations. Several of these bioactions are accomplished through the ligand-receptor pairing between 20-HETE and its high-affinity receptor, GPR75. This 20-HETE axis is at the root of various vascular pathologies and processes including ischemia induced angiogenesis, arteriogenesis, septic shock, hypertension, atherosclerosis, myocardial infarction and cardiometabolic diseases including diabetes and insulin resistance. Pharmacologically, several preclinical tools have been developed to disrupt the 20-HETE axis including 20-HETE synthesis inhibitors (DDMS and HET0016), synthetic 20-HETE agonist analogues (20-5,14-HEDE and 20-5, 14-HEDGE) and 20-HETE receptor blockers (AAA and 20-SOLA). Systemic or cell-specific therapeutic targeting of the 20-HETE-GPR75 axis continues to be an invaluable approach as studies examine the molecular underpinnings activated by 20-HETE under various physiological settings. In particular, the development and characterization of 20-HETE receptor blockers look to be a promising new class of compounds that can provide a considerable benefit to patients suffering from these cardiovascular pathologies.
1. Introduction
20-Hydroxyeicosatetraenoic acid (20-HETE) is a potent vasoactive eicosanoid shown to be instrumental in the regulation of endothelial function, vascular tone, vascular remodeling and blood pressure; working alone and in concert with an array of mediators that influence vascular function. The molecular mechanisms associated with 20-HETE are varied and dependent on the associated target cells, receptors, and tissues. Elucidation of these diverse arrays of signaling pathways is vital for the understanding of the 20-HETE-axis and development of 20-HETE targeted therapeutics for the treatment of vascular inflammation/dysfunction, hypertension and cardiometabolic disease.
2. 20-HETE biosynthesis
20-HETE synthesis occurs intracellularly across a variety of cell types including smooth muscle cells (Gebremedhin et al., 1998; Harder et al., 1994; Miyata & Roman, 2005; Parmentier, Lavrentyev, Falck, Capdevila, & Malik, 2005; Singh et al., 2007; Williams, Murphy, Burke, & Roman, 2010; Xu, Falck, Ortiz de Montellano, & Kroetz, 2004), platelets (Tsai, Croft, Puddey, Beilin, & Barden, 2011), neutrophils (Tsai et al., 2011), endothelial progenitor cells (Chen et al., 2014) (EPC) and in mature endothelial cells under hypoxic conditions (Chen et al., 2014). Interestingly, endothelial cells under normoxic conditions have yet to be identified as a source of 20-HETE. 20-HETE is a product of the ω-hydroxylation of arachidonic acid (AA), a polyunsaturated fatty acid and major component of membrane phospholipids, by distinct enzymes of the cytochrome P450, CYP4A and CYP4F sub-families. The ω-hydroxylation reaction inserts a hydroxyl group at the terminal sp3 carbon group of AA (O’Donnell, Maskrey, & Taylor, 2009). The human CYP isoforms responsible for the majority of 20-HETE include CYP4A11 and CYP4F2, while CYP4F11 and CYP4F3 have limited capacities to generate 20-HETE. The dominant 20-HETE synthase in mice is the CYP4a12 isoform; in rats, CYP4A1, CYP4A2, CYP4A3, CYP4F1, and CYP4F2 are the prevailing 20-HETE synthases (Waldman, Peterson, Arad, & Hochhauser, 2016). In general, the CYP4 enzymes share a conserved sequence homology and catalytic properties. Interestingly, these enzymes are distinctly distributed across tissues and tightly regulated by various factors, including being subjected to transcriptional regulation by nuclear receptors including peroxisomal proliferator-activated receptor α (PPARα) and the androgen receptor (AR) (Hiratsuka, Matsuura, Watanabe, Sato, & Suzuki, 1996; Holla et al., 2001; Thomas et al., 2015; Vasudevan, Yuen, & McNeill, 2012), post-translational regulation by microRNAs (Hutcheson et al., 2013; Rocic & Schwartzman, 2018) and upregulated by various autacoids including angiotensin II (Ang II), serotonin [5-hydroxytryptamine (5-HT)] (Cambj-Sapunar, Yu, Harder, & Roman, 2003) and endothelin 1 (ET-1) (Tsai et al., 2011). These are all responsible for stimulating CYP4A elevations throughout the kidney (Rong et al., 2018; Zhou et al., 1864), vasculature (Ito, Omata, Ito, Hoagland, & Roman, 2001) and beyond. Experimentally, 20-HETE synthesis is inhibited by nitric oxide (NO) and can be increased through the use of nitric oxide synthase (NOS) inhibitors (Oyekan & McGiff, 1998). Moreover, elevations in 20-HETE are correlated with various pathologies including hypertension (Pascale, Lucchesi, & Garcia, 2021; Rocic & Schwartzman, 2018; Wang et al., 2022; Williams et al., 2010), chronic kidney disease (Dreisbach et al., 2014), atherosclerosis (Auguet et al., 2018), obesity/body mass index (BMI) (Gilani et al., 2018; Issan et al., 2013), and liver/hepatic fibrosis (Rocic & Schwartzman, 2018) across human studies and various animal models.
3. 20-HETE receptors
Recent discoveries have uncovered the presence of receptors that pair with 20-HETE and elicit an array of biological effects. Presently, these receptors can be stratified as high- and low-affinity receptors of 20-HETE, i.e., GPR75 and GPR40/FFAR1, respectively.
4. G protein-coupled receptor 75 (GPR75)
GPR75 is a G-protein coupled receptor (GPCR) and member of the Gq-coupled Rhodopsin Class A family of GPCRs. 20-HETE is recognized as a high-affinity ligand for GPR75 (Pascale et al., 2021). The 20-HETE-GPR75 pairing is associated with activation of the receptor complex via dissociation of the Gαq/11 G protein, changes in intracellular Ca2+, IP-1 accumulation, activation of downstream signal cascades and β-arrestin recruitment (Fan & Roman, 2017; Garcia et al., 2017; Pascale et al., 2021). The aforementioned signaling cascades include the transactivation of the epidermal growth factor receptor (EGFR) followed by a MAPK-IKKβ-NF㮫- signaling program that enhances the transcriptional activation of angiotensin converting enzyme (ACE), increasing circulating angiotensin II levels (Garcia et al., 2015a, 2017; Garcia, Shkolnik, Milhau, Falck, & Schwartzman, 2016). Moreover, the activation of the IKK-complex results in the uncoupling of endothelial nitric oxide synthase (eNOS), reducing nitric oxide (NO) bioavailability, further driving endothelial dysfunction across the vasculature (Fig. 1A) (Cheng et al., 2008, 2012). In prostate cancer cells (PC-3), the 20-HETE-GPR75 pairing is associated with increasing epithelial-mesenchymal transition (EMT), as well as metalloproteinase-2 (MMP-2) activity ostensibly involving HIC-5, EGFR, NF-κB, AKT, p-AKT, p-38 and PKCα (Cardenas et al., 1865). Moreover, the pairing of 20-HETE and GPR75 has been shown to influence the transcription androgen receptor (AR) correlated with changes in AR-driven gene expression (CREB3L2, ELL2, FKBP5, GREB1, HPGD, IGF1, KLK3, NDRG1, NFKB1, PRKCA, TMPRSS2, TWIST1) in LNCaP cells (Cardenas et al., 2023). The relationship between 20-HETE-GPR75 and cancer is not limited to the prostate cancer as a clear association between 20-HETE-GPR75 and non-small cell lung cancer (NSCLC) has recently been uncovered (Chen et al., 2022; Van Ginderachter, 2022). In fact, a 20-HETE-GPR75-STAT3-c-Jun axis has been shown to influence NSCLC immune checkpoints like PD-L1 and elicit the production of proangiogenic factors like IL6 and TGFβ (Chen et al., 2022). Interestingly, the activation of GPR75 via a 20-HETE mimetic in the context of septic shock is closely associated with a signaling cascade involving Gαq/11, GPR kinase interactor 1 (GIT1), proto-oncogene tyrosine-protein kinase (c-Src), PKCα and the MaxiK² channel; all of which protect against hypotension and the consequent tachycardia associated with vascular hyporeactivity (see septic shock) (Tunctan et al., 2022).
Fig. 1.
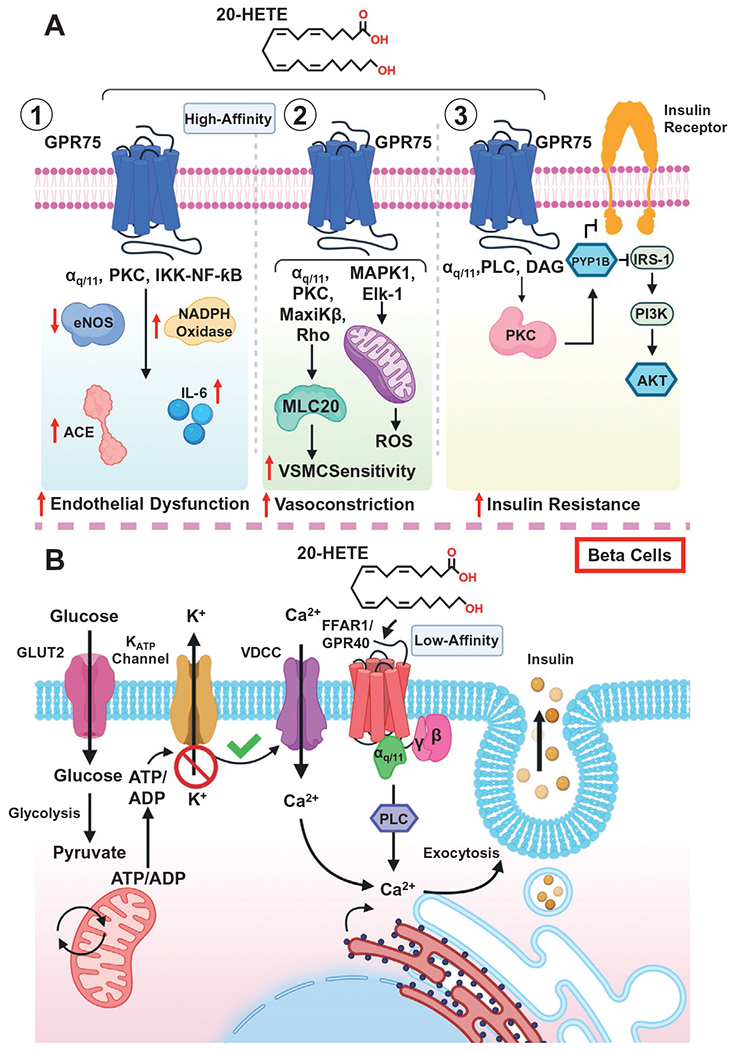
20-HETE receptor signaling. (A) Molecular mechanisms and pathways associated with the pairing of 20-HETE and GPR75 across (1) endothelial cells, (2) vascular smooth muscle cells and (3) adipose tissues. (B) The signaling pathways associated with the pairing of 20-HETE and the free fatty acid receptor (FFAR/GPR40). Glucose drives changes in intracellular calcium that alongside 20-HETE, promotes and enhance increases in glucose-stimulated insulin secretion through the activation of FFAR1/GPR40 in beta cells of the pancreas.
5. G protein-coupled receptor 40 (GPR40)/free fatty acid receptor-1 (FFAR-1)
GPR40, also recognized as free fatty acid receptor-1 (FFAR-1), is a Gq/G11-coupled receptor expressed across various cell types including pancreatic β-cells, human and bovine endothelial cells, smooth muscle cells, arteries, hepatocytes and macrophages (Park et al., 2018; Tomita, Hosoda, Fujikura, Inagaki, & Nakao, 2014; Tunaru et al., 2018). Interestingly, studies have demonstrated GPR40’s ability to utilize long-chain fatty acids as ligands, including various eicosanoids (Park et al., 2018). Most notably, arachidonic acid, 11,12-EET, 14,15-EET and 20-HETE act as GPR40 ligands, albeit with low-affinity (Park et al., 2018; Tunaru et al., 2018). With respect to 20-HETE, studies have illustrated its ability to promote glucose-stimulated insulin secretion (GSIS) via the presence and activation of GPR40 across β-cells of the pancreas (Tunaru et al., 2018). These cells are essential for the production of insulin and impairments to the pancreas and β-cell function can result in the development of type 1 and type 2 diabetes depending on the severity of the attack or dysfunction (Dirice et al., 2019). This current model suggests that glucose elicits changes in intracellular calcium via GLUT2 followed by a sequence of intracellular changes that are coupled alongside the pairing of 20-HETE to GPR40 to drive insulin secretion (Fig. 1B) (Tunaru et al., 2018). Further studies are necessary to fully uncover the vascular consequences associated with the pairing of 20-HETE and GPR40 across endothelial and vascular smooth muscle cells.
6. Vascular bioactions of 20-HETE
6.1. Endothelial cells
20-HETE promotes an array of actions across the vasculature, particularly in endothelial and vascular smooth muscle cells. Specifically, 20-HETE elicits an inflammatory signaling cascade inducing reactive oxygen species (ROS), cytokine production, adhesion molecules and enzymes which promote endothelial dysfunction and promote hypertension (Pascale, Lucchesi, & Garcia, 2021). 20-HETE increases oxidative stress through the stimulation of superoxide formation and activation of NADPH oxidase across endothelial cells (Cheng et al., 2008; Medhora et al., 2008). Moreover, 20-HETE-mediated increases in ROS stimulate vascular endothelial growth factor (VEGF) synthesis and promote cell proliferation (Guo et al., 2007, 2009). Additionally, 20-HETE serves as a nonhypoxic regulator of HIF-1α, a key transcription factor in the regulation of cellular responses to oxygen levels/concentrations (Guo et al., 2009). Changes in the expression of adhesion molecules such as intracellular cell adhesion molecule 1 (ICAM-1 (Ishizuka et al., 2008)) and increases in cytokines (IL-6 (Cheng et al., 2014), IL-8, IL-13, and IL-4 (Ishizuka et al., 2008)) have also been observed in endothelial cells exposed to 20-HETE at varying concentrations. In conjunction with changes in oxidative stress, 20-HETE promotes the uncoupling of eNOS from its chaperone protein HSP90, resulting in reductions in nitric oxide (NO) synthesis and bioavailability, further exacerbating endothelial dysfunction (Cheng et al., 2008). This uncoupling event is initiated by the same MAPK-IKKβ-NFκB molecular association that drives increases in ACE expression (Fig. 1A) (Garcia et al., 2016).
7. Vascular smooth muscle cells
20-HETE is recognized as an activator and sensitizer of vascular smooth muscle cells (VSMC) in response to constrictor stimuli (Rocic & Schwartzman, 2018). This occurs through the coordinated activation of various receptors, kinases, and channels. In VSMCs, the 20-HETE-mediated activation of PKC, MAPK, tyrosine kinase, and Rho kinase culminates in the phosphorylation and inhibition of the Ca2+-activated K+ channels (BKca) (Fan et al., 2013, 2016; Rocic & Schwartzman, 2018; Zou et al., 1996). This propagates changes in VSMC depolarization resulting in elevations in cytosolic [Ca2+] via the L-type Ca2+ channels (Gebremedhin et al., 1998; Zeng et al., 2010). Additionally, activation of Rho kinase and subsequent phosphorylation of myosin light chain (MLC20) via 20-HETE increases the sensitivity of the contractile apparatus (Agostinucci et al., 2022; Randriamboavonjy, Busse, & Fleming, 2003; Rocic & Schwartzman, 2018). In VSMC, the activation of GPR75 via 20-HETE promotes a GIT1-mediated PKC-stimulated phosphorylation of the MaxiKβ (BKca channel β subunit) that can additionally facilitate vasoconstriction (Garcia et al., 2017). In conjunction with the inhibition of channels and activation of L-type Ca+2 channels, it has also been observed that transient receptor potential cation channel 6 (TRPC6) (a non-voltage-gated Ca2+ entry/depolarization channel) (Basora, Boulay, Bilodeau, Rousseau, & Payet, 2003) and transient receptor potential vanilloid 1 (TRPV1) (a non-selective cation channel) (Wen et al., 2012) are influenced by 20-HETE, which further mobilize Ca2+ and amplifies 20-HETE’s pro-vasoconstrictor effects via VSMCs (Fig. 1A) (Inoue et al., 2009). These mechanisms work in concert to prime the vasculature to produce pronounced constrictor responses. In addition to the various pro-vasoconstrictive signals, 20-HETE also elicits profound changes to oxidative stress throughout VSMCs. 20-HETE increases VSMC mitochondrial superoxide production through a MAPK1-Elk-1-dependent pathway (Lakhkar et al., 2016). This mechanism echoes the dynamic changes to ROS via 20-HETE observed in ECs.
8. Blood pressure and vascular tone
The 20-HETE-mediated changes to the vascular endothelium and smooth muscle set the stage for dynamic changes in blood pressure and vascular tone. Various animal models have illustrated 20-HETE’s sensitization of vessels to constrictor stimuli such as phenylephrine, endothelin-1 and Ang II5. Of note, Ang II, a potent driver of blood pressure in its own right, promotes the synthesis and release of 20-HETE from microvessels (Croft, McGiff, Sanchez-Mendoza, & Carroll, 2000). In turn, the released 20-HETE promotes increases to endothelial ACE protein levels and activity, which elevates circulating Ang II levels through the proteolytic conversion of Ang I to Ang II, further driving changes in blood pressure through a feed forward mechanism (Fig. 1A) (Hoopes, Garcia, Edin, Schwartzman, & Zeldin, 2015). Various animal models have confirmed the relationships associated with 20-HETE myogenic tone and blood pressure. These models include the androgen-induced hypertension model, wherein administration of 5α-dihydrotestosterone (DHT) to mice elicits increases in systolic blood pressure, an effect that can be blocked or reversed by 20-HETE antagonists (Ding et al., 2013; Garcia et al., 2015b; Wu, Gupta, Garcia, Ding, & Schwartzman, 2014). In the spontaneously hypertensive rat (SHR) model, co-treatment with an epoxyeicosatrienoic acid (EET) analogue (EET-A) and a 20-HETE receptor blocker (AAA) has been shown to exhibit antihypertensive effects and increase nitric oxide metabolite excretion, an indicator of restored or improved endothelial function (Baranowska et al., 2021). Genetic models globally overexpressing Cyp4a12 or CYP4A/F isoforms have also clearly illustrated 20-HETE’s ability to enhance myogenic responses, increase vascular reactivity, drive vascular remodeling and increase blood pressure (Garcia et al., 2015a; Liu et al., 2009). Additionally, the tissue-specific overexpression of 20-HETE synthases across the endothelium [CYP4A2 (EC-targeted (VECAD-lentivirus) and CYP4F2 (EC-targeted (Tie2)] or VSMC [CYP4A1 (VSMC-targeted SM22alpha adenoviruses) and Cyp4a12 (VSMC-targeted Myh11-Cre)] are also correlated with increased contractility, reduced vasodilatory capacity, and increased systolic blood pressure (Agostinucci et al., 2022; Cheng et al., 2014; Inoue et al., 2009; Jiang et al., 2004). Lastly, in humans, various CYP4A single nucleotide polymorphisms (SNP), particularly to CYP4A11 (−845G/A, rs1126742/T8590C and rs4660980) and CYP4F2 (rs3093100/G421C, rs2108622/c.1347G→A, rs1558139) are closely associated with hypertension and elevated 20-HETE levels(Wu et al., 2014).
9. Vascular remodeling
Persistent elevations in blood pressure are associated with increased inflammation, endothelial dysfunction, vascular contractility, end organ damage and vascular remodeling of resistance vessels across the vasculature. This remodeling is characterized by dynamic changes to media thickness, increased media: lumen ratio and cross-sectional area (Fig. 4) (Garcia et al., 2015a). Interestingly, elevated 20-HETE levels are intimately associated with the remodeling of the renal microvasculature (Agostinucci et al., 2022; Ding et al., 2013; Garcia et al., 2015a). Moreover, the remodeling process is independent of increased blood pressure and RAS activation, suggesting that vascular remodeling of resistance arteries is induced by persistent elevated systemic 20-HETE levels even when blood pressure is controlled with ACE inhibition or Ang II receptor blockade (Ding et al., 2013; Garcia et al., 2015a). Lastly, the 20-HETE-associated changes in vascular remodeling can be prevented by either administration of 20-HETE biosynthesis blockers, antagonism of 20-HETE or during deficiencies in the 20-HETE receptor, GPR75 (Agostinucci et al., 2022; Ding et al., 2013; Garcia et al., 2015a). While the precise mechanism associated with the 20-HETE-dependent and blood pressure-independent changes in vascular remodeling remain unclear, the pairing of 20-HETE to its receptor, GPR75, is a prime candidate. The endothelial dysfunction driven by the 20-HETE-GPR75 pairing results in decreases in endothelial derived NO bioavailability and reductions in endothelial-dependent vasorelaxation, which have clearly been shown to regulate and promote increases in vascular remodeling (Yu et al., 2012). Furthermore, reductions in NO are associated with a host of pro-fibrotic and inflammatory genes including plasminogen activator inhibitor-1 (PAI-1). PAI-1 is not only considered a major inhibitor of fibrinolysis; it is also intimately linked to fibrosis, SMC proliferation, vascular remodeling, endothelial dysfunction and accelerated aging (Ji et al., 2016; Vaughan, Rai, Khan, Eren, & Ghosh, 2017; Weisberg et al., 2005). Interestingly, PAI-1 has also been shown to be a negative regulator of eNOS, further impairing endothelial function (Garcia et al., 2020). 20-HETE-mediated reductions in NO may contribute to changes in PAI-1 to further promote RAS-independent increases to vascular remodeling. Additional studies are necessary to elucidate the array of molecular processes activated by the 20-HETE-GPR75 pairing that contribute to vascular remodeling.
Fig. 4.
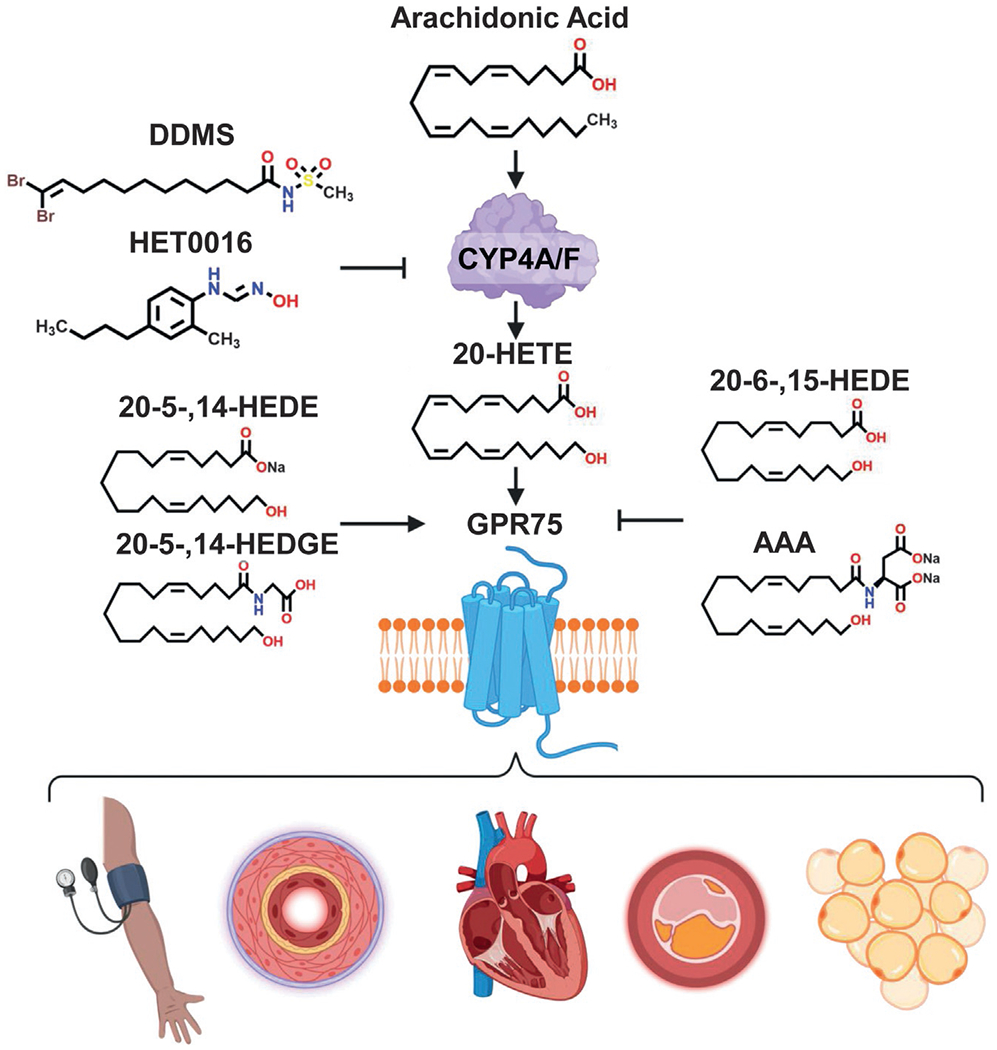
The 20-HETE-GPR75 axis. Systemic elevations in 20-HETE and the activation of its receptor, GPR75, drive various vascular pathologies, i.e., hypertension, vascular remodeling, myocardial infarction/heart failure, atherosclerosis and cardiometabolic disease. The 20-HETE-GPR75 axis can be augmented by the use of 20-HETE synthesis inhibitors (DDMS, HET0016), 20-HETE agonist analogues (20-5,14-HEDE, 20-5,14-HEDGE) or GPR75 receptor blockers (AAA, 20-SOLA (SOLA)).
10. Angiogenesis vs Arteriogenesis
Several investigations have shown 20-HETE’s ability to participate and promote angiogenesis. Early studies focused on 20-HETE’s ability to stimulate endothelial sprouting and capillary formation in renal arterial blood vessels (Jiang et al., 2004). Additionally, the use of 20-HETE synthesis inhibitors (HET0016 and DDMS) have been shown to disrupt and reduce corneal neovascularization and angiogenic responses (Chen et al., 2005; Orozco et al., 2013; Roman & Fan, 2018). 20-HETE stimulates the proliferation, migration and tube formation of endothelial cells and endothelial progenitor cells (EPC); key cells involved in ischemia-induced angiogenesis (Chen, Ackerman, & Guo, 2012; Chen et al., 2016; Guo et al., 2007, 2009). Subsequently, ischemia was shown to increase Cyp4a12 in a time-dependent manner, followed by increases in stromal cell-derived factor-1α (SDF-1α), EPC mobilization from the bone marrow and EPC homing to the site of ischemia (Chen et al., 2019). Interestingly, inflammatory neutrophil-derived myeloperoxidase (MPO) and hypochlorous acid (HOCl) have been shown to increase the endothelial cell expression of CYP4A11 and synthesis of 20-HETE thereby driving postischemic compensatory angiogenesis (Azcona et al., 2022). 20-HETE’s ability to participate in ischemia-induced angiogenesis is linked to changes across several key signaling mediators including HIF-1alpha, VEGF, VEGFR2, SDF-1α, ERK1/2 of MAPK, and Akt (Chen et al., 2016). Additionally, 20-HETE-induced increases in EPC Oct4, Sox2, and Nanog gene expression, which are indicative of increased EPC stemness (Fig. 2) (Chen et al., 2019). These changes, moreover, have been shown to be 20-HETE-dependent through the pharmacological blockade of 20-HETE synthesis and a 20-HETE antagonist, like 20-6,15-HEDGE (Chen et al., 2019).
Fig. 2.
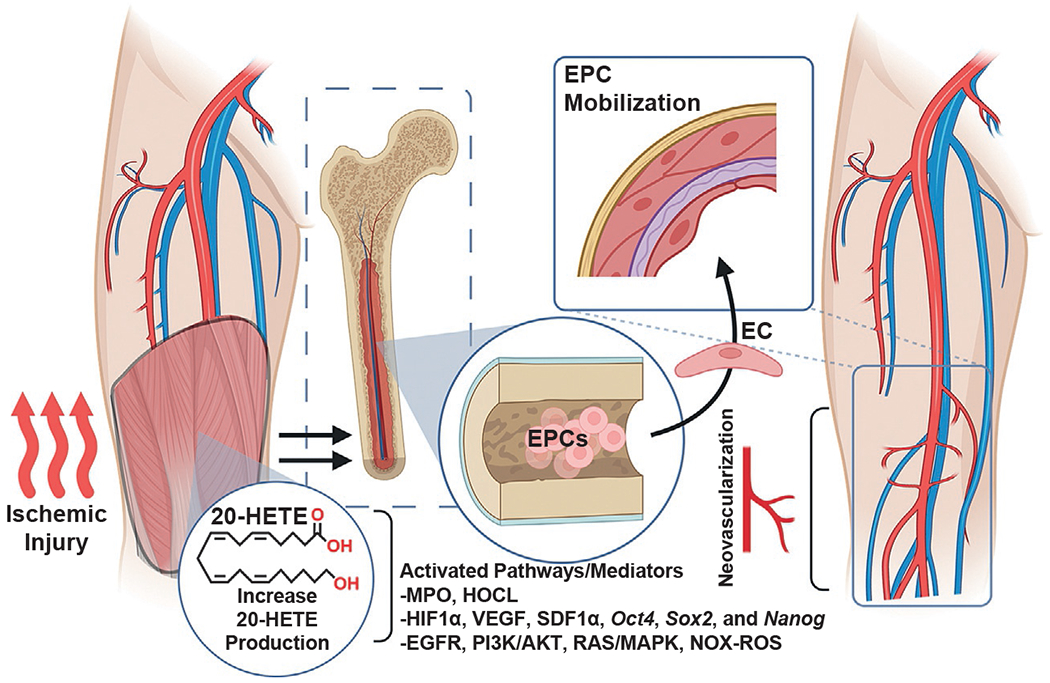
20-HETE regulates the neovascularization of ischemic tissue. Schematic illustrating the role of 20-HETE as a regulator of angiogenesis and neovascularization during ischemic injury. 20-HETE promotes the mobilization of endothelial progenitor cells (EPCs) from the bone marrow to the circulation and target site of angiogenesis.
Arteriogenesis, also known as coronary collateral growth (CCG) is the growth and enlargement of pre-existing collateral arterioles into fully developed arteries, triggered primarily from physical forces like fluid shear stress (Heil, Eitenmuller, Schmitz-Rixen, & Schaper, 2006). 20-HETE’s ability to disrupt endothelial function and alter inflammatory mediators is at the heart of the 20-HETE-mediated disruptions in arteriogenesis observed across animal models. Specifically, in a model of metabolic syndrome (with impaired CCG), 20-HETE was found to be elevated due to increased CYP4A expression (Joseph et al., 2017). The majority of 20-HETE production came from local neutrophils in the artery, while the rest was produced from endothelial cells and fibroblasts (Joseph et al., 2017). These cells all work in tandem towards arteriogenesis which shows the varied and complex actions of 20-HETE in a given micro-environment, and how it can have such strong and divergent effects. Strikingly, 20-HETE antagonism alone completely restored normal arteriogenesis in this model (Joseph et al., 2017). Mechanistically, the development of CCG and subsequent arteriogenesis differ substantially from the establish proangiogenic mechanisms associated with 20-HETE. Further studies are necessary to dissect additional drivers of arteriogenesis influenced by 20-HETE and its receptor in order to expand our understanding and the importance of these processes.
10.1. Septic shock
Septic shock occurs in a subset of patients with sepsis due to uncontrolled inflammatory and immune system dysregulations causing organ damage which results in hyperlactatemia and concurrent hypotension, which if left untreated leads to mortality (Hotchkiss et al., 2016). Experimentally in rodents, Escherichia coli derived lipopolysaccharide (LPS) serves as the agent to elicit septic shock. In rat and mouse models of LPS induced septic shock, the 20-HETE mimetic, N-(20-hydroxyeicosa-5(Z),14(Z)-dienoyl) glycine (20-5,14-HEDGE) offered protection against the nitric oxide (NO) mediated vascular hyporeactivity, hypotension, and tachycardia, preventing the mortality of endotoxemic mice (Cuez et al., 2010; Tunctan et al., 2013). Mechanistically, the protective effects associated with 20-5,14-HEDGE are due to reductions in iNOS protein expression, prevention of vasodilatory prostanoid formation, suppression of the iNOS-sGC-PKG-dependent pathway and COX-2, CYP4A1 and gp91(phox) participation (Cuez et al., 2010; Tunctan et al., 2013). Moreover, these changes are dependent on the activation of the 20-HETE receptor, GPR75, which sets in motion a Gα q/11 /PKCα/MaxiKβ, GIT1/PKCα/MaxiKβ, GIT1/c-Src/MaxiKβ, and GIT1/c-Src/EGFR signaling pathway that may serve to protect against the aforementioned changes in blood pressure and heart rate, characteristic of septic shock (Tunctan et al., 2022).
10.2. Myocardial infarction and atherosclerosis
The infrastructure of the heart is rich with CYP4A and 4F isoforms necessary for 20-HETE synthesis (Gross et al., 2004; Joseph et al., 2017; Nithipatikom et al., 2004; Rocic & Schwartzman, 2018). Interestingly, in humans, the incidence rate of myocardial infarction (MI) is closely associated with elevations in 20-HETE levels (Fu et al., 2013). In animal studies, the blockade of 20-HETE synthesis (17-ODYA and DMMS) was correlated with a marked reduction in myocardial infarct size (Gross et al., 2005; Nithipatikom et al., 2004). Likewise, the antagonism of 20-HETE has been shown to prevent 20-HETE’s ability to exacerbate cardiac injury (Nithipatikom et al., 2006). Animal studies using a repetitive ischemia protocol revealed marked changes in 20-HETE synthesis across the heart, with ischemic collateral-dependent zones (CZ) of the heart exhibiting elevated 20-HETE production as opposed to nonischemic zones (NZ) (Joseph et al., 2017). Interestingly, the microRNA, miR-145, is described as a potential upstream regulator of cardiac 20-HETE directly influencing CYP4F expression (Joseph et al., 2017). Previous ischemia reperfusion studies have also observed significant increases in 20-HETE levels throughout coronary venous plasma (Nithipatikom et al., 2001). Studies coupling this ischemic protocol alongside a rat model of metabolic syndrome (JCR: LA-cp, JCR) observed even greater elevations to 20-HETE across the ischemic CZ (Fig. 3). Additional studies exploring the JCR rat model have shown 20-HETE functions as a driver of large artery stiffness and decreased arterial compliance due to increased elastin degradation, MMP12 activation and pronounced systolic hypertension (Soler et al., 2018).
Fig. 3.
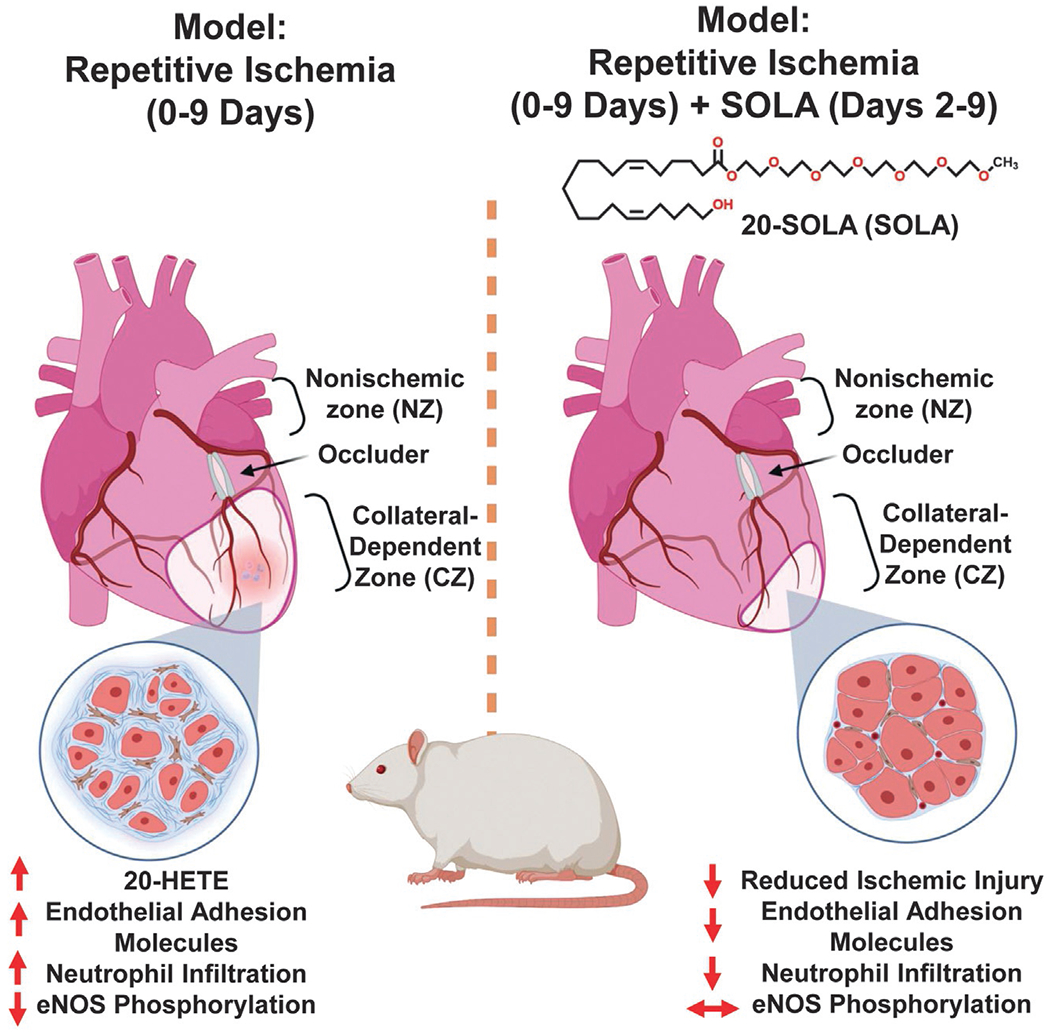
20-HETE increases myocardial ischemia and it impairs coronary collateral growth in the heart. Illustration highlighting the role of 20-HETE in the rat model of metabolic syndrome (JCR) as a driver of inflammation, endothelial dysfunction, apoptosis and neutrophil infiltration blocking the generation of coronary collateral growth across collateral-dependent zones (CZ) compared to normal zones (NZ). The pharmacological antagonism of 20-HETE via 20-SOLA restores the generation of coronary collateral growth and alleviates the heart from the pro-inflammatory bioactions associated with 20-HETE.
Aside from the changes associated with 20-HETE during myocardial infarction and ischemia, 20-HETE has been shown to dramatically disrupt cardiac myocytes. Studies examining the response of neonatal cardiac myocytes to 20-HETE exposed the dissipation of myocyte mitochondrial membrane potential alongside increases in the expression of pro-apoptotic Bax and elevated caspase 3 activity (Bao et al., 2011). Ang II-induced apoptosis of myocytes relies closely on 20-HETE and, in conjunction, these mediators disrupt the permeability of the mitochondria and promote the productions of ROS (Zhao et al., 2015). 20-HETE also participates in beta-adrenergic induced cardiac myocyte apoptosis via a calmodulin dependent kinase pathway (Jiang et al., 2017). Lastly, the 20-HETE-dependent activation of the NADPH oxidase-2 is associated with cardiac myocyte apoptosis and glycation end products in diabetes-induced heart failure (Wang et al., 2019).
Atherosclerosis is characterized by a medley of changes to the vasculature including the deposition and localization of low-density lipoproteins (LDLs) across the vascular wall, inflammatory response/processes coupled to lipid-laden macrophages developing into foam cells, disruptions in immune cell clearing, dense regions of accumulated lipids and cells; culminating in a cycle of inflammation, necrosis, fibrosis, and calcification to establish the formation of a plaque (Bentzon, Otsuka, Virmani, & Falk, 2014; Witztum & Lichtman, 2014). In a study of males undergoing carotid endarterectomy, there was a significant association between the levels of 20-HETE and carotid atheroma plaques (Auguet et al., 2018). Moreover, this study also established that 20-HETE levels were correlated with body mass index (BMI) and diastolic blood pressure across this cohort of patients (Auguet et al., 2018). Interestingly, signaling molecules like Ang II have been denoted to directly aggravate atherosclerosis through increasing oxidative stress, suggesting 20-HETE-driven increases in Ang II may also contribute to atherosclerosis (Schiffrin, 2002). Additional studies are required to elucidate the precise role of 20-HETE, if any, to plaque formation.
10.3. Cardiometabolic disease
Cardiometabolic disease (CMD) is a multifaceted condition that is often characterized by several underlying health complications including hypertension, elevated fasting blood sugar, elevated triglycerides, dyslipidemia, and obesity. The unchecked progression of these complications often leads to the development of type 2 diabetes mellitus, cardiovascular complications and end organ damage (Vincent et al., 2017). CMD is not only the culmination of various disease states; it is also directly influenced and altered by behavior, socioeconomic status, environmental factors and genetics (Loos & Yeo, 2022). In addition to the intimate associations between 20-HETE and hypertension, 20-HETE is closely correlative with BMI and metabolic syndrome (Issan et al., 2013; Peterson et al., 2016; Ward, Hodgson, Puddey, Beilin, & Croft, 2006). In humans, CYP4F2 polymorphisms are associated with metabolic syndrome and weight management (Fava et al., 2012; Ward et al., 2014). In a CYP4F2 transgenic mouse model, elevated urinary 20-HETE levels coincided with elevated fasting blood sugar in the absence of altered plasma insulin levels (Lai, Wu, Liu, & Zhao, 2012). Animal studies have shown that a high-fat diet in conjunction with elevations in 20-HETE drive a rapid and pronounce obesity phenotype alongside hypertension and insulin resistance (Gilani et al., 2018). Mechanistically, 20-HETE impairs insulin-stimulated vasodilation via inhibition of the IRS-1/PI3K/Akt/eNOS axis and phosphorylation of IRS-1 on (Ser-616 and Ser-307) as well as reductions in phosphorylation of the insulin receptor on Tyr-972 across various tissues (Fig. 1A) (Li et al., 2014). Interestingly, in an animal model of type 2 diabetes (Non-obese, type 2 diabetic MKR mice), activation of the CYP-20-HETE axis is associated with diabetic peripheral neuropathy brought about by 20-HETE-induced oxidative injury/stress, altered Beclin-1 and LC3 protein levels and AMPK inactivation (Haddad et al., 2022),
In a world-wide multi-ethnic exome sequencing of over 640,000 individuals, several GPR75 loss of function (pLOF) variants were identified and shown to be associated with leanness and a protective phenotype against obesity in humans and mice (Akbari et al., 2021; Powell et al., 2022). More specifically, individuals exhibiting these GPR75 mutations exhibited a 1.8kg per square meter lower BMI, were roughly 12 lbs. lighter and had a 54% lower likelihood of obesity (Akbari et al., 2021). The genetic deletion of GPR75 in mice resulted in a robust resistance to weight gain and sustained glycemic control in a high-fat diet (HFD) model (Akbari et al., 2021). While 20-HETE levels are closely correlated with BMI, caloric restriction, has been shown to reduce 20-HETE levels and protect mice from acute kidney injury (Hoyer-Allo et al., 2022). Taken together, these studies clearly implicate 20-HETE and its receptor in the pathogenesis of diabetes, insulin resistance, hypertension, renal damage and diet-induced obesity.
11. Drug development: 20-HETE synthesis inhibitors, agonists and receptor blockers
Several key pharmacological tools have been essential in uncovering 20-HETE’s bioactions across the vasculature and beyond. The compounds, 17-octadecynoicacid(17-ODYA),N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016) and dibromo-dodecenyl-methylsulfimide (DDMS), disrupt the synthesis of 20-HETE; with DDMS considered the most 20-HETE-synthase specific inhibitor across experimental studies (Alonso-Galicia, Drummond, Reddy, Falck, & Roman, 1997; Chen et al., 2019; Jain et al., 2017; Miyata et al., 2001). This class of 20-HETE synthesis inhibitors continues to evolve as new compounds are being developed for preclinical studies including selective CYP4A11/4F2 compounds (Kawamura et al., 2022). 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (20-5,14-HEDE) and N-(20-hydroxyeicosa-5(Z),14(Z)-dienoyl)glycine (20-5,14-HEDGE) are two potent and synthetic 20-HETE mimetics/analogues used experimentally to recapitulate the vascular and pro-inflammatory effects associated with 20-HETE and its receptor GPR75(Fig. 4) (Tunctan et al., 2022; Williams et al., 2010).
Prior to the discovery of GPR75 as a 20-HETE receptor, 20-HETE receptor blockers were conventionally referred to as “functional or biological antagonists”. This class of compounds include 20-hydroxyeicosa-6 (Z),15(Z)-dienoic acid (20-HEDE), N-[20-hydroxyeicosa-6(Z),15(Z)-dienoyl]glycine (20-HEDGE), [2,5,8,11,14,17-hexaoxanonadecan-19-yl 20-hydroxyicosa-6(Z),15(Z)-dienoate] (20-SOLA) and N-disodium succinate- 20-hydroxyeicosa-6(Z),15(Z)-diencarboxamide (AAA) (Garcia et al., 2015a; Pascale, Lucchesi, & Garcia, 2021). They have been shown to fully antagonize and prevent 20-HETE’s bioactions across the vasculature, including 20-HETE’s ability to promote inflammation, increase endothelial dysfunction, increase RAS activation and regulate blood pressure (Fig. 4). Additionally, the pharmacological characteristics of these drugs suggest a direct competition with 20-HETE, particularly evident throughout in vitro studies (Garcia et al., 2015a, 2016, 2017). 20-SOLA and AAA were developed as water soluble compounds with increased stability suitable for extended in vitro studies and preclinical in vivo experiments wherein the drugs can be administered in drinking water (Gangadhariah et al., 2015; Joseph et al., 2017; Savas et al., 2016; Sedlakova et al., 2018). 20-SOLA, the first water soluble 20-HETE antagonist, was shown to greatly ameliorate changes in blood pressure and renal injury associated with a streptozotocin (STZ)-diabetic mouse model (Gangadhariah et al., 2015). Moreover, 20-SOLA facilitates the restoration of CCG after ischemic injury, preventing endothelial dysfunction and apoptosis (Joseph et al., 2017).
AAA, has been shown to bind directly to GPR75 and prevent the increases in intracellular Ca2+, IP-1 and β-arrestin associated with the 20-HETE-GPR75 pairing (Pascale et al., 2021). In cancer, HET0016 and AAA were also shown to disrupt the malignant transformation of PC-3 cells towards a more aggressive phenotype (Cardenas et al., 1865). In the Cyp1a1-Ren-2 transgenic rat, a model of ang II-dependent malignant hypertension, AAA prevents the development of malignant hypertension and reverses the established increase in systolic BP. AAA has also been shown to reduce and normalize elevated blood pressure, shift phenylephrine- and acetylcholine-induced responses and reverse changes to media: lumen in the Myh11-4a12 mice mouse model (Fig. 4) (Agostinucci et al., 2022). This new class of 20-HETE receptor blockers (20HRBs) are invaluable tools for studies dissecting 20-HETE’s bioactions across multitude of biological systems.
12. Conclusion
20-HETE’s involvement in the regulation of endothelial cell function, vascular smooth muscle cell processes and its role as a contributing force in the pathogenesis of hypertension, vascular remodeling, septic shock, angiogenesis, myocardial infarction and beyond underscore the importance of developing and characterizing 20-HETE receptor blockers. Specifically, the observations that 20-HETE can elicit increases in vascular remodeling independent of blood pressure highlight that harm to the vasculature can persist even when blood pressure is normalized. Interestingly, the use of 20-HETE receptor (GPR75) agonists looks to be a promising approach for the ambulatory treatment of septic shock wherein synthetic 20-HETE analogues could counterbalance the septic shock-induced changes across the vasculature. The recent discoveries of high- and low-affinity 20-HETE receptors, each with their own unique signaling cascades and net outcomes, further highlight the complex nature of not just 20-HETE, but the greater class of eicosanoids known to have pleiotropic effects across various target organs. The advent and access to new and more sophisticated biochemical tools and assays looks to facilitate new research ventures with exciting discoveries on the horizon.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) grants PO1034300 and HL139793 (MLS), diversity supplement to VG (HL139793-1S), Sinsheimer Scholar Award (VG), Juvenile Diabetes Research Foundation (JDRF) SRA (3-SRA-2022-1163-S-B) (ED), NIH DK126452 (JRF) and by the Robert A. Welch Foundation (I-0011) (JRF).
Non-standard Abbreviations and Acronyms
- 20-HETE
20-hydroxy-5,8,11,14-eicosatetraenoic acid
- AA
arachidonic acid
- AAA
N-disodium succinate-20-hydroxyeicosa-6(Z),15(Z)-diencarboxamide
- ACE
angiotensin converting enzyme
- Ang II
angiotensin II
- AR
androgen receptor
- CYP
cytochrome P450
- DDMS
dibromo-dodecenyl-methylsulfimide
- DHT
5α-dihydrotestosterone
- EET
epoxyeicosatrienoic acid
- EGFR
epidermal growth factor receptor
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cells
- ET-1
endothelin 1
- FFAR-1
free fatty acid receptor-1
- GPCR
G-protein coupled receptor
- NO
nitric oxide
- PAI-1
plasminogen activator inhibitor-1
- PPARα
peroxisomal proliferator-activated receptor α
- SHR
spontaneously hypertensive rat
- VSMC
vascular smooth mucle cell
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
References
- Agostinucci K, et al. (2022). Blockade of 20-hydroxyeicosatetraenoic acid receptor lowers blood pressure and alters vascular function in mice with smooth muscle-specific overexpression of CYP4A12-20-HETE synthase. Journal of Hypertension, 40, 498–511. 10.1097/HJH.0000000000003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari P, et al. (2021). Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science, 373. 10.1126/science.abf8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, & Roman RJ (1997). Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension, 29, 320–325. 10.1161/01.hyp.29.T320. [DOI] [PubMed] [Google Scholar]
- Auguet T, et al. (2018). Targeted metabolomic approach in men with carotid plaque. PLoS One, 13, e0200547. 10.1371/journal.pone.0200547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcona JA, et al. (2022). Neutrophil-derived myeloperoxidase and hypochlorous acid critically contribute to 20-Hydroxyeicosatetraenoic acid increases that drive postischemic angiogenesis. The Journal of Pharmacology and Experimental Therapeutics, 381, 204–216. 10.1124/jpet.121.001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, et al. (2011). 20-Hydroxyeicosatetraenoic acid induces apoptosis in neonatal rat cardiomyocytes through mitochondrial-dependent pathways. Journal of Cardiovascular Pharmacology, 57, 294–301. 10.1097/FJC.0b013e3182073c78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska I, et al. (2021). Epoxyeicosatrienoic acid analog and 20-HETE antagonist combination prevent hypertension development in spontaneously hypertensive rats. Frontiers in Pharmacology, 12, 798642. 10.3389/fphar.2021.798642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basora N, Boulay G, Bilodeau L, Rousseau E, & Payet MD (2003). 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. The Journal of Biological Chemistry, 278, 31709–31716. 10.1074/jbc.M304437200. [DOI] [PubMed] [Google Scholar]
- Bentzon JF, Otsuka F, Virmani R, & Falk E (2014). Mechanisms of plaque formation and rupture. Circulation Research, 114, 1852–1866. 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- Cambj-Sapunar L, Yu M, Harder DR, & Roman RJ (2003). Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke, 34, 1269–1275. 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- Cardenas S, et al. (1865). GPR75 receptor mediates 20-HETE-signaling and metastatic features of androgen-insensitive prostate cancer cells. Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids, 158573, 2020. 10.1016/j.bbalip.2019.158573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas S, et al. (2023). 20-HETE/GPR75 pairing modulates the expression and transcriptional activity of the androgen receptor in androgen-sensitive prostate cancer cells. Molecular and Cellular Endocrinology, 559, 111784. 10.1016/j.mce.2022.111784. [DOI] [PubMed] [Google Scholar]
- Chen L, Ackerman R, & Guo AM (2012). 20-HETE in neovascularization. Prostaglandins & Other Lipid Mediators, 98, 63–68. 10.1016/j.prostaglan-dins.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Chen P, et al. (2005). Inhibitors of cytochrome P450 4A suppress angiogenic responses. The American Journal of Pathology, 166, 615–624. 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. (2014). 20-HETE regulates the angiogenic functions of human endothelial progenitor cells and contributes to angiogenesis in vivo. The Journal of Pharmacology and Experimental Therapeutics, 348, 442–451. 10.1124/jpet.113.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. (2016). 20-HETE contributes to ischemia-induced angiogenesis. Vascular Pharmacology, 83, 57–65. 10.1016/j.vph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. (2019). CYP4A/20-HETE regulates ischemia-induced neovascularization via its actions on endothelial progenitor and preexisting endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 316, H1468–H1479. 10.1152/ajpheart.00690.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. (2022). CYP4F2-catalyzed metabolism of arachidonic acid promotes stromal cell-mediated immunosuppression in non-small cell lung Cancer. Cancer Research, 82, 4016–4030. 10.1158/0008-5472.CAN-21-4029. [DOI] [PubMed] [Google Scholar]
- Cheng J, et al. (2008). 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. American Journal of Physiology. Heart and Circulatory Physiology, 294, H1018–H1026. 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- Cheng J, et al. (2012). Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology, 32, 1917–1924. 10.1161/ATVBAHA.112.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, et al. (2014). Vascular characterization of mice with endothelial expression of cytochrome P450 4F2. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 28, 2915–2931. 10.1096/fj.13-241927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft KD, McGiff JC, Sanchez-Mendoza A, & Carroll MA (2000). Angiotensin II releases 20-HETE from rat renal microvessels. American Journal of Physiology. Renal Physiology, 279, F544–F551. 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- Cuez T, et al. (2010). A synthetic analogue of 20-HETE, 5,14-HEDGE, reverses endotoxin-induced hypotension via increased 20-HETE levels associated with decreased iNOS protein expression and vasodilator prostanoid production in rats. Basic & Clinical Pharmacology & Toxicology, 106, 378–388. 10.1111/j.1742-7843.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, et al. (2013). 20-HETE induces remodeling of renal resistance arteries independent of blood pressure elevation in hypertension. American Journal of Physiology. Renal Physiology, 305, F753–F763. 10.1152/ajprenal.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirice E, et al. (2019). Human duct cells contribute to beta cell compensation in insulin resistance. JCI insight, 4. 10.1172/jci.insight.99576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach AW, et al. (2014). Urinary CYP eicosanoid excretion correlates with glomerular filtration in African-Americans with chronic kidney disease. Prostaglandins & Other Lipid Mediators, 113-115, 45–51. 10.1016/j.prostaglandins.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, & Roman RJ (2017). GPR75 identified as the first 20-HETE receptor: A chemokine receptor adopted by a new family. Circulation Research, 120, 1696–1698. 10.1161/CIRCRESAHA.117.311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, et al. (2013). 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One, 8, e82482. 10.1371/journal.pone.0082482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, et al. (2016). Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed), 21, 1427–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava C, et al. (2012). The functional variant V433M of the CYP4F2 and the metabolic syndrome in swedes. Prostaglandins & Other Lipid Mediators, 98, 31–36. 10.1016/j.prostaglandins.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Fu Z, et al. (2013). A novel polymorphism of the CYP4A11 gene is associated with coronary artery disease. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, 19, 60–65. 10.1177/1076029611436197. [DOI] [PubMed] [Google Scholar]
- Gangadhariah MH, et al. (2015). Hypertension is a major contributor to 20-hydroxyeicosatetraenoic acid-mediated kidney injury in diabetic nephropathy. Journal of the American Society of Nephrology: JASN, 26, 597–610. 10.1681/ASN.2013090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Shkolnik B, Milhau L, Falck JR, & Schwartzman ML (2016). 20-HETE activates the transcription of angiotensin-converting enzyme via nuclear factor-kappaB translocation and promoter binding. The Journal of Pharmacology and Experimental Therapeutics, 356, 525–533. 10.1124/jpet.115.229377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, et al. (2015a). Angiotensin II receptor blockade or deletion of vascular endothelial ACE does not prevent vascular dysfunction and remodeling in 20-HETE-dependent hypertension. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 309, R71–R78. 10.1152/ajpregu.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, et al. (2015b). Androgen-induced hypertension in angiotensinogen deficient mice: Role of 20-HETE and EETS. Prostaglandins & Other Lipid Mediators, 116–117, 124–130. 10.1016/j.prostaglandins.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, et al. (2017). 20-HETE signals through G-protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circulation Research, 120, 1776–1788. 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, et al. (2020). Unbiased proteomics identifies plasminogen activator inhibitor-1 as a negative regulator of endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America, 117, 9497–9507. 10.1073/pnas.1918761117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, et al. (1998). Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. The Journal of Physiology, 507(Pt 3), 771–781. 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani A, et al. (2018). High-fat diet-induced obesity and insulin resistance in CYP4a14(−/−) mice is mediated by 20-HETE. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 315, R934–R944. 10.1152/ajpregu.00125.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ER, et al. (2004). Cytochrome P450 omega-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. Journal of Molecular and Cellular Cardiology, 37, 1245–1249. 10.1016/j.yjmcc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Gross GJ, et al. (2005). Cytochrome P450 and arachidonic acid metabolites: Role in myocardial ischemia/reperfusion injury revisited. Cardiovascular Research, 68, 18–25. 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Guo AM, et al. (2007). Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. The Journal of Pharmacology and Experimental Therapeutics, 321, 18–27. 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- Guo AM, et al. (2009). 20-HETE can act as a nonhypoxic regulator of HIF-1alpha in human microvascular endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 297, H602–H613. 10.1152/ajpheart.00874.2008. [DOI] [PubMed] [Google Scholar]
- Haddad M, et al. (2022). Activation of 20-HETE synthase triggers oxidative injury and peripheral nerve damage in type 2 diabetic mice. The Journal of Pain, 23, 1371–1388. 10.1016/j-jpain.2022.02.011. [DOI] [PubMed] [Google Scholar]
- Harder DR, et al. (1994). Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. The American Journal of Physiology, 266, H2098–H2107. [DOI] [PubMed] [Google Scholar]
- Heil M, Eitenmuller I, Schmitz-Rixen T, & Schaper W (2006). Arteriogenesis versus angiogenesis: Similarities and differences. Journal of Cellular and Molecular Medicine, 10, 45–55. 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka M, Matsuura T, Watanabe E, Sato M, & Suzuki Y (1996). Sex and strain differences in constitutive expression of fatty acid omega-hydroxylase (CYP4A-related proteins) in mice. Journal of Biochemistry, 119, 340–345. 10.1093/oxfordjournals.jbchem.a021245. [DOI] [PubMed] [Google Scholar]
- Holla VR, et al. (2001). Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proceedings of the National Academy of Sciences of the United States of America, 98, 5211–5216. 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes SL, Garcia V, Edin ML, Schwartzman ML, & Zeldin DC (2015). Vascular actions of 20-HETE. Prostaglandins & Other Lipid Mediators, 120, 9–16. 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, et al. (2016). Sepsis and septic shock. Nature Reviews. Disease Primers, 2, 16045. 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Allo KJR, et al. (2022). Caloric restriction reduces the pro-inflammatory eicosanoid 20-hydroxyeicosatetraenoic acid to protect from acute kidney injury. Kidney International, 102, 560–576. 10.1016/j.kint.2022.04.033. [DOI] [PubMed] [Google Scholar]
- Hutcheson R, et al. (2013). MicroRNA-145 restores contractile vascular smooth muscle phenotype and coronary collateral growth in the metabolic syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology, 33, 727–736. 10.1161/ATVBAHA.112.301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, et al. (2009). Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. American Journal of Physiology. Renal Physiology, 297, F875–F884. 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, et al. (2008). 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. The Journal of Pharmacology and Experimental Therapeutics, 324, 103–110. 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- Issan Y, et al. (2013). Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins & Other Lipid Mediators, 100–101, 15–21. 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito O, Omata K, Ito S, Hoagland KM, & Roman RJ (2001). Effects of converting enzyme inhibitors on renal P-450 metabolism of arachidonic acid. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 280, R822–R830. 10.1152/ajpregu.2001.280.3.R822. [DOI] [PubMed] [Google Scholar]
- Jain M, et al. (2017). Intravenous formulation of HET0016 decreased human glioblastoma growth and implicated survival benefit in rat xenograft models. Scientific Reports, 7, 41809. 10.1038/srep41809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, et al. (2016). Pharmacological targeting of plasminogen activator Inhibitor-1 decreases vascular smooth muscle cell migration and neointima formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 36, 2167–2175. 10.1161/ATVBAHA.116.308344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, et al. (2004). Smooth muscle- -specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circulation Research, 94,167–174. 10.1161/01.RES.0000111523.12842.FC. [DOI] [PubMed] [Google Scholar]
- Jiang S, et al. (2017). beta-adrenergic receptor-stimulated cardiac myocyte apoptosis: Role of cytochrome P450 omega-hydroxylase. Journal of Cardiovascular Pharmacology, 70, 94–101. 10.1097/FJC.0000000000000499. [DOI] [PubMed] [Google Scholar]
- Joseph G, et al. (2017). Elevated 20-HETE impairs coronary collateral growth in metabolic syndrome via endothelial dysfunction. American Journal of Physiology. Heart and Circulatory Physiology, 312, H528–H540. 10.1152/ajpheart.00561.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, et al. (2022). Discovery of novel Pyrazolylpyridine derivatives for 20-Hydroxyeicosatetraenoic acid synthase inhibitors with selective CYP4A11/4F2 inhibition. Journal of Medicinal Chemistry, 65, 14599–14613. 10.1021/acs.jmedchem.2c01089. [DOI] [PubMed] [Google Scholar]
- Lai G, Wu J, Liu X, & Zhao Y (2012). 20-HETE induces hyperglycemia through the cAMP/PKA-PhK-GP pathway. Molecular Endocrinology, 26, 1907–1916. 10.1210/me.2012-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhkar A, et al. (2016). 20-HETE-induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose-6-phosphate dehydrogenase inhibition. American Journal of Physiology. Heart and Circulatory Physiology, 310, H1107–H1117. 10.1152/ajpheart.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. (2014). 20-Hydroxyeicosatetraenoic acid impairs endothelial insulin signaling by inducing phosphorylation of the insulin receptor substrate-1 at Ser616. PLoS One, 9, e95841. 10.1371/journal.pone.0095841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. (2009). Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure. Kidney International, 75, 1288–1296. 10.1038/ki.2009.67. [DOI] [PubMed] [Google Scholar]
- Loos RJF, & Yeo GSH (2022). The genetics of obesity: From discovery to biology. Nature Reviews. Genetics, 23, 120–133. 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, et al. (2008). 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 294, L902–L911. 10.1152/ajplung.00278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata N, & Roman RJ (2005). Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. Journal of smooth muscle research =. Nihon Heikatsukin Gakkai kikanshi, 41, 175–193. 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- Miyata N, et al. (2001). HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. British Journal of Pharmacology, 133, 325–329. 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithipatikom K, et al. (2001). Determination of cytochrome P450 metabolites of arachidonic acid in coronary venous plasma during ischemia and reperfusion in dogs. Analytical Biochemistry, 292, 115–124. 10.1006/abio.2001.5044. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, et al. (2004). Inhibition of cytochrome P450omega-hydroxylase: A novel endogenous cardioprotective pathway. Circulation Research, 95, e65–e71. 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, et al. (2006). Effects of selective inhibition of cytochrome P-450 omega-hydroxylases and ischemic preconditioning in myocardial protection. American Journal of Physiology. Heart and Circulatory Physiology, 290, H500–H505. 10.1152/ajpheart.00918.2005. [DOI] [PubMed] [Google Scholar]
- O’Donnell VB, Maskrey B, & Taylor GW (2009). Eicosanoids: Generation and detection in mammalian cells. Methods in Molecular Biology, 462, 5–23. [PubMed] [Google Scholar]
- Orozco LD, et al. (2013). 20-Hydroxyeicosatetraenoic acid inhibition attenuates balloon injury-induced neointima formation and vascular remodeling in rat carotid arteries. The Journal of Pharmacology and Experimental Therapeutics, 346, 67–74. 10.1124/jpet.113.203844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyekan AO, & McGiff JC (1998). Functional response of the rat kidney to inhibition of nitric oxide synthesis: Role of cytochrome p450-derived arachidonate metabolites. British Journal of Pharmacology, 125, 1065–1073. 10.1038/sj.bjp.0702171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, et al. (2018). GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells. The Journal of Biological Chemistry, 293, 10675–10691. 10.1074/jbc.RA117.001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier JH, Lavrentyev EN, Falck JR, Capdevila JH, & Malik KU (2005). Evaluation of cytochrome P450 4 family as mediator of phospholipase D activation in aortic vascular smooth muscle cells. Life Sciences, 77, 1015–1029. 10.1016/j.lfs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pascale JV, Lucchesi PA, & Garcia V (2021). Unraveling the role of 12- and 20-HETE in cardiac pathophysiology: G-protein-coupled receptors, pharmacological inhibitors, and transgenic approaches. Journal of Cardiovascular Pharmacology, 77, 707–717. 10.1097/FJC.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale JV, et al. (2021). Uncovering the signalling, structure and function of the 20-HETE-GPR75 pairing: Identifying the chemokine CCL5 as a negative regulator of GPR75. British Journal of Pharmacology, 178, 3813–3828. 10.1111/bph.15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SJ, et al. (2016). Oxidized HDL is a potent inducer of adipogenesis and causes activation of the ang-II and 20-HETE systems in human obese females. Prostaglandins & Other Lipid Mediators, 123, 68–77. 10.1016/j.prostaglandins.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Powell DR, et al. (2022). Mice lacking Gpr75 are Hypophagic and thin. Diabetes, metabolic syndrome and obesity : targets and therapy, 15, 45–58. 10.2147/DMSO.S342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamboavonjy V, Busse R, & Fleming I (2003). 20-HETE-induced contraction of small coronary arteries depends on the activation of rho-kinase. Hypertension, 41, 801–806. 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- Rocic P, & Schwartzman ML (2018). 20-HETE in the regulation of vascular and cardiac function. Pharmacology & Therapeutics, 192, 74–87. 10.1016/j.pharmthera.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ, & Fan F (2018). 20-HETE: Hypertension and beyond. Hypertension, 72, 12–18. 10.1161/HYPERTENSIONAHA.118.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, et al. (2018). Angiotensin II upregulates CYP4A isoform expression in the rat kidney through angiotensin II type 1 receptor. Prostaglandins & Other Lipid Mediators, 139, 80–86. 10.1016/j.prostaglandins.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Savas U, et al. (2016). 20-Hydroxyeicosatetraenoic acid (HETE)-dependent hypertension in human cytochrome P450 (CYP) 4A11 transgenic mice: NORMALIZATION OF BLOOD PRESSURE BY SODIUM RESTRICTION, HYDROCHLOROTHIAZIDE, OR BLOCKADE OF THE TYPE 1 ANGIOTENSIN II RECEPTOR. The Journal of Biological Chemistry, 291, 16904–16919. 10.1074/jbc.M116.732297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL (2002). Beyond blood pressure: The endothelium and atherosclerosis progression. American Journal of Hypertension, 15, 115S–122S. 10.1016/s0895-7061(02)03006-6. [DOI] [PubMed] [Google Scholar]
- Sedlakova L, et al. (2018). 20-Hydroxyeicosatetraenoic acid antagonist attenuates the development of malignant hypertension and reverses it once established: A study in Cyp1a1-ren-2 transgenic rats. Bioscience Reports, 38. 10.1042/BSR20171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, et al. (2007). Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension, 50, 123–129. 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- Soler A, et al. (2018). Elevated 20-HETE in metabolic syndrome regulates arterial stiffness and systolic hypertension via MMP12 activation. Journal of Molecular and Cellular Cardiology, 117, 88–99. 10.1016/j.yjmcc.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, et al. (2015). Peroxisome proliferator-activated receptor alpha, PPARalpha, directly regulates transcription of cytochrome P450 CYP2C8. Frontiers in Pharmacology, 6, 261. 10.3389/fphar.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Hosoda K, Fujikura J, Inagaki N, & Nakao K (2014). The G-protein-coupled long-chain fatty acid receptor GPR40 and glucose metabolism. Frontiers in Endocrinology, 5, 152. 10.3389/fendo.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai IJ, Croft KD, Puddey IB, Beilin LJ, & Barden A (2011). 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. American Journal of Physiology. Heart and Circulatory Physiology, 300, H1194–H1200. 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- Tunaru S, et al. (2018). 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nature Communications, 9, 177. 10.1038/s41467-017-02539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunctan B, et al. (2013). Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91(phox) to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock. Nitric Oxide : Biology and Chemistry, 33, 18–41. 10.1016/j.niox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunctan B, et al. (2022). Activation of GPR75 signaling pathway contributes to the effect of a 20-HETE mimetic, 5,14-HEDGE, to prevent hypotensive and tachycardic responses to lipopolysaccharide in a rat model of septic shock. Journal of Cardiovascular Pharmacology, 80, 276–293. 10.1097/FJC.0000000000001265. [DOI] [PubMed] [Google Scholar]
- Van Ginderachter JA (2022). The heat is on: 20-HETE instructs an immunosuppressive phenotype in Cancer-associated fibroblasts. Cancer Research, 82, 3882–3883. 10.1158/0008-5472.CAN-22-2774. [DOI] [PubMed] [Google Scholar]
- Vasudevan H, Yuen VG, & McNeill JH (2012). Testosterone-dependent increase in blood pressure is mediated by elevated Cyp4A expression in fructose-fed rats. Molecular and Cellular Biochemistry, 359, 409–418. 10.1007/s11010-011-1035-7. [DOI] [PubMed] [Google Scholar]
- Vaughan DE, Rai R, Khan SS, Eren M, & Ghosh AK (2017). Plasminogen activator Inhibitor-1 is a marker and a mediator of senescence. Arteriosclerosis, Thrombosis, and Vascular Biology, 37, 1446–1452. 10.1161/ATVBAHA.117.309451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GE, et al. (2017). Improving cardiometabolic health with diet, physical activity, and breaking up sitting: What about sleep? Frontiers in Physiology, 8, 865. 10.3389/fphys.2017.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman M, Peterson SJ, Arad M, & Hochhauser E (2016). The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins & Other Lipid Mediators, 125, 108–117. 10.1016/j.prostaglandins.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Wang R, et al. (2019). Specific inhibition of CYP4A alleviates myocardial oxidative stress and apoptosis induced by advanced glycation end-products. Frontiers in Pharmacology, 10, 876. 10.3389/fphar.2019.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. (2022). Fine particulate matter and vasoactive 20-hydroxyeicosatetraenoic acid: Insights into the mechanisms of the prohypertensive effects of particulate air pollution. The Science of the Total Environment, 806, 151298. 10.1016/j.scitotenv.2021.151298. [DOI] [PubMed] [Google Scholar]
- Ward NC, Hodgson JM, Puddey IB, Beilin LJ, & Croft KD (2006). 20-Hydroxyeicosatetraenoic acid is not associated with circulating insulin in lean to overweight humans. Diabetes Research and Clinical Practice, 74, 197–200. 10.1016/j.diabres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ward NC, et al. (2014). The effect of a single nucleotide polymorphism of the CYP4F2 gene on blood pressure and 20-hydroxyeicosatetraenoic acid excretion after weight loss. Journal of Hypertension, 32, 1495–1502. discussion 1502, 10.1097/HJH.0000000000000208. [DOI] [PubMed] [Google Scholar]
- Weisberg AD, et al. (2005). Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 365–371. 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- Wen H, et al. (2012). 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. The Journal of Biological Chemistry, 287, 13868–13876. 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Murphy S, Burke M, & Roman RJ (2010). 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. Journal of Cardiovascular Pharmacology, 56, 336–344. 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum JL, & Lichtman AH (2014). The influence of innate and adaptive immune responses on atherosclerosis. Annual Review of Pathology, 9, 73–102. 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Gupta T, Garcia V, Ding Y, & Schwartzman ML (2014). 20-HETE and blood pressure regulation: Clinical implications. Cardiology in Review, 22, 1–12. 10.1097/CRD.0b013e3182961659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Falck JR, Ortiz de Montellano PR, & Kroetz DL (2004). Catalytic activity and isoform-specific inhibition of rat cytochrome p450 4F enzymes. The Journal of Pharmacology and Experimental Therapeutics, 308, 887–895. 10.1124/jpet.103.059626. [DOI] [PubMed] [Google Scholar]
- Yu J, et al. (2012). Endothelium derived nitric oxide synthase negatively regulates the PDGF-survivin pathway during flow-dependent vascular remodeling. PLoS One, 7, e31495. 10.1371/journal.pone.0031495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, et al. (2010). 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. American Journal of Physiology. Heart and Circulatory Physiology, 299, H1109–H1117. 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. (2015). 20-Hydroxyeicosatetraenoic acid is a key mediator of angiotensin II-induced apoptosis in cardiac myocytes. Journal of Cardiovascular Pharmacology, 66, 86–95. 10.1097/FJC.0000000000000248. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. (1864). Induction of cytochrome P450 4A14 contributes to angiotensin II-induced renal fibrosis in mice. Biochimica et Biophysica Acta - Molecular Basis of Disease, 860–870, 2018. 10.1016/j.bbadis.2017.12.028. [DOI] [PubMed] [Google Scholar]
- Zou AP, et al. (1996). 20-HETE is an endogenous inhibitor of the large-conductance ca(2+)-activated K+ channel in renal arterioles. The American Journal of Physiology, 270, R228–R237. 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]


