Abstract
Aim: Age is a risk factor for constipation. Constipation is common in patients with ulcerative colitis (UC) and has been positively associated with disease activity, but evidence is limited. This study aimed to assess the association between disease activity and constipation in patients with UC. Methods: The study subjects consisted of 290 Japanese UC patients. The definition of constipation was based on Rome I criteria and/or medication for constipation. Information on and lifestyle habits was obtained from a self-administered questionnaire. Mucosal healing (MH) was defined as Mayo endoscopic subscore 0. Clinical remission (CR) was defined as both the absence of rectal bleeding and no abnormally high stool frequency (<3 times per day). Results: The prevalence of constipation is not associated with MH, CR, duration of UC and disease extent of UC. The prevalence of constipation among age groups, <40, 40–49 years, 50–59 years, 60–69 years, and >70 years was 10.0%, 5.8%, 15.7%, 11.8%, and 25.6%, respectively. >70 years was independently and positively associated with the prevalence of constipation (adjusted odds ratio 3.64 [95% confidence interval 1.26, 10.95], p for trend: .001). Conclusions: Aging was independently and positively associated with the prevalence of constipation in UC.
Keywords: ulcerative colitis, constipation, mucosal healing, aging, inflammatory bowel disease
Introduction
Ulcerative colitis (UC) is a disease of repeated relapses and remissions, and the number of UC patients is increasing worldwide, including Japan (Asakura et al., 2009; Cosnes et al., 2011; Murakami et al., 2020). In the clinical setting, maintaining mucosal healing (MH) among patients with UC was an established treatment goal in several previous studies (Colombel et al., 2011; Frøslie et al., 2007; Lichtenstein & Rutgeerts, 2010; Peyrin-Biroulet et al., 2011; Sandborn et al., 2016; Shah et al., 2016).
Constipation, a common gastrointestinal symptom, might worsen quality of life (Belsey et al., 2010; Koloski et al., 2013; Wald et al., 2007) and negatively impact social labor productivity (Sun et al., 2011). Additionally, constipation is associated with chronic kidney disease (Sumida et al., 2017), incidence of cardiovascular disease (Salmoirago-Blotcher et al., 2011), and all-cause mortality (Sumida et al., 2019). In a Japanese population-based study, lower defecation frequency was associated with cardiovascular disease mortality (Honkura et al., 2016). In a United States (US) study, female gender and aging was positively associated with the prevalence of constipation (Choung et al., 2007). Similarly, in Japan, according to the 2019 Comprehensive Survey of Living Conditions, the prevalence of constipation is 34.8% in total study population and 68.6% in 65 years and older (Ministry of Health, Labour and Welfare, 2019). The prevalence of constipation increases with age in both men and women (Choung et al., 2007; Higgins & Johanson, 2004; Roque & Bouras, 2015).
Previous studies showed the close association between UC and constipation. Approximately one-third to one-half of patients with UC suffered from symptoms of constipation (James et al., 2018; Lennard-Jones et al., 1962). Disease activity is positively associated with the prevalence of constipation (OR 5.56 [1.96, 16.67]), while age, disease duration, or treatment was not associated with constipation (James et al., 2018). Despite the adverse effects of constipation on various diseases, the evidence of constipation in UC is modest. In addition, relevant evidence of constipation in Japanese patients with UC is lacking. Therefore, we aimed to evaluate the association between UC disease activity and constipation in Japanese patients.
Methods
Study Design
This was a cross-sectional study that used baseline data from a prospective cohort study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was approved by the Institutional Review Board of the Ehime University Graduate School of Medicine (No. 1505011). Well-trained staff obtained written informed consent from all enrolled patients. The study was registered at each hospital between 2015 and 2019.
Study Population
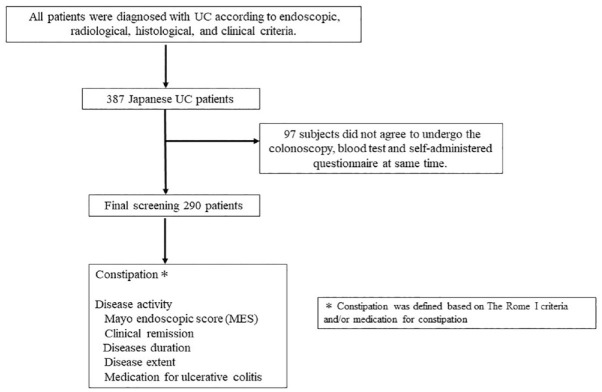
The study subjects consisted of 387 Japanese patients with UC seen at the Department of Gastroenterology and Metabology of the Ehime University Graduate School of Medicine, and at several affiliated hospitals and clinics in Ehime prefecture. All patients were diagnosed with UC according to endoscopic, radiological, histological, and clinical criteria. Consecutive outpatients and inpatients with UC who could understand our study were candidates. Even after being informed about the study and agreeing to participate, 97 subjects did not agree to undergo the colonoscopy and blood test; thus, some data are missing. After these 97 patients were excluded due to incomplete data, the final analysis sample in this study consisted of 290 patients (Figure 1).
Figure 1.
Flow chart of the present study.
Measurements
Information on duration of UC, medication for UC, and disease extent was collected from medical records. Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters.
Definition of Constipation
The Rome I criteria for constipation, as recommended by an international workshop on constipation management, were used in this study (Whitehead et al., 1991). Constipation was assessed using four questions that pertained to the last 12 months and were as follows: (1) Do you experience straining during bowel movements?; (2) Do you feel a sense of incomplete evacuation after bowel movements?; (3) How often do you experience hard stools?; and (4) How frequently do you have bowel movements each week? Respondents could choose from four answer options for questions 1–3: never, sometimes (<25% of the time), often (>25% of the time), and always. Constipation was defined as meeting two or more of the four criteria, with an answer of “often” or “always” for questions 1–3 and less than three bowel movements per week for question 4. Information regarding medication for constipation was obtained using self-administered questionnaire. In this study, the definition of constipation was based on Rome I criteria and/or medication for constipation.
Definition of Mucosal Healing and Clinical Remission
A certified endoscopist evaluated mucosal status by total colonoscopy. Endoscopy was performed as required by the attending physician. In patients with newly diagnosed UC, colonoscopy was performed regardless of the severity of symptoms. Complete MH was defined as Mayo endoscopic score (MES) category 0 (Schroeder et al., 1987). Clinical remission (CR) was defined as no rectal bleeding and no abnormally high stool frequency (<3 times per day) at the time of this study’s research. One endoscopic specialist was responsible for evaluating MES, CR, and MH, and was blind to constipation.
Statistical Analysis
Duration of UC was divided into two groups: (1) <7 years and (2) ≥7 years. Disease extent was divided into two groups: (1) pancolitis and (2) non-pancolitis. Age was divided into five groups of 10 years each: (1) Under 40 years old, (2) 40–49 years old, (3) 50–59 years old, (4) 60–69 years old, and (5) 70 years old and over. Multiple logistic regression analyses were used to adjust for potential confounding factors. Sex, age, BMI, current drinking, and current smoking were selected as confounding factors. Trend of association was assessed using a logistic regression model assigning consecutive integers to the categories of the age variables. Statistical analyses were mainly performed using the SAS software package ver. 9.4 (SAS Institute, Cary, NC, USA). All probability values for statistical tests were two-tailed, and a p-value of < .05 was considered statistically significant.
Results
Table 1 shows the characteristics of the 290 study participants. The percentage of men was 57.6% in this study population. The mean age and BMI were 50.4 years and 22.54 kg/m2, respectively. The percentage of <40 years, 40–49 years, 50 to 59 years, 60 to 69 years, and >70 years was 29.0%, 23.8%, 17.6%, 17.6%, and 13.5%, respectively. The prevalence of constipation, complete MH (MES 0), and CR was 12.4%, 25.2%, and 57.6%, respectively.
Table 1.
Clinical Characteristics of 290 Study Participants.
| Variables | Mean ± SD or n (%) |
|---|---|
| Age (years) | 50.4 ± 16.1 |
| <40 years | 80 (29.0) |
| 40–49 years | 69 (23.8) |
| 50–59 years | 51 (17.6) |
| 60–69 years | 51 (17.6) |
| ≥70 years | 39 (13.5) |
| Male (%) | 167 (57.6) |
| Disease extent (n; pancolitis/left-sided/proctitis/other) | 120/79/88/7 |
| Duration of UC (years) | 8.1 ± 8.3 |
| BMI (kg/m2) | 22.54 ± 4.51 |
| Current smoking | 21 (7.2) |
| Current drinking | 118 (40.7) |
| Medication for UC | |
| 5-Aminosalicylates | 264 (91.0) |
| Prednisolone | 58 (20.0) |
| Thiopurines | 42 (14.5) |
| TNF-α monoclonal antibody | 15 (5.2) |
| Mayo Endoscopic Subscore (MES) | 1.22 ± 0.91 |
| Mucosal healing (MES 0) | 73 (25.2) |
| Clinical remission | 167 (57.6) |
| Constipation (%) | 36 (12.4) |
| Medication for constipation (%) | 31 (10.7) |
Note. BMI = body mass index; UC = ulcerative colitis; SD = standard deviation; TNF = tumor necrosis factor; Other: right-sided, segmental colitis, and postoperative patients (lack of any preoperative medical records for postoperative patients).
Table 2 shows the crude and adjusted ORs and 95% CIs for the association between UC disease activity and constipation. The prevalence of constipation among MH (MES 0), CR, long duration (>7 years), and pancolitis was 8.2%, 13.4%, 12.9%, and 12.5%, respectively. MH, CR, long duration, and pancolitis was not associated with the prevalence of constipation. The prevalence of constipation among age groups, <40, 40–49 years, 50–59 years, 60–69 years, and >70 years was 10.0%, 5.8%, 15.7%, 11.8%, and 25.6%, respectively. Age groups were independently associated with constipation (adjusted OR with age groups, <40, 40–49 years, 50–59 years, 60–69 years, and >70 years: aOR 0.59 [95% CI 0.15, 2.00], aOR 1.65 [95% CI 0.56, 4.87], aOR 1.45 [95% CI 0.44, 0.4], and aOR 3.64 [95% CI 1.26, 10.95], p for trend .001). Only >70 years was independently positively associated with the prevalence of constipation (p = .018).
Table 2.
Crude and Adjusted Odds Ratios and 95% Confidence Intervals for the Associations Between Ulcerative Colitis (UC) Disease Activity and Constipation.
| Variable | Prevalence, n/n (%) | Crude OR [95% CI] | p-value | Adjusted OR [95% CI] | p-value |
|---|---|---|---|---|---|
| Constipation | |||||
| MH (MES 0) | |||||
| No | 30/217 (13.8) | 1.00 | 1.00 | ||
| Yes | 6/73 (8.2). | 0.56 [0.20, 1.32] | .214 | 0.45 [0.16, 1.10] | .102 |
| Clinical remission | |||||
| No | 13/118 (11.0) | 1.00 | 1.00 | ||
| Yes | 23/172 (13.4) | 1.25 [0.61, 2.64] | .551 | 1.02 [0.48, 2.22] | .962 |
| Long duration of UC | |||||
| <7 years | 20/166 (12.1) | 1.00 | 1.00 | ||
| ≥7 years | 16/124 (12.9) | 1.08 [0.53, 2.18] | .827 | 0.94 [0.45, 1.95] | .866 |
| Pancolitis | |||||
| No | 21/170 (12.4) | 1.00 | 1.00 | ||
| Yes | 15/120 (12.5) | 1.01 [0.49, 2.05] | .970 | 0.98 [0.46, 2.03] | .946 |
| Age | |||||
| <40 years | 8/80 (10.0) | 1.00 | 1.00 | ||
| 40–49 years | 4/69 (5.8) | 0.55 [0.14, 1.85] | .353 | 0.59 [0.15, 2.00] | .411 |
| 50–59 years | 8/51 (15.7) | 1.67 [0.58, 4.87] | .336 | 1.65 [0.56, 4.87] | .359 |
| 60–69 years | 6/51 (11.8) | 1.20 [0.37, 3.68] | .750 | 1.45 [0.44, 4.62] | .531 |
| ≥70 years | 10/39 (25.6) | 3.10 [1.12, 8.90] | .030 | 3.64 [1.26, 10.95] | .018 |
| p for trend | .001 | ||||
Note. Odds ratios were adjusted for sex, age, body mass index, current drinking, and current smoking. OR = odds ratio; CI = confidence interval; MH = mucosal healing; MES = Mayo Endoscopic Score.
Discussion
This is the first study to investigate the association between disease activity and the prevalence of constipation in Japanese patients with UC. In the present study, aging was significantly positively associated with the prevalence of constipation. However, no association between disease activity and constipation was found in this study population.
Bloody stools and diarrhea are well-known UC-related gastrointestinal symptoms, but some patients also suffer from constipation (James et al., 2018; Lennard-Jones et al., 1962). However, the association between constipation and UC remains inconsistent. The prevalence of constipation was similar between UC and the general population in a US study (Lee et al., 2017). In the present study, the prevalence of constipation might be lower than that in previous research (12.4% vs. 32%–46%) (James et al., 2018; Lennard-Jones et al., 1962). However, the discrepancies between our study and previous studies may be explained, at least in part, by differences in sex, age distribution, definition of constipation, BMI, and treatment of constipation.
In general, aging is associated with constipation (Choung et al., 2007; Higgins & Johanson, 2004; Roque & Bouras, 2015). The findings in the present study are consistent with the results of previous general population studies. Recently, the number of elderly patients with UC has been increasing in Japan (Higashiyama et al., 2021; Shimodaira et al., 2021). Thus, the prevalence of constipation in patients with UC might be increasing in the future. As constipation is associated with poor quality of life, several diseases, and all-cause mortality, healthcare professionals should be aware that UC is associated with a high frequency of constipation, particularly in older patients with UC, regardless of disease activity. In an Australian study of 125 patients with UC, however, age was not associated with constipation (James et al., 2018). It is of note that there are several differences between our study and the previous Australian study, including the ratio of males (57.6%vs. 48.8%), mean age (50.4vs. 47 years), definition of constipation (Rome I criteria and/or medication vs. Rome III criteria), and sample size (n = 290vs. n = 125).
Although the underlying mechanism linking aging and constipation remains unclear, there are several biological plausible possibilities. Age-related changes in physical activity, fluid and food intake, and microbiota (Simrén et al., 2013) may lead to constipation. In previous studies, slow transit constipation represents 15% to 37% of constipated patients (Bassotti et al., 2004; Probert et al., 1994; Surrenti et al., 1995). Older adults have slower colonic transit than younger adults, but there is no difference in gastric emptying capacity or small bowel transit (Madsen JL et al., 2004). In addition, the rectal sensory threshold of older healthy individuals is higher than that of younger healthy individuals (Lagier E et al., 1999). Higher rectal sensory thresholds have also been associated with constipation (Ratuapli et al., 2013). Slower colonic transit and higher rectal sensory thresholds in the elderly may be associated with constipation. Slower colonic transit and higher rectal sensory thresholds in the elderly may be associated with constipation. Thus, aging might cause constipation via some of the mechanisms described above.
This study has several limitations. First, it was a cross-sectional analysis, and therefore, we cannot conclude that there is a causal association between age and constipation. Second, the exclusion rate for this study population was high, resulting in a small sample size. Third, the definition of constipation differed from other papers, as it was extracted using a self-administered questionnaire. However, many other papers have also reported unclear definitions. Fourth, in this study, information was obtained using self-administrated questionnaire but not interviews. These different methods of information acquisition might affect the association between disease activity and constipation. Finally, the subjects of the present study might not be representative of Japanese patients with UC. Nevertheless, the use of prednisolone and biologics was similar between the present study and a Japanese national study based on UC claims data in 2016 (15.5% and 9.0%, respectively) (Matsuoka et al., 2021).
Conclusion
Age may be independently and positively associated with constipation among Japanese patients with UC. In this study, disease activity was not associated with constipation. However, the evidence on this issue is still limited and further larger studies and longitudinal studies are needed in the future.
Acknowledgments
The authors would like to thank Kenichiro Mori, Keitarou Kawasaki, Yuji Mizukami, Satoshi Imamine, Masamoto Torisu, Harumi Yano, Makoto Yano, Masato Murakami, Aki Hasebe, Masumi Hino, and Tomo Kogama.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was approved by the Institutional Review Board of the Ehime University Graduate School of Medicine (No. 1505011). Well-trained staff obtained written informed consent from all enrolled patients. The study was registered at each hospital between 2015 and 2019.
ORCID iD: Shinya Furukawa  https://orcid.org/0000-0002-0041-7688
https://orcid.org/0000-0002-0041-7688
References
- Asakura K., Nishiwaki Y., Inoue N., Hibi T., Watanabe M., Takebayashi T. (2009). Prevalence of ulcerative colitis and Crohn’s disease in Japan. Journal of Gastroenterology, 44(7), 659–665. [DOI] [PubMed] [Google Scholar]
- Bassotti G., de Roberto G., Sediari L., Morelli A. (2004). Toward a definition of colonic inertia. World Journal of Gastroenterology, 10(17), 2465–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsey J., Greenfield S., Candy D., Geraint M. (2010). Systematic review: Impact of constipation on quality of life in adults and children. Alimentary Pharmacology & Therapeutics, 31(9), 938–949. [DOI] [PubMed] [Google Scholar]
- Choung R. S., Locke G. R., 3rd, Schleck C. D., Zinsmeister A. R., Talley N. J. (2007). Cumulative incidence of chronic constipation: A population-based study 1988-2003. Alimentary Pharmacology & Therapeutics, 26(11-12), 1521–1528. [DOI] [PubMed] [Google Scholar]
- Colombel J. F., Rutgeerts P., Reinisch W., Esser D., Wang Y., Lang Y., Marano C. W., Strauss R., Oddens B. J., Feagan B. G., Hanauer S. B., Lichtenstein G. R., Present D., Sands B. E., Sandborn W. J. (2011). Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology, 141(4), 1194–1201. [DOI] [PubMed] [Google Scholar]
- Cosnes J., Gower-Rousseau C., Seksik P., Cortot A. (2011). Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology, 140(6), 1785–1794. [DOI] [PubMed] [Google Scholar]
- Frøslie K. F., Jahnsen J., Moum B. A., Vatn M. H.; IBSEN Group. (2007). Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology, 133(2), 412–422. [DOI] [PubMed] [Google Scholar]
- Higashiyama M., Komoto S., Suzuki Y., Watanabe M., Hibi T., Miura S., Hokari R. (2021). Relation of geriatric nutritional risk index with clinical risks in elderly-onset ulcerative colitis. Journal of Gastroenterology and Hepatology, 36(1), 163–170. [DOI] [PubMed] [Google Scholar]
- Higgins P. D., Johanson J. F. (2004). Epidemiology of constipation in North America: A systematic review. The American Journal of Gastroenterology, 99(4), 750–759. [DOI] [PubMed] [Google Scholar]
- Honkura K., Tomata Y., Sugiyama K., Kaiho Y., Watanabe T., Zhang S., Sugawara Y., Tsuji I. (2016). Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis, 246, 251–256. [DOI] [PubMed] [Google Scholar]
- James S. L., van Langenberg D. R., Taylor K. M., Gibson P. R. (2018). Characterization of ulcerative colitis-associated constipation syndrome (proximal constipation). JGH Open, 2(5), 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloski N. A., Jones M., Wai R., Gill R. S., Byles J., Talley N. J. (2013). Impact of persistent constipation on health-related quality of life and mortality in older community-dwelling women. The American Journal of Gastroenterology, 108(7), 1152–1158. [DOI] [PubMed] [Google Scholar]
- Lagier E., Delvaux M., Vellas B., Fioramonti J., Bueno L., Albarede J., L., Frexinos J. (1999). Influence of age on rectal tone and sensitivity to distension in healthy subjects. Neurogastroenterology and Motility, 11(2), 100–107. [DOI] [PubMed] [Google Scholar]
- Lee A. D., Spiegel B. M., Hays R. D., Melmed G. Y., Bolus R., Khanna D., Khanna P. P., Chang L. (2017). Gastrointestinal symptom severity in irritable bowel syndrome, inflammatory bowel disease and the general population. Neurogastroenterology and Motility, 29(5), e13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard-Jones J. E., Cooper G. W., Newell A. C., Wilson C. W., Jones F. A. (1962). Observations on idiopathic proctitis. Gut, 3(3), 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein G. R., Rutgeerts P. (2010). Importance of mucosal healing in ulcerative colitis. Inflammatory Bowel Diseases, 16(2), 338–346. [DOI] [PubMed] [Google Scholar]
- Madsen J., L., Graff J. (2004). Effects of ageing on gastrointestinal motor function. Age and Ageing, 33(2), 154–159. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Igarashi A., Sato N., Isono Y., Gouda M., Iwasaki K., Shoji A., Hisamatsu T. (2021). Trends in corticosteroid prescriptions for ulcerative colitis and factors associated with long-term corticosteroid use: Analysis using Japanese claims data from 2006 to 2016. Journal of Crohn's and Colitis, 15(3), 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare. (2019). Summary report of comprehensive survey of living conditions 2019, https://www.mhlw.go.jp/english/database/db-hss/dl/report_gaikyo_2019.pdf
- Murakami Y., Nishiwaki Y., Oba M. S., Asakura K., Ohfuji S., Fukushima W., Suzuki Y., Nakamura Y. (2020). Correction to: Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2015: An analysis of a nationwide survey. Journal of Gastroenterology, 55(1), 131. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Ferrante M., Magro F., Campbell S., Franchimont D., Fidder H., Strid H., Ardizzone S., Veereman-Wauters G., Chevaux J. B., Allez M., Danese S., Sturm A.; Scientific Committee of the European Crohn's and Colitis Organization. (2011). Results from the 2nd scientific workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. Journal of Crohn's and Colitis, 5(5), 477–483. [DOI] [PubMed] [Google Scholar]
- Probert C. S., Emmett P. M., Cripps H. A., Heaton K. W. (1994). Evidence for the ambiguity of the term constipation: The role of irritable bowel syndrome. Gut, 35(10), 1455–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratuapli S. K., Bharucha A. E., Noelting J., Harvey D. M., Zinsmeister A. R. (2013). Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology, 144(2), 314–322.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque M. V., Bouras E. P. (2015). Epidemiology and management of chronic constipation in elderly patients. Clinical Interventions in Aging, 10, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmoirago-Blotcher E., Crawford S., Jackson E., Ockene J., Ockene I. (2011). Constipation and risk of cardiovascular disease among postmenopausal women. The American Journal of Medicine, 124, 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W. J., Panés J., Zhang H., Yu D., Niezychowski W., Su C. (2016). Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology, 150(1), 96–102. [DOI] [PubMed] [Google Scholar]
- Schroeder K. W., Tremaine W. J., Ilstrup D. M. (1987). Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine, 317(26), 1625–1629. [DOI] [PubMed] [Google Scholar]
- Shah S. C., Colombel J. F., Sands B. E., Narula N. (2016). Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: A systematic review and meta-analysis. Clinical Gastroenterology and Hepatology, 14(9), 1245–1255.e8. [DOI] [PubMed] [Google Scholar]
- Shimodaira Y., Watanabe K., Iijima K. (2021). Clinical course of ulcerative colitis associated with an age at diagnosis: A recent Japanese database survey. The Tohoku Journal of Experimental Medicine, 255(1), 33–39. [DOI] [PubMed] [Google Scholar]
- Simrén M., Barbara G., Flint H. J., Spiegel B. M., Spiller R. C., Vanner S., Verdu E. F., Whorwell P. J., Zoetendal E. G.; Rome Foundation Committee. (2013). Intestinal microbiota in functional bowel disorders: A Rome Foundation report. Gut, 62(1), 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida K., Molnar M. Z., Potukuchi P. K., Thomas F., Lu J. L., Obi Y., Rhee C. M., Streja E., Yamagata K., Kalantar-Zadeh K., Kovesdy C. P. (2017). Prognostic significance of pre-end-stage renal disease serum alkaline phosphatase for post-end-stage renal disease mortality in late-stage chronic kidney disease patients transitioning to dialysis. Turkish Nephrology Dialysis Transplantation, 33(2), 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida K., Molnar M. Z., Potukuchi P. K., Thomas F., Lu J. L., Yamagata K., Kalantar-Zadeh K., Kovesdy C. P. (2019). Constipation and risk of death and cardiovascular events. Atherosclerosis, 281, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. X., Dibonaventura M., Purayidathil F. W., Wagner J. S., Dabbous O., Mody R. (2011). Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: An analysis of the national health and wellness survey. Digestive Diseases and Sciences, 56(9), 2688–2695. [DOI] [PubMed] [Google Scholar]
- Surrenti E., Rath D. M., Pemberton J. H., Camilleri M. (1995). Audit of constipation in a tertiary referral gastroenterology practice. The American Journal of Gastroenterology, 90, 1471–1475. [PubMed] [Google Scholar]
- Wald A., Scarpignato C., Kamm M. A., Mueller-Lissner S., Helfrich I., Schuijt C., Bubeck J., Limoni C., Petrini O. (2007). The burden of constipation on quality of life: Results of a multinational survey. Alimentary Pharmacology & Therapeutics, 26(2), 227–236. [DOI] [PubMed] [Google Scholar]
- Whitehead W., Chaussade S., Corazziari E., Kumar D. (1991). Report of an international workshop on management of constipation. Clinics in Gastroenterology, 4, 99–113. [Google Scholar]