Abstract
Background
More than half of the patients with locally advanced low rectal cancer exhibit no or minor response to nCRT. It is important to investigate the predictive and prognostic values of potential biomarkers in patients with locally advanced low rectal cancer receiving nCRT.
Materials and Methods
This retrospective study included 162 patients with locally advanced low rectal cancer who underwent nCRT, followed by total mesorectal excision (TME) between 2016 and 2019. Cytokeratin 7 (CK7) expression and mismatch repair (MMR) status were determined by immunohistochemistry (IHC). Categorical variables were compared using the chi-square test. Overall survival (OS) and disease-free survival (DFS) curves were estimated using the Kaplan–Meier and Cox methods.
Results
There were predominance significant differences in distance from anus margin (P < .0001) and circumferential extent of the tumor (P < .0001).CK7 positive expression was detected in 21 of the 162 patients (13%). The univariate and multivariate analysis revealed that patients whose tumors had CK7 positive expression had significantly shorter OS (HR = 3.878, P = .038; HR = 1.677, P = .035) and DFS (HR = 3.055, P = .027;HR = 3.569, P = .038) than those with CK7 negative expression. While patients with CK7 positive expression had a higher proportion of worse TRG compared with CK7 negative patients (P = .001). Patients with deficient mismatch repair (dMMR) just occupied a small proportion (8.6%), but there was still a close connection between the MMR status and recurrence after TME (P = .045). MMR status was an independent risk factor affecting the OS (HR = .307, P < .0001; HR = .123, P < .0001) and DFS (HR = .288, P < .0001; HR = .286, P < .0001) by univariate and multivariate analysis. But no significant difference in the proportion of TRG was observed between patients with dMMR and pMMR (P = .920).
Conclusions
The result confirms negative prognostic role of CK7-positive and dMMR statuses, which have potential predictive value for neoadjuvant chemoradiotherapy response. This provides opportunity to modify individualized treatment strategies for patients with different CK7 expression levels and dMMR statuses.
Keywords: low rectal cancer, cytokeratin 7, deficient mismatch repair, neoadjuvant chemoradiotherapy
Introduction
Relative to cancer-related morbidity and mortality, colorectal carcinoma (CRC) is the second most common malignancy in the world. Approximately 862,000 deaths occur annually with a slowly decreasing mortality rate annually. 1 Some patients with low rectal cancer require removal of the anus to ensure complete excision of the tumor. 2 Based on clinical treatment guidelines, total mesorectal excision (TME) after neoadjuvant chemoradiotherapy (nCRT) is the primary treatment for patients with locally advanced low rectal cancer. nCRT not only reduces local recurrence in patients with low rectal cancer 3 but also increases the chance of preserving the anus by reducing tumor size and downgrading tumors. 4
Many studies have shown that the responses to nCRT are highly variable. 5 More than half of the patients exhibit no or minor response to nCRT, and develop metastasis and recurrence in over 25% of the cases.6-8 nCRT can increase the risk of toxicity without any apparent benefits. 9 It is important to identify these patients and formulate an individualized neoadjuvant therapy strategy to reduce perioperative complications and recurrence rates. Therefore, identifying biomarkers as predictors to identify these patients prior to treatment has become an important clinical challenge; however, none of these identified protein markers or signatures have been independently verified in the clinic. Thus, the present study aimed to investigate the predictive and prognostic values of potential biomarkers in patients with locally advanced low rectal cancer receiving nCRT.
Materials and Methods
Study Population
A total of 162 rectal cancer cases receiving nCRT followed by TME were selected. This study was retrospectively analyzed from consecutively collected patient data. The written informed consents were obtained for the use of the tissue specimens for research purposes, as approved by the ethics committees of Tianjin Union Medical Center. This study was conducted in one center. This study was a retrospective analysis based on patient information for which data were consecutively collected. The grade and stage of the tumors were recorded based on the medical records from our institution. Staging was assigned according to the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system (8th edition). The four categories of AJCC tumor regression grading (TRG) system were classified as following: grade 0 (complete response), no remaining viable cancer cells; grade 1 (moderate response), only small cluster or single cancer cells remaining; grade 2 (minimal response), residual cancer remaining, but with predominant fibrosis; grade 3 (poor response), minimal or no tumor regression with extensive residual cancer. 10 Medical records from the pathology department provided 162 randomly selected cases of histopathologically verified rectal adenocarcinoma that were surgically treated between 2016 and 2019, including distance from anus margin and circumferential extent of the tumor, which were measured by pathologists during pathological examination. Patients with synchronous multiple primary cancers, inflammatory bowel disease, or familial adenomatous polyposis were excluded from this study.
Immunohistochemistry Staining and Assessment
Paraffin-embedded tissue samples were sectioned and stained using an immunohistochemistry autostainer (BenchMark, Roche, Basel, Switzerland). Primary monoclonal antibodies against cytokeratin 7(CK7) (clone EP16; Zhong Shan Jinqiao, Beijing, China), mutL homolog1 (MLH1) (clone OTI4H4; Zhong Shan Jinqiao), mutShomolog2 (MSH2) (clone OTIR1B12; Zhong Shan Jinqiao), mutS homolig6 (MSH6) (clone UMAB258; Zhong Shan Jinqiao), and postmeiotic segregation increased2 (PMS2) (clone EP51; Zhong Shan Jinqiao) were used.
Treatment and Follow-Up
All patients underwent chemoradiotherapy before total mesorectal excision, which defined as neoadjuvant chemoradiotherapy, including chemotherapy regimens with fluorouracil/oxaliplatin, and radiotherapy was administered at 2.0 Gy in 25 fractions over 5 weeks, with a total dose of 50 Gy (24–26). Physical examinations and computed tomography scans were performed every 3–6 months for the first 1 year and every 6 months for the following 2 years after TME as a routine follow-up strategy for all patients. The data were updated in May 2022.
Microscopic Observation
All immunohistochemical examinations were performed under a microscope by two experienced pathologists. For CK7 IHC staining, the distribution of CK7 positive cells was scattered in some cases, and even less than 10% of the positive cells for CK7 IHC staining in one slide, it was also considered as immunoreaction. Tumors with any obvious nuclear staining for MSH2, MSH6, PMS2, or MLH1 were considered MMR-proficient (pMMR). Tumors with negative nuclear staining and positive control in the normal adjacent epithelium and lymphocytes were considered MMR-deficient (dMMR).
Ethical Statement
This study was reviewed and approved by the ethics committees of Tianjin Union Medical Center in Tianjin, China (the ethical approval number was 2023 (B74) and the ethical committee name was the Ethics Committees of Tianjin Union Medical Center), and was conducted in accord with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The reporting of this study conforms to the REMARK guidelines 11 and all patient information was de-identified. Written informed consent for their data to be used was obtained from all of the patients.
Statistical Analysis
Categorical variables were compared using the chi-square test. Overall survival (OS) and disease-free survival (DFS) curves were estimated using the Kaplan–Meier method and Cox proportional hazards regression model with P values for prognostic factors. Variables with P values <.05 in the univariate analysis or multivariate analysis were considered statistically significant. All statistical analyses were performed using the SPSS software.
Results
Clinicopathological Data
A total of 162 patients were enrolled in this study. Among them, CK7 expression and MMR status were detected by IHC, 21 patients had CK7 positive expression, while dMMR tumors were found in 14 patients. No significant differences were observed between the CK7-positivity and CK7-negativity group in terms of age, sex, pT or pN stage, number of lymph nodes and recurrence after TME (P > .05) (Table 1). There were statistically significant differences in distance from anus margin (P < .0001) and circumferential extent of the tumor (P < .0001) (Table 1). As to the different MMR status groups, significant differences were found in distance from anus margin (P < .0001) and circumferential extent of the tumor (P < .0001) either (Table 2). Meanwhile, dMMR patients were more easily happened to recur after operation (P = .045) (Table 2).
Table 1.
The Clinicopathologic Characteristics of Patients after CRT based on CK7 Expression.
| Variable | Total (n) | CK7 Expression | P | |
|---|---|---|---|---|
| CK7 +(n) | CK7 -(n) | |||
| Sex | .542 | |||
| Male | 117 | 14 | 103 | |
| Female | 45 | 7 | 38 | |
| Age | .896 | |||
| <60 y | 87 | 11 | 76 | |
| ≥60 y | 75 | 10 | 65 | |
| Distance from annus margin | .007 | |||
| <5 cm | 84 | 12 | 72 | |
| 5-10 cm | 69 | 5 | 64 | |
| >10 cm | 9 | 4 | 5 | |
| Circumferential extent of the tumor | .050 | |||
| <1/2 | 108 | 10 | 98 | |
| ≥1/2 | 54 | 11 | 43 | |
| pT | .863 | |||
| T0 | 1 | 0 | 1 | |
| T1 | 6 | 0 | 6 | |
| T2 | 25 | 3 | 22 | |
| T3 | 115 | 14 | 101 | |
| T4 | 15 | 0 | 11 | |
| pN | .246 | |||
| N0 | 97 | 12 | 85 | |
| N1 | 44 | 4 | 40 | |
| N2 | 21 | 5 | 16 | |
| Number of lymph nodes | .931 | |||
| <12 | 45 | 6 | 39 | |
| ≥12 | 117 | 15 | 102 | |
| Recurrence after TME | .342 | |||
| Yes | 38 | 7 | 31 | |
| No | 104 | 13 | 91 | |
| Censored | 20 | 1 | 19 | |
| TRG | .001 | |||
| 0 | 1 | 0 | 1 | |
| 1 | 16 | 1 | 15 | |
| 2 | 78 | 3 | 75 | |
| 3 | 76 | 17 | 50 | |
Table 2.
The Clinicopathologic Characteristics of Patients after CRT based on MMR Status.
| Variable | Total (n) | MMR Status | P | ||
|---|---|---|---|---|---|
| dMMR(n) | pMMR(n) | ||||
| Sex | .945 | ||||
| Male | 117 | 10 | 107 | ||
| Female | 45 | 4 | 41 | ||
| Age | .394 | ||||
| <60 y | 87 | 6 | 81 | ||
| ≥60 y | 75 | 8 | 67 | ||
| Distance from annus margin | .000 | ||||
| <5 cm | 84 | 4 | 80 | ||
| 5-10 cm | 69 | 6 | 63 | ||
| >10 cm | 9 | 4 | 5 | ||
| Circumferential extent of the tumor | .000 | ||||
| <1/2 | 108 | 3 | 105 | ||
| ≥1/2 | 54 | 11 | 43 | ||
| pT | .432 | ||||
| T0 | 1 | 0 | 1 | ||
| T1 | 6 | 1 | 5 | ||
| T2 | 25 | 1 | 24 | ||
| T3 | 115 | 9 | 106 | ||
| T4 | 15 | 3 | 12 | ||
| pN | .191 | ||||
| N0 | 97 | 7 | 90 | ||
| N1 | 44 | 3 | 41 | ||
| N2 | 21 | 4 | 17 | ||
| Number of lymph nodes | .488 | ||||
| <12 | 45 | 5 | 40 | ||
| ≥12 | 117 | 9 | 108 | ||
| CK7 expression | .877 | ||||
| Positivity | 21 | 2 | 19 | ||
| Negativity | 141 | 12 | 129 | ||
| Recurrence after TME | .045 | ||||
| Yes | 38 | 5 | 33 | ||
| No | 104 | 5 | 99 | ||
| Censored | 20 | 4 | 16 | ||
| TRG | .920 | ||||
| 0 | 1 | 0 | 1 | ||
| 1 | 16 | 2 | 14 | ||
| 2 | 78 | 6 | 72 | ||
| 3 | 67 | 6 | 61 | ||
CK7 Expression was Associated with Survival and Tumor Responses to Neoadjuvant Therapy
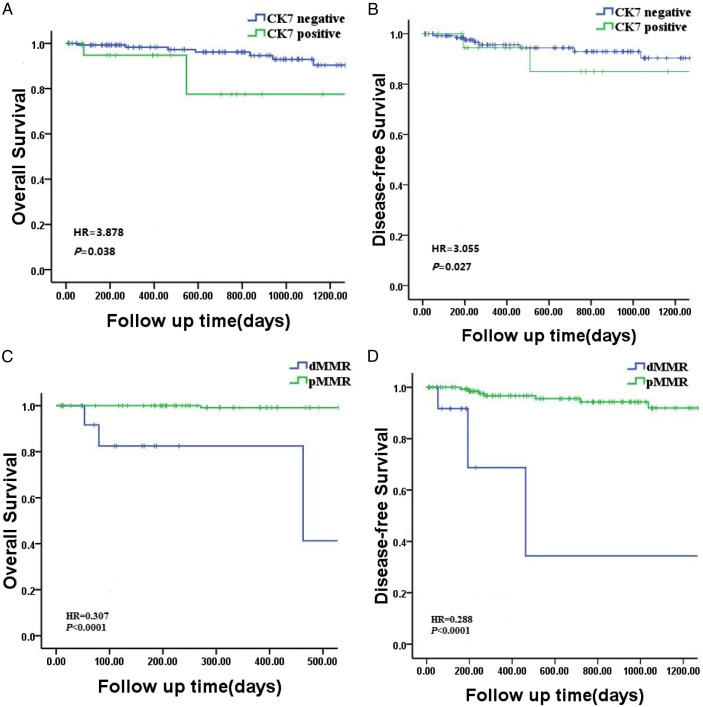
Among the 162 enrolled patients, 21 CK7 positive and 141 CK7 negative cases were summarized in Table 1. Univariate analysis revealed that CK7 expression was associated with OS (HR = 3.878, P = .038) and DFS (HR = 3.055, P = .027)after nCRT of rectal cancer (Table 3).After neoadjuvant therapy, patients with CK7 positive expression had a lower OS(HR = 1.677, P = .035) (Figure 1(A)), and DFS(HR = 3.569, P = .038) (Figure 1(B)) in the multivariate analysis (Table 4). After nCRT, patients with CK7 positive expression had a higher proportion of worse TRG compared with CK7 negative patients (P = .001) (Table 1).
Table 3.
Univariate Analysis of Overall survival (OS) and Disease Free Survival (DFS) in Patients With low Rectal Cancer After Neoadjuvant Therapy.
| Variable | OS | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Distance from annus margin (<5vs.5-10vs.>10 cm) | 4.216 (1.460–12.168) | .000 | 3.585 (1.408–9.127) | .000 |
| Circumferential extent of the tumor (<1/2 vs ≥1/2) | 5.612 (3.752–8.393) | .000 | 4.176 (2.832–6.158) | .000 |
| CK7 (positive vs negative) | 3.878 (1.080–13.926) | .038 | 3.055 (1.145–6.460) | .027 |
| MMR status (dMMR vs pMMR) | .307 (.146–.641) | .000 | .288 (.138–.604) | .000 |
Figure 1.
Survival by CK7 expression and mismatch repair status. (A) OS and (B) DFS in patients by CK7 expression; (C) OS and (D) DFS in patients by mismatch repair status.
Table 4.
Multivariate Analysis of Overall survival (OS) and Disease Free Survival (DFS) in Patients With low Rectal Cancer After Neoadjuvant Therapy.
| Variable | OS | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Distance from annus margin (<5vs.5–10vs.>10 cm) | 6.324 (2.383–16.786) | .000 | 4.371 (1.828–10.449) | .001 |
| Circumferential extent of the tumor (<1/2 vs≥1/2) | 8.764 (2.258–34.018) | .002 | 4.878 (1.388–17.147) | .013 |
| CK7 (positive vs negative) | 1.677 (1.037–2.712) | .035 | 3.569 (1.073–11.872) | .038 |
| MMR status (dMMR vs pMMR) | .123 (.062–.246) | .000 | .286 (.152.537) | .000 |
MMR Status was Associated with Survival and Tumor Responses to Neoadjuvant Therapy
The associations of different clinicopathologic characteristics between dMMR and pMMR groups are listed in Table 2. Deficient MMR was defined as loss of expression of more than one of the four proteins (MSH2, MSH6, MLH1, and PMS2) in tumors by IHC. With a median follow-up period of 25.1 months, a statistically significant difference was observed in MMR status and local recurrence rates (P = .045) (Table 2).Univariate analysis showed that MMR status was associated with OS (HR = .307, P < .0001) and DFS (HR = .288, P < .0001) after nCRT of rectal cancer (Table 3). For patients who received nCRT had disappointing OS (HR = .123, P < .0001) (Figure 1(C)) and DFS (HR = .286, P < .0001) (Figure 1(D)) were observed in the presence of a dMMR status by multivariate analysis (Table 4).No significant difference in the proportion of TRG was observed between patients with dMMR and pMMR (P = .920). But patients with pMMR had a better response to nCRT as higher proportion of TRG 3 was confirmed in patients with dMMR (Table 2).
Survival and Tumor Responses to nCRT According to Other Clinical Findings
Univariate analysis showed that the distance from anus margin (HR = 4.216, P < .0001) and circumferential extent of the tumor (HR = 5.612, P < .0001) were related to OS after nCRT (Table 3). The distance from anus margin (HR = 3.585, P < .0001) and circumferential extent of the tumor (HR = 4.176, P < .0001) were also related to DFS after nCRT for rectal cancer (Table 3).
Multivariate Cox analysis showed that the distance from anus margin (HR = 6.324, P < .0001) and the circumferential extent of the tumor (HR = 4.371, P = .001) were independent predictors of OS after nCRT (Table 4). The distance from anus margin (HR = 8.764, P = .002) and the circumferential extent of the tumor (HR = 4.878, P = .013) were independent predictors of DFS after nCRT for rectal cancer (Table 4).
Discussion
The correlation between the protein levels of specific genes and the response to nCRT in rectal cancer has been extensively investigated using immunohistochemical analysis. The identified protein biomarkers are involved in diverse biological processes such as DNA repair, oncogenic signaling, and apoptosis.12-14 Here, we analyzed whether the application of protein markers could predict the response to nCRT and found that CK7 expression and MMR status were important factors affecting the prognosis of patients undergoing nCRT.
CRC is usually associated with a CK7 negative and CK20 positive profile. CK7 is considered a good marker for primary lung cancer and metastatic CRC; however, not all CRCs lack CK7 expression. Studies have shown that the rate of CK7-positivity can vary from 0 to 22%.15,16 In terms of TRG, the response to nCRT of patients with CK7 positive expression was worse than that of CK7 negative patients in our study, while less research between CK7 and nCRT was done. A main finding of our study was that CK7 expression in low rectal cancer with nCRT was an independent, strong, and negative prognostic predicator. Bayrak et al. described more frequent expression of CK7 in CRCs with regional lymph node metastasis. 17 Hernandez et al. reported a higher prevalence of CK7 positivity in advanced-stage CRCs than early-stage cancers. 18 Fei et al. reported a trend toward an increased risk of positive lymph nodes and high-grade tumors in patients with high CK7 expression. 19 Moreover, Loupakis et al. reported that CK7 expression signifies poor survival. 20 In our study, CK7 positivity was independently associated with worse survival in the multivariate analysis of nCRT for OS and DFS. A possible reason for the poor survival and tumor response is that CK7 positivity has a particular molecular phenotype in CRC with higher invasiveness. Fei et al. established a close association between CK7 positive and polyploid giant cancer cells, which are associated with tumor budding, vascular invasion, and micropapillary patterns, by observing more CK7 positive in tumor budding cells. 19 Furthermore, according to the previous studies, CK7 expression may be regarded as a negative prognostic factor with a relatively high recurrence, which is linked to epithelial-mesenchymal transition that gives the tumor more aggressive capacity. 17 However, no significant differences were observed between the CK7-positivity and recurrence after nCRT in our study. A possible reason may be that the CK7 positive group was small in CRC, with a rate varying from 0 to 22%, and the number of cases available for nCRT clinical studies is even smaller.
Tumors are typically classified as either pMMR or dMMR, 21 the latter can lead to microsatellite instability (MSI), which could result in the accumulation of insertion or deletion mutations within microsatellite DNA regions. Deficient MMR can result from the inheritance of a germline mutation in the MMR gene (MLH1, MSH2, MSH6, or PMS2) that is often associated with the CpG island methylator phenotype. 22 Our finding might imply that patients with dMMR respond worse to neoadjuvant therapy in terms of TRG than patients with pMMR, as a higher proportion of TRG 3 was confirmed in patients with dMMR. Previous studies have evaluated the tumor response and survival effect of MMR status in rectal cancer patients with conflicting results. Meillan et al. reported that dMMR was correlated with worse TRG. 23 Wu et al. showed that pMMR patients had a better response, and dMMR is a good prognostic marker for DFS in ypStage II/III patients after nCRT. 24 Conversely, Chen et al. found there were better overall survival rates for LARC patients with dMMR status after nCRT. 25 Kim JH et al. also demonstrated the prognostic features of MSI-H CRCs include a favorable survival of patients. 26 There may be two main reasons for this: One possible explanation for the converse result involves different nCRT regimens, such as consolidation chemotherapy with Capeox or mFOLFOX6 regimens can significantly improve survival,27,28 while a limited effects of fluoropyrimidines may happened during nCRT. 29 On the other hand, compared with other carcinomas, dMMR status just occurs in less than 5% of rectal cancer, a smaller number of dMMR group was available for the correlation clinical trials. Our data also showed that in low rectal cancer patients who received nCRT, dMMR status was an independent and significant prognostic marker for OS and DFS. While a close connection was observed between the local recurrence rates and dMMR status in our study. Cercek et al. found 29% of dMMR/MSI locally advanced rectal tumors had disease progression on nCRT, 30 an alarmingly high rate compared to no progression in pMMR rectal tumors. One possible explanation for that is a process lacking a normal repair system may occur as the presence of dMMR, resulting in dMMR resistance to 5-FU; hence, a lower effect is induced by fluorouracil-based chemotherapy with dMMR status.
As for the two indicators discussed above, Landau MS et al. documented a significant association between CK7 expression and microsatellite stability, as a more expression of CK7 in BRAF-mutated MSS colorectal carcinoma. 31 This may be due to the high mutation pathway and genomic instability in MSI CRC, which lead to the gaining of non-intestinal markers. The association between CK7 positive expression and dMMR status was corroborated in our study, unfortunately the difference did not reach statistical significance, even both of them exhibited a worse prognosis.
In addition, clinical factors that may be involved in prediction have been identified, such as tumor size, TNM stage, radiation dose, and the time interval between nCRT and surgery. For decades, an N-staging system based on the number of lymph nodes has been used to guide adjuvant treatment. 32 Previous studies have reported that preoperative therapy may decrease LN yield. Amajoyi et al. observed that the mean number of lymph nodes obtained was lower in patients treated with nCRT than in surgery alone. 33 In the present study, even with no statistically significant differences, a high proportion of less lymph nodes harvested from nCRT samples was observed (27.8%), except the well-known direct effect on reducing the size of primary tumors.
Several studies have investigated the correlation between the tumor size and pCR of rectal cancer after preoperative nCRT.34,35 In general, large tumors always increase the possibility of hypoxic status, which might be tightly associated with radioresistance and poor response to nCRT. Suwanthanma et al. reported that tumor length >4 cm was associated with a low rate of pCR in rectal cancer followed by CRT. 36 Hsu et al. revealed that, for patients with locally advanced rectal cancer receiving preoperative nCRT, tumor compactness is a useful radiomic parameter for improving the volumetric-based prediction model. 37 Multivariate analysis in our study showed that a larger proportion of tumors in the intestinal lumen (≥1/2) were an independent factor influencing OS and DFS. Similarly, as described above, the smaller the tumor volume, the less the tumor occupies the intestinal lumen, and the higher the OS and DFS obtained due to a better response to nCRT. In this study, the multivariate analysis also showed that the distance from anus margin was an unfavorable independent prognostic factor for OS and DFS, which partly associated with unsatisfactory radiation treatment fields.
Conclusions
Our study provides interesting results indicating that in low rectal carcinoma with nCRT, CK7-positivity expression and dMMR status are predictive and independent markers of poor prognosis. There is great benefit in identifying biomarkers to predict the response to nCRT in locally advanced low rectal cancer, which may help establish individualized treatment strategies. However, this study has several limitations. First, it was a retrospective study, and selection bias cannot be avoided, although we used propensity score matching to reduce it. Second, the database did not include some important clinicopathological characteristics such as the interval between nCRT and TME, baseline levels of tumor biomarkers, and patient compliance with nCRT. A potential bias may have been induced by the lack of information. Third, the MMR status was determined by IHC. However, owing to the possible presence of non-functional proteins, the sensitivity and specificity of IHC alone could not be on par with those of PCR testing in a few cases of MSI with rare missense mutations. The most important challenge is integrating the identified markers to facilitate clinical implementation.
Acknowledgments
We acknowledge editage service for the manuscript language edit.
Appendix
Abbreviations
- CRC
colorectal carcinoma
- TME
total mesorectal excision
- nCRT
neoadjuvant chemoradiotherapy
- IHC
immunohistochemistry
- CK7
cytokeratin 7
- MMR
mismatch repair
- pMMR
MMR proficient
- dMMR
MMR deficient
- OS
overall survival
- DFS
disease-free survival
- TRG
tumor regression grading
- AJCC
the American Joint Committee on Cancer.
Footnotes
Author Contributions: Conceptualization and supervision: Shiwu Zhang; Methodology: Songli Shi, Mingming Zhou, Gang Wang, and Jinling Xu; Validation: Dan Zhang; Review and editing, Shiwu Zhang; Funding acquisition, Shiwu Zhang. All authors have read and agreed to the published version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the National Science Foundation of China (#82173283 and #82103088), Foundation of the committee on science and technology of Tianjin (#21JCZDJC00230, 21JCYBJC00190, and 21JCYBJC01070), and Foundation of Tianjin Union Medical Center (2019YJ009).The funder had no roles in the design of the study, data collection, analysis and interpretation, or decision to write and publish the work. The funders had no roles in the design of the study, data collection, analysis and interpretation, or decision to write and publish the work.
Ethical Statement
Ethical Approval
This study was approved by the Ethics Committee of Tianjin Union Medical Center, Tianjin, China. All the patients anonymity was maintained in accordance with the Declaration of Helsinki. The ethical approval statement include the name (the Ethics Committee of Tianjin Union Medical Center) and location of the review board (Tianjin Union Medical Center), the approval number (2023 (B74) of rapid review), and the date of approval to your manuscript (2016–2023).
ORCID iD
Shiwu Zhang https://orcid.org/0000-0002-5052-2283
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2.Lin H, Wang L, Zhong X, Zhang X, Shao L, Wu J. Meta-analysis of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for locally advanced rectal cancer. World J Surg Oncol. 2021;19:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coraglio MF, Eleta MA, Kujaruk MR, et al. Analysis of long-term oncological results of clinical versus pathological responses after neoadjuvant treatment in locally advanced rectal cancer. World J Surg Oncol. 2020;18:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dossa F, Acuna SA, Rickles AS, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol. 2018;4:930-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Jiang T, Xiao L, et al. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis. Oncol. 2021;26:e1555-e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley AM, Lynam-Lennon N, O’Neill H, O’Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol. 2020;17:298-313. [DOI] [PubMed] [Google Scholar]
- 7.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4:e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Sluis FJ, Couwenberg AM, de Bock GH, et al. Population-based study of morbidity risk associated with pathological complete response after chemoradiotherapy for rectal cancer. Br J Surg. 2020;107:131-139. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari L, Fichera A. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (Oxf). 2015;3:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HY, Feng LL, Li M, et al. College of American pathologists tumor regression grading system for long-term outcome in patients with locally advanced rectal cancer. Oncol. 2021;26:e780-e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou X, Wang R, Meng X, et al. The prognostic role of TCF4 expression in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy. Cancer Biomark. 2015;15:181-188. [DOI] [PubMed] [Google Scholar]
- 13.Yan X, Chen J, Meng Y, et al. RAD18 may function as a predictor of response to preoperative concurrent chemoradiotherapy in patients with locally advanced rectal cancer through caspase-9-caspase-3-dependent apoptotic pathway. Cancer Med. 2019;8:3094-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaanan A, Park JM, Tougeron D, et al. Association of beclin 1 expression with response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal carcinoma. Int J Cancer. 2015;137:1498-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Maghrabi J, Emam E, Gomaa W. Immunohistochemical staining of cytokeratin 20 and cytokeratin 7 in colorectal carcinomas: Four different immunostaining profiles. Saudi J Gastroenterol. 2018;24:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrudka J, Fiserova H, Jelinkova K, Matej R, Waldauf P. Cytokeratin 7 expression as a predictor of an unfavorable prognosis in colorectal carcinoma. Sci Rep. 2021;11:17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayrak R, Yenidunya S, Haltas H. Cytokeratin 7 and cytokeratin 20 expression in colorectal adenocarcinomas. Pathol Res Pract. 2011;207:156-160. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez BY, Frierson HF, Moskaluk CA, et al. CK20 and CK7 protein expression in colorectal cancer: Demonstration of the utility of a population-based tissue microarray. Hum Pathol. 2005;36:275-281. [DOI] [PubMed] [Google Scholar]
- 19.Fei F, Li C, Cao Y, et al. CK7 expression associates with the location, differentiation, lymph node metastasis, and the Dukes’ stage of primary colorectal cancers. J Cancer. 2019;10:2510-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loupakis F, Biason P, Prete AA, et al. CK7 and consensus molecular subtypes as major prognosticators in (V600E)BRAF mutated metastatic colorectal cancer. Br J Cancer. 2019;121:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olave MC, Graham RP. Mismatch repair deficiency: the what, how and why it is important. Genes Chromosomes Cancer. 2022;61:314-321. [DOI] [PubMed] [Google Scholar]
- 22.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meillan N, Vernerey D, Lefevre JH, et al. Mismatch repair system deficiency is associated with response to neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2019;105:824-833. [DOI] [PubMed] [Google Scholar]
- 24.Wu Z, Hu H, Wang C, et al. The prognostic and predictive value of mismatch repair status in patients with locally advanced rectal cancer following neoadjuvant therapy. Ann Transl Med. 2022;10:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Yang X, Zhang Y, et al. Survival outcomes analysis according to mismatch repair status in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy. Front Oncol. 2022;12:920916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014;20:4230-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Huang Y, Sun G, et al. Rectal cancer patients with downstaging after neoadjuvant chemoradiotherapy and radical resection do not benefit from adjuvant chemotherapy. Ann Transl Med. 2020;8:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [DOI] [PubMed] [Google Scholar]
- 29.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cercek A, Dos Santos Fernandes G, Roxburgh CS, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. 2020;26:3271-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landau MS, Kuan SF, Chiosea S, Pai RK. BRAF-mutated microsatellite stable colorectal carcinoma: An aggressive adenocarcinoma with reduced CDX2 and increased cytokeratin 7 immunohistochemical expression. Hum Pathol. 2014;45:1704-1712. [DOI] [PubMed] [Google Scholar]
- 32.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: Rectal cancer, version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [DOI] [PubMed] [Google Scholar]
- 33.Amajoyi R, Lee Y, Recio PJ, Kondylis PD. Neoadjuvant therapy for rectal cancer decreases the number of lymph nodes harvested in operative specimens. Am J Surg. 2013;205:289-292. [DOI] [PubMed] [Google Scholar]
- 34.Appelt AL, Ploen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambregts DM, Rao SX, Sassen S, et al. MRI and diffusion-weighted MRI volumetry for identification of complete tumor Responders after preoperative chemoradiotherapy in patients with rectal cancer: A Bi-institutional validation study. Ann Surg. 2015;262:1034-1039. [DOI] [PubMed] [Google Scholar]
- 36.Suwanthanma W, Kitudomrat S, Euanorasetr C. Clinical outcome of neoadjuvant chemoradiation in rectal cancer treatment. Medicine (Baltim). 2021;100:e27366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu CY, Wang CW, Kuo CC, et al. Tumor compactness improves the preoperative volumetry-based prediction of the pathological complete response of rectal cancer after preoperative concurrent chemoradiotherapy. Oncotarget. 2017;8:7921-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]