Abstract
Sodium-ion batteries (SIBs) are seen as an emerging force for future large-scale energy storage due to their cost-effective nature and high safety. Compared with lithium-ion batteries (LIBs), the energy density of SIBs is insufficient at present. Thus, the development of high-energy SIBs for realizing large-scale energy storage is extremely vital. The key factor determining the energy density in SIBs is the selection of cathodic materials, and the mainstream cathodic materials nowadays include transition metal oxides, polyanionic compounds, and Prussian blue analogs (PBAs). The cathodic materials would greatly improve after targeted modulations that eliminate their shortcomings and step from the laboratory to practical applications. Before that, some remaining challenges in the application of cathode materials for large-scale energy storage SIBs need to be addressed, which are summarized at the end of this Outlook.
Short abstract
The reasonable structural design and scalable preparation of cathode materials are crucial factors for the successful transition of sodium-ion batteries from the lab to industry.
1. Introduction
Sodium-ion batteries (SIBs) offer safer and more environmentally sustainable solutions to lithium-ion batteries (LIBs) with comparable performance.1 Despite great potential in applications for high-power energy storage systems, current SIBs still suffer from drawbacks, such as an inferior charge and discharge rate (low power density), lower specific capacity (low energy density), and short cycle life.2−4 Cathode materials play a central role in determining the electrochemical performance of SIBs. However, the current SIB cathodes face challenging issues, including undesirable phase changes along cycling, sluggish Na ion mobility, and unfavorable interphase formation between electrode and electrolytes, which are mainly associated with the larger Na ions compared to Li ions.5,6 Hence, the selection of electrode materials, especially cathode materials featuring high energy densities and prolonged cycle life, that could buffer the repeated Na+ (de)insertion is quite crucial.
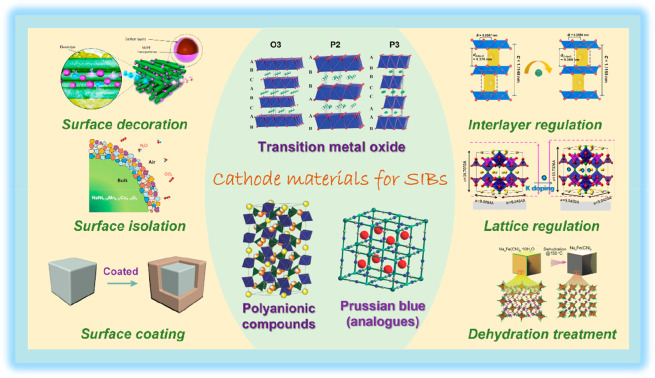
Up to now, three categories of materials have been explored as cathodic alternatives for SIBs: transition metal oxides, polyanionic compounds, and Prussian blue analogs (PBAs). Each category of the cathode materials has their own features and inherent problems. The transition layered oxides with large spacers for Na+ storage have high reversible specific capacities, high energy densities, and excellent rate capabilities combined with susceptibly convertible technologies. However, such a layered structure is prone to collapse when accommodating large-radius Na+ for (de)insertion, resulting in an unsatisfactory cycle lifespan; besides, most layered oxides are sensitive to the moisture in the air and the absorbent, thus bringing about storage difficulties.3,78 Polyanionic compounds possess high working voltages and excellent thermal/cyclic stability but suffer from inferior intrinsic electronic conductivity that causes a low specific capacity and poor rate capability.9−11 Prussian blue and its analogs have the advantages of low cost, great rate performance, and adjustable working voltage, but the stubborn lattice water is difficult to remove and reduces the chemical stability and structural stability of the PBA material.12 Effective improvement strategies have been proposed to address the shortcomings of different cathode materials, such as surface modification (isolation or coating), structural design, and lattice or interlayer modulation, in order to realize the high energy density, superior rate capability, and long service lifespan of SIBs (Figure 1).
Figure 1.
Overview of cathodic materials and their effective modification strategies. Top left image reproduced with permission from ref (182). Copyright 2016 American Chemical Society. Middle left image reproduced with permission from ref (44). Copyright 2018 American Chemical Society. Bottom left image reproduced with permission from ref (138). Copyright Wiley-VCH. Top center image reproduced from (6). Copyright 2020 Wiley-VCH. Bottom-left center image reproduced with permission from ref (12). Copyright 2018 Wiley-VCH. Bottom-right center image reproduced with permission from ref (9). Copyright 2018 Wiley-VCH. Top right image reproduced with permission from ref (68). Copyright 2022 Springer Nature. Middle right image reproduced with permission from ref (183). Copyright 2019 Elsevier. Bottom right image reproduced with permission from ref (184). Copyright 2016 Wiley-VCH.
In this Outlook, we summarized the recent progress of the major cathodic materials for SIBs, introducing their crystal structures, physicochemical properties, and electrochemical applications. Finally, the remaining challenges in the application of these cathode materials for future large-scale energy storage SIBs are discussed. We hope this Outlook can make a guiding contribution to the development of cathode materials for high-energy SIBs.
2. Transition Metal Oxides
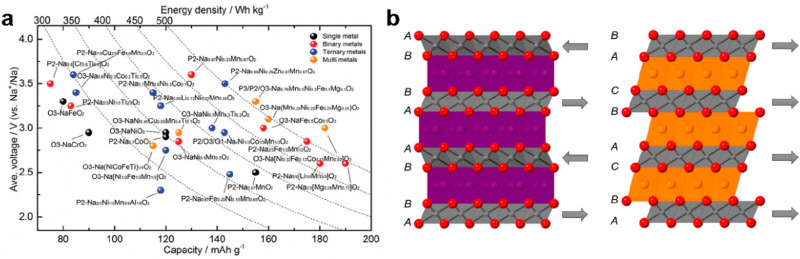
The composition and structure of current cathodes for SIBs are mostly inherited from the LIB cathode analogs, while it is observed that the insertion/alloying of larger Na ions into/with the electrodes leads to distinctive crystal structures, in other words, offering increased structural versatility. In general, the materials that have been investigated as cathodes for SIBs include layered- and tunnel-structured transition metal oxides, polyanion compounds, and Prussian blue analogs (PBAs). Among them, layered transition metal oxides are considered as the most important cathodic alternatives for SIBs. Figure 2a compares the voltages, capacities, and energy densities of the layered metal oxide cathodes composed of single, binary, ternary and multicomponent metal ions. Typical Na-based layered transition oxides, i.e., NaMO2 (M = Ni, Co, Mn, Fe, Cr, V, etc.), exist in different crystal structures denoted as P2, P3, O2, and O3 according to Delmas’ notation.13 O and P indicate the coordination environment of Na+, in which O represents the Na occupancy at the octahedron sites surrounded by six oxygens and P represents the Na occupancy at the center of prism sites surrounded by six oxygens. Among them, O3-phase and P2-phase are most widely investigated as cathodes for SIBs, and their crystal structures are illustrated in Figure 2b. Typical examples of O3-type metal oxides include NaFeO2,14 NaNiO2,15 and NaNi1/2Mn1/2O2,16 and those for P2-type metal oxides include Na2/3MnO2,17 Na0.7CoO2,18 and Na2/3Ni1/3Mn2/3O2.19 Generally, the P2 structure renders the best power performance for SIBs, as Na ion diffuse through rectangular faces between adjacent trigonal prismatic environments, which is unavailable in LIBs. The O3-structured materials are outstanding in capacity, as they have the highest sodium stoichiometries. In recent years, hybrid P2/O3-structured materials have attracted extraordinary attention for simultaneous optimization of the power and energy density for SIBs.
Figure 2.
(a) Summary of the electrochemical properties of various layered oxide cathodes. (b) Illustration of P2 (left) and O3 (right) crystal structures. Reproduced with permission from from ref (20). Copyright 2021 IOP.
2.1. Na-Free Transition Metal Oxides
Vanadium oxides have been investigated as the cathodic materials for SIBs due to their features of high capacity and low cost. VO2(A) is unstable during electrochemical reactions,21 but VO2(B) is considered a more suitable cathode for SIBs because its layered structure allows for rapid Na ion diffusion; the corresponding theoretical capacity is as high as 322 mA h g–1, which is associated with one e– transfer during the (de)sodification process.22,23 However, VO2(B) is metastable and less conductive, so rapid capacity fading was often observed for VO2(B) because of the drastic volume expansion, dissolution, and aggregation of the electrode material. In comparison, V2O5 shows higher chemical and thermal stability with layered and orthorhombic structures, both of which are electrochemically active. The theoretical capacity of V2O5 varies depending on the number of transfer electrons involved in the redox reaction (capacities of 294 and 441 mA h g–1 corresponding to 2e– and 3e– participating in the reaction, respectively). The electrochemical process of V2O5, however, starts to degrade with the morphology change or crystal structure collapse upon cycling, which is also known as “lattice breathing”.24 The stability of V2O5 electrodes can be enhanced by inserting larger cations (Na+, NH4+) or water molecules into the crystal interlayers.25,26,27 Constructing V2O5 aerogels with highly porous 3D networks has also proved to be an efficient strategy for enhancing their performance.28 Apart from the crystallized structure, the amorphous V2O5 also demonstrates a Na storage property.29 Furthermore, manganese oxides, such as α-MnO2 and β-MnO2, show promise as cathodes for SIBs, and they exhibit a theoretical Na+ storage capability of 308 mA h g–1.30 In particular, the compact tunnels in β-MnO2 along the [001] direction are favorable for the Na+ insertion/extraction and contribute to the better Na storage capacity. Introduction of exchangeable guest cations into the MnO2 framework can modulate the Na storage performance, though the exact role and effects of the guest ions still needs further investigation.31
2.2. Layered Sodium Monometallic Oxides
The sodium monometallic oxides often suffer from poor stability and rapid degradation due to the continuous phase changes of the oxides, especially at high voltages. For example, α-NaFeO2 is a O3-type cathode material with excellent thermal stability with an active Fe3+/Fe4+ redox couple. Under higher voltages, their electrochemical performance degrades mainly due to the Jahn–Teller distortion and polarization. Fe4+ is reduced to Fe3+ at the charged state, and the excessive Fe3+ will migrate and block the diffusion pathways of Na ions, causing the degradation of performance.32 A recent finding also points out both oxygen reactivity and the Fe3+/Fe4+ contribute to the electrochemical activity of NaFeO2, evidenced by the diminished Fe3+ ions under high voltages.33 Partially substituting Fe with Ni3+ ions or Co3+ ions, reducing the particle sizes, or surface modification can help enhance the stability of NaFeO2.34 NaxCoO2 exists in both the P2 and O3 phase, where the P2 phase is relatively more stable without gliding of the CoO6 slabs during the charge and discharge process.35,36 The stability of this material is mainly affected by the Na+/vacancy ordering, which can be relieved by partial substitution of the Co ions with Ni3+, Mn2+, and Ti4+.36,37,38 Similar to MnO2, NaxMnO2 suffers from severe volume change induced by the Jahn–Teller distortion and dissolution of Mn species during the electrochemical reactions.39,40 The disproportion of Mn3+ into Mn4+ and Mn2+ results in the dissolution of Mn species into the electrolyte. High-temperature quenching can remove the Mn vacancies and suppress their dissolution, while also creates more Mn3+ and leads to more severe Jahn–Teller distortion.17 An alternative strategy is to quench the electrode using liquid N2 which can eliminate the Mn vacancies without creating extra Mn3+ ions.41 Although Ni has been widely used as a doping metal, NaxNiO2 electrodes show inferior performance and poor stability when used as cathodes for SIBs. Decay of a high-Ni cathode is mainly associated with the insertion of water and carbonate ions between the TMO2 slabs and oxidation of the electrodes.42−44 Washing with ethanol, reducing interlayer spacing, and using proper electrolyte are effective strategies to enhance their stability.45 NaVO2 shows a similar structure to O3 a-NaFeO2, while the pure-phase NaVO2 is difficult to synthesize. NaVO2 can only be reversibly cycled in the narrow working window of 1.4–2.5 V.46 When a higher voltage was applied, the composition underwent continuous variation with the emergence of many potential plateaus. NaCrO2 has a theoretical capacity of ∼250 mA h g–1, but it faces a similar issue of poor irreversibility at high voltages, just like NaVO2.47 It has been reported that partial substitution of Cr by Ru and Ca ions can be effective in obtaining a more stable NaCrO2 electrode.48,49 The Ru substitution can possibly improve the working plateau (presenting an extra high voltage plateau at 3.8 V) and shows an excellent cycling performance (80.7% capacity retention after 1100 cycles). When Ca is doped in NaCrO2, it can improve the cycling performance (76% for 500 cycles) and air stability (slight change observed after exposure for a month). For layered sodium monometallic oxides, element doping is primarily applied to restrict the influence of Na+/vacancy ordering and the Jahn–Teller effect in NaxMO2 (M = Fe, Co, Mn, Ni, etc.), as well as to improve the structural stability (air-stability). In this regard, some inactive elements such as Ti, Ru, and Ca have shown effective results in improving the aforementioned effects.
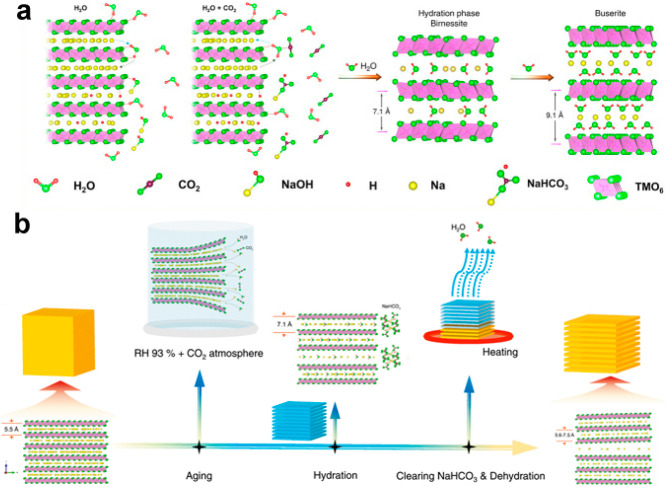
Another challenge with the layered metal oxides is their hygroscopic nature, as they tend to uptake water and CO2 from air, which results in fast capacity decay and dissolution of the electrodes (as illustrated in Figure 3a).42,50,51 Surface modification by coating ZrO2, Na2TiO7, or AlF3 can be effective in promoting ion diffusion, enhancing air stability, and preventing infiltration of the electrolyte, therefore improving the stability of the cathode materials.44,52,53 The hygroscopic nature of NaxMnO2 has been utilized to expand the interlayer spacing between Na layers, which can facilitate the Na ion transport and suppress the phase transformations during the electrochemical reactions. As illustrated in Figure 3b, the continuous aging and hydration process allows full uptake and insertion of CO2 and water molecules, leading to significantly enlarged Na+ layer spacing in the P2–Na0.67MnO2.54
Figure 3.
(a) Schematic illustration of the hydration and CO2 uptake process when exposing layered metal oxides in air. Reproduced with permission from ref (51). Copyright 2023 American Chemical Society. (b) Water-mediated synthetic process that can expand the interlayer spacing between the Na+ layers. Reproduced with permission from ref (54). Copyright 2021 Springer Nature.
2.3. Layered Sodium Multimetallic Oxides
Nowadays, most of the research attention has been devoted to the development of multimetallic oxide cathodes for high-performance SIBs. With the cooperative benefits from different metal ions, the common issues encountered by the monometallic oxide cathodes, such as the Jahn–Teller distortion, undesired Na+/vacancy ordering, and structural instability, can be effectively addressed. Substituting Mn ions with Cu2+ ions can modulate the air and water sensitivity of NaxMnO2.55 Partial substitution of Cr3+ with Ti4+ in Na2/3–xCr2/3Ti1/3O2 endowed the electrode with a higher operating voltage.56 The presence of Ru in the Na0.88Cr0.88Ru0.12O2 suppressed the irreversible migration of Cr ions and elevated the operating voltage.48 In particular, forming a O3/P2 hybrid structure has become a popular strategy to achieve cathode materials with both high capacity and high stability. Typical O3/P2 hybrids include the O3-type NaNi0.5Mn0.5O2 mixed with minor P2 phases, such as Na0.66Li0.18Mn0.71Ni0.21Co0.08O2,57 Na0.67Mn0.55Ni0.25Ti0.2–xLixO2,58 and Nax[Ni0.2Fex–0.4Mn1.2–x]O2 (x = 0.7–1.0).59 These hybrid-structured electrodes generally showed smoother charge–discharge profiles, reduced polarizations, and higher capacities during the cycling process.
Metal substitution is effective in suppressing the phase transition during the reaction process. For example, Al and Fe substitution can suppress the undesired phase transition in Na0.67Al0.1Fe0.05Mn0.9O2.41 The Jahn–Teller distortion in NaxMnO2 can be suppressed by the introduction of Li+, Mg2+, Fe3+, Ni3+, and Ti4+ ions.,60,61 The reason for Li+ and Mg2+ substitution is that they can oxidize Mn3+ into Mn4+ and thus reduce the Jahn–Teller distortion.62,63 Besides cation doping, doping with fluorine has lowered the energy barrier for Na ion diffusion in Na0.46Mn0.93Al0.07O1.79F0.21.64 A honeycomb-ordered O3–Na3Ni2Sb6O6 has demonstrated a high capacity and stability upon cycling.65 The enhanced stability originated from the presence of the honeycomb-ordered Ni2SbO6 slabs. Substituting 1/3 of Ni with Sb led to the formation of the Ni6-ring structure inside NaNiO2, which degenerated the electronic orbitals and increased the redox potential of the cathode. Partial substituting Ni2+ with inactive cations such as Zn2+, Mg2+, and Ca2+ resulted in the formation of the nanodomains composed of intergrown P3–O1 phases within the crystal structure of Na0.2Ni0.45Zn0.05Mn0.4Ti0.1O2, which not only fully strengthened the potential capacity of the metal oxide electrode but also suppressed the undesired phase transition and structural degradation upon cycling.66 Potassium ions were introduced into the P2–K0.5Mn0.7Fe0.2Ti0.1O2 and served as pillar ions to expand the lattice for Na ion insertion and deinsertion and stabilize the crystal structure.67
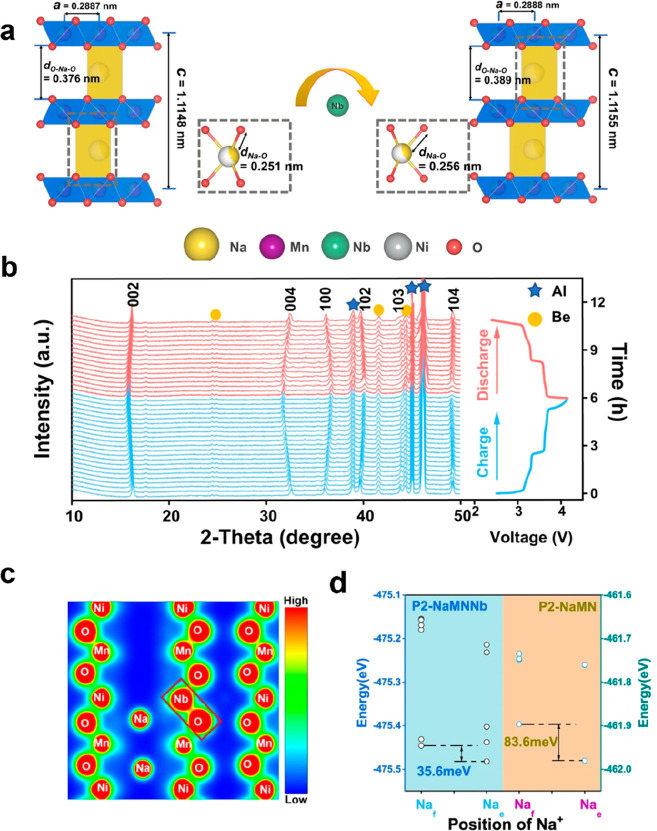
To address the irreversible structural changes or phase transitions of P2–Na2/3Mn2/3Ni1/3O2 aroused by severe interfacial transition and metal dissolution, Nb-doped P2–Na0.78Ni0.31Mn0.67Nb0.02O2 with proper surface modifications enabled fast Na+ (de)intercalation for efficient battery cycling even at low temperatures such as −40 °C, showing a high specific capacities of 83.6 and 62.9 mA h g –1 at 920 and 1.84 A g–1, respectively. Besides, superior long-term cyclability at low temperatures is demonstrated by the high capacity retention of 76% at 368 mA g–1 over 1800 cycles.68 As shown in the refined crystal structure in Figure 4a, Nb doping can expand the spacing between the TM layers from 0.376 to 0.389 nm and extend the Na–O bond from 0.251 to 0.256 nm, endowing Na+ with enhanced (de)intercalation capabilities. The in situ X-ray diffraction (XRD) spectra shown in Figure 4b illustrate that all the characteristic diffraction peaks revert to their original initial positions without the appearance of any new phase after a charge/discharge cycle. The charge density distribution of the Nb-doped Na2/3Mn2/3Ni1/3O2 reflects that the interaction between TM and O is more intense than that between Na and O (Figure 4c), and the energy calculation implies that the Na hopping is easier when Nb is doped in (Figure 4d).
Figure 4.
(a) Variation of the crystal structures in Na2/3Mn2/3Ni1/3O2 with Nb doping. (b) In situ XRD patterns of the electrodes during the charge and discharge process. (c) Charge density distribution in the Nb-doped Na2/3Mn2/3Ni1/3O2.. (d) Calculated energy difference between different Na sites with and without Nb doping. Reproduced with permission from ref (68). Copyright 2022 from Springer Nature.
2.4. Novel Metal Oxide Cathodes
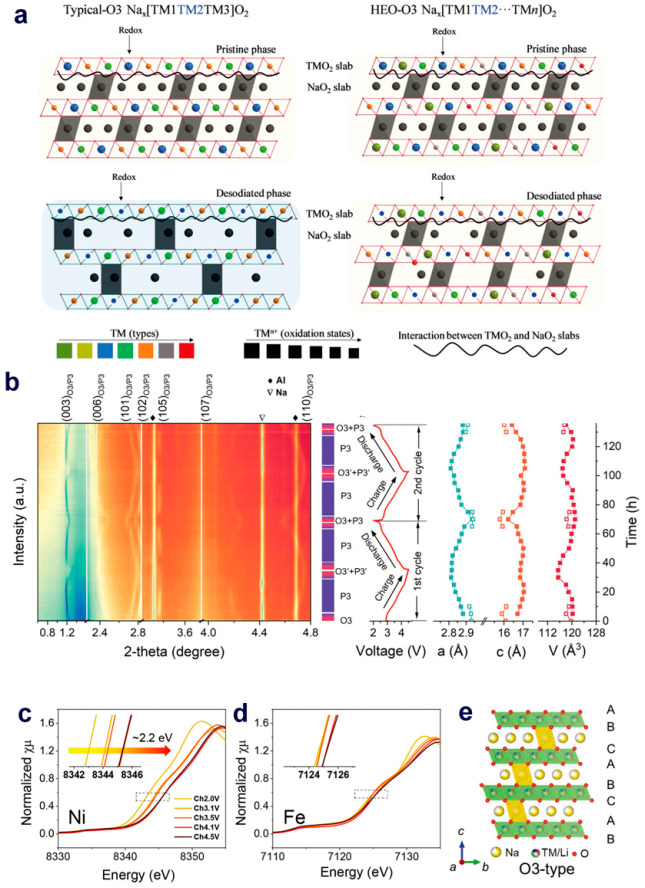
Apart from the conventional layered metal oxides cathodes, metal oxides cathodes that adopt novel compositions and crystal structures or employ a novel Na storage mechanism has also been explored. Middle-entropy oxides (MEOs) and high-entropy oxides (HEOs) are novel categories of multimetallic single-phase solid solution oxides with multiple metals sharing the crystallographic sites and stabilizing the host structure through the “entropy-stabilization effect”.69 In addition, the oxygen vacancies generated among the metal ions can effectively promote the Na ion diffusion. For example, the multicomponent in O3-type NaNi0.12Cu0.12Mg0.12Fe0.15Co0.15Mn0.1Ti0.1Sn0.1Sb0.04O2 results in different local interactions between elements in TMO2 slabs and Na in NaO2 slabs and achieves entropy stabilization on the host (illustrated in Figure 5a),70 which has suppressed the phase transition and benefited the long-term cycling of the high-entropy metal oxides electrodes.
Figure 5.
(a) Illustration of the mechanisms of traditional metal oxides and multicomponent HEO in stabilizing the O3-type structure. Reproduced with permission from ref (70). Copyright 2019 Wiley-VCH. (b) In situ high-energy XRD patterns during the first charge–discharge cycles of Na2/3Li1/6Fe1/6Co1/6Ni1/6Mn1/3O2 cathode. XANES spectra of the (c) Ni K edge and (d) Fe K edge. (e) O3-structure with a superlattice. Reproduced with permission from ref (71). Copyright 2022 Wiley-VCH.
A high-entropy Na2/3Li1/6Fe1/6Co1/6Ni1/6Mn1/3O2 cathode with a superlattice structure with Li/transition metal ordering presented excellent electrochemical performance.71 The as-prepared cathode shows high reverse capacities of 172.3 mA h g–1 in the first cycle at 0.1 C and 78.2 mA h g–1 at 10 C, demonstrating its superior rate capacity. Excellent cycling stability with the retention of 63.7% after 300 cycles at a current density of 5 C was also validated. In situ high-energy XRD confirmed the O3-type structure of the original cathode, which underwent a fast O3–P3 phase transition at the initial stage of charging (Figure 5b). X-ray absorption spectroscopy (XAS) analysis (Figure 5c, d) reveals that the Ni2+/Ni3+/Ni4+ and Fe3+/Fe4+ redox couples jointly contributed to the high reversible capacity, while Co doping enhanced the electronic conductivity. Moreover, the superlattice structure of the electrode maintained stable even after long cycles, as illustrated in Figure 5e.
Another emerging type of cathode material is metal oxides involving anionic redox reactions (ARRs) for ion storage. Some of the metal oxides are anionic redox-active intrinsically. For example, NaVO3 and Na3RuO4 are intrinsic ARR materials with both cathodic and anionic redox couples that contribute to the high capacity.72,73 With proper engineering over the structure and composition, ARR electrodes can be created through metal ion doping. The Zn-doped P2–Na2/3Mn1–yZnyO2 electrode showed high oxygen redox activity associated with nonbonding O(2p) orbitals.74 Doping metal ions causes ionic bonding, such that the electrons fully localized on the oxygen anions and the TM deficiency were the key to activate the oxygen anion redox activity. Metal ions that can form ionic bonds with oxygen, e.g., Li–O, Na–O, and Mg–O bonds, can be doped to create O(2p) nonbonding orbitals so that the electrons are fully localized on the oxygen anions. On the other hand, the P2 structure in ARR Na0.72[Li0.24Mn0.76]O2 could be stabilized even when 0.93 Na was extracted.75 The change in the oxygen radii and charges carried by the oxygen ions resulted in a decrease in oxygen repulsion around the empty Na layer and hence stabilized the structure. Besides, with the double redox reaction from both Ni2+/Ni4+ and O2–/On–, the higher redox potential of Na[Mn0.5Ni0.5]O2 compared to that of NaMnO2 with a single redox reaction was expected.76 However, it was also noticed that the ARR electrodes suffer from the structural degradation in complex phase transitions and loss of oxygen during the cycling process.
2.5. Scalable Preparation of Transition Metal Oxides
The reports on the scalable preparation of transition metal oxides mainly center on the coprecipitation method. It has the ability to achieve layered oxide cathode materials with a smooth surface, uniform particle size distribution, and high compaction density by controlling reaction conditions, making this method more suitable for industrial production. Sun and coworkers proposed a nickel-rich Na(Ni0.65Co0.08Mn0.27)O2 material with a core–shell structure, which was prepared through coprecipitation followed by milling at a rotational speed of 1000 r min–1 at 50 °C.52 Its first discharge specific capacity was 168 mA h g–1 measured at 0.5 C within the voltage range of 1.5–4.0 V, and the capacity retention rate after 50 cycles was found to be 77%. Ding et al. synthesized a novel Ni-rich O3-type Na[Ni0.60Fe0.25Mn0.15]O2 cathode for SIBs via the industrially feasible hydroxide coprecipitation method followed by high-temperature calcination.77 By reducing the charge voltage from 4.2 to 4.0 V (i.e., eliminating the high-voltage O3″ phase), the electrode exhibited an excellent overall performance, including the high reversible capacity of 152 mA h g–1 and a superior capacity retention of ∼84% after 200 cycles at 0.5 C.
3. Polyanionic Compounds
Among varous cathode materials, the polyanionic-type cathodes also attract much attention due to their high working potential and great structural stability. The general formula of polyanionic-type cathode materials in SIBs is NaMx(XOy)z·nH2O, where M represents a transition mental element, such as V, Fe, Mn, Cr, Ni, Ti, etc., and X is nonmetal element like P, S, Si, As, Mo, or W.9,10 According to the different type of polyanion, polyanionic cathode can be divided into the following categories: phosphate, sulfate, silicate, borate, and mixed-polyanion materials. The high induction effect brought by the polyanionic XO4 can effectively increase the working voltage of the cathode, and the polyhedral connection of XO4 and MO6 makes the structure stable, which can withstand repeatedly Na+ (de)insertion, prolonging the working life of the batteries.78 Nevertheless, polyanionic materials also face certain problems, including intrinsically inferior electronic conductivity, causing lower specific capacity and poor rate performance. In order to solve these problems, it is essential to have a comprehensive understanding of polyanionic materials, ranging from the crystal structures, their basic physicochemical properties, the Na storage mechanism, and current advances of polyanionic cathodes for SIBs.
3.1. Characteristics of Polyanionic Compounds
3.1.1. Phosphates
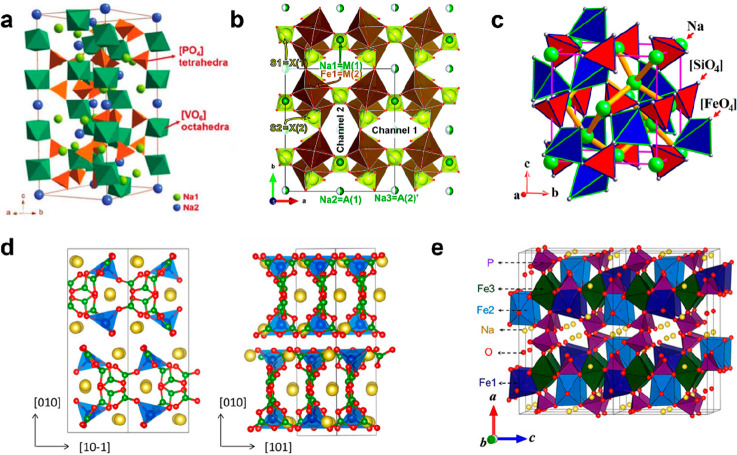
The phosphate-based materials in SIBs can be divided to three categories: orthophosphate NaMPO4 (M = Fe, Mn, Ni), NASICON-type NaxMy(PO4)3 (M = V, Fe, Mn, Ti), and pyrophosphate Na2MP2O7 (M = V, Fe, Co, Mn).79 As a representative of NaMPO4, the crystal structures of NaFePO4 are mainly olivine type (o-NaFePO4) and maricite type (m-NaFePO4). o-NaFePO4 consists of FeO6 octahedra and PO4 tetrahedra forming a spatial skeleton, with Na+ occupying the cosided octahedra and forming a long chain along the b-axis direction (its theoretical capacity is 156 mA h g–1 based on 2e– transportation); in contrast, the positions of Na+ and Fe2+ in m-NaFePO4 are reversed and the position of PO43– remains unchanged, blocking the Na+ diffusion channel and resulting in poor or even inactive electrochemical performance. With the structural stability of m-NaFePO4, methods for stimulating its electrochemical activity are being continuously studied. As shown in Figure 6a, the classical NASICON-type Na3V2(PO4)3 belonging to NaxMy(PO4)3 (M = V, Fe, Mn) has two MO6 octahedra and three PO4 tetrahedra sharing oxygen atoms for linkage, with the Na+ occupying two unequal Wyckoff sites, one Na+ at the 6b site (M1) and the other at the 18e site (M2). The pyrophosphate-type materials are represented as Na2MP2O7 (M = V, Fe, Co), with P2O75– having a higher inductive effect than PO43– that can greatly increase working voltage. In the crystal structure of Na2FeP2O7, the Fe2O11 copolymer connected by two FeO6 octahedra coangularly and P2O7 connected by two PO4 tetrahedra coangularly are bridged together in a coedge or coangle to form a 3D twisted zig-zag-type Na+ transport channel, and five Na sites with different occupancy degrees are generated. The redox reaction of Fe2+/Fe3+ occurs at a suitable voltage window for the reversible extraction/insertion corresponding to one Na+ with a theoretical specific capacity of 97 mA h g–1, and two clear plateaus of 2.5 and 3.0 V can be observed.
Figure 6.
(a) Crystal structure of Na3V2(PO4)3. Reproduced with permission from ref (87). Copyright 2019 Royal Society of Chemistry. (b) Crystal structure of Na2Fe2(SO4)3. Reproduced with permission from ref (88). Copyright 2019 Royal Society of Chemistry. (c) Crystal structure of Na2FeSiO4. Reproduced with permission from ref (82). Copyright 2016 American Chemical Society. (d) Crystal structure of Na3FeB5O10. Reproduced with permission from ref (83). Copyright 2016 American Chemical Society. (e) Crystal structure of Na4Fe3(PO4)2(P2O7). Reproduced with permission from ref (89). Copyright 2012 American Chemical Society.
3.1.2. Sulfates
The thermodynamic stability of the SO42– group in sulfate polyanionic compounds Na2M(SO4)2·nH2O (M = Fe, Mn, Co, Ni, etc.) is inferior, and its decomposition temperature is lower than 400 °C. When exposed to temperatures above the decomposition temperature, SO2 gas is easily released, resulting in low chemistry purity and toxic substances, so the low-temperature solid-phase method is often used for sulfate synthesis. According to the Pauling electromagnetic principle, the bonding of S–O is stronger than that of P–O, leading to the strong induction of sulfate. Thus, the energy level cleavage resulting from the hybridization of the d orbitals of transition metal ions with O 2p orbitals is intensified, making the redox potential of the material high. Since the charge-to-mass ratio of sulfate is significantly lower than that of phosphate, the theoretical specific capacity of sulfate materials is thus lower. Although in terms of practical applications, sulfate materials can hardly be comparable to commercialized LiCoO2, LiFePO4, NCM-811, and so on, they can unleash their own unique advantages in the field of low-cost energy storage. In 2014, Yamada et al. successfully prepared Na2Fe2(SO4)3, which belongs to monoclinic crystal system with the P21/c space group.80 It possesses a three-dimensional skeleton structure and Na+ diffusion channels along the c-axis direction for Na+ migration, delivering a theoretical capacity of 120 mA h g–1 with a high working platform of 3.8 V. As illustrated in Figure 6b, the FeO6 octahedra form an isolated Fe2O10 dimer by coedging and bridging with the SO4 tetrahedra through the vertices, thus resulting in a three-dimensional (3D) skeletal structure with large ion channels along the c-axis. More specifically, Na+ occupies three different Na sites in the 3D skeleton structure, where the Na2 and Na3 sites have 1D Na+ diffusion channels along the c-axis and the Na+ located at these two sites can easily diffuse along their respective channels.
3.1.3. Silicates
Silicates, represented as Na2MSiO4 (M = Fe, Mn, Co, Ni), are promising cathode candidates for large-scale energy storage because they possess the cost-effectiveness and resourcefulness of Na, Fe, and Si raw materials on Earth. Take Na2FeSiO4 as an example to dissect the structure of a silicate-based polyanionic compound. In monoclinic Na2FeSiO4 materials (space group Pn), the FeO4 tetrahedron and SiO4 tetrahedron are joined alternately to form a solid framework, while the sodium is hexacoordinated and forms a sublattice alone; Na ions are in a relatively disordered state in this structure (Figure 6c). Due to the large ion gap in the structure framework, Na+ has greater freedom of motion, leading to a high Na+ diffusion coefficient. Thus, high Na+ diffusion in Na2FeSiO4 can still be achieved even without the fast ion transport channels, which also applies to Co-based and Mn-based silicate cathodes.81 Na2FeSiO4 with a high theoretical capacity of ∼278 mA h g–1 corresponds to reversible insertion/extraction of two Na+ per unit. Although theoretical studies showed the possibility of 2e– reactions in silicate electrode materials, current studies show the occurrence of only the 1e– reaction owing to electrolyte decomposition at potentials required to insert/extract the second Na+.82
3.1.4. Borates
Reports on borate-based materials are much rarer. Borate polyanionic electrode materials have also received some attention from researchers because of their small molar mass, abundant resources, and environmental friendliness. Boron atoms can be sp2- and sp3-hybridized to form various groups, such as [BO3]3–, [BO4]5–, and [B2O4]4–, that can be condensed or polycondensed to form islands, chains, layers and skeletal groups, leading to a variety of boronate crystal structures. Compared with other polyanionic compounds, borates have higher theoretical capacities but a much lower operating voltages due to the weak induction. The pentaborate polyanionic cathode material of Na3MB5O10 (M stands for V, Fe, Mn, Co, etc.) can be easily fabricated by the solid-phase method. As illustrated in Figure 6d, Na3FeB5O10 belonging to the orthogonal crystal structure (space group Pbca) consists of four vertices of FeO4 tetrahedra connected to the [B5O10]5– unit, with the FeO4–B5O10 network aggregated into layers in the ab-plane and stacked along the c-axis; Na+ occuplies the interlamination positions.83 Additionally, its theoretical capacity is 78 mA h g–1 based on the reversible intercalation of one Na+ per formula unit.
3.1.5. Mixed-Polyanion Materials
A series of hybrid polyanionic cathode materials with novel structures, such as a phosphate–pyrophosphate hybrid, a phosphate–carbonate hybrid, and fluorinated phosphate, can be obtained by taking advantage of their mutual compatibility. The phosphate–pyrophosphate hybrid polyanionic cathode material can be expressed as Na4M3(PO4)2(P2O7) (M = Fe, Ni, Mn, Co, etc.). The crystal structure of Na4M3(PO4)2(P2O7) is rhombohedral with a space group of Pn21a, according to Figure 6e, and the MO6 octahedra and PO4 tetrahedra form a double chain by covertex connections, which is further bridged to a laminar structure by P–O–P bonding of pyrophosphate.84 The Na4Fe3(PO4)2(P2O7) first reported by Kang’s group exhibited excellent Na storage performance as a cathode for SIBs, with a high reversible specific capacity of 129 mA h g–1 with operating voltage of ∼3.2 V, achieving energy density of 412.8 W h kg–1.89
In addition, a series of crucial fluorinated polyanionic materials with high voltages can be prepared by replacing part of the polyanion with high electronegativity fluorine, thereby developing NaVPO4F, Na3V2(PO4)2F3 (NVPF), Na3V2(PO4)2O2F (NVPOF), NaFeSO4F, and other fluorinated materials. The tetragonal NVPF with a space group of P42/mnm was composed of [V2O8F3] bioctahedra and [PO4] tetrahedra that interconnected by angle sharing, and the occupancy ratio between Na(1) sites and Na(2) sites is 2:1. Owing to the high electronegativity of fluorine, the fluorinated material has a high working plateau of ∼3.9 V with a theoretical capacity of ∼130 mA h g–1 (corresponding to a theoretical energy density of ∼500 W h kg–1), thus being quite suitable for high-energy SIBs.85,86
3.2. Promising Polyanionic Cathodes
3.2.1. Na3V2(PO4)3
As one of the most widely studied polyanionic materials, Na3V2(PO4)3 (NVP) possesses high ionic conductivity, excellent cycling stability and great thermal stability. Employed as the cathode for SIBs, Na3V2(PO4)3 has a theoretical capacity of 117.6 mA h g–1 and a working plateau of 3.4 V, which originated from the V3+/V4+ redox couple corresponding to two Na+ ionsinvolved in (de)sodiation.87,90 Unfortunately, the NVP cathode material characterized by sluggish diffusion kinetics and low electronic conductivity (∼10–12 cm2 s–1) has an unsatisfactory specific capacity and rate capability, and effective modifications are desired. At this stage, modification methods, including surface coating, morphological construction, and lattice modulation, have been developed. Recently, Xiong and coworkers proposed a polymer-stabilized droplet template strategy to synthesize a novel porous single-crystal-structured Na3V2(PO4)3 compound (Figure 7a), and selected area electron diffraction (SAED) confirmed its single-crystal structure. The phase diagram in Figure 7b summarizes the pore structures at the mesoscale and macroscopic scales under various reaction conditions. When less volatile solvents combine with high-molecular-weight polyvinylpyrrolidone (PVP), hierarchically meso/macroporous structured NVP could be synthesized. Compared with the macroporous and mesoporous structures, the hierarchically porous structure with the 3D interlinked channel provides faster Na+ transport paths and a larger contact area, effectively accelerating the Na+ transportation. Another advantage is that Na+ can migrate along the smooth solid–liquid interface in the HP-NVP. As a consequence, an outstanding rate capability of 61 mA h g–1 at ultrahigh rate of 100 C and a prolonged lifespan of 10000 cycles at 20 C without capacity fading can be achieved (Figure 7c). Specially, HP-NVP was assembled to form a symmetric cell, which exhibits a specific capacity of 47 mA h g–1 at 50 C and stable cycling at 10 C for 700 cycles.91 Xu et al. proposed a spray drying method for synthesizing NVP/rGO HSs. Owing to the unique porous hollow architecture effectively shortening the Na+/e– diffusion path, the synthesized NVP/rGO HSs compound manifested a high reversible capacity of 116 mA h g–1 at 1 C (98% of theoretical capacity), an outstanding high-rate capability of 98.5 mA h g–1 at 20 C, as well as a stable cycling performance of 73.1 mA h g–1 over 1000 cycles at 10 C. To explore the practical application of NVP/rGO Hs cathode, the full cells assembled with NVP-HSs cathode and S-CMTs anode exhibit a capacity retention of 84.2 mA h g–1 after 100 cycles at 1 C. Assembling a high-performance sodium-ion full battery (SIFB) requires overall matching between the cathode, anode and electrolyte. Wei et al. proposed an excellent SIFB integrated with an optimized NVP@C@carbon nanotube (NVP@C@CNTs) cathode, a mesocarbon microbead (MCMB) anode, and a Na+–diglyme electrolyte. The as-synthesized NVP@C@CNT cathode displays a high electronic conductivity, reducing the overpotential and charge transfer resistance and leading to a superior rate capability at a high rate of 80 A g–1. Besides, it demonstrated a discharge capacity of 70 mA h g–1 with extraordinary stability over ultralong 20 000 cycles at a high current density of 20 A g–1. Furthermore, the NVP@C@CNTs||MCMB full cell obtained high energy density of 88 W h kg–1 at ∼10 kW kg–1 and 58 W h kg–1 at ∼23 kW kg–1. Besides, superior cyclability with 72.7% capacity retention for 5000 cycles at 5 A g–1 could be achieved (Figure 7d). Both the high conductivity of NVP@C@CNT cathode and the expanded ion diffusion paths at the anode resulted from the initial pseudocapacitive cointercalation, which contributed to this high rate capability and excellent cyclability.92
Figure 7.
(a) TEM of the hierarchically porous Na3V2(PO4)3 materials and (b) ternary diagram of pore structure of porous NVP. (c) Rate capacities of the Na3V2(PO4)3 materials with different pore morphologies. Reproduced with permission from ref (91). Copyright 2021 Wiley-VCH. (d) Cycling performance of NVP@C@CNTs||NaPF6 in Diglyme||MCMB at 5 A g–1. Reproduced with permission from ref (92). Copyright 2022 Wiley-VCH.
In addition to morphological construction and surface coating, lattice regulation is also beneficial for improving NVP performance. Most studies primarily concentrate on introducing inactive elements into the V site. Liang et al. fabricated Mo-doped 1D NVP nanowires (MNVP@C NWs) as a multifunctional cathode for Na storage and fully explored their practicality.93 The electron energy loss spectroscopy (EELS) confirmed the uniform distribution of external Mo within the nanowires rather than surface doping. This cathode obtained a discharge capacity of 116.8 mAh g–1 at 0.1 C and displayed a capacity retention of 85.7% after 8000 cycles at 5 C. Additionally, the constructed pocket-flexible SIBs demonstrated a large energy density of 262.4 W h kg–1 and an ultrahigh rate capability of 77 mA h g–1 at 150 C. This is because when higher valence Mo6+ was introduced into the NVP, Na+ vacancies would be generated due to valence equilibrium, which enhanced the electronic conductivity and ion diffusion kinetics of the electrode due to the smaller Na+ migration barrier. Besides, Shi et al. developed a cathode of bismuth-doped NVP enwrapped with carbon nanotubes.94 The optimized Na3V1.97Bi0.03(PO4)3/C@CNTs sample displayed a reversible capacity of 97.8 mA h g–1 and maintained a capacity of 80.6 mA h g–1 over a prolonged 9000 cycles at 12 C. Even when cycled at an ultrahigh rate of 80 C, the cathode also exhibited a high capacity of 84.3 mA h g–1 and achieved 87% of its capacity after 6000 cycles. These excellent rate capability and outstanding cyclability can be attributed to the doped Bi3+ that acted as the pillar of NVP crystal structure, buffering crystal deformation and enhancing the structural stability.
3.2.2. Na3VM(PO4)3
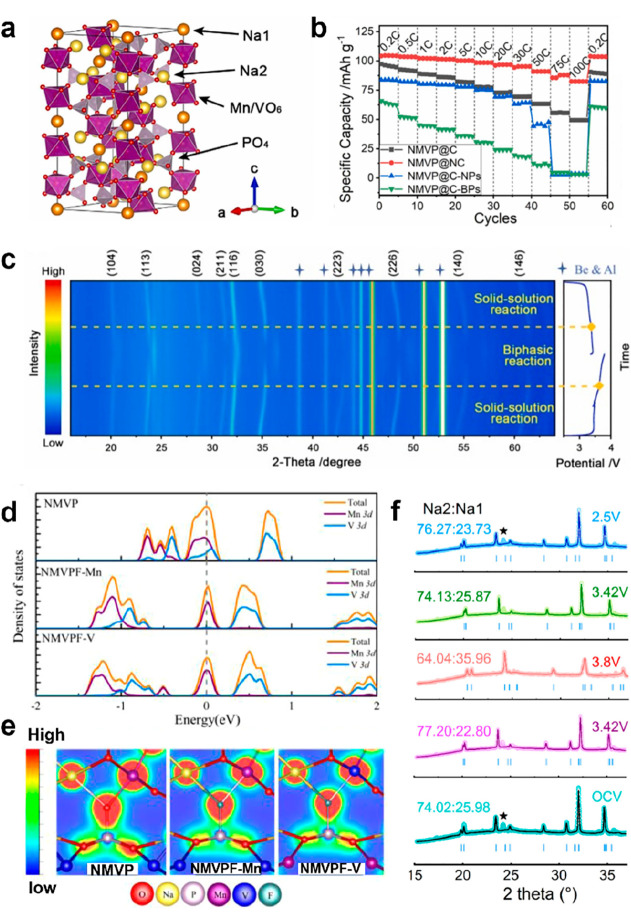
Vanadium-based materials profiting from multivalence states and rich resource of vanadium are some of the preferred electrodes for batteries, but vanadium has high toxicity. Thus, cost-efficient and environment-friendly elements (e.g., Fe, Mn) are doped into the V-site in Na3V2(PO4)3, producing Na4VFe(PO4)3 and Na4VMn(PO4)3. As illustrated in Figure 8a, Na4VMn(PO4)3 is constructed by MnO6/VO6 octahedra sharing all the corners with PO4 tetrahedra, and it possesses a theoretical capacity of 111 mA h g–1 and voltage plateaus of 3.6 (Mn2+/Mn3+) and 3.3 V (V3+/V4+) corresponding to reversible insertion/extraction of two Na+. Except for suffering from low electron migration kinetics, the John–Teller effect of Mn3+ will lead to Mn digestion and structural instability, shortening the lifespan of the Na3VM(PO4)3 electrode.95 The most facile and effective way to improve the electronic conductivity of Na3VM(PO4)3 is to coat it with conductive materials. Recently, Zhu et al. designed a unique hierarchical bayberry-like NMVP@NC material as a cathode for SIBs via facile ball-milling and subsequent calcination. Even cycled at an ultrahigh rate of 100 C, the NMVP@NC cathode can still deliver a high discharge capacity of 82.4 mA h g–1 (Figure 8b), which is far superior to other NMVP-based electrodes. When assembled with commercial soft carbon as the anode, the full cell could deliver 94 mA h g–1 at 0.1 C and 57 mA h g–1 at 10 C. Figure 8c reveals the structural evolution of the NMVP@NC cathode during the first electrochemical cycle. The NMVP@NC cathode underwent a solid-solution reaction when charged to 3.6 V and a biphasic reaction in the interval of 3.6–3.8 V. Besides, peaks during the discharge and the charge process appear symmetric, confirming the high reversibility of the electrochemical reactions. The NMVP@NC cathode is unique: (i) the ultrasmall sizes of nanoparticles render a short diffusion distance for Na+ and provide a larger electrode/electrolyte contact area, (ii) the 3D N-doped carbon network availably improves the electrical conductivity of NMVP, and (iii) the robust structure suppresses the volumetric expansion during the repeated Na+ insertion/extraction, giving rise to superior cyclability.96
Figure 8.
(a) Crystal structure of Na4MnV(PO4)3, (b) rate performance of the NMVP-based cathode, and (c) in situ XRD pattern of Na4MnV(PO4)3@NC. Reproduced with permission from ref (96). Copyright 2022 Elsevier. (d) Total and projected density of states and (e) side-view of the electron density difference of NMVP and NMVPF-Mn/V samples. (f) Rietveld refinement of partial in situ XRD patterns of NMVPF. Reproduced with permission from ref (98). Copyright 2021 Elsevier.
To mitigate the malignant effects caused by Mn3+, more modification of Na4MnV(PO4)3 associated with heteroatomic doping of Al3+, Mg2+, and other elements has been explored. The substitution by heteroatoms is aimed at reducing the concentration of Mn3+ in the NVMP cathode so that the Jahn–Teller distortion is suppressed and structural stability is enhanced with increased covalency by inducing shorter (V/Mn/Mg/Al)–O bond lengths. Moreover, the substitution of inert Al3+ into the NVMP structure would generate abundant Na vacancies, which are expected to reduce the activation energy and enhance the Na+ mobility.97 In addition to doping at the vanadium sites, doping at the polyanion sites has also been studied. The innovative Na-deficient Na3.85□0.15MnV(PO3.95F0.05)3 material was fabricated by partially doping F into the NMVP. Electron density differences shown in Figure 8d and e prove that the change of electron density caused by the substituted F near Mn or V atoms and the weak Coulomb interaction induced by the Na vacancy effectively promotes the Na+ diffusion dynamics. Executing ex situ 23Na NMR and in situ XRD (Figure 8f) characterization of NMVPF electrodes at different states of (dis)charge revealed the higher Na+ extraction rate from the Na2 site.98
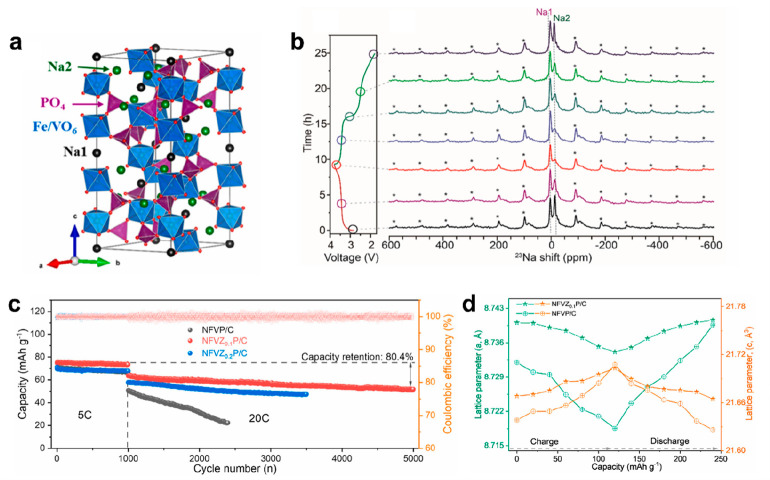
Na4FeV(PO4)3 belonging to the Na4VM(PO4)3 type is another promising cathode for SIBs with a similar framework as Na4MnV(PO4)3. It is constructed by FeO6/VO6 octahedra with PO4 tetrahedra (Figure 9a). Lu et al. proposed a novel Na4FeV(PO4)3@C cathode synthesized via a combined ball-milling, sol–gel, and calcination process. The as-prepared Na4FeV(PO4)3@C exhibited specific capacities of 100 mA h g–1 at 0.1 C and 80.6 mA h g–1 at 10 C when tested at the wide voltage window of 1.3–3.8 V. In charge–discharge curves, two plateaus located at 2.5 and 3.5 V can be ascribed to Fe2+/Fe3+ and V3+/V4+ redox couples, respectively. Besides, the cathode exhibited great cycling stability, with 96.8% capacity retention after 800 cycles at 5 C, surpassing the original Na4FeV(PO4)3 cathode that rapidly decays after only 400 cycles. The solid-state 23Na nuclear magnetic resonance revealed that the Na+ stand at Na2 sites exhibited faster insertion/extraction dynamics upon cycling (Figure 9b). XRD and time-of-flight neutron powder diffraction illustrated that the electrochemical process undergoes a reversible solid-solution reaction, confirming its stable framework structure.99 Wang et al. reported a bicarbon-decorated NFVP@rGO@CNT material as the cathode for SIBs. A high discharge capacity of 156 mA h g–1 at 0.1 C could be achieved in the operating window of 2.0–4.4 V. Besides, a rate capacity of 60 mA h g–1 at 30 C and 71% capacity retention over 600 cycles at 2 C were realized. Such great rate performance and cyclability benefit from the double carbon layer (CNTs and rGO) accelerating the electron transfer. Even when fabricated with a high mass loading of 6.2 mg cm–2, the cathode exhibited excellent rate capability and cyclability (58.7 mA h g–1 at 30 C and 72.1% capacity retention after 1000 cycles at 10 C), confirming its practical potential.100 Ma et al. reported a heteroatomic doping strategy in Na4FeV(PO4)3 materials, which generate extra Na vacancies to boost the electroconductivity. The optimized Na3.9FeV0.9Zr0.1(PO4)3/C electrode exhibited a high discharge capacity (114 mA h g–1 at 0.1 C), superior rate capability (66.7 mA h g–1 at 40 C), and remarkable cyclability of 82.4% capacity retention over 4000 cycles at 20 C (Figure 9c). As illustrated in Figure 9d, the NFVZ0.1P/C cathode showed a smaller volume change (ΔV/Vpristine) of ∼5.21% during electrochemical cycling compared with the undoped sample. Its excellent structural stability benefited from the pillar support from Zr. Furthermore, the assembled NFVZr0.1P/C||HC full cell exhibited a superior rate capacity of 56.4 mA h g–1 at 1000 mA g–1 and 97% capacity retention over 100 cycles at 200 mA g–1.101
Figure 9.
(a) Crystal structure of Na4FeV(PO4)3. (b) 23Na magic-angle-spinning (MAS) NMR of NFVP@C at different cycle states. Reproduced with permission from ref (99). Copyright 2022 Elsevier. (c) Cycling performance of NFVP/C with varying Zr-content (0–0.2). (d) Variation of the lattice parameters of NFVP/C and NFVZ0.1P/C electrodes. Reproduced with permission from ref (101). Copyright 2022 Elsevier.
More recently, a high-entropy crystal substitution strategy for promoting polyanionic materials was proposed by Li and coworker, and Na3VAl0.2Cr0.2Fe0.2In0.2Ga0.2(PO4)3 (denoted as NVMP) was developed via a facile sol–gel method and explored for SIBs at both ambient and low temperatures. Benefiting from the doping of high-entropy crystals, the activity of V4+/V5+ electron couples is activated, enabling a highly reversible capacity of 102 mA h g–1 at 0.1 C. Besides, the NMVP half-cell also showed outstanding cyclability over 5000 cycles at 20 C. Even tested at −20 °C, the NVMP cathode could still demonstrate prolonged cyclability with 94.2% capacity retention over 1000 cycles at a high rate of 5 C. For a real application, the constructed NVMP||HC full batteries could deliver 81 mA h g–1 at 0.2 C and steadily cycle for 50 cycles when paired with hard carbon.102
The element doping strategy for polyanionic compounds, particularly vanadium-based phosphates, focuses on using more cost-effective transition metal elements (e.g., Mn, Fe, etc.) to replace V, achieving a lower vanadium content while maintaining similar electrochemical performance. Mn substitution brings about higher voltage plateaus (3.6 V), but it also generates an unfavorable Jahn–Teller effect. To address this, additional atoms such as nonactive metal elements like Al, Mg, Ce, and Cr are introduced to suppress the adverse effects caused by Mn3+.103 The introduction of Fe stabilizes the lattice further, but the overall decreased voltage (∼3.0 V) limits its application in high-energy-density SIBs, making it less favorable. On the other hand, the introduction of highly electronegative fluorine at polyanion sites has proven to be an effective strategy for promoting the working voltage of polyanionic compounds. However, it is crucial to note that excessive F content hinders the transmission of Na+ in the lattice, necessitating strict control over its introduction amount. Furthermore, utilizing high-entropy crystal substitute and activating electron redox with higher valence states are promising avenues for future research.
3.3. Scalable Preparation of Polyanionic Compounds
The scalable preparation of polyanionic materials is also of great concern. Qi et al. proposed a groundbreaking synthesis route for the scalable production (150 g per batch) of multishelled Na3(VOPO4)2F microspheres using in situ generated bubbles as soft templates at room temperature.104 In this method, raw materials were extracted from vanadium slag and NVPOF microspheres were formed during the coprecipitation process with the appropriate reaction time. The large-scale prepared Na3(VOPO4)2F exhibited an outstanding rate capacity of 81 mA h g–1 and remarkable cycling stability, with 70% capacity remaining over 3000 cycles at 15 C. This room-temperature scalable production strategy paves the way for the commercialization of SIBs. Similarly, Shen and coworker developed a rapid synthesis route for Na3V2(PO4)2O2F using a solvent-free room-temperature solid-phase mechanochemical method, and they rigorously verified the feasibility of production using ten different types of vanadium raw materials.105 The optimized NVPOF@8%KB demonstrated a high initial capacity (142.2 mA h g–1 at 0.1 C), superior rate capability (112.8 mA h g–1 at 20 C), and remarkable cyclability (maintaining 98% capacity over 10 000 cycles at 20 C). To confirm the feasibility of large-scale production of sodium vanadium fluorophosphate using mechanochemical methods, a kilogram-scale preparation was executed. Subsequently, the large-scale synthesized NVPOF materials were matched with a hard carbon anode to fabricate a 26650 cylindrical battery, which delivered a high capacity of 1500 mA h g–1 and an energy density of ∼90 Wh kg–1. The successful kilogram-scale production and the excellent electrochemical performance of the large-scale synthesized product further validate the feasibility of the mechanochemical method for the commercial SIB cathode materials.
4. Prussian Blue Cathodes
Hexacyanoferrates (HCFs)/Prussian blue (PB) and its analogues (PBAs) are promising cathode candidates for SIBs owing to their low cost, easy preparation, and open framework structure for Na+ accommodation.6,106,107 The chemical formulas of PBAs could be denoted as NaxM1[M2(CN)6]y□1–y·zH2O (0 ≤ x ≤ 2, 0 ≤ y ≤ 1), where M1 = Fe, Mn, Ni, Cu, Co, Zn, etc.; M2 = Fe, Mn, Co; and □ represents a transition metal coordinated with N and C atoms and [M2(CN)6] vacancies inside the crystal structure, respectively.108 The crystal structures of PBAs can be cubic, monoclinic, rhombohedral, and trigonal, which vary according to the number of Na+ ions and the content of water. Generally, the alkaline-deficient PBAs present a cubic structure, while alkaline-rich PBAs show a monoclinic phase.109 After dehydration treatment, the phase structure will be transformed to rhombohedral or trigonal for the reduced amount of water.110 The specific capacities of PBAs depend on chemical compositions when applied as cathode materials for SIBs (e.g., 85 mA h g–1 for a single-electron redox-active site (SE-PBAs M1 = Zn, Ni) and 170 mA h g–1 for double-electron redox-active sites (DE-PBAs, M1 = Mn, Fe, Co)). Taking into account the high average discharging voltage (above 3.0 V vs Na+/Na), the theoretical energy density of DE-PBAs could reach 510 Wh kg–1, which is competitive with commercial LiFePO4 employed in LIBs.111
Typically, PBAs are prepared by simple coprecipitation of sodium hexacyanoferrate and transition metal salts in water. However, the obtained PBAs present a random distribution of H2O and [M2(CN)6] vacancies because of the rapid reaction between the hexacyanoferrate ligand and transition metal ions.12 Additionally, the H2O in PBAs can be divided into three species: (i) H2O adsorbed on the surface, (ii) interstitial or zeolite H2O located at the alkali metal ion sites, and (iii) coordinated H2O chemically bonded with transition metals for the absence of [M2(CN)6]. The H2O/vacancies in PBAs would cause lattice distortion and even structure collapse during (de)sodiation processes, leading to rapid capacity degradation.112 Meanwhile, the irreversible phase transition during charging and discharging process also contributes to the short cycle lifespan. Further, low electronic conductivity for poor rate performance of PBAs is another obstacle should be overcome for practical application.113 As a consequence, strategies aiming at preparing PBAs with low levels of water and vacancies, mitigated phase transitions during cycling, and enhanced electronic conductivity are imperative and challenging.
4.1. Modification of Prussian Blue Materials
Although many metals are capable of occupying the M1 and M2 sites, the Fe-based (M1 = M2 = Fe) and Mn-based (M1 = Mn, M2 = Fe) PBAs with two redox-active centers are the most investigated for their high theoretical specific capacity and low cost. Besides of the high content of H2O/[Fe(CN)6] vacancies and low electronic conductivity, Fe-based PBAs also suffer from a low practical specific capacity for the irreversible electrochemical reaction of low-spin Fe coordinated with C,114 and the Mn-based PBAs suffer from the Jahn–Teller effect of Mn3+ and the dissolution of Mn2+.115,116 Crystal structure control, nonaqueous preparation/dehydration treatment, compositing with conductive carbon, surface coating, and cationic doping are effective approaches to prepare PBAs with low H2O/vacancy contents, high crystallinity, high electronic conductivity, and improved the electrochemical performance according to the previous studies, which are summarized in this section. In addition, the scalable preparation of PBAs and its practical application in full-cells were also introduced.
4.1.1. Crystal Structure Control
[Fe(CN)6] vacancies inside the PBA crystal were generated during the fast coprecipitation between metal salts and sodium hexacyanoferrate. The vacancies occupied by coordinated water and interstitial water reduce the amount of extractable sodium, hinder the migration of Na+, and decrease the practical specific capacity.117,118 Additionally, the crystal structure of defect-rich PBAs tends to collapse during (de)insertion of Na+ due to the absence of bulky [Fe(CN)6], which deteriorates the electrochemical performance.15 It is documented that the critical point for decreasing the [Fe(CN)6] vacancies and enhancing the crystallization of PBAs is slowing down the reaction rate of coprecipitation. Pioneer researchers have put forward several effective strategies to reduce the rate of coprecipitation: (i) Implementing chelating agent/surfactant-assisted precipitation. A chelating agent, such as sodium citrates (Na3Cit),119 ethylenediaminetetraacetic acid disodium (Na2EDTA),120 diethylenetriaminepentaacetic acid disodium (Na2DTPA),121 and pyrophosphoric salts (Na4P2O7)122,123 having high complexation with transition metal salts could slow down the release rate of metal ions and the crystallization ratio. (ii) Lowering the precipitation temperature for Fe-based PBAs. Our group124 found that Fe-based PBAs prepared below 0 °C or iced conditions exhibited fewer [Fe(CN)6] vacancies than those synthesized at room or high temperature for the decreased reaction rate, which was consistent with the result of Ma’s group.125 (iii) Preparing a high salt concentration. Guo’s group reported Mn-PBAs fabricated using a saturated Na4Fe(CN)6 solution displayed only 4% vacancies (24% at traditional condition), and vacancy-free Mn-PBAs could be obtained once the sample was aged at 80 °C for 20 h.126
4.1.2. Nonaqueous Preparation/Dehydration Treatment
Although several reports demonstrated that the interstitial water located at body-centered sites can stably exist in PBAs for structural stabilization,127 most researchers believed that the deteriorated electrochemical properties of PBAs are due to the side reactions between water and the nonaqueous electrolyte.128 It was found in our group that the water content of the PBA cathode at the discharging state could reach the ultrahigh value about 20 ppm, which was fivefold higher than those in Na3V2(PO4)3, and resulted in the swell of pouch cell.112 Therefore, it is imperative to solve the water problem of PBAs to promote their commercial application. Normally, preparing PBAs in a nonaqueous solution and performing a dehydration treatment after primary drying are effective methods to reduce the water content.
4.1.2.1. Nonaqueous Preparation
As we discussed above, PBAs were prepared in an aqueous solution, making it difficult to completely eliminate the crystal water (10–15 wt %). Substitution of partial/whole water by organic solvents has been proven effective to suppress crystal water growth in PBAs. You’s group demonstrated that high-crystallinity PBAs with a low water content (7.90%) could be synthesized by the solvothermal method using an ethylene glycol/water mixed solvent to minimize water content in the reaction environment and decrease the crystal nucleation rate.129 However, water in the reaction was necessary to dissolve Na4Fe(CN)6 precursors. To improve the solubility of the precursor and accelerate the reactions in organic solutions, He’s group developed a microwave-assisted solvothermal approach with anhydrous ethanol as the solvent. The microwave supplies external energy, and PBAs could be synthesized at a slightly elevated temperature within hours.130 As a result, the content of interstitial water in obtained samples is only 4.34–5.13 wt %, and a high discharging specific capacity of 150 mA h g–1 could be reached, suggesting organic solvents are alternative mediums for the preparation of PBAs. Recently, our group proposed a “water-in-salt” nanoreactor strategy to prepare highly crystallized Mn-based PBAs with a decreased water content (10.1% vs 18.5% prepared by coprecipitation), higher volume yield, and enhanced electrochemical performance over a wide temperature range from −10 to 50 °C, indicating it was a promising route to achieve the large-scale production of PBAs.131
4.1.2.2. Dehydration Treatment
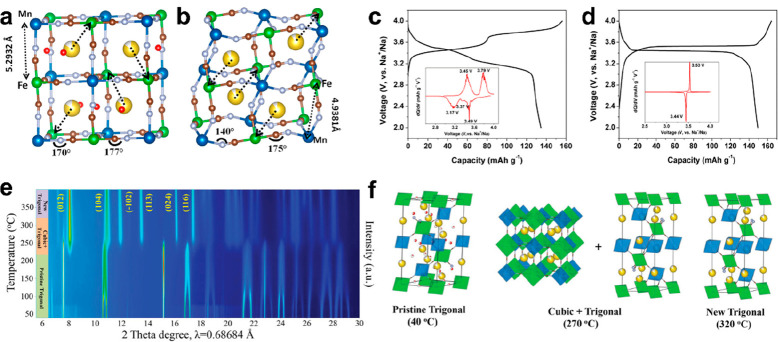
Goodenough’s group first proposed the post-dehydration of Mn-based PBAs at low temperature (100 °C) heating under high-vacuum (15 mTorr) for the removal of interstitial water.110 After the dehydration treatment, the water content in Na2−δMnHCF decreased from 12% to 2%, indicating the z value was only 0.3 in dehydrated Na2−δMnHCF. Meanwhile, the monoclinic Na2−δMnHCF (M-Na2−δMnHCF) converted to the rhombohedral phase (R-Na2−δMnHCF) after the dehydration treatment due to lattice shrinking and distortion, as shown in Figure 10a and b. The electrochemical behavior was also changed after the removal of interstitial water. The M-Na2−δMnHCF electrode show two pairs redox peaks located at 3.17/3.45 and 3.49/3.79 V, and a discharging capacity of 137 mA h g–1 could be delivered (Figure 10c), while R-Na2−δMnHCF displayed an apparently single flat plateau at 3.44/3.53 V and a higher initial discharging capacity of 150 mA h g–1 (Figure 10d). Additionally, the dehydrated Na2−δMnHCF exhibited promising cycling performance (75% capacity retention after 500 cycles) and rate capability (81% capacity retention at 20 C). A similar conclusion has been drawn by Younesi’s group, that is, the phase structure of Fe-PBAs converted from monoclinic to rhombohedral after dehydration.132 Furthermore, the relationship between the water content and phase structure of sodium-rich Na2–xFeFe(CN)6 was systematically studied in our group.112 As depicted in Figure 10e and f, the pristine trigonal phase was maintained when adsorbed water was removed (<150 °C), while cubic and new high-temperature trigonal phases could be found from 220 to 300 °C; the trigonal phase dominated at 270 °C, at which the interstitial and coordinated water faded away. The cubic phase disappeared at temperatures higher than 300 °C, and the new trigonal phase was stable up to 400 °C. After dehydration at 270 °C under Ar, the low-spin Fe2+/Fe3+ redox reaction at ≈3.4 V was activated and the specific capacities were improved. Moreover, the dehydrated Na2–xFeFe(CN)6 exhibited excellent cycling performance (98.9% capacity retention after 2000 cycles at 100 mA g–1), evidencing that post-dehydration was an effective strategy to reduce the water content and improve the electrochemical performance of PBAs.
Figure 10.
Local structures of (a) M-Na2−δMnHCF and (b) R-Na2−δMnHCF and galvanostatic initial charge and discharge profiles of (c) M-Na2−δMnHCF and (d) R-Na2−δMnHCF. Reproduced with permission from ref (110). Copyright 2015 ACS publications. (e) 2D contour map of in situ high-temperature synchrotron X-ray diffraction patterns of as-prepared Na2–xFeFe(CN)6 samples under Ar and (f) corresponding crystal structures at 40, 270, and 320 °C. Reproduced with permission from ref (112). Copyright 2022 Wiley-VCH.
4.1.3. Compositing with Conductive Carbon
Although the 3D open framework inside the PBAs facilitated Na+ diffusion, the rate capability of PBAs is below expectation for their limited electronic conductivity. Compositing PBAs with conductive carbon has been considered as an effective strategy to improve their rate performance. Hence, considerable research has been devoted to building mesoscopic or nanoscopic interactions between conductive carbon (carbon nanotubes (CNT),113 Ketjen black (KB),133 graphene,134 ordered mesoporous carbon (CMK-3),135 and 3D N-doped ultrathin carbon (3DNC),136 among others, and PBAs particles by in situ growth or chemical coprecipitation. Goodenough’s group constructed monodispersed PBAs nanocubes nucleating on a CNT conductive network (PB/CNT).113 The “built-in” CNT network accelerated the electron transport and thus the sodiation reaction of PBAs. As a result, PB/CNT delivered a high discharge capacity of 142 mA h g–1 at 0.1 C under subzero temperatures (−25 °C), corresponding to 85% capacity retention compared with that at 25 °C. A reversible capacity of 52 mA h g–1 at 6 C could be obtained at −25 °C, while it is only 2 mA h g–1 for bare PBAs. After that, Dou and coworkers synthesized a PB@C composition through a facile and in situ solution method, with NaFeHCF directly grown on KB chains.133 Despite the degraded electrochemical activity of FeLS(C) caused by [Fe(CN)6] vacancies, the perfectly shaped PB@C composition with a lower vacancy content (7% vs 15% for bare PB) and fast charge/Na+ diffusion exhibited a higher reversible capacity (130 mA h g–1 vs 90 mA h g–1 at 0.5 C, 1C = 100 mA g–1) and unprecedented rate capacity (77.5 mA h g–1 at 90 C). Owing to the large specific surface and superior electrical conductivity, 3DNC networks were considered as an ideal skeleton for loading redox-active materials. Zhao’s group found that the Na+ adsorption energy at interfaces was decreased and Fe 3d charges were more delocalized after the introduction of 3DNC (8.26 wt %) into NaK-MnHCF, contributing to the better rate performance of the NaK-MnHCF@3DNC composite compared to the bare NaK-MnHCF. Therefore, compositing affords a simple solution to resolve the low electrical conductivity for PBAs.136
4.1.4. Surface Coating
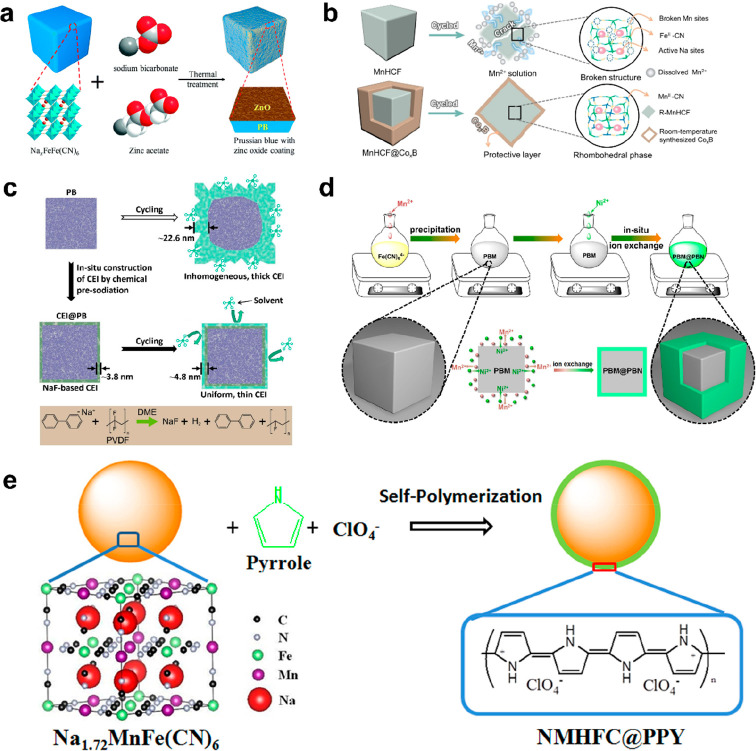
It is well acknowledged that PBA cathodes suffer from serious capacity fading due to the transition metal dissolution and side reactions between the electrode materials and organic electrolytes. Thus, surface coating has been used to protect the PBAs from metal dissolution and unwanted side reactions. Considering the instability of PBAs at temperatures higher than 350 °C, however, only low-temperature surface coating is suitable for PBAs. Up to now, inorganic materials, stable SE-PBAs materials (Ni-HCF), and conductive carbon/polymers have been applied as protective layers for electrochemical performance enhancement, as illustrated in Figure 11.
Figure 11.
Coating layers for PBAs: (a) ZnO (reproduced from ref (137), copyright 2019 Royal Society of Chemistry), (b) CoxB (reproduced from ref (138), copyright 2023 Wiley-VCH), (c) artificial NaF-rich CEI (reproduced from ref (139), copyright 2022 Elsevier), (d) Ni-HCF (reproduced from ref (115), copyright 2021 Elsevier), and (e) PPY (reproduced from ref (140), copyright 2015 Elsevier).
4.1.4.1. Inorganic Materials
Liu’s group constructed a semiconducting and chemically stable ZnO layer (∼50 nm) on the surface of NaxFeFe(CN)6 (PB@ZnO) via a thermal treatment at 200 °C under N2 (Figure 11a), which helped reduce the charge-transfer resistance and prohibit the decomposition of the PB lattice.137 In order to suppress the microstructural degradation and undesirable Jahn–Teller effect, Hu’s group recently created a magical CoxB on the MnHCF surface through a facile wet-chemical coating method (Figure 11b).138 Owing to the whole coverage of CoxB, the optimal MnHCF-5%CoxB cathode displayed limited Mn dissolution and reduced intergranular cracks, thereby contributing to the outstanding cycling performance (74% capacity retention over 2500 cycles at 10 C, 1 C= 170 mA g–1). In addition, Ma’s group reported the creation of a Na3(VOP4)2F (NVOPF) coated NaMnHCF composite (PBM@NVOPF) through solution precipitation.141 The NASICON-type NVOPF with high chemical stability can undoubtably protect NaMnHCF from the corrosion of HF formed in the electrolyte and inhibit the dissolution of active materials. Hence, excellent electrochemical performance at both room temperature (84.3% capacity retention after 500 cycles) and 55 °C (78.8% capacity retention after 200 cycles) could be acquired at the current density of 100 mA g–1.
Moreover, the degradation of PBAs would induce cracks and even the collapse of the cathode–electrolyte interface (CEI), and the newly exposed surface could trigger new CEI formation. Eventually, a thick and uneven CEI was formed and the electrolyte was used up, leading to the death of SIBs. Therefore, it is of great significance to construct a stable and homogeneous CEI on the surface of PBAs to enhance the cycling stability of SIBs. Lately, Li’s group created an artificial NaF-rich CEI via chemical presodiation between metallic Na, biphenyl, and 1,2-dimethoxyethane (DME), as illustrated in Figure 11c.139 The Na+-conducting NaF-rich CEI effectively prevents CEI@PB from attacking organic solvents and contributes to the longer lifespan of coated PBAs compared to those of bare PBAs. More importantly, the uniformity was maintained, and the thickness of the CEI was approximately 4.8 nm after cycling, which was much smaller than that of PB (∼22.6 nm).
4.1.4.2. Stable SE-PBAs Materials
Although the aforementioned inorganic coating layers have been proven effective, a lattice mismatch between PBAs and coating exists. Coating PBAs with compounds of similar lattice parameters will eliminate lattice mismatches to a greater extent. Among various PBAs with different transition metal ions, Ni-based PBAs (Ni-HCF) have been proven the most chemical/electrochemical stable materials with “zero strain” during cycling.142 Therefore, most research focused on preparing a core–shell composite with Ni-HCF as the outer shell to suppress lattice distortion, transition metal dissolution, and the side reactions between PBAs and the organic electrolyte, thus enhancing the electrochemical performance. Generally, the following three strategies have been adopted for Ni-HCF coating: (i) In situ deposition of Ni-HCF on the surface of PBAs through coprecipitation.143,144 (ii) In situ ion exchange. Given the fact that the Mn—C≡N—Fe group has a higher solubility constant than Ni-HCF, it is feasible to coat sodium nickel hexacyanoferrate (PBN) on the surface of sodium manganese hexacyanoferrate (PBM), as shown in Figure 11d.115 (iii) One-pot synthesis to obtain epitaxial core–shell PBAs because of the unequal formation and stability constants of citrate anion for Ni2+ and Mn2+. Our group found that Ni2+ tends to be released only when Mn2+ is completely consumed, resulting in an epitaxial growth of NiPB on the already formed MnPB template.145 Benefiting from the highly matched lattice parameters, NiPB exerted a stabilizing counterbalancing strain on the Jahn–Teller-distorted MnN6 octahedra. As a result, the MnNiPB-4xcit with an optimized equivalent of Na3Cit possessed an appropriate thickness (9% of thickness of MnPB) to imbue the material with phase stability and an ultrahigh capacity retention of 96% after 500 cycles.
4.1.4.3. Conductive Carbon/Polymers
Besides inorganic materials and Ni-HCF, conductive carbons/polymers, including reduced graphene oxide (rGO),146,147 polypyrrole (PPY),140,148 polydopamine (PDA),149 polyaniline (PANI),150 and poly(3,4-ethylene dioxythiophene) (PEDOT),151 could be applied as the coating layers. The conductive carbon/polymer coating has multiple merits: enhancing the overall electrical conductivity, suppressing the chemical dissolution, preventing unexpected attack of organic electrolyte, reducing the partially oxidized trivalent Fe3+/Mn3+ by in situ polymerization, and offering additional redox sites to increase the capacity of electrodes by some of the carbonaceous materials, such as PPY and PANI (Figure 11e).
4.1.5. Cationic Doping
Cationic doping (elemental substitution) has been proven to be effective strategy to enhance the capacity, working voltage, and cycling performance for cathode materials in SIBs. The same concept could be used for PBAs with partial substitution of the transition metal coordinated with a N atom (Fe atom coordinated with C is fixed in most cases) or an alkali element. It is confirmed that the species or amount of doping metal has a significant influence on the structural stability and electrochemical behavior of PBAs. Therefore, ingenious regulation of the doping level is crucial for electrochemical performance improvement.
The studies on transition metal doping are mainly focused on DE-PBAs (Fe-HCFs and Mn-HCFs) with high specific capacities but insufficient cycling performances, in which the N-coordinated Fe/Mn could be partially substituted by one or multiple elements to adjust the crystal structure and redox behaviors. For Fe-HCF, the low-spin FeLS redox at the higher voltage is hard to activate due to the existence of [Fe(CN)6] vacancies. Through divalent Ni,152,153 Zn,154 Cu,155,156 or Mn157 doping, the capacity contribution of the low spin FeLS redox could be elevated to decrease the energy barriers of Na+ migration. It was found that 3% Ni substitution in Fe-HCF could increase the low spin FeLS capacity contribution from 28% (27 mA h g–1) to 43% (50 mA h g–1).152 Meanwhile, Yang’s group reported that 11% Zn substitution in FeZn-PB delivered a higher low spin FeLS capacity of 60.5 mA h g–1, which was higher than that of Fe-PB (50 mA h g–1) at current density of 20 mA g–1.154 In addition, it was demonstrated in our group that a sample with 36% Zn substitution shows minor lattice distortion for the simplified and reversible phase transition from cubic to tetragonal.158 As for Mn-HCF, a dramatic capacity decay is observed due to the Jahn–Teller distortion of Mn3+, and a 10% decrease in Mn–N distances could be detected after a full charge.159,160 The effects of doping Fe, Co, and Ni for the cycling and rate performance of Mn-HCF have been investigated in Shibata’s group.161 They found that the lifespan and the capacity retention at high rate were significantly increased due to the suppressed Jahn–Teller distortion of Mn3+. After that, He and coworkers reported that the phase structure of Mn-HCF would change from rhombohedral to cubic after Sn4+ doping, and enhanced capacity retention after 100 cycles at 240 mA g–1 was obtained (80.5% vs 54.0% for bare Mn-HCF).162 Lately, Jahn–Teller distortion was found by Shao’ group to be repressed through employing Mn vacancies (VMn) in combination with Ni doping.163
In order to further enhance the electrochemical performance, multication lattice substitution has been employed.164,165 High quality (HQ)-NixCo1–x[Fe(CN)6] PBAs were synthesized through a chelating agent (trisodium citrate)/surfactant (polyvinylpyrrolidone, PVP) coassisted crystallization method with fewer [Fe(CN)6] vacancies and water molecules in Han’s group.166 As a result, the optimized sample (x = 0.3) exhibited a high specific capacity of 145 mA h g–1 and prolonged cyclability of 90% capacity retention after 600 cycles. After that, a ternary NiCoFe-PB sample with Co and Fe at the Ni site was prepared by Yang and coworkers. In such a unique electrode material, Co doping enhanced the redox activity of FeLS; meanwhile, Fe doping enhanced the redox activity of CoHS.167 Additionally, FeCo-co-doping could reduce the Na+ diffusion resistance within the solid electrolyte interface; thus, an ultralow capacity fading rate of 0.0044% per cycle has been obtained. Moreover, high-entropy PBAs cathode materials with FeMnNiCuCo sharing the N-coordinated M1 site for SIB were first reported by Brezesinski’s group.168 The equimolar fractions of above five metal cations increased the structural stability and configurational entropy and suppressed the degradation of PBAs cathodes at high voltages. After that, a link between the high-entropy effect and the observed energy storage capabilities of Mn-HCF was established for the first time. By systematic comparison of the structural and chemical properties of high-, medium-, and low-entropy Mn-HCFs, Brezesinki and coworkers concluded that the electrochemical performance enhancement could be ascribed to the entropy-mediated suppression of the Jahn–Teller distortion.169 Inspired by the disordered Rubik’s cube, our group synthesized a high-entropy PBA sample as a “proof-of-concept” to demonstrate its application in energy storage devices.170 It was revealed that the increased configuration entropy could promote thermal/air stability and afford a zero-strain two-phase (cubic ↔ tetragonal) Na+ storage mechanism. As a consequence, the ultralong cycling lifespan over 50 000 cycles (a capacity retention of 79.2%) was achieved.
Owing to the large family of PBAs, the reversible (de)insertion of an alkali ion can be allowed, and K+ insertion has been confirmed to exhibit the best reversibility with the highest potential.171 Therefore, most researchers are forced on K+ doping to improve the structural and electrochemical stability.172,173 A low concentration of K+ in NaxKyFeHCF samples would expand the PBA framework structure and provide a larger cell volume for Na+ intercalation.174 In addition, K+ could be reinserted at 8c sites before Na+, providing extra specific capacity and preventing the phase transition and lattice expansion. Then, the synthesis of a series of KxNayMnFe(CN)6 (x + y ≤ 2, KNMF) samples through coprecipitation route was proposed by Qiao’s group, where adjustive sodium citrate was used as sodium resource and organic additive.175 KNMF-3 (x = 1.59, y = 0.25) exhibited better electrochemical performance with good crystallinity, a high Na+ content, and nanocubic morphology compared to the sample without K-doping. A facile “potassium-ions assisted” strategy was developed by our group to prepare highly crystallized Fe-based PBAs by controlling the crystal phase orientation.176 The optimized product NKPB-3 (Na0.28K1.55Fe[Fe(CN)6]·1.53H2O) displayed a stable structure-orientating (220) plane with fewer [Fe(CN)6]4 vacancies and a lower water content. Attributed to the highly crystal structure and pillar effect of K+, the as-obtained electrode delivered a high initial specific capacity of 147.9 mA h g–1 and 83.5% capacity retention after 300 cycles.
In order to effectively enhance the doping measures for Fe-HCF materials, it is important to increase the redox activity of FeLS and the capacity contribution of the low spin FeLS. This can be achieved by incorporating alternative elements, such as Ni, Co, Zn, etc. For Mn-HCF, the key issue is to use the doped elements to limit the Jahn–Teller effect of Mn3+ and enhance the structural stability. In this strategy, elements such as Fe, Co, Ni, and Sn were adopted. It was worth noting the introduction of both Co and Ni, as they had dual effects, while taking into account their intake/cost. Furthermore, doping K+ into the alkali site is an excellent practice that greatly improves the structural and electrochemical stability. Continuous regulation of the introduced solubility is necessary to achieve the best effect for future development.
4.2. Scalable Preparation
Up to now, several groups have begun to prepare PBAs cathode materials on a kilogram-scale. Xie’s group synthesized the Ni and Fe-codoped manganese hexacyanoferrate PB (MnFeNi-PB) via a Na3Cit-assisted coprecipitation method.177 They realized kilogram-scale MnFeNi-PB using a 100 L reactor, and 3.2 kg of sample could be obtained, as shown in Figure 12a and b. The as-prepared MnFeNi-PB exhibited a long cycle life at room temperature (65.5% capacity after 2000 cycles, 5 C, 1 C = 150 mA g–1), 45 °C (83.5% capacity retention, 300 cycles, 1 C), and −20 °C (92.1% capacity retention, 700 cycles, 1 C). After that, they synthesized Mn/Ni binary PBAs in a high precursor salt concentration of 0.5 mol L–1 (Mn0.5Ni0.5-0.5), resulting in a higher yield for mass production.178 The result showed that the cycling performance and discharge specific capacity were better than those of the sample prepared at a lower salt concentration. Moreover, Mn0.5Ni0.5-0.5 displayed excellent cycling performance at overcharge to 4.8 V (91.8% retention) and overdischarge to 1.2 V (89.1% retention) after 300 cycles, demonstrating that it exhibited a satisfactory tolerance for deep charge/discharge. In addition, sodium-rich Na2–xFeFe(CN)6 has been successfully prepared in our group by a scale-up precipitation route with Na3Cit as a chelating agent and sodium supplement (yield of 5 kg per 100 L).179 It was concluded that Na3Cit could play the most important role in crystal growth. With the increase of the Na3Cit concentration, the morphology of Na2–xFeFe(CN)6 turned to a single microcube compared to irregular particles at low concentration. Subsequently, A 5 Ah pouch full cell with an as-prepared Na2–xFeFe(CN)6 cathode and hard carbon anode has been assembled, and excellent electrochemical behavior has been achieved (Figure 12c). Additionally, no sodium compensation was added because the sodium atomic ration in this Fe-PBA cathode reached up to 1.73. As shown in Figure 12d and e, an obvious plateau at 2.9 V was observed, and 78% capacity retention could be obtained after 1000 cycles. Such significant work can pave the way for scaled-up preparation of PBAs in the future.
Figure 12.
(a and b) Kilogram-grade preparation of MnFeNi-PB. Reproduced with permission from ref (177). Copyright 2022 Elsevier. (c) Working mechanism of a 5 Ah pouch full cell-based Na2–xFeFe(CN)6 cathode and hard carbon anode, (d) charge–discharge curves, and (e) cycling performance of the 5 Ah pouch full cell. Reproduced with permission from ref (179). Copyright 2020 Springer Nature.
5. Outlook and Perspectives
In summary, great progress has been made in developing cathode materials for SIBs in recent years in spite of the many challenges remaining. The commonly used cathode materials for SIBs include transition metal oxides, polyanion compounds, and Prussian blue (analogs), which have different physical and chemical properties and electrochemical performance due to their versatile compositions and crystal structures. The transition metal oxides can be simply prepared and demonstrate high specific capacity and good rate capability, but they are prone to collapse during repeated Na insertion/extraction owing to their fragile crystal structures, which is usually resolved by lattice regulation. Polyanionic materials have high working voltages and excellent thermal/cyclic stability due to from their stable crystal structure and strong X–O polar bonds, but they suffer from inferior electronic conductivity. Surface modification and morphology/lattice regulation have been explored to improve the performance of polyanionic cathode. Prussian blue and its analogs have the advantages of low cost, great rate performance, and adjustable working voltages, but the stubborn crystal water causes their chemical and structural instability. In conclusion, there are remaining issues that need to be addressed before the above three categories of cathode materials showcase their grandeur in the field of large-scale energy storage, as discussed in below (Figure 13).
-
(i)
Developing large-scale synthesis techniques: Anode materials, such as hard carbon and silicon, can be easily mass produced with high consistency due to their simple compositions and abundant raw materials, while syntheses of cathodic materials containing complex components and expensive raw materials (e.g., V-, Cu-, and Co-based compositions) usually involve cumbersome synthesis steps, making the mass production of cathode materials highly challenging. Synthesis techniques such as the coprecipitation method and solid-state ball milling can be extended to large scale production with their product yields and batch stability further improved.
-
(ii)
In-depth understanding of the Na storage mechanism: The studies on the in situ structure/component evolution of the electrodes and the genesis of a cathode electrolyte interface (CEI) film during cycling are essential to illustratethe Na storage mechanism and guiding the development of the high performance cathode materials. Therefore, advanced characterization techniques, such as in situ neutron diffraction, in situ X-ray absorption spectroscopy (XAS), and in situ electrochemical monitoring, should be more intensively adopted.
-
(iii)
Seeking matching anodes for full-cell study: The assemble of a Na full cell must consider the matching between the cathode and the anode. High specific capacity is the ultimate pursuit in selecting anode materials, so traditional hard carbon anode materials with limited theoretical capacity cannot meet the needs of high-energy SIBs. Carbon-based anode materials with high specific capacities, such as graphene and carbon nanotubes, have been developed and applied to full cells of SIBs. In addition, alloy–carbon composites and sodium metal anodes are also under consideration, expecting the high expansion of the alloy anode and severe dendritic growth of the Na metal anode will be resolved in the near future.
-
(iv)
Optimizing the construction of SIBs: The bipolar electrode design using inexpensive aluminum as a shared current collector can help achieve efficient recycling of electrode materials, and the absence of alloying reaction between Na and Al is the foundation of this design. Although the construction of SIBs with a bipolar electrode may render a lot of advantages, including higher specific (volumetric) energy density, excellent high-rate performance, and reduced cell resistance, potential electrolyte leakage and subsequent intermixing may cause exhaustive failure of the batteries. The validity and reliability of a variety of battery constructions have yet to be examined.
Figure 13.
Figure for the development prospects of cathode materials in SIBs. Top left image reproduced with permission from ref180. Copyright 2023 Springer Nature. Top right (center) image reproduced with permission from ref (181). Copyright 2019 Wiley-VCH. Far right image reproduced with permission from ref (185). Copyright 2021 Royal Society of Chemistry. Bottom left (center) image reproduced with permission from ref (186). Copyright 2023 American Chemical Society.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 51925207, 52161145101, U1910210, 52250710680, 51971124, 52171217, 52101265, 52202284, and 22209123), the “Transformational Technologies for Clean Energy and Demonstration” Strategic Priority Research Program of Chinese Academy of Sciences (Grant XDA21000000), the National Synchrotron Radiation Laboratory (KY2060000173), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (Grant. YLU-DNL Fund 2021002), the Zhejiang Natural Science Foundation (LQ23E020002), and the Wenzhou Natural Science Foundation (G20220019).
Author Contributions
∇ S.X. and H.D. contributed equally to this work.
The authors declare no competing financial interest.
References
- Xu M.; Liu M.; Yang Z.; Wu C.; Qian J. Research Progress on Presodiation Strategies for High Energy Sodium-Ion Batteries. Acta Phys. -Chim. Sin. 2022, 39, 2210043. 10.3866/PKU.WHXB202210043. [DOI] [Google Scholar]
- Li Q.; Yang D.; Chen H.; Lv X.; Jiang Y.; Feng Y.; Rui X.; Yu Y. Advances in Metal Phosphides for Sodium-Ion Batteries. SusMat 2021, 1, 359–392. 10.1002/sus2.19. [DOI] [Google Scholar]
- Zhao C. L.; Wang Q. D.; Yao Z. P.; Wang J. L.; Sanchez-Lengeling B.; Ding F. X.; Qi X. G.; Lu Y. X.; Bai X. D.; Li B. H.; et al. Rational Design of Layered Oxide Materials for Sodium-Ion Batteries. Science 2020, 370, 708–711. 10.1126/science.aay9972. [DOI] [PubMed] [Google Scholar]
- Gabriel E.; Ma C.; Graff K.; Conrado A.; Hou D.; Xiong H. Heterostructure Engineering in Electrode Materials for Sodium Ion Batteries: Recent Progress and Perspectives. eScience 2023, 100139. 10.1016/j.esci.2023.100139. [DOI] [Google Scholar]
- Xia X.; Xu S.; Tang F.; Yao Y.; Wang L.; Liu L.; He S.; Yang Y.; Sun W.; Xu C.; et al. A Multifunctional Interphase Layer Enabling Superior Sodium-Metal Batteries under Ambient Temperature and −40 degrees C. Adv. Mater. 2023, 35, 2209511. 10.1002/adma.202209511. [DOI] [PubMed] [Google Scholar]
- Liu Q. N.; Hu Z.; Chen M. Z.; Zou C.; Jin H. L.; Wang S.; Chou S. L.; Liu Y.; Dou S. X. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. 10.1002/adfm.201909530. [DOI] [Google Scholar]
- Song T.; Wang C.; Lee C. Structural Degradation Mechanisms and Modulation Technologies of Layered Oxide Cathodes for Sodium-Ion Batteries. Carbon Neutralization 2022, 1, 68–92. 10.1002/cnl2.7. [DOI] [Google Scholar]
- Wang Q.; Chu S.; Guo S. Progress on Multiphase Layered Transition Metal Oxide Cathodes of Sodium Ion Batteries. Chin. Chem. Lett. 2020, 31, 2167–2176. 10.1016/j.cclet.2019.12.008. [DOI] [Google Scholar]
- Barpanda P.; Lander L.; Nishimura S.-i.; Yamada A. Polyanionic Insertion Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703055. 10.1002/aenm.201703055. [DOI] [Google Scholar]
- Jin T.; Li H.; Zhu K.; Wang P. F.; Liu P.; Jiao L. Polyanion-Type Cathode Materials for Sodium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. 10.1039/C9CS00846B. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Zhao Y.; Xu M. Manganese-Based Polyanionic Cathodes for Sodium-Ion Batteries. Carbon Neutralization 2023, 2, 150–168. 10.1002/cnl2.48. [DOI] [Google Scholar]
- Qian J. F.; Wu C.; Cao Y. L.; Ma Z. F.; Huang Y. H.; Ai X. P.; Yang H. X. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619. 10.1002/aenm.201702619. [DOI] [Google Scholar]
- Gan L.; Yuan X.; Han J.; Li J.; Zheng L.; Yao H. The Synergy of Dis-/ordering Ensures the Superior Comprehensive Performance of P2-Type Na-Based Layered Oxide Cathodes. Carbon Neutralization 2023, 2, 235–244. 10.1002/cnl2.53. [DOI] [Google Scholar]
- Susanto D.; Cho M. K.; Ali G.; Kim J.-Y.; Chang H. J.; Kim H.-S.; Nam K.-W.; Chung K. Y. Anionic Redox Activity as a Key Factor in the Performance Degradation of NaFeO2 Cathodes for Sodium Ion Batteries. Chem. Mater. 2019, 31, 3644–3651. 10.1021/acs.chemmater.9b00149. [DOI] [Google Scholar]
- Wang L.; Wang J.; Zhang X.; Ren Y.; Zuo P.; Yin G.; Wang J. Unravelling the Origin of Irreversible Capacity Loss in NaNiO2 for High Voltage Sodium Ion Batteries. Nano Energy 2017, 34, 215–223. 10.1016/j.nanoen.2017.02.046. [DOI] [Google Scholar]
- Yabuuchi N.; Yano M.; Yoshida H.; Kuze S.; Komaba S. Synthesis and Electrode Performance of O3-Type NaFeO2-NaNi1/2Mn1/2O2 Solid Solution for Rechargeable Sodium Batteries. J. Electrochem. Soc. 2013, 160, A3131. 10.1149/2.018305jes. [DOI] [Google Scholar]
- Kumakura S.; Tahara Y.; Kubota K.; Chihara K.; Komaba S. Sodium and Manganese Stoichiometry of P2-Type Na2/3MnO2. Angew. Chem., Int. Ed. 2016, 55, 12760–12763. 10.1002/anie.201606415. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Yu X. Y.; Lou X. W. A Practical High-Energy Cathode for Sodium-Ion Batteries Based on Uniform P2-Na0.7CoO2 Microspheres. Angew. Chem., Int. Ed. 2017, 56, 5801–5805. 10.1002/anie.201702024. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Yan Z.; Luo W.; Hu Z.; Liu G.; Zhang L.; Yang X.; Ou M.; Liu W.; Huang L.; et al. Vitalization of P2-Na2/3Ni1/3Mn2/3O2 at High-Voltage Cyclability via Combined Structural Modulation for Sodium-Ion Batteries. Energy Storage Mater. 2020, 29, 182–189. 10.1016/j.ensm.2020.04.012. [DOI] [Google Scholar]
- Tapia-Ruiz N.; Armstrong A. R.; Alptekin H.; Amores M. A.; Au H.; Barker J.; Boston R.; Brant W. R.; Brittain J. M.; Chen Y.; et al. 2021 Roadmap for Sodium-Ion Batteries. J. Phys. Energy 2021, 3, 031503. 10.1088/2515-7655/ac01ef. [DOI] [Google Scholar]
- Hu X.; Zhao Z.; Wang L.; Li J.; Wang C.; Zhao Y.; Jin H. VO2 (A)/Graphene Nanostructure: Stand up to Na Ion Intercalation/Deintercalation for Enhanced Electrochemical Performance as a Na-Ion Battery Cathode. Electrochim. Acta 2019, 293, 97–104. 10.1016/j.electacta.2018.10.010. [DOI] [Google Scholar]
- Murphy D.; Christian P. Solid State Electrodes for High Energy Batteries. Science 1979, 205, 651–656. 10.1126/science.205.4407.651. [DOI] [PubMed] [Google Scholar]
- Popuri S. R.; Miclau M.; Artemenko A.; Labrugere C.; Villesuzanne A.; Pollet M. l. Rapid Hydrothermal Synthesis of VO2 (B) and Its Conversion to Thermochromic VO2 (M1). Inorg. Chem. 2013, 52, 4780–4785. 10.1021/ic301201k. [DOI] [PubMed] [Google Scholar]
- Wei Q.; Liu J.; Feng W.; Sheng J.; Tian X.; He L.; An Q.; Mai L. Hydrated Vanadium Pentoxide with Superior Sodium Storage Capacity. J. Mater. Chem. A 2015, 3, 8070–8075. 10.1039/C5TA00502G. [DOI] [Google Scholar]
- Dong Y.; Li S.; Zhao K.; Han C.; Chen W.; Wang B.; Wang L.; Xu B.; Wei Q.; Zhang L.; et al. Hierarchical Zigzag Na1.25V3O8 Nanowires with Topotactically Encoded Superior Performance for Sodium-Ion Battery Cathodes. Energy Environ. Sci. 2015, 8, 1267–1275. 10.1039/C5EE00036J. [DOI] [Google Scholar]
- Kang H.; Liu Y.; Shang M.; Lu T.; Wang Y.; Jiao L. NaV3O8 Nanosheet@Polypyrrole Core-Shell Composites with Good Electrochemical Performance as Cathodes for Na-Ion Batteries. Nanoscale 2015, 7, 9261–9267. 10.1039/C5NR02064F. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Oh S. M.; Park B.; Ye B. U.; Lee N. S.; Baik J. M.; Hwang S. J.; Kim M. H. Unidirectional Growth of Single Crystalline β-Na0.33V2O5 and α-V2O5 Nanowires Driven by Controlling the pH of Aqueous Solution and Their Electrochemical Performances for Na-Ion Batteries. CrystEngComm 2017, 19, 5028–5037. 10.1039/C7CE00781G. [DOI] [Google Scholar]
- Moretti A.; Maroni F.; Osada I.; Nobili F.; Passerini S. V2O5 Aerogel as a Versatile Cathode Material for Lithium and Sodium Batteries. ChemElectroChem. 2015, 2, 529–537. 10.1002/celc.201402394. [DOI] [Google Scholar]
- Liu S.; Tong Z.; Zhao J.; Liu X.; Wang J.; Ma X.; Chi C.; Yang Y.; Liu X.; Li Y. Rational Selection of Amorphous or Crystalline V2O5 Cathode for Sodium-Ion Batteries. Phys. Chem. Chem. Phys. 2016, 18, 25645–25654. 10.1039/C6CP04064K. [DOI] [PubMed] [Google Scholar]
- Su D.; Ahn H. J.; Wang G. Hydrothermal Synthesis of α-MnO2 and β-MnO2 Nanorods as High Capacity Cathode Materials for Sodium Ion Batteries. J. Mater. Chem. A 2013, 1, 4845–4850. 10.1039/c3ta00031a. [DOI] [Google Scholar]
- Lu K.; Hu Z.; Xiang Z.; Ma J.; Song B.; Zhang J.; Ma H. Cation Intercalation in Manganese Oxide Nanosheets: Effects on Lithium and Sodium Storage. Angew. Chem. 2016, 128, 10604–10608. 10.1002/ange.201605102. [DOI] [PubMed] [Google Scholar]
- Silván B.; Gonzalo E.; Djuandhi L.; Sharma N.; Fauth F.; Saurel D. On the Dynamics of Transition Metal Migration and Its Impact on the Performance of Layered Oxides for Sodium-Ion Batteries: NaFeO2 as a Case Study. J. Mater. Chem. A 2018, 6, 15132–15146. 10.1039/C8TA02473A. [DOI] [Google Scholar]
- Lee E.; Brown D. E.; Alp E. E.; Ren Y.; Lu J.; Woo J.-J.; Johnson C. S. New Insights into the Performance Degradation of Fe-Based Layered Oxides in Sodium-Ion Batteries: Instability of Fe3+/Fe4+ Redox in α-NaFeO2. Chem. Mater. 2015, 27, 6755–6764. 10.1021/acs.chemmater.5b02918. [DOI] [Google Scholar]
- Thorne J.; Zheng L.; Lee C. L.; Dunlap R.; Obrovac M. Synthesis and Electrochemistry of O3-Type NaFeO2-NaCo0. 5Ni0. 5O2 Solid Solutions for Na-Ion Positive Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 22013–22022. 10.1021/acsami.8b03336. [DOI] [PubMed] [Google Scholar]
- Kaufman J. L.; Van der Ven A. NaxCoO2 Phase Stability and Hierarchical Orderings in the O3/P3 Structure Family. Phys. Rev. Mater. 2019, 3, 015402. 10.1103/PhysRevMaterials.3.015402. [DOI] [Google Scholar]
- Bianchini M.; Wang J.; Clément R.; Ceder G. A First-Principles and Experimental Investigation of Nickel Solubility into the P2 NaxCoO2 Sodium-Ion Cathode. Adv. Energy Mater. 2018, 8, 1801446. 10.1002/aenm.201801446. [DOI] [Google Scholar]
- Carlier D.; Cheng J.; Berthelot R.; Guignard M.; Yoncheva M.; Stoyanova R.; Hwang B.; Delmas C. The P2-Na2/3Co2/3Mn1/3O2 Phase: Structure, Physical Properties and Electrochemical Behavior as Positive Electrode in Sodium Battery. Dalton Trans. 2011, 40, 9306–9312. 10.1039/c1dt10798d. [DOI] [PubMed] [Google Scholar]
- Braconnier J. J.; Delmas C.; Fouassier C.; Hagenmuller P. Comportement Electrochimique Des Phases NaxCoO2. Mater. Res. Bull. 1980, 15, 1797–1804. 10.1016/0025-5408(80)90199-3. [DOI] [Google Scholar]
- Clément R. J.; Bruce P. G.; Grey C. P. Manganese-Based P2-Type Transition Metal Oxides as Sodium-Ion Battery Cathode Materials. J. Electrochem. Soc. 2015, 162, A2589. 10.1149/2.0201514jes. [DOI] [Google Scholar]
- Mendiboure A.; Delmas C.; Hagenmuller P. Electrochemical Intercalation and Deintercalation of NaxMnO2 Bronzes. J. Solid State Chem. 1985, 57, 323–331. 10.1016/0022-4596(85)90194-X. [DOI] [Google Scholar]
- Liu X.; Zhong G.; Xiao Z.; Zheng B.; Zuo W.; Zhou K.; Liu H.; Liang Z.; Xiang Y.; Chen Z.; et al. Al and Fe-Containing Mn-Based Layered Cathode with Controlled Vacancies for High-Rate Sodium Ion Batteries. Nano Energy 2020, 76, 104997. 10.1016/j.nanoen.2020.104997. [DOI] [Google Scholar]
- Buchholz D.; Chagas L. G.; Vaalma C.; Wu L.; Passerini S. Water Sensitivity of Layered P2/P3-NaxNi0.22Co0.11Mn0.66O2 Cathode Material. J. Mater. Chem. A 2014, 2, 13415–13421. 10.1039/C4TA02627F. [DOI] [Google Scholar]
- Lu Z.; Dahn J. Intercalation of Water in P2, T2 and O2 Structure Az[CoxNi1/3-xMn2/3]O2. Chem. Mater. 2001, 13, 1252–1257. 10.1021/cm000721x. [DOI] [Google Scholar]
- You Y.; Dolocan A.; Li W.; Manthiram A. Understanding the Air-Exposure Degradation Chemistry at a Nanoscale of Layered Oxide Cathodes for Sodium-Ion Batteries. Nano Lett. 2019, 19, 182–188. 10.1021/acs.nanolett.8b03637. [DOI] [PubMed] [Google Scholar]
- Zheng L.; Li L.; Shunmugasundaram R.; Obrovac M. Effect of Controlled-Atmosphere Storage and Ethanol Rinsing on NaNi0.5Mn0.5O2 for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38246–38254. 10.1021/acsami.8b14209. [DOI] [PubMed] [Google Scholar]
- Guignard M.; Didier C.; Darriet J.; Bordet P.; Elkaïm E.; Delmas C. P2-NaxVO2 System as Electrodes for Batteries and Electron-Correlated Materials. Nat. Mater. 2013, 12, 74–80. 10.1038/nmat3478. [DOI] [PubMed] [Google Scholar]
- Delmas C.; Fouassier C.; Hagenmuller P. Structural Classification and Properties of the Layered Oxides. Physica B+C 1980, 99, 81–85. 10.1016/0378-4363(80)90214-4. [DOI] [Google Scholar]
- Xi K.; Chu S.; Zhang X.; Zhang X.; Zhang H.; Xu H.; Bian J.; Fang T.; Guo S.; Liu P.; et al. A high-Performance Layered Cr-Based Cathode for Sodium-Ion Batteries. Nano Energy 2020, 67, 104215. 10.1016/j.nanoen.2019.104215. [DOI] [Google Scholar]
- Zheng L.; Bennett J. C.; Obrovac M. Stabilizing NaCrO2 by Sodium Site Doping with Calcium. J. Electrochem. Soc. 2019, 166, A2058. 10.1149/2.1041910jes. [DOI] [Google Scholar]
- Nam K. W.; Kim S.; Yang E.; Jung Y.; Levi E.; Aurbach D.; Choi J. W. Critical Role of Crystal Water for a Layered Cathode Material in Sodium Ion Batteries. Chem. Mater. 2015, 27, 3721–3725. 10.1021/acs.chemmater.5b00869. [DOI] [Google Scholar]
- Zuo W.; Innocenti A.; Zarrabeitia M.; Bresser D.; Yang Y.; Passerini S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-economics. Acc. Chem. Res. 2023, 56, 284–296. 10.1021/acs.accounts.2c00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. H.; Hwang J. Y.; Yoon C. S.; Heller A.; Mullins C. B. Capacity Degradation Mechanism and Cycling Stability Enhancement of AlF3-Coated Nanorod Gradient Na[Ni0. 65Co0. 08Mn0. 27]O2 Cathode for Sodium-Ion Batteries. ACS Nano 2018, 12, 12912–12922. 10.1021/acsnano.8b08266. [DOI] [PubMed] [Google Scholar]
- Li J.; Hu H.; Wang J.; Xiao Y. Surface Chemistry Engineering of Layered Oxide Cathodes for Sodium-Ion Batteries. Carbon Neutralization 2022, 1, 96–116. 10.1002/cnl2.19. [DOI] [Google Scholar]
- Zuo W.; Liu X.; Qiu J.; Zhang D.; Xiao Z.; Xie J.; Ren F.; Wang J.; Li Y.; Ortiz G. F.; et al. Engineering Na+-Layer Spacings to Stabilize Mn-Based Layered Cathodes for Sodium-Ion Batteries. Nat. Commun. 2021, 12, 4903. 10.1038/s41467-021-25074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L. W.; Lu Y. X.; Wang Y. S.; Liu L. L.; Qi X. G.; Zhao C. L.; Chen L. Q.; Hu Y. S. A high-Temperature β-Phase NaMnO2 Stabilized by Cu Doping and Its Na Storage Properties. Chin. Phys. Lett. 2018, 35, 048801. 10.1088/0256-307X/35/4/048801. [DOI] [Google Scholar]
- Tsuchiya Y.; Takanashi K.; Nishinobo T.; Hokura A.; Yonemura M.; Matsukawa T.; Ishigaki T.; Yamanaka K.; Ohta T.; Yabuuchi N. Layered NaxCrxTi1-xO2 as Bifunctional Electrode Materials for Rechargeable Sodium Batteries. Chem. Mater. 2016, 28, 7006–7016. 10.1021/acs.chemmater.6b02814. [DOI] [Google Scholar]
- Guo S.; Liu P.; Yu H.; Zhu Y.; Chen M.; Ishida M.; Zhou H. A Layered P2-and O3-Type Composite as a High-Energy Cathode for Rechargeable Sodium-Ion Batteries. Angew. Chem., Int. Ed. 2015, 54, 5894–5899. 10.1002/anie.201411788. [DOI] [PubMed] [Google Scholar]
- Li Z. Y.; Zhang J.; Gao R.; Zhang H.; Zheng L.; Hu Z.; Liu X. Li-Substituted Co-Free Layered P2/O3 Biphasic Na0.67Mn0.55Ni0.25Ti0.2-xLixO2 as High-Rate-Capability Cathode Materials for Sodium Ion Batteries. J. Phys. Chem. C 2016, 120, 9007–9016. 10.1021/acs.jpcc.5b11983. [DOI] [Google Scholar]
- Qi X.; Liu L.; Song N.; Gao F.; Yang K.; Lu Y.; Yang H.; Hu Y. S.; Cheng Z. H.; Chen L. Design and Comparative Study of O3/P2 Hybrid Structures for Room Temperature Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 40215–40223. 10.1021/acsami.7b11282. [DOI] [PubMed] [Google Scholar]
- Konarov A.; Choi J. U.; Bakenov Z.; Myung S. T. Revisit of Layered Sodium Manganese Oxides: Achievement of High Energy by Ni Incorporation. J. Mater. Chem. A 2018, 6, 8558–8567. 10.1039/C8TA02067A. [DOI] [Google Scholar]
- Wang P. F.; Yao H. R.; Liu X. Y.; Yin Y. X.; Zhang J. N.; Wen Y.; Yu X.; Gu L.; Guo Y. G. Na+/Vacancy Disordering Promises High-Rate Na-Ion Batteries. Sci. Adv. 2018, 4, eaar6018 10.1126/sciadv.aar6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi N.; Hara R.; Kajiyama M.; Kubota K.; Ishigaki T.; Hoshikawa A.; Komaba S. New O2/P2-type Li-Excess Layered Manganese Oxides as Promising Multi-Functional Electrode Materials for Rechargeable Li/Na Batteries. Adv. Energy Mater. 2014, 4, 1301453. 10.1002/aenm.201301453. [DOI] [Google Scholar]
- Hemalatha K.; Jayakumar M.; Bera P.; Prakash A. Improved Electrochemical Performance of Na0.67MnO2 through Ni and Mg Substitution. J. Mater. Chem. A 2015, 3, 20908–20912. 10.1039/C5TA06361B. [DOI] [Google Scholar]
- Chae M. S.; Kim H. J.; Lyoo J.; Attias R.; Gofer Y.; Hong S. T.; Aurbach D. Anomalous Sodium Storage Behavior in Al/F Dual-Doped P2-Type Sodium Manganese Oxide Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2002205. 10.1002/aenm.202002205. [DOI] [Google Scholar]
- Yuan D.; Liang X.; Wu L.; Cao Y.; Ai X.; Feng J.; Yang H. A Honeycomb-Layered Na3Ni2SbO6: A high-rate and cycle-stable cathode for sodium-ion batteries. Adv. Mater. 2014, 26, 6301–6306. 10.1002/adma.201401946. [DOI] [PubMed] [Google Scholar]
- Mariyappan S.; Marchandier T.; Rabuel F.; Iadecola A.; Rousse G.; Morozov A. V.; Abakumov A. M.; Tarascon J.-M. The Role of Divalent (Zn2+/Mg2+/Cu2+) Substituents in Achieving Full Capacity of Sodium Layered Oxides for Na-Ion Battery Applications. Chem. Mater. 2020, 32, 1657–1666. 10.1021/acs.chemmater.9b05205. [DOI] [Google Scholar]
- Xu Y.-S.; Gao J. C.; Tao X.-S.; Sun Y. G.; Liu Y.; Cao A. M.; Wan L. J. High-Performance Cathode of Sodium-Ion Batteries Enabled by A Potassium-Containing Framework of K0.5Mn0.7Fe0.2Ti0.1O2. ACS Appl. Mater. Interfaces 2020, 12, 15313–15319. 10.1021/acsami.0c02157. [DOI] [PubMed] [Google Scholar]
- Shi Q.; Qi R.; Feng X.; Wang J.; Li Y.; Yao Z.; Wang X.; Li Q.; Lu X.; Zhang J.; et al. Niobium-Doped Layered Cathode Material for High-Power and Low-Temperature Sodium-Ion Batteries. Nat. Commun. 2022, 13, 3205. 10.1038/s41467-022-30942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost C. M.; Sachet E.; Borman T.; Moballegh A.; Dickey E. C.; Hou D.; Jones J. L.; Curtarolo S.; Maria J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. 10.1038/ncomms9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.; Ding F.; Lu Y.; Chen L.; Hu Y. S. High-Entropy Layered Oxide Cathodes for Sodium-Ion Batteries. Angew. Chem., Int. Ed. 2020, 59, 264–269. 10.1002/anie.201912171. [DOI] [PubMed] [Google Scholar]
- Yao L.; Zou P.; Wang C.; Jiang J.; Ma L.; Tan S.; Beyer K. A.; Xu F.; Hu E.; Xin H. L. High-Entropy and Superstructure-Stabilized Layered Oxide Cathodes for Sodium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201989. 10.1002/aenm.202201989. [DOI] [Google Scholar]
- Su B.; Wu S.; Liang H.; Zhou W.; Liu J.; Goonetilleke D.; Sharma N.; Sit P. H.-L.; Zhang W.; Yu D. Y. High-Performance NaVO3 with Mixed Cationic and Anionic Redox Reactions for Na-Ion Battery Applications. Chem. Mater. 2020, 32, 8836–8844. 10.1021/acs.chemmater.0c02244. [DOI] [Google Scholar]
- de Boisse B. M.; Reynaud M.; Ma J.; Kikkawa J.; Nishimura S.-i.; Casas-Cabanas M.; Delmas C.; Okubo M.; Yamada A. Coulombic Self-Ordering upon Charging A Large-Capacity Layered Cathode Material for Rechargeable Batteries. Nat. Commun. 2019, 10, 2185. 10.1038/s41467-019-09409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X.; Sathiya M.; Mendoza-Sánchez B.; Iadecola A.; Vergnet J.; Dedryvère R.; Saubanère M.; Abakumov A. M.; Rozier P.; Tarascon J. M. Anionic Redox Activity in a Newly Zn-Doped Sodium Layered Oxide P2-Na2/3Mn1-yZnyO2 (0 < y < 0.23). Adv. Energy Mater. 2018, 8, 1802379. 10.1002/aenm.201802379. [DOI] [Google Scholar]
- Rong X.; Hu E.; Lu Y.; Meng F.; Zhao C.; Wang X.; Zhang Q.; Yu X.; Gu L.; Hu Y. S.; et al. Anionic Redox Reaction-Induced High-Capacity and Low-Strain Cathode with Suppressed Phase Transition. Joule 2019, 3, 503–517. 10.1016/j.joule.2018.10.022. [DOI] [Google Scholar]
- Kim D.; Lee J. Anionic Redox Reactions in Manganese-Based Binary Layered Oxides for Advanced Sodium-Ion Batteries. Chem. Mater. 2020, 32, 5541–5549. 10.1021/acs.chemmater.0c00415. [DOI] [Google Scholar]
- Ding F. X.; Zhao C. L.; Zhou D.; Meng Q. S.; Xiao D. D.; Zhang Q. Q.; Niu Y. S.; Li Y. Q.; Rong X. H.; Lu Y. X.; et al. A Novel Ni-Rich O3-Na[Ni0.60Fe0.25Mn0.15]O2 Cathode for Na-Ion Batteries. Energy Storage Mater. 2020, 30, 420–430. 10.1016/j.ensm.2020.05.013. [DOI] [Google Scholar]
- Masquelier C.; Croguennec L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. 10.1021/cr3001862. [DOI] [PubMed] [Google Scholar]
- Cao X.; Zhou J.; Pan A.; Liang S. Recent Advances in Phosphate Cathode Materials for Sodium-ion Batteries. Acta Phys. Chim. Sin. 2020, 36, 1905018. 10.3866/PKU.WHXB201905018. [DOI] [Google Scholar]
- Barpanda P.; Oyama G.; Nishimura S.; Chung S. C.; Yamada A. A 3.8-V Earth-Abundant Sodium Battery Electrode. Nat. Commun. 2014, 5, 4358. 10.1038/ncomms5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini F.; Fjellvag H.; Vajeeston P. First-Principles Study of the Structural Stability and Electrochemical Properties of Na2MSiO4 (M = Mn, Fe, Co and Ni) Polymorphs. Phys. Chem. Chem. Phys. 2017, 19, 14462–14470. 10.1039/C7CP01395G. [DOI] [PubMed] [Google Scholar]
- Li S.; Guo J.; Ye Z.; Zhao X.; Wu S.; Mi J. X.; Wang C. Z.; Gong Z.; McDonald M. J.; Zhu Z.; et al. Zero-Strain Na2FeSiO4 as Novel Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 17233–17238. 10.1021/acsami.6b03969. [DOI] [PubMed] [Google Scholar]
- Strauss F.; Rousse G.; Sougrati M. T.; Dalla Corte D. A.; Courty M.; Dominko R.; Tarascon J. M. Synthesis, Structure, and Electrochemical Properties of Na3MB5O10 (M = Fe, Co) Containing M2+ in Tetrahedral Coordination. Inorg. Chem. 2016, 55, 12775–12782. 10.1021/acs.inorgchem.6b02070. [DOI] [PubMed] [Google Scholar]
- Li J. J.; Kuang Q.; Wen N.; Yao H.; Wu J.; Fan Q. H.; Dong Y. Z.; Zhao Y. M. Dual-Carbon Decorated Na3Mn2(P2O7)(PO4) Nanocomposite via Freeze Drying: A Zero-Strain Cathode Material for Sodium Ion Batteries. J. Power Sources 2022, 521, 230927. 10.1016/j.jpowsour.2021.230927. [DOI] [Google Scholar]
- Xu S.; Yang Y.; Tang F.; Yao Y.; Lv X.; Liu L.; Xu C.; Feng Y.; Rui X.; Yu Y. Vanadium Fluorophosphates: Advanced Cathode Materials for Next-Generation Secondary Batteries. Mater. Horiz. 2023, 10, 1901–1923. 10.1039/D3MH00003F. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Wang H.; Sun D.; Tang Y.; Wang H. A Comprehensive Review on the Fabrication, Modification and Applications of Na3V2(PO4)2F3 Cathodes. J. Mater. Chem. A 2020, 8, 21387–21407. 10.1039/D0TA07872G. [DOI] [Google Scholar]
- Zhang X.; Rui X.; Chen D.; Tan H.; Yang D.; Huang S.; Yu Y. Na3V2(PO4)3: An Advanced Cathode for Sodium-Ion Batteries. Nanoscale 2019, 11, 2556–2576. 10.1039/C8NR09391A. [DOI] [PubMed] [Google Scholar]
- Jungers T.; Mahmoud A.; Malherbe C.; Boschini F.; Vertruyen B. Sodium Iron Sulfate Alluaudite Solid Solution for Na-Ion Batteries: Moving towards Stoichiometric Na2Fe2(SO4)3. J. Mater. Chem. A 2019, 7, 8226–8233. 10.1039/C9TA00116F. [DOI] [Google Scholar]
- Kim H.; Park I.; Seo D. H.; Lee S.; Kim S. W.; Kwon W. J.; Park Y. U.; Kim C. S.; Jeon S.; Kang K. New Iron-Based Mixed-Polyanion Cathodes for Lithium and Sodium Rechargeable Batteries: Combined First Principles Calculations and Experimental Study. J. Am. Chem. Soc. 2012, 134, 10369–10372. 10.1021/ja3038646. [DOI] [PubMed] [Google Scholar]
- Rui X.; Sun W.; Wu C.; Yu Y.; Yan Q. An Advanced Sodium-Ion Battery Composed of Carbon Coated Na3V2(PO4)3 in a Porous Graphene Network. Adv. Mater. 2015, 27, 6670–6676. 10.1002/adma.201502864. [DOI] [PubMed] [Google Scholar]
- Xiong H. L.; Sun G.; Liu Z. L.; Zhang L.; Li L.; Zhang W.; Du F.; Qiao Z. A. Polymer Stabilized Droplet Templating towards Tunable Hierarchical Porosity in Single Crystalline Na3V2(PO4)3 for Enhanced Sodium-Ion Storage. Angew. Chem., Int. Ed. 2021, 60, 10334–10341. 10.1002/anie.202100954. [DOI] [PubMed] [Google Scholar]
- Wei Q. L.; Chang X. Q.; Wang J.; Huang T. Y.; Huang X. J.; Yu J. Y.; Zheng H. F.; Chen J. H.; Peng D. L. An Ultrahigh-Power Mesocarbon Microbeads|Na+-Diglyme|Na3V2(PO4)3 Sodium-Ion Battery. Adv. Mater. 2022, 34, 2108304. 10.1002/adma.202108304. [DOI] [PubMed] [Google Scholar]
- Liang L. W.; Li X. Y.; Zhao F.; Zhang J. Y.; Liu Y.; Hou L. R.; Yuan C. Z. Construction and Operating Mechanism of High-Rate Mo-Doped Na3V2(PO4)(3)@C Nanowires toward Practicable Wide-Temperature-Tolerance Na-Ion and Hybrid Li/Na-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100287. 10.1002/aenm.202100287. [DOI] [Google Scholar]
- Shi H.; Chen Y.; Li J.; Guo L. Outstanding Long Cycle Stability Provide by Bismuth Doped Na3V2(PO4)3 Enwrapped with Carbon Nanotubes Cathode for Sodium-Ion Batteries. J. Colloid Interface Sci. 2023, 652, 195–207. 10.1016/j.jcis.2023.08.067. [DOI] [PubMed] [Google Scholar]
- Li H.; Jin T.; Chen X.; Lai Y.; Zhang Z.; Bao W.; Jiao L. Rational Architecture Design Enables Superior Na Storage in Greener NASICON Na4MnV(PO4)3 Cathode. Adv. Energy Mater. 2018, 8, 1801418. 10.1002/aenm.201801418. [DOI] [Google Scholar]
- Zhu L.; Zhang M. J.; Yang L. X.; Zhou K. X.; Wang Y.; Sun D.; Tang Y. G.; Wang H. Y. Engineering Hierarchical Structure and Surface of Na4MnV(PO4)3 for Ultrafast Sodium Storage by a Scalable Ball Milling Approach. Nano Energy 2022, 99, 107396. 10.1016/j.nanoen.2022.107396. [DOI] [Google Scholar]
- Ghosh S.; Barman N.; Senguttuvan P. Impact of Mg2+ and Al3+ Substitutions on the Structural and Electrochemical Properties of NASICON-NaxVMn0.75M0.25(PO4)3 (M = Mg and Al) Cathodes for Sodium-Ion Batteries. Small 2020, 16, 2003973. 10.1002/smll.202003973. [DOI] [PubMed] [Google Scholar]
- Hou J.; Hadouchi M.; Sui L.; Liu J.; Tang M.; Kan W. H.; Avdeev M.; Zhong G.; Liao Y.-K.; Lai Y.-H.; et al. Unlocking Fast and Reversible Sodium Intercalation in NASICON Na4MnV(PO4)3 by Fluorine Substitution. Energy Storage Mater. 2021, 42, 307–316. 10.1016/j.ensm.2021.07.040. [DOI] [Google Scholar]
- Lu F. Q.; Wang J. H.; Chang S. Q.; He L. H.; Tang M. X.; Wei Q.; Mo S. Y.; Kuang X. J. New-type NASICON-Na4FeV(PO4)3 Cathode with High Retention and Durability for Sodium Ion Batteries. Carbon 2022, 196, 562–572. 10.1016/j.carbon.2022.05.033. [DOI] [Google Scholar]
- Wang S.; Zheng J. Q.; He L.; Zhang L. Y.; Ge X. C.; Li S. H.; Wang X.; Zhang Z. Carbon-Modified NASICON Na4FeV(PO4)3 Cathode with Enhanced Kinetics for High-Performance Sodium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 9616–9624. 10.1021/acsaem.2c01278. [DOI] [Google Scholar]
- Ma X. D.; Yu X.; Li X. W.; Liu Q.; Liu Y. Modulating Na Vacancies of Na4FeV(PO4)3 via Zr-Substitution: Toward a Superior Rate and Ultrastable Cathode for Sodium-Ion Batteries. J. Power Sources 2022, 541, 231727. 10.1016/j.jpowsour.2022.231727. [DOI] [Google Scholar]
- Li M.; Sun C.; Ni Q.; Sun Z.; Liu Y.; Li Y.; Li L.; Jin H. B.; Zhao Y. J. High Entropy Enabling the Reversible Redox Reaction of V4+/V5+ Couple in NASICON-Type Sodium Ion Cathode. Adv. Energy Mater. 2023, 13, 2203971. 10.1002/aenm.202203971. [DOI] [Google Scholar]
- Liu X.; Lai W.; Peng J.; Gao Y.; Zhang H.; Yang Z.; He X.; Hu Z.; Li L.; Qiao Y.; et al. A NASICON-typed Na4Mn0.5Fe0.5Al(PO4)3 cathode for low-cost and high-energy sodium-ion batteries. Carbon Neutralization 2022, 1, 49–58. 10.1002/cnl2.6. [DOI] [Google Scholar]
- Qi Y.; Tong Z.; Zhao J.; Ma L.; Wu T.; Liu H.; Yang C.; Lu J.; Hu Y.-S. Scalable Room-Temperature Synthesis of Multi-shelled Na3(VOPO4)2F Microsphere Cathodes. Joule 2018, 2, 2348–2363. 10.1016/j.joule.2018.07.027. [DOI] [Google Scholar]
- Shen X.; Zhou Q.; Han M.; Qi X.; Li B.; Zhang Q.; Zhao J.; Yang C.; Liu H.; Hu Y. S. Rapid Mechanochemical Synthesis of Polyanionic Cathode with Improved Electrochemical Performance for Na-Ion Batteries. Nat. Commun. 2021, 12, 2848. 10.1038/s41467-021-23132-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Zheng M.; Zhu W.; Pang H. Hollow Structures Prussian Blue, Its Analogs, and Their Derivatives: Synthesis and Electrochemical Energy-Related Applications. Carbon Neutralization 2023, 2, 271–299. 10.1002/cnl2.64. [DOI] [Google Scholar]
- Ren J.; Zhu H.; Fang Y.; Li W.; Lan S.; Wei S.; Yin Z.; Tang Y.; Ren Y.; Liu Q. Typical Cathode Materials for Lithium-Ion and Sodium-Ion Batteries: From Structural Design to Performance Optimization. Carbon Neutralization 2023, 2, 339–377. 10.1002/cnl2.62. [DOI] [Google Scholar]
- Xie B.; Sun B.; Gao T.; Ma Y.; Yin G.; Zuo P. Recent Progress of Prussian Blue Analogues as Cathode Materials for Nonaqueous Sodium-Ion Batteries. Coordin. Chem. Rev. 2022, 460, 214478. 10.1016/j.ccr.2022.214478. [DOI] [Google Scholar]
- Wang B.; Han Y.; Wang X.; Bahlawane N.; Pan H.; Yan M.; Jiang Y. Prussian Blue Analogs for Rechargeable Batteries. iScience 2018, 3, 110–133. 10.1016/j.isci.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Wang L.; Lu Y.; Liu J.; Guo B.; Xiao P.; Lee J. J.; Yang X. Q.; Henkelman G.; Goodenough J. B. Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery. J. Am. Chem. Soc. 2015, 137, 2658–2664. 10.1021/ja512383b. [DOI] [PubMed] [Google Scholar]
- Peng J.; Zhang W.; Liu Q. N.; Wang J. Z.; Chou S. L.; Liu H. K.; Dou S. X. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. 10.1002/adma.202108384. [DOI] [PubMed] [Google Scholar]
- Wang W.; Gang Y.; Peng J.; Hu Z.; Yan Z.; Lai W.; Zhu Y.; Appadoo D.; Ye M.; Cao Y.; et al. Effect of Eliminating Water in Prussian Blue Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2111727. 10.1002/adfm.202111727. [DOI] [Google Scholar]
- You Y.; Yao H. R.; Xin S.; Yin Y. X.; Zuo T. T.; Yang C. P.; Guo Y. G.; Cui Y.; Wan L. J.; Goodenough J. B. Subzero-Temperature Cathode for a Sodium-Ion Battery. Adv. Mater. 2016, 28, 7243–7248. 10.1002/adma.201600846. [DOI] [PubMed] [Google Scholar]
- Guo J.; Feng F.; Zhao S.; Shi Z.; Wang R.; Yang M.; Chen F.; Chen S.; Ma Z. F.; Liu T. High FeLS(C) Electrochemical Activity of an Iron Hexacyanoferrate Cathode Boosts Superior Sodium Ion Storage. Carbon Energy 2023, 5, e314. 10.1002/cey2.314. [DOI] [Google Scholar]
- Feng F.; Chen S.; Zhao S.; Zhang W.; Miao Y.; Che H.; Liao X.; Ma Z.-F. Enhanced Electrochemical Performance of MnFe@NiFe Prussian Blue Analogue Benefited from the Inhibition of Mn Ions Dissolution for Sodium-Ion Batteries. Chem. Eng. J. 2021, 411, 128518. 10.1016/j.cej.2021.128518. [DOI] [Google Scholar]
- Shang Y.; Li X.; Song J.; Huang S.; Yang Z.; Xu Z. J.; Yang H. Y. Unconventional Mn Vacancies in Mn-Fe Prussian Blue Analogs: Suppressing Jahn-Teller Distortion for Ultrastable Sodium Storage. Chem. 2020, 6, 1804–1818. 10.1016/j.chempr.2020.05.004. [DOI] [Google Scholar]
- Peng J.; Ou M.; Yi H.; Sun X.; Zhang Y.; Zhang B.; Ding Y.; Wang F.; Gu S.; López C. A.; et al. Defect-Free-Induced Na+ Disordering in Electrode Materials. Energy Environ. Sci. 2021, 14, 3130–3140. 10.1039/D1EE00087J. [DOI] [Google Scholar]
- Liu X.; Cao Y.; Sun J. Defect Engineering in Prussian Blue Analogs for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2202532. 10.1002/aenm.202202532. [DOI] [Google Scholar]
- Wu X.; Wu C.; Wei C.; Hu L.; Qian J.; Cao Y.; Ai X.; Wang J.; Yang H. Highly Crystallized Na2CoFe(CN)6 with Suppressed Lattice Defects as Superior Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5393–5399. 10.1021/acsami.5b12620. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Xu Y.; Xu Z.; Sun Y.; Xu X.; Tu J.; Xie J.; Liu S.; Zhao X. Long-Life Na-Rich Nickel Hexacyanoferrate Capable of Working under Stringent Conditions. J. Mater. Chem. A 2021, 9, 21228–21240. 10.1039/D1TA05998J. [DOI] [Google Scholar]
- Jiang Y.; Shen L.; Ma H.; Ma J.; Yang K.; Geng X.; Zhang H.; Liu Q.; Zhu N. A Low-Strain Metal Organic Framework for Ultra-Stable and Long-Life Sodium-Ion Batteries. J. Power Sources 2022, 541, 231701. 10.1016/j.jpowsour.2022.231701. [DOI] [Google Scholar]
- Xu Y.; Ou M.; Liu Y.; Xu J.; Sun X.; Fang C.; Li Q.; Han J.; Huang Y. Crystallization-Induced Ultrafast Na-Ion Diffusion in Nickel Hexacyanoferrate for High-Performance Sodium-Ion Batteries. Nano Energy 2020, 67, 104250. 10.1016/j.nanoen.2019.104250. [DOI] [Google Scholar]
- Xu Y.; Wan J.; Huang L.; Xu J.; Ou M.; Liu Y.; Sun X.; Li S.; Fang C.; Li Q.; et al. Dual Redox-Active Copper Hexacyanoferrate Nanosheets as Cathode Materials for Advanced Sodium-Ion Batteries. Energy Storage Mater. 2020, 33, 432–441. 10.1016/j.ensm.2020.08.008. [DOI] [Google Scholar]
- Peng J.; Zhang W.; Hu Z.; Zhao L.; Wu C.; Peleckis G.; Gu Q.; Wang J. Z.; Liu H. K.; Dou S. X.; et al. Ice-Assisted Synthesis of Highly Crystallized Prussian Blue Analogues for All-Climate and Long-Calendar-Life Sodium Ion Batteries. Nano Lett. 2022, 22, 1302–1310. 10.1021/acs.nanolett.1c04492. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Liu E.; Yan X.; Ma C.; Wen W.; Liao X. Z.; Ma Z. F. Influence of Structural Imperfection on Electrochemical Behavior of Prussian Blue Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2117–A2123. 10.1149/2.0031610jes. [DOI] [Google Scholar]
- Yu Z. E.; Cheng H.; Lyu Y.; Liu Y.; Zhou J.; Chen R.; Guo B. A Vacancy-Free Sodium Manganese Hexacyanoferrate as Cathode for Sodium-Ion Battery by High-Salt-Concentration Preparation. J. Alloys Compd. 2021, 887, 161388. 10.1016/j.jallcom.2021.161388. [DOI] [Google Scholar]
- Hu J.; Tao H.; Chen M.; Zhang Z.; Cao S.; Shen Y.; Jiang K.; Zhou M. Interstitial Water Improves Structural Stability of Iron Hexacyanoferrate for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 12234–12242. 10.1021/acsami.1c23762. [DOI] [PubMed] [Google Scholar]
- Ojwang D. O.; Svensson M.; Njel C.; Mogensen R.; Menon A. S.; Ericsson T.; Haggstrom L.; Maibach J.; Brant W. R. Moisture-Driven Degradation Pathways in Prussian White Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 10054–10063. 10.1021/acsami.0c22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Qin M.; Huang M.; Huang X.; Li Q.; You Y. Organic Solvothermal Method Promoted Monoclinic Prussian Blue as a Superior Cathode for Na-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 6927–6935. 10.1021/acsaem.2c00536. [DOI] [Google Scholar]
- Geng W.; Zhang Z.; Yang Z.; Tang H.; He G. Non-Aqueous Synthesis of High-Quality Prussian Blue Analogues for Na-Ion Batteries. Chem. Commun. 2022, 58, 4472–4475. 10.1039/D2CC00699E. [DOI] [PubMed] [Google Scholar]
- Peng J.; Gao Y.; Zhang H.; Liu Z.; Zhang W.; Li L.; Qiao Y.; Yang W.; Wang J.; Dou S. Ball Milling Solid-State Synthesis of Highly Crystalline Prussian Blue Analogue Na2-xMnFe(CN)6 Cathodes for All-Climate Sodium-Ion Batteries. Angew. Chem., Int. Ed. 2022, 61, e202205867. 10.1002/anie.202205867. [DOI] [PubMed] [Google Scholar]
- Brant W. R.; Mogensen R.; Colbin S.; Ojwang D. O.; Schmid S.; Häggström L.; Ericsson T.; Jaworski A.; Pell A. J.; Younesi R. Selective Control of Composition in Prussian White for Enhanced Material Properties. Chem. Mater. 2019, 31, 7203–7211. 10.1021/acs.chemmater.9b01494. [DOI] [Google Scholar]
- Jiang Y.; Yu S.; Wang B.; Li Y.; Sun W.; Lu Y.; Yan M.; Song B.; Dou S. Prussian Blue@C Composite as an Ultrahigh-Rate and Long-Life Sodium-Ion Battery Cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. 10.1002/adfm.201600747. [DOI] [Google Scholar]
- Bu F.; Feng X.; Jiang T.; Shakir I.; Xu Y. One Versatile Route to Three-Dimensional Graphene Wrapped Metal Cyanide Aerogels for Enhanced Sodium Ion Storage. Chemistry 2017, 23, 8358–8363. 10.1002/chem.201700742. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Huang Y.; Chu D.; Li C.; Zhang Y.; Wu F.; Li L.; Xie M.; Huang J.; Chen R. Continuous Conductive Networks Built by Prussian Blue Cubes and Mesoporous Carbon Lead to Enhanced Sodium-Ion Storage Performances. ACS Appl. Mater. Interfaces 2021, 13, 38202–38212. 10.1021/acsami.1c06634. [DOI] [PubMed] [Google Scholar]
- Mao Y.; Chen Y.; Qin J.; Shi C.; Liu E.; Zhao N. Capacitance Controlled, Hierarchical Porous 3D Ultra-Thin Carbon Networks Reinforced Prussian Blue for High Performance Na-Ion Battery Cathode. Nano Energy 2019, 58, 192–201. 10.1016/j.nanoen.2019.01.048. [DOI] [Google Scholar]
- Qiao Y.; Wei G.; Cui J.; Zhang M.; Cheng X.; He D.; Li S.; Liu Y. Prussian Blue Coupling with Zinc Oxide as a Protective Layer: An Efficient Cathode for High-Rate Sodium-Ion Batteries. Chem. Commun. 2019, 55, 549–552. 10.1039/C8CC07951J. [DOI] [PubMed] [Google Scholar]
- Xu C.; Ma Y.; Zhao J.; Zhang P.; Chen Z.; Yang C.; Liu H.; Hu Y. S. Surface Engineering Stabilizes Rhombohedral Sodium Manganese Hexacyanoferrates for High-Energy Na-Ion Batteries. Angew. Chem., Int. Ed. 2023, 62, e202217761. 10.1002/anie.202217761. [DOI] [PubMed] [Google Scholar]
- Ye M.; You S.; Xiong J.; Yang Y.; Zhang Y.; Li C. C. In-Situ Construction of A NaF-Rich Cathode-Electrolyte Interface on Prussian Blue toward a 3000-Cycle-Life Sodium-Ion Battery. Mater. Today Energy 2022, 23, 100898. 10.1016/j.mtener.2021.100898. [DOI] [Google Scholar]
- Li W. J.; Chou S. L.; Wang J. Z.; Wang J. L.; Gu Q. F.; Liu H. K.; Dou S. X. Multifunctional Conducing Polymer Coated Na1+xMnFe(CN)6 Cathode for Sodium-Ion Batteries with Superior Performance via a Facile and One-Step Chemistry Approach. Nano Energy 2015, 13, 200–207. 10.1016/j.nanoen.2015.02.019. [DOI] [Google Scholar]
- Peng F.; Yu L.; Yuan S.; Liao X. Z.; Wen J.; Tan G.; Feng F.; Ma Z. F. Enhanced Electrochemical Performance of Sodium Manganese Ferrocyanide by Na3(VOPO4)2F Coating for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 37685–37692. 10.1021/acsami.9b12041. [DOI] [PubMed] [Google Scholar]
- You Y.; Wu X.-L.; Yin Y.-X.; Guo Y.-G. A Zero-Strain Insertion Cathode Material of Nickel Ferricyanide for Sodium-Ion Batteries. J. Mater. Chem. A 2013, 1, 14061–14065. 10.1039/c3ta13223d. [DOI] [Google Scholar]
- Wan M.; Tang Y.; Wang L.; Xiang X.; Li X.; Chen K.; Xue L.; Zhang W.; Huang Y. Core-Shell Hexacyanoferrate for Superior Na-Ion Batteries. J. Power Sources 2016, 329, 290–296. 10.1016/j.jpowsour.2016.08.059. [DOI] [Google Scholar]
- Yin J.; Shen Y.; Li C.; Fan C.; Sun S.; Liu Y.; Peng J.; Qing L.; Han J. In Situ Self-Assembly of Core-Shell Multimetal Prussian Blue Analogues for High-Performance Sodium-Ion Batteries. ChemSusChem 2019, 12, 4786–4790. 10.1002/cssc.201902013. [DOI] [PubMed] [Google Scholar]
- Gebert F.; Cortie D. L.; Bouwer J. C.; Wang W.; Yan Z.; Dou S. X.; Chou S. L. Epitaxial Nickel Ferrocyanide Stabilizes Jahn-Teller Distortions of Manganese Ferrocyanide for Sodium-Ion Batteries. Angew. Chem., Int. Ed. 2021, 60, 18519–18526. 10.1002/anie.202106240. [DOI] [PubMed] [Google Scholar]
- Luo J.; Sun S.; Peng J.; Liu B.; Huang Y.; Wang K.; Zhang Q.; Li Y.; Jin Y.; Liu Y.; et al. Graphene-Roll-Wrapped Prussian Blue Nanospheres as a High-Performance Binder-Free Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 25317–25322. 10.1021/acsami.7b06334. [DOI] [PubMed] [Google Scholar]
- Yang D.; Xu J.; Liao X. Z.; Wang H.; He Y. S.; Ma Z. F. Prussian Blue/RGO with Less Coordinated Water as Superior Cathode Material for Sodium-Ion Batteries. Chem. Commun. 2022, 59, 211–214. 10.1039/D2CC04702K. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Zhang W.; Xue L.; Ding X.; Wang T.; Liu X.; Liu J.; Li X.; Huang Y. Polypyrrole-Promoted Superior Cyclability and Rate Capability of NaxFe[Fe(CN)6] Cathodes for Sodium-Ion Batteries. J. Mater. Chem. A 2016, 4, 6036–6041. 10.1039/C6TA00876C. [DOI] [Google Scholar]
- Liu Y.; He D.; Cheng Y.; Li L.; Lu Z.; Liang R.; Fan Y.; Qiao Y.; Chou S. A Heterostructure Coupling of Bioinspired, Adhesive Polydopamine, and Porous Prussian Blue Nanocubics as Cathode for High-Performance Sodium-Ion Battery. Small 2020, 16, 1906946. 10.1002/smll.201906946. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Fu L.; Luan J.; Huang X.; Tang Y.; Xie H.; Wang H. Surface Engineering Induced Core-Shell Prussian Blue@Polyaniline Nanocubes as a High-Rate and Long-Life Sodium-Ion Battery Cathode. J. Power Sources 2018, 395, 305–313. 10.1016/j.jpowsour.2018.05.085. [DOI] [Google Scholar]
- Wang X.; Wang B.; Tang Y.; Xu B. B.; Liang C.; Yan M.; Jiang Y. Manganese Hexacyanoferrate Reinforced by PEDOT Coating towards High-Rate and Long-Life Sodium-Ion Battery Cathode. J. Mater. Chem. A 2020, 8, 3222–3227. 10.1039/C9TA12376H. [DOI] [Google Scholar]
- Fu H.; Liu C.; Zhang C.; Ma W.; Wang K.; Li Z.; Lu X.; Cao G. Enhanced Storage of Sodium Ions in Prussian Blue Cathode Material through Nickel Doping. J. Mater. Chem. A 2017, 5, 9604–9610. 10.1039/C7TA00132K. [DOI] [Google Scholar]
- Wang B.; Han Y.; Chen Y.; Xu Y.; Pan H.; Sun W.; Liu S.; Yan M.; Jiang Y. Gradient Substitution: An Intrinsic Strategy towards High Performance Sodium Storage in Prussian Blue-Based Cathodes. J. Mater. Chem. A 2018, 6, 8947–8954. 10.1039/C8TA02291G. [DOI] [Google Scholar]
- Zhang L. L.; Chen Z.-Y.; Fu X. Y.; Yan B.; Tao H. C.; Yang X. L. Effect of Zn-Substitution Induced Structural Regulation on Sodium Storage Performance of Fe-Based Prussian Blue. Chem. Eng. J. 2022, 433, 133739. 10.1016/j.cej.2021.133739. [DOI] [Google Scholar]
- Chen Z. Y.; Fu X. Y.; Zhang L. L.; Yan B.; Yang X. L. High-Performance Fe-Based Prussian Blue Cathode Material for Enhancing the Activity of Low-Spin Fe by Cu Doping. ACS Appl. Mater. Interfaces 2022, 14, 5506–5513. 10.1021/acsami.1c23793. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Huang Y.; Luo R.; Wu F.; Li L.; Xie M.; Huang J.; Chen R. Ion-Exchange Synthesis of High-Energy-Density Prussian Blue Analogues for Sodium Ion Battery Cathodes with Fast Kinetics and Long Durability. J. Power Sources 2019, 436, 226868. 10.1016/j.jpowsour.2019.226868. [DOI] [Google Scholar]
- Xi Y.; Lu Y. Electrochemically Active Mn-Doped Iron Hexacyanoferrate as the Cathode Material in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 39022–39030. 10.1021/acsami.2c07779. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Peng J.; Li L.; Zhao Y.; Gao Y.; Wang J.; Cao Y.; Dou S.; Chou S. Low-Cost Zinc Substitution of Iron-Based Prussian Blue Analogs as Long Lifespan Cathode Materials for Fast Charging Sodium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2210725. 10.1002/adfm.202210725. [DOI] [Google Scholar]
- Mullaliu A.; Asenbauer J.; Aquilanti G.; Passerini S.; Giorgetti M. Highlighting the Reversible Manganese Electroactivity in Na-Rich Manganese Hexacyanoferrate Material for Li- and Na-Ion Storage. Small Methods 2020, 4, 1900529. 10.1002/smtd.201900529. [DOI] [Google Scholar]
- Li W.; Han C.; Wang W.; Xia Q.; Chou S.; Gu Q.; Johannessen B.; Liu H.; Dou S. Stress Distortion Restraint to Boost the Sodium Ion Storage Performance of a Novel Binary Hexacyanoferrate. Adv. Energy Mater. 2020, 10, 1903006. 10.1002/aenm.201903006. [DOI] [Google Scholar]
- Moritomo Y.; Urase S.; Shibata T. Enhanced Battery Performance in Manganese Hexacyanoferrate by Partial Substitution. Electrochim. Acta 2016, 210, 963–969. 10.1016/j.electacta.2016.05.205. [DOI] [Google Scholar]
- Li J.; He X.; Ostendorp S.; Zhang L.; Hou X.; Zhou D.; Yan B.; Meira D. M.; Yang Y.; Jia H.; et al. Tin Modification of Sodium Manganese Hexacyanoferrate as a Superior Cathode Material for Sodium Ion Batteries. Electrochim. Acta 2020, 342, 135928. 10.1016/j.electacta.2020.135928. [DOI] [Google Scholar]
- El-Hady D. A.; Lyu Y.; Zhan S.; Yang J.; Wang Y.; Yang F.; Zhao Q.; Gu M.; Shao M. Vacancy and Composition Engineering of Manganese Hexacyanoferrate for Sodium-Ion Storage. ACS Appl. Energy Mater. 2022, 5, 8547–8553. 10.1021/acsaem.2c01085. [DOI] [Google Scholar]
- Xie B.; Zuo P.; Wang L.; Wang J.; Huo H.; He M.; Shu J.; Li H.; Lou S.; Yin G. Achieving Long-Life Prussian Blue Analogue Cathode for Na-Ion Batteries via Triple-Cation Lattice Substitution and Coordinated Water Capture. Nano Energy 2019, 61, 201–210. 10.1016/j.nanoen.2019.04.059. [DOI] [Google Scholar]
- Zhu Y.; Zhang Z.; Bao J.; Zeng S.; Nie W.; Chen P.; Zhou Y.; Xu Y. Multi-Metal Doped High Capacity and Stable Prussian Blue Analogue for Sodium Ion Batteries. Int. J. Energy Res. 2020, 44, 9205–9212. 10.1002/er.5576. [DOI] [Google Scholar]
- Peng J.; Wang J.; Yi H.; Hu W.; Yu Y.; Yin J.; Shen Y.; Liu Y.; Luo J.; Xu Y.; et al. A Dual-Insertion Type Sodium-Ion Full Cell Based on High-Quality Ternary-Metal Prussian Blue Analogs. Adv. Energy Mater. 2018, 8, 1702856. 10.1002/aenm.201702856. [DOI] [Google Scholar]
- Zhang L.; Wei C.; Fu X.; Chen Z.; Yan B.; Sun P. P.; Chang K.; Yang X. Ternary Ni-Based Prussian Blue Analogue with Superior Sodium Storage Performance Induced by Synergistic Effect of Co and Fe. Carbon Energy 2021, 3, 827–839. 10.1002/cey2.142. [DOI] [Google Scholar]
- Ma Y.; Ma Y.; Dreyer S. L.; Wang Q.; Wang K.; Goonetilleke D.; Omar A.; Mikhailova D.; Hahn H.; Breitung B. High-Entropy Metal-Organic Frameworks for Highly Reversible Sodium Storage. Adv. Mater. 2021, 33, e2101342. 10.1002/adma.202101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Hu Y.; Pramudya Y.; Diemant T.; Wang Q.; Goonetilleke D.; Tang Y.; Zhou B.; Hahn H.; Wenzel W.; et al. Resolving the Role of Configurational Entropy in Improving Cycling Performance of Multicomponent Hexacyanoferrate Cathodes for Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2202372. 10.1002/adfm.202202372. [DOI] [Google Scholar]
- Peng J.; Zhang B.; Hua W.; Liang Y.; Zhang W.; Du Y.; Peleckis G.; Indris S.; Gu Q.; Cheng Z. A Disordered Rubik’s Cube-Inspired Framework for Sodium-Ion Batteries with Ultralong Cycle Lifespan. Angew. Chem., Int. Ed. 2023, 62, e202215865. 10.1002/anie.202215865. [DOI] [PubMed] [Google Scholar]
- Zhou A.; Cheng W.; Wang W.; Zhao Q.; Xie J.; Zhang W.; Gao H.; Xue L.; Li J. Hexacyanoferrate-Type Prussian Blue Analogs: Principles and Advances Toward High-Performance Sodium and Potassium Ion Batteries. Adv. Energy Mater. 2021, 11, 2000943. 10.1002/aenm.202000943. [DOI] [Google Scholar]
- Wei C.; Fu X.-Y.; Zhang L.-L.; Liu J.; Sun P.-P.; Gao L.; Chang K.-J.; Yang X.-L. Structural Regulated Nickel Hexacyanoferrate with Superior Sodium Storage Performance by K-Doping. Chem. Eng. J. 2021, 421, 127760. 10.1016/j.cej.2020.127760. [DOI] [Google Scholar]
- Zhao C.; Yao Z.; Zhou D.; Jiang L.; Wang J.; Murzin V.; Lu Y.; Bai X.; Aspuru-Guzik A.; Chen L.; et al. Constructing Na-Ion Cathodes via Alkali-Site Substitution. Adv. Funct. Mater. 2020, 30, 1910840. 10.1002/adfm.201910840. [DOI] [Google Scholar]
- Liao J.-Y.; Hu Q.; Zou B.-K.; Xiang J.-X.; Chen C.-H. The Role of Potassium Ions in Iron Hexacyanoferrate as a Cathode Material for Hybrid Ion Batteries. Electrochim. Acta 2016, 220, 114–121. 10.1016/j.electacta.2016.10.062. [DOI] [Google Scholar]
- Liu Y.; He D.; Han R.; Wei G.; Qiao Y. Nanostructured Potassium and Sodium Ion Incorporated Prussian Blue Frameworks as Cathode Materials for Sodium-Ion Batteries. Chem. Commun. 2017, 53, 5569–5572. 10.1039/C7CC02303K. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Gao Y.; Peng J.; Fan Y.; Zhao L.; Li L.; Xiao Y.; Pang W. K.; Wang J.; Chou S. L. Prussian Blue Analogues with Optimized Crystal Plane Orientation and Low Crystal Defects toward 450 Wh kg–1 Alkali-Ion Batteries. Angew. Chem., Int. Ed. 2023, 62, e202303953. 10.1002/anie.202303953. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Sun Y.; Xie J.; Nie Y.; Xu X.; Tu J.; Shen C.; Jin Y.; Li Y.; Lu Y.; et al. High-Performance Ni/Fe-Codoped Manganese Hexacyanoferrate by Scale-up Synthesis for Practical Na-Ion Batteries. Mater. Today Sustain. 2022, 18, 100113. 10.1016/j.mtsust.2022.100113. [DOI] [Google Scholar]
- Xu Z.; Sun Y.; Xie J.; Nie Y.; Xu X.; Tu J.; Zhang J.; Qiu L.; Zhu T.; Zhao X. Scalable Preparation of Mn/Ni Binary Prussian Blue as Sustainable Cathode for Harsh-Condition-Tolerant Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 13277–13287. 10.1021/acssuschemeng.2c02377. [DOI] [Google Scholar]
- Wang W.; Gang Y.; Hu Z.; Yan Z.; Li W.; Li Y.; Gu Q. F.; Wang Z.; Chou S. L.; Liu H. K.; et al. Reversible Structural Evolution of Sodium-Rich Rhombohedral Prussian Blue for Sodium-Ion Batteries. Nat. Commun. 2020, 11, 980. 10.1038/s41467-020-14444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J.; Shi F. F.; Glossmann T.; Burnett C.; Liu Z. From Laboratory Innovations to Materials Manufacturing for Lithium-Based Batteries. Nat. Energy 2023, 8, 329–339. 10.1038/s41560-023-01221-y. [DOI] [Google Scholar]
- Liu D. Q.; Shadike Z.; Lin R. Q.; Qian K.; Li H.; Li K. K.; Wang S. W.; Yu Q. P.; Liu M.; Ganapathy S.; et al. Review of Recent Development of In Situ/Operando Characterization Techniques for Lithium Battery Research. Adv. Mater. 2019, 31, 1806620. 10.1002/adma.201806620. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Meng X.; Wei Z.; Wang D.; Gao Y.; Wei Y.; Du F.; Chen G. Core/Double-Shell Structured Na3V2(PO4)2F3@C Nanocomposite as the High Power and Long Lifespan Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8 (46), 31709–31715. 10.1021/acsami.6b11372. [DOI] [PubMed] [Google Scholar]
- Li L.; Liu X.; Tang L.; Liu H.; Wang Y.-G. Improved electrochemical performance of high voltage cathode Na3V2(PO4)2F3 for Na-ion batteries through potassium doping. J. Alloys Compd. 2019, 790, 203–211. 10.1016/j.jallcom.2019.03.127. [DOI] [Google Scholar]
- Su D.; Cortie M.; Wang G. Fabrication of N-doped Graphene-Carbon Nanotube Hybrids from Prussian Blue for Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602014 10.1002/aenm.201602014. [DOI] [Google Scholar]
- Li X.; Jiang S.; Li S.; Yao J.; Zhao Y.; Bashir T.; Zhou S.; Yang S.; Li W.; Zhu W.; Liu T.; Zhao J.; Gao L. Overcoming the rate-determining kinetics of the Na3V2O2(PO4)2F cathode for ultrafast sodium storage by heterostructured dual-carbon decoration. J. Mater. Chem. A 2021, 9, 11827–11838. 10.1039/D1TA02250D. [DOI] [Google Scholar]
- Qiao S.; Zhou Q.; Ma M.; Liu H. K.; Dou S. X.; Chong S. Advanced Anode Materials for Rechargeable Sodium-Ion Batteries. ACS Nano 2023, 17 (12), 11220–11252. 10.1021/acsnano.3c02892. [DOI] [PubMed] [Google Scholar]