ABSTRACT
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by a lack of structural or biochemical abnormalities. The current diagnosis of IBS is based on the Rome IV criteria, and it is recommended to approach IBS patients using a multidimensional clinical profile (MDCP). The pathophysiology of IBS is multifactorial and involves motility disorders, genetic factors, immune responses, visceral hypersensitivity, brain–gut dysregulation, and altered intestinal microbiota. The management of IBS includes both nonpharmacologic and pharmacologic therapies. Nonpharmacologic therapy options include physical activity, low fermentable oligosaccharides, disaccharides, monosaccharides, and polyol diet, as well as cognitive behavioral therapy. Pharmacologic therapy options include probiotics, antidepressants, antispasmodics, and new agents. In clinical practice, a multidisciplinary strategy, including nonpharmacologic or/and pharmacologic treatment for IBS, is emphasized. Therefore, clinicians should carefully consider the underlying pathophysiology before selecting an appropriate therapeutic option for the treatment of IBS. In other words, individualized treatment plans are necessary for managing IBS.
KEYWORDS: Irritable bowel syndrome, Management, Pathophysiology
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by symptoms such as abdominal pain or discomfort and changes in bowel habits, including stool consistency or frequency. These symptoms occur without any detectable structural or biochemical abnormalities. IBS is more commonly seen in females and younger individuals. A recent meta-analysis report indicated that the global prevalence of IBS was 9.2% based on the Rome III criteria and 3.8% based on the Rome IV criteria [1]. Interestingly, a recent epidemiological study from Taiwan showed a decreasing trend in the incidence and prevalence of IBS from 2012 to 2018 [2]. However, it is important to note that these data were obtained from the National Health Insurance Research Database using the ICD-9 coding, rather than based on the Rome criteria, raising doubts about the accurate diagnosis of IBS.
IBS has a significant impact on health-related quality of life and is often accompanied by psychological comorbidities such as anxiety and depression [3-6]. In Taiwan, female IBS patients with a history of sexual abuse had a higher prevalence of anxiety, depression, and sleep disturbances [7]. Furthermore, IBS results in substantial direct and indirect costs worldwide [8]. IBS patients often visit doctors more frequently and undergo extensive diagnostic investigations [9,10]. They also experience increased absenteeism and impaired work productivity [11,12]. Therefore, it is crucial to enhance our understanding of IBS, as this can help reduce unnecessary medical examinations and provide better care for patients.
PATHOPHYSIOLOGY
Biopsychosocial model
Drossman DA first introduced a biopsychosocial model to enhance our understanding of this condition [13]. This model emphasizes the interconnections among biology, cognition, behavior, and the environment. Figure 1 illustrates how these factors interact and contribute to the development of IBS.
Figure 1.
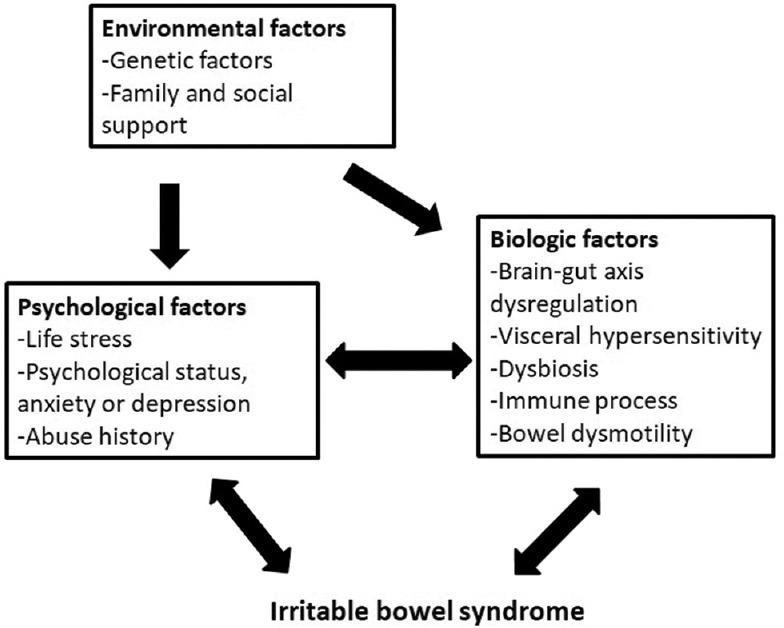
The biopsychosocial model of irritable bowel syndrome
Visceral hypersensitivity and brain–gut axis dysregulation
IBS patients exhibit lower pain thresholds in their colon compared to healthy controls, as evidenced by several studies [14-17]. These studies have demonstrated that IBS patients have lower sensory thresholds during rectal balloon distension. In addition, a meta-analysis study revealed that IBS patients display an increased activation of the brain regions associated with emotional arousal and endogenous pain modulation in response to rectal balloon stimulation, in comparison to healthy controls [17]. Therefore, the brain’s processing of noxious stimuli from the gut and the bidirectional communication between the brain and gut are crucial factors in the pathophysiology of IBS. Furthermore, several studies have indicated a high prevalence of comorbid anxiety and depression disorders among individuals with IBS [3,18-20].
Gastrointestinal microbiota
Postinfectious IBS has been considered one of the etiologies of IBS. As a result, altered fecal microbiota has been proposed as one possible cause of IBS [21-24]. In a systemic review, Pittayanon et al. showed that IBS patients had increased an abundance of family Enterobacteriaceae (phylum Proteobacteria), family Lactobacillaceae, and genus Bacteroides, but a decreased abundance of uncultured Clostridiales I, genus Faecalibacterium (including Faecalibacterium prausnitzii), and genus Bifidobacterium [25].
Bowel dysmotility
Altered bowel transit is a distinctive feature observed in different subtypes of IBS. For example, diarrhea-predominant IBS (D-IBS) is characterized by rapid bowel transit, whereas constipation-predominant IBS (C-IBS) is associated with sluggish bowel transit [26]. Serotonin (5-HT) in the bowel plays a multifaceted role in regulating motor and sensory functions through the enteric nervous system, thereby influencing intestinal motility [27,28]. Plasma levels of 5-HT vary between D-IBS and C-IBS, with higher levels detected in D-IBS and lower levels observed in C-IBS [29,30].
Immune process
IBS patients exhibit distinct immune system characteristics compared to healthy controls, including cytokine imbalances, immune cell activation, and increased gastrointestinal membrane permeability. A meta-analysis study revealed that IBS patients had elevated levels of the pro-inflammatory cytokine tumor necrosis factor-α, while the levels of the anti-inflammatory cytokine interleukin (IL)-10 did not show significant differences [31]. Moreover, IBS patients demonstrated an increased presence of inflammatory cells in the intestinal mucosa, indicating a potential state of low-grade inflammation. In addition, IBS patients exhibited heightened gastrointestinal membrane permeability. Colonic mucosal mast cell counts and increased membrane permeability were found to be correlated with abdominal pain and disease severity [32].
Genetic factors
IBS may have a genetic component, as indicated by twin studies that have demonstrated a higher concordance rate in monozygotic twins compared to dizygotic twins [33-35]. Among the genes investigated, the serotonin transporter gene polymorphism (5-HTTLPR) has been one of the most extensively studied in relation to IBS [36-39]. However, the findings of these studies have yielded conflicting results [37].
DIAGNOSIS
The current diagnosis of IBS is based on the Rome IV criteria [40,41]. According to these criteria, patients must experience recurrent abdominal pain, occurring at least once per week for the past 3 months, with symptom onset at least 6 months before diagnosis. The pain should be associated with two or more of the following: changes in defecation, stool frequency, or stool appearance (form). It is also important to employ a multiple dimensional clinical profile (MDCP) approach when treating IBS patients [42]. Before diagnosing IBS, it is crucial to thoroughly investigate warning signs [43]. These include the onset of symptoms after the age of 50, unintentional weight loss, overt gastrointestinal bleeding, recent changes in bowel habits, palpable abdominal mass or lymphadenopathy, and a family history of colon cancer or inflammatory bowel disease.
Based on the predominant bowel habits during days with abnormal bowel movements, IBS can be classified into four subtypes: D-IBS, C-IBS, mixed (M-IBS), and unclassified (U-IBS). The diagnostic criteria for IBS according to the Rome III and Rome IV are provided in Table 1 [44]. In the Rome IV, the term “abdominal discomfort” has been omitted from the definition due to its imprecise nature. Abdominal pain should occur at least once per week during the past 3 months. The phrase “improvement with” defecation has been revised to “related to” defecation as some patients may experience an increase or maintenance of pain. In addition, the term “onset” has been removed from the associated changes in stool frequency and appearance.
Table 1.
The Rome III and Rome IV diagnostic criteria for irritable bowel syndrome
| Rome III Recurrent abdominal pain or discomfort at least 3 days/month associated with two or more of the following criteria | Rome IV Recurrent abdominal pain at least once/week associated with two or more of the following criteria |
|---|---|
| Improvement after defecation | Related with defecation |
| Onset associated with a change in the frequency of stool | Associated with a change in the frequency of stool |
| Onset associated with a change in the form (appearance) of stool | Associated with a change in the form (appearance) of stool |
Symptoms onset at least 3 months in the past 6 months
The sensitivity and specificity of the Rome IV criteria are 62.7% and 97.1%, respectively, while those of the Rome III criteria are 73.1% and 93.1%, respectively [45]. The estimated prevalence of IBS is significantly lower when using the Rome IV criteria (5.7%) compared to the Rome III criteria (10.7%) [45]. This aids in identifying true IBS patients and avoiding overdiagnosis.
MANAGEMENT
Table 2 presents the treatment strategies for IBS.
Table 2.
Treatment strategies for irritable bowel syndrome
| Nonpharmacologic | Pharmacologic |
|---|---|
| Patient reassurance and education | First-line Antidiarrheals (D-IBS) |
| Stress management | Laxatives (C-IBS) |
| Low-FODMAP diet probiotics | Antispasmodics |
| Physical activity | Antidepressants (TCAs and SSRIs) Second-line new agents |
| Cognitive behavior therapy | Linaclotide (C-IBS) |
| Rifaximin (D-IBS) | |
| Eluxadoline (D-IBS) | |
| Asimadoline (D-IBS) |
FODMAP: Fermentable oligosaccharides, disaccharides, monosaccharides, and polyol, D-IBS: Diarrhea-predominant irritable bowel syndrome, C-IBS: Constipation-predominant irritable bowel syndrome, TCAs: Tricyclic antidepressants, SSRIs: Selective serotonin reuptake inhibitors
Dietary and lifestyle advice
Patient reassurance and stress reduction
IBS is a benign disease without a reduction of life expectancy or any correlation with the development of organic diseases [46]. Therefore, patient education and reassurance are warranted, and these will ease their anxiety. A positive patient–physician relationship can improve symptom control [46]. Stress reduction also has been shown beneficial effects on IBS symptoms [47,48].
Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyol diet
The low fermentable oligosaccharides, disaccharides, monosaccharides, and polyol (FODMAP) diet involve reducing the consumption of poorly absorbed short-chain carbohydrates and low gas-producing foods. Unabsorbed fructose, polyols, and lactose can increase small intestinal water content, while indigestible fructans and galacto-oligosaccharides may lead to microbial fermentation in the colon, triggering symptoms in IBS patients [49]. The low FODMAP diet is a dietary therapy that should be supervised by a nutritionist. Halmos et al. demonstrated that a low FODMAP diet can improve bloating, abdominal pain, and flatulence in IBS patients [50]. In a recent meta-analysis of 13 randomized controlled trials (RCTs), a low FODMAP diet was found to be the most effective dietary therapy for reducing abdominal pain severity and abdominal bloating in IBS patients [49]. Ideally, the low FODMAP diet should be followed for 4–6 weeks, as long-term restrictions may increase the risk of inadequate nutrition.
Probiotics
Probiotics have been shown to have a beneficial effect on global symptoms, abdominal pain, bloating, and flatulence in IBS patients [51]. However, the specific species and strains of probiotics that are most effective for treating IBS remain uncertain.
Physical activity
In 2011, Johannesson et al. reported that physical activity can improve symptoms of IBS [52]. IBS patients experienced relief from abdominal distress and extracolonic symptoms after engaging in 12 weeks of physical activity. Their subsequent study revealed the long-term positive effects of physical activity on IBS symptoms [53].
Pharmacological treatments
Antidiarrheals and laxatives
Basically, we can choose drugs according to the predominant symptoms of IBS. For instance, antidiarrheals including loperamide and bile acid sequestrants such as cholestyramine were prescribed for D-IBS, while laxatives were used for C-IBS [54]. Treatment goals targeted to decrease the symptoms and improve the quality of life. Therefore, these medications could be temporarily stopped and should be evaluated if patients still needed them.
Antispasmodics
Antispasmodics are smooth muscle relaxants that act on the gastrointestinal tract, including antimuscarinic, anticholinergic agents, and calcium channel blockers. They can improve symptoms of IBS, particularly relieving cramping distress, due to their spasmolytic effects. Ford et al. demonstrated that antispasmodics, specifically otilonium and hyoscine, can improve IBS symptoms [55]. In Taiwan, Chang et al. revealed that otilonium is as effective as mebeverine in improving IBS symptoms, including abdominal pain, flatulence, and bloating [56].
Tricyclic antidepressants and selective serotonin reuptake inhibitors
Due to the pathophysiology of IBS mentioned earlier, antidepressants such as tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been shown to have pain-modulating effects on both the central and peripheral nervous systems [57,58]. In a systematic review and meta-analysis conducted by Ford et al., it was found that both TCAs and SSRIs can alleviate IBS symptoms, with an estimated number needed to treat of 4.5 and 5, respectively [59]. The most commonly reported adverse effects of these antidepressants are drowsiness and dry mouth.
Second-line new agents
Rifaximin is a nonsystemic antibiotic that possesses gut-modulatory and anti-inflammatory effects [60]. In May 2015, it was approved by the US Food and Drug Administration for the treatment of D-IBS. Several studies have demonstrated its efficacy and safety profiles [61-64]. Linaclotide, a guanylate cyclase-C agonist, acts as a secretagogue in the gastrointestinal tract. Some studies have shown its beneficial effects on C-IBS [65-68]. Eluxadoline and asimadoline, both targeting opioid receptors, are utilized for the treatment of D-IBS [69-73]. However, it is important to note that these newer agents are currently not available in Taiwan.
Psychological intervention
Cognitive behavioral therapy (CBT) is a psychological therapy that incorporates various components, including psychoeducation, relaxation strategies, cognitive restructuring, problem-solving skills, and exposure techniques [74]. A meta-analysis and systematic review of 20 RCTs demonstrated that CBT can improve gastrointestinal symptoms in both short-term and long-term for individuals with IBS [75]. However, the widespread use of CBT is limited due to the scarcity of well-trained gastrointestinal psychology therapists. In Taiwan, Internet-delivered CBT for young female IBS patients showed a positive impact on reducing anxiety and depression, although it did not have a significant effect on IBS symptoms [76].
CONCLUSIONS
IBS is a complex disease with no specific etiology. The possible pathophysiological mechanisms include visceral hypersensitivity, dysregulation of the brain–gut axis, dysbiosis of intestinal microbiota, bowel dysmotility, immune responses, and genetic factors. The biopsychosocial model provides a comprehensive framework for understanding this disease. In clinical practice, a multidisciplinary approach that incorporates nonpharmacological and/or pharmacological treatments is emphasized for managing IBS. Therefore, health-care providers should carefully consider the underlying pathophysiology before selecting an appropriate therapeutic option. In other words, individualized treatment plans are necessary for effectively managing IBS.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:908–17. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 2.Lai YT, Chen CY, Bair MJ. Epidemiology, clinical features, and prescribing patterns of irritable bowel syndrome in Taiwan. Front Pharmacol. 2021;12:788795. doi: 10.3389/fphar.2021.788795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:132–43. doi: 10.1111/apt.15325. [DOI] [PubMed] [Google Scholar]
- 4.Trindade IA, Melchior C, Törnblom H, Simrén M. Quality of life in irritable bowel syndrome: Exploring mediating factors through structural equation modelling. J Psychosom Res. 2022;159:110809. doi: 10.1016/j.jpsychores.2022.110809. [DOI] [PubMed] [Google Scholar]
- 5.Cassar GE, Youssef GJ, Knowles S, Moulding R, Austin DW. Health-related quality of life in irritable bowel syndrome: A systematic review and meta-analysis. Gastroenterol Nurs. 2020;43:E102–22. doi: 10.1097/SGA.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 6.Schauer B, Grabe HJ, Ittermann T, Lerch MM, Weiss FU, Mönnikes H, et al. Irritable bowel syndrome, mental health, and quality of life: Data from a population-based survey in Germany (SHIP-Trend-0) Neurogastroenterol Motil. 2019;31:e13511. doi: 10.1111/nmo.13511. [DOI] [PubMed] [Google Scholar]
- 7.Lee HF, Liu PY, Wang YP, Tsai CF, Chang FY, Lu CL. Sexual abuse is associated with an abnormal psychological profile and sleep difficulty in patients with irritable bowel syndrome in Taiwan. J Neurogastroenterol Motil. 2018;24:79–86. doi: 10.5056/jnm17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm. 2013;19:755–64. doi: 10.18553/jmcp.2013.19.9.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canavan C, West J, Card T. Review article: The economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023–34. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 10.Schmulson MJ. Limited diagnostic testing can decrease the direct economic impact of irritable bowel syndrome. Rev Med Chil. 2008;136:1398–405. doi: 10.4067/s0034-98872008001100005. [DOI] [PubMed] [Google Scholar]
- 11.Frändemark Å, Törnblom H, Jakobsson S, Simrén M. Work productivity and activity impairment in irritable bowel syndrome (IBS): A multifaceted problem. Am J Gastroenterol. 2018;113:1540–9. doi: 10.1038/s41395-018-0262-x. [DOI] [PubMed] [Google Scholar]
- 12.Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11:S17–26. [PubMed] [Google Scholar]
- 13.Drossman DA. Presidential address: Gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998;60:258–67. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–92. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 16.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–12. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636–43. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 18.Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–60. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- 19.Sibelli A, Chalder T, Everitt H, Workman P, Windgassen S, Moss-Morris R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065–80. doi: 10.1017/S0033291716001987. [DOI] [PubMed] [Google Scholar]
- 20.Midenfjord I, Polster A, Sjövall H, Törnblom H, Simrén M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil. 2019;31:e13619. doi: 10.1111/nmo.13619. [DOI] [PubMed] [Google Scholar]
- 21.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome –A meta-analysis. Am J Gastroenterol. 2006;101:1894–9. doi: 10.1111/j.1572-0241.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: Post-infectious irritable bowel syndrome after travellers'diarrhoea. Aliment Pharmacol Ther. 2015;41:1029–37. doi: 10.1111/apt.13199. [DOI] [PubMed] [Google Scholar]
- 23.Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: Role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil. 2012;18:258–68. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundin J, Rangel I, Fuentes S, Heikamp-de Jong I, Hultgren-Hörnquist E, de Vos WM, et al. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharmacol Ther. 2015;41:342–51. doi: 10.1111/apt.13055. [DOI] [PubMed] [Google Scholar]
- 25.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut microbiota in patients with irritable bowel syndrome-a systematic review. Gastroenterology. 2019;157:97–108. doi: 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120–30. doi: 10.5056/jnm.2010.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Raskov H, Burcharth J, Pommergaard HC, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365–83. doi: 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–70. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black CJ, Ford AC. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473–86. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 31.Bashashati M, Rezaei N, Shafieyoun A, McKernan DP, Chang L, Öhman L, et al. Cytokine imbalance in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036–48. doi: 10.1111/nmo.12358. [DOI] [PubMed] [Google Scholar]
- 32.Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–52. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 33.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: Heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 34.Saito YA, Petersen GM, Larson JJ, Atkinson EJ, Fridley BL, de Andrade M, et al. Familial aggregation of irritable bowel syndrome: A family case-control study. Am J Gastroenterol. 2010;105:833–41. doi: 10.1038/ajg.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–7. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 36.Geeraerts B, van Oudenhove L, Tack J. Serotonin transporter gene polymorphisms in irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:957–9. doi: 10.1111/j.1365-2982.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang ZF, Duan ZJ, Wang LX, Yang D, Zhao G, Zhang L. The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: A meta-analysis of 25 studies. BMC Gastroenterol. 2014;14:23. doi: 10.1186/1471-230X-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grzesiak M, Beszłej JA, Waszczuk E, Szechiński M, Szewczuk-Bogusławska M, Frydecka D, et al. Serotonin-related gene variants in patients with irritable bowel syndrome and depressive or anxiety disorders. Gastroenterol Res Pract. 2017;2017:4290430. doi: 10.1155/2017/4290430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghoshal UC, Singh R. Pathogenesis of irritable bowel syndrome: Is it really in the gene?J Neurogastroenterol Motil. 2014;20:284–6. doi: 10.5056/jnm14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.032. S0016-5085(16)00223-7. [DOI] [PubMed] [Google Scholar]
- 41.Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.014. S0016-5085(16)00180-3. [DOI] [PubMed] [Google Scholar]
- 42.Lucak S, Chang L, Halpert A, Harris LA. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Therap Adv Gastroenterol. 2017;10:253–75. doi: 10.1177/1756283X16663396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99. doi: 10.3390/jcm6110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drossman DA. Functional gastrointestinal disorders: What's new for Rome IV? Lancet Gastroenterol Hepatol. 2016;1:6–8. doi: 10.1016/S2468-1253(16)30022-X. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead WE, Palsson OS, Simrén M. Irritable bowel syndrome: What do the new Rome IV diagnostic guidelines mean for patient management?Expert Rev Gastroenterol Hepatol. 2017;11:281–3. doi: 10.1080/17474124.2017.1292130. [DOI] [PubMed] [Google Scholar]
- 46.Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: Long-term prognosis and the physician-patient interaction. Ann Intern Med. 1995;122:107–12. doi: 10.7326/0003-4819-122-2-199501150-00005. [DOI] [PubMed] [Google Scholar]
- 47.Exarchopoulou K, Papageorgiou A, Bacopoulou F, Malisiova EK, Vlachakis D, Chrousos GP, et al. A biofeedback-assisted stress management program for patients with irritable bowel syndrome: A randomised controlled trial. EMBnet J. 2021;26:e980. doi: 10.14806/ej.26.1.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw G, Srivastava ED, Sadlier M, Swann P, James JY, Rhodes J. Stress management for irritable bowel syndrome: A controlled trial. Digestion. 1991;50:36–42. doi: 10.1159/000200738. [DOI] [PubMed] [Google Scholar]
- 49.Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut. 2022;71:1117–26. doi: 10.1136/gutjnl-2021-325214. [DOI] [PubMed] [Google Scholar]
- 50.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. e5. [DOI] [PubMed] [Google Scholar]
- 51.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–61. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 52.Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. Am J Gastroenterol. 2011;106:915–22. doi: 10.1038/ajg.2010.480. [DOI] [PubMed] [Google Scholar]
- 53.Johannesson E, Ringström G, Abrahamsson H, Sadik R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J Gastroenterol. 2015;21:600–8. doi: 10.3748/wjg.v21.i2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cashman MD, Martin DK, Dhillon S, Puli SR. Irritable bowel syndrome: A clinical review. Curr Rheumatol Rev. 2016;12:13–26. doi: 10.2174/1573397112666151231110521. [DOI] [PubMed] [Google Scholar]
- 55.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: Systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang FY, Lu CL, Luo JC, Chen TS, Chen MJ, Chang HJ. The evaluation of otilonium bromide treatment in Asian patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:402–10. doi: 10.5056/jnm.2011.17.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–7. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol. 2003;284:G130–7. doi: 10.1152/ajpgi.00266.2002. [DOI] [PubMed] [Google Scholar]
- 59.Ford AC, Lacy BE, Harris LA, Quigley EM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21–39. doi: 10.1038/s41395-018-0222-5. [DOI] [PubMed] [Google Scholar]
- 60.Xu D, Gao J, Gillilland M, 3rd, Wu X, Song I, Kao JY, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146:484–96. doi: 10.1053/j.gastro.2013.10.026. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fumi AL, Trexler K. Rifaximin treatment for symptoms of irritable bowel syndrome. Ann Pharmacother. 2008;42:408–12. doi: 10.1345/aph.1K345. [DOI] [PubMed] [Google Scholar]
- 62.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: A systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 63.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 64.Schey R, Rao SS. The role of rifaximin therapy in patients with irritable bowel syndrome without constipation. Expert Rev Gastroenterol Hepatol. 2011;5:461–4. doi: 10.1586/egh.11.50. [DOI] [PubMed] [Google Scholar]
- 65.Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–12. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- 66.Lacy BE, Levenick JM, Crowell MD. Linaclotide: A novel therapy for chronic constipation and constipation-predominant irritable bowel syndrome. Gastroenterol Hepatol (N Y) 2012;8:653–60. [PMC free article] [PubMed] [Google Scholar]
- 67.Macdougall JE, Johnston JM, Lavins BJ, Nelson LM, Williams VS, Carson RT, et al. An evaluation of the FDA responder endpoint for IBS-C clinical trials: Analysis of data from linaclotide phase 3 clinical trials. Neurogastroenterol Motil. 2013;25:481–6. doi: 10.1111/nmo.12089. [DOI] [PubMed] [Google Scholar]
- 68.Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714–24. doi: 10.1038/ajg.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242–53. doi: 10.1056/NEJMoa1505180. [DOI] [PubMed] [Google Scholar]
- 70.Adler AI. NICE guidance on eluxadoline for treating irritable bowel syndrome with diarrhoea. Lancet Gastroenterol Hepatol. 2017;2:779–80. doi: 10.1016/S2468-1253(17)30267-4. [DOI] [PubMed] [Google Scholar]
- 71.Cash BD, Lacy BE, Schoenfeld PS, Dove LS, Covington PS. Safety of eluxadoline in patients with irritable bowel syndrome with diarrhea. Am J Gastroenterol. 2017;112:365–74. doi: 10.1038/ajg.2016.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, et al. Clinical trial: Asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239–49. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 73.Mangel AW, Hicks GA. Asimadoline and its potential for the treatment of diarrhea-predominant irritable bowel syndrome: A review. Clin Exp Gastroenterol. 2012;5:1–10. doi: 10.2147/CEG.S23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: Current insights. Psychol Res Behav Manag. 2017;10:231–7. doi: 10.2147/PRBM.S120817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:937–47. doi: 10.1016/j.cgh.2015.11.020. e4. [DOI] [PubMed] [Google Scholar]
- 76.Lee TY, Hsieh TC, Sung HC, Chen WL. Internet-delivered cognitive behavior therapy for young Taiwanese female nursing students with irritable bowel syndrome-a cluster randomized controlled trial. Int J Environ Res Public Health. 2019;16:708. doi: 10.3390/ijerph16050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


