ABSTRACT
Objectives:
Alopecia is a soft but meaningful complaint affecting women’s physical and psychological health. Female alopecia (FA) has diverse etiologies. Nonetheless, FA is stereotyped as female pattern hair loss, also known as female androgenetic alopecia, and has not been thoroughly investigated. This study aimed to identify the etiologies of FA at a tertiary medical center in Eastern Taiwan.
Materials and Methods:
This retrospective study enrolled female patients with hair loss who visited the dermatology department of (blinded information). A complete history taking was obtained, including the onset and duration of alopecia, menstruation, gynecologic diseases, psychological stress, underlying diseases, vaccination, and dietary habits, etc., Blood tests were performed, including hemoglobin (Hb), ferritin, Zn, autoimmune and thyroid profiles, etc., Iron deficiency (ID) was defined as serum ferritin level <60 ng/mL. The hair condition, ferritin, and Hb levels were monitored every 3 months after supplementation.
Results:
A total of 155 patients were recruited. The etiologies of FA were diverse; the top five etiologies were nutrient deficiencies (83.9%), autoimmune (14.8%) and thyroid (7.7%) diseases, psychological stress (12.3%), and coronavirus disease 2019 (COVID-19) vaccination (6.5%). ID accounted for 70.3% of cases. The disease duration was an important prognostic factor for the improvement of serum ferritin. Patients with subjective improvement of hair regrowth also had more increase of ferritin levels after iron supplementation. The corresponding ferritin level for female anemia (Hb: 12.0 g/dL) was 5.1 ng/mL, lower than the adequate level for hair growth (40–60 ng/mL), the corresponding Hb level of which was 13.1–13.8 g/dL.
Conclusion:
The causes of FA varied, including nutrient deficiencies, autoimmune diseases, psychological stress, thyroid diseases, and COVID-19 vaccination, etc., Therefore, a complete survey before treatment is essential. Seventy percentage of FA cases were ID-FA. We suggest to redefine the serum ferritin level ≥60 ng/mL, with the corresponding Hb ≥13.0 g/dL as the normal range for early diagnosis. Initiation of iron supplementation within 6 months would result in a better prognosis.
KEYWORDS: Alopecia, Female alopecia, Iron deficiency, Iron deficiency-related female alopecia
INTRODUCTION
Alopecia is a common complaint among women visiting dermatological clinics and often affects their quality of life [1]. Alopecia was regarded as a nonspecific complaint and was previously not included in the criteria for systemic lupus erythematosus (SLE). In 2012, nonscarring alopecia was incorporated into the Systemic Lupus International Collaborating Clinics criteria [2]. Such evolution illustrates that alopecia is a “soft but meaningful” complaint. It has become a serious physical and psychological health issue with diverse etiologies.
The hair growth cycle comprises of three major phases – namely, anagen (growth), catagen (involution), and telogen (resting phase before shedding). Approximately 85%–90% of hair follicles are in the anagen phase. Each phase of the hair growth cycle is regulated by multiple factors. Hair loss may result from the shortening of the anagen phase, an early shift from the anagen phase to the telogen phase, or the premature detachment of telogen hair [3]. Androgenetic alopecia (AGA) is a well-known type of alopecia characterized by progressive hair thinning that develops under the influence of dihydrotestosterone in genetically predisposed patients. Hair thinning at the vertex region and anterior hairline recession occur in men, whereas women present with diffuse hair thinning. Nonetheless, the role of androgens in the pathogenesis of hair loss in women remains unclear, and the oversimplified term “female pattern hair loss (FPHL)” has accordingly been proposed for female AGA. FPHL is defined as gradual loss of terminal hair and follicular miniaturization to vellus hair fibers on the scalp in a characteristic distribution [4]. Most patients with female alopecia (FA) are not thoroughly surveyed or treated effectively and are oversimplified as FPHL, which is stereotyped as female AGA, leading to the patients’ depression and frustration. Therefore, we desired to conduct an in-depth investigation on the etiologies of FA.
FA is triggered by various etiologies, including hormonal dysregulation, thyroid disorders, connective tissue diseases, malignancy, infection, environmental stress, drugs, and deficiencies in nutrients such as iron, zinc, selenium, amino acids, proteins, fatty acids, Vitamin B3, Vitamin D, Vitamin A, Vitamin E, biotin, and folic acid [5-8]. FA is usually clinically diagnosed based on history, laboratory tests, and physical examination. While the mean serum ferritin levels have been reported by previous studies to be significantly lower in patients with hair loss than in healthy individuals [9,10], no observational study has investigated the factors associated with the therapeutic response following iron supplementation. Moreover, the relationship between hemoglobin (Hb) and serum ferritin levels has not been elucidated.
Therefore, the present study aimed to identify the diverse etiologies of FA at a tertiary medical center in Eastern Taiwan, analyze the factors associated with iron deficiency-related alopecia (ID-FA) in women, and evaluate the response to iron supplementation with respect to serum ferritin levels and hair growth.
MATERIALS AND METHODS
Research ethics and study design
This study was conducted in accordance with the principles embodied in the Declaration of Helsinki and was approved by the Institutional Review Board of (blinded information) (approval number: IRB110-122-B).
This retrospective study was conducted from January 1, 2017, to August 31, 2022, and enrolled 155 female patients with a chief complaint of hair loss who visited the dermatology outpatient department of (blinded information). A complete history was obtained, including the onset and duration of alopecia; whether alopecia was acute (<6 months) or chronic (>6 months); menstruation (regularity, cycle length and frequency, and amount of bleeding in menstruation); gravidity and parity; gynecologic diseases (e.g. endometriosis, uterine myoma, and polycystic ovary syndrome [PCOS]); psychological stress, defined by the World Health Organization and the American Psychological Association as a state of worry or mental tension due to a difficult situation, which disturbs the day-to-day functioning; dietary habits (vegetarian/meat eaters); a family history of alopecia or other hereditary diseases; autoimmune diseases (e.g., alopecia areata [AA], sicca syndrome, vitiligo, SLE, rheumatoid arthritis [RA]); thyroid diseases (e.g., hyperthyroidism, hypothyroidism, Graves’ disease, and Hashimoto’s thyroiditis); gastrointestinal (GI) diseases (e.g., peptic ulcer, gastroesophageal reflux disease [GERD], and lower GI bleeding); solid organ or hematologic malignancy; and metabolic syndrome, defined as having three or more of the following factors: systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, fasting blood glucose ≥110 mg/dL, fasting blood triglyceride ≥150 mg/dL, fasting blood high-density lipoprotein <50 mg/dL, and waist circumference >35 inches [11]. During the coronavirus disease 2019 (COVID-19) pandemic, alopecia related to COVID-19 infection or vaccination have appeared. Alharbi reported that two-thirds of participants receiving COVID-19 vaccines experienced postvaccination hair falls [12], with mRNA vaccines from Pfizer and Moderna being commonly reported [13,14]. Therefore, the patients’ history of vaccination was documented in the present study.
COVID-19 infection or vaccination and psychological stress-related alopecia are due to telogen effluvium with a positive pull test, which usually occurs 3 months after the event that causes hair loss, and lasts up to 6 months [15]. Therefore, we defined these etiologies, appearing within 6 months before the onset of hair loss as associated etiologies of alopecia. Besides, we defined those underlying diseases reported in previous literatures, including nutrient deficiencies, autoimmune diseases, thyroid diseases, malignancy, metabolic syndrome, and hereditary diseases, which appeared before the onset of alopecia and were persistent during the clinical course of alopecia as associated endogenous etiologies [7,16-19].
Blood tests were performed, including complete and differential blood cell counts, serum ferritin and zinc levels, thyroid profiles, antinuclear antibodies, and complement components 3 and 4. ID was defined as serum ferritin level <60 ng/mL [20]. Iron supplementation with ferric hydroxide polymaltose complex (100 mg/tablet) was applied. In addition, zinc gluconate (78 mg/tablet) was administered when the serum zinc level was <700 μg/L (normal serum zinc level in adults: 700–2500 μg/L [21]). The hair condition and serum ferritin, zinc, and Hb levels were monitored every 3 months after supplementation.
Statistical analysis
Data were analyzed using the Stata version 16.0 (StataCorp, College Station, Texas, USA) and SPSS Statistics for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). The continuous and categorical variables were expressed as mean ± standard deviation and count (percentage), respectively. The mean difference in continuous variables was analyzed using an independent t-test, whereas the association among categorical variables was examined using the Chi-squared test or Fisher’s exact test. Logistic regression was used to evaluate the risk factors associated with improvement in serum ferritin levels. Linear regression was applied to assess the association between the duration of ID-FA and difference in serum ferritin levels and between the serum ferritin and Hb levels. Statistical significance was set at a two-sided P < 0.05.
RESULTS
Diverse etiologies of FA
A total of 155 female patients with alopecia were recruited. The etiologies of FA were diverse and included nutrient deficiencies (ID, zinc deficiency, and both ID and zinc deficiency), autoimmune diseases, psychological stress, thyroid diseases, COVID-19 vaccination or infection, malignancy, metabolic syndrome, and hereditary diseases [Table 1]. ID was the most prevalent condition, accounting for 70.3% of cases. Furthermore, 39.4% of patients had zinc deficiency, whereas 25.8% of patients presented with concurrent ID and zinc deficiency. Underlying autoimmune diseases, including AA (9%), sicca syndrome (3.9%), vitiligo (1.9%), RA (0.6%), and SLE (0.6%), also played an important role in FA, accounting for 14.8% of cases. Psychological stress-related alopecia was also identified, accounting for 12.3% of cases. Thyroid diseases such as hyperthyroidism and hypothyroidism were important underlying diseases in 7.7% of patients. COVID-19 vaccination (7 cases of mRNA-1273 [Moderna] and 3 cases of BNT162b2 [Pfizer–BioNTech]) represented a newly identified trigger factor for alopecia, accounting for 6.5%. COVID-19 infection accounted for 3.2%. Other etiologies were malignancy (5.2%), metabolic syndrome (4.5%), and hereditary diseases (thalassemia and G6PD deficiency) (1.3%).
Table 1.
Summary of etiological investigation of female alopecia (n=155)
| Disease category | n (%) |
|---|---|
| Nutrient deficiency | 130 (83.9) |
| ID | 109 (70.3) |
| Zinc deficiency | 61 (39.4) |
| Iron and zinc deficiency | 40 (25.8) |
| Autoimmune disease | 23 (14.8) |
| AA | 14 (9.0) |
| Sicca syndrome | 6 (3.9) |
| Vitiligo | 3 (1.9) |
| RA | 1 (0.6) |
| SLE | 1 (0.6) |
| Psychological stress | 19 (12.3) |
| Thyroid disease | 12 (7.7) |
| Hypothyroidism | 8 (5.2) |
| Hyperthyroidism | 4 (2.6) |
| COVID-19 vaccination | 10 (6.5) |
| Malignancy | 8 (5.2) |
| Metabolic syndrome | 7 (4.5) |
| COVID-19 infection | 5 (3.2) |
| Hereditary disease (thalassemia, G6PD deficiency) | 2 (1.3) |
AA: Alopecia areata, RA: Rheumatoid arthritis, SLE: Systemic lupus erythematosus, G6PD: Glucose-6-phosphate dehydrogenase, ID: Iron deficiency, COVID-19: Coronavirus disease 2019
Iron deficiency-associated underlying diseases: Obstetric and gynecologic and gastrointestinal diseases were the most prevalent underlying causes of iron deficiency
In our study, the majority of women with ID-FA were premenopausal, with a mean age of 34.4 (7–77) years old. Women were quite alert to hair loss and two-thirds of patients with ID-FA visited the dermatology outpatient department within 6 months after alopecia onset [Table 2]. Underlying causes in 44.1% of patients with ID-FA were obstetric and gynecologic (OBS/GYN) diseases, GI diseases, and dietary restrictions. Among them, OBS/GYN diseases (e.g., hypermenorrhea, pregnancy, and irregular menstruation) and GI diseases (e.g., GERD, lower GI bleeding, and peptic ulcers) were the most frequent causes of blood loss. Hypermenorrhea occurred in 10.1% of patients with ID-FA and pregnancy-related ID-FA accounted for 9.2% of cases. GERD (6.4%) and lower GI bleeding (6.4%) were the main GI diseases that caused acute or chronic hemorrhage. ID-FA was also detected in four patients (3.7%) with PCOS. PCOS-related obesity might interfere with iron absorption. Patients with long-term dietary restrictions had inadequate nutritional iron intake, leading to ID-FA [Table 2]. ID of the other 55.9% patients with ID-FA is possibly due to the Asian dietary habits, including the low intake of foods rich in heme iron, such as red meat, and the high phytate content, which inhibits the absorption of iron [22].
Table 2.
Iron deficiency-related female alopecia (n=109)
| n (%) | |
|---|---|
| Demographics of ID-FA | |
| Age (years) | 34.4±12.7 (7–77) |
| Disease duration (months) (n=103) | 26.2±56.9 |
| Type (n=103) | |
| Acute (≤6 months) | 67 (65.1) |
| Chronic (>6 months) | 36 (34.9) |
| Underlying diseases in ID-FA | |
| OBS/GYN diseases | 32 (29.4) |
| Hypermenorrhea | 11 (10.1) |
| Pregnancy-related | 10 (9.2) |
| Irregular menstruation | 7 (6.4) |
| PCOS | 4 (3.7) |
| GI disease | 15 (13.8) |
| GERD | 7 (6.4) |
| Lower GI bleeding | 7 (6.4) |
| Peptic ulcer | 5 (4.6) |
| Dietary restriction | 1 (0.9) |
OBS/GYN: Obstetric and gynecologic, PCOS: Polycystic ovary syndrome, GI: Gastrointestinal, GERD: Gastroesophageal reflux disease, ID-FA: Iron deficiency-related female alopecia
Most patients with iron deficiency-FA had comorbidities other than iron deficiency
Among patients with ID-FA, 62.4% had comorbidities other than ID, including zinc deficiency (36.7%), psychological stress (11.9%), autoimmune diseases (8.3%), COVID-19 vaccination (8.3%), thyroid diseases (6.4%), malignancy (6.4%), COVID-19 infection (4.6%), metabolic syndrome (4.6%), and hereditary diseases (thalassemia and G6PD deficiency) (1.8%) [Figure 1].
Figure 1.
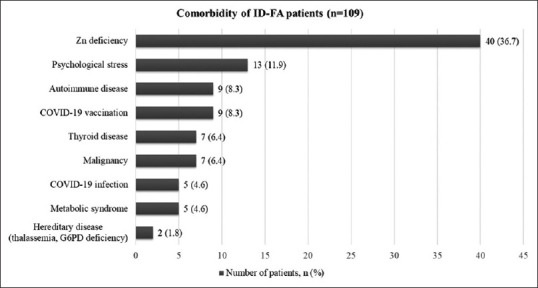
Comorbidities in patients with iron deficiency-related female alopecia (ID-FA). Overall, 109 patients with ID-FA had various comorbidities, including zinc deficiency, psychological stress, autoimmune diseases, COVID-19 vaccination or infection, thyroid diseases, malignancy, metabolic syndrome, and hereditary diseases (thalassemia and G6PD deficiency). ID-FA: Iron deficiency, COVID-19: Coronavirus disease 2019
Association between disease duration and serum ferritin levels after iron supplementation
In this study, we defined the improvement in ferritin levels as an increase in ferritin values by >40% after iron supplementation, as compared with ferritin values before iron supplementation. The duration of alopecia was defined as the time from the onset of hair loss to the date of visit. Logistic regression was used to evaluate the risk factors associated with the improvement in serum ferritin levels. By adjusting for “disease duration” and “disease type (chronic or acute),” we found that long disease duration and chronic alopecia >6 months were poor prognostic factors for ID-FA [Table 3] and that age, comorbidity count, and free T4 levels were not associated with the prognosis of ID-FA. Besides, we observed that the shorter the duration, particularly <6 months (i.e., the acute phase), the greater the improvement in serum ferritin levels after iron supplementation [Figure 2a]. However, even when the duration was longer than 20 years, serum ferritin levels could still be corrected after iron supplementation [Figure 2b].
Table 3.
Factors associated with the improvement of serum ferritin level (>40%) (n=109)
| Item | Crude | Adjusted (Model 1) | Adjusted (Model 2) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.99 (0.95-1.03) | 0.598 | 0.97 (0.90-1.04) | 0.337 | 0.98 (0.92-1.04) | 0.465 |
| Disease duration | 0.98 (0.96-0.99) | 0.029* | 0.97 (0.94-0.99) | 0.017* | ||
| Type (Chronic/Acute) | 0.27 (0.09-0.81) | 0.019* | 0.15 (0.04-0.56) | 0.005* | ||
| Comorbidity count | 1.12 (0.59-2.12) | 0.729 | 2.03 (0.76-5.45) | 0.159 | 1.26 (0.54-2.94) | 0.593 |
| Free T4 | 0.70 (0.12-3.91) | 0.681 | 1.63 (0.20-13.23) | 0.649 | 1.13 (0.15-8.29) | 0.904 |
*P<0.05 is considered statistically significant. Free T4: Free thyroxine, OR: Odds ratio, CI: Confidence interval
Figure 2.
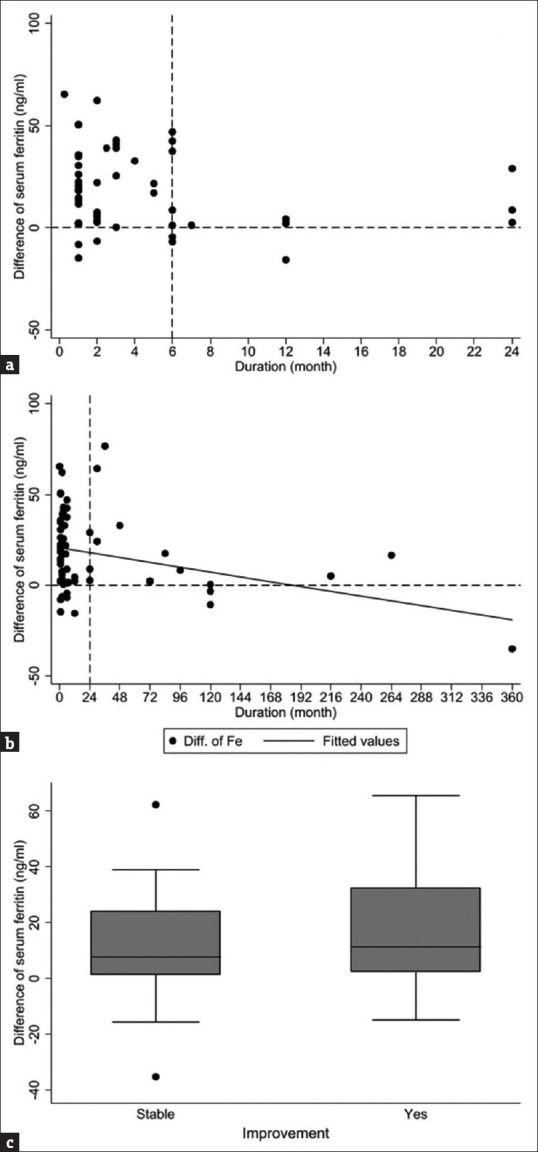
Association of “difference of serum ferritin” with the “duration of hair loss” and “subjective improvement”. The definition of “difference of serum ferritin” is the difference between the value of serum ferritin detected on the last follow-up after iron supplementation and the value of serum ferritin detected before iron supplementation. (a) The shorter the duration, particularly <6 months (the acute phase), the greater the improvement in serum ferritin levels after iron supplementation. (b) However, the serum ferritin levels in few patients with a duration of >2 years could still be corrected after iron supplementation. (c) Patients with subjective improvement after taking iron supplements had higher ferritin differences. Therefore, the difference in serum ferritin levels may predict subsequent subjective improvements
Patients with ID-FA with subjective improvement after iron supplementation had more increase of ferritin levels. The elevation in serum ferritin levels appeared earlier than hair regrowth. Therefore, regular follow-up of serum ferritin levels may aid in predicting subsequent subjective improvements [Figure 2c].
Ferritin was a more sensitive marker than hemoglobin for the early detection of iron deficiency
Ferritin is not a routine parameter in general blood tests, whereas Hb is often included in routine complete blood counts. Accordingly, we performed a linear regression analysis to evaluate the association between serum ferritin and Hb levels [Figure 3a]. The corresponding serum ferritin level for the current definition of female anemia (Hb: 12.0 g/dL) was 5.1 ng/mL, which was lower than the adequate ferritin level for hair growth (40–60 ng/mL). In this study, we found that the corresponding Hb value for a ferritin level of 40–60 ng/mL was 13.1–13.8 g/dL, which was higher than the level for the current definition of female anemia (Hb: 12.0 g/dL), implying that ferritin was a more sensitive marker than Hb for the early detection of ID [Figure 3b]. The current criteria for anemia in women have traditionally been based on the levels necessary to support hematopoiesis rather than normal hair growth. Therefore, we suggest increasing the normal female Hb level from 12.0 to 13.0 g/dL.
Figure 3.
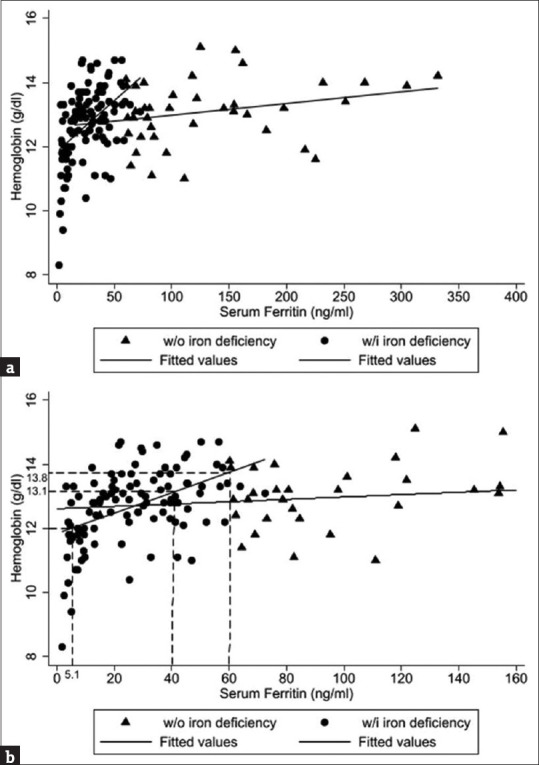
Association between serum ferritin and hemoglobin (Hb) levels. (a) There was a positive relationship between Hb and serum ferritin levels. (b) The corresponding serum ferritin level for the current definition of female anemia (Hb: 12.0 g/dL) was 5.1 ng/mL, which was lower than the adequate ferritin level for hair growth (40–60 ng/mL). The corresponding Hb value for a ferritin level of 40–60 ng/mL was 13.1–13.8 g/dL, which was higher than the level for the current definition of female anemia (Hb: 12.0 g/dL), implying that ferritin was a more sensitive marker than Hb for the early detection of ID. Hb: Hemoglobin
DISCUSSION
Previous studies reported diverse etiologies of FA, including nutrient deficiency (iron, zinc, and Vitamin D deficiencies), thyroid disorders, AA, PCOS, metabolic syndrome, telogen effluvium, postpartum hair loss, psychoemotional stress, weight loss, crash dieting, starvation, malignancy, human immunodeficiency virus, and drugs (such as lithium) [7,16-19]. In this study, we found that ID was the most prevalent etiology of FA, accounting for 70.3% of cases. In Dr. Chang’s clinics in the tertiary medical centers in Kaohsiung and Taichung, ID is also the most common etiology of female hair loss. Therefore, complete clinical and laboratory work-ups are essential before deciding on therapeutic strategies [23,24].
Du et al. conducted animal studies and showed that mutational inactivation of TMPRSS6 lowered the iron content in the bodies of mice [25]. Ohyama et al. identified 58 genes in the bulge of the outer root sheath of the human hair follicle that were selectively upregulated or downregulated, as compared with the sub-bulge region [26]. St Pierre reported that CDC2, NDRG1, ALAD, RRM2, Decorin, and DCT could be regulated by iron and speculated that ID might alter the normal progression of the hair cycle [27].
Iron is a cofactor for ribonucleotide reductase, an enzyme involved in DNA synthesis and is crucial for physiological and cellular processes. Hair follicle matrix cells are the most rapidly dividing cells and are extremely sensitive to even a minor decrease in iron availability [28]. Prolonged ID can lead to alopecia, fatigue, dizziness, tinnitus, pallor, koilonychia, and atrophic glossitis. In the USA, 15% of premenopausal women and 18% of pregnant women have been reported to have ID [29,30]. The causes of ID include inadequate nutritional iron intake, inadequate nutritional iron absorption (use of antacids or proton-pump inhibitors, Helicobacter pylori infection, inflammatory bowel disease, and obesity), increased iron requirements (growth or pregnancy), and blood loss (reflux esophagitis, gastric ulcers, duodenal ulcers, hemorrhoids, and gynecologic bleeding). Blood loss-induced ID is clinically more common in women; therefore, we analyzed the underlying causes of iron loss.
ID is a continuum of iron depletion, iron-deficient erythropoiesis, and ID anemia. Ferritin is a protein complex that plays an important role in iron storage and is the main iron-binding protein in non-erythroid cells. Serum ferritin levels are directly related to total body iron stores [28]. Decreased serum ferritin levels are significantly associated with diffuse and patterned hair loss, especially in nonmenopausal women [31-33].
Various cut-off values of serum ferritin for ID have been reported in previous studies. A cut-off value of 10–15 ng/mL yielded a sensitivity of only 59% and a specificity of 99%, whereas a cut-off value of 41 ng/mL yielded a higher sensitivity (98%) and specificity (98%) [28]. Other studies also considered that the appropriate serum ferritin level for diagnosing ID was 60–70 ng/mL [20]. However, in previous studies, the ranges considered to be “normal” had traditionally been based on levels necessary to support hematopoiesis. Additionally, no study has addressed the minimal iron levels necessary for normal hair growth.
The effect of iron supplementation on ID-FA remains controversial according to previous studies. In a cohort study of 194 patients conducted by Sinclair, low iron stores were found in 12 patients (6%). However, no iron supplementation led to the cessation of hair loss or improvement in hair density [34]. From the perspective of our study, the unsatisfactory response to iron replacement may be associated with a longer disease duration, especially in those with a clinical course >6 months (chronic type), which may affect the regeneration of hair follicle stem cells [35].
ID and zinc deficiency have been shown to coexist, because zinc deficiency may downregulate the expression of iron transporters, such as ferroportin-1, which inhibits the mobilization of iron from the liver or intestines to the circulation. On the contrary, zinc repletion may trigger the mobilization of iron from storage form, which contributes to the utilization of iron for erythropoiesis [36,37]. Therefore, we recommend to evaluate zinc and iron levels at the same time. Psychological stress is also a common comorbidity in ID-FA. Chronic stress inhibited hair growth and led to perifollicular inflammation through stress-mediating substances, such as cortisol [38,39].
This study has two limitations. First, the variable quality of medical records affected the results because of the retrospective nature of this study. Second, the evaluation of therapeutic response was subjective, and no objective measurement of the amount of hair was conducted in this study.
CONCLUSION
The causes of FA varied, including nutrient deficiencies, autoimmune diseases, psychological stress, thyroid diseases, and COVID-19 vaccination, etc., 70% of FA cases were ID-FA, 62.4% of which had comorbidities, including zinc deficiency, psychological stress, autoimmune diseases, COVID-19 vaccination, thyroid diseases, malignancy, COVID-19 infection, metabolic syndrome, and hereditary diseases. Given that the etiologies of FA are diverse, associated with multiple comorbidities, the oversimplified and stereotypical term, “female AGA” should be abandoned. Treatments for AGA are not suitable for most FA, which are commonly misused for FA without detailed evaluation. A complete survey of etiologies and comorbidities is essential, which guides an accurate and comprehensive therapeutic strategy. We suggest to redefine the serum ferritin level ≥60 ng/mL, with the corresponding Hb ≥13.0 g/dL as the normal range. Initiation of iron supplementation within 6 months would contribute to a better prognosis of ID-FA.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Chung-Hsing Chang, the editorial board member at Tzu Chi Medical Journal, played no role in the peer review process or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
Acknowledgment
We are grateful to the patients and our colleagues, who provided insight and expertise that greatly assisted the research.
REFERENCES
- 1.Su LH, Chen LS, Chen HH. Factors associated with female pattern hair loss and its prevalence in Taiwanese women: A community-based survey. J Am Acad Dermatol. 2013;69:e69–77. doi: 10.1016/j.jaad.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Concha JSS, Werth VP. Alopecias in lupus erythematosus. Lupus Sci Med. 2018;5:e000291. doi: 10.1136/lupus-2018-000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandrini A, Bruni F, Piraccini BM, Starace M. Common causes of hair loss –Clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629–40. doi: 10.1111/jdv.17079. [DOI] [PubMed] [Google Scholar]
- 4.Olsen EA, Messenger AG, Shapiro J, Bergfeld WF, Hordinsky MK, Roberts JL, et al. Evaluation and treatment of male and female pattern hair loss. J Am Acad Dermatol. 2005;52:301–11. doi: 10.1016/j.jaad.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Sadick N, Arruda S. Understanding causes of hair loss in women. Dermatol Clin. 2021;39:371–4. doi: 10.1016/j.det.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Saini K, Mysore V. Role of vitamin D in hair loss: A short review. J Cosmet Dermatol. 2021;20:3407–14. doi: 10.1111/jocd.14421. [DOI] [PubMed] [Google Scholar]
- 7.Harfmann KL, Bechtel MA. Hair loss in women. Clin Obstet Gynecol. 2015;58:185–99. doi: 10.1097/GRF.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 8.Nanda S, De Bedout V, Miteva M. Alopecia as a systemic disease. Clin Dermatol. 2019;37:618–28. doi: 10.1016/j.clindermatol.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407–11. doi: 10.5114/ada.2020.96251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salinas M, Leiva-Salinas M, Flores E, López-Garrigós M, Leiva-Salinas C. Alopecia and iron deficiency: An interventional pilot study in primary care to improve the request of ferritin. Adv Hematol. 2020:7341018. doi: 10.1155/2020/7341018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Y, Zhou X, Fu S, Luo S, Li Y. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv00645. doi: 10.2340/actadv.v101.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alharbi M. Telogen effluvium after COVID-19 vaccination among public in Saudi Arabia. J Family Med Prim Care. 2022;11:6056–60. doi: 10.4103/jfmpc.jfmpc_377_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen B, Tosti A. Alopecia areata after COVID-19 infection and vaccination: A cross-sectional analysis. J Eur Acad Dermatol Venereol. 2023;37:e7–8. doi: 10.1111/jdv.18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aryanian Z, Balighi K, Hatami P, Afshar ZM, Mohandesi NA. The role of SARS-CoV-2 infection and its vaccines in various types of hair loss. Dermatol Ther. 2022;35:e15433. doi: 10.1111/dth.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharquie KE, Jabbar RI. COVID-19 infection is a major cause of acute telogen effluvium. Ir J Med Sci. 2022;191:1677–81. doi: 10.1007/s11845-021-02754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbrocini G, Cantelli M, Masarà A, Annunziata MC, Marasca C, Cacciapuoti S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203–11. doi: 10.1016/j.ijwd.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaworski J, Delannoy PY, Boussekey N, Thellier D, Georges H, Leroy O. Lithium:one drug, five complications. J Intensive Care. 2017;5:70. doi: 10.1186/s40560-017-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deo K, Sharma YK, Wadhokar M, Tyagi N. Clinicoepidemiological observational study of acquired alopecias in females correlating with anemia and thyroid function. Dermatol Res Pract. 2016;2016:6279108. doi: 10.1155/2016/6279108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druschky K, Bleich S, Grohmann R, Burda K, Frieling H, Hillemacher T, et al. Severe hair loss associated with psychotropic drugs in psychiatric inpatients-data from an observational pharmacovigilance program in German-speaking countries. Eur Psychiatry. 2018;54:117–23. doi: 10.1016/j.eurpsy.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Ong KH, Tan HL, Lai HC, Kuperan P. Accuracy of various iron parameters in the prediction of iron deficiency in an acute care hospital. Ann Acad Med Singap. 2005;34:437–40. [PubMed] [Google Scholar]
- 21.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: A review of the evidence. Br J Nutr. 2008;99((Suppl 3)):S14–23. doi: 10.1017/S0007114508006818. [DOI] [PubMed] [Google Scholar]
- 22.Hu PJ, Ley SH, Bhupathiraju SN, Li Y, Wang DD. Associations of dietary, lifestyle, and sociodemographic factors with iron status in Chinese adults: A cross-sectional study in the China Health and Nutrition Survey. Am J Clin Nutr. 2017;105:503–12. doi: 10.3945/ajcn.116.136861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HJ, Yeh JW, Chang YF, Wu JS, Yang CC. Comorbid laboratory abnormalities in female pattern hair loss patients. Dermatol Sin. 2022;40:174. [Google Scholar]
- 24.Yorulmaz A, Hayran Y, Ozdemir AK, Sen O, Genc I, Gur Aksoy G, et al. Telogen effluvium in daily practice: Patient characteristics laboratory parameters and treatment modalities of 3028 patients with telogen effluvium. J Cosmet Dermatol. 2022;21:2610–7. doi: 10.1111/jocd.14413. [DOI] [PubMed] [Google Scholar]
- 25.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–92. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–60. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Pierre SA, Vercellotti GM, Donovan JC, Hordinsky MK. Iron deficiency and diffuse nonscarring scalp alopecia in women: More pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070–6. doi: 10.1016/j.jaad.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 28.Trost LB, Bergfeld WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol. 2006;54:824–44. doi: 10.1016/j.jaad.2005.11.1104. [DOI] [PubMed] [Google Scholar]
- 29.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–43. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 30.Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397:233–48. doi: 10.1016/S0140-6736(20)32594-0. [DOI] [PubMed] [Google Scholar]
- 31.Ali SY, Fatima U, Fazal SN. Serum ferritin levels and diffuse hair loss –A correlation. IP Indian J Clin Exp Dermatol. 2020;6:274–6. [Google Scholar]
- 32.Aslam MF, Khalid M, Amad Aslam M. The association of serum ferritin levels with non-scarring alopecia in women. Cureus. 2022;14:e32123. doi: 10.7759/cureus.32123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Park SY, Na SY, Kim JH, Cho S, Lee JH. Iron plays a certain role in patterned hair loss. J Korean Med Sci. 2013;28:934–8. doi: 10.3346/jkms.2013.28.6.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinclair R. There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. Br J Dermatol. 2002;147:982–4. doi: 10.1046/j.1365-2133.2002.04997.x. [DOI] [PubMed] [Google Scholar]
- 35.Yuan AR, Bian Q, Gao JQ. Current advances in stem cell-based therapies for hair regeneration. Eur J Pharmacol. 2020;881:173197. doi: 10.1016/j.ejphar.2020.173197. [DOI] [PubMed] [Google Scholar]
- 36.Kondaiah P, Palika R, Mashurabad P, Singh Yaduvanshi P, Sharp P, Pullakhandam R. Effect of zinc depletion/repletion on intestinal iron absorption and iron status in rats. J Nutr Biochem. 2021;97:108800. doi: 10.1016/j.jnutbio.2021.108800. [DOI] [PubMed] [Google Scholar]
- 37.Kondaiah P, Yaduvanshi PS, Sharp PA, Pullakhandam R. Iron and zinc homeostasis and interactions: Does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients. 2019;11:1885. doi: 10.3390/nu11081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thom E. Stress and the hair growth cycle: Cortisol-induced hair growth disruption. J Drugs Dermatol. 2016;15:1001–4. [PubMed] [Google Scholar]
- 39.Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30((Supp l)):S41–7. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


