Abstract
Scrub typhus is an infectious disease caused by Orientia tsutsugamushi, a bacterium within the family Rickettsiaceae. The clinical symptoms are usually acute and are characterized by fever, eschar formation or ulceration, local or generalized lymphadenopathy, and rash. Because of the extensive damage to small blood vessels throughout the body, scrub typhus can involve multiple systems and organs, causing damage to the respiratory, digestive, and nervous systems and inducing kidney and liver dysfunction. Death can occur in severe cases. We herein report two cases of scrub typhus with liver damage and intracranial infection. Among patients with scrub typhus, the risk of death is significantly higher in those who develop liver injury and intracranial infection. However, there are few reports on the treatment of patients with liver injury and intracranial infection caused by scrub typhus, and relevant treatment experience is thus lacking. Our clinical case report helps to fill the knowledge gap in this area.
Keywords: Eschar, intracranial infection, liver injury, scrub typhus, multiple organ dysfunction, metagenomic next-generation sequencing
Introduction
Tsutsugamushi disease, also known as jungle typhus or scrub typhus, is an acute infectious disease caused by the Orientia tsutsugamushi bacterium, which is commonly found in forested regions. This naturally occurring epidemic predominantly emerges in jungle environments.1–3 It is mainly transmitted through the bite of chiggers (larval mites), resulting in a diverse and complex array of clinical manifestations often accompanied by various comorbidities.4,5 These manifestations often lead to multi-organ damage with the characteristic presence of an eschar. Scrub typhus is widely endemic in the Asia-Pacific region, with a prominent distribution in China’s southeastern coastal areas.6,7 About 1 million individuals worldwide are affected by this disease every year, making it a serious threat to human health. 8
The mortality rate of liver injury combined with intracranial infection in patients with scrub typhus is high, and there is a lack of consensus on relevant treatment. We herein report two cases of liver injury combined with intracranial infection and their successful treatment. We expect the information in this case report to be helpful in clinical practice.
Case report
Case 1
A 70-year-old man was admitted to the hospital with a 1-week history of fever and 5-day history of abdominal pain. The patient’s fever was recurrent, with a maximum body temperature of 39°C. He took antipyretic medication, which helped to control the fever. Five days before presentation, the patient developed tolerable pain throughout his whole abdomen, loose stools, and an incomplete feeling of defecation. He had no vomiting, mucopurulent or bloody stools, back pain, urinary frequency or urgency, urinary pain, hematuria, or other symptoms.
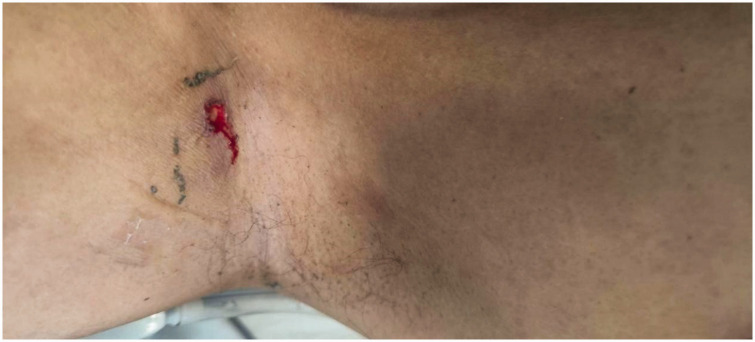
Initial testing for novel coronavirus yielded negative results. Upon examination, the patient showed clear consciousness, pharyngeal hyperemia, and no tonsillar swelling. A round skin lesion measuring approximately 1.0 × 1.0 cm and surrounded by a red halo was observed in the right axilla. Abdominal assessment revealed a flat and soft abdomen with no abdominal muscle tension, tenderness, rebound tenderness, or mass. No liver, spleen, or subcostal abnormalities were palpated. Abdominal percussion produced a resonant sound, and bowel sounds were detected at a rate of four times per minute. Pathological signs were absent, and no neck rigidity was noted. No superficial lymph nodes were palpable. No other abnormalities were found during the physical examination. Laboratory testing revealed a white blood cell count of 10.98 × 109/L (reference range, 4–10 × 109/L), procalcitonin (PCT) concentration of 14.36 ng/mL (reference range, 0–0.05 ng/mL), amylase concentration of 103.1 U/L (reference range, 39–117 U/L), and lipase concentration of 106.9 U/L (reference range, 0–60 U/L). Liver function testing revealed an alanine aminotransferase concentration of 97 U/L (reference range, 9–50 U/L), aspartate aminotransferase concentration of 56 U/L (reference range, 15–40 U/L), and total bilirubin concentration of 63.4 µmol/L (reference range, 2–20.4 µmol/L). Additionally, the blood creatinine concentration was 148 mg/L (reference range, 59–104 µmol/L) and platelet count was 73 × 109/L (reference range, 100–300 × 109/L). Abdominal computed tomography showed no pancreatic swelling or peripancreatic exudation. Leptospira and adenovirus antibodies were negative. Overall, these test results excluded acute pancreatitis, Leptospira infection, and adenovirus infection. The PCT index was significantly increased, however, and we considered that the possibility of bacterial infection was high, particularly considering the presence of multiple organ dysfunction and an eschar in the right axilla (Figure 1). Based on the patient’s history of pastoral work before the onset of illness, scrub typhus was considered a possible diagnosis.
Figure 1.
Right axillary skin lesion, 0.8 × 0.6 cm (Case 1).
A treatment regimen of doxycycline at 100 mg twice daily was initiated. By the second day, the patient’s condition had deteriorated, leading to gradual loss of consciousness and limb convulsions. As a result, the patient was transferred to the intensive care unit, intubated, and provided respiratory support. A cerebrospinal fluid (CSF) sample obtained via lumbar puncture exhibited a pale yellow color with significantly elevated trace protein levels (Table 1). CSF and blood cultures revealed no pathogenic bacteria. Metagenomic next-generation sequencing (mNGS) identified 26 sequences of Orientia scrub typhus in the blood and CSF, confirming the diagnosis of scrub typhus. Liver injury and intracranial infection were also evident.
Table 1.
Cerebrospinal fluid examination results (Case 1).
| Normal value/reference range | Day 2 of illness | Day 9 of illness | |
|---|---|---|---|
| Color | Colorless | Faint yellow | Clear |
| Total cell count, ×109/L | 0–8 | 109 | 9 |
| White blood cell count, ×109/L | 0–8 | 88 | 9 |
| Pandy’s test | Negative | Positive | Positive |
| Trace protein, mg/dL | 8–43 | 287.2 | 111.2 |
| Chlorine, mmol/L | 120–132 | 123 | 134 |
| Glucose | 3.6–4.5 | 2.5 | 6.23 |
In formulating our treatment plan, we considered the need to penetrate the blood–brain barrier and the potential toxic effects of certain medications on the liver. Therefore, we initiated treatment with moxifloxacin, which is characterized by slightly better blood–brain barrier penetration and less liver damage than other medications, at a dosage of 0.4 g once daily. On hospitalization day (HOD) 5, the patient’s fever subsided and his limb convulsions were controlled. On HOD 7, the patient regained consciousness, although without limb movement. Pain stimulation elicited responses. On HOD 9, the tracheal tube was removed, and the patient was able to perform coordinated actions such as leg lifting and fist clenching. Subsequent CSF analysis indicated a significantly reduced total cell count, white blood cell count, and trace protein concentration (Table 1). Brain magnetic resonance imaging (MRI) revealed no abnormalities. Upon discharge on HOD 19, the patient’s limb muscle strength had largely returned to normal.
Case 2
An 83-year-old male farmer had a history of engaging in farmland and outdoor activities before the onset of his illness. He was admitted to the hospital because of an 8-day history of fever and abdominal pain. The patient had fever, chills, and occasional coughing with production of small amounts of phlegm and white sticky sputum. During this period, he additionally developed abdominal pain and vomiting of stomach contents, but no lower back pain, diarrhea, or bloody stools. He also experienced a headache, mainly on the top of the head, although the duration was unclear. The patient had taken painkillers on his own, but the effect was unsatisfactory. His symptoms progressively worsened, and he presented to our hospital for treatment.
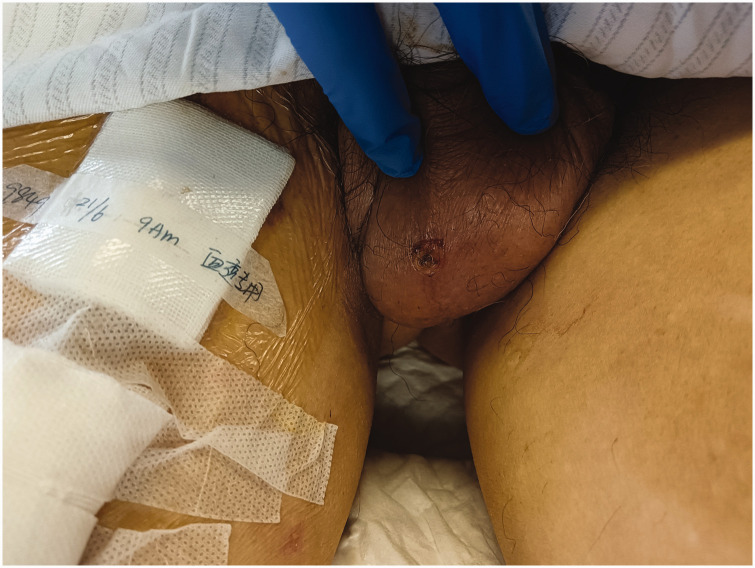
On admission, he showed confusion, fever, and diffusely flushed skin across the entire body. His skin had a slightly yellow color and showed scattered red congestive rashes over the whole body. Conjunctival congestion and edema were also observed. Abdominal examination revealed an increased abdominal wall tone, and a positive Murphy sign was suspected. The patient exhibited tenderness in the left abdomen but had no rebound tenderness. Bowel sounds were not audible, although abdominal ultrasound indicated bowel movements. No superficial lymph nodes were palpable. A round black scab with a diameter of approximately 1 cm was seen on the right scrotum, raising suspicion of an eschar (Figure 2). Laboratory tests showed multi-organ insufficiency (Table 2).
Figure 2.
Circular black scab with a diameter of approximately 1 cm on the right scrotum (Case 2).
Table 2.
Laboratory test results (Case 2).
| Test | Reference range | Day of illness |
||||
|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 9 | 16 | ||
| WBC count, ×109/L | 4–10 | 10.19 | 8.6 | 6 | 8.32 | 7.6 |
| PLT count, ×109/L | 100–300 | 97 | 52 | 86 | 236 | 276 |
| ALT, U/L | 9–50 | 110 | 124 | 634 | 214 | 149 |
| AST, U/L | 15–40 | 175 | 206 | 409 | 65 | 48 |
| TB, µmol/L | 2–20.4 | 52.7 | 106.3 | 73.3 | 41.9 | 34.4 |
| PCT, ng/mL | 0–0.05 | 5.31 | 2.46 | 0.77 | 0.43 | 0.54 |
| IL-6, pg/mL | 0–7 | 433 | 46.5 | 14.22 | 10.73 | 11.6 |
| Cr, µmol/L | 59–104 | 417 | 238 | 362 | 341 | 292 |
| Amylase, U/L | 39–117 | 136.3 | 296 | |||
| Lipase, U/L | 0–60 | 15.4 | 243 | |||
WBC, white blood cell; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; PCT , procalcitonin; IL-6, interleukin-6; Cr , creatinine.
Computed tomography examination of the chest and abdomen revealed consolidation of dorsal strips in both lungs, suggestive of pulmonary inflammation. The gallbladder was enlarged and the gallbladder wall was blurred, indicating cholecystitis. No pancreatic enlargement or peripancreatic exudation was present. The patient’s blood amylase concentration was slightly high, but the lipase index was not high (Table 2). We ruled out acute pancreatitis based on the laboratory and computed tomography findings. Initially, the patient received empirical treatment comprising doxycycline at 100 mg twice daily combined with meropenem at 1 g three times daily to control infection. His urine output was 30 to 50 mL/hour, and he showed a positive balance of intake and output. Arterial blood gas analysis indicated metabolic acidosis, and severe infection and acute renal insufficiency were evident. Therefore, continuous venovenous hemofiltration was performed six times. The patient’s urine output returned to 100 to 150 mL/hour. Dynamic monitoring showed a gradual decrease in the blood creatinine concentration, and arterial blood gas analysis showed that the metabolic acidosis had been corrected.
On HOD 2, blood mNGS identified 67 sequences of Orientia scrub typhus. With the diagnosis confirmed, the anti-infection regimen was adjusted to doxycycline at 100 mg twice daily and moxifloxacin at 0.4 g once daily to control infection. Despite numerous blood and bronchoalveolar lavage fluid cultures, no pathogenic bacteria were detected. Antibodies against both epidemic hemorrhagic fever virus and Leptospira were negative; thus, epidemic hemorrhagic fever and leptospirosis were ruled out. Antibodies against adenovirus were also negative, and the patient had not undergone measles-related tests. He did not have a typical epidemic blood history or high white blood cell count, but he had a high PCT concentration (Table 2). Based on our clinical judgment, he had a low possibility of adenovirus infection or measles.
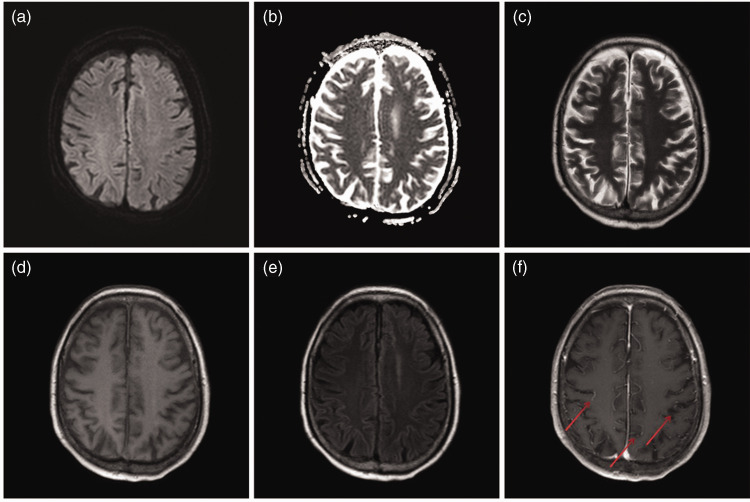
On HOD 3, the patient became unconscious. A lumbar puncture was conducted on HOD 6, with consent from his family members. The puncture revealed a significant increase in both the total CSF cell count and leukocyte count along with an elevated CSF microprotein concentration (Table 3). One sequence of Orientia scrub typhus was identified in the CSF through mNGS. No pathogenic bacteria were found in CSF culture. On HOD 10, ventilator assistance was discontinued. Enhanced brain MRI suggested meningitis as evidenced by local thickening and enhancement of the meninges (Figure 3). On HOD 15, the patient regained consciousness but showed weakness in the limbs, with grade 2 muscle strength and low muscle tension. On HOD 16, mNGS examination yielded no evidence of Orientia scrub typhus in the blood, bronchoalveolar lavage fluid, or CSF. A review of the CSF showed significant reductions in the total cell count, leukocyte count, and trace protein concentration (Table 3). On HOD 39, the patient was discharged from the hospital. At the time of discharge, he was conscious and able to eat, experienced occasional coughing, and showed grade 3 limb muscle strength with normal muscle tone. One week after discharge, the patient’s condition had considerably improved; he was able to walk a distance of 4 to 5 m using crutches, and his cough had significantly subsided.
Table 3.
Cerebrospinal fluid examination results (Case 2).
| Normal value/reference range | Day 7 of illness | Day 26 of illness | |
|---|---|---|---|
| Color | Colorless | Yellow | Transparent |
| Total cell count, × 109/L | 0–8 | 40 | 31 |
| White blood cell count, × 109/L | 0–8 | 35 | 23 |
| Pandy’s test | Negative | Positive | Weakly positive |
| Trace protein, mg/dL | 8–43 | 386.8 | 71.1 |
| Chlorine, mmol/L | 120–132 | 117 | 135 |
| Glucose, mmol/L | 3.6–4.5 | 3.33 | 4.31 |
Figure 3.
Brain magnetic resonance imaging results on day 10 of hospitalization (Case 2)
The patients’ families provided informed written consent for all treatments described in this case report, and both the patients and their families provided written approval for publication of the report. The reporting of this study was approved by the ethics committee of our hospital (Zhongshan City People’s Hospital Clinical Research and Animal Experiment Ethics Committee) and conformed to the Declaration of Helsinki. The reporting of this study conforms to the CARE guidelines. 9
Discussion
Scrub typhus, caused by a bacterium within the family Rickettsiaceae, leads to widespread small vessel vasculitis due to the release of toxins. This results in multi-organ hyperemia, edema, exudation, interstitial inflammation, and cell degeneration. 10 The clinical manifestations of the disease are complex and result in many complications, potentially affecting multiple systems of the body. The exact mechanism behind infection of the central nervous system in patients with scrub typhus remains unclear. Some scholars believe that the rickettsial bacteria enter the human body through the bite of chiggers, initially multiplying at the bite site to form an eschar or ulcer. The bacteria then enter the bloodstream, causing rickettsial disease. The toxins released by the pathogens play a significant role in disease onset because a substantial quantity of these toxins can invade the brain parenchyma, causing inflammatory and degenerative lesions.5,11 Another plausible explanation is that the rickettsial bacteria multiply within vascular endothelial cells, causing rickettsial vasculitis. This process increases vascular permeability, resulting in the extrusion of intravascular substances with resultant ischemia, edema, and disruption of brain tissue metabolism. 12
The clinical manifestations of central nervous system involvement in scrub typhus are diverse, with seizures being the most common. Other manifestations, such as myoclonus, myelitis, acute disseminated encephalomyelitis, and nerve palsy, have also been reported.13–15 In one study, a distinct eschar was absent in 30.8% of patients with scrub typhus-associated meningoencephalitis. 16 In patients who lack a typical eschar, the clinical manifestations are diverse and nonspecific. Traditional diagnostic techniques have limitations that make clear identification of pathogenic infections challenging. Hence, misdiagnosis or delayed diagnosis can easily occur, delaying or preventing optimal treatment.
Both patients described in this report presented with fever and altered mental states, with the CSF revealing signs of bacterial inflammation. While one patient showed no abnormality on brain MRI, the other showed changes indicative of meningitis. Ultimately, the application of mNGS led to the identification of Orientia scrub typhus in both cases. Chen et al. 17 also reported a case of severe scrub typhus in which infection with Orientia scrub typhus was detected via blood mNGS on HOD 6 despite the absence of an eschar. The accurate diagnosis guided precise treatment, ultimately yielding satisfactory results.
Clinically, the manifestations of scrub typhus can closely resemble those of leptospirosis and certain intracranial infectious diseases, making differentiation challenging. The initially prescribed antibiotic treatment might not effectively cover the specific pathogenic bacteria. Moreover, the issue of bacterial resistance caused by inappropriate empirical antibiotic therapy has received increasing attention. Early identification of the causative bacteria can not only improve the success rate of treatment and reduce mortality rates but can also reduce the development of bacterial resistance caused by improper empirical administration of antibiotics. mNGS technology has the advantages of higher accuracy, provision of more information, faster results, and increased sensitivity compared with traditional microbial detection methods. 18
Multi-organ dysfunction is common in scrub typhus, with liver damage affecting 34% to 87% of patients.19–21 Meningoencephalitis has also been extensively reported,22–24 with incidence rates ranging from 14.0% to 23.3%.25,26 The risk of death in patients with concurrent intracranial infection is also significantly increased. Chaudhry et al. 27 reported five patients with scrub typhus, among whom four had concurrent intracranial infection. Despite initial treatment with doxycycline, all patients ultimately died. 27 Few reports have described the treatment of concurrent liver injury and intracranial infection in patients with scrub typhus, and there is currently no consensus on relevant treatments. Effective drugs for the clinical treatment of scrub typhus include doxycycline, tetracycline, chloramphenicol, and macrolide agents; among these, doxycycline is preferred. However, for patients with concomitant liver injury and meningoencephalitis, the ability of doxycycline to penetrate the blood–brain barrier is limited. Very few drugs that can treat scrub typhus are able to pass through the blood–brain barrier; moreover, the effect of such drugs on liver function must be considered. Clinically, moxifloxacin has demonstrated good blood–brain barrier permeability.28,29 We administered a combination of doxycycline and moxifloxacin to our two patients with scrub typhus-induced multi-organ dysfunction and meningoencephalitis. This treatment improved the patients’ central nervous system symptoms and restored their liver function, ultimately yielding favorable treatment outcomes.
Conclusion
In clinical practice, the absence of an eschar or the presence of an atypical eschar may pose a challenge to using traditional methods to diagnose scrub typhus. mNGS can help doctors identify the causative agent of clinical infections. Scrub typhus often leads to liver injury and intracranial infection. The combined regimen of doxycycline and moxifloxacin has shown good efficacy.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Supplemental material, sj-pdf-4-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Acknowledgements
The authors thank all colleagues who contributed to the study by assisting with the data collection.
Authors’ contributions: JianWei Li and MiaoLian Chen conceived this study. Li Lei, Haiming Niu, and Jiezhang Yang assisted with the data collection. HongKui Sun edited the manuscript. All authors read and approved the final manuscript.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ORCID iD: MiaoLian Chen https://orcid.org/0000-0002-0306-5999
Data availability statement
All relevant data are included within the manuscript.
Declaration of competing interests
The authors declare that there are no conflicts of interest.
References
- 1.Luo L, Guo Z, Lei Z, et al. Epidemiology of tsutsugamushi disease and its relationship with meteorological factors in Xiamen city, China. PLoS Negl Trop Dis 2020; 14: E0008772. doi: 10.1371/journal.pntd.0008772. PMID: 33057334; PMCID: PMC7591240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahk YY, Jun H, Park SH, et al. Surveillance of chigger mite vectors for Tsutsugamushi disease in the Hwaseong area, Gyeonggi-do, Republic of Korea, 2015. Korean J Parasitol 2020; 58: 301–308. doi: 10.3347/kjp.2020.58.3.301. Epub 2020 Jun 26. PMID: 32615743; PMCID: PMC7338901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee A, Kulkarni S. Orientia tsutsugamushi: the dangerous yet neglected foe from the East. Int J Med Microbiol 2021; 311: 151467. doi: 10.1016/j.ijmm.2020.151467; PMID: 33338890. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara S, Shimizu T. A typical case of scrub typhus (tsutsugamushi disease). BMJ Case Rep 2014; 2014: bcr2014207824. doi: 10.1136/bcr-2014-207824; PMID: 25355755; PMCID: PMC4216889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paris DH, Shelite TR, Day NP, et al. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 2013; 89: 301–307. doi: 10.4269/ajtmh.13-0064. PMID: 23926142; PMCID: PMC3741252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Li M, Xu W, et al. Analysis of the clinical characteristics of severe tsutsugamushi disease in Yunnan Province from 2017 to 2018. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019; 31: 1018–1023. Chinese. doi: 10.3760.2095-4352.2019.08.021. PMID: 31537231. [DOI] [PubMed] [Google Scholar]
- 7.Musa TH, Ahmad T, Wana MN, et al. The epidemiology, diagnosis and management of scrub typhus disease in China. Hum Vaccin Immunother 2021; 17: 3795–3805. doi: 10.1080.2021.1934355. Epub 2021 Jun 14. PMID: 34124995; PMCID: PMC8437466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu G, Walker DH, Jupiter D, et al. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 2017; 11: E0006062. doi: 10.1371/journal.pntd.0006062. PMID: 29099844; PMCID: PMC5687757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep 2013; 2013: bcr2013201554. doi: 10.1136/bcr-2013-201554. PMID: 24155002; PMCID: PMC3822203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soong L. Dysregulated Th1 immune and vascular responses in scrub typhus pathogenesis. J Immunol 2018; 200: 1233–1240. doi: 10.4049/jimmunol.1701219. PMID: 29431689; PMCID: PMC5812358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YC, Chen PC, Lee KF, et al. Scrub typhus cases in a teaching hospital in Penghu, Taiwan, 2006-2010. Vector Borne Zoonotic Dis 2013; 13: 154–159. doi: 10.1089/vbz.2012.1059. Epub 2013 Feb 19. PMID: 23421889. [DOI] [PubMed] [Google Scholar]
- 12.Varghese GM, Raj D, Francis MR, et al. Epidemiology & risk factors of scrub typhus in south India. Indian J Med Res 2016; 144: 76–81. doi: 10.4103/0971-5916.193292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KL, Lee JK, Yim YM, et al. Acute transverse myelitis associated with scrub typhus: case report and a review of literatures. Diagn Microbiol Infect Dis 2008; 60: 237–239. doi: 10.1016/j.diagmicrobio.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Chiou YH, Yang CJ, Lai TH. Scrub typhus associated with transient parkinsonism and myoclonus. J Clin Neurosci 2013; 20: 182–183. doi: 10.1016/j.jocn.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Chen PH, Hung KH, Cheng SJ, et al. Scrub typhus-associated acute disseminated encephalomyelitis. Acta Neurol Taiwan 2006; 15: 251–254. [PubMed] [Google Scholar]
- 16.Misra UK, Kalita J, Mani VE. Neurological manifestations of scrub typhus. J Neurol Neurosurg Psychiatry 2015; 86: 761–766. doi: 10.1136/jnnp-2014-308722. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Zheng XD, Dai QH, et al. Diagnosis of severe scrub typhus infection by next-generation sequencing: a case report. BMC Infect Dis 2020; 20: 270. doi: 10.1186/s12879-020-04991-y. PMID: 32264829; PMCID: PMC7137524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Cai Q, Miao Q, et al. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods 2021; 5: 2000792. doi: 10.1002/smtd.202000792. Epub 2020 Dec 13. PMID: 33614906; PMCID: PMC7883231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahajan SK, Rolain JM, Kashyap R, et al. Scrub typhus in Himalayas. Emerg Infect Dis 2006; 12: 1590–1592. doi: 10.3201/eid1210.051697. PMID: 17176580; PMCID: PMC3290934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrispal A, Boorugu H, Gopinath KG, et al. Scrub typhus: an unrecognized threat in South India – clinical profile and predictors of mortality. Trop Doct 2010; 40: 129–133. doi: 10.1258/td.2010.090452. Epub 2010 Apr 1. PMID: 20360426. [DOI] [PubMed] [Google Scholar]
- 21.Mathai E, Rolain JM, Verghese GM, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci 2003; 990: 359–364. doi: 10.1111/j.1749-6632.2003.tb07391.x. PMID: 12860654. [DOI] [PubMed] [Google Scholar]
- 22.Alam AM, Gillespie CS, Goodall J, et al. Neurological manifestations of scrub typhus infection: a systematic review and meta-analysis of clinical features and case fatality. PLoS Negl Trop Dis 2022; 16: E0010952. doi: 10.1371/journal.pntd.0010952. PMID: 36441812; PMCID: PMC9731453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyaya A, Alam MR, Raeen AA, et al. Scrub typhus meningoencephalitis: an overlooked entity. Cureus 2022; 14: E28989. doi: 10.7759/cureus.28989. PMID: 36133506; PMCID: PMC9471502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaba S, Garg S, Gupta M, et al. Haemorrhagic encephalitis in the garb of scrub typhus. BMJ Case Rep 2020; 13: E235790. doi: 10.1136/bcr-2020-235790. PMID: 32859623; PMCID: PMC7454241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis 2014; 23: 39–43. doi: 10.1016/j.ijid.2014.02.009. Epub 2014 Mar 21. PMID: 24661931. [DOI] [PubMed] [Google Scholar]
- 26.Vivekanandan M, Mani A, Priya YS, et al. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 2010; 58: 24–28. PMID: 20649095. [PubMed] [Google Scholar]
- 27.Chaudhry R, Thakur CK, Gupta N, et al. Mortality due to scrub typhus – report of five cases. Indian J Med Res 2019; 149: 790–794. doi: 10.4103/ijmr.IJMR_1314_18. PMID: 31496533; PMCID: PMC6755786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mensa J, Gatell JM, García-Sánchez JE, et al. Guía de terapéutica antimicrobiana. 27th ed. Barcelona: Antares; 2017. [Google Scholar]
- 29.Cabrera-Maqueda JM, Fuentes Rumí L, Valero López G, et al. Difusión de los antibióticos en el sistema nervioso central. Antibiotic diffusion to central nervous system. Rev Esp Quimioter 2018; 31: 1–12. Spanish. Epub 2018 Jan 31. PMID: 29390599; PMCID: PMC6159365. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Supplemental material, sj-pdf-4-imr-10.1177_03000605231214503 for Clinical treatment of patients with scrub typhus-induced liver injury and intracranial infection by HongKui Sun, Li Lei, JianWei Li, Haiming Niu, Jiezhang Yang and MiaoLian Chen in Journal of International Medical Research
Data Availability Statement
All relevant data are included within the manuscript.