Abstract
Purpose:
To assess short-term efficacy of a single injection of brolucizumab in neovascular AMD.
Methods:
This was a multicenter, retrospective chart review of 25 eyes of 25 patients who received a single injection of brolucizumab. Visual acuity (VA) and optical coherence tomography (OCT) features such as central subfield thickness (CSFT), subretinal fluid (SRF), intraretinal fluid, and pigment epithelial detachment (PED) were recorded at baseline, first month, and third month.
Results:
Of the 25 eyes, 14 eyes were treatment-naïve and 11 eyes had received previous injections. VA improved from 0.68 ± 0.59 log MAR at baseline to 0.31 ± 0.43 log MAR at the end of 3 months. SRF height in first and third month was significantly reduced from baseline (P < 0.001). Subretinal hyperreflective material height significantly reduced from baseline (P value 0.008 at first month and 0.01 at third month, respectively). CSFT was 464.16 ± 247.97 microns at baseline and showed a significant reduction in first month (P < 0.001) and third month (P < 0.001). There was a significant reduction of PED height from baseline at both follow-ups. None of the eyes showed a recurrence of fluid at the end of 3 months.
Conclusion:
Our study demonstrated sustained improvement in VA and OCT parameters after a single injection of brolucizumab at 3 months. A longer follow-up may demonstrate even farther effects of a single injection.
Keywords: Brolucizumab, neovascular age-related macular degeneration, pigment epithelial detachment, subretinal fluid, subretinal hyperreflective material, type 1 macular neovascularization
Neovascular age-related macular disease (nAMD) is a chronic and progressive macular disease that causes major irreversible vision loss. Biologic agents that inhibit vascular endothelial growth factor (anti-VEGF) are the standard of care for the management of nAMD.[1] Anti-VEGFs need to be administered at frequent intervals and continued indefinitely depending on disease patterns and responsiveness to treatment for optimal outcomes.[2] There is a large amount of evidence on the safety and efficacy of bevacizumab, ranibizumab, and aflibercept, both from trials and real-world settings. There is also growing evidence about treatment regimens such as monthly dosing, treat and extend (TREX), and pro- re- nata (PRN).[3] TREX and PRN dosing regimens are instituted after three loading doses. Loading doses followed by monthly injections are impractical in real-world scenarios. Studies have shown the noninferiority of initiating TREX and PRN after a single injection when compared to instituting after three loading doses.[4] Molecules such as aflibercept and brolucizumab have the distinct advantage of having a longer treatment-free interval.[5,6]
The HAWK and HARRIER trials have shown the noninferiority of brolucizumab 6 milligram (mg)/0.05 milliliter (mL) compared to aflibercept (2 mg/0.05 mL). These two studies showed that nearly 50% of eyes maintained 12-weekly dosing after loading doses through week 48.[6] Brolucizumab has been marketed as Pagenax (Novartis India Ltd, Mumbai, India) since October 2020. Real-world data have shown that fewer injections of brolucizumab are needed in nAMD with a good safety profile. Herein, we describe the sustained effects of a single injection of brolucizumab in an Indian cohort on visual acuity and micromorphometric parameters at the end of three months.
Methods
This was a multicentric, retrospective, consecutive, nonrandomized and uncontrolled study. The study was approved by the institutional review board of both the tertiary centers and was in adherence to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients for data collection.
Patients who received intravitreal brolucizumab (Pagenax (6 mg/0.05 mL), Novartis India ltd, Mumbai, India) between November 2020 and February 2022 were included. Patients with treatment naïve nAMD or those who had received multiple anti-VEGF treatments (switch treatment) in the past with either bevacizumab, ranibizumab, or aflibercept for recalcitrant fluid (<100 ù reduction in fluid) or worsening of fluid on spectral domain optical coherence tomography on serial OCT scans were included. Eyes which had received bevacizumab and ranibizumab injections prior to 4 weeks and aflibercept injections prior to 8 weeks were included for switch treatment with brolucizumab. The presence or absence of subretinal fluid (SRF), intraretinal fluid, subretinal hyperreflective material (SHRM) and pigment epithelial detachment (PED) were noted. SRF height, SHRM height, PED height, and central subfield thickness (CSFT) was measured in the central 1-millimeter (mm) with the built-in caliper function. In eyes with multiple PEDs or multiple lobules, the tallest PED in the foveal line scan was taken into consideration. Only those eyes with nAMD (on OCT, fluorescein angiography and OCT angiography) were included, and polypoidal choroidal vasculopathy was excluded using indocyanine green angiography. Eyes with media opacities, coexisting vitreoretinal pathologies, history of retinal surgeries, and systemic vasculitis were excluded. Brolucizumab was used in patients who freely opted for it after providing a choice of all anti-VEGF agents. Injections were given under sterile technique. No perioperative drops were used. Patients were advised to follow-up in case of ocular or systemic side effects. Patients with at least two follow-up visits at 1 month and 3 months were included. None of these patients could come for follow up at 2 months, explaining the lack of data in these eyes at that time point. Patients who followed-up only till the second month were excluded. At follow-ups, standard ocular examination with best-corrected visual acuity (BCVA), intraocular pressure, slit lamp bio microscopy, indirect ophthalmoscopy, and OCT (Spectralis HRA-2, Heidelberg Engineering, Heidelberg, Germany) was performed.
Outcome measures
Change in BCVA and improvement in CSFT (1 mm) from baseline was noted. Qualitative analysis of micromorphometric features, like the presence or absence of SRF, IRF, and PED were noted. Change in CSFT, SRF height, and PED height was noted.
Statistical analysis was performed by SPSS 21.0 version (SPSS inc. Chicago III, USA). Continuous variables were described as mean ± standard deviation. Paired t-test was used for comparing means of follow-up values with baseline. Categorical variables were described by percentages; paired data in comparison with baseline was analyzed using Mc Nemar test. Variables with a P value < 0.05 was considered statistically significant.
Results
A total of 54 eyes of 54 patients received injection brolucizumab (Pagenax, 6 mg/0.05 mL) at two centers. Of the 54 eyes, 12-week follow-up data were available in 25 eyes. There were 11 females and 14 males. Mean age was 65.36 ± 12.09 years (Range: 28–84 years). The left eye was involved in 13 patients and the right eye in 12 patients. Type 1 macular neovascularization (MNV) was seen in all eyes. Fourteen eyes were treatment-naïve. Eleven eyes had received previous injections (mean number of injections- 4.52 ± 6.15, range- 0- 21, median – 2). The mean BCVA at presentation was 0.68 ± 0.59. At first month follow-up, BCVA was 0.46 ± 0.40 and 0.31 ± 0.43 at third month follow-up. At first month, there was no significant difference from baseline; however, significant improvement in vision was seen at third month (p. 0.001). SRF was present at baseline in 80% eyes (21/25), at first month in 40% eyes (n = 10, P < 0.001)) and 16% eyes at third month (n = 4, p. 0.004) [Figs. 1 and 2]. At baseline, IRF was present in nine eyes (36%) and in 6 eyes at first month. None of the eyes had IRF at third-month follow-up month [Fig. 3]. SHRM was seen in nine eyes at baseline (36%), six eyes at first visit (24%), and no eyes had SHRM at the end of 3 months. At baseline, PED was present in 22/25 eyes (88%) at baseline and persisted in only one eye at the first month. None of the eyes had PED at the end of 3 months. [Table 1]
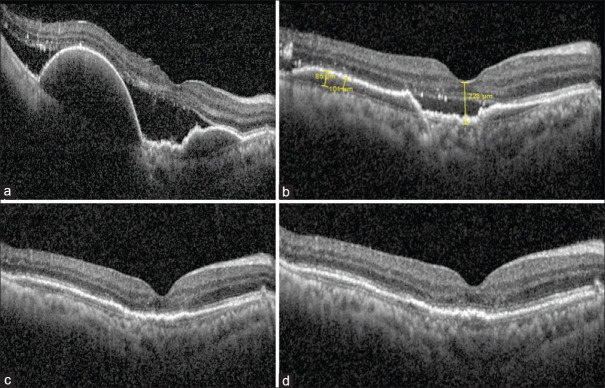
Figure 1.
(a) A 51-year-old patient with two large PEDs in the right eye with a flat irregular PED (FIPED) connecting the two, along with subretinal fluid received a single injection of Brolucizumab. (b) At first month, there was marked reduction of PED height as well as SRF. (c) At 2 months, there was resolution of the SRF with persistence of a flat irregular PED continuous with the pre-existing “bridging” FIPED. (d) At end of 3 months also, this effect was maintained with a visual acuity of 6/9 N6
Figure 2.
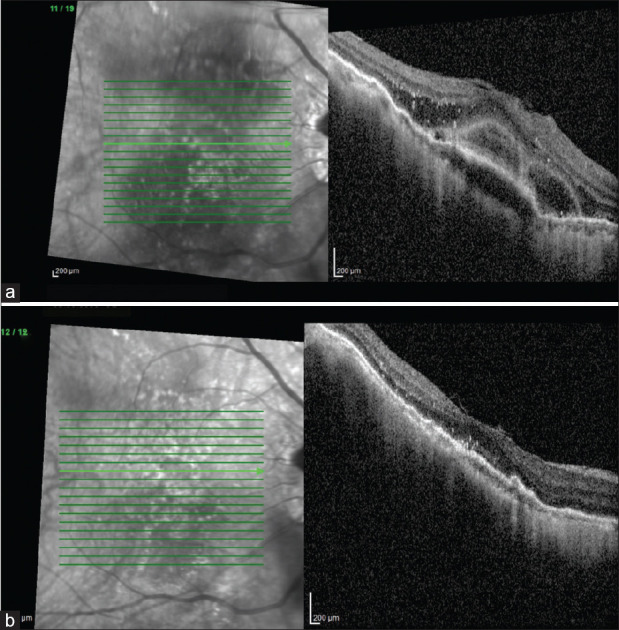
(a) A 66-year-old woman undergoing switch treatment with brolucizumab at baseline had subretinal fluid with bacillary layer detachment, PED, intraretinal hyper reflective foci (HRF), and spongy edema. (b) At the end of 3 months, the resolution of SRF and IRF and reduction of PED and HRF were maintained with a visual acuity of 6/12, N10
Figure 3.
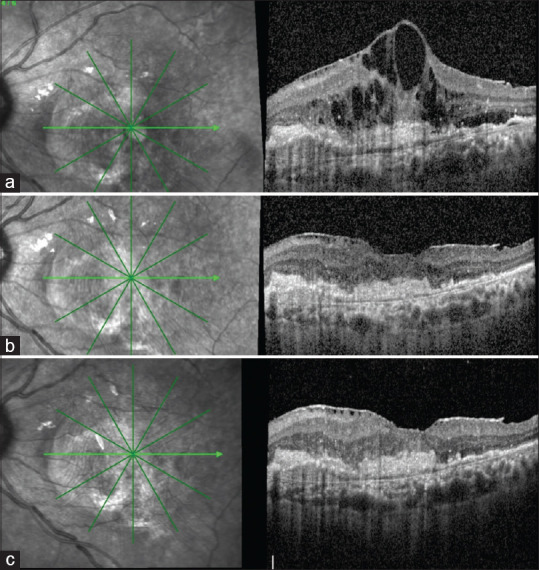
A 65-year-old male patient underwent switch treatment with brolucizumab after receiving 13 injections in the past. (a) Before injection with brolucizumab, there were large cystoid spaces at the fovea, SHRM, and FIPED in the nasal parafovea. (b) At first month, there was near-complete resolution of the cystoid spaces, reduction in SHRM height, and a concurrent epiretinal membrane (ERM). (c) At third month, the effect was maintained with a slight increase in the height of the nasal FIPED. The vision at 3 months improved remarkably to 6/6 N6
Table 1.
Comparison of qualitative variables between baseline, 1st visit, and 3rd visit
| Baseline | 1st visit | 2nd Visit | McNemar Test | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Frequency | Percent | Frequency | Percent | Frequency | Percent | Baseline vs 1st Visit | Baseline vs 2nd Visit | |
| SRF | ||||||||
| Absent | 4 | 16.0 | 12 | 48.0 | 21 | 84.0 | 0.004* | <0.001* |
| Present | 21 | 84.0 | 10 | 40.0 | 4 | 16.0 | ||
| IRF | ||||||||
| Absent | 15 | 60.0 | 16 | 64.0 | 21 | 84.0 | 0.25 (NS) | 0.15 (NS) |
| Present | 10 | 40.0 | 6 | 24.0 | 4 | 16.0 | ||
| SHRM | ||||||||
| Absent | 16 | 64.0 | 17 | 68.0 | 0.25 (NS) | - | ||
| Present | 9 | 36.0 | 6 | 24.0 | ||||
| PED | ||||||||
| Absent | 3 | 12.0 | 22 | 88.0 | <0.001* | - | ||
| Present | 22 | 88.0 | 1 | 4.0 | ||||
SRF height was 210.56 ± 209.50 μ (range 0–1001) microns at baseline; it was 24.26±±47.82 μ at first month and 54.04 ± 94.09 μ at third month. SRF height at first and third month was significantly reduced from baseline (P < 0.001). SHRM height was 114.28 ± 187.33 μ at baseline. At first and third month, SHRM height was 44.57 ± 90.82 and 51.16 ± 100.92 μ, respectively. SHRM height significantly reduced from baseline (p. 0.008 and 0.01 first and third month, respectively). CMT was 464.16 ± 247.97 μ at baseline. At first visit, CMT was 305.04 ± 99.7 μ, which was significantly reduced from baseline (P value < 0.001). At third month, CMT was 301.20 ± 141.89 μ which was also significantly reduced from baseline (P < 0.001). PED height at baseline was 329.04 ± 298.86 μ. PED height reduced to 140.70 ± 139.40 μ at first month (P < 0.001). However, at third month, PED height increased to 190.60 ± 188.88 μ. This value was still significantly lesser than the baseline PED height (P < 0.001). [Table 2]
Table 2.
Comparison of quantitative variables between baseline 1st visit, and 3rd visit
| n | Mean | SD | Min | Max | Percentiles | Wilcoxon Sign rank test | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Q1 | Median | Q3 | Baseline vs 1st Visit | Baseline vs 3rd Visit | ||||||
| Distance | ||||||||||
| Baseline | 25 | 0.68 | 0.59 | 0 | 2 | 0.2 | 0.5 | 1.15 | 0.18 (NS) | 0.001* |
| 1st Visit | 23 | 0.46 | 0.46 | 0 | 1.7 | 0.2 | 0.3 | 0.6 | ||
| 3rd Visit | 25 | 0.31 | 0.43 | 0 | 1.25 | 0 | 0 | 0.55 | ||
| PED height (microns) | ||||||||||
| Baseline | 25 | 329.04 | 298.86 | 0 | 1181 | 122 | 245 | 403 | <0.001* | <0.001* |
| 1st Visit | 23 | 140.70 | 139.40 | 0 | 538 | 39 | 120 | 182 | ||
| 3rd Visit | 25 | 190.60 | 188.88 | 0 | 850 | 71 | 145 | 271.5 | ||
| SRF height | ||||||||||
| Baseline | 25 | 210.56 | 209.50 | 0 | 1001 | 96.5 | 148 | 289 | <0.001* | 0.001* |
| 1st Visit | 23 | 24.26 | 47.82 | 0 | 200 | 0 | 0 | 33 | ||
| 3rd Visit | 25 | 54.04 | 94.09 | 0 | 313 | 0 | 0 | 76.5 | ||
| SHRM height | ||||||||||
| Baseline | 25 | 114.28 | 187.33 | 0 | 705 | 0 | 0 | 214.5 | 0.008* | 0.01* |
| 1st Visit | 23 | 44.57 | 90.82 | 0 | 343 | 0 | 0 | 47 | ||
| 3rd Visit | 25 | 51.16 | 100.92 | 0 | 335 | 0 | 0 | 42.5 | ||
| CMT (microns) | ||||||||||
| Baseline | 25 | 464.16 | 247.97 | 170 | 1113 | 276 | 403 | 586 | <0.001* | <0.001* |
| 1st Visit | 23 | 305.04 | 99.70 | 170 | 571 | 228 | 305 | 347 | ||
| 3rd Visit | 25 | 301.20 | 141.89 | 120 | 738 | 209 | 257 | 350.5 | ||
Discussion
Anti-VEGF therapy is the standard of care for MNV. There are distinct advantages of each agent. Bevacizumab is used off-label but is the most economical choice.[7] Ranibizumab has the highest evidence in literature and is FDA-approved for nearly 15 years.[8] Aflibercept has the advantage of a bimonthly dosing as it acts against multiple targets, namely, VEGF-A, VEGF-B, and placental growth factor.[9] Brolucizumab is long-acting owing to its low molecular weight, the resultant high molar dosing, and ≥12-week dosing interval. Brolucizumab by virtue of being a much smaller 26 k-DA anti-VEGF antibody allows for packing of a higher molecular concentration into 0.05 mL volume.[10]
HAWK and HARRIER were two similar phase 3 trials designed to demonstrate noninferiority of brolucizumab to fixed-dose aflibercept in terms of BCVA change from baseline to week 48. Eyes were randomized 1:1:1 to brolucizumab 3 mg, brolucizumab 6 mg or aflibercept 2 mg (HAWK) or to 1:1 to brolucizumab 6 mg or aflibercept 2 mg (HARRIER). Apart from BCVA change, key end points also included the percentage of patients who maintained q12w dosing through week 48 and other micromorphometric parameters. At week 48, each brolucizumab arm demonstrated noninferiority in mean BCVA change from baseline +6.6 (6 mg) and +6.1 (3 mg) letters with brolucizumab vs. +6.8 letters with aflibercept (HAWK); +6.9 (brolucizumab 6 mg) vs. 7.6 letters (aflibercept) (HARRIER) (P < 0.001 for each comparison). Greater than 50% of brolucizumab 6-mg treated eyes were maintained on q12w dosing through week 48 (56% [HAWK] and 51% [HARRIER]). At week 16, disease activity was seen in lesser eyes treated with brolucizumab 6 mg versus aflibercept in both HAWK and HARRIER. CSFT reductions from baseline to week 48 were observed with brolucizumab 6 mg versus aflibercept in HAWK (LS mean – 172.8 microns vs. 143.7 microns; P = 0.001) and HARRIER (LS mean -193.8 microns versus -143.9 microns, P < 0.001). Anatomical reductions in the fluid also favored brolucizumab over aflibercept. The proportion of study eyes that gained ≥15 letters at week 48 was 25.2% (brolucizumab 3 mg), 33.6% (brolucizumab 6 mg), and 25.4% (aflibercept 2 mg) in HAWK and 29.3% and 29.9% (brolucizumab 6 mg and aflibercept 2 mg, respectively) in HARRIER. In both HAWK and HARRIER, at matched 16-week assessment, i.e. 8 weeks after completion of loading doses in all patients, fewer brolucizumab eyes had disease activity (presence of IRF and/or SRF) versus aflibercept.[6]
BREW study – Sharma et al.[11] presented one of the earliest real-world data of brolucizumab in previously treated nAMD eyes. In their series of 42 eyes, 28 eyes received a single injection, nine eyes received two injections, three eyes had three injections, and one eye had four injections. Of the rest, 15 eyes received aflibercept and one eye received ranibizumab after brolucizumab (PRN). There was improvement in vision in all eyes at a mean follow-up of 7.2 ± 3.6 weeks. There was a significant reduction in CST (P = 0.0027). There was an effective resolution of SRF (approx. 40%); and IRF (approx. 37%) and reduction SRF and IRF in 45% and 42% eyes, respectively. There was a significant reduction in PED in about 50% of cases.
Pro re nata brolucizumab for exudative AMD (PROBE study) was a retrospective observational study from India, wherein outcomes of treatment-naive patients of nAMD in 27 eyes treated with PRN brolucizumab were studied. Patients had a minimum follow-up of 10 months. Seven eyes out of 27 showed completely resolved exudation after one injection, 13/27 eyes showed complete resolution after 2 injections, and only 7 eyes needed 3 or more injections. Recurrence was seen in 23/27 eyes before the end of the last follow-up and at a mean 3.7 ± 1.2 months after last injection. At the end of follow- up, 14/27 eyes retained BCVA.[12]
BRAILLE study was a retrospective study in treatment-naïve as well as previously treated eyes (either ranibizumab or aflibercept) for recalcitrant fluid in nAMD. A total of 94 eyes of 94 patients were included in this study of which 20 were treatment-naïve and remaining 74 eyes underwent switch therapy. The eyes that received switch therapy had received a mean of 8.63 ± 4.74 injections (range 3–44). A total of 126 injections were given. Of the total study population, 65 received a single injection, 24 eyes received two injections, and 5 eyes received three injections of brolucizumab. Final visual acuity in the treatment-naïve group was not statistically significant (baseline Log MAR BCVA 0.41 ± 0.35; final Log MAR BCVA: 0.36 ± 0.41; p. 0.36). However, switch group showed a significant improvement in final BCVA (baseline log MAR BCVA: 0.91 ± 0.49; final BCVA log MAR: 0.73 ± 0.51; P < 0.00001). There was a significant reduction in CST values in both treatment-naïve and the switch group. Complete resolutions of SRF, IRF, and PED were observed in 15.5%, 39.29%, and 23.81% eyes, respectively.[13] The authors suggested that a ceiling effect in patients with better baseline acuity could account for the visual improvement not being significant. However, SHIFT and BREW studies that looked at real-world data on brolucizumab after switching found no significant improvements.[11,14]
Haensli et al.[15] found the beneficial effect of brolucizumab in terms of increased reading acuity, in eyes undergoing anti-VEGF treatment with treatment intervals ≤6 weeks. Seven eyes completed the 6-month follow-up and had received 4.4 ± 0.5 injections in approximately 28 weeks. BCVA improved by approximately four letters. Reading acuity improved from 0.48 ± 0.15 to 0.31 ± 0.17 log MAR and CST reduced from 422 ± 97.3 to 353.6 ± 100.9 u. Although, a sustained reduction in CST after 6 months was not noted, a sustained improvement in reading acuity was seen.
Less intensive treatment regimens such as PRN and TREX have somewhat circumvented the need for fixed-interval dosing by fulfilling objectives of visual gains and an anatomically dry macula.[3,16] Studies have shown that the efficacy of a single dose followed by PRN provides outcomes comparable to regimens that included loading doses followed by PRN.[17] ALTAIR study has shown that four-weekly extensions can be attempted as opposed to standard two-weekly extensions.[18] Our study has demonstrated a sustained improvement in vision and OCT parameters at the end of the third month. A ”treat and observe” regimen may, thus, be permissible up to at least 12 weeks, even after a single dose. This review of literature is summarized in Table 3.
Table 3.
Review of literature – at a glance
| Sample | Methodology | Outcome | |
|---|---|---|---|
| HAWK | 1078 treatment- naïve nAMD eyes | 3 monthly loading doses, followed by q8w or q12w dosing based on disease activity assessment (brolucizumab) compared with q8w aflibercept. | Noninferiority of brolucizumab in mean BCVA change from baseline to week 48 |
| HARRIER | 739 eyes of treatment-naïve nAMD | 3 monthly loading doses of brolucizumab, followed by q8w or q12w dosing based on disease activity assessment (brolucizumab) versus q8w dosing (aflibercept) | Noninferiority of brolucizumab in mean BCVA change from baseline to week 48 |
| BREW | 42 previously treated eyes of nAMD | Brolucizumab given at baseline and then injected on PRN basis | Brolucizumab safe and effective in stabilizing BCVA in previously treated eyes |
| PROBE | 27 eyes of treatment naïve nAMD | PRN dosing, repeated if persistent fluid noted at weeks after first injection | >50% eyes with >10-15 letters visual gain. Recurrence noted at around 3.7 months. Eyes received an average of 2.2 injections per year |
| BRAILLE | 94 (20 treatment naïve eyes and 74 previously treated eyes of nAMD) | One injection of brolucizumab followed by PRN regimen | Efficacy noted with respect to BCVA, SRF, IRF, and PED with a mean retreatment interval of 10 weeks |
| Haensli et al. | 12 eyes (switch group) | Brolucizumab at baseline (within 6 weeks of previous Ranibizumab or Aflibercept injection), at 4 weeks and then 2 week incremental intervals based on morphological features on OCT | Benefit of switching to brolucizumab TREX regimen in terms of increased reading speed and increased treatment interval in eyes that were insufficiently responding to previous anti-VEGF agents in intervals of ≤6 weeks |
| Present Study | 25 (14 treatment- naïve and 11 previously treated eyes of nAMD) | Single injection of Brolucizumab assessed at end of 3 months for BCVA and micromorphometry | Sustained improvement in BCVA and reduction in OCT parameters such as SRF, IRF, PED, and SHRM at the end of 3 months |
Conclusion
Our study demonstrated sustained improvement in VA and sustained reductions of OCT parameters at 3 months. We suggest that loading doses are not needed with brolucizumab, and a single injection would be sufficient for showing sustained action for 3 months. A longer follow-up may demonstrate even farther effects of a single injection. The increase in the dosing interval will translate to better patient compliance and reduce cost burden in middle-income countries.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019;4:e000398. doi: 10.1136/bmjophth-2019-000398. doi: 10.1136/bmjophth-2019-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarthy U, Armendariz BG, Fauser S. 15 years of anti-VEGF treatment for nAMD: Success or failure or something in between? Eye. 2022;36:2232–3. doi: 10.1038/s41433-022-02153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;5:CD012208. doi: 10.1002/14651858.CD012208.pub2. doi: 10.1002/14651858.CD012208.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1:314–21. doi: 10.1016/j.oret.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 7.van Asten F, Michels CTJ, Hoyng CB, van der Wilt GJ, Klevering BJ, Rovers MM, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration—A cost-effectiveness analysis from a societal perspective. PLoS One. 2018;13:e0197670. doi: 10.1371/journal.pone.0197670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 9.Weber M, Dominguez M, Coscas F, Faure C, Baillif S, Kodjikian L, et al. Impact of intravitreal aflibercept dosing regimens in treatment-naïve patients with neovascular age-related macular degeneration: 2-year results of RAINBOW. BMC Ophthalmol. 2020;20:206. doi: 10.1186/s12886-020-01468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen QD, Das A, Do DV, Dugel PU, Gomes A, Holz FG, et al. Brolucizumab: Evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020;127:963–76. doi: 10.1016/j.ophtha.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Kumar N, Parachuri N, Sadda SR, Corradetti G, Heier J, et al. Brolucizumab-early real-world experience: BREW study. Eye (Lond) 2021;35:1045–7. doi: 10.1038/s41433-020-1111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilgic A, Kodjikian L, Srivastava S, Dwivedi S, Banker AS, Abukashabah A, et al. Initial pro re nata brolucizumab for exudative AMD: The PROBE study. J Clin Med. 2021;10:4153. doi: 10.3390/jcm10184153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty D, Maiti A, Sheth JU, Boral S, Mondal S, Nandi K, et al. Brolucizumab in neovascular age-related macular degeneration – Indian real-world experience: The BRAILLE study. Clin Ophthalmol. 2021;15:3787–95. doi: 10.2147/OPTH.S328160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulirsch LM, Saßmannshausen M, Nadal J, Liegl R, Thiele S, Holz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2022;106:1288–94. doi: 10.1136/bjophthalmol-2020-318672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haensli C, Pfister IB, Garweg JG. Switching to brolucizumab in neovascular age-related macular degeneration incompletely responsive to ranibizumab or aflibercept: Real-Life 6 month outcomes. J Clin Med. 2021;10:2666. doi: 10.3390/jcm10122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: A comparative study. Graefes Arch Clin Exp Ophthalmol. 2019;257:1889–95. doi: 10.1007/s00417-019-04404-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Yuan Y, Wang L, Ye X, Zhao J, Shen M, et al. One-year outcomes of 1 dose versus 3 loading doses followed by pro re nata regimen using ranibizumab for neovascular age-related macular degeneration: The ARTIS trial. J Ophthalmol 2019. 2019:7530458. doi: 10.1155/2019/7530458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR. Adv Ther. 2020;37:1173–87. doi: 10.1007/s12325-020-01236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]