Abstract
Filariae of animals, especially those of mammals, often infect humans and typically produce cryptic infections. These “zoonotic” infections have been reported from virtually all parts of the world including temperate zones. Infections may be symptomatic or not, and the parasites are found in surgical tissue biopsy specimens or, more rarely, are removed intact from superficial sites such as the orbit or conjuctivae. Typically, these worms tend to occupy tissue sites similar to those occupied in the natural animal host, with the exception of the eyes. Many kinds of filariae have been isolated from humans, including species of Dirofilaria, Brugia, Onchocerca, Dipetalonema, Loaina and Meningonema. Worms have been found in subcutaneous tissues, the heart and lungs, lymphatics, the eye, and the central nervous system. Specific identification of these filariae is based on their morphological features in histologic sections. Unfortunately, some of these worms cannot be identified even at the generic level. There are other species of filariae, presumed to be zoonotic, which produce patent infections in humans but are poorly and incompletely known. These include Microfilaria semiclarum and Microfilaria bolivarensis. It is probable that almost any filaria parasitizing animals can, under proper circumstances, infect humans and undergo some degree of development. Undoubtedly, additional species of filariae will continue to be isolated from humans in the future.
Human infections with filariae of animals, referred to as zoonotic filariasis, occur worldwide. First reported in modern literature more than 100 years ago (2), the numbers of cases and parasite species involved have steadily increased. Many widely different species of filariae have been identified as agents of infection (47, 62). Although various species of filariae can cause common infections in birds, reptiles, and amphibians, to date only filariae which are natural parasites of mammals have been recorded as causing zoonotic infections. All of the filariae utilize bloodsucking insects as biological vectors, so that humans are infected by zooanthropophilic species which fed previously, in an appropriate time frame, on an animal with a patent filaria infection (7).
The infective larvae of these filariae invade a variety of human tissues and elicit little or no discernible response from the host during the course of their development unless they enter exquisitely sensitive tissues such as the conjunctivae. However, when these parasites die in the tissues, the host mounts a foreign body response to their presence. It is unclear in these cases whether the parasite becomes moribund and the host responds to the dying worm or whether the host ultimately mounts a response which kills the worm. Inasmuch as most of these infections persist for months without a detectable host response, it seems likely that at some level, the worm finds itself in an unnatural host and succumbs and that this is followed by a tissue reaction to the dying worm. This argument is further strengthened by the observation that in their natural hosts, filariae are typically long-lived, living often several years or more. Zoonotic infections are typically cryptic; i.e., only in rare instances are circulating microfilariae found (45, 57, 80).
In a majority of instances, the parasites are found in tissue biopsy specimens; less frequently, they are removed from the tissues intact. Typically, only one (sometimes two, but rarely more) worm is removed from an individual; removal is therapeutic.
Because the parasites are found most frequently in sections of tissue, their identification depends on a knowledge of the micromorphologic features of the individual parasite species. Often, many of these filariae can be accurately identified at the generic level from the morphology of the body wall. It is possible to determine the sexual maturity and reproductive state of female worms by examination of the contents of the reproductive tubes, especially the ovaries, seminal receptacles, and uterine branches either in the intact worms or in transverse sections (67). Some parasites are well described and characterized, while others are not. Consequently, species identifications are often difficult if not impossible.
In this review, we discuss the species of filariae recovered from humans on the basis of the tissue locations in which they are found. The behavior, biology, and morphologic features of the parasites in their gross and microscopic aspects based on current knowledge are discussed.
PARASITES BY TISSUE LOCATION
Skin and Subcutaneous Tissues
Dirofilaria spp.
The zoonotic filariae recovered most commonly from humans are found in the subcutaneous tissues; the vast majority belong to the genus Dirofilaria. The dirofilarias are natural parasites of a great variety of animals; with the exception of the heartworms, they live in the subcutaneous tissues of their hosts, produce microfilariae which circulate in the blood, and in most instances are transmitted by species of mosquitoes. Development in the mosquito requires about 2 weeks; these parasites require several months to reach sexual maturity in their natural definitive hosts (60). Since the worms recovered from humans are usually sexually mature, they must have undergone development for an extended period without any significant challenge to their presence from the host. Symptoms which signal their presence include transitory inflammatory swellings or nodules (Fig. 1), which may or may not be tender or painful. If living worms enter the conjunctiva, they may cause acute symptoms, but the affected individual usually seeks immediate medical attention. However, worms are recovered more frequently in excised nodules (Fig. 2) than in the intact living state (Fig. 3). Female worms are typically infertile, although gravid female worms have been found (51, 71, 73); however, patent infections are extremely rare (57).
The earliest documented report of Dirofilaria infection in humans dates back to the report of Addario (in 1885), who removed a worm from the eyelid of a woman in Milan, Italy (2). However, Dirofilaria infection in humans may go back more than 400 years in history. Amatus Lusitanus reported a clinical case of ocular filariasis in a 3-year-old child in southern France that in all likelihood can be attributed to Dirofilaria repens (72). This report and later commentary by Aldravando suggest that similar cases were not uncommon in southern France at that time (4). These worms were referred to early on as Filaria conjunctivae and later as D. conjunctivae because of their frequent association with the orbit (2, 28). Later, when the worms were subjected to critical examination and species identification, the previous artificial taxon was dropped in favor of the actual species (19). In the United States, the earliest reports of human dirofilariasis did not appear until the 1950s (37, 38).
In the United States, particularly the southeast, Dirofilaria tenuis, a common parasite of raccoons, is regarded as the most common agent of human infections (62). The gross and microscopic features of the parasite were described in detail by Orihel and Beaver in 1965, so identification of intact worms removed from human tissues is not particularly difficult (63). The isolation of intact worms is especially useful in judging their state of maturation. D. tenuis worms recovered from human tissues measure about 10 cm in length and have an average diameter of approximately 300 μm (Fig. 3). The surface of the cuticle, except at the extremities, bears longitudinal ridges which grossly have a beaded appearance; this is readily evident in gross specimens (Fig. 4) and in histologic sections (Fig. 5) (63).
More important are the morphologic features seen in transverse sections of the worms. The cuticle is multilayered, about 5 to 8 μm thick in living worms; the longitudinal ridges are low, smoothly rounded, and about 10 μm apart (Fig. 6). Approximately 90 ridges are seen at the midbody level. On the inner surface of the cuticle in the lateral fields, there is a conspicuous cuticular ridge that protrudes into the inner surface of the lateral chords (Fig. 7). In dead, degenerating worms, the cuticle typically is swollen and thickened, the internal ridges are much enlarged, and the longitudinal ridges may remain conspicuous (Fig. 8). Lateral chords are a prominent morphological feature, and these also become swollen and vacuolated in the dead or dying worm (Fig. 8). Muscle cells are the coelomyarian type and are well developed; there are about 17 to 22 per quadrant at the midbody level (Fig. 6). They degenerate quickly in the dead worm and lose their normal architecture (Fig. 7 to 9). Not much attention is given to the number of muscle cells usually seen in transverse sections of the worm, but comparison of species suggests that there may be sufficient differences among some species to warrant further study of this feature. The number of reproductive tubes and their contents (eggs, microfilariae, and sperm) help identify the sex of the worm (Fig. 7 to 9). However, these soft tissues degenerate quickly in the dead worm, and often only the connective tissue of their outer wall remains (Fig. 9). Rarely, sections through the posterior end of a male worm reveal caudal alae, papillae, and spicules (Fig. 10).
Other species of Dirofilaria isolated from humans include Dirofilaria ursi, which parasitizes bears, and Dirofilaria subdermata, a parasite of porcupines in the northern United States and Canada. Unlike the other dirofilarias, D. ursi utilizes blackflies (Simulium species) rather than mosquitoes as vectors (7). These two Dirofilaria species are very similar in morphology and even more difficult to differentiate in transverse sections. Consequently, specimens recovered from human tissues are usually designated “D. ursi-like.” At least 10 cases of D. ursi-like infections have been reported in the northern United States and Canada (21). Although D. ursi has wider geographical distribution (Alaska, Japan, and Russia) in its natural host, human infections have not been reported in these other locations as far as we know. In the reported infections, the worms were found in sectioned granulomatous lesions removed from the scalp and upper body of the individuals. The worms were characterized as having the basic morphological features of Dirofilaria but with a small diameter (80 to 260 μm) and with approximately 65 to 90 tall, sharply crested, widely spaced ridges on the body circumference (Fig. 11). The ridges seem to be equally evident in living and necrotic worms (21, 47).
Dirofilaria striata, a natural parasite of felids (bobcats, pumas, panthers, etc.) in North and South America, has been isolated once from a human—a 9-year-old boy from North Carolina (68). A 28-cm-long female worm was removed intact from the orbit. This species is much larger than most other dirofilarias (females are 25 to 28 cm long by 440 to 550 μm in diameter; males are 8 to 8.5 cm long by 350 to 380 μm in diameter) and easily recognized by this feature. In transverse sections, the longitudinal ridges are relatively inconspicuous (Fig. 12). A key defining feature is the presence of small, rounded lateral alae on the surface of the cuticle (Fig. 13).
In Europe and parts of Africa and Asia, D. repens is a common subcutaneous parasite of dogs and, in some areas, of cats and wild carnivores as well. This filaria is an important agent of human subcutaneous filariasis. Its geographical distribution may overlap with that of Dirofilaria immitis in some areas (73).
Recently, Pampiglione et al. made an extensive review of human infections with D. repens (73). They enumerated more than 400 cases from 30 countries in Europe, Asia, and Africa. The largest number (181 cases) were from Italy. The majority of the worms (76%) presented in nodules in subcutaneous and subconjunctival tissues on the upper half of the body, with the largest number localized around the eyes, in the eyelids, or under the conjunctiva. On the lower half of the body, nodules were found on the torso, the extremities, and the male sexual organs (scrotum, penis, and testicles). In deeper locations, worms were found in the peritoneal cavity, omentum, and ligaments. Interestingly, several cases involved the lungs and pulmonary blood vessels. These will be discussed below. Female worms were found with greater frequency than males.
Because adult D. repens worms have been recovered from various natural definitive hosts in different parts of the world, there is a wide variation in the reported sizes of males and females. Compared to other dirofilarias, D. repens is a robust parasite; mature female worms have a maximum length of 17 cm and a diameter of 650 μm, whereas males are about 7 cm long by 450 μm in maximum diameter (6).
Worms recovered intact from the tissues do not pose significant problems in identification, since one can rely on documented descriptions of the gross features of the species (6). Identification of the parasite in transverse sections is more difficult. Emphasis is placed on size and the features of the body wall (Fig. 14 and 15). Parasites from human tissues identified as D. repens measure up to 660 μm in maximum diameter (46, 73). Female worms are invariably larger, even in the immature state, than are comparable worms of related species including D. tenuis, D. ursi, and D. immitis (55). Gutierrez states that key features useful in identification of the species include “… longitudinal ridges separated by a distance wider than the ridge itself, 95–105 ridges on the circumference of the body and 2–5 chord nuclei in each cross section of worm” (47). Our own experience suggests that the shape, height, and interridge distances are actually quite variable at different levels of the body in the same worm or even within a single transverse section and hence do not constitute reliable criteria (Fig. 15).
It should be pointed out that identification and/or confirmation of the species is ultimately closely correlated with the geographical area where the infection was believed to have been acquired, i.e., where the individual lives or possibly has traveled to.
Since our knowledge of the key diagnostic morphological features of many dirofilarias in sections of tissues is limited and since investigators find worms that do not match any of those that are most familiar to them, these will often be reported as D. tenuis-like, D. ursi-like, or D. repens-like.
All of the described species of Dirofilaria which live in the subcutaneous tissues of their natural hosts, with the exception of Dirofilaria lutrae, have longitudinal ridges on the surface of the cuticle. Most dirofilarias recovered from human subcutaneous tissues also have these cuticular features. When worms with smooth cuticles are found in the subcutaneous tissues, identification becomes a problem. Among the dirofilarias, only the heartworm, i.e., D. immitis (= D. spectans), and D. lutrae have a smooth cuticle. Only D. immitis is typically found in the heart, pulmonary arteries, or other blood vessels of humans. D. lutrae has not been identified in human tissues. Some investigators have concluded that the early developmental stages of D. immitis may in fact be recovered from subcutaneous tissues, since this is where early development occurs in the natural host (48). Consequently, dirofilarias with smooth cuticles are often designated D. immitis-like (48).
Onchocerca spp.
Another group of filariae, members of the genus Onchocerca, have caused zoonotic infections on rare occasions. Many species of Onchocerca are natural parasites of animals, particularly bovines and equines worldwide, and one species (Onchocerca volvulus) infects humans in Africa and Central and South America. Six cases of human infections with the onchocercas of animals have been reported outside the areas of endemic human onchocerciasis infection, namely, in the United States, Canada, Switzerland, the Russian Crimea, and Japan. Infections presented as firm subcutaneous nodules that were painful or not and were located in a tendon of an eye muscle, the knee, the wrists (in two patients), and the sole of the foot (in one patient) (5, 9, 17, 22, 79, 82). The cases reported to date all involved a single female worm. In their natural definitive hosts, Onchocerca parasites have a marked predilection for connective tissue and typically are highly coiled in discrete nodules; this is true of the zoonotic infections as well (Fig. 16).
Species of Onchocerca have distinctive morphological features that are evident grossly and in tissue sections of the parasites and that allow an accurate identification to the genus level. Typically, the female worm has a thick, multilayered cuticle which bears prominent annular ridges on the external surface and transverse striae in the underlying layer (Fig. 17). The number of striae between consecutive annular ridges is useful in identifying species in many cases (17, 30). In transverse sections of female worms, the cuticle varies widely in thickness; this is attributed to the annular ridges on the outer surface (Fig. 18 and 19). In male worms, the cuticle is thin, not visibly divided into layers, and less ornate than that of the female. Although there are marked annular striations in the cuticle of male worms, these are not seen in transverse section. In addition to the cuticle, the musculature and hypodermis have equally distinctive features. It is not unusual for the muscle cells, even in living worms, and particularly in female worms, to appear weak, poorly developed, and somewhat atrophied (Fig. 18 and 19). In contrast, the cuticle is much thinner and the musculature is much better organized and developed in male worms (Fig. 20). Generally, individual muscle cells are coelomyarian type, in which the contractile portion has a loose, fibrillar appearance; there are usually fewer than 12 cells per hemisphere, with those in the ventral region being much taller than those in the dorsal hemisphere. The hypodermis is usually very conspicuous, underlying the bands of muscles, and the lateral chords are equally large and conspicuous. The gut is vestigial and is often overlooked because of its small size and weak nature. Generally, the morphological features of onchocercas are sufficiently characteristic to allow recognition at the genus level. However, assigning an accurate species designation is difficult and often impossible.
Interestingly, in the published cases, the morphological features of all six worms were strikingly similar, suggesting that they were the same species; they most closely resembled Onchocerca gutturosa, a parasite of cattle with worldwide distribution.
Heart and Pulmonary and Other Blood Vessels
Dirofilaria spp.
Reports of “heartworms” in humans date back to before the turn of the century. These have been described as species of Dirofilaria, i.e., D. magalhaesi (23) and D. louisianensis (39). Another parasite, D. spectans, has been found in blood vessels on at least one occasion (41). It is probable that all of these are D. immitis, the common heartworm of dogs.
D. immitis (heartworm) is a common parasite of dogs and other canids in many regions of the world and on occasion has been isolated from other animals such as seals, beavers, horses, domestic and wild cats, bears, nutrias, and muskrats. The adult worms live in the right side of the heart (Fig. 21) and produce microfilariae, which circulate in the peripheral blood; the parasite is transmitted by mosquitoes. In the dog, the parasite undergoes its early development in the subcutaneous tissues for about 3 months before migrating to the right side of the heart (58). The large number of mosquito species that can transmit the parasite and the shared environment of humans and dogs probably accounts for the frequency with which humans are infected. Human infections with D. immitis have been reported in many parts of the world and are found wherever the parasite is enzootic.
Although adult D. immitis worms have been found on several occasions in the heart and major vessels of humans at necropsy (1, 39, 44), the usual finding is for immature worms to be located in partially or completely occluded small pulmonary arteries, where the obstruction has produced an infarct and eventually a well-circumscribed, granulomatous, coin lesion (Fig. 22) containing the worm (19). The lesions, which measure about 1 to 3 cm in diameter, are frequently found on routine chest X rays (Fig. 23). Because these lesions mimic neoplasms and other pathologic conditions (including tuberculosis, fungal infections, and hamartomas), they typically receive immediate medical attention. As often as not, individuals with coin lesions in the lung are completely asymptomatic. When symptoms are associated with the lesion, they include cough, chest pains, moderate eosinophilia, and occasionally hemoptysis and fever (24). Most individuals harbor a single nodule, but two and three nodules have been reported (3, 27).
Identification of the intact adult worms in these cases is straightforward and is rarely a problem. Their presence in the heart chamber or adjacent great vessels, their exceptionally large size and unique morphological features, and a history of residence in an area of enzootic infection usually suffice for making an identification.
The microanatomy of worms in these zoonotic infections is, as expected, similar to that of the worms in their natural host, and the microscopic morphologic features of the worms render their identification in sections of tissues equally straightforward. D. immitis has a thick, multilayered, smooth cuticle with prominent, internal, cuticular ridges. The hypodermis is well demarcated, and the lateral chords are large and prominent. Muscle cells are the coelomyarian type, tall and very slender; they are numerous, with about 25 being present in each quadrant of the body (Fig. 24). Sexes are determined by the number of reproductive tubes present in the body cavity, typically two in females and only one in males (Fig. 24).
Female worms in granulomas are typically immature and usually measure up to 325 μm in diameter. Because the worms apparently die before or at the time they occlude the blood vessel, they are usually in some stage of degeneration (Fig. 25). Often, the cuticle is the most recognizable part of the worm, and it is usually enormously thickened (Fig. 26). The intestine and genital tubes are recognized only by their basal lamellae; the epithelium and contents of the tubes have totally disappeared. The hypodermis, including the large lateral chords, degenerates rapidly as well and often has a frothy appearance. Muscle cells retain a semblance of their normal architecture longer than other elements of the body. Their large number usually remains evident, but this depends on the degree of degeneration that has taken place. On rare occasions, gravid female worms have been found, as evidenced by the presence of microfilariae in utero (38). Patent infections have not been reported.
An adult male worm, identified as D. spectans, was removed from the digital artery of a woman in Rio de Janeiro, Brazil (41). This parasite species was originally isolated from the heart and pulmonary blood vessels of Brazilian otters (40). However, there are few features that clearly distinguish it from D. immitis, so its validity as a separate species remains in question.
In their 1995 review of D. repens infections, Pampiglione et al. documented a total of nine infections in which worms were located “… inside a thrombotic arterial vessel on the periphery of the lung which gives rise to a small area of infarction” (Fig. 27) (73). Seven of the nine cases were from Italy. The worms were easily identified as D. repens on the basis of their typical dirofilarid anatomical features and the presence of longitudinal ridges on the surface of the cuticle (Fig. 28).
Other genera.
Other kinds of filariae have been found in pulmonary blood vessels. In 1971, Beaver et al. identified a mature but infertile female filaria associated with a pulmonary infarct in a jute mill operator in India as a Brugia species closely resembling Brugia malayi, a natural lymphatic-dwelling parasite of humans (Fig. 29) (16). Later, Beaver and Cran reported a living, sexually mature but infertile female filaria associated with a pulmonary infarct in an individual who had recently returned from service in Singapore (15). The worm closely resembled Wuchereria bancrofti, a natural lymphatic parasite of humans (Fig. 30). More recently, Beaver et al. described a small, mature, but infertile female filaria from a necrotic, peripheral, subcutaneous artery of a young boy in Costa Rica (14). The worm could not be identified to genus or species level, but the authors were certain that it was not a Dirofilaria, Onchocerca, Wuchereria, or Brugia species.
Lymphatic System
Brugia spp.
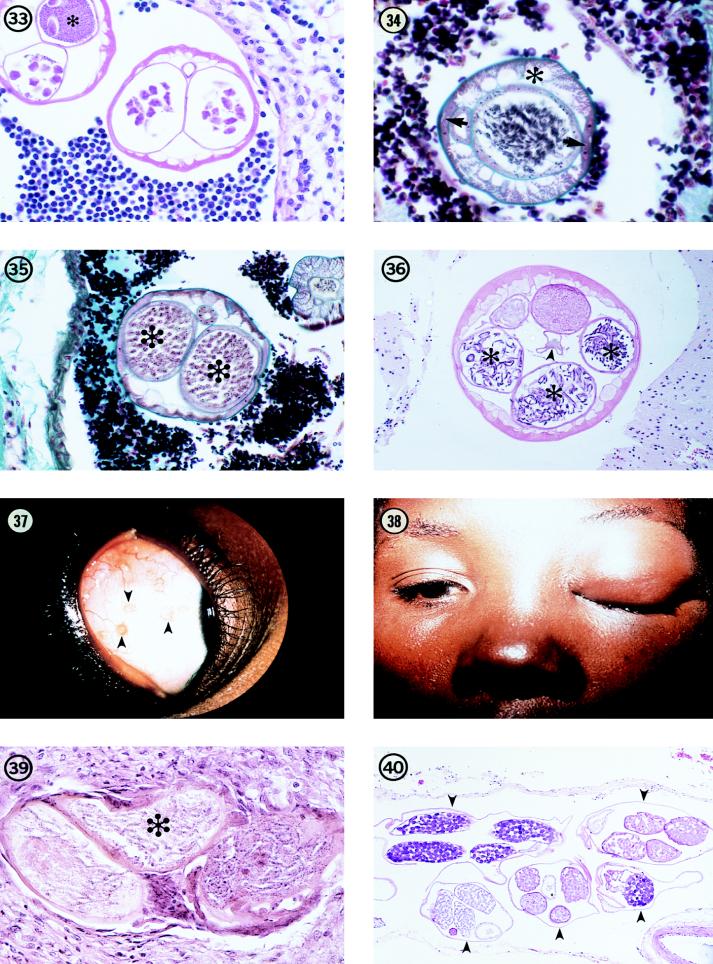
There are several species of Brugia which infect a wide variety of animals ranging from tree shrews and monkeys in Southeast Asia to raccoons and rabbits in the United States (31, 59, 69). The adult parasites typically inhabit the lymphatic system of their hosts, and the microfilariae circulate in the peripheral blood. In humans and monkeys, Brugia microfilariae typically exhibit nocturnal periodicity, but in lower animals, there is usually no evidence of any periodicity. In all instances, they utilize mosquitoes as vectors. Development in the vector requires about 10 days or more. Development in the final host usually takes at least 60 to 90 days. In experimental rodent hosts, adult worms often are found in heart chambers and subcutaneous tissues (8).
The first published report of human infection with a zoonotic Brugia sp. occurred in 1962 in a resident of New York City, who presented with painless swelling of inguinal lymph nodes (76). Although this disorder was initially diagnosed as lymphoma, examination of histopathologic material revealed the presence of three small filarial worms identified as Brugia-like, although the individual had never visited or lived in an area where brugian filariasis was endemic. Since that time, there have been at least 29 recognized cases in the United States: 8 from New York; 3 each from Connecticut, Massachusetts, Pennsylvania, and Rhode Island; and 1 each from California, Florida, Louisiana, Michigan, Mississippi, North Carolina, Ohio, Oklahoma, and New Jersey (32, 35, 64). Furthermore, an additional five cases have been documented outside the United States: two in Colombia and one each in Brazil, Peru, and Ethiopia (10, 56, 64). Of these cases, about one-third involve lymph nodes in the groin, one-third involve the neck or head region, and the remainder involve other sites including the breast, arm, conjunctiva, and torso. In two instances, the diagnosis was based on the presence of microfilariae in peripheral blood (56, 80); in one of these patients, microfilariae were observed in the blood at different times over a period of several days (80). In all the other cases, the diagnosis was based on an examination of histologic sections of the affected lymph node (Fig. 31 and 32). In two of these cases, one from Colombia and one from Peru, the female worm was gravid (Fig. 33), indicating that at least one additional worm, a male, was present and that mating had occurred.
Because of the limited number of nematode parasites that are known to occur typically in lymphoid tissues, the presence of a small filarial worm in or associated with a lymph node is virtually pathognomonic for one of two genera, Wuchereria or Brugia. These two parasites are very similar in their morphologic features, but in general Wuchereria, a natural human parasite, is much larger at a comparable stage of development than is Brugia. Both have a thin, smooth cuticle which is perceptibly thickened over the lateral chords, three or four well-developed, low, broad muscle cells per quadrant, and relatively broad, flat lateral chords (Fig. 29 to 35). In individuals who have resided in or previously traveled in an area of bancroftian filariasis endemic infection, distinguishing these two parasites would be very difficult except for their sizes.
In the zoonotic Brugia infections documented to date in the United States, the worms were all very small, ranging between 45 and 75 μm in greatest diameter, including female worms that were gravid. Male worms were appreciably smaller than females. The worms recovered from individuals in South America tended to be somewhat larger than those recovered from individuals in North America; for example, female worms were approximately 100 μm in diameter. This difference is undoubtedly a reflection of the different species of Brugia involved in these geographical areas.
The ability to make a species identification in any of these cases of Brugia infection has been hampered by our limited knowledge of the micromorphologic features of each species and the marked morphological similarity of these parasites in transverse sections. Only one species found in animals (with the exception of Brugia malayi), namely, Brugia beaveri, has been studied and described in any detail (49).
Based on the descriptions of the brugias drawn from case reports and the experimental studies on B. beaveri, the following morphologic features characterize the zoonotic Brugia parasites (Fig. 29 and 31 to 35). The cuticle is smooth and thin, 1 to 2 μm thick, except over the lateral chords, where it thickens to 3 to 5 μm. In contrast to many other filariae, no distinct layering of the cuticle is evident. The lateral chords are usually large, occupying up to one-third of the circumference of the worm. There are three to five muscle cells per body quadrant; these cells are rather flat and broad, and each is about equally divided into cytoplasmic and contractile portions. The intestine is a simple tube with a small diameter that is often difficult to see or find. In female worms, the uterine tubes are large and generally fill the pseudocelomic cavity even when the worms are nongravid. The tubes usually contain various numbers of unsegmented ova; in gravid worms, the tubes contain developing eggs and microfilariae (Fig. 35). Male worms, always smaller in diameter than comparably aged females, have a single reproductive tube that contains developing spermatozoa (Fig. 34). Depending on the level of the body, one may see portions of the muscular and glandular elements of the esophagus (anterior extremity) or, in the posterior end of the body, sections through the spicules, cloaca, and, on the ventral surface of the body, genital papillae.
The current state of knowledge about human infections with species of Brugia and the identification of the species involved is undeniably limited. Because the worms are typically lodged in host lymphatic tissues, they are always seen in histologic material, never (to date) in the intact state. Worms are easily recognized at the genus level on the basis of their morphology in human tissues. As stated above, species level features have not been ascertained for any of the described species. We know that there are probably many undescribed species in local animals. For example, human infections with Brugia spp. have been described in geographical areas where Brugia species have not yet been found in animals. We assume that human cases in the United States are probably due to B. beaveri, a parasite of raccoons, or Brugia lepori, found in rabbits, but this is not based on the morphologic features of these species. It is obvious that much remains to be learned about zoonotic brugian filariasis before we are comfortable in our understanding of this infection.
Eyes
Worms presumed to be filariae or accurately identified as such have been found on numerous occasions in the eye. In 1989, Beaver reviewed the subject in depth and found reports of 56 such cases from all over the world during the period from 1771 to 1989 (13). Some were described from visual observations of the eye, and others represented worms that appeared and then disappeared spontaneously from the field of view during clinical examinations. Actually, only six worms were removed from the eye, described, and identified (13).
In a number of instances, these infections were attributed to filariae, such as W. bancrofti and Loa loa, that are natural parasites of humans, although descriptions of the worms generally were not provided in the reports (13). Other cases were attributed to species of Dirofilaria including D. immitis, D. repens, and D. roemeri. In a few of these cases, the worms were clearly of zoonotic origin. They were removed intact (or broken) from a chamber of the eye and identified on the basis of gross morphologic features.
Dipetalonema spp.
Worms in cases isolated from three individuals in western Oregon have been identified tentatively as either Dipetalonema arbuta, a natural parasite of the porcupine, or D. sprenti, from the beaver (18). The worms, being females, had no unique, distinguishing, gross, morphologic features that allowed unequivocal identification; however, other morphologic features such as the structure of the cuticle, position of the nerve ring and vulva, and general arrangement of the reproductive system strongly support the diagnoses. In general, these worms have a smooth cuticle devoid of ridges or striae and an esophagus that is long and clearly divided into muscular and glandular parts. The tail is long and bears two or more papillae. The internal morphology as seen in microsections is not well described, but on the basis of what is known about this group, one expects to see prominent lateral, internal, cuticular ridges and numerous, tall, coelomyarian muscle cells. The Dipetalonema group has undergone extensive revision over the years since the term “Dipetalonema-like” was coined. Currently, the species sprenti and arbuta are placed in the genus Molinema. Species in this genus are transmitted by mosquitoes (7).
Loaina spp.
In 1984, Eberhard and Orihel erected a new genus, Loaina, to accommodate certain species of Dirofilaria, i.e., Dirofilaria uniformis and Dirofilaria scapiceps, that were clearly unrelated to that taxon based on both biological and morphologic features of the microfilaria and adult worms (34). They also noted that D. roemeri most probably should be included in the genus Loaina as well. Loaina uniformis and L. scapiceps are natural parasites of rabbits in the United States, although their geographical distribution is incompletely known, while L. roemeri is a natural parasite of kangaroos in Australia. In 1984, a Loaina-like worm was recovered from the eye of a patient in Colombia, South America (25). Interestingly, the source of the infection was not determined, since this parasite has not been recorded in the animals in Colombia. It should be said, however, that this is probably due primarily to the lack of knowledge of the parasites of the local fauna. Limited studies of Loaina in histologic sections indicate that this parasite is readily distinguished from species of Dirofilaria as well as other filariae (34). The cuticle is comparatively thin, lateral chords are large, and there are only a few muscle cells per body quadrant (Fig. 36). Additionally, the arrangement of the reproductive system within the pseudocelom is unique and characteristic of Loa and Loaina (Fig. 36) (33). L. scapiceps is distinguished easily from L. uniformis by the presence of large lateral cuticular alae. It should be noted that in 1986 Bartlett and Greiner chose to place D. scapiceps and D. roemeri, but not D. uniformis, in the genus Pelicitus (12). With the exception of cuticular bosses, Loaina shares numerous morphologic features with Loa, a parasite of humans which has a very restricted geographical distribution. Under any circumstances, the identification of Loaina in humans, whether in the intact state or in tissues, should be straightforward.
Before going any further, it should be emphasized that a wide variety of filarial worms, including natural parasites of humans as well as a far greater number of zoonotic species, may be found in the chambers of the eye.
Bung-eye disease.
Since 1932, a condition called “bung-eye” or “bulge-eye” has been reported in East Africa, particularly Uganda. In 1932, Owen and Hennessey first described the presence of small, yellowish nodules in the bulbar conjunctiva (Fig. 37), often causing edema of the lids and proptosis (Fig. 38) (70). Although examination of microsections through the nodules revealed the presence of small filaria-like worms (Fig. 39), exact identification of the worm remained unclear, in large part because the worms were usually dead and too necrotic to be identified. In 1973, Poltera examined a larger series of cases and concluded that the worm might be Loa loa (74). However, the size and morphological features of the worm suggest a Mansonella species. Baird et al. presented a much more in-depth study of new material in 1988 and suggested, based on morphologic features and comparison to available slides, that the bung-eye worm most closely resembled Mansonella perstans (11). Although it is not possible to state with absolute certainty that M. perstans, in an aberrant location, is responsible for these interesting and unusual cases, it would appear that a worm in the Mansonella (Esslingeria) group is the most likely candidate. Whether this represents a zoonotic infection with a species such as M. vanhoofi, M. rodhaini, or M. leopoldi of apes, an aberrant location for a known human species such as M. perstans or M. streptocerca, or a yet unrecognized species cannot be answered with certainty at present. It is known that the infection is relatively common in some locations and seems to overlap the geographical distribution of M. perstans and that a small filarial worm is responsible. The parasite is small (frequently less than 100 μm in diameter), the females are gravid with fully developed microfilariae in utero, the cuticle is smooth and relatively thick in relation to the overall size of the worm, and the muscle cells are low and numerous (8 to 10 per quadrant). Baird et al. noted the presence of pigmented granules in the lateral chords and the occurrence of the same feature in microsections of M. perstans (11). These features, alone or in combination, would serve to clearly distinguish these worms, even if not seen in a native of East Africa, from other types of zoonotic filariae such as Dirofilaria or Loaina.
Central Nervous System
Meningonema peruzzii.
One of the more interesting zoonotic filarial infections, because of the location of the adult worms and morphologic features of the parasite, is that attributed to Meningonema peruzzii. This worm is a parasite of African monkeys, and the normal habitat for the adult worm is the subarachnoid spaces along the dorsum of the brain stem at the level of the medulla oblongata (Fig. 40) (66). It is likely that various species of cercopithecid monkey are hosts and that the geographical distribution spans much of Central Africa. The microfilariae, which are sheathed, are found in the peripheral blood in monkey hosts but have been recovered from the cerebrospinal fluid in zoonotic human infections (26, 29, 61). Morphologically, the microfilariae resemble quite closely those of Mansonella perstans, with the exception of minor size differences and the presence of a sheath in M. peruzzii. It has been noted previously that the sheath may be very tightly applied and difficult to visualize. For this reason, it is likely that zoonotic infections with M. peruzzii are frequently overlooked or misdiagnosed as M. perstans infections.
Various neurologic disorders have been attributed to this filarial infection. Two cases of cerebral filariasis described by Dukes et al. are of particular interest (29). Initially, these cases were thought to be caused by M. perstans. Upon subsequent examination and in light of the recognition of the occurrence of M. peruzzii in monkeys, it was possible to determine that these human cases were actually caused by M. peruzzii (61). Recently, Boussinesq et al. recovered an immature female M. peruzzii worm from the cerebrospinal fluid of a man in Cameroon, further confirming the zoonotic potential of the parasite (26).
One of the more interesting morphologic features of this parasite, in addition to its similarity to M. perstans, is the fact that the female worm has four uterine branches (quadridelphic) rather than the two uterine branches seen in all other filariae known to infect humans in either zoonotic or natural infections. This feature is readily evident in histologic preparations. Other salient features observed in histologic sections include an extremely thin body wall, very weak musculature confined to less than one-third of the body diameter, and broad, flat lateral chords, which occupy up to two-thirds of the body circumference (Fig. 40). As in other filariae, the intestine is unremarkable in appearance with a low cuboidal epithelium.
The recovery of juvenile or adult filarial worms from cerebrospinal fluid or the observation of worms lying in the subarachnoid spaces in histologic sections should alert the pathologist or clinician to suspect M. peruzzii. Recovery of microfilariae from cerebrospinal fluid is not diagnostic for M. peruzzii, since there are reports of other species of microfilariae being recovered from this fluid, but again, the clinician should consider the possibility of infection with Meningonema.
MICROFILARIAE OF PRESUMED ZOONOTIC ORIGIN
On occasion, microfilariae not identified as natural human parasites have been found in human blood or skin samples. Sometimes these represent a single case; at other times a larger series of cases are detected. All of these infections are believed to represent zoonotic infections. However, in instances when a number of individuals have been shown to be infected and clustered geographically, it is difficult to establish conclusively whether the infections are zoonotic or have become established in a human population. In all of these cases, the adult worms have not been recovered, and consequently it has not been possible to assign a generic name.
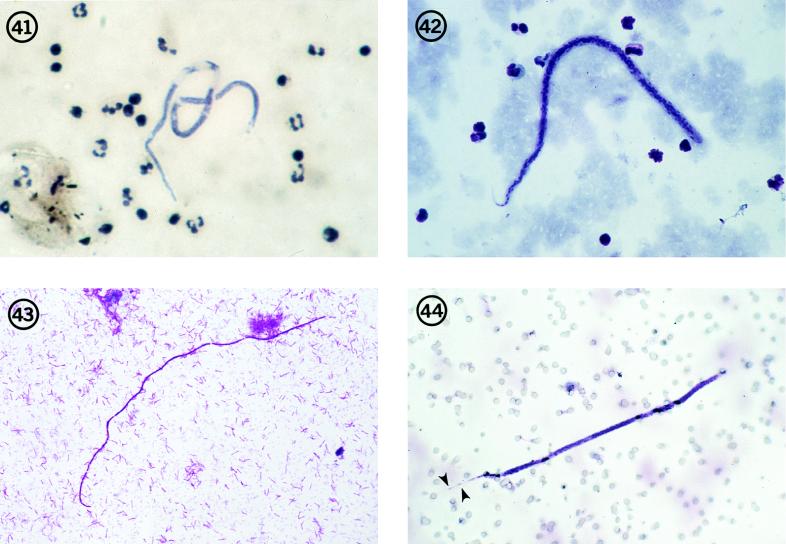
Microfilaria semiclarum.
In 1974, Fain reported the occurrence of an unknown microfilaria in the blood of Zairian villagers in Equateur Province (36). He named the microfilaria Dipetalonema semiclarum because of a pronounced clear space in the body of the microfilaria (Fig. 41). The adult stages have never been recovered; hence, the generic name is inappropriate. The filaria is best designated Microfilaria semiclarum. The morphology of the microfilaria is not similar to that of any recognized animal filaria reported from that region of Africa. The microfilariae measure about 220 μm in length by 5 μm in width and resemble, superficially, the microfilariae of M. perstans, with the exception of the clear spaces in the posterior half of the body (Fig. 41).
Microfilaria species.
In 1978, Greene et al. reported finding Dipetalonema-like microfilariae in the blood of a child in Alabama (45). Although the species could not be positively established, it appeared most like Mansonella interstitium, a natural parasite of squirrels.
Microfilaria bolivarensis.
In 1980, Microfilaria bolivarensis was found in Amerindian villages along the Orinoco river in Venezuela (43). There appeared to be little if any contact between the villages, and it is unclear how widely distributed this infection may be. Again, the microfilaria does not correspond to any known animal filaria in the area. The microfilariae in stained blood smears measure 250 μm in length by 7 to 8 μm in width (Fig. 42). In Knott samples, the microfilariae measure about 300 by 8 μm and have a characteristically flexed body and tail (43).
Mansonella rodhaini.
In 1982, microfilariae indistinguishable from Mansonella rodhaini were recovered from skin snips of 14 villagers in Gabon (75). M. rodhaini is recognized as a natural parasite in chimpanzees in Central Africa and is probably transmitted by species of the genus Culicoides. This would seem to be a clear case of zoonotic infection with a known animal filaria. The microfilariae of M. rodhaini are distinctive because of their unusual length and width (Fig. 43), being approximately 320 μm long by only 2 to 2.5 μm wide.
Brugia spp.
As previously noted in the section on worms in the lymphatics, microfilariae clearly belonging to the genus Brugia have been found on two occasions in persons residing in areas not known to harbor endemic human brugian filariasis. The first was the report of microfilariae detected in the blood of a young child in Oklahoma (Fig. 44) (80), and the second was from an adult Ethiopian (56). In neither of these cases could the microfilaria be ascribed to a known species of either animal or human origin.
DISCUSSION
Filarial infections are among the most common zoonotic infections of humans worldwide, especially in the United States. To date, most infections have been found in the temperate zone rather than in the tropical areas, where it would seem that environmental conditions might be more favorable for parasite transmission. It is not possible at present to establish whether zoonotic infections are equally common in the tropics and simply not recognized or reported. Undoubtedly, recognition of zoonotic infections in tropical, less developed areas of the world is influenced by the level of health care available and the pressure of dealing with other, more significant infectious diseases. Actually, it is impossible to determine how common infections are even in the United States or Europe, mostly because symptoms are mild and may go unrecognized and untreated unless worms migrate to highly sensitive tissues such as the orbit or lymphatics. In addition, in some places, including the United States, specific infections are seen so frequently that unless there is some unique aspect, such as more than one worm or the presence of microfilariae, cases are regarded as too commonplace to warrant the publication of case reports (73). The unavailability of reliable serologic assays has precluded epidemiologic serosurveys that would help ascertain the true prevalence of these infections.
Environmental conditions relating to parasite vectors as well as human behavioral activities are important in the epidemiology of these infections. Infections appear to be more common in areas with long mosquito breeding seasons and where people spend a lot of time out of doors in conjunction with work or recreational activities. In the United States, south Florida may be a good example of such an area. The largest number of human cases has been reported in this area. The climate is warm for much of the year, the mosquito breeding season is extended, there is a large wild-animal population with filarial infections, and people engage in a variety of outdoor recreational activities. Interestingly, human infection with D. ursi-like parasites has been documented primarily in trappers.
Zoonotic filariae typically tend to settle into a tissue habitat similar to or the same as that in their natural hosts, e.g., subcutaneous tissues, the vascular system, and the lymphatics. The number of species which have been isolated from the chambers of the eye and from pulmonary arteries is unusually high. For the former, this is unexpected since we do not recognize that this occurs in natural infections with these parasites. Although this behavior is frequently observed, we have no definitive explanation for it. Perhaps for worms in the pulmonary arteries, however, this is not that unusual. For example, as stated above, species of Brugia, when inoculated into experimental rodent hosts, often migrate to the heart and pulmonary arteries.
We surmise that many people are exposed and probably many more are infected than is recognized. It is likely that in most of these infections, the larvae die or are killed very quickly. Curiously, in some instances, infective larvae that invade the tissues are able to develop to sexual maturity without an apparent challenge from the host. Whether they die naturally or are destroyed by the host is not known. Gravid female worms are sometimes recovered, but patency does not necessarily occur (10, 51, 52, 71). Yet, in a few instances as mentioned above, microfilaremia has been reported. This phenomenon occurs with some frequency even in natural and experimental infections in animals, but the nature and dynamics of the host parasite relationship remain to be elucidated.
Human infections typically include only one or, at the most, a few worms. Why so few? There are many possibilities. In all likelihood, only a few infective larvae are transmitted by the vector at any one time. Another possibility is that most larvae die at an early age; it is likely that the death of very young larvae would not stimulate a significant host response and therefore would go unnoticed. Most worms recovered from the tissues are dead but sexually mature or nearly so. This raises the question, which cannot be answered adequately at present, whether the parasite reached the end of its sojourn and died or whether the host ultimately mounted an effective response and killed the worm. Both are likely possibilities, and in any given case, one or the other may have been the primary initiating event. Not being able to accurately gauge the age (duration) of the infection hinders our understanding, but it is well recognized from experimental studies and limited observations of natural infections in humans and animals that filariae are long-lived (42, 65). This might argue that in most cases, the host ultimately mounts a response that leads to the death of the worm. It might even be argued that unmated worms may lose their vitality and die sooner than mated worms.
Why are most of the female worms recovered sexually mature but not gravid? These worms have not been inseminated by a male worm because none were present. A few studies have investigated this phenomenon. It has been observed in studies with Acanthocheilonema viteae and Loa loa that if male worms are present, they have a remarkable ability to find unmated females (20, 65).
The ability to assign zoonotic filariae to broad generic groupings, as outlined in this section, is generally straightforward. However, attempting to more precisely identify species in histopathologic specimens is hindered by a variety of circumstances. The material available for study typically is very limited and inadequate for reconstruction of even small parts of the parasite. The worm is frequently dead, so important diagnostic features are obscured. Our knowledge of the microanatomical features of the various species is extremely limited. Although structure and features of the cuticle offer key morphologic characteristics, other key morphologic features, such as the morphology and number of muscle cells, have not been adequately studied and described. When it is necessary to distinguish among similar species, the application of molecular technology could have a great impact. The development of species-specific probes would allow an identification where a microscopic diagnosis is virtually impossible. However, because the number of specific probes needed to accomplish the task is large and the application is limited, it is unlikely that a significant number will be generated and available for routine use.
In recent years, there have been some marked improvements in serodiagnostic techniques for filarial infections, but these have been restricted primarily to natural infections, specifically W. bancrofti and O. volvulus infections in humans and D. immitis infections in dogs. Naturally enough, these efforts tend to focus on the development of antigen assays that are species specific; such assays will not be useful for the detection of infections with unrelated or even closely related species. These assays have permitted significant gains in our understanding of the epidemiology of human filariasis, but currently they hold little promise of being useful in detecting or identifying zoonotic infections. Detection of filaria-specific antibodies is more promising for use in serodiagnostic assays for zoonotic infections, particularly in distinguishing filarial infections from other tissue-dwelling helminth infections. There appears to be enough cross-reactivity between filarial species to warrant further efforts to develop such assays. As currently formatted, these assays do suffer from lack of specificity and hence are of no help in determining the genus or species of the parasite causing the infection. Another major problem has been the inability of serologic assays to distinguish between exposure and active infection. However, it has been demonstrated that antifilarial immunoglobulin G4 responses are elevated in persons with active infection, which should permit active infections to be distinguished from past infections or exposure (50, 53, 54). It should be noted that it is not clear whether zoonotic infections stimulate the production of immunoglobulin G4 antibody responses. Two groups, working in Spain and Japan, reportedly have developed good assays for detection of Dirofilaria infections in humans (77, 78, 81). However, the serodiagnosis of zoonotic filarial infections is complicated by the large number of diverse species potentially involved and the fact that a large segment of the population may be exposed (and hence may mount antibody responses) but only a small number of individuals are actively infected at any given point in time. One area in which serologic assays have provided some useful information about zoonotic infections has been in monitoring patients after removal of worms. At least in zoonotic Brugia infections, levels of antifilarial antibodies have been documented to decline rapidly over a 3- to 6-month period following surgical removal of the worms (32, 35).
Although the dirofilarias are the most common, the most extensively studied, and the best-characterized group of zoonotic filariae, our knowledge of these species is still rather sketchy. Intact worms are the most easily identified at the species level because of the number of morphologic features that can be evaluated. It cannot be stressed too often that when intact worms are recovered from the tissues, they should be fixed and prepared for study in that state rather than sectioned for histopathologic study. It should be emphasized that the ends of the worm, male or female, contain the greatest amount of morphologic information and every effort should be made to collect and preserve the entire specimen. The clinician who removes an intact worm from the tissues is immediately faced with the dilemma of making an accurate identification. Calling upon a well-trained parasitologist with a good knowledge of the literature and the use of taxonomic keys can be very helpful. Such an individual usually can make a preliminary diagnosis and may even want to seek the assistance of someone especially knowledgeable about the zoonotic filarial worms for confirmation of the diagnosis.
The zoonotic filariae in sectioned tissues can be identified to the genus level for most of the groups that currently are known to infect humans. The composition of the body wall, i.e., cuticle, hypodermis, and muscles, provides important diagnostic features. For example, thickness, layering, and ornamentation of the cuticle distinguish the major groups (Dirofilaria, Brugia, and Onchocerca). Surface ornamentation (ridges, alae, or smooth surface) separate some of the species of Dirofilaria. The number and type of muscle cells also differ among species in these groups. The reproductive systems in these worms offer little if any assistance in separating species but do separate male and female worms and, for females, provide key information about their sexual maturity. Size can never be overlooked because of the often significant differences in the sizes of these worms, e.g., Dirofilaria and Brugia. There are several excellent publications to which the pathologist can refer to get detailed descriptions and the necessary information to aid in making an identification (47, 62).
Especially intriguing are the circumstances under which humans acquire such infections as Mansonella rodhaini, Meningonema peruzzii, and Microfilaria bolivarensis and Microfilaria semiclarum. For the first two, we know that there are animal reservoirs of these infections and that transmission to the human host would not be difficult. It is not clear whether M. rodhaini is now established in the human population or whether infection depends on ape-to-human transmission. On the other hand, based on current knowledge, Meningonema infection is transmitted directly from primates to humans via an unknown arthropod vector. Microfilaria semiclarum, a filaria which we know virtually nothing about, has been established in the human population in isolated villages in Zaire. Considering the extent to which people are infected, it seems likely that the cycle is maintained in and by the human population. The same is probably true of Microfilaria bolivarensis. The isolation of these foci of infection has severely restricted our ability to conduct the kinds of studies that would better define the circumstances of transmission.
Zoonotic filarial infections will undoubtedly continue to be recognized with increasing frequency, in new geographical areas, in different locations in the host, and as a result of different species of parasites. Clinicians, pathologists, and parasitologists will have to continue to be alert to the possibility of these infections, both in currently recognized clinical presentations and in unusual presentations. To date, we are not aware of clusters of infections (outbreaks), but the possibility of multiple persons being infected at or about the same time cannot be excluded. Probably the greatest mystery surrounding zoonotic filarial infections is why some individuals become infected whereas the majority of individuals who are exposed do not become infected or the infective larvae die at a very early stage. In individuals in whom the worm survives, it does so without a noticeable host response, often for months or years (42). Future studies of natural filarial infections in humans or experimental infections in laboratory animals may shed light on this question. For whatever reasons, zoonotic filarial infections will continue to intrigue the medical and scientific community for some time to come.
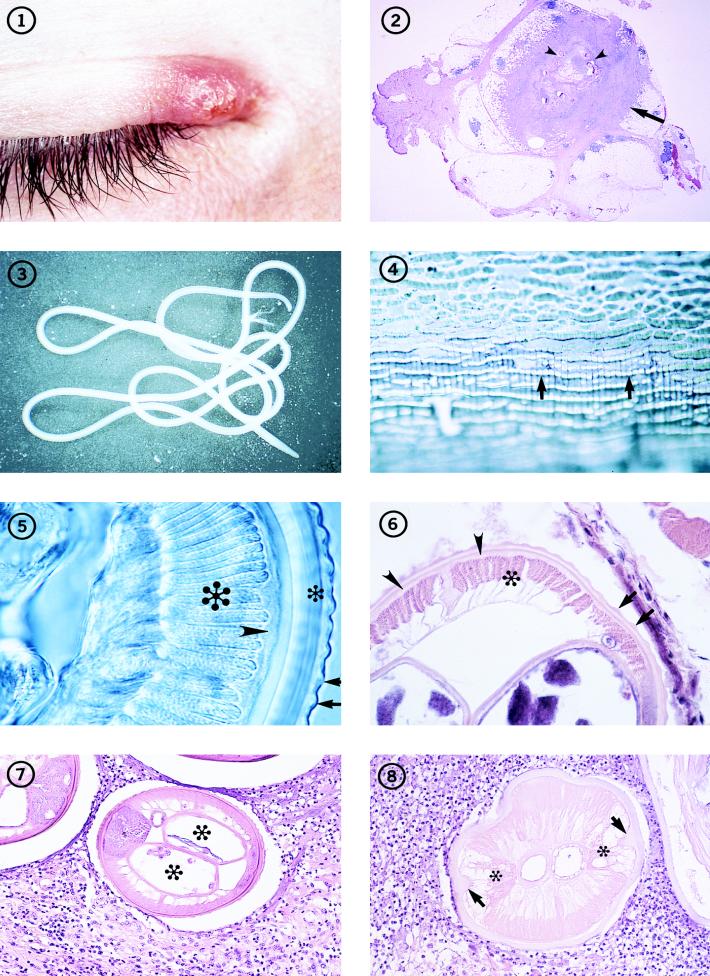
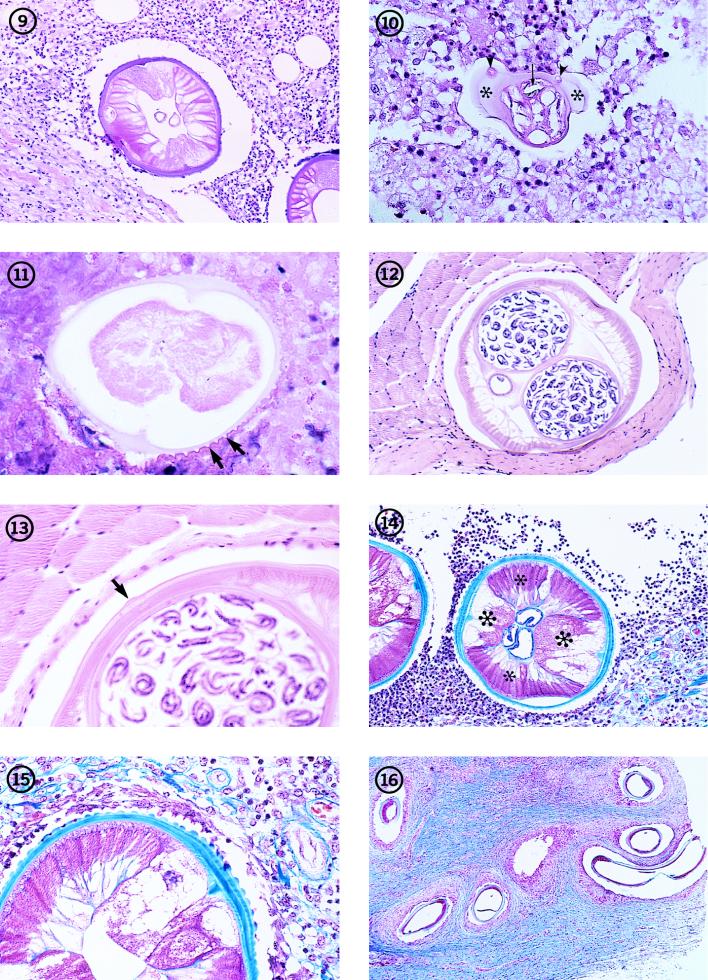
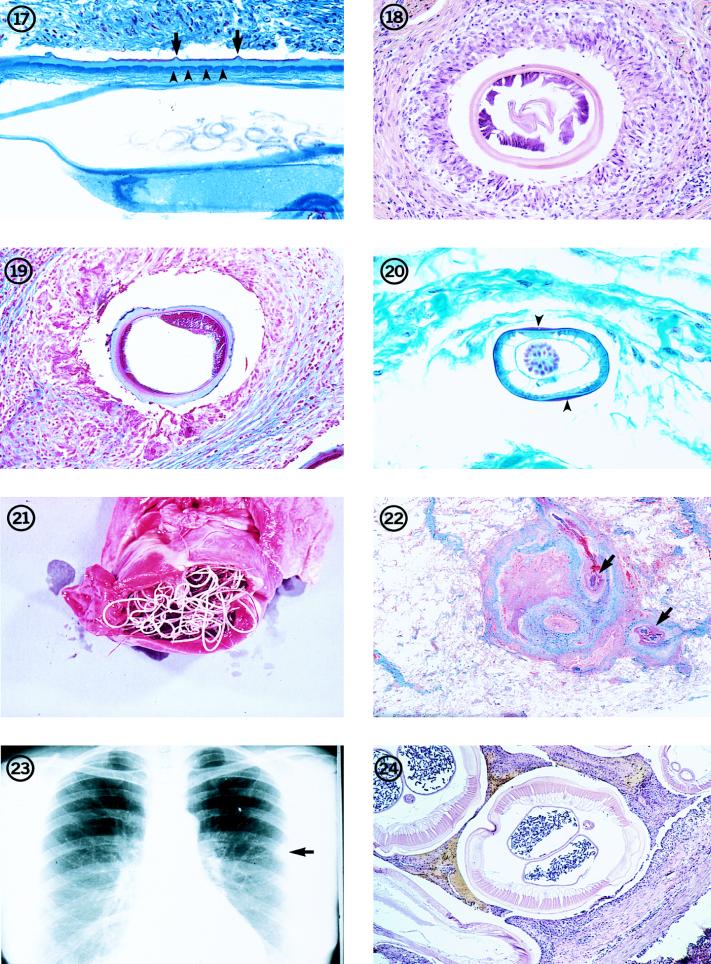
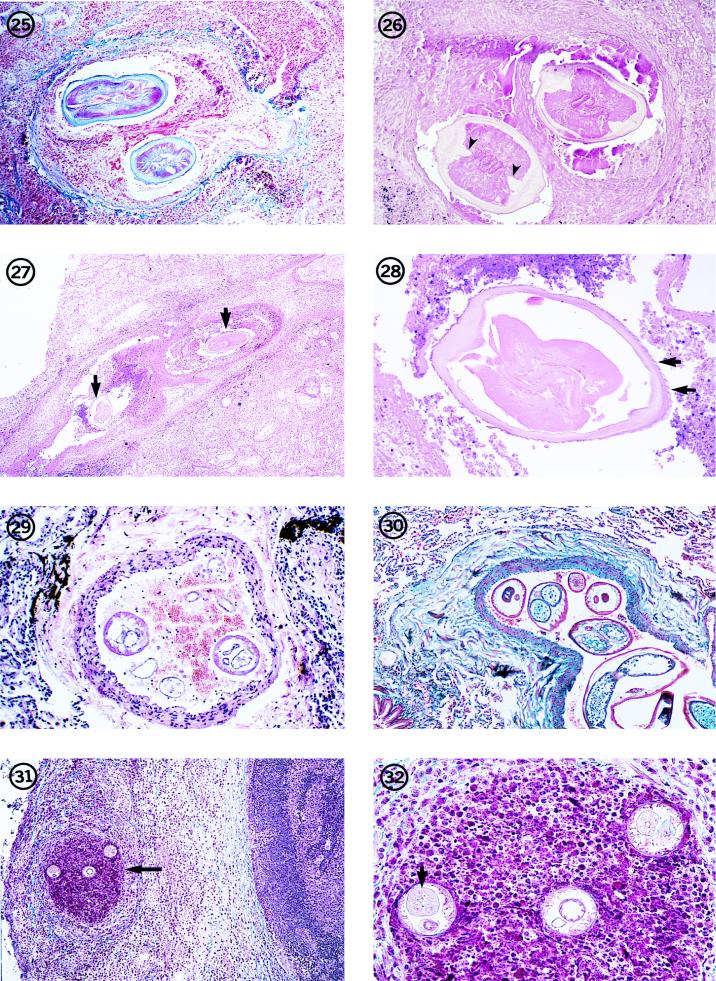
FIG. 1-8.
Individual with an inflammatory lesion at the inner canthus of the eye from which an immature adult D. tenuis worm was removed.
Fig. 2 Subcutaneous nodule (arrow) removed from a patient in Florida, containing several sections of a D. tenuis worm (arrowheads). Hematoxylin and eosin stain; magnification, ×4.5.
Fig. 3 An immature adult female Dirofilaria species that measured more than 12 cm in length, removed intact from human subcutaneous tissues.
Fig. 4 The surface of the cuticle of D. repens, illustrating the beaded appearance of the longitudinal ridges (arrows). The worm was preserved and mounted in glycerin. Magnification, ×182.
Fig. 5 Section through an adult D. repens worm, illustrating the appearance of the longitudinal ridges (arrows) in transverse section, as well as the thick, multilayered cuticle (small asterisk), hypodermis (arrowhead), and muscle cells (large asterisk). The worm is unstained but cleared in glycerin. Magnification, ×391.
Fig. 6 Portion of a transverse section of D. tenuis, showing the longitudinal ridges (arrows) on the surface of the multilayered cuticle, the thin hypodermis (arrowheads), and the coelomyarian muscles (asterisk). Hematoxylin and eosin stain; magnification, ×227.
Fig. 7 Transverse section through a female D. tenuis worm removed from human subcutaneous tissues, illustrating its characteristic morphologic features. The paired uterine tubes (asterisks) contain unsegmented eggs. Hematoxylin and eosin stain; magnification, ×121.
Fig. 8 Dead, degenerating female Dirofilaria worm, with a thickened cuticle, prominent lateral internal cuticular ridges (arrows), and frothy, vacuolated lateral chords (asterisks). In the pseudocelom, the tube to the left is the vagina; adjacent to it is the intestine. This section is cut in the anterior one-fifth of the worm. Hematoxylin and eosin stain; magnification, ×164.
FIG. 9-16.
Transverse section through a dead, degenerating male D. tenuis worm in human subcutaneous tissues. Note the two tubes in the body cavity (pseudocelom). One is the intestine; the other is the reproductive tube. Hematoxylin and eosin stain; magnification, ×121.
Fig. 10 Section through the tail of a dead, male D. tenuis worm. Note the large caudal alae (asterisks) in which caudal papillae are embedded (arrowheads). Note also the presence (in transverse section) of one of the cuticularized spicules (arrow). Hematoxylin and eosin stain; magnification, ×136.
Fig. 11 D. ursi-like worm in an advanced state of degeneration in human subcutaneous tissues. Note especially the sharply crested, longitudinal ridges on the surface of the cuticle (arrows). Hematoxylin and eosin stain; magnification, ×573.
Fig. 12 Transverse section through a gravid, female D. striata worm from the tissues of a bobcat (Lynx rufus), illustrating its typical dirofilariid morphologic features and especially the absence of conspicuous longitudinal ridges on the surface of the cuticle. Hematoxylin and eosin stain; magnification, ×82.
Fig. 13 Higher magnification of the worm in Fig. 12. The relatively inconspicuous lateral ala on the surface of the cuticle is clearly evident (arrow). Hematoxylin and eosin stain; magnification, ×227.
Fig. 14 Dead female D. repens worm removed from human subcutaneous tissues. Note the conspicuous longitudinal ridges, the enlarged, frothy lateral chords (large asterisks), and the strong musculature (small asterisks), which retains some of its normal architecture. Trichrome stain; magnification, ×64.
Fig. 15 Higher magnification of the worm in Fig. 14. Note the multilayered structure of the cuticle and the irregular spacing of the longitudinal ridges on its surface. Trichrome stain; magnification, ×191.
Fig. 16 Section of nodule removed from a patient in Illinois and containing a zoonotic Onchocerca worm. The highly coiled nature of the worm and the dense connective tissue surrounding the worm are evident. Trichrome stain; magnification, ×191. (From the case reported in reference 17.)
FIG. 17-24.
Longitudinal section through a female O. cervicalis worm from a naturally infected horse, illustrating the morphologic features of the cuticle, including the outer circular ridges (arrows) and the inner striae (arrowheads). There are four striae per ridge. Trichrome stain; magnification, ×209.
Fig. 18 Transverse section through a female Onchocerca worm removed from a human in Japan. The variable thickness of the cuticle is evident, as is the weak musculature. Two uterine reproductive tubes are present in the body cavity. Hematoxylin and eosin stain; magnification, ×114. (From the case presented in reference 22.)
Fig. 19 Transverse section of the female Onchocerca worm illustrated in Fig. 16. Note the unevenly thick cuticle and the low, weak, poorly developed musculature. Trichrome stain; magnification, ×114. (From the case reported in reference 17.)
Figure 20 Section through a male O. gutturosa worm from a naturally infected cow, illustrating the thin cuticle which thickens laterally (arrowheads), small number of muscle cells, and large testis, which fills the body cavity. The intestine is the small, collapsed tube adjacent to the testis. Trichrome stain; magnification, ×328.
Fig. 21 Adult D. immitis in the right ventricle of the heart of its natural host, the domestic dog. Reprinted from reference 62 with permission of the publisher.
Fig. 22A coin lesion in a human lung, involving a small pulmonary artery. Sections of an immature D. immitis worm (arrows) can be seen in the obstructed vessel. Hematoxylin and eosin stain; magnification, ×3.6.
Fig. 23 Chest X ray showing a typical coin lesion (arrow) in a human lung as a result of a D. immitis infection.
Fig. 24 Transverse section of an adult female D. immitis worm, illustrating the characteristic morphologic features of this parasite. Hematoxylin and eosin stain; magnification, ×45.
FIG. 25-32.
Dead D. immitis worm in an occluded small pulmonary artery in a human lung. The parasite can be identified by its morphologic features, including its size and the thick but smooth cuticle with prominent lateral, internal cuticular ridges. Hematoxylin and eosin stain; magnification, ×45.
Fig. 26 Dead, degenerating, female D. immitis worm in a small pulmonary nodule. Note the thick, swollen cuticle with prominent internal cuticular ridges (arrowheads). The three tubes in the pseudocelom (two reproductive tubes and an intestine) indicate that it is a female worm. Hematoxylin and eosin stain; magnification, ×91.
Fig. 27 Low-power view of sections of D. repens (arrows) in an obstructed pulmonary artery of an individual in Italy. Hematoxylin and eosin stain; magnification, ×11.4. Reprinted from reference 62 with permission of the publisher.
Fig. 28 Higher magnification of one of the sections of the worm in Fig. 27. The parasite is in an advanced state of degeneration as indicated by the swollen cuticle and degeneration of the soft tissues of the worm. However, longitudinal ridges can be seen on the cortical surface of the cuticle (arrows). Hematoxylin and eosin stain; magnification, ×91. Reprinted from reference 62 with permission of the publisher.
Fig. 29 Section through a small pulmonary artery removed from an individual who had lived in India, showing two transverse sections through a Brugia-like worm. Hematoxylin and eosin stain; magnification, ×91. Reprinted from reference 62 with permission of the publisher.
Fig. 30 W. bancrofti-like worm lying in a small pulmonary artery of a soldier who had served in Singapore. Trichrome stain; magnification, ×45. (From the case presented in reference 15.)
Fig. 31 Section through a peripheral lymph node from the upper arm of a patient in Ohio. Three sections of a small Brugia worm are present in an occluded lymph channel (arrow) lying inside the nodular capsule. The medullary portion of the lymph node is visible to the right of the nodule. Hematoxylin and eosin stain; magnification, ×59.
Fig. 32 Higher magnification of the worm in Fig. 31. Two sections through the anterior end at the level of the esophagus and ovejector (right and left sections) and one section through the region of the intestine and vagina uterina (middle section) are evident. The thin body wall, triradiate esophagus (arrow), few muscle cells per quadrant, and the muscular vagina vera and uterina are evident. Hematoxylin and eosin stain; magnification, ×237.
FIG. 33-40.
Two sections of a gravid female Brugia from a zoonotic infection in Peru. In both sections, the paired uterine tubes are evident and contain developing eggs or, in the upper left-hand section, sperm (asterisk), indicating that a male worm was present and that mating had occurred. Hematoxylin and eosin stain; magnification, ×455. (From the case reported in reference 64.)
Fig. 34 Transverse section through a male B. beaveri worm isolated from a naturally infected raccoon in Louisiana. This section clearly illustrates the typical morphology of Brugia, including the thin cuticle which thickens slightly over the lateral chords, the prominent lateral chords (arrows), the well-developed musculature (asterisk), which is composed of few muscle cells per quadrant and individual muscle cells that are broad and low. The epithelial cell lining of the gut and testis are evident, as are the developing spermatozoa. Trichrome stain; magnification, ×455.
Fig. 35 Transverse section through a female B. beaveri worm recovered from a naturally infected raccoon in Louisiana. The paired uterine tubes (asterisks) containing packed microfilariae fill most of the body cavity. Most of the other morphologic features are as represented in Fig. 34, except that the lateral chords are much broader and flatter than in the male worm. Trichrome stain; magnification, ×182. Reprinted from reference 62 with permission of the publisher.
Fig. 36 Transverse section of a female L. uniformis worm removed from the tissues of a naturally infected rabbit in Louisiana. The morphologic features of the parasite, including the relatively thin cuticle, the large, broad muscle cells, and the arrangement of the reproductive tubes, are clearly evident. Three sections of one uterine tube (asterisks) contain well-developed microfilariae, while the other two sections represent the second uterine tube. These are cut at the level of the seminal receptacle and contain sperm. The thin-walled, poorly developed intestine is seen lying in the middle of the body cavity (arrowhead). Hematoxylin and eosin stain; magnification, ×91.
Fig. 37 Several small nodules (arrowheads) in the bulbar conjunctiva of a resident of Uganda, Africa, illustrating the typical location of the parasite responsible for bung-eye disease.
Fig. 38 Edema of the eyelid in a resident of Uganda, showing the clinical presentation of bung-eye.
Fig. 39 Section through a gravid female worm in a nodule removed from the conjunctiva of a bung-eye patient, illustrating the small size of the worm, the presence of microfilariae in utero (asterisk), and the thin body wall. The worm has undergone extensive degeneration. Hematoxylin and eosin stain; magnification, ×246.
Fig. 40 Five sections (arrowheads) of an adult M. peruzzii worm in the meninges of its natural host, a talapoin monkey (Miopithecus talapoin). Hematoxylin and eosin stain; magnification, ×73.
FIG. 41-44.
Microfilaria semiclarum in a thick blood smear from an individual in Equateur Province, Zaire. Note its superficial resemblance to the microfilariae of M. perstans. Hematoxylin stain; magnification, ×250.
Fig. 42 Microfilaria bolivarensis in a thick blood smear from an Amerind in Bolivar State, Venezuela. This is a robust microfilaria that, in stained blood smears, often shows a flexed tail. Giemsa stain; magnification, ×200.
Fig. 43 Microfilaria of M. rodhaini in a skin snip from a resident of Gabon, West Africa. Giemsa stain; magnification, ×250.
Fig. 44 Microfilaria of a Brugia species in a blood sample collected on a Nuclepore filter from a patient in Oklahoma. The terminal and subterminal nuclei in the tail (arrowheads), diagnostic of Brugia species, are clearly evident. Giemsa stain; magnification, ×250. (From the case reported in reference 80.)
ADDENDUM IN PROOF
An immature female filaria, tentatively identified as Onchocerca cervicalis, was removed from the cornea of a resident in Colorado. This is the first reported case of zoonotic Onchocerca in the eye and only the second case overall in the United States (W. E. Burr, M. F. Brown, and M. L. Eberhard, Ophthalmology, in press).
REFERENCES
- 1.Abadie H, Swartzwelder J C, Holman R L. A human case of Dirofilaria immitis infection. Am J Trop Med Hyg. 1965;14:117–118. doi: 10.4269/ajtmh.1965.14.117. [DOI] [PubMed] [Google Scholar]
- 2.Addario C. Su un nematode dell’occhio umano. Ann Ottalmolog. 1885;13:135–147. [Google Scholar]
- 3.Akashi M, Tashiro T, Got J, Nasu M, Itoga T, Tsuboi M, Araki K. A case of pulmonary dirofilariasis. Jpn J Thorac Dis. 1983;21:1228–1232. [PubMed] [Google Scholar]
- 4.Aldravando U. Boloniae apud Ioan Bapt. Bellagambaum; 1602. De animalibus inssectis libri septem cum singulorum iconibus ad vivum expressis; pp. 655–656. [Google Scholar]
- 5.Ali-Khan Z. Tissue pathology and comparative microanatomy of Onchocerca from a resident of Ontario and other enzootic Onchocerca species from Canada and the USA. Ann Trop Med Parasitol. 1977;71:469–482. doi: 10.1080/00034983.1977.11687213. [DOI] [PubMed] [Google Scholar]
- 6.Anderson R C. Description and relationships of Dirofilaria ursi Yamaguti, 1941, and a review of the genus Dirofilaria Railliet and Henry, 1911. Trans R Can Inst. 1952;29:35–64. [Google Scholar]
- 7.Anderson R C. Nematode parasites of vertebrates. Their development and transmission. Wallingford, England: CAB International; 1992. [Google Scholar]
- 8.Ash L R, Riley J M. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J Parasitol. 1970;56:969–973. [PubMed] [Google Scholar]
- 9.Azarova N S, Miretskij O Y, Sonin M D. The first discovered case of nematode parasitism in the USSR in a human being (genus Onchocerca Diesing, 1841) Med. Parazytol (Moscow) 1965;34:156–158. . (In Russian.) [PubMed] [Google Scholar]
- 10.Baird K J, Neafie R C. South American brugian filariasis: report of a human infection acquired in Peru. Am J Trop Med Hyg. 1988;39:185–188. doi: 10.4269/ajtmh.1988.39.185. [DOI] [PubMed] [Google Scholar]
- 11.Baird J K, Neafie R C, Connor D H. Nodules in the conjunctiva, bung-eye, and bulge-eye in Africa caused by Mansonella perstans. Am J Trop Med Hyg. 1988;38:553–557. doi: 10.4269/ajtmh.1988.38.553. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett C M, Greiner E C. A revision of Pelicitus Railliet & Henry, 1910 (Filarioidea, Dirofilariinae) and evidence for the “capture” by mammals of filarioids from birds. Bull Mus Nat Hist Nat. 1986;8:47–99. [Google Scholar]
- 13.Beaver P C. Intraocular filariasis: a brief review. Am J Trop Med Hyg. 1989;40:40–45. doi: 10.4269/ajtmh.1989.40.40. [DOI] [PubMed] [Google Scholar]
- 14.Beaver P C, Brenes R, Solano G V. Zoonotic filaria in a subcutaneous artery of a child in Costa Rica. Am J Trop Med Hyg. 1984;33:583–585. doi: 10.4269/ajtmh.1984.33.583. [DOI] [PubMed] [Google Scholar]
- 15.Beaver P C, Cran I R. Wuchereria-like filaria in an artery, associated with pulmonary infarction. Am J Trop Med Hyg. 1974;23:869–876. doi: 10.4269/ajtmh.1974.23.869. [DOI] [PubMed] [Google Scholar]
- 16.Beaver P C, Fallon M, Smith G H. Pulmonary nodule caused by a living Brugia malayi-like filaria in an artery. Am J Trop Med Hyg. 1971;20:661–666. doi: 10.4269/ajtmh.1971.20.661. [DOI] [PubMed] [Google Scholar]
- 17.Beaver P C, Horner G S, Bilos J Z. Zoonotic onchocercosis in a resident of Illinois and observations on the identification of Onchocerca species. Am J Trop Med Hyg. 1974;23:595–607. doi: 10.4269/ajtmh.1974.23.595. [DOI] [PubMed] [Google Scholar]
- 18.Beaver P C, Meyers E A, Jarroll E L, Rosenquist R C. Dipetalonema from the eye of a man in Oregon. Am J Trop Med Hyg. 1980;29:369–372. doi: 10.4269/ajtmh.1980.29.369. [DOI] [PubMed] [Google Scholar]
- 19.Beaver P C, Orihel T C. Human infection with filaria of animals in the United States. Am J Trop Med Hyg. 1965;14:1010–1029. doi: 10.4269/ajtmh.1965.14.1010. [DOI] [PubMed] [Google Scholar]
- 20.Beaver P C, Orihel T C, Johnson M H. Dipetalonema viteae in the experimentally infected jird, Meriones unguiculatus. II. Microfilaremia in relation to worm burden. J Parasitol. 1974;60:310–315. [PubMed] [Google Scholar]
- 21.Beaver P C, Wolfson J S, Waldron M A, Evans G W, Adler J. Dirofilaria ursi-like parasites acquired by humans in the northern United States and Canada: report of two cases and brief review. Am J Trop Med Hyg. 1987;37:357–362. doi: 10.4269/ajtmh.1987.37.357. [DOI] [PubMed] [Google Scholar]
- 22.Beaver P C, Yoshimura H, Takayasu S, Hashimoto H, Little M D. Zoonotic Onchocerca in a Japanese child. Am J Trop Med Hyg. 1989;40:298–300. doi: 10.4269/ajtmh.1989.40.298. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard R A E. Parasites animaux. Traite Pathol Gen. 1896;2:649–810. [Google Scholar]
- 24.Boreham P F L. Dirofilariasis in man. In: Boreham P F L, Atwell R B, editors. Dirofilariasis. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 217–226. [Google Scholar]
- 25.Botero D, Aguledo L M, Uribe F J, Esslinger J H, Beaver P C. Intraocular filaria, a Loaina species, from man in Colombia. Am J Trop Med Hyg. 1984;33:578–582. doi: 10.4269/ajtmh.1984.33.578. [DOI] [PubMed] [Google Scholar]
- 26.Boussinesq M, Bain O, Chabaud A G, Gardon-Wendel N, Kamgno J, Chippaux J P. A new zoonosis of the cerebrospinal fluid of man probably caused by Meningonema peruzzii, filaria of the central nervous system of Cercopithecidae. Parasite. 1995;2:173–176. doi: 10.1051/parasite/1995022173. [DOI] [PubMed] [Google Scholar]
- 27.Ciferi F. Human pulmonary dirofilariasis in the United States: a critical review. Am J Trop Med Hyg. 1982;31:302–308. doi: 10.4269/ajtmh.1982.31.302. [DOI] [PubMed] [Google Scholar]
- 28.Desportes C. –1940. Filaria conjunctivae Addario, 1885, parasite accidental de l’homme, est un Dirofilaria. Ann Parasitol. 1939;17:380–515. [Google Scholar]
- 29.Dukes D C, Gelfand M, Gadd K G, Clarke V de V, Goldsmid J M. Cerebral filariasis caused by Acanthocheilonema perstans. Cent Afr J Med. 1968;14:21–27. [PubMed] [Google Scholar]
- 30.Eberhard M L. Studies on the Onchocerca (Nematoda: Filarioidea) found in cattle in the United States. I. Systematics of O. gutturosa and O. lienalis with a description of O. stilesi sp.n. J Parasitol. 1979;65:379–388. [PubMed] [Google Scholar]
- 31.Eberhard M L. Brugia lepori sp.n. (Filarioidea: Onchocercidae) from rabbits (Sylvilagus aquaticus, S. floridanus) in Louisiana. J Parasitol. 1984;70:576–579. [PubMed] [Google Scholar]
- 32.Eberhard M L, DeMeester L J, Martin B W, Lammie P J. Zoonotic Brugia infection in Western Michigan. Am J Surg Pathol. 1993;17:1058–1061. doi: 10.1097/00000478-199310000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Eberhard M L, Orihel T C. Loa loa: development and larval morphology of Loa loa in experimental primate hosts. J Parasitol. 1981;67:556–564. [PubMed] [Google Scholar]
- 34.Eberhard M L, Orihel T C. Loaina gen. n. (Filarioidea: Onchocercidae) for the filariae parasitic in rabbits in North America. Proc Helminthol Soc Wash. 1984;51:49–53. [Google Scholar]
- 35.Elenitoba-Johnson K S J, Eberhard M L, Dauphinais R M, Lammie P J, Khorsand J. Zoonotic brugian lymphadenitis: an unusual case with florid monocytic B-cell proliferation. Am J Clin Pathol. 1996;105:384–387. doi: 10.1093/ajcp/105.4.384. [DOI] [PubMed] [Google Scholar]
- 36.Fain A. Dipetalonema semiclarum sp. nov. from the blood of man in the Republic of Zaire (Nematoda: Filarioidea) Ann Soc Belge Med Trop. 1974;54:195–207. [PubMed] [Google Scholar]
- 37.Faust E C. Human infections with species of Dirofilaria. Z Tropenmed Parasitol. 1957;8:59–68. [PubMed] [Google Scholar]
- 38.Faust E C, Agosin M, Garcia-Laverde A, Sayad W Y, Johnson V M, Murray N A. Unusual findings of filarial infections in man. Am J Trop Med Hyg. 1952;1:239–249. doi: 10.4269/ajtmh.1952.1.239. [DOI] [PubMed] [Google Scholar]
- 39.Faust E C, Thomas E P, Jones J. Discovery of human heartworm infection in New Orleans. Parasitology. 1941;27:115–122. [Google Scholar]
- 40.Freitas J F T, Lent H. Nova Dirofilaria parasita do aparelho circulatorio de ariranha (Nematoda: Filarioidea) Rev Bras Biol. 1949;9:377–380. [PubMed] [Google Scholar]
- 41.Freitas J F T, Mayall R D. Fenomeno de Raynaud na mao esquerda, provocado por Dirofilaria spectans. Rev Bras Med. 1953;10:463–467. [PubMed] [Google Scholar]
- 42.Gardiner C H, Oberdorfer C E, Reyes J E, Pinkus W H. Infection of man by Dirofilaria repens. Am J Trop Med Hyg. 1978;27:1279–1281. doi: 10.4269/ajtmh.1978.27.1279. [DOI] [PubMed] [Google Scholar]
- 43.Godoy G A, Orihel T C, Volcan G S. Microfilaria bolivarensis: a new species of filaria from man in Venezuela. Am J Trop Med Hyg. 1980;29:545–547. doi: 10.4269/ajtmh.1980.29.545. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein J D, Smith D R. Dirofilaria immitis in a portal shunt. Hum Pathol. 1985;16:1172–1173. doi: 10.1016/s0046-8177(85)80190-8. [DOI] [PubMed] [Google Scholar]
- 45.Greene B M, Otto G F, Greenough W B., III Circulating non-human microfilaria in a patient with systemic lupus erythematosus. Am J Trop Med Hyg. 1978;27:905–909. doi: 10.4269/ajtmh.1978.27.905. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez Y. Diagnostic features of zoonotic filariae in tissue sections. Hum Pathol. 1984;15:514–525. doi: 10.1016/s0046-8177(84)80004-0. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez Y. Diagnostic pathology of parasitic infections with clinical correlations. Philadelphia, Pa: Lea & Febiger; 1990. [Google Scholar]
- 48.Gutierrez Y, Catallaer M, Wicker D L. Extrapulmonary Dirofilaria immitis-like infections in the Western Hemisphere. Am J Surg Pathol. 1996;20:299–305.50. doi: 10.1097/00000478-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Harbut C L, Orihel T C. Brugia beaveri: microscopic morphology in host tissues and observations on its life history. J Parasitol. 1995;81:239–243. [PubMed] [Google Scholar]
- 50.Hitch W L, Hightower A W, Eberhard M L, Lammie P J. Analysis of isotype-specific anti-filarial antibody levels in a Haitian pediatric population. Am J Trop Med Hyg. 1991;44:161–167. doi: 10.4269/ajtmh.1991.44.161. [DOI] [PubMed] [Google Scholar]
- 51.Jung R C, Espenan P H. A case of infection in man with Dirofilaria. Am J Trop Med Hyg. 1967;16:172–174. doi: 10.4269/ajtmh.1967.16.172. [DOI] [PubMed] [Google Scholar]
- 52.Kozek W J, Reyes M A, Ehrman J, Garrido F, Nieto M. Enzootic Brugia infection in a two-year-old Colombian girl. Am J Trop Med Hyg. 1984;33:65–69. doi: 10.4269/ajtmh.1984.33.65. [DOI] [PubMed] [Google Scholar]
- 53.Kwan-Lim G E, Forsyth K P, Maizels R M. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J Immunol. 1990;145:4298–4305. [PubMed] [Google Scholar]
- 54.Lal R B, Ottesen E A. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J Infect Dis. 1988;158:1034–1037. doi: 10.1093/infdis/158.5.1034. [DOI] [PubMed] [Google Scholar]
- 55.MacDougall L T, Magoon C C, Fritsche T R. Dirofilaria repens manifesting as a breast nodule. Diagnostic problems and epidemiologic considerations. Am J Clin Pathol. 1992;97:625–630. doi: 10.1093/ajcp/97.5.625. [DOI] [PubMed] [Google Scholar]
- 56.Menendez M C, Bouza M. Brugia species in a man from western Ethiopia. Am J Trop Med Hyg. 1988;39:189–190. doi: 10.4269/ajtmh.1988.39.189. [DOI] [PubMed] [Google Scholar]
- 57.Nozais J P, Bain O, Gentilini M. A case of subcutaneous Dirofilaria (Nochtiella) repens with microfilaremia originating in Corsica. Bull Soc Pathol Exot. 1994;87:183–185. [PubMed] [Google Scholar]
- 58.Orihel T C. Morphology of the larval stages of Dirofilaria immitis in the dog. J Parasitol. 1961;47:251–262. [PubMed] [Google Scholar]
- 59.Orihel T C. Brugia tupaiae sp.n. (Nematoda: Filarioidea) in tree shrews (Tupaia glis) from Malaysia. J Parasitol. 1966;52:162–165. [PubMed] [Google Scholar]
- 60.Orihel T C. Dirofilaria corynodes (Linstow, 1899): morphology and life history. J Parasitol. 1969;55:94–103. [PubMed] [Google Scholar]
- 61.Orihel T C. Cerebral filariasis in Rhodesia—a zoonotic infection? Am J Trop Med Hyg. 1973;22:596–599. doi: 10.4269/ajtmh.1973.22.596. [DOI] [PubMed] [Google Scholar]
- 62.Orihel T C, Ash L R. Parasites in human tissues. Chicago, Ill: American Society of Clinical Pathologists; 1995. [Google Scholar]
- 63.Orihel T C, Beaver P C. Morphology and relationship of Dirofilaria tenuis and Dirofilaria conjunctivae. Am J Trop Med Hyg. 1965;14:1030–1043. doi: 10.4269/ajtmh.1965.14.1030. [DOI] [PubMed] [Google Scholar]
- 64.Orihel T C, Beaver P C. Zoonotic Brugia infections in North and South America. Am J Trop Med Hyg. 1989;40:638–647. doi: 10.4269/ajtmh.1989.40.638. [DOI] [PubMed] [Google Scholar]
- 65.Orihel T C, Eberhard M L. Loa loa: development and course of patency in experimentally-infected primates. Tropenmed Parasitol. 1985;36:215–224. [PubMed] [Google Scholar]
- 66.Orihel T C, Esslinger J H. Meningonema peruzzii gen. et sp. n. (Nematoda: Filarioidea) from the central nervous system of African monkeys. J Parasitol. 1973;59:437–441. [PubMed] [Google Scholar]
- 67.Orihel T C, Helentjaris D, Alger J. Subcutaneous dirofilariasis: single inoculum, multiple worms. Am J Trop Med Hyg. 1997;56:452–455. doi: 10.4269/ajtmh.1997.56.452. [DOI] [PubMed] [Google Scholar]
- 68.Orihel T C, Isbey E K. Dirofilaria striata infection in a North Carolina child. Am J Trop Med Hyg. 1990;42:124–126. doi: 10.4269/ajtmh.1990.42.124. [DOI] [PubMed] [Google Scholar]
- 69.Orihel T C, Pacheco G. Brugia malayi in the Philippine macaque. J Parasitol. 1966;52:394. [Google Scholar]
- 70.Owen H B, Hennessey R S F. A note on some ocular manifestations of helminthic origin occurring in natives of Uganda. Trans R Soc Trop Med Hyg. 1932;25:267–273. [Google Scholar]
- 71.Pacheco G, Schofield H L., Jr Dirofilaria tenuis containing microfilariae in man. Am J Trop Med Hyg. 1968;14:180–182. doi: 10.4269/ajtmh.1968.17.180. [DOI] [PubMed] [Google Scholar]
- 72.Pampiglione S. Dirofilariosi umana sottoconguintivale: su di un probabile caso osservato in Francia da Amato Lusitano nel XVI Secolo. Parassitologia. 1995;37:75–78. [PubMed] [Google Scholar]
- 73.Pampiglione S, Canestri Trotti G, Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: a review of world literature. Parassitologia. 1995;37:149–193. [PubMed] [Google Scholar]
- 74.Poltera A A. The histopathology of ocular loiasis in Uganda. Trans R Soc Trop Med Hyg. 1973;67:819–829. doi: 10.1016/0035-9203(73)90010-2. [DOI] [PubMed] [Google Scholar]
- 75.Richard-Lenoble D, Kombila M, Bain O. Foyer de filariose humaine au Gabon a microfilaire dermique indifferenciable de Microfilaria rodhaini. Ann Parasitol. 1982;57:506. [PubMed] [Google Scholar]
- 76.Rosenblatt P, Beaver P C, Orihel T C. A filarial infection apparently acquired in New York City. Am J Trop Med Hyg. 1962;11:641–645. doi: 10.4269/ajtmh.1962.11.641. [DOI] [PubMed] [Google Scholar]
- 77.Santamaria B, Cordero M, Muro A, Simon F. Evaluation of Dirofilaria immitis excretory/secretory products for seroepidemiological studies on human dirofilariasis. Parasite. 1995;2:269–273. doi: 10.1051/parasite/1995023269. [DOI] [PubMed] [Google Scholar]
- 78.Santamaria B, DiSacco B, Muro A, Genchi C, Simon F, Cordero M. Serological diagnosis of subcutaneous dirofilariasis. Clin Exp Dermatol. 1995;20:19–21. doi: 10.1111/j.1365-2230.1995.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 79.Siegenthaler R, Gubler R. Paraarticulares Nematodengranulom (einheimische Onchocerca) Schweiz Med Wochenschr. 1965;95:1102–1104. [PubMed] [Google Scholar]
- 80.Simmons C F, Winter H S, Berde C, et al. Zoonotic filariasis with lymphedema in an immunodeficient infant. N Engl J Med. 1974;310:1243–1245. doi: 10.1056/NEJM198405103101908. [DOI] [PubMed] [Google Scholar]
- 81.Sun S, Sugane K. Immunodiagnosis of human dirofilariasis by enzyme-linked immunosorbent assay using recombinant DNA-derived fusion protein. J Helminthol. 1992;66:220–226. doi: 10.1017/s0022149x00014590. [DOI] [PubMed] [Google Scholar]
- 82.Takaoka H, Bain O, Tajimi S, Kashima K, Nakayama I, Korenaga M, Aoki C, Otsuka Y. Second case of zoonotic Onchocerca infection in a resident of Oita in Japan. Parasite. 1996;3:179–182. doi: 10.1051/parasite/1996032179. [DOI] [PubMed] [Google Scholar]