Abstract
Giant cell arteritis (GCA) is a granulomatous inflammation involving medium and large vessels that can lead to serious clinical manifestations associated with tissue ischemia. Temporal artery biopsy (TAB) is currently the gold standard method for the diagnosis of GCA, with a specificity of 100% and a sensitivity of 77%. However, the false-negative rate for TAB ranges from 9% to 61%. False negatives may be related to the timing of biopsy, the length of specimen, and the existence of “skip lesions.” We reviewed the relevant evidence for methods to improve the sensitivity and reduce the false-negative rate for TAB. To reduce the false-negative rate for TAB, it is recommended to perform TAB within 1 week of starting corticosteroid therapy. Although there is currently no consensus, we suggest that the temporal artery is cut to a length of 20‒30 mm and to prepare serial pathological sections. It is necessary to attach great importance to patients suspected of having GCA, and complete TAB should be performed as soon as possible while starting corticosteroid therapy promptly. We also discuss the clinical value of non-invasive vascular imaging technologies, such as DUS, CTA, MRA, and 18F-FDG-PET/CT, as auxiliary methods for GCA diagnosis that could partially replace TAB.
Keywords: Diagnostic methods, giant cell arteritis, temporal artery biopsy, vasculitis
Giant cell arteritis (GCA) is a type of autoimmune inflammation involving the medium and large vessels that leads to proliferation of the vascular intima, thickening of the vascular wall, narrowing and blocking of the lumen, and exacerbation of tissue ischemia. GCA is common in elderly people in Western countries. It is usually accompanied by headache, scalp tenderness, mastication discomfort, fatigue, anorexia, fever, weight loss, and other systemic symptoms.[1] GCA is considered to be a clinical emergency in ophthalmology and neurology. In prior studies, 14%‒70% of GCA patients had eye involvement, which can lead to acute and rapidly progressive vision loss, and 20% of patients had partial or total permanent vision loss in one eye or both eyes.[2] Arteritic anterior ischemic optic neuropathy (A-AION) is the most common cause of visual dysfunction caused by GCA, accounting for 81.2% of cases of permanent vision loss due to GCA.[3,4] Beyond serious ocular manifestations, GCA-related stroke was reported in 2.7%‒7.4% of cases, and it may be fatal in severe cases.[5,6]
In 1990, the American College of Rheumatology (ACR) published diagnostic criteria for GCA comprising the following five factors[7]: I, age at onset ≥50 years; II, onset of a new headache; III, temporal artery tenderness or reduced pulsation; IV, elevated erythrocyte sedimentation rate (ESR) (≥ 50 mm/h using the Westergren method); and V, histological evidence of necrotizing arteritis in the temporal artery with predominantly mononuclear cell infiltration or granulomatous inflammation usually with multinucleate giant cells. The diagnosis of GCA is made if patients satisfy at least three of these five criteria. In 2016, the ACR published updated diagnostic criteria for GCA and quantified the relative contribution of each criterion to the diagnosis.[8] Notably, the importance of histological evidence was not weakened.
It is reported that the clinical diagnostic sensitivity of the 1990 GCA classification diagnostic criteria is 93.5% and the specificity is 91.2%.[9] Some studies have shown that the diagnostic accuracy rate for GCA patients based on these criteria is just 51.4% without temporal artery biopsy (TAB), but the accuracy rate increased to 73% when combined with pathological diagnosis,[10] suggesting that TAB plays an important role in the diagnosis of GCA. The diagnosis of GCA using TAB is highly specific. The site of the temporal artery showing tenderness or containing nodules should be selected for biopsy. Positive pathological results can confirm the presence of GCA, but negative results cannot exclude its diagnosis.[11] In a recent meta-analysis, the sensitivity of TAB for the diagnosis of GCA was 77%.[12] In prior studies, the false-negative rate ranged from 9% to 61%.[13-15] False negatives may be due to the timing of biopsy, the length of the specimen, or the presence of so-called “skip lesions.”[1]
In this review, we discuss the relevant evidence for methods to improve the sensitivity and reduce the false-negative rate, and we describe the clinical value of noninvasive vascular imaging technology as an auxiliary method for the diagnosis of GCA that can partially replace TAB.
Histopathology of TAB
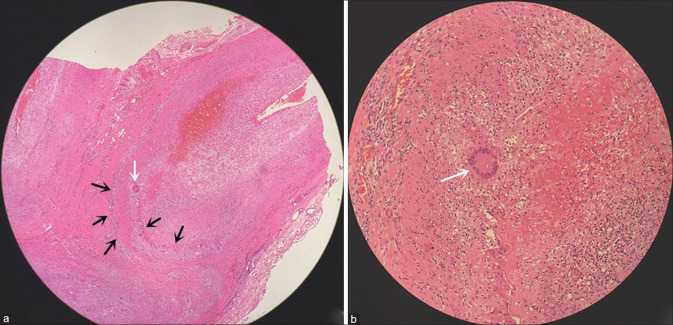
Although TAB is currently the gold standard method for the diagnosis of GCA,[11] there are no standards for the histopathology of GCA. The main diagnostic histopathological feature is angiogranulomatous inflammation comprising epithelioid cells, T lymphocytes, macrophages, and giant cells. Plasma cells, eosinophils, and fibroblasts can also be found. The presence of giant cells is not essential and, in fact, these cells are absent in 18%‒50% of specimens.[16] Other features include narrowing and occlusion of the arterial lumen, intimal proliferation, rupture of the internal elastic layer, and lumen thrombosis [Fig. 1]. These pathological manifestations can be either continuous or segmental,[17] and segmental “skipping lesions” was observed in 12%‒28% of cases.[16,17]
Figure 1.
Histopathology of temporal artery showing infiltration of lymphocytes, plasma cells, and histiocytes (black arrow), and multinucleated giant cells (white arrow) (hematoxylin and eosin staining; original magnification, 40× in a and 200× in b)
Timing of TAB
Early recognition and timely intervention of GCA are very important for the prevention of vision loss. For patients with suspected GCA, TAB should be performed as soon as possible to obtain biopsy samples.[18] Because A-AION usually leads to irreversible visual impairment, timely steroid treatment is valuable for preventing further visual deterioration, reducing disease recurrence, and reducing the incidence of GCA in the fellow eye. Biousse et al.[19] reported that ≥50% of untreated patients had visual impairment in the fellow eye shortly after the onset of visual impairments in the first eye. Beri et al.[20] reported that 19 of the 20 patients with bilateral A-AION showed involvement of both eyes at the first visit. In the other patient, the fellow eye developed A-AION symptoms within 2 days after starting systemic corticosteroid treatment. In addition, GCA patients without ocular involvement at the initial visit were followed up for many years, but no signs of A-AION were observed after starting corticosteroid therapy. These findings indicate that systemic corticosteroid therapy is effective for preventing A-AION. However, it takes some time for corticosteroids to block the development of arteritis in the posterior ciliary artery. Therefore, some patients may develop A-AION a few days after starting corticosteroid therapy.
Chaudhuri reported that TAB remained positive for 32 months after starting corticosteroid therapy.[21] However, Allison reported that the positive rate using TAB decreased from 82% before corticosteroid therapy to 60% at 1 week after corticosteroid intervention; therefore, it was recommended to perform TAB within 1 week after starting corticosteroid therapy.[22] Narvaez reported that the positive rate using TAB was 78% after corticosteroid therapy for 2 weeks, 65% after corticosteroid therapy for 2‒4 weeks, and only 40% after corticosteroid therapy for ≥4 weeks.[23] For patients with suspected GCA or A-AION, TAB should be performed as soon as possible in order to confirm the diagnosis and avoid delaying treatment, and corticosteroid treatment should not be delayed.[24]
Length of the specimen
Due to the segmental distribution of lesions in GCA, there is no consensus on the minimum length of the temporal artery that should be biopsied to achieve the best sensitivity.[1] In order to avoid negative results caused by “skip lesions,” Murchison suggested that a fixed specimen length of 20 mm should be used as the minimum sample length.[25] Several studies have shown that the mean length of positive specimens was greater than that of negative specimens.[26,27] Oh reviewed 545 TAB specimens and found that, compared with vessel specimens <15 mm long, the positive rate for vessel specimens ≥15 mm long was 2.25 times greater, and the positive rate of TAB increased by 3.4% for each 1 mm increase in specimen length.[28] Breuer reported that the positive rate for TAB was 19% for vessel specimens ≤5 mm long, 71%‒79% for lengths of 6‒20 mm, and 89% for lengths >20 mm.[29] The British Society of Rheumatology (BSR) guidelines recommend an arterial biopsy length of 10 mm.[30] In the TABUL study, 7.3% of the specimens <10 mm long did not contain any arterial tissue, suggesting that shorter specimens and greater difficulty in obtaining a specimen increased the false-negative rate for pathological examination.[13] However, Mahr reported that a fixed specimen length of ≥5 mm could be sufficient for diagnosis.[31] Grossman reported that the specimen length was not related to the positive rate for TAB after comparing the lengths of specimens with different pathological results in GCA patients.[32] A study with temporal artery biopsies over an 11-year period from Mayo Clinic also indicated that biopsy length was uniformly noted to have no significant effect on biopsy positivity.[33] The authors explained that the reasons may be due to rich clinical experience and bilateral TAB to increase the positive rate.
Management strategies for TAB-negative patients
For patients with suspected GCA, unilateral TAB is recommended first. Although TAB is positive in most cases of cranial GCA, the negative rate for TAB is about 40% in patients with extracranial/large vessel GCA (LV-GCA).[34,35] Butendieck examines 603 patients with a bilateral biopsy for the diagnosis of GCA in Mayo Clinic and found 7% patients had a negative initial biopsy followed by a positive result on the contralateral side.[33] Mehta reviewed 310 patients who underwent bilateral TAB and observed a 12.1% discordance rate between pathology results.[36] The discordance rates in TAB specimens suggest that performing a bilateral TAB offers additional clinical benefit, such as increasing the positive rate of TAB and reducing the missed diagnosis rate of GCA. If the result of unilateral biopsy is negative, even after corticosteroid therapy, contralateral TAB should be performed to confirm the diagnosis.[18] Furthermore, for patients with a negative result for the initial TAB, no studies have evaluated the effects of steroid or immunosuppressive therapy on the results of subsequent TAB.
In an autopsy study of GCA patients, it was found that the temporal artery was not always involved and that other arteries may be involved.[37] In patients with negative results of bilateral TAB and there is a strong clinical indication for another biopsy, the temporal artery bifurcation or the posterior temporal branch are suitable sites. Other studies proposed biopsy of other branches of the external carotid artery, such as the occipital artery or maxillary artery, but these sites are more difficult to access and there is a risk of complications, especially nerve injury.[38]
Because of the seriousness of GCA, negative TAB reports should not change the clinical management if GCA is highly suspected based on the patient’s clinical manifestations. Sait proposed a simple management plan based on the revised ACR 2016 criteria for GCA: (1) biopsy may not be required if the score does not exceed 2, as these patients are unlikely to have GCA; (2) TAB is not required in patients with a score of ≥5, as they are likely to have GCA and should continue corticosteroid therapy; and (3) biopsy is necessary for cases with a score of 3 and 4, as the results of TAB show the greatest variability in these patients.[39]
Possible alternatives to TAB
TAB is a traumatic procedure with a risk of complications such as parotid gland injury, surgical site hematoma, local infection or sepsis, and overlapping infection.[40] The utility of noninvasive diagnostic methods as alternatives to TAB remains widely debated.[41,42] Patients and many doctors have welcomed novel imaging techniques for GCA in clinical practice that avoid the risks associated with invasive interventions. The European League Against Rheumatism (EULAR) 2018 guidelines recommend that the diagnosis of GCA should be confirmed by imaging or histology.[43] Doppler ultrasound (DUS), computed tomography angiography (CTA), magnetic resonance angiography (MRA), and 18F-deoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) are currently the most commonly used alternative methods.
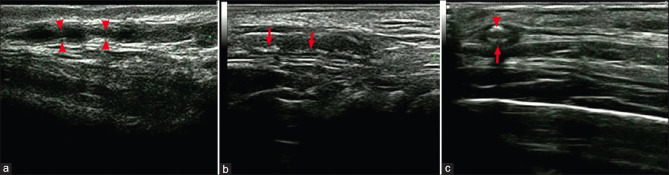
DUS is recommended as a first-line method in the EULAR guidelines.[43] The typical manifestation of GCA observed on DUS is the halo sign, a low echo ring around the arterial lumen, depicting edematous thickening of the arterial wall owing to inflammation.[44] Segmental or diffuse thickening, stenosis, and occlusion of the vascular wall can also be detected by DUS [Fig. 2].[45] In a recent meta-analysis, the sensitivity and specificity of temporal artery DUS were 67% and 95%, respectively, based on the clinical diagnosis.[46] DUS can also be used to guide TAB in terms of determining the path of the temporal artery, the side of the branch that should be collected, and whether the involved side is unilateral or asymmetric. However, the interpretation of DUS is highly dependent on the radiologist’s diagnostic skills because they need special training to provide accurate reports.[13] Moreover, the halo signals related to GCA can also be caused by significant atherosclerosis, leading to false-positive results.[47] Furthermore, there are some differences between countries and regions in terms of judging the effectiveness of DUS for the diagnosis of GCA.[36,42,48]
Figure 2.
Superficial temporal artery ultrasound. (a) Segmental stenosis (arrows); (b) segmental thickening with calcification (arrows), and (c) hypoechoic halo (large arrow) with calcification (small arrow). (Cited from Chen Q, et al. Front Med (Lausanne) 2022.)
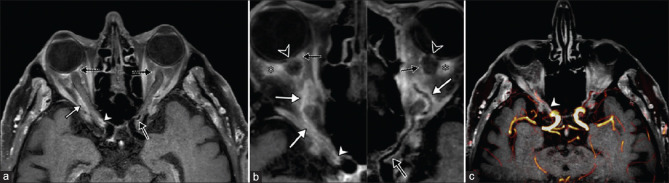
Although magnetic resonance imaging (MRI) is mainly used to exclude differential diagnoses, such as compressive optic neuropathy, it has recently been used to identify the positive signs of GCA-related A-AION, for which typical features include circular vessel wall thickening and strong contrast enhancement of the vessel wall [Fig. 3].[49-51] Previous studies have determined the sensitivity (68%‒89%) and specificity (73%‒97%) of high-resolution MRI for the evaluation of cranial GCA.[52-55] Siemonsen used 3T MRI to study intracranial vessels and reported a medium sensitivity (50%) but high specificity (100%) for the diagnosis of GCA.[56] Mohammed-Brahim used 3T high-resolution vascular wall MRI to accurately determine the inflammatory changes in the ophthalmic artery, and the sensitivity, specificity, positive predictive value, and negative predictive value were all 100%. That method was also more likely to detect posterior ciliary arteritis in patients with A-AOIN.[57] Sommer used high-resolution black blood MRI to evaluate posterior ciliary arteritis in GCA patients and reported sensitivity and specificity of 92.9% and 92.3%, respectively.[51] Other studies have reported that high-resolution MRA of the temporal artery is a reliable method for the diagnosis of cranial GCA, with a sensitivity of 88.7% and a specificity of 75.0%.[54,58] High-resolution vascular wall (HR-VW) MRI and extracranial artery MRI can also be used for the accurate diagnosis of GCA showing typical inflammatory changes.[54,59,60]
Figure 3.
High-resolution vessel wall (HR-VW) imaging in a patient with bilateral A-AION. MRI reformatted in an axial plane (a) and in a curvilinear plane along the axis of each ophthalmic artery (b) showing marked wall thickening and strong mural enhancement with perivascular inflammatory infiltration (white arrow) in the whole course of the right artery, with severe involvement of its proximal part (white arrowhead), and in the left artery wall thickening and substantial mural enhancement (black arrow). MRI also shows bilateral enhancement of the optic disc (black arrowhead) and of the orbital fat (asterisk), as well as inflammatory changes of posterior ciliary arteries (dashed black arrows). Fused magnetic resonance angiography and HR-VW imaging (c) showing stenosis of the proximal part of the ophthalmic artery, due to the severe arteritis-related inflammatory changes (white arrowhead). (Cited from Mohammed-Brahim N, et al. Invest Radiol 2019)
18F-FDG-PET/CT has been proposed to assist in the diagnosis of GCA. The high metabolic activity of inflammatory cells, such as macrophages and lymphocytes, causes high uptake of FDG at arterial inflammatory sites [Fig. 4].[61,62] Compared with TAB, the coincidence rate of 18F-FDG-PET/CT in GCA was 69.2%.[61] Extracranial vasculitis, such as arteritis, can also be evaluated by 18F-FDG-PET/CT, which is helpful to clarify the diagnosis of vasculitis.[63,64] However, it is often difficult to perform MRA or 18F-FDG-PET/CT in a timely manner in some hospitals, and the specificities of these imaging modalities are lower than that of TAB. In addition, the sensitivity rapidly decreases after only a few days of high dose steroid exposure.[65]
Figure 4.
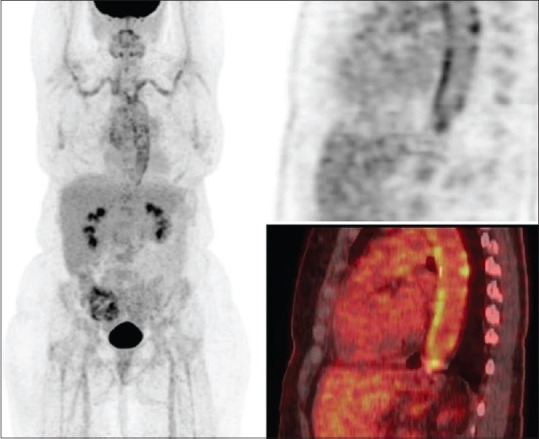
FDG-PET whole-body MIP image (left) and sagittal PET (upper right) and fused PET/CT (lower right) images of the aorta shows increased FDG uptake in the thoracic aorta, subclavian arteries and axillary arteries. FDG uptake is clearly higher than liver uptake and thus categorized as pathologic. Note nodular calcification in the aorta without FDG uptake. This finding together with the diffuse FDG uptake along the vascular wall also points to vasculitis rather than atherosclerosis as the underlying etiology for FDG uptake. (Cited from Emamifar A, et al. ACR Open Rheumatol 2020.)
Dejaco expressed support for the recently published EULAR proposal for large vessel vasculitis imaging in clinical practice.[64] The authors suggested that GCA could be diagnosed without biopsy or further imaging in patients with high clinical suspicion of GCA and positive DUS results. PET and/or MRI were more valuable in patients with cranial GCA without head symptoms or signs. It is worth noting that EULAR emphasized the need for TAB if the clinical, laboratory, and imaging results could not confirm or exclude the presence of GCA. In addition, TAB remains the preferred diagnostic method if vascular imaging expertise is in doubt.[64]
Conclusion
In conclusion, TAB remains an important tool for the diagnosis of GCA. It is necessary to attach great importance to patients suspected of having GCA, and complete TAB should be performed as soon as possible while starting corticosteroid therapy promptly. To reduce the false-negative rate for TAB, it is recommended to perform TAB within 1 week of starting corticosteroid therapy. Although there is currently no consensus, when we consider that tissues contract during fixation, we suggest that the temporal artery is cut to a length of 20‒30 mm and to prepare serial pathological sections. New imaging methods, particularly DUS, CTA, MRA, and 18F-FDG-PET/CT, can partially replace TAB to assist in the diagnosis of GCA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Simon S, Ninan J, Hissaria P. Diagnosis and management of giant cell arteritis: Major review. Clin Exp Ophthalmol. 2021;49:169–85. doi: 10.1111/ceo.13897. [DOI] [PubMed] [Google Scholar]
- 2.Ciofalo A, Gulotta G, Iannella G, Pasquariello B, Manno A, Angeletti D, et al. Giant Cell Arteritis (GCA) pathogenesis, clinical aspects and treatment approaches. Curr Rheumatol Rev. 2019;15:259–68. doi: 10.2174/1573397115666190227194014. [DOI] [PubMed] [Google Scholar]
- 3.Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009;28:34–62. doi: 10.1016/j.preteyeres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Pereira LS, Yoon MK, Hwang TN, Hong JE, Ray K, Porco T, et al. Giant cell arteritis in Asians: A comparative study. Br J Ophthalmol. 2011;95:214–6. doi: 10.1136/bjo.2009.177220. [DOI] [PubMed] [Google Scholar]
- 5.Parreau S, Dumonteil S, Montoro FM, Gondran G, Bezanahary H, Palat S, et al. Giant cell arteritis-related stroke in a large inception cohort: A comparative study. Semin Arthritis Rheum. 2022;55:152020. doi: 10.1016/j.semarthrit.2022.152020. [DOI] [PubMed] [Google Scholar]
- 6.Samson M, Jacquin A, Audia S, Daubail B, Devilliers H, Petrella T, et al. Stroke associated with giant cell arteritis: A population-based study. J Neurol Neurosurg Psychiatry. 2015;86:216–21. doi: 10.1136/jnnp-2014-307614. [DOI] [PubMed] [Google Scholar]
- 7.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 8.Salehi-Abari I. 2016 ACR revised criteria for early diagnosis of Giant Cell (Temporal) Arteritis. Autoimmune Dis Ther Approaches. 2016;3:1. [Google Scholar]
- 9.Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: Revisiting the concept of the disease. Rheumatology (Oxford) 2017;56:506–15. doi: 10.1093/rheumatology/kew273. [DOI] [PubMed] [Google Scholar]
- 10.Murchison AP, Gilbert ME, Bilyk JR, Eagle RC, Pueyo V, Sergott RC, et al. Validity of the American College of Rheumatology criteria for the diagnosis of giant cell arteritis. Am J Ophthalmol. 2012;154:722–9. doi: 10.1016/j.ajo.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Prieto-González S, Arguis P, García-Martínez A, Espígol-Frigolé G, Tavera-Bahillo I, Butjosa M, et al. Large vessel involvement in biopsy-proven giant cell arteritis: Prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71:1170–6. doi: 10.1136/annrheumdis-2011-200865. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein E, Maldini C, Gonzalez-Chiappe S, Chevret S, Mahr A. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: A systematic literature review and meta-analysis. Rheumatology. 2020;59:1011–20. doi: 10.1093/rheumatology/kez385. [DOI] [PubMed] [Google Scholar]
- 13.Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Health Technol Assess. 2016;20:1–238. doi: 10.3310/hta20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomassen I, den Brok AN, Konings CJ, Nienhuijs SW, van de Poll MC. Steroid use is associated with clinically irrelevant biopsies in patients with suspected giant cell arteritis. Am Surg. 2012;78:1362–8. [PubMed] [Google Scholar]
- 15.Grossman C, Barshack I, Bornstein G, Ben-Zvi I. Is temporal artery biopsy essential in all cases of suspected giant cell arteritis? Clin Exp Rheumatol. 2015;33:S-84-9. [PubMed] [Google Scholar]
- 16.Wang AL, Raven ML, Surapaneni K, Albert DM. Studies on the histopathology of temporal arteritis. Ocul Oncol Pathol. 2017;3:60–5. doi: 10.1159/000449466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein RG, Campbell RJ, Hunder GG, Carney JA. Skip lesions in temporal arteritis. Mayo Clin Proc. 1976;51:504–10. [PubMed] [Google Scholar]
- 18.Maz M, Chung SA, Abril A, Langford CA, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021;73:1349–65. doi: 10.1002/art.41774. [DOI] [PubMed] [Google Scholar]
- 19.Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372:2428–36. doi: 10.1056/NEJMra1413352. [DOI] [PubMed] [Google Scholar]
- 20.Beri M, Klugman MR, Kohler JA, Hayreh SS. Anterior ischemic optic neuropathy. VII. Incidence of bilaterality and various influencing factors. Ophthalmology. 1987;94:1020–8. doi: 10.1016/s0161-6420(87)33350-0. [DOI] [PubMed] [Google Scholar]
- 21.Ray-Chaudhuri N, Kiné DA, Tijani SO, Parums DV, Cartlidge N, Strong NP, et al. Effect of prior steroid treatment on temporal artery biopsy findings in giant cell arteritis. Br J Ophthalmol. 2002;86:530–2. doi: 10.1136/bjo.86.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison MC, Gallagher PJ. Temporal artery biopsy and corticosteroid treatment. Ann Rheum Dis. 1984;43:416–7. doi: 10.1136/ard.43.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narváez J, Bernad B, Roig-Vilaseca D, García-Gómez C, Gómez-Vaquero C, Juanola X, et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum. 2007;37:13–9. doi: 10.1016/j.semarthrit.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Rahman W, Rahman FZ. Giant cell (temporal) arteritis: An overview and update. Surv Ophthalmol. 2005;50:415–28. doi: 10.1016/j.survophthal.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Murchison AP, Bilyk JR, Eagle RC, Jr, Savino PJ. Shrinkage revisited: How long is long enough? Ophthalmic Plast Reconstr Surg. 2012;28:261–3. doi: 10.1097/IOP.0b013e31824ee720. [DOI] [PubMed] [Google Scholar]
- 26.Aghdam KA, Sanjari MS, Manafi N, Khorramdel S, Alemzadeh SA, Akbar Navahi RA. Temporal artery biopsy for diagnosing giant cell arteritis: A ten-year review. J Ophthalmic Vis Res. 2020;15:201–9. doi: 10.18502/jovr.v15i2.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor-Gjevre R, Vo M, Shukla D, Resch L. Temporal artery biopsy for giant cell arteritis. J Rheumatol. 2005;32:1279–82. [PubMed] [Google Scholar]
- 28.Oh LJ, Wong E, Gill AJ, McCluskey P, Smith JEH. Value of temporal artery biopsy length in diagnosing giant cell arteritis. ANZ J Surg. 2018;88:191–5. doi: 10.1111/ans.13822. [DOI] [PubMed] [Google Scholar]
- 29.Breuer GS, Nesher R, Nesher G. Effect of biopsy length on the rate of positive temporal artery biopsies. Clin Exp Rheumatol. 2009;27:S10–13. [PubMed] [Google Scholar]
- 30.Mackie SL, Dejaco C, Appenzeller S, Camellino D, Duftner C, Gonzalez-Chiappe S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis: Executive summary. Rheumatology (Oxford) 2020;59:487–94. doi: 10.1093/rheumatology/kez664. [DOI] [PubMed] [Google Scholar]
- 31.Mahr A, Saba M, Kambouchner M, Polivka M, Baudrimont M, Brochériou I, et al. Temporal artery biopsy for diagnosing giant cell arteritis: The longer, the better? Ann Rheum Dis. 2006;65:826–8. doi: 10.1136/ard.2005.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grossman C, Ben-Zvi I, Barshack I, Bornstein G. Association between specimen length and diagnostic yield of temporal artery biopsy. Scand J Rheumatol. 2017;46:222–5. doi: 10.1080/03009742.2016.1196501. [DOI] [PubMed] [Google Scholar]
- 33.Butendieck R, Jr, Calamia K, Sandin A. A study of temporal artery biopsy for the diagnosis of giant cell arteritis. Clin Rheumatol. 2023;42:159–66. doi: 10.1007/s10067-022-06371-0. [DOI] [PubMed] [Google Scholar]
- 34.Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42:311–7. doi: 10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Allsop CJ, Gallagher PJ. Temporal artery biopsy in giant-cell arteritis. A reappraisal. Am J Surg Pathol. 1981;5:317–23. doi: 10.1097/00000478-198106000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Mehta K, Eid M, Gangadharan A, Pritchard A, Lin CC, Goodney P, et al. The utility of the bilateral temporal artery biopsy for diagnosis of giant cell arteritis. J Vasc Surg. 2022;76:1704–9. doi: 10.1016/j.jvs.2022.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch Neurol. 1972;27:378–91. doi: 10.1001/archneur.1972.00490170010003. [DOI] [PubMed] [Google Scholar]
- 38.Noumegni SR, Hoffmann C, Bressollette L, Jousse-Joulin S, Cornec D. Technique et valeur diagnostique de la biopsie de l'artère temporale. Revue du Rhumatisme Monographies. 2020;87:189–93. [Google Scholar]
- 39.Sait MR, Lepore M, Kwasnicki R, Allington J, Balasubramanian R, Somasundaram SK, et al. The 2016 revised ACR criteria for diagnosis of giant cell arteritis –Our case series: Can this avoid unnecessary temporal artery biopsies? Int J Surg Open. 2017;9:19–23. [Google Scholar]
- 40.Parreau S, Liozon E, Chen JJ, Curumthaullee MF, Fauchais AL, Warrington KJ, et al. Temporal artery biopsy: A technical guide and review of its importance and indications. Surv Ophthalmol. 2023;68:104–12. doi: 10.1016/j.survophthal.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies CG, May DJ. The role of temporal artery biopsies in giant cell arteritis. Ann R Coll Surg Engl. 2011;93:4–5. doi: 10.1308/003588411X12851639107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moiseev SV, Smitienko I, Bulanov N, Novikov PI. The role of temporal artery biopsy in patients with giant-cell arteritis is debated. Ann Rheum Dis. 2019;78:e31. doi: 10.1136/annrheumdis-2018-213282. [DOI] [PubMed] [Google Scholar]
- 43.Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. doi: 10.1136/annrheumdis-2019-215672. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337:1336–42. doi: 10.1056/NEJM199711063371902. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, Chen W, Feng C, Gong D, Zhang J, Bi Y, et al. Giant cell arteritis presenting with ocular symptoms: Clinical characteristics and multimodal imaging in a Chinese case series. Front Med (Lausanne) 2022;9:885463. doi: 10.3389/fmed.2022.885463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebastian A, Coath F, Innes S, Jackson J, van der Geest KSM, Dasgupta B. Role of the halo sign in the assessment of giant cell arteritis: A systematic review and meta-analysis. Rheumatol Adv Pract. 2021;5:rkab059. doi: 10.1093/rap/rkab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ing E. Temporal artery biopsy versus imaging in patients with cranial giant cell arteritis. Radiol Med. 2020;125:902–3. doi: 10.1007/s11547-020-01166-2. [DOI] [PubMed] [Google Scholar]
- 48.Maldini C, Dépinay-Dhellemmes C, Tra TT, Chauveau M, Allanore Y, Gossec L, et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: Analysis of 77 subjects. J Rheumatol. 2010;37:2326–30. doi: 10.3899/jrheum.100353. [DOI] [PubMed] [Google Scholar]
- 49.Remond P, Attyé A, Lecler A, Lamalle L, Boudiaf N, Aptel F, et al. The central bright spot sign: A potential new MR imaging sign for the early diagnosis of anterior ischemic optic neuropathy due to giant cell arteritis. Am J Neuroradiol. 2017;38:1411–5. doi: 10.3174/ajnr.A5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geiger J, Ness T, Uhl M, Lagrèze WA, Vaith P, Langer M, et al. Involvement of the ophthalmic artery in giant cell arteritis visualized by 3T MRI. Rheumatology. 2008;48:537–41. doi: 10.1093/rheumatology/kep011. [DOI] [PubMed] [Google Scholar]
- 51.Sommer NN, Treitl KM, Coppenrath E, Kooijman H, Dechant C, Czihal M, et al. Three-dimensional high-resolution black-blood magnetic resonance imaging for detection of arteritic anterior ischemic optic neuropathy in patients with giant cell arteritis. Invest Radiol. 2018;53:698–704. doi: 10.1097/RLI.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 52.Bley TA, Weiben O, Uhl M, Vaith P, Schmidt D, Warnatz K, et al. Assessment of the cranial involvement pattern of giant cell arteritis with 3T magnetic resonance imaging. Arthritis Rheum. 2005;52:2470–7. doi: 10.1002/art.21226. [DOI] [PubMed] [Google Scholar]
- 53.Bley TA, Uhl M, Carew J, Markl M, Schmidt D, Peter H-H, et al. Diagnostic value of high-resolution MR imaging in giant cell arteritis. Am J Neuroradiol. 2007;28:1722–7. doi: 10.3174/ajnr.A0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klink T, Geiger J, Both M, Ness T, Heinzelmann S, Reinhard M, et al. Giant cell arteritis: Diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis—results from a multicenter trial. Radiology. 2014;273:844–52. doi: 10.1148/radiol.14140056. [DOI] [PubMed] [Google Scholar]
- 55.Geiger J, Bley T, Uhl M, Frydrychowicz A, Langer M, Markl M. Diagnostic value of T2- weighted imaging for the detection of superficial cranial artery inflammation in giant cell arteritis. J Magn Reson Imaging. 2010;31:470–4. doi: 10.1002/jmri.22047. [DOI] [PubMed] [Google Scholar]
- 56.Siemonsen S, Brekenfeld C, Holst B, Kaufmann-Buehler A-K, Fiehler J, Bley TA. 3T MRI reveals extra- and intracranial involvement in giant cell arteritis. Am J Neuroradiol. 2015;36:91–7. doi: 10.3174/ajnr.A4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohammed-Brahim N, Clavel G, Charbonneau F, Duron L, Picard H, Zuber K, et al. Three tesla 3D high-resolution vessel wall MRI of the orbit may differentiate arteritic from nonarteritic anterior ischemic optic neuropathy. Invest Radiol. 2019;54:712–8. doi: 10.1097/RLI.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 58.Junek M, Hu A, Garner S, Rebello R, Legault K, Beattie K, et al. Contextualizing temporal arterial magnetic resonance angiography in the diagnosis of giant cell arteritis: A retrospective cohort study. Rheumatology (Oxford) 2021;60:4229–37. doi: 10.1093/rheumatology/keaa916. [DOI] [PubMed] [Google Scholar]
- 59.Goll C, Thormann M, Hofmüller W, Friebe B, Behrens-Baumann W, Bley TA, et al. Feasibility study: 7 T MRI in giant cell arteritis. Graefes Arch Clin Exp Ophthalmol. 2016;254:1111–6. doi: 10.1007/s00417-016-3337-7. [DOI] [PubMed] [Google Scholar]
- 60.Rhéaume M, Rebello R, Pagnoux C, Carette S, Clements-Baker M, Cohen-Hallaleh V, et al. High-resolution magnetic resonance imaging of scalp arteries for the diagnosis of giant cell arteritis: Results of a prospective cohort study. Arthritis Rheumatol. 2017;69:161–8. doi: 10.1002/art.39824. [DOI] [PubMed] [Google Scholar]
- 61.Emamifar A, Ellingsen T, Hess S, Gerke O, Hviid Larsen R, Ahangarani Farahani Z, et al. The utility of 18F-FDG PET/CT in patients with clinical suspicion of polymyalgia rheumatica and giant cell arteritis: A prospective, observational, and cross-sectional study. ACR Open Rheumatol. 2020;2:478–90. doi: 10.1002/acr2.11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nienhuis PH, Sandovici M, Glaudemans AW, Slart RH, Brouwer E. Visual and semiquantitative assessment of cranial artery inflammation with FDG-PET/CT in giant cell arteritis. Semin Arthritis Rheum. 2020;50:616–23. doi: 10.1016/j.semarthrit.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Slart RHJAJA, Glaudemans AWJM, Chareonthaitawee P, Treglia G, Besson FL, Bleyd TA, et al. FDG-PET/CT (A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45:1250–69. doi: 10.1007/s00259-018-3973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–43. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 65.Buttgereit F, Matteson EL, Dejaco C. Polymyalgia rheumatic and giant cell arteritis. JAMA. 2020;324:993–4. doi: 10.1001/jama.2020.10155. [DOI] [PubMed] [Google Scholar]