Abstract
Background:
Retinal artery occlusion (RAO) may lead to irreversible blindness. For acute RAO, intravenous thrombolysis (IVT) can be considered as treatment. However, due to the rarity of RAO, data about IVT safety and effectiveness is limited.
Methods:
From the multicenter database ThRombolysis for Ischemic Stroke Patients (TRISP), we retrospectively analyzed visual acuity (VA) at baseline and within 3 months in IVT and non-IVT treated RAO patients. Primary outcome was difference of VA between baseline and follow up (∆VA). Secondary outcomes were rates of visual recovery (defined as improvement of VA ⩾ 0.3 logMAR), and safety (symptomatic intracranial hemorrhage (sICH) according to ECASS II criteria, asymptomatic intracranial hemorrhage (ICH) and major extracranial bleeding). Statistical analysis was performed using parametric tests and a linear regression model adjusted for age, sex and baseline VA.
Results:
We screened 200 patients with acute RAO and included 47 IVT and 34 non-IVT patients with complete information about recovery of vision. Visual Acuity at follow up significantly improved compared to baseline in IVT patients (∆VA 0.5 ± 0.8, p < 0.001) and non-IVT patients (∆VA 0.40 ± 1.1, p < 0.05). No significant differences in ∆VA and visual recovery rate were found between groups at follow up. Two asymptomatic ICH (4%) and one (2%) major extracranial bleeding (intraocular bleeding) occurred in the IVT group, while no bleeding events were reported in the non-IVT group.
Conclusion:
Our study provides real-life data from the largest cohort of IVT treated RAO patients published so far. While there is no evidence for superiority of IVT compared to conservative treatment, bleeding rates were low. A randomized controlled trial and standardized outcome assessments in RAO patients are justified to assess the net benefit of IVT in RAO.
Keywords: Retinal artery occlusion, IVT, thrombolysis, central retinal artery, visual acuity
Introduction
Retinal artery occlusion (RAO) is an ophthalmologic emergency. Irreversible damage to the retina may develop within 90–240 min after thrombotic or embolic occlusion of the retinal artery. 1 The classical clinical presentation of RAO is acute, painless, unilateral vision loss. Risk factors are similar to other cardiovascular diseases.2,3 As spontaneous recanalization of the central retinal artery is rare, rapid diagnosis and treatment is imperative. 4 Treatment options include conservative therapy, local intraarterial thrombolysis and systemic intravenous thrombolysis (IVT). However, currently no treatment option for RAO is generally recommended by the American Academy of Ophthalmology. 5 Intravenous acetazolamide, mannitol, topical beta-blockade, and anterior chamber paracentesis are conservative treatment options. However, there is little evidence supporting the efficacy of conservative therapies, and data from a patient-level meta-analysis suggest that conservative treatments may be futile or even harmful.6,7 Local intraarterial thrombolysis, despite positive results in prospective case series and retrospective studies, did not improve outcome in the randomized controlled EAGLE trial (European Assessment Group for Lysis in the Eye), and is therefore no longer recommended. 8 There is no randomized controlled study to prove the effectiveness of IVT in RAO so far. Larger randomized control trials are currently ongoing but have yet to be completed. 9 The above-mentioned patient-level meta-analysis by Schrag et al. 7 showed a significantly higher chance of good visual outcome in patients treated with IVT compared to conservative treatment. Like in non-RAO ischemic stroke, IVT was most effective when started within 4.5 h after symptom onset. This was confirmed in an updated meta-analysis in 2020. 10 Concerning safety, less data is available. In a cohort study, 1 out of 25 RAO patients (4%) treated with IV alteplase developed asymptomatic intracranial hemorrhage (asICH), and in another safety and feasibility study, 1 out of 30 IVT treated patients (3%) developed asICH. 11
As total RAO patient numbers are low and mostly derived from smaller individual studies, we aimed to assess the safety and effectiveness of IVT in RAO patients within a larger multicenter cohort. To this end, we retrospectively analyzed the outcome of RAO patients treated with IVT from 10 European stroke centers within the multicenter IVT collaboration ThRombolysis in Ischemic Stroke Patients (TRISP).
Methods
Study population
The data for this study was obtained from the TRISP (ThRombolysis in Ischemic Stroke Patients) collaboration containing >10,000 prospectively collected data sets of IVT treated patients from 20 European stroke centers.12,13 The locally obtained data was anonymized at the respective center, and then sent and pooled for analysis by the study coordinators in Zurich.
We included data from all IVT treated RAO patients within the TRISP database and collected additional data of conservatively treated RAO patients in each center if available. Patients were included based on the diagnosis of RAO within the medical records, which was based on clinical parameters and ophthalmological exam performed by a trained ophthalmologist.
Exclusion criteria were missing information about visual acuity (VA) at baseline and/or missing follow-up VA, as well as visual impairment at baseline of less than 0.3 logMAR, that is, patients with amaurosis fugax.
Patient data
We analyzed baseline demographics, medical history and prior medication, RAO etiology, baseline and follow-up VA, safety parameters as well as symptom-to-needle time for the IVT group. VA was analyzed in logMAR equivalences. LogMAR is the logarithm of the Minimum Angle of Resolution. It is either derived directly by use of an Early Treatment Diabetic Retinopathy Study (ETDRS) vision test chart (also called logMAR chart) as opposed to the commonly used Snellen chart, or can be calculated from the results of measurement of the VA with a Snellen chart. In logMAR notation, lower numbers correspond to better vision, and higher numbers reflect a worse VA. A VA of six-sixths in the Snellen chart (i.e. being able to read six lines from 6 m on a Snellen chart) corresponds to a MAR of 1′ (1 min of arc) and would correspond to a VA in logMAR of zero.14,15 Lower, semi-quantitative VA values, including counting fingers (CF), hand motion (HM), light perception (LP) and blindness (BL) were translated into logMAR approximates based on previous work who could derive VA values in logMAR from these semi-quantitative scales by using the Freiburg Visual Acuity Test (FrACT) (CF = logMAR 2.0, HM = logMAR 2.3, LP = logMAR 2.6, BL = logMAR 2.9).10,16 Follow-up VA was obtained from medical records. Median follow-up VA was 90 days after RAO in both groups (median (IQR): 90 (87.0) days for IVT patients, 90 (83.5) days for non-IVT patients. In the IVT group, 45/47 (96%) patients had a short-term follow-up VA assessment (within 7 days), while 23/47 patients (49%) had a long-term follow up VA ( > 7 days up to 3 months) assessment after RAO. In the non-IVT group 25/34 patients (74%) had a short-term follow up VA and 22/34 patients (65%) had a long-term follow up VA. If VA values at multiple follow-up time points were available, the latest follow-up was used for analysis.
Outcome parameters and statistical analysis
The primary study outcome was difference in VA at follow-up (in logMAR) compared to baseline (∆VA). Secondary outcomes were rate of visual recovery (defined as improvement of ⩾0.3 logMAR) and safety in IVT and non-IVT patients. Safety parameters were symptomatic intracranial hemorrhage (sICH) according to Second European-Australasian Acute Stroke Study (ECASS II), 17 any intraparenchymal hemorrhage (any ICH), subdural hematoma (SDH) or subarachnoid hemorrhage (SAH)), and major extracranial bleeding according to the criteria of the International Society on Thrombosis and Hemostasis (ISTH). 18
Baseline characteristics were reported as mean ± SD, mean (Minimum/Maximum) or numbers (%). Comparisons of baseline and follow-up visual acuity were performed with a paired sample t-test for each group. Comparisons between groups were done with an independent samples t-test and recovery rate was compared using a chi-squared test. Additionally, analysis of covariance (ANCOVA) was conducted to determine the difference between IVT and non-IVT patients on VA at follow up controlling for age, sex, time-to-treatment, and baseline VA.
Ethics
The present study was approved by the Cantonal Ethics Committee Zurich, Switzerland (PREDICT, BASEC-Nr. PB_2016-01751). Additional local ethics approval was obtained by participating centers if required.
Results
Patient population and baseline characteristics
Within the TRISP cohort, we identified 59 IVT patients with RAO. In addition, participating centers provided data of 142 non-IVT RAO patients. We excluded 109 patients (10 IVT, 99 non-IVT patients) due to missing information about VA. Eleven patients (two IVT, nine non-IVT) were excluded due to VA impairment of less than 0.3 at baseline. Finally, we included 81 patients (47 IVT and 34 non-IVT patients) in the present analysis (Figure 1).
Figure 1.
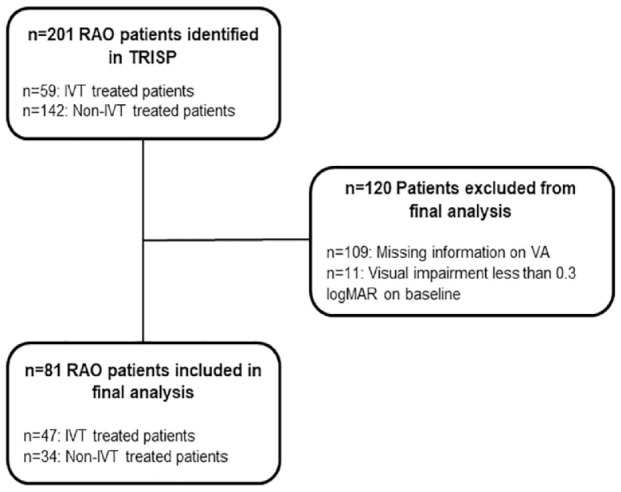
Flow chart of included and excluded patients. IVT: intravenous thrombolysis; RAO: retinal artery occlusion; VA: visual acuity; logMAR: logarithm of minimum angle of resolution.
Mean age of the included patients was 65 years (standard deviation (SD) ±11.6 years) in the IVT group and 71 years (SD ±18.4 years) in the non-IVT group. Most common cardiovascular risk factors in both groups were hypertension, dyslipidemia, and diabetes. Rates of antiplatelet or anticoagulation therapy prior to the event did not differ significantly between groups. Initial systolic and diastolic blood pressure or laboratory results at baseline were similar as well. Available baseline characteristics of the 101 excluded patients were similar to the included patients (Supplemental Table 1). The median onset-to-needle time of the included patients was 262 min in the IVT group (min. 30 min, max. 865 min). Conservative treatment (isovolemic hemodilution, ocular massage, topical beta-blockers and/or IV acetazolamide was used in 9/47 (19%) IVT patients and 8/34 non-IVT patients (24%) (Table 1).
Table 1.
Baseline characteristics. Summary of baseline characteristics of all included patients.
| IVT n = 47 | Non-IVT n = 34 | |
|---|---|---|
| Age, mean (SD) | 65.0 (11.6) | 71.2 (18.4) |
| Sex, female (%) | 19 (40.4) | 16 (47.1) |
| Atrial fibrillation (%) | 5 (10.6) | 3 (8.8) |
| Diabetes (%) | 12 (25.5) | 5 (14.7) |
| Arterial hypertension (%) | 30 (63.8) | 23 (67.6) |
| Dyslipidemia (%) | 33 (70.2) | 23 (67.6) |
| Current smoker (%) | 10 (21.3) | 2 (5.9) |
| Coronary artery disease (%) | 7 (14.9) | 8 (23.5) |
| Prior ischemic stroke (%) | 10 (21.3) | 4 (11.8) |
| >50% extracranial ICA stenosis (%) | 11 (23.4) | 9 (26.5) |
| SBP, mmHg, mean (SD) | 156 (27) | 150 (24) |
| DBP, mmHg, mean (SD) | 88 (14) | 81 (13) |
| Prior antiplatelet therapy (%) | 15 (31.9) | 9 (26.5) |
| Prior anticoagulation (%) | 3 (6.4) | 6 (17.6) |
| Glucose, mmol/l, mean (SD) | 7.8 (9.4) | 6.3 (1.7) |
| CRP, mg/l, mean (SD) | 14.1 (38.2) | 10.4 (26.7) |
| Creatinine, μmol/l, mean (SD) | 82.2 (22.5) | 96.6 (35.5) |
| Onset-to-needle time, median (Min., Max.) | 262 (30, 865) | |
| Conservative treatment | 9 (19) | 8 (24) |
CRP: C-reactive protein; DBP: diastolic blood pressure; ICA: internal carotid artery; IVT: intravenous thrombolysis; SBP: systolic blood pressure; SD: standard deviation.
Most common etiologies of RAO were large-artery atherosclerosis and undetermined etiology in both groups. Etiologies did not differ significant between IVT and non-IVT patients (Supplemental Table 2).
40/81 patients (49%) received a CT scan only during the acute phase, 16/81 patients (20%) received an MRI scan only, 13/81 patients received both an MRI and CT scan (16%). 12/81 patients received no cerebral imaging (all in the non-IVT group). Concomitant ischemia was found in 24/69 patients (35%) who had a cerebral imaging. 36/47 RAO patients in our study received IVT within 4.5 h while 10/47 received IVT later than 4.5 h, up to 14.4 h after symptom onset. In one patient IVT time could not be deducted from medical records. Mean VA on admission was similar in both groups (2.2 ± 0.6 logMAR in IVT vs 2.1 ± 0.6 logMAR in non-IVT patients) (Table 2).
Table 2.
Baseline visual acuity. Mean baseline VA and distribution of VA at admission of all patients.
| IVT n = 47 | Non-IVT n = 34 | |
|---|---|---|
| VA on admission, mean (logMAR, SD) | 2.2 (±0.6) | 2.1 (±0.8) |
| Distribution of VA on admission | ||
| VA 0.0–0.9 (%) | 2 (4.2) | 5 (14.6) |
| VA 1.0–1.9 (%) | 3 (6.4) | 4 (11.7) |
| CF (%) | 14 (29.8) | 3 (8.8) |
| HM (%) | 10 (21.3) | 9 (26.5) |
| LP (%) | 7 (14.9) | 5 (14.7) |
| BL (%) | 11 (23.4) | 8 (23.5) |
IVT: intravenous thrombolysis; logMAR: logarithm of minimum angle of resolution; SD: standard deviation; CF: counting fingers; HM: hand motion; LP: light perception; BL = blindness.
Outcomes
VA improved significantly at follow up compared to baseline in IVT patients (∆VA 0.5 ± 0.8, p < 0.001) and non-IVT patients (∆VA 0.40 ± 1.1, p < 0.05) (Figure 2(a)). We did not find significant differences in ∆VA and visual recovery rate when comparing IVT and non-IVT patients at follow up (0.5 ± 0.8vs 0.4 ± 1.1, p = 0.196 for ∆VA and 21 (44.7%) vs 13 (38.2%), p = 0.562 for recovery rate) (Figure 2(b) and (c) and Table 3).
Figure 2.
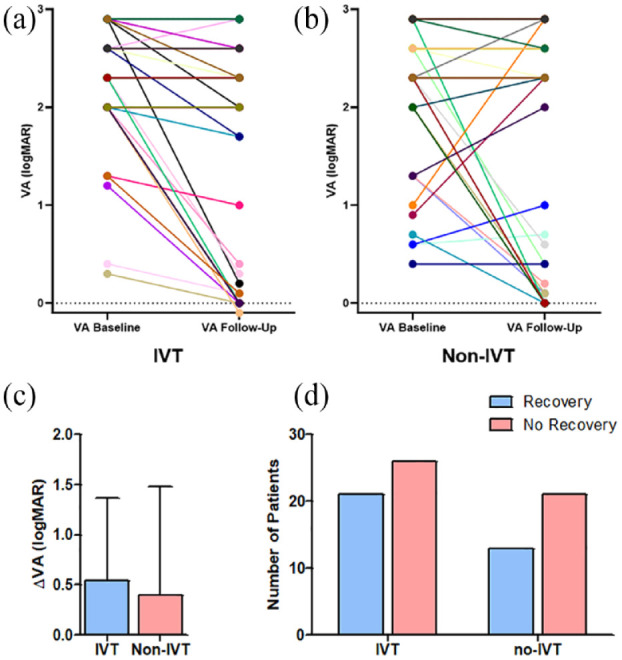
Visual acuity at baseline and follow-up. VA of each individual patient at baseline and follow up (a and b). Please note that data from more than one patient with similar VA course may overlay. IVT patients as well as non-IVT patients had a significantly improved VA at follow-up compared to baseline (∆VA 0.5 ± 0.8, p < 0.001) for IVT patients, ∆VA 0.40 ± 1.1, p < 0.05 for non-IVT patients). When comparing the ∆VA (c) and number of patients with visual recovery at follow-up (d), no significant differences between IVT and non-IVT patients were found (∆VA 0.5 ± 0.8 for the IVT group vs 0.4 ± 1.1 for the non-IVT group, p = 0.196, 21/47 (44.7%) IVT patients with visual recovery vs 13/34 (38.2%) non-IVT patients with visual recovery, p = 0.562). IVT: intravenous thrombolysis; logMAR: logarithm of minimum angle of resolution; VA: visual acuity.
Table 3.
Visual Outcome. Changes in VA between baseline and follow-up and number of patients with visual recovery (Defined as improvement of VA ⩾ 0.3 or).
| n | Baseline VA | Follow-up VA | ∆VA | Visual recovery | p-Value* | |
|---|---|---|---|---|---|---|
| All patients | 81 | 2.2 ± 0.7 | 1.7 ± 1.1 | 0.5 ± 0.9 | 47/81 (58.0%) | <0.001 |
| IVT | 47 | 2.2 ± 0.6 | 1.7 ± 1.1 | 0.5 ± 0.8 | 21/47 (44.7%) | <0.001 |
| Non-IVT | 34 | 2.1 ± 0.8 | 1.7 ± 1.2 | 0.4 ± 1.1 | 13/34 (38.2%) | <0.05 |
| p-Value** | - | 0.509 | 0.562 |
IVT: intravenous thrombolysis; VA: visual acuity.
p-Value was calculated by a paired sample t-test.
p-Value was calculated by an independent sample t-test for ∆VA and chi-squared test for visual recovery between IVT and non-IVT group.
In ANCOVA, there was no significant effect of IVT on VA at follow up after controlling for VA at baseline, time-to-treatment, age, and sex (Table 4).
Table 4.
Standard ANCOVA Standard ANCOVA adjusted for IVT treatment, age, sex, time-to-treatment, and baseline VA. No evidence for IVT being efficacious after adjusting for age, sex, time-to-treatment and baseline VA.
| Estimate | SE | t-Value | p-Value* | |
|---|---|---|---|---|
| IVT treatment | 0.00228 | 0.14260 | 0.016 | 0.987 |
| Age | 0.16162 | 0.15635 | 1.034 | 0.307 |
| Sex | −0.01784 | 0.22000 | −0.081 | 0.936 |
| Time-to-treatment | −0.19076 | 0.11011 | −1.732 | 0.091 |
| VA baseline | 0.70090 | 0.14373 | 4.876 | <0.001 |
IVT: intravenous thrombolysis; SE: standard error; VA: visual acuity.
Concerning safety, two asymptomatic ICH (4%) and one (2%) major extracranial bleeding (intraocular bleeding) occurred in the IVT group. No ICH was registered in the non-IVT group (Table 5).
Table 5.
Safety parameters. Two ICHs and one major extracranial bleeding (intraocular bleeding) occurred in the IVT group, while no sICH, fatal ICH, or other ICH were observed. No bleeding events occurred in the non-IVT group.
| IVT n = 47 | Non-IVT n = 34 | |
|---|---|---|
| Any ICH (%) | 2 (4.3) | 0 |
| sICH (%) | 0 | 0 |
| Fatal ICH (%) | 0 | 0 |
| Other ICH (SDH, SAH) (%) | 0 | 0 |
| Major extracranial bleeding (%) | 1 (2.1) | 0 |
IVT: intravenous thrombolysis; ICH: intracranial hemorrhage; sICH: symptomatic intracranial hemorrhage; SDH: subdural hematoma; SAH: subarachnoid hemorrhage.
Discussion
We investigated the safety and effectiveness of IVT in RAO. In this large, multicenter cohort of RAO patients, we found that patients receiving IVT had a significantly improved VA at follow-up compared to baseline. However, when comparing ∆VA and visual recovery rate of IVT and non-IVT treated RAO patients; there were no differences between groups. In the ANCOVA corrected for age, sex and baseline VA, we found no evidence supporting the hypothesis that IVT is superior to conservative treatment in RAO. We observed a good safety profile of IVT, with no occurrences of symptomatic or fatal intracranial bleedings.
Limitations of our study are (i) the retrospective design, which could cause selection bias regarding treatment (ii) the non-standardized time-point of clinical outcome assessment and (iii) the high number of excluded patients due to missing information on VA, potentially affecting our results. This highlights the importance of a standardized follow-up exam including assessment of VA in RAO patients, like in ischemic stroke where outcome measures like the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin scale (mRS) are routinely obtained at a 3 months follow up.
Strengths of our study include the large multicenter sample size (47 IVT-RAO patients), which is the largest IVT treated RAO cohort so far, the presence of a comparison group (34 non-IVT RAO patients), the inclusion of patient characteristics and safety parameters, and the fact that our study reflects a multicenter IVT cohort of 10 experienced stroke centers.
Our study contains the largest cohort of IVT treated RAO patients published so far. Other retrospective studies and non-randomized prospective studies contained between 11 and 30 IVT treated RAO patients.10,11,19–23 There is one small randomized controlled trial with eight IVT and eight control patients, showing that only 25% of IVT treated patients improved VA by at least three lines in the Snellen chart (corresponding to an improvement of ⩾0.3 logMAR in our study). 24 However, in this study, IVT was administered up to 24 h after RAO onset, exceeding the usual IVT time window of 4.5 h. In our multicenter European cohort, we found a visual recovery rate of 44.7% in the IVT group with a mean onset-to-needle time of 262 min. Thus, visual recovery rates of IVT treated patients in our study are comparable to previous reports.11,23
Non-IVT patients in our study, however, reached higher rates of visual recovery as previously reported. In two recent meta-analyses, non-IVT patients had a much lower visual recovery rate (13.1% and 12% compared to the 35% in our study).10,19 The reason for better visual outcome of the non-IVT group in our cohort remains open. More patients in the non-IVT group had to be excluded due to missing information on visual acuity, which could lead to a potential bias. It is possible that patients with a normalized VA where more likely to cancel follow-up appointments due to the fact that they had no residual deficits, on the other hand, patients with severely impaired VA might have preferred their follow up at their local ophthalmologists instead of the stroke center. Another source of bias could be treatment selection. Patients who reported a spontaneous partial recovery of VA on admission could have been less likely to receive IVT, as they were expected to achieve a better clinical outcome regardless of treatment. However, this cannot be verified in our cohort, as the individual reasons for treatment decisions were not documented. As our primary outcome was visual acuity, which depends on the function of the macula, it is also possible that IVT improves the visual field, which was not systematically tested in our patient cohort. When comparing the baseline characteristics of the patients in our study, on average, non-IVT patients tended to be older and had higher rates of prior intake of anticoagulation, which was, however, not significant. Cardiovascular risk factors were similarly distributed between groups, with a trend for a higher number of smokers and presence of diabetes in the IVT group, which did not reach statistical significance. Differences due to comorbidities such as cancer as potential contraindication of IVT were not systematically assessed. However, when comparing our patient characteristics to other studies, both IVT and non-IVT treated patients had a similar age and cardiovascular risk profile.10,23
The effectiveness of IVT in RAO was previously shown to be time dependent, with the highest recovery rate in the subset of patients treated within 1.5 h after symptom onset.7,10 In our data, we did not find an effect of time on IVT effectiveness. Mean onset-to-needle time in our study was 262 min, which is at the upper end of the 4.5 h window, and only a small subset of patients received IVT within 1.5 h. This demonstrates the difficulty of applying IVT early in a real-life setting, as delays from onset, first medical contact, diagnosis of RAO, and referral to the hospital are likely to be higher than in ischemic stroke. 25
Despite receiving treatment with IVT, more than half of the patients in the IVT group did not achieve a significant visual recovery at follow-up. One reason could be the delay until treatment. Second, it is unclear whether visual recovery is solely dependent on achieving recanalization of the retinal artery. Late complications of RAO such as neovascularization may negatively alter the visual outcome despite initial treatment. 26 Analyzing visual outcome at later time points after RAO and the use of additional diagnostic tools such as fluorescence angiography to assess recanalization after IVT could be helpful to deduct whether IVT reduces these complications and if recanalization of the retinal artery is a useful prognostic tool for a good visual outcome. Additionally, most studies, including ours, use VA as the main outcome parameter, as this parameter is best documented in clinical routine. However, as VA measurements are derived from only a small part of the retina, additional outcome parameters such as peripheral vision to assess other areas of the retina, might also be helpful to deduce whether treatment is beneficial (Supplemental Table 3). These parameters should be modified according to local infrastructure and should be discussed and adapted jointly by ophthalmologist and neurologists.
Two of the 47 (4%) IVT treated patients had asymptomatic ICHs, while no sICH occurred in non-IVT patients. Our results are comparable to other retrospective studies and meta-analyses,11,19 with lower rates of hemorrhagic complications compared to ischemic stroke patients, where sICH rates are between 2% and 7% depending on patient population and definition of sICH.27,28 Both asymptomatic ICHs occurred in patients who received IVT within 4.5 h. However, both patients were found to have concomitant ischemia on cerebral imaging. This finding suggest that cerebral imaging should be performed before IVT in RAO patients, and more data should be gathered if concomitant brain ischemia indicates higher risk of ICH.
Conclusion
Our study provides real-life data from a large European cohort of IVT treated RAO patients. On follow up 3 months after RAO, vision was improved in 44.7% of IVT and 38.2% of non-IVT patients. While VR was not superior in IVT versus non-IVT patients, there is scope for benefit of IVT in RAO-patients. To facilitate further studies, outcome assessment in RAO patients should be standardized like for ischemic stroke in the brain. Based on our data, a randomized controlled trial assessing the benefit of IVT in RAO seems justified and participation in current clinical trials should be offered to RAO patients whenever possible.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231185895 for Safety and effectiveness of IV Thrombolysis in retinal artery occlusion: A multicenter retrospective cohort study by Philipp Baumgartner, Lucas Kook, Valerian L Altersberger, Henrik Gensicke, Elena Ardila-Jurado, Georg Kägi, Alexander Salerno, Patrik Michel, Kiran M Gopisingh, Paul J Nederkoorn, Jan F Scheitz, Christian H Nolte, Mirjam R Heldner, Marcel Arnold, Charlotte Cordonnier, Lucie Della Schiava, Christian Hametner, Peter A. Ringleb, Ronen R Leker, Hamza Jubran, Andreas R Luft, Stefan T Engelter and Susanne Wegener in European Stroke Journal
Acknowledgments
We thank all participating centers and collaborators of TRISP and all patients who contributed their data to research.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PB: Research funds by the Gottfried and Julia Bangerter-Rhyner Foundation and Swiss Academy of Medical Sciences and funding for travel and conference fees from BMS/Pfizer. SW: Research funds by the Swiss National Science Foundation, the UZH Clinical research priority program (CRPP) stroke, the Swiss Heart foundation, the Zurich Neuroscience Center (ZNZ), speaker honoraria from Springer, Teva Pharma and consultancy fees from Bayer and Novartis. STE has received funding for travel or speaker honoraria from Bayer, Boehringer Ingelheim and Daiichi-Sankyo. He has served on scientific advisory boards for Bayer, Boehringer Ingelheim, BMS/Pfizer, and MindMaze and on the editorial board of Stroke. His institutions have received an educational grant from Pfizer, compensation from Stago for educational efforts and research support from Daiichi-Sankyo, the Science Funds [Wissenschaftsfonds] of the University Hospital Basel, the University Basel, from the “Wissenschaftsfonds Rehabilitation” of the University Department for Geriatric Medicine Felix Platter, the “Freiwillige Akademische Gesellschaft Basel,” the Swiss Heart Foundation, and the Swiss National Science Foundation. PR has received honoraria for advisory board participation from Boehringer Ingelheim and Pfizer and lecture fees from Boehringer Ingelheim, Bayer, Pfizer and Daiichi Sankyo. His institution has received a grant from Boehringer Ingelheim for the ECASS-4 Study. RRL received funding for speaker honoraria from Pfizer, Boehringer Ingelheim, Biogen and Iscehma View. He has served on scientific advisory boards for Novo-Nordisk and Bayer.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Swiss National Science Foundation (SNSF) PP00P3_202663, the Olga Mayenfish foundation, and the UZH Clinical Research Priority Program (CRPP) Stroke.
Ethical approval: Ethical approval for this study was obtained from the cantonal ethics committee Zurich, Switzerland (PREDICT, BASEC-Nr.: PB_2016-01751) Additional local ethics approval was obtained by participating centers if required.
Informed consent: Informed consent was not sought for the present study because it was not required from the local ethics committee due to the study design.
Guarantor: SW
Contributorship: PB researched literature, conceived the study, obtained data, performed data analysis and wrote the first draft of the manuscript. SW researched literature, conceived the study, and gained ethical approval. LK performed data analysis, VA, HG, EJ, GK, AS, PM, KG, PN, JS, CN, MH, MA, CC, LS, CH, PR, RL, HJ, AL, SE obtained local data. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Philipp Baumgartner  https://orcid.org/0000-0003-1525-2019
https://orcid.org/0000-0003-1525-2019
Alexander Salerno  https://orcid.org/0000-0001-8494-5527
https://orcid.org/0000-0001-8494-5527
Jan F Scheitz  https://orcid.org/0000-0001-5835-4627
https://orcid.org/0000-0001-5835-4627
Christian H Nolte  https://orcid.org/0000-0001-5577-1775
https://orcid.org/0000-0001-5577-1775
Ronen R Leker  https://orcid.org/0000-0003-4794-0334
https://orcid.org/0000-0003-4794-0334
Susanne Wegener  https://orcid.org/0000-0003-4369-7023
https://orcid.org/0000-0003-4369-7023
Supplemental material: Supplemental material for this article is available online.
References
- 1. Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. Retinal survival time. Exp Eye Res 2004; 78: 723–736. [DOI] [PubMed] [Google Scholar]
- 2. Lavin P, Patrylo M, Hollar M, et al. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol 2018; 196: 96–100. [DOI] [PubMed] [Google Scholar]
- 3. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century. Stroke 2013; 44: 2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta N, Marco RD, Goldhardt R, et al. Central retinal artery occlusion: acute management and treatment. Curr Ophthalmol Rep 2017; 5: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal and ophthalmic artery occlusions preferred practice pattern®. Ophthalmology 2020; 127: 259–287. [DOI] [PubMed] [Google Scholar]
- 6. Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev 2009; 2009: CD001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schrag M, Youn T, Schindler J, et al. Intravenous fibrinolytic therapy in central retinal artery occlusion a patient-level meta-analysis. JAMA Neurol 2015; 72: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 8. Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010; 117: 1367–1375.e1. [DOI] [PubMed] [Google Scholar]
- 9. Schultheiss M, Spitzer MS, Hattenbach L-O, et al. Update intravenöse Lysetherapie. Der Ophthalmologe 2021; 118: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 10. Mac Grory B, Nackenoff A, Poli S, et al. Intravenous fibrinolysis for central retinal artery occlusion: A cohort study and updated patient-level meta-analysis. Stroke 2020; 51: 2018–2025. [DOI] [PubMed] [Google Scholar]
- 11. Préterre C, Godeneche G, Vandamme X, et al. Management of acute central retinal artery occlusion: intravenous thrombolysis is feasible and safe. Int J Stroke 2017; 12: 720–723. [DOI] [PubMed] [Google Scholar]
- 12. Scheitz JF, Gensicke H, Zinkstok SM, et al. Cohort profile: thrombolysis in ischemic stroke patients (TRISP): a multicentre research collaboration. BMJ Open 2018; 8: e023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nordanstig A, Curtze S, Gensicke H, et al. EndoVAscular treatment and ThRombolysis for ischemic stroke patients (EVA-TRISP) registry: Basis and methodology of a pan-European prospective ischaemic stroke revascularisation treatment registry. BMJ Open 2021; 11: e042211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daiber HF, Gnugnoli DM. Visual acuity. Treasure Island, FL: StatPearls Publishing, 2023. [PubMed] [Google Scholar]
- 15. Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS thesis). Trans Am Ophthalmol Soc 2009; 107: 311–324. [PMC free article] [PubMed] [Google Scholar]
- 16. Lange C, Feltgen N, Junker B, et al. Resolving the clinical acuity categories ‘hand motion’ and ‘counting fingers’ using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol 2009; 247: 137–142. [DOI] [PubMed] [Google Scholar]
- 17. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). J Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 18. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 19. Huang L, Wang Y, Zhang R. Intravenous thrombolysis in patients with central retinal artery occlusion: a systematic review and meta-analysis. J Neurol 2022; 269: 1825–1833. [DOI] [PubMed] [Google Scholar]
- 20. Wu X-J, Gao F, Liu X, et al. Observation on therapeutic efficacy of rt-PA intravenous thrombolysis combined with compound anisodine injection on central retinal artery occlusion. Exp Ther Med 2016; 12: 2617–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nedelmann M, Graef M, Weinand F, et al. Retrobulbar spot sign predicts thrombolytic treatment effects and etiology in central retinal artery occlusion. Stroke 2015; 46: 2322–2324. [DOI] [PubMed] [Google Scholar]
- 22. Hattenbach L-O, Kuhli-Hattenbach C, Scharrer I, et al. Intravenous thrombolysis with low-dose recombinant tissue plasminogen activator in central retinal artery occlusion. Am J Ophthalmol 2008; 146: 700–706.e1. [DOI] [PubMed] [Google Scholar]
- 23. Schultheiss M, Härtig F, Spitzer MS, et al. Intravenous thrombolysis in acute central retinal artery occlusion – A prospective interventional case series. PLoS One 2018; 13: e0198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CS, Lee AW, Campbell B, et al. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion: report from a randomized, controlled trial. Stroke 2011; 42: 2229–2234. [DOI] [PubMed] [Google Scholar]
- 25. Ardila Jurado E, Sturm V, Brugger F, et al. Central retinal artery occlusion: current practice, awareness and prehospital delays in Switzerland. Front Neurol 2022; 13: 888456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol 2010; 20: 1042–1046. [DOI] [PubMed] [Google Scholar]
- 27. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e343–e361. [DOI] [PubMed] [Google Scholar]
- 28. Seet RCS, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis 2012; 34: 106–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231185895 for Safety and effectiveness of IV Thrombolysis in retinal artery occlusion: A multicenter retrospective cohort study by Philipp Baumgartner, Lucas Kook, Valerian L Altersberger, Henrik Gensicke, Elena Ardila-Jurado, Georg Kägi, Alexander Salerno, Patrik Michel, Kiran M Gopisingh, Paul J Nederkoorn, Jan F Scheitz, Christian H Nolte, Mirjam R Heldner, Marcel Arnold, Charlotte Cordonnier, Lucie Della Schiava, Christian Hametner, Peter A. Ringleb, Ronen R Leker, Hamza Jubran, Andreas R Luft, Stefan T Engelter and Susanne Wegener in European Stroke Journal


