Abstract
Purpose:
To propose a consensus-based definition and framework for motor rehabilitation after stroke.
Methods:
An expert European working group reviewed the literature, attaining internal consensus after external feedback.
Findings:
Motor rehabilitation is defined as a process that engages people with stroke to benefit their motor function, activity capacity and performance in daily life. It is necessary for people with residual motor disability whose goal is to enhance their functioning, independence and participation. Motor rehabilitation operates through learning- and use-dependent mechanisms. The trajectory of motor recovery varies across patients and stages of recovery. Early behavioral restitution of motor function depends on spontaneous biological mechanisms. Further improvements in activities of daily living are achieved by compensations. Motor rehabilitation is guided by regular assessment of motor function and activity using consensus-based measures, including patient-reported outcomes. Results are discussed with the patient and their carers to set personal goals. During motor rehabilitation patients learn to optimize and adapt their motor, sensory and cognitive functioning through appropriately dosed repetitive, goal-oriented, progressive, task- and context-specific training. Motor rehabilitation supports people with stroke to maximize health, well-being and quality of life. The framework describes the International Classification of Functioning, Disability and Health in the context of stroke, describes neurobiological mechanisms of behavioral restitution and compensation, and summarizes recommendations for clinical assessment, prediction tools, and motor interventions with strong recommendations from clinical practice guidelines (2016–2022).
Conclusions:
This definition and framework may guide clinical educators, inform clinicians on current recommendations and guidelines, and identify gaps in the evidence base.
Keywords: Review, motor rehabilitation, motor recovery, assessment, prediction, intervention
Introduction
Stroke is the third leading cause of death and disability worldwide 1 and a leading cause of adult disability in Europe. 2 Forecasts for Europe from 2017 to 2047 predict a 17% decrease in stroke mortality, but a 27% increase in stroke prevalence. 2 These trends are expected to increase demand for stroke rehabilitation services.
The European Stroke Organisation (ESO) has released a European Stroke Action Plan (ESAP) 3 and defined 30 targets and 72 research priorities within seven domains to improve stroke services. One of these domains is stroke rehabilitation for improving management, outcome and quality of life after stroke in 2030. 3 Rehabilitation was defined following the WHO as “a set of measures that assist individuals, who experience or are likely to experience disability, to achieve and maintain optimal functioning in interaction with their environments.” 4 This general definition encompasses several neurological domains such as motor function, cognition and communication, but specific principles of motor rehabilitation were not addressed.
This article presents a definition of “motor rehabilitation” developed by expert consensus. The agreed definition is supported by a framework that synthesizes key literature to provide a state-of-the-art overview of the stroke motor rehabilitation domain. This framework is intended to guide educators who train stroke rehabilitation clinicians, to update clinicians about current recommendations and guidelines, and to enable researchers to identify gaps in the evidence base.
Development of a consensus-based definition of motor rehabilitation after stroke
The ESO Guideline Board invited a panel of 16 experts to collaborate on a definition of post-stroke motor rehabilitation, using standard ESO operating procedures. 5 The panel engaged in a three-round process. The first round consisted of online meetings in April and May 2022 where the panel agreed to structure the definition as a paragraph. A first draft of the definition paragraph was presented to panelists through an online survey, and panelists had the options to agree, suggest changes, request additional elements, or disagree with the paragraph. Results were collated (available from GV) and presented to the expert panel for discussion and revision at the end of June 2022. A second-round survey was held in July–August 2022, and panelists could agree or disagree with each part of the definition. A 75% agreement threshold was defined a priori for acceptance of each part of the definition, similar to formal Delphi processes.6,7 Results were collated (also available from GV) and presented in September to the expert panel, with further discussion and fine-tuning. The topic sentence at the beginning of the definition paragraph reached 91.7% agreement. The next three supporting sentences provide further explanation of the concept and reached 75%, 91.7%, and 81.8% agreement, respectively. The final concluding sentence reached 91.7% agreement. Along with the definition, a separate glossary related to motor rehabilitation was compiled (Box 1).
Box 1.
Glossary and definitions.
|
Behavioral adaptation: occurs when the movement or task is executed with the impaired body part but an alternative atypical movement pattern is used. Behavioral adaptation results in deviating quality of movement compared to non-disabled individuals.a,b
Behavioral compensation: occurs as adaptation, in which the impaired body part is used in an atypical way to accomplish a movement or a motor task; or as substitution, in which different atypical body part(s) or body segment(s) are used to accomplish a task.a,b Behavioral restitution: a return toward more normal patterns of motor control with the impaired body part(s) as seen in pre-stroke state.a,b International Classification of Functioning, Disability and Health (ICF) terminologyc • Body functions: the physiological functions of body systems. • Body structures: anatomical parts of the body such as organs, limbs and their components. • Impairments: problems (the negative term) in body functions and structures. • Activities: the execution of a task(s) or action(s). • Activity limitations: difficulties (the negative term) in executing tasks and activities. • Activity capacity: relates to what an individual can do in a “standardized” environment. • Activity performance: what the person actually does in his or her “current” (usual) environment. • Participation: involvement in a life situation. • Participation restriction: problems (the negative term) an individual may experience in involvement in life situations. • Functioning: an umbrella term for body function, body structures, activities and participation. It denotes the positive or neutral aspects of the interaction between a person’s health condition(s) and that individual’s contextual factors (environmental and personal factors). • Disability: an umbrella term for impairments, activity limitations and participation restrictions. It denotes the negative aspects of the interaction between a person’s health condition(s) and that individual’s contextual factors (environmental and personal factors). Motor control: the process whereby the central nervous system produces purposeful coordinated movements to interact with the rest of the body and the environment.d Motor function: body functions related to muscle force and endurance, control over and coordination of voluntary movements, and movement patterns associated with walking, running or other whole body movements.c Motor learning: the changes, associated with practice or experience, in internal processes, that determine a person’s capability for producing a motor skill.e Motor recovery: the extent to which motor functions and activities have returned to their pre-stroke state.a Motor skill: a skill for which the primary determinant of success is the quality of movement that the performer produces.e Motor skill acquisition: the processes by which an individual acquires the ability to identify an appropriate movement goal given a particular task context, select the correct action given a sensory stimulus and/or the current state of the body and the world, and execute that action with accuracy and precision.f Multidisciplinary rehabilitation: defined by the World Health Organization (WHO) as the coordinated delivery of multidimensional rehabilitation intervention provided by two or more disciplines (such as nursing, physiotherapy, occupational therapy, social work, psychology and other allied health), in conjunction with medical professionals (rehabilitation physician, neurologist; oncologist; palliative physician), which aims to improve patient symptoms and maximize functional independence and participation (social integration) using a holistic biopsychosocial model, as defined by the ICF.c Neural plasticity: structural or functional changes (or both) within neurons that affect the connectivity of neurons with each other in a network serving a function. These changes are usually in response to a change in neuronal input or firing patterns induced by this input, such as during learning (use- and experience-dependent) or after injury.g Phases of stroke recovery:a • Hyperacute from 0 to 24 h post stroke onset • Acute between 1 and 7 days • Early subacute between 7 days and 3 months • Late subacute between 3 and 6 months • Chronic phase beyond 6 months post stroke Spontaneous neurological recovery: improvement in function and activities that is independent of specific targeted treatment and occurs within a restricted time-window of the first 3 months after stroke onset. It is considered an endogenous repair process that presumably relies on residual intact neural architecture as a template for reorganization.g,h Quality of movement (QoM): operationally defined by comparing an individual’s motor task execution to a reference population of non-disabled age-matched individuals. The closer the movement matches to those seen in non-disabled individuals, the better the quality of their movement.i References: a. Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. International journal of stroke : official journal of the International Stroke Society. 2017;12(5):444–450. b. Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23(4):313–319. c. World Health Organization (WHO). International Classification of Functioning Disability and Health (ICF). Geneva: WHO, 2001. d. Latash ML, Levin MF, Scholz JP, Schöner G. Motor control theories and their applications. Medicina (Kaunas). 2010;46(6):382–92. PMID: 20944446; PMCID: PMC3017756. e. Schmidt, R. A. & Wrisberg, C. A. Motor learning and performance: a situation-based learning approach. 4th ed, Human Kinetics, 2008. f. Krakauer JW, Hadjiosif AM, Xu J, Wong AL, Haith AM. Motor learning. Compr Physiol. 2019;9(2):613–663. doi:10.1002/cphy.c170043. g. Joy MT, Carmichael ST. Encouraging an excitable brain state: mechanisms of brain repair in stroke. Nat Rev Neurosci. 2021;22(1):38–53. doi:10.1038/S41583-020-00396-7. h. Krakauer JW & Carmichael ST. Broken Movement: The Neurological recovery after stroke MIT Express, Cambridge 2017: p. 85. i. Kwakkel G, Van Wegen E, Burridge JH, et al. Standardized measurement of quality of upper limb movement after stroke: Consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. International journal of stroke: official journal of the International Stroke Society. 2019;14(8):783–791. |
Expert panel members presented the agreed definition to a convenience sample of clinicians working in stroke rehabilitation through in-person and online consultation. Feedback was collated (available from GV) and discussed by the expert group in December 2022 with fine-tuning of the definition. The final agreed definition on motor rehabilitation after stroke is presented in Box 2. The definition was also discussed with two persons with lived experience of stroke, who confirmed that all elements of the definition were highly relevant and important for motor rehabilitation. They emphasized including maximizing health, well-being and quality of life, and the need to communicate that patients can improve, even long-term after stroke.
Box 2.
Agreed, expert-based definition of motor rehabilitation after stroke.
| Motor rehabilitation is a process that engages people with stroke in order to benefit their motor function, activity capacity and performance in daily life. It is necessary for all people with residual motor disability whose goal is to enhance their functioning, independence and participation. Motor rehabilitation strives to reduce motor impairments and improve functioning in activities through learning- and use-dependent mechanisms. The trajectory of motor and functional recovery varies between patients and stages of recovery. At early stages, behavioral restitution of motor function depends on the underlying mechanisms of spontaneous neurological recovery. At later stages, further functional improvements can be achieved by compensations. Motor rehabilitation is guided by regular assessment of motor function and activity using consensus-based measures, including patient-reported outcomes. Results are discussed with the patient and their carers in order to set personal goals. The core element of motor rehabilitation incorporates principles of motor control in which patients learn to optimize and adapt their motor, sensory and cognitive functioning through appropriately dosed, repetitive, goal-oriented, progressive, task- and context-specific training. Motor rehabilitation supports people with stroke to maximize health, well-being and quality of life. |
The definition paragraph describes key concepts. Further details are needed when using the definition in education, clinical practice and research. We addressed this by developing a framework that elaborates on each component of the definition. This framework is presented below to contextualize the motor rehabilitation definition.
Motor rehabilitation after stroke framework
Overarching concept: The International Classification of Functioning, Disability and Health
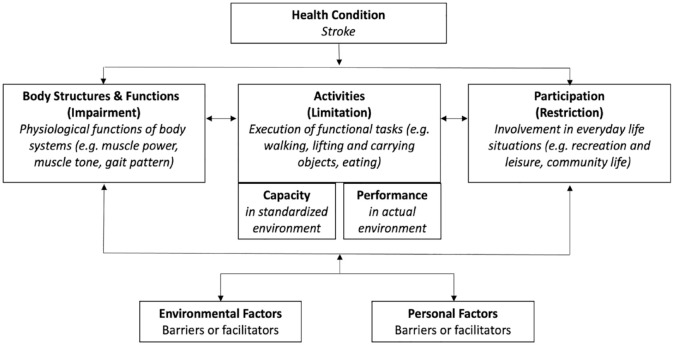
The first sentence of the definition positions it with respect to the International Classification of Functioning, Disability and Health (ICF). The ICF conceptualizes interactions between body functions and structures, activities, and participation in relation to environmental and personal factors (Figure 1). 8 Body functions can be impaired, activities can be limited, and participation can be restricted. Assessments in the body functions domain evaluate physiological functions of body systems, such as muscle strength and synergies, coordination, pain, and muscle tone. Assessments in the activity domain evaluate the execution of tasks such as reaching and grasping, self-care, mobility and walking. The primary goal for many patients is to return to participation in their home environment and life roles. Therefore, assessments in the participation domain evaluate involvement in everyday life situations such as shopping, working, and socializing. Capacity and performance are two constructs used in the activity and participation domains. Capacity relates to what an individual can do in a standardized environment or test situation, while performance relates to what a person actually does in their habitual environment. 8 Environmental and personal factors can be barriers or facilitators of the person’s functioning. Figure 1 illustrates the ICF in the context of motor rehabilitation after stroke.
Figure 1.
The international classification of functioning, disability and health. 8
Regaining motor function is critical for independence in activities of daily living, 9 which is associated with satisfactory quality of life. The concept of “Quality of Life” was not originally part of the ICF framework, however it has been defined by the World Health Organization 10 Quality of Life Group as an “individual’s perception of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns” (p. 11). It can be thought of as an overarching concept related to all domains of the ICF. 11
It is important to note that the components of the ICF are interrelated in a non-linear way. An impairment in body function, such as leg muscle weakness, does not necessarily produce a limitation in walking or a restriction in participation. Furthermore, motor functions are closely related to and influenced by other body functions such as sensation, pain, cognition, mood and fatigue. All these aspects of functioning need to be considered in post-stroke motor rehabilitation.
Motor recovery
The second section of the definition reflects the biology of recovery and distinguishes between improvements at early versus later stages after stroke. Motor recovery probably occurs through a combination of spontaneous biological processes and “use-dependent” processes that include motor learning and skill acquisition.12,13 The interactions between spontaneous biological processes at molecular, cellular, and physiological levels and the mechanisms of learning in the first months post-stroke are still poorly understood. 13
Most stroke rehabilitation studies use the term “recovery” as a general expression of “change” or “improvement,” without distinguishing between behavioral restitution or compensation.12,14,15 In general, the term “motor recovery” indicates restitution of behavior after stroke, where movements or tasks are performed as they were before stroke. This “true recovery” is thought to reflect spontaneous biological recovery processes occurring during the initial days and weeks after stroke. In contrast, the term “compensation” encompasses performing movements or tasks with atypical movement patterns at the expense of movement quality, or through performing tasks with a different limb altogether. Adaptation and compensation are thought to result from motor learning processes that can continue indefinitely after stroke.
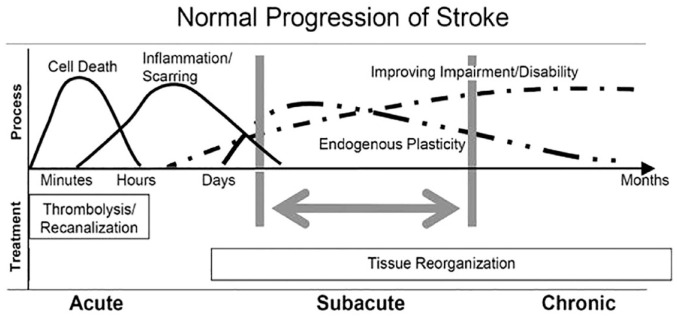
Findings from several longitudinal studies show that for most stroke patients spontaneous neurological recovery and behavioral restitution follow a logistic pattern that plateaus within the first 10 weeks post-stroke, regardless of their age or the type and amount of therapy they complete (Figure 2).9,16–18 Spontaneous neurological recovery is observed for the upper limb19–21 and lower limb,22,23 as well as somatosensory,24,25 visuospatial, 26 and language functions. 27 Patients with initially mild to moderate motor impairment typically exhibit early spontaneous neurological recovery of upper and lower limb motor functions. Some patients with initially more severe motor impairment can also exhibit early or slightly delayed spontaneous recovery, 21 while others remain severely impaired.17,20
Figure 2.
Patterns, processes and treatment opportunities post-stroke (adapted from Dobkin and Carmichael, 28 used with permission).
Spontaneous neurological recovery is initiated by the cascade of neurochemical processes resulting from focal ischemic brain injury. These involve bio-energetic failure leading to excitotoxicity and oxidative stress, mitochondrial failure, and ultimately to apoptosis and cell death. 29 These pathologic processes start within minutes after stroke onset, 30 expand quickly within hours, and may continue for days even if perfusion is restored. 29 Ultimately, the cascade of pathophysiological processes leads to permanent loss of neurons, microglia, astrocytes and endothelial cells in the infarcted area that includes damage to the blood-brain barrier as well as transsynaptic degeneration in remote, anatomically connected brain areas. 16
The mechanisms responsible for behavioral restitution are less well understood.13,31,32 The following time-restricted, overlapping processes contribute to behavioral restitution and compensation post-stroke.
Autoregulation of vascular collaterals can compensate for focal hypoperfusion and support the survival of penumbral tissue. A larger number of collaterals is associated with smaller final infarct volumes, faster and more extensive spontaneous neurological recovery in the first week post-stroke 33 and ultimately better outcomes. 34
Neuronal plasticity in perilesional areas is enhanced by a cascade of post-ischemic inflammation processes in the initial days and weeks post-stroke. At a cellular level, neuronal networks are (re)modeled by competition and selection in response to training and experience. 13 The sensitivity of cortical mapping with non-invasive serial neuroimaging 35 or transcranial magnetic stimulation36,37 techniques to these neuronal plasticity mechanisms is unclear.
Gradual peripheral effects can influence and constrain behavioral restitution and force patients to compensate for their motor performance after stroke. These include neural components such as spasticity, and mechanical effects such as loss of muscle volume and serial sarcomere number38,39 altering the length-tension relationship, 40 as well as higher passive stiffness in tendons and soft tissues.41,42
Behavioral restitution is driven by poorly understood mechanisms of spontaneous neurological recovery and plateaus within 10 weeks after stroke. 43 Unfortunately, there is currently insufficient evidence in humans that rehabilitation therapies interact with the biological mechanisms responsible for behavioral restitution.17,20 Fine-grained biomechanical measures of movement quality indicate that therapy-induced improvements are mainly adaptive, as patients learn to optimize the use of their limbs to accomplish a standardized task. For example, hand transport and orientation for grasping with the paretic upper limb is characterized by increased muscle synergies between shoulder abduction and elbow flexion.44,45 To compensate for the limitations imposed by these muscle synergies, patients learn to flex and rotate their trunk to expand their limited workspace and improve hand orientation.43,44,46 Similarly, faster walking speeds are achieved via compensation with the less-affected side post-stroke, and without significant improvements in paretic intralimb coordination. 47 Overall, the evidence indicates that improvements at the level of activities result from a combination of behavioral restitution of impaired body functions occurring mainly in the first 10 weeks post-stroke, 17 as well as learning to deal with residual impairments by using compensatory movement strategies. These findings support the growing point of view that motor rehabilitation interventions mainly help patients learn to optimize their performance by adaptation and compensation through appropriately dosed, repetitive, goal-oriented, progressive, task- and context-specific training. 48
Assessment of motor function and activity
The third section of the definition is supported by widely recommended motor assessments and prediction tools. Repeated motor assessments, preferably at specific timepoints, result in a better understanding of recovery. International consensus recommends early assessment within 1 week, followed by assessments at 4 weeks, and 3 and 6 months after stroke, as these times relate to key transitions in the biological processes underlying recovery. 16 Standardized assessments at specific times post-stroke provide transparency in stroke care pathways and allow comparison of stroke rehabilitation outcomes at the national, regional and global level. Aggregated standardized assessment data are also expected to reveal which interventions are effective in clinical practice, including information about dose, timing, and setting. 49
Recent evidence- and consensus-based recommendations for motor assessments after stroke have several common features and are summarized in Table 1. In clinical practice, motor assessment should be performed at least before and after rehabilitation programs, using standardized measures with sound psychometric properties and established validity, reliability, responsiveness, and interpretability.49–51 The Fugl-Meyer motor assessment, Action Research Arm Test, and 10 m walk test are commonly recommended for both clinical practice and research. Standardized measurement of upper limb movement quality using kinematic methods has also been recommended for both clinical practice 50 and research. 51
Table 1.
Recent consensus-based recommendations for motor assessments in stroke rehabilitation.
| Source | Aim | Focus | Time post stroke | Recommended assessments |
|---|---|---|---|---|
| CAULIN
50
2021 |
Clinical practice | Upper limb | Within first week, 3-, 6- and 12-months; prior to discharge or transfer; before, during and after a rehabilitation program | FMA-UE, ARAT Extended: Kinematics, BBT, CAHAI, WMFT, NHPT, ABILHAND Supplementary: MI, CMSA, STREAM, FAT, MAS, sensor-based use of the upper limb |
| Core set
52
2020 |
Clinical practice | Motor assessment | Day 2 ± 1 and 7, week 2 and 4, month 3, 6 and 12, and every following 6 months | FMA, ARAT, 10MWT, TUG, BBS, SIS |
| SRRR-2
53
2019 |
Research | Upper limb quality of movement | Within first week, 3-, 6- and 12-months, 4 and 8 weeks recommended | Performance assays (2D reaching, finger individuation, grip/pinch strength) and 3D functional drinking task |
| SRRR-1
54
2017 |
Research | Stroke recovery | Within first week, 3-months, 6- and 12-months recommended | NIHSS, FMA-UE and FMA-LE, ARAT, ability to walk, 10MWT, mRS and EQ-5D |
CAULIN: Clinical Assessment of Upper Limb in Neurorehabilitation; FMA: Fugl-Meyer Assessment; ARAT: Action Research Arm Test; BBT: Box & Block Test; CAHAI: Chedoke Arm and Hand Activity Inventory; WMFT: Wolf Motor Function Test; NHPT: Nine-Hole Peg Test; MI: Motricity Index; CMSA: Chedoke-McMaster Stroke Assessment; STREAM: Stroke Rehabilitation Assessment of Movement; FAT: Frenchay Arm Test; MAS: Modified Ashworth Scale; 10MWT: 10-Meter Walk Test; TUG: Timed Up & Go; BBS: Berg Balance Scale; SIS: Stroke Impact Scale; SRRR: Stroke Recovery and Rehabilitation Roundtable; NIHSS: National Institutes of Health Stroke Scale; UE: upper Extremity; LE: Lower Extremity; mRS: Modified Rankin Scale; EQ-5D: European Quality of Life 5 Dimensions.
Patients’ actual functioning should be assessed at different ICF levels, encompassing impaired motor function, limited activity capacity, and limited activity performance. 11 Patient-reported outcome measures relating to activity and participation should be considered to complement data collected by technology or clinician observation. 50
Agreed assessments are important for predicting and monitoring recovery and outcomes at different levels of the ICF. 8 Motor assessment results should be discussed with patients and their caregivers, together with assessments of other domains, such as cognition and communication, to establish a shared understanding of the patient’s current status. Assessment results can also be used to gauge the patient’s likely outcomes, and these expectations can be combined with the patient’s personal goals to agree on the rehabilitation plan. 55
The treatment plan for post-acute stroke patients is influenced by clinicians’ expectations of motor recovery and outcome.55,56 Patients with initially moderate to severe motor impairment are the most difficult to make accurate predictions for based on clinical assessments alone, yet accurate prognosis is most important for these patients. 57 Clinicians’ prognoses are a source of variation that can produce inequitable access to rehabilitation services.55,57 Variation can be reduced when clinicians use objective prediction tools that combine standardized assessments to predict an individual patient’s likely outcome. Prediction tools can be used to guide rehabilitation goal setting and tailor therapy, and doing so may improve rehabilitation efficiency.58,59
Several tools have been developed to predict motor activity after stroke. 55 Validated tools that predict outcomes at specific time points for individual patients are summarized in Table 2. Most predict the probability of achieving a categorical activity capacity outcome for the upper limb or walking, defined using recommended clinical assessments. There are also tools available that can be used to predict recovery trajectories at any time in the first year post-stroke, for upper limb activity capacity 60 and independence in ADLs. 61 Implementation, 62 clinical impact, 59 and long-term accuracy 63 have been evaluated for one tool that predicts upper limb activity outcome. 64 Impact studies are needed to investigate the clinical benefits of prediction tools, 65 and evidence-based strategies are needed to support their successful and sustainable implementation. 62
Table 2.
Cross-validated tools that predict outcomes at specific time points for individual patients.
| Prediction tool | Domain | Predicted outcome | When prediction is made post-stroke | When outcome is measured post-stroke | Type of tool | Predictor variables |
|---|---|---|---|---|---|---|
| PUPPI 66 | Body function UL impairment |
Binarized NIHSS arm score, <2 and 2–4 | 24 h | 3 months | Scoring system | Age NIHSS |
| PREP2 64 | Activity UL capacity |
One of four categories of UL activity capacity: Excellent, Good, Limited, Poor | 3–10 days | 3 months | Decision tree | SAFE Age MEP status a NIHSS a |
| EPOS – Upper Limb 67 | Activity UL capacity |
Probability of achieving at least 10 out of 57 points on the ARAT | 2–10 days | 3 months | Multi-variable equation | FMA-UE finger extension, MI shoulder abduction |
| EPOS – Walking 67 | Activity Walking capacity |
Probability of independent walking, FAC score >3/5 | 3–10 days | 3 months | Multi-variable equation | TCT sitting MI leg |
| TWIST 68 | Activity Walking capacity |
Probability of independent gait, FAC score >3/5 | 7 days | 4, 6, 9, 16 and 26 weeks | Scoring system | Age Knee extension strength BBS |
| Kwah 69 | Activity Walking capacity |
Probability of independent gait, MAS item 5 score >2/6 | Within 7 days | 6 months | Multi-variable equation | Age NIHSS |
PUPPI: Persistent Upper Extremity Impairment; UL: Upper Limb; PREP: Predict Recovery Potential; SAFE: Shoulder Abduction and Finger Extension; MEP: Motor Evoked Potential; NIHSS: National Institutes of Health Stroke Scale; EPOS: Early Prediction of Functional Outcome after Stroke; ARAT: Action Research Arm Test; FMA: Fugl-Meyer Assessment Upper Extremity; MI: Motricity Index; FAC: Functional Ambulation Categories; TCT: Trunk Control Test; TWIST: Tenecteplase in Wake-up Ischemic Stroke Trial; BBS: Berg Balance Scale; MAS: Motor Assessment Scale.
Variable required for a subset of patients.
Standardized assessments and prediction tools are expected to inform shared decision-making and goal-setting between clinicians, patients, and families. They can be used to guide the rehabilitation plan so that it maximizes the patient’s chances of the best possible outcome. Standardized assessment of motor function at baseline is an essential element of all available prediction tools and needs to be repeated during recovery to understand progress toward predicted outcomes. Standardized assessment schedules are therefore required for consistently effective rehabilitation planning and monitoring.
Motor rehabilitation interventions
The fourth section of the definition is supported by strongly recommended interventions from recent motor rehabilitation guidelines. National clinical practice guidelines for stroke rehabilitation were reviewed to summarize the current evidence-base on interventions targeting motor rehabilitation. We included guidelines written in English and Dutch since three of the core writing group members were Dutch-speaking. Guidelines needed to contain a section that specifically addressed “rehabilitation after stroke” to be considered. An overview of existing stroke guidelines was recently published summarizing “strong” recommendations for the broad field of stroke care. 70 The definition and framework here focus on motor rehabilitation and summarize motor-specific recommendations.
Five high-quality, evidence-based clinical practice guidelines published between 2016 and 202271–76 were reviewed to identify common strong recommendations. The Australian and New Zealand guidelines 75 were developed using the GRADE methodology (Grading of Recommendations, Assessment, Development and Evaluation) 77 and strong recommendations mean that the evidence supports a clear balance toward a desirable effect. The Canadian guidelines 73 defined strong recommendations as evidence from a meta-analysis of randomized controlled trials or consistent findings from two or more randomized controlled trials. The American guidelines 76 made strong recommendations based on multiple randomized clinical trials or meta-analyses. The National Clinical Guidelines of the UK 72 did not adopt a hierarchical grading system for the “strength” of recommendations. Instead, a formal consensus approach was used to identify the key recommendations in terms of their wider impact on stroke. Last, the guidelines from the Netherlands71,74 made strong recommendations based on RCTs with good methodological quality, sufficient size and consistency, 78 and systematic reviews with at least some RCTs that met the aforementioned criteria.
Interventions for motor rehabilitation with strong recommendations were extracted independently by two researchers (BE, MMN), compiled, and discussed in case of disagreement. Recommendations “in favor of” that were included in at least three of the screened guidelines are presented in Table 3, providing an overview of strongly recommended interventions for the motor rehabilitation domain with international consensus. The recommendations presented in Table 3 cover: (i) timing of rehabilitation delivery, (ii) general principles of motor control and motor learning, (iii) interventions for functions and activities involving the lower limb, postural control, and walking and (iv) upper limb functions and activities. Guidelines and recommendations are continuously updated based on available evidence, and therefore it is important for clinicians to consult recent and updated or living guidelines.
Table 3.
Overview of motor interventions with strong recommendations in at least three international clinical practice guidelines published between 2016 and 2022.
| Strongly recommended | Guideline origin | Specific information |
|---|---|---|
| Time | ||
| Commencement of rehabilitation | ||
| Commence mobilization (out-of-bed-activity) within 48 h of stroke onset unless otherwise contraindicated (e.g. receiving end-of-life care). | AU/NZ, CA, NL, UK, US |
AU/NZ:
• Patients with baseline NIHSS scores above 4 and below 7 have higher odds of favorable outcome when mobilized >1x per day and spend less than 13.5 min per day in out-of-bed activities. • As patients tolerate more out-of-bed activity, better to increase frequency of sessions than duration of each session. • Particular care taken to avoid durations out of bed in people >76y and with more severe strokes (NIHSS > 7). • No rationale for restricting people to bed rest if they can move independently. CA: excluded if medically unstable, severe cognitive impairment preventing from participating in therapy, inappropriate behavior putting self or others at risk, not willing to participate in program. NL: patients with severe stroke (NIHSS > 16) should be mobilized shortly (not longer than 10 min) and frequently (minimally 2 to 3 times a day) in the first days after their stroke. Other patients should be mobilized according to their ability. UK: patients with difficulty moving early after stroke who are medically stable should be offered frequent, short daily mobilizations (sitting out of bed, standing or walking) by appropriately trained staff, typically beginning between 24 and 48 h of stroke onset. US: It is recommended that early rehabilitation for hospitalized stroke patients be provided in environments with organized, interprofessional stroke care. |
| Early supported discharge services | ||
| Offered to patients with mild to mod disability if appropriate home-based coordinated stroke services are available. | AU/NZ, CA, US |
AU/NZ: Where appropriate home-based coordinated stroke services are available, early supported discharge (ESD) services should be offered to stroke patients with mild to moderate disability. CA: ESD services, designed to reduce length of hospital stay and still provide same intensity of inpatient rehabilitation, are an acceptable form of rehabilitation and should be offered to a select group of patients when available and provided by a well-resourced, coordinated specialized team. Criteria for ESD candicacy include mild to moderate disability, ability to participate in rehabilitation from the point of discharge, and being medically stable. US: ESD services may be reasonable for people with mild to moderate disability. |
| Principles | ||
| Intensity (amount of rehabilitation) | ||
| Rehab is structured to provide as much scheduled therapy (OT and PT) as possible. | AU/NZ, CA, NL |
AU/NZ: therapist should maximize the amount of active task practice during therapy sessions. Use of objective measurement of activity should be considered. CA: once deemed to be medically and neurologically stable, more therapy results in better outcomes. NL (in all phases): intensifying exercise therapy (more hours) compared to fewer hours leads to faster recovery of the dissociated movement, comfortable walking speed, maximum walking speed, walking distance, muscle tone, sitting and standing balance, the performance of basic activities of daily living, quality of life and degree of depression and feelings of anxiety. |
| Mobility | ||
| General considerations | ||
| Patients should participate in training that is meaningful, engaging, progressively adaptive, intensive, task-specific and goal-oriented in an effort to improve transfer skills and mobility. | CA, UK, US |
CA: Therapy should include repetitive and intense use of patient-valued tasks that challenge the patient to acquire the necessary skills needed to perform functional tasks and activities. UK: People with loss of movement should be taught task-specific, repetitive, intensive exercises or activities that will increase strength. US: Intensive, repetitive, mobility-task training if gait limitations. |
| Specific therapies | ||
| Weakness | ||
| Progressive resistance training to improve strength | AU/NZ | AU/NZ: For stroke survivors with reduced strength in their arms or legs, progressive resistance training should be provided to improve strength. |
| Balance | ||
| Balance training | NL, UK, US |
NL (examined in ER, LR and RC): balance training during different activities improves sit- and standing balance and basic ADL activities. UK: People with significant impairment of their balance and walking ability after stroke should receive progressive balance training. US: Individuals with stroke who have poor balance, low balance confidence, and fear of falls or are at risk for falls should be provided with a balance training program. |
| Walking | ||
| Circuit class therapy (with a focus on overground walking practice) | AU/NZ, NL, US |
AU/NZ: Stroke survivors with difficulty walking should be given the opportunity to undertake tailored repetitive practice of walking (or components of walking) as much as possible, whereby circuit class therapy may be used (with a focus on overground walking practice). NL: circuit class training focused on walking and other mobility-related functions and activities improves the walking distance/walking speed (ER, LR, RC), sit- and standing balance (ER, LR, RC), walking ability (ER, LR, RC) and reduced the inactivity (LR, RC). US: Group therapy with circuit training is a reasonable approach to improve walking. |
| Task and goal-oriented training that is repetitive and progressively adapted | AU/NZ, CA, NL, US |
AU/NZ: Stroke survivors with difficulty walking should be given the opportunity to undertake tailored repetitive practice of walking (or components of walking) as much as possible. CA: Task and goal-oriented training that is repetitive and progressively adapted should be used to improve performance of selected lower-extremity tasks such as sit to stand, walking distance and walking speed. NL: It is plausible that functional exercise therapy that can be performed in an environment as relevant to the patient as possible (task- and context-specific) has a positive effect on the skill to be learned skill itself. Elements of variation and sufficient repetition (repetition-without-repetition) appear to be important aspects for an effective learning process. US: Intensive, repetitive, mobility-task training is recommended for all individuals with gait limitations after stroke. |
| Exercises which aim to improve aerobic fitness and/or muscle strength | NL, UK, US |
NL (examined in ER, LR and RC): training of the aerobic endurance in combination with strength training improves the dissociated movements, muscle strength of the paretic leg, comfortable and maximal walking speed, walking distance, maximal oxygen absorption capacity, heart frequency during exercise, balance, amount of physical activity in daily life, and quality of life. UK: People with stroke, including those who use wheelchairs or have poor mobility, should be advised to participate in exercise with the aim of improving aerobic fitness and/or muscle strength unless there are contraindications. US: Incorporating cardiovascular exercise and strengthening interventions is reasonable to consider for recovery of gait capacity and gait-related mobility tasks. |
| Treadmill training with or without bodyweight support (BWS) | AU/NZ, CA, NL, UK, US |
AU/NZ: Stroke survivors with difficulty walking should be given the opportunity to undertake tailored repetitive practice of walking (or components of walking) as much as possible, for which treadmill training with or without body weight support may be used. CA: Treadmill-based gait training (with or without body weight support) should be used to enhance walking speed, and distance walked as an adjunct to over-ground training or when over-ground training is not available or appropriate. NL (examined in ER and RC): treadmill training with BWS improves comfortable walking speed and walking distance, but was not proven to be more effective than control condition for sitting- and standing balance. NL (examined in ER, LR and RC): treadmill training without BWS is more effective for improving maximal walking speed and walking width than conventional walking training. UK: People who are able to walk independently after stroke should be offered treadmill training with or without body weight support or other walking-orientated interventions at a higher intensity than usual care and as an adjunct to other treatments. US: Practice walking with a treadmill (with or without body-weight support) may be reasonable for recovery of walking function. |
| Overground walking exercise training combined with conventional rehabilitation | AU/NZ, CA, NL, UK, US |
AU/NZ: Stroke survivors with difficulty walking should be given the opportunity to undertake tailored repetitive practice of walking (or components of walking) as much as possible through circuit class training focused on overground walking practice. CA: Over-ground training should be used to enhance walking speed, and distance walked, with treadmill-based gait training as an adjunct or when over-ground training is not available or appropriate. NL (examined in RC): more effective than treadmill training to improve walking distance and reduce fear for patients who can walk without physical support. UK: People with stroke who are able to walk with or without assistance should undergo task-specific walking training with a cardiorespiratory and/or muscle strength focus at sufficient intensity to improve endurance and walking speed. US: Practice walking with overground walking exercise training combined with conventional rehabilitation may be reasonable for recovery of walking function. |
| Robot-assisted movement training in combination with conventional therapy | CA, NL, UK, US |
CA: Electromechanical (robotic) assisted gait training devices could be considered for patients who would not otherwise practice walking. They should not be used in place of conventional gait therapy. NL: improves, in patients who cannot walk independently, the comfortable walking speed (ER), maximal walking speed (ER and RC), walking distance (ER and RC), heartrate (ER), sit- and standing balance (ER), walking ability (ER) and performing basic ADL activities (ER and RC). UK: for people who cannot walk independently. US: for non-ambulatory or low ambulatory early after stroke. |
| Ankle foot orthosis (AFO) to improve walking and balance, evaluated and individually fitted before long-term use | CA, NL, UK, US | CA, NL, UK, US: for people who have compromised ankle/foot stability and/or reduced ability to dorsiflex the foot (“foot-drop”) that impedes safe and efficient walking should be offered an ankle-foot orthosis to improve walking and balance. |
| Functional electrical stimulation (FES) | CA, NL, UK, US | CA, NL, UK, US: in people with reduced ability to dorsiflex the foot (“foot-drop”) should be offered FES to improve their gait as alternative to AFO, but effects may not be sustained. |
| Arm activity | ||
| Specific therapies | ||
| Traditional or modified constraint-induced movement therapy ((m)CIMT) | AU/NZ, CA, NL, UK, US | if 20° active wrist extension and 10° active finger extension in the affected hand AU/NZ: min. 2 h /a/ therapy/d for 2 w plus restraint for at least 6 h/d. Active, intensive task practice is key component, no evidence for use of restraint alone, CIMT only relevant for people with no or minimal cognitive deficits. CA: Traditional or modified constraint-induced movement therapy (CIMT) should be considered for a select group of patients who demonstrate at least 20 degrees of active wrist extension and 10 degrees of active finger extension, with minimal sensory deficits and normal cognition. NL: original CIMT (LR), low intensity mCIMT (ER, LR, RC) and high intensity mCIMT (ER, RC) improve arm-hand skills, experience use of arm and hand and quality of movement. Low-intensity CIMT further improves basic ADL activities, and original CIMT quality of life. US: CIMT or its modified version is reasonable to consider for eligible stroke survivors. UK: People with stroke who have 20 degrees of active wrist extension and 10 degrees of active finger extension in the affected hand should be considered for constraint-induced movement therapy. |
| Mental practice as an adjunct to conventional therapy | CA, UK, US |
CA: Following assessment to determine if they are suitable candidates, patients should be encouraged to engage in mental imagery to enhance upper-limb, sensorimotor recovery. UK: People with stroke who have been assessed as cognitively suitable to participate in mental practice of an activity should be trained and encouraged to use it to improve arm function, as an adjunct to conventional therapy. US: Mental practice is reasonable to consider as an adjunct to upper extremity rehabilitation services. |
| Robot-assisted movement therapy as an adjunct to conventional therapy | NL, UK, US |
NL: unilateral robot-assisted training of paretic shoulder and elbow improves dissociated movements and arm strength (ER, LR, RC) and reduces atypical pain in the paretic arm (ER, LR). Bilateral robot-assisted training of elbow and wrist improves dissociated movements and arm strength (ER, RC). UK: People with reduced arm function after a stroke should only be offered robot-assisted movement therapy as an adjunct to conventional therapy in the context of a clinical trial. US: Robotic therapy is reasonable to consider to deliver more intensive practice for individuals with moderate to severe upper limb paresis. |
| NMES as an adjunct to conventional therapy | CA, NL, UK, US |
CA: FES targeted at the wrist and forearm muscles should be considered to reduce motor impairment and improve function. NL: NMES of paretic wrist- and finger-flexors and –extensors improves dissociated movements and muscle strength (ER), NMES paretic shoulder muscles improves glenohumeral subluxation (ER, LR, RC), EMG-NMES paretic wrist- and finger-extensors improves dissociated movements, active ROM and arm-hand skills (ER, RC). UK: People with reduced arm function after a stroke should only be offered neuromuscular electrical stimulation as an adjunct to conventional therapy in the context of a clinical trial. US: for individuals with minimal volitional movement within the first few months after stroke or individuals with shoulder luxation. |
| Activities of daily living (participation restrictions) | ||
| Community-dwelling stroke survivors with confirmed difficulties in personal or extended ADL: should have specific therapy from a trained clinician (e.g. task-specific practice and training in the use of appropriate aids) | AU/NZ, NL, UK, US |
AU/NZ: Community-dwelling stroke survivors with confirmed difficulties in personal or extended activities of daily living should have specific therapy from a trained clinician (e.g. task-specific practice and training in the use of appropriate aids) to address these issues. NL (examined in ER): positive effect on participation in leisure activity. NL (examined in ER and REC): not more effective for quality of life, mood and depression, motoric functions, ADL activities compared to other interventions. UK: People with limitations of personal activities of daily living after stroke should be referred to an occupational therapist with experience in neurological disability, be assessed within 72 h of referral, and be offered treatment for identified problems (e.g. feeding, toileting) by the occupational therapist, who should also involve other members of the specialist multidisciplinary team. US: All individuals with stroke should receive (I)ADL training tailored to individual needs and eventual discharge setting. |
AUS & NZ: Australia and New Zealand; CA: Canada; UK: United Kingdom; US: United States of America; NL: Netherlands; NIHSS: National Institutes of Health Stroke Scale; OT: Occupational Therapy; PT: Physical Therapy; NMS: Neuromuscular Electrostimulation; TENS: Transcutaneous Electrical Nerve Stimulation; ADL: Activities of Daily Living; VR: Virtual Reality; BWS: Body Weight Support; AFO: Ankle Foot Orthosis; CIMT: Constraint-Induced Movement Therapy; mCIMT: modified constraint-induced movement therapy; FES: Functional Electrical Stimulation; EMG: Electromyography; rTMS: Repetitive Transcranial Magnetic Stimulation; HAR: Hyperacute Rehabilitation phase (0–24 h post-stroke); ER: Early Rehabilitation phase (24 h–3 months post-stroke); LR: Late Rehabilitation phase (3–6 months); RC: Rehabilitation in the Chronic phase (> 6 months).
Discussion and conclusion
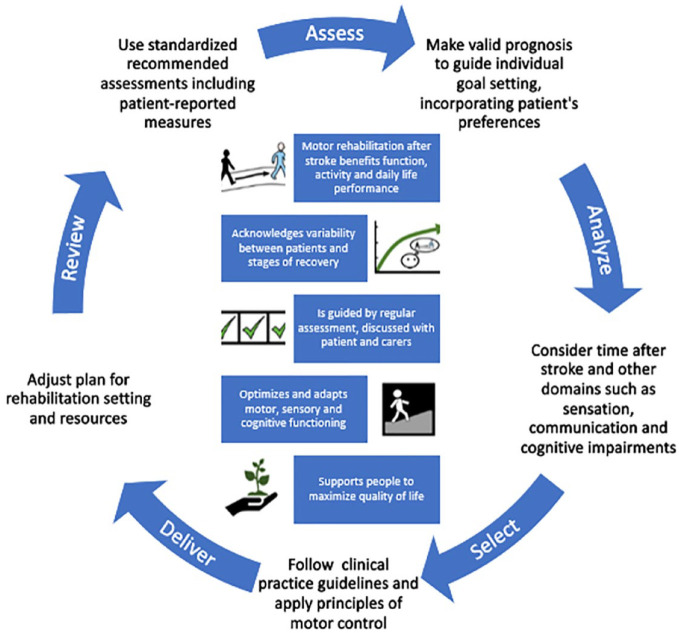
Motor rehabilitation is a key element of the stroke care pathway for people with persisting movement and mobility deficits. An expert panel agreed on the first definition of motor rehabilitation after stroke, which is supported by a contemporary motor rehabilitation framework for clinicians, educators and researchers. Figure 3 presents the definition and core elements of the motor rehabilitation process after stroke in an abbreviated pictorial format.
Figure 3.
Pictorial abbreviated motor rehabilitation after stroke definition (center) and summarized process of motor rehabilitation after stroke.
The strengths of this work include the expertise of the panel, the three-round process used for producing the definition, and the consultation with clinical stakeholders, integrating their feedback in final group discussions. Working group members were primarily European, in response to the assignment provided by the ESO, and also included colleagues from three global regions. The definition focuses on fundamental concepts which are likely similar across countries. The WHO rehabilitation definition is one sentence, 4 however the panel constructed a definition paragraph which is more appropriate for multi-faceted concepts such as motor rehabilitation after stroke. It is rare for one definition to serve all stakeholders. Here we focused on the needs of educators, clinicians and researchers, rather than laypersons. Nevertheless, we obtained feedback from two people with lived experience, and a lay version with visual supporting elements could help to communicate the concepts to patients and carers.
The formal definition of motor rehabilitation forms a base for future research and guideline development in the domain of motor rehabilitation. The working group is now developing motor rehabilitation guidelines based on the summary of interventions with strong recommendations presented here. Interdisciplinary rehabilitation teams need to follow evidence-based recommendations and integrate the views of patients and carers to improve life after stroke.
Acknowledgments
The working group would like to acknowledge the valuable feedback received from all clinicians consulted in various clinical settings. More specifically, we are grateful for the input from: The stroke unit and neurorehabilitation ward team from Sahlgrenska University Hospital Sweden; 13 physiotherapists, 8 occupational therapists and 7 physicians; Neurologist and specialized neuro physiotherapist from University Hospital Brussels, Belgium; Neurological physiotherapists at Auckland City Hospital, New Zealand; Two clinician physiotherapists, specialized in subacute and chronic stroke care from the Hospital de Santa Creu de Vic and Clínica de Neurorehabilitación, Spain; Three specialized neuro physiotherapists working at the acute stroke unit and three at the rehabilitation unit from University Hospitals Leuven, Belgium; Highly specialized occupational therapist (1) and physiotherapists (3) from stroke services at Gateshead, Northumbria and South Tyneside and Sunderland NHS Foundation Trusts, UK; Two physiatrists from Department of physical medicine and rehabilitation, University Hospital Mostar, Bosnia and Herzegovina; Physiotherapist (1) from Unit of Occupational Medicine, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Physiotherapists (2) from Department of Neurology, University Hospital of Zurich, and cereneo Center for Neurology and Rehabilitation, Vitznau, Switzerland; Physicians (2) from Service de médecine physique et réadaptation, CHU de Toulouse, Toulouse, France. Special thanks to the patient partners Anders Andersson and Johanna Persson who provided valuable input to the definition.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Not applicable.
Ethical approval: Not applicable.
Guarantor: G.V.
Contributorship: Geert Verheyden (GV) and Margit Alt Murphy (MAM) are co-chairs of this European Stroke Organisation (ESO) working group and supported by two junior faculty; Bea Essers (BE) and Maria Munoz Novoa (MMN). Together with Gert Kwakkel and Cathy Stinear, they formed the core writing group as part of the working group, preparing the draft manuscript of this review, revising and finalizing the work based on the reviewer comments received. The working group further consists of Meret Branscheidt, Rosa Cabanas-Valdés, Sandra Lakičević, Sofia Lampropoulou, Andreas R Luft, Philippe Marque, Sarah A Moore, John M Solomon, Eva Swinnen and Andrea Turolla. GV, MAM, BE and MMN prepared all the meetings and the surveys, collected the results and led the discussions about the survey results and definition with all working group members.
All working group members reviewed and commented upon the manuscript, leading to the final draft before submission. Date received: 27 March 2023; accepted: 16 July 2023.
ORCID iDs: Rosa Cabanas-Valdés  https://orcid.org/0000-0002-5255-2494
https://orcid.org/0000-0002-5255-2494
Andrea Turolla  https://orcid.org/0000-0002-1609-8060
https://orcid.org/0000-0002-1609-8060
Geert Verheyden  https://orcid.org/0000-0003-3095-8175
https://orcid.org/0000-0003-3095-8175
References
- 1. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke 2022; 17: 18–29. [DOI] [PubMed] [Google Scholar]
- 2. Wafa HA, Wolfe CD, Emmett E, et al. Burden of stroke in Europe: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke 2020; 51: 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018-2030. Eur Stroke J 2018; 3: 309–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO), World Bank. World report on disability. Geneva: WHO, 2011. [Google Scholar]
- 5. Steiner T, Dichgans M, Norrving B, et al. European Stroke Organisation (ESO) standard operating procedure for the preparation and publishing of guidelines. Eur Stroke J 2021; 6: CXXII–CXXXIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014; 67: 401–409. [DOI] [PubMed] [Google Scholar]
- 7. Niederberger M, Spranger J. Delphi technique in health sciences: a map. Front Public Health 2020; 8: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO). Towards a common language for functioning, disability and Health. Geneva: ICF, 2002. [Google Scholar]
- 9. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011; 377: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organisation (WHO). WHOQOL user manual. Geneva: WHO, 1998. [Google Scholar]
- 11. McDougall J, Wright V, Rosenbaum P. The ICF model of functioning and disability: incorporating quality of life and human development. Dev Neurorehabil 2010; 13(3): 204–211. [DOI] [PubMed] [Google Scholar]
- 12. Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 2009; 23: 313–319. [DOI] [PubMed] [Google Scholar]
- 13. Joy MT, Carmichael ST. Encouraging an excitable brain state: mechanisms of brain repair in stroke. Nat Rev Neurosci 2021; 22: 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor Neurol Neurosci 2004; 22: 281–299. [PubMed] [Google Scholar]
- 15. Jones TA. Motor compensation and its effects on neural reorganization after stroke. Nat Rev Neurosci 2017; 18(5): 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 17. van der Vliet R, Selles RW, Andrinopoulou ER, et al. Predicting upper limb motor impairment recovery after stroke: a mixture model. Ann Neurol 2020; 87: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldsmith J, Kitago T, Garcia de la Garza A, et al. Arguments for the biological and predictive relevance of the proportional recovery rule. eLife 2022; 11:e80458. DOI: 10.7554/ELIFE.80458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008; 22: 64–71. [DOI] [PubMed] [Google Scholar]
- 20. Byblow WD, Stinear CM, Barber PA, et al. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015; 78: 848–859. [DOI] [PubMed] [Google Scholar]
- 21. Winters C, van Wegen EE, Daffertshofer A, et al. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair 2015; 29: 614–622. [DOI] [PubMed] [Google Scholar]
- 22. Smith MC, Byblow WD, Barber PA, et al. Proportional recovery from lower limb motor impairment after stroke. Stroke 2017; 48: 1400–1403. [DOI] [PubMed] [Google Scholar]
- 23. Veerbeek JM, Winters C, van Wegen EEH, et al. Is the proportional recovery rule applicable to the lower limb after a first-ever ischemic stroke? PLoS One 2018; 13: e0189279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boccuni L, Meyer S, Kessner SS, et al. Is there full or proportional somatosensory recovery in the upper limb after stroke? Investigating behavioral outcome and neural correlates. Neurorehabil Neural Repair 2018; 32(8): 691–700. [DOI] [PubMed] [Google Scholar]
- 25. Zandvliet SB, Kwakkel G, Nijland RHM, et al. Is recovery of somatosensory impairment conditional for upper-limb motor recovery early after stroke? Neurorehabil Neural Repair 2020; 34: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winters C, van Wegen EE, Daffertshofer A, et al. Generalizability of the maximum proportional recovery rule to visuospatial neglect early poststroke. Neurorehabil Neural Repair 2017; 31: 334–342. [DOI] [PubMed] [Google Scholar]
- 27. Lendrem W, Lincoln NB. Spontaneous recovery of language in patients with aphasia between 4 and 34 weeks after stroke. J Neurol Neurosurg Psychiatry 1985; 48: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobkin BH, Carmichael ST. The specific requirements of neural repair trials for stroke. Neurorehabil Neural Repair 2016; 30: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 2009; 111: 483–495. [DOI] [PubMed] [Google Scholar]
- 30. Saver JL. Time is brain–quantified. Stroke 2006; 37: 263–266. [DOI] [PubMed] [Google Scholar]
- 31. Ward NS. Restoring brain function after stroke - bridging the gap between animals and humans. Nat Rev Neurol 2017; 13: 244–255. [DOI] [PubMed] [Google Scholar]
- 32. Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 2008; 63: 272–287. [DOI] [PubMed] [Google Scholar]
- 33. Berkhemer OA, Jansen IG, Beumer D, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke 2016; 47: 768–776. [DOI] [PubMed] [Google Scholar]
- 34. Liebeskind DS, Tomsick TA, Foster LD, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014; 45: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ward NS, Brown MM, Thompson AJ, et al. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003; 126: 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wittenberg GF. Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis 2010; 37: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beaulieu LD, Milot MH. Changes in transcranial magnetic stimulation outcome measures in response to upper-limb physical training in stroke: a systematic review of randomized controlled trials. Ann Phys Rehabil Med 2018; 61: 224–234. [DOI] [PubMed] [Google Scholar]
- 38. Nelson CM, Murray WM, Dewald JPA. Motor impairment-related alterations in biceps and triceps brachii fascicle lengths in chronic hemiparetic stroke. Neurorehabil Neural Repair 2018; 32: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adkins AN, Dewald JPA, Garmirian LP, et al. Serial sarcomere number is substantially decreased within the paretic biceps brachii in individuals with chronic hemiparetic stroke. Proc Natl Acad Sci USA 2021; 118: e2008597118. DOI: 10.1073/PNAS.2008597118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paudyal A, Degens H, Baan GC, et al. Changes in muscle-tendon unit length-force characteristics following experimentally induced photothrombotic stroke cannot be explained by changes in muscle belly structure. Eur J Appl Physiol 2021; 121: 2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dias CP, Freire B, Goulart NBA, et al. Impaired mechanical properties of Achilles tendon in spastic stroke survivors: an observational study. Top Stroke Rehabil 2019; 26: 261–266. [DOI] [PubMed] [Google Scholar]
- 42. Zhang LQ, Chung SG, Ren Y, et al. Simultaneous characterizations of reflex and nonreflex dynamic and static changes in spastic hemiparesis. J Neurophysiol 2013; 110: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Kordelaar J, van Wegen EE, Nijland RH, et al. Understanding adaptive motor control of the paretic upper limb early poststroke: the EXPLICIT-stroke program. Neurorehabil Neural Repair 2013; 27: 854–863. [DOI] [PubMed] [Google Scholar]
- 44. Sukal TM, Ellis MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res 2007; 183: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ellis MD, Sukal-Moulton T, Dewald JP. Progressive shoulder abduction loading is a crucial element of arm rehabilitation in chronic stroke. Neurorehabil Neural Repair 2009; 23: 862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saes M, Mohamed Refai MI, van Beijnum BJF, et al. Quantifying quality of reaching movements longitudinally post-stroke: a systematic review. Neurorehabil Neural Repair 2022; 36: 183–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buurke JH, Nene AV, Kwakkel G, et al. Recovery of gait after stroke: what changes? Neurorehabil Neural Repair 2008; 22: 676–683. [DOI] [PubMed] [Google Scholar]
- 48. Kwakkel G, Veerbeek JM, van Wegen EE, et al. Constraint-induced movement therapy after stroke. Lancet Neurol 2015; 14: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burridge J, Alt Murphy M, Buurke J, et al. A systematic review of international clinical guidelines for rehabilitation of people with neurological conditions: what recommendations are made for upper limb assessment? Front Neurol 2019; 10: 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prange-Lasonder GB, Alt Murphy M, Lamers I, et al. European evidence-based recommendations for clinical assessment of upper limb in neurorehabilitation (CAULIN): data synthesis from systematic reviews, clinical practice guidelines and expert consensus. J Neuroeng Rehabil 2021; 18: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kwakkel G, Van Wegen E, Burridge JH, et al. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2019; 14: 783–791. [DOI] [PubMed] [Google Scholar]
- 52. Pohl J, Held JPO, Verheyden G, et al. Corrigendum: consensus-based core set of outcome measures for clinical motor rehabilitation after stroke-a Delphi study. Front Neurol 2021; 12: 697935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kwakkel G, van Wegen EEH, Burridge JH, et al. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair 2019; 33: 951–958. [DOI] [PubMed] [Google Scholar]
- 54. Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int J Stroke 2017; 12: 451–461. [DOI] [PubMed] [Google Scholar]
- 55. Stinear CM, Smith MC, Byblow WD. Prediction tools for stroke rehabilitation. Stroke 2019; 50: 3314–3322. [DOI] [PubMed] [Google Scholar]
- 56. Cormier DJ, Frantz MA, Rand E, et al. Physiatrist referral preferences for postacute stroke rehabilitation. Medicine 2016; 95: e4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 2017; 16: 826–836. [DOI] [PubMed] [Google Scholar]
- 58. Stinear CM, Lang CE, Zeiler S, et al. Advances and challenges in stroke rehabilitation. Lancet Neurol 2020; 19: 348–360. [DOI] [PubMed] [Google Scholar]
- 59. Stinear CM, Byblow WD, Ackerley SJ, et al. Predicting recovery potential for individual stroke patients increases rehabilitation efficiency. Stroke 2017; 48: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 60. Selles RW, Andrinopoulou ER, Nijland RH, et al. Computerised patient-specific prediction of the recovery profile of upper limb capacity within stroke services: the next step. J Neurol Neurosurg Psychiatry 2021; 92: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Douiri A, Grace J, Sarker SJ, et al. Patient-specific prediction of functional recovery after stroke. Int J Stroke 2017; 12: 539–548. [DOI] [PubMed] [Google Scholar]
- 62. Connell LA, Smith MC, Byblow WD, et al. Implementing biomarkers to predict motor recovery after stroke. NeuroRehabilitation 2018; 43: 41–50. [DOI] [PubMed] [Google Scholar]
- 63. Smith MC, Ackerley SJ, Barber PA, et al. PREP2 algorithm predictions are correct at 2 years poststroke for most patients. Neurorehabil Neural Repair 2019; 33: 635–642. [DOI] [PubMed] [Google Scholar]
- 64. Stinear CM, Byblow WD, Ackerley SJ, et al. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol 2017; 4: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moons KG, Altman DG, Vergouwe Y, et al. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009; 338: b606–1490. [DOI] [PubMed] [Google Scholar]
- 66. de Havenon A, Heitsch L, Sunmonu A, et al. Accurate prediction of persistent upper extremity impairment in patients with ischemic stroke. Arch Phys Med Rehabil 2022; 103: 964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Veerbeek JM, Pohl J, Luft AR, et al. External validation and extension of the early prediction of functional outcome after stroke (EPOS) prediction model for upper limb outcome 3 months after stroke. PLoS One 2022; 17: e0272777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith MC, Barber AP, Scrivener BJ, et al. The TWIST tool predicts when patients will recover independent walking after stroke: an observational study. Neurorehabil Neural Repair 2022; 36: 461–471. [DOI] [PubMed] [Google Scholar]
- 69. Langerak AJ, McCambridge AB, Stubbs PW, et al. Externally validated model predicting gait independence after stroke showed fair performance and improved after updating. J Clin Epidemiol 2021; 137: 73–82. [DOI] [PubMed] [Google Scholar]
- 70. Mead GE, Sposato LA, Sampaio Silva G, et al. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int J Stroke 2023; 18: 499–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koninklijk Nederlands Genootschap voor Fysiotherapie. KNGF-Richtlijn Beroerte, www.kngfrichtlijnen.nl (2017, accessed 20 March 2023).
- 72. Royal College of Physicians. National clinical guideline for stroke. 5th ed. London: Intercollegiate Stroke Working Party, 2016. https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines
- 73. Canadian Stroke Best Practices. Rehabilitation, recovery and community participation following stroke, https://www.strokebestpractices.ca/recommendations/stroke-rehabilitation (2019, accessed 20 March 2023).
- 74. Federatie Medisch Specialisten. Herseninfarct En Hersenbloeding, https://richtlijnendatabase.nl/richtlijn/herseninfarct_en_hersenbloeding/startpagina_herseninfarct_-bloeding.html (2019, accessed 20 March 2023).
- 75. Stroke Foundation. Clinical guidelines for stroke management. Chapter 5 of 8: Rehabilitation. https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management (2021, accessed 20 March 2023).
- 76. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98–e169. [DOI] [PubMed] [Google Scholar]
- 77. Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. [Google Scholar]
- 78. PEDro scale. http://www.pedro.org.au/wp-content/uploads/PEDro_scale.pdf (1999, accessed 27 June 2023).