Abstract
Introduction:
The best therapeutic strategy for patients with mechanical heart valves (MHVs) having acute ischemic stroke during treatment with vitamin K antagonists (VKAs) remain unclear. Being so, we compared the outcomes for: (i) full dose heparin along with VKA (bridging therapy group) and (ii) restarting VKA without heparin (nonbridging group).
Patients and methods:
For this multicenter observational cohort study, data on consecutive acute ischemic stroke patients with MHV was retrospectively collected from prospective registries. Propensity score matching (PSM) was adopted to adjust for any treatment allocation confounders. The primary outcome was the composite of stroke, systemic embolism, symptomatic cerebral bleeding, and major extracerebral bleeding at 90 days.
Results:
Overall, 255 out of 603 patients (41.3%) received bridging therapy: 36 (14.1%) had combined outcome, compared with 28 (8.0%) in the nonbridging group (adjusted OR 1.83; 95% CI 1.05–3.18; p = 0.03). Within the bridging group, 13 patients (5.1%) compared to 12 (3.4%) in the nonbridging group had an ischemic outcome (adjusted OR 1.71; 95% CI 0.84–3.47; p = 0.2); major bleedings were recorded in 23 (9.0%) in the bridging group and 16 (4.6%) in the nonbridging group (adjusted OR 1.88; 95% CI 0.95–3.73; p = 0.07). After PSM, 36 (14.2%) of the 254 bridging patients had combined outcome, compared with 23 (9.1%) of 254 patients in the nonbridging group (OR 1.66; 95% CI 0.95–2.85; p = 0.07).
Conclusion:
Acute ischemic stroke patients with MHV undergoing bridging therapy had a marginally higher risk of ischemic or hemorrhagic events, compared to nonbridging patients.
Keywords: Mechanical heart valve, acute stroke, prevention, oral anticoagulants
Introduction
Patients with mechanical heart valves (MHV) require lifelong anticoagulation with vitamin K antagonists (VKAs) to reduce the risk of ischemic stroke and systemic embolism. In the case of such an event, the risk of bleeding is higher whenever anticoagulation is continued, whereas, the risk of thromboembolism is generally higher after anticoagulation has been interrupted. Therefore, to judiciously manage these risks, currently a treating physician can: (1) continue anticoagulation with VKA; (2) discontinue VKA and resume it only after excluding for the presence of a hemorrhagic transformation by a second brain CT scan or an MRI performed 24–72 h from index event; (3) discontinue VKA and initiate early anticoagulation therapy using bridge therapy with a full dose of a short-acting anticoagulant (e.g. low molecular weight heparin) along with VKA, until the therapeutic international normalized ratio (INR) is achieved. Regarding acute stroke patients with atrial fibrillation (AF), past study results have suggested that using bridging therapy is associated with higher risks of both early ischemic recurrence and hemorrhagic transformation, compared to nonbridging patients.1,2 In patients with acute stroke and MHV, the benefit and safety profile associated with bridge therapy remains unclear. In fact, results from a meta-analysis, indicate that early anticoagulation with heparins in patients with cardioembolic stroke was associated with a non-significant reduction in the recurrence of ischemic stroke, but the study also observed an increased intracranial bleeding rate. However, in this meta-analysis, the majority of the patients had AF. 3
In light of this, we compared the clinical profiles and outcomes of patients with acute ischemic stroke and MHV receiving and not receiving bridging therapy.
Methods
In this multicenter observational cohort study, data of consecutive acute ischemic stroke patients with MHV hospitalized between January 2007 and September 2022 were collected from prospective registries using prespecified variables and collection methods. Patients were enrolled from 43 Stroke Units across Europe and Asia (18 from teaching hospitals and 25 from nonteaching hospitals) all with high expertise on the management of patients with stroke. These registries were part of an established network that had been collaborating since 2007 to collect data on acute stroke patients. Over these years, the number of centers has progressively increased with only centers that have demonstrated the ability to collect reliable data have been included. The enrollment period of consecutive patients was determined by each participating center, depending on local registry characteristics. Patients were excluded if they had not had a follow-up brain imaging at 24–72 h, and if they had had contraindications for continuing anticoagulation therapy including a high risk for malignant brain edema or presence of early hemorrhagic transformation.
On admission, stroke severity was assessed using the National Institute of Health Stroke Scale (NIHSS). A non-contrast cerebral computed tomography (CT) or cerebral magnetic resonance imaging (MRI) scan was performed at admission on all patients to exclude for intracranial hemorrhage. Intravenous thrombolysis and/or endovascular thrombectomy treatments were delivered according to international guidelines. Standard stroke unit care, monitoring, and treatment were provided at all participating centers according to international recommendations for acute ischemic stroke according to the period of inclusion. All patients were monitored for blood pressure, temperature, glucose level, and heart rate over the first days after stroke. Attending physicians were free to make decisions on the types of therapy (bridging or not) to be used and its initiation day. A second brain CT scan or magnetic resonance was performed 24–72 h from stroke onset on all patients. Hemorrhagic transformation (HT) was defined on CT scan as any degree of hyperdensity within the area of low attenuation and was classified as either hemorrhagic infarction or parenchymal hematoma.4,5 On magnetic resonance imaging, HT was defined as hypointensity on axial T1-weighted or T2-weighted images. HT was considered to be symptomatic if it was associated with an increase of ⩾4 points on the NIHSS score or death. 6 We assessed for the presence of white matter changes (leukoaraiosis defined on the first computed tomography examination as ill-defined and moderately hypodense areas of ⩾5 mm according to published criteria). 7 Leukoaraiosis in the deep white matter was dichotomized into absent versus present (mild, moderate, or severe).
The sites and sizes of the qualifying infarcts were classified based on standard templates8,9 as (1) small, when a lesion was ⩽1.5 cm in the anterior or posterior circulation; (2) medium, when a lesion was in a superficial cortical branch of the middle cerebral artery (MCA), in the MCA deep branch, in the internal border zone territories, in a cortical superficial branch of posterior cerebral artery, in a cortical superficial branch of the anterior cerebral artery; (3) large when a lesion involved the entire territory of MCA, posterior cerebral artery, or anterior cerebral artery, in 2 superficial cortical branches of MCA, in a cortical superficial branch of MCA associated to the MCA deep branch, or in >1 artery territory (e.g. MCA associated to anterior cerebral artery territories) or when a lesion was ⩾1.5 cm in the brain stem or cerebellum. 10
For the purpose of this analysis, bridging therapy was defined as any temporary full dose of LMWH (e.g. 100 UI/kg of enoxaparin twice a day) or unfractionated heparin (aPTT ratio 1.5–2.0) started combined or before with VKAs, in order to guarantee the required time to achieve its therapeutic effect 11 or any full dose (given for at least 24 h) of LMWH prior to the use of VKAs.
This study was approved by the pertinent institutional review boards when required. Informed consent was obtained in accordance with local requirements.
This study was designed adhering STROBE statement recommendations (Strengthening the Reporting of Observational Studies in Epidemiology) (Supplemental Material).
Baseline data
The following baseline variables were investigated: age, sex, history of hypertension (blood pressure of ⩾140/90 mmHg at least twice before stroke or treatment with antihypertensive drugs), history of diabetes mellitus (fasting glucose level ⩾126 mg/ dL preprandial on 2 measurements, glucose level ⩾200 mg/dL post-prandial, or HbA1c ⩾6.5%, or diabetic treatment), current cigarette smoking, hyperlipidemia (total cholesterol ⩾200 mg/dL or triglyceride ⩾140 mg/ dL or lipid lowering therapy), history of symptomatic ischemic heart disease (myocardial infarction, angina, existence of multiple lesions on thallium heart isotope scan or evidence of coronary disease on coronary angiography), current alcohol abuse (⩾300 g per week), history of previous stroke/transient ischemic attack, presence of AF classified as paroxysmal when episodes terminated spontaneously within 7 days, type of MHV (mitral, aortic or both), history of symptomatic peripheral arterial disease (intermittent claudication of presumed atherosclerotic origin; or ankle/arm systolic blood pressure ratio <0.85 in either leg at rest; or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty), presence of a pacemaker, intracardiac thrombus on echocardiography (transthoracic and/or transesophageal), INR on admission, creatinine clearance (calculated by Cockcroft-Gault equation) and the day of starting anticoagulant treatment after index stroke. Any concurrent antiplatelet use prior to and/or after the index stroke was also recorded.
Evaluation of outcomes
Patients were followed up prospectively through face-to-face or telephone interviews. Study outcomes at 90 days were (1) recurrent ischemic stroke or symptomatic systemic embolisms; (2) symptomatic cerebral bleedings or major extracerebral bleedings. The primary study outcome was the composite of stroke, systemic embolism, symptomatic cerebral bleeding, and major extracerebral bleeding. HTs found on neuroimaging 24–72 h after onset were not considered outcome events unless classified as symptomatic. Stroke was defined as the sudden onset of a new focal neurological deficit of vascular origin in a site consistent with the territory of a major cerebral artery and categorized as ischemic or hemorrhagic. Systemic embolism was defined as an acute vascular occlusion of an extremity or organ confirmed by imaging, surgery, or autopsy. Major extracerebral bleeding was defined as a reduction in the hemoglobin level of at least 2 g/dL, requiring blood transfusion of at least 2 units, or symptomatic bleeding in a critical area or organ. 12
Disability and mortality at 90 days were also assessed using the modified Rankin Scale (mRS). Nondisabling functional outcome was defined as a mRS score of 0–2.
Statistical analysis
The analysis was performed to identify predictors of composite outcome events. Chi-square test or Fisher’s exact test with Yate’s correction, when appropriate, for categorical variables or the Mann-Whitney U test for continuous variables were used to compare patients with combined events to those without events, according to the presence of risk factors for stroke, as well as the type of anticoagulation therapy (bridging or nonbridging therapy). Multivariable logistic regression analysis was performed to identify variables that would be associated with combined outcome event. The variables of interest included in this analysis were selected from the univariate analysis, using backward stepwise analysis with a 0.1 level as a screening criterion for the selection of candidate variables. The day of starting anticoagulant treatment was inserted into the models as a continuous variable. Furthermore, for outcome events, survival and empirical cumulative hazard functions were estimated via the Kaplan-Meier estimator for the two groups. Patients were censored at the time of an outcome event or death.
Propensity score matching (PSM) was adopted to control for factors driving treatment allocation. For the treatment group this score was calculated including all of the study variables. Matching was then done with a 1:1 ratio across the groups, without replacement, and with a forced preservation of bridging cases. After PSM, survival function and empirical cumulative hazard function were independently utilizing the Kaplan-Meier estimator for the two groups. Patients were censored at the time of an outcome event, death, or if they had been lost to follow-up. Data were analyzed using the SPSS/PC Win package 25.0 and R v3.3.1.
Sample size calculation
To perform a logistic regression analysis, we needed at least 10 patients with outcome for each variable included in the model. 13 The expected primary outcome event rate at 3 months was estimated to be 10%. 3 In light of the above, to evaluate the predictors of the primary outcome event, it was calculated that 600 patients would have been needed, so no more than 6 variables were required for inclusion in the model to reach a sufficient level of confounding.
Results
A total of 627 patients with acute stroke and MHV were enrolled in the study and 24 patients were excluded from the analysis because anticoagulation therapy was considered contraindicated or not indicated: 16 had malignant middle cerebral artery syndromes, 5 early severe hemorrhagic transformations, 2 severe extracranial bleedings on admission and 1 severe pancytopenia. In Table S1 on Supplemental Material are reported the number of patients included in each participating center. After index acute ischemic stroke, 255 of 603 patients (41.3%) underwent bridging therapy (239 with LMWH and 16 with unfractionated heparin) (bridging group), 77 (12.8%) continued VKA and 271 (44.9%) interrupted VKA that was restarted subsequently. These last two groups together comprised the nonbridging group.
Clinical characteristics of the bridging and nonbridging groups
The bridging and nonbridging groups differed for sex, presence of AF, history of stroke, the percentage of small-sized lesions, revascularization therapy, and for NIHSS and INR ⩾ 2.5 on admission (Table 1).
Table 1.
Characteristics of the patients treated with or without bridging therapy.
| Bridging therapy N = 255 |
No bridging therapy N = 348 |
p | |
|---|---|---|---|
| Age (mean, years) | 68.9 ± 13.3 | 69.9 ± 12.0 | 0.2 |
| NIHSS on admission (mean, median) | 10.1 ± 7.4 9 (IQR 12) |
7.2 ± 6.5 5 (IQR 9) |
<0.0001 |
| Sex male | 156 (61.2%) | 181 (52.0%) | 0.02 |
| Diabetes Mellitus | 64 (25.1%) | 78 (22.4%) | 0.4 |
| Hypertension | 178 (69.8%) | 252 (72.4%) | 0.5 |
| Hyperlipidemia | 130 (51.0%) | 151 (43.4%) | 0.07 |
| Atrial fibrillation | 118 (46.3%) | 198 (56.9%) | 0.01 |
| History of stroke | 69 (27.1%) | 126 (36.2%) | 0.02 |
| Smoking (ongoing) | 53 (20.8%) | 59 (16.9%) | 0.2 |
| Alcoholism | 34 (13.3%) | 29 (8.3%) | 0.06 |
| Congestive heart failure | 52 (20.4%) | 75 (21.5%) | 0.7 |
| History of myocardial infarction | 46 (18.0%) | 62 (17.8%) | 1.0 |
| Peripheral artery disease | 31 (12.1%) | 32 (9.2%) | 0.3 |
| Pacemaker | 32 (12.5%) | 48 (13.8%) | 0.7 |
| Lesion size | 0.002 | ||
| Small | 96 (37.6%) | 181 (52.0%) | |
| Medium | 91 (35.7%) | 101 (29.0%) | |
| Large | 68 (26.7%) | 66 (19.0%) | |
| Hemorrhagic transformation (24–72 h) | 44 (17.2%) | 54 (15.5%) | 0.6 |
| Intracardiac thrombus | 19 (7.4%) | 17 (4.9%) | 0.2 |
| Mechanical mitral valve | 115 (45.1%) | 144 (41.4%) | 0.5 |
| Mechanical aortic valve | 114 (44.7%) | 172 (49.4%) | 0.3 |
| Mechanical mitral and aortic valves | 26 (10.2%) | 33 (9.5%) | 0.8 |
| rtPA | 43 (16.9%) | 29 (8.3%) | 0.002 |
| rtPA and/or mechanical thrombectomy | 97 (38.0%) | 59 (16.9%) | <0.0001 |
| Antiplatelet on admission | 85 (33.3%) | 94 (27.0%) | 0.1 |
| INR ⩾ 2.5 on admission | 45 (17.6%) | 106 (30.4%) | <0.0001 |
| Creatinine clearance | 77.2 ± 36.6 | 67.9 ± 25.4 | <0.0001 |
| Antiplatelet at discharge | 12 (4.7%) | 30 (8.6%) | 0.08 |
| Timing of anticoagulant therapy initiation (mean, days) | 3.3 ± 2.6 | 3.5 ± 7.6 | 0.6 |
IQR: interquartile range.
The treatment with anticoagulant was initiated after a mean of 3.3 ± 2.6 days in the bridging group compared to 3.7 ± 5.3 days in the nonbridging group (p = 0.6).
On admission, 85 of 255 patients (33.3%) in the bridging group were simultaneously taking an antiplatelet agent (either aspirin, 100–300 mg per day, or clopidogrel, 75 mg per day), whereas in the nonbridging group, patients under antiplatelet therapy were 94 of 348 (27.0%), being statistically similar (p = 0.1).
At multivariable analysis, NIHSS on admission (odds ratio [OR], 1.05 for each point increase; 95% CI, 1.01–1.08; p = 0.003), history of hyperlipidemia (OR 1.52; 95% CI 1.04–2.21; p = 0.03) and revascularization therapy with rtPA (OR 2.36; 95% CI, 1.53–3.65; p = 0.001) were correlated with the use of bridging therapy. Presence of AF (OR 0.65; 95% CI 0.44–0.96; p = 0.03) and INR ⩾ 2.5 on admission (OR 0.54; 95% CI 0.35–0.83; p = 0.005) were inversely correlated with the use of bridging therapy.
Outcomes in the bridging and nonbridging groups
Overall, 36 of 255 bridging patients (14.1%) had combined outcome, compared with 28 of 348 (8.0%) in the nonbridging group (OR 1.88; 95% CI 1.11–3.17; p = 0.02) (Table 2). Within the bridging group, 13 of 36 (5.1% of all patients) versus 12 of 28 (3.4% of all patients) in the nonbridging group had an ischemic outcome (OR 1.50; 95% CI 0.67–3.35; p = 0.4); specifically, 12 ischemic strokes and 1 systemic embolism in the bridging group and 10 ischemic strokes, 2 systemic embolisms in the nonbridging group. Major bleedings occurred in 23 of 36 patients (9.0% of all patients) in the bridging group and 16 of 28 (4.6% of all patients) in the nonbridging group (OR 2.06; 95% CI 1.06–3.98; p = 0.04); specifically, 11 symptomatic cerebral bleedings and 12 severe extracranial bleedings in the bridging group and 14 symptomatic cerebral bleedings and 2 severe extracranial bleedings in the nonbridging group.
Table 2.
Outcome events in patients treated with or without bridging therapy.
| Bridging therapy N = 255 |
No bridging therapy N = 348 |
Odds Ratio (95% CI) | p | |
|---|---|---|---|---|
| Combined outcome events | 36 (14.1%) | 28 (8.0%) | 1.88 (1.11–3.17) adj. 1.97 (1.12–3.47)* |
0.02 0.02 |
| Ischemic outcome events | 13 (5.1%) | 12 (3.4%) | 1.50 (0.67–3.35) adj. 1.68 (0.69–4.08)* |
0.4 0.2 |
| Hemorrhagic outcome events | 23 (9.0%) | 16 (4.6%) | 2.06 (1.06–3.98) adj. 2.05 (1.02–4.13)* |
0.04 0.04 |
| Mortality | 24 (9.4%) | 23 (6.6%) | 1.46 (0.80–2.66) adj. 1.34 (0.70–2.56)** |
0.2 0.3 |
| Ischemic stroke | 12 (4.7%) | 10 (2.9%) | 1.67 (0.71–3.93) | 0.2 |
| Systemic embolism | 1 (0.4%) | 2 (0.6%) | 0.68 (0.06–7.55) | 0.7 |
| Symptomatic intracerebral bleeding | 11 (4.3%) | 14 (4.0%) | 1.08 (0.48–2.41) | 0.8 |
| Severe extracranial bleeding | 12 (4.7%) | 2 (0.6%) | 8.54 (1.89–38.52) | 0.0008 |
adj = adjusted.
Adjusted for age, lesion size, history of stroke, clearance of creatinine and timing of initiation of anticoagulant therapy.
Adjusted for age, sex, history of hypertension, atrial fibrillation, congestive heart failure, lesion size and intracardiac thrombus.
Table 3 summarizes the characteristics of the patients with and without combined outcome events.
Table 3.
Characteristics of the patients having or not combined outcome event.
| Combined outcome event N = 64 |
No outcome event N = 539 |
p | |
|---|---|---|---|
| Age (mean, years) | 72.4 ± 11.6 | 69.2 ± 12.7 | 0.05 |
| NIHSS on admission (mean) | 13.3 ± 7.1 | 7.9 ± 6.8 | <0.0001 |
| Sex male | 39 (60.9%) | 297 (55.1%) | 0.4 |
| Diabetes Mellitus | 20 (31.2%) | 122 (22.6%) | 0.2 |
| Hypertension | 49 (76.6%) | 380 (70.5%) | 0.3 |
| Hyperlipidemia | 27 (42.2%) | 254 (47.1%) | 0.5 |
| Atrial fibrillation | 38 (59.4%) | 278 (51.6%) | 0.3 |
| History of stroke | 30 (46.9%) | 165 (30.6%) | 0.01 |
| Smoking (ongoing) | 16 (25.0%) | 95 (17.6%) | 0.2 |
| Alcoholism | 8 (12.5%) | 55 (10.2%) | 0.5 |
| Congestive heart failure | 19 (29.7%) | 108 (20.0%) | 0.1 |
| History of myocardial infarction | 16 (25.0%) | 92 (17.1%) | 0.1 |
| Peripheral artery disease | 11 (17.2%) | 51 (9.5%) | 0.07 |
| Pacemaker | 14 (21.9%) | 66 (12.2%) | 0.04 |
| Lesion size | <0.0001 | ||
| Small | 12 (18.8%) | 266 (49.4%) | |
| Medium | 24 (37.5%) | 168 (31.1%) | |
| Large | 28 (43.7%) | 105 (19.5%) | |
| Leukoaraiosis | 38 (59.4%) | 283 (52.5%) | 0.3 |
| Intracardiac thrombus | 6 (9.4%) | 30 (5.6%) | 0.2 |
| rtPA | 10 (15.6%) | 62 (11.5%) | 0.3 |
| rtPA and/or mechanical thrombectomy | 20 (31.2%) | 135 (25.0%) | 0.3 |
| INR⩾2.5 | 18 (28.1%) | 133 (24.7%) | 0.3 |
| Timing of anticoagulant therapy initiation (mean, days) | 5.6 ± 14.5 | 3.2 ± 3.9 | 0.003 |
From multivariable analysis, bridging therapy was associated with combined outcome (OR 1.97; 95% CI, 1.12–3.47) and hemorrhagic events (OR 2.05; 95% CI 1.02–4.13) but not with ischemic event (OR 1.50; 95% CI 0.67–3.35).
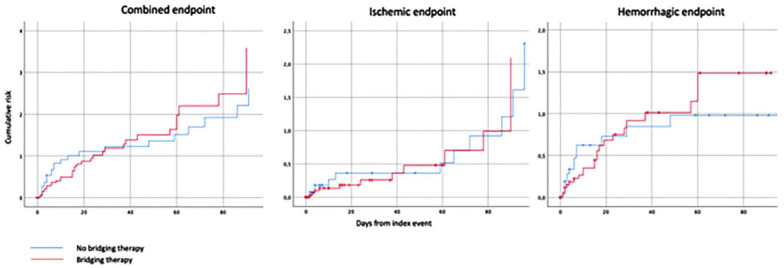
The Kaplan-Meier curves that compared the overall outcome events in those patients with and without bridging therapy are shown in Figure 1.
Figure 1.
Cumulative risk of combined, ischemic or hemorrhagic endpoint events in patients receiving or not bridging therapy.
Excluding the 77 patients from the nonbridging group who had continued VKA therapy (Table S2 on Supplemental Material), the rates of outcome events in the remaining 271 patients in the nonbridging group who had their VKA therapy interrupted were the following: combined outcome events 23 (8.5%), hemorrhagic outcome events 13 (4.8%) and ischemic outcome events 10 (3.9%) (Table S3 on Supplemental Material). From multivariable analysis, the bridging therapy group (255 patients) compared with the nonbridging therapy group where the patients had interrupted their VKA therapy (271 patients), was associated with combined outcome (adjusted OR 1.89; 95% CI 1.03–3.47, p = 0.03) and hemorrhagic events (adjusted OR 2.17; 95% CI 1.03–4.60, p = 0.04).
After PSM, the 254 patients who did not have bridging therapy were compared to the 254 who did. In Table S4 on Supplemental Material, the characteristics of the patients after PSM are reported. Matching allowed to compare similar rates of cardiovascular risk factors across the cohorts, as well as mitigation of the differences in stroke severity and hyperacute treatment, of which the latter two variables were higher among patients who had underwent bridging. Regarding outcome events, 36 (14.2%) bridging patients had a combined outcome event, compared with 23 (9.1%) nonbridging patients (OR 1.66 [95% CI, 0.95–2.85]; p = 0.07). In the bridging group, 13 of 36 (5.1% of all patients) compared to 10 of 23 (4.0% of all patients) in the nonbridging group, had an ischemic outcome (OR 1.31; 95% CI 0.56–3.03; p = 0.5). Major bleedings occurred in 13 out of 23 patients (5.1% of all patients) in the nonbridging group and 23 out of 36 (9.1% of all patients) in the bridging group (OR 1.85; 95% CI 0.91–3.70; p = 0.08).
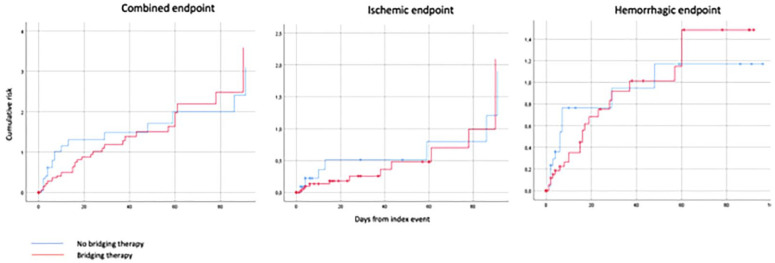
The Kaplan-Meier curves that compared the combined outcome events (ischemic stroke, systemic embolism, intracranial hemorrhage, and major extracranial bleeding) between the two treatment groups after PSM are reported in Figure 2.
Figure 2.
Cumulative risk of combined, ischemic or hemorrhagic endpoint events in patients receiving or not bridging therapy after Propensity Score Matching.
As 30.4% of patients in the nonbridging group and 17.6% in the bridging group had INR ⩾ 2.5 on admission, we performed a sensitivity analysis with the aim of testing the robustness of the results by restricting the cohort to patients with INR < 2.5 on admission when bridging therapy would have been more appropriate. Overall, 27 of the 210 patients (12.8%) in the bridging group had combined outcomes, compared to 18 of the 242 patients (7.3%) in the nonbridging group (OR 1.84; 95% CI 0.98–3.44, p = 0.059). After adjustment for age and sex, bridging therapy was associated with combined outcome events (OR 1.88; 95% CI 1.00–3.54, p = 0.05).
At 90 days, 110 (43.1%) patients treated with bridging therapy were deceased or disabled (mRS score ⩾3); of those, 24 (9.4%) were deceased while 138 (39.6%) patients treated with nonbridging therapy were deceased or disabled; of those 23 (6.6%) were deceased (p = 0.2 and p = 0.3, respectively). Variables associated with mortality or disability were age (OR 1.04 for each year increase; 95% CI 1.02–1.06; p = 0.0001), presence of AF (OR 1.57; 95% CI 1.06–2.33; p = 0.02), history of stroke (OR 1.90; 95% CI 1.26–2.86; p = 0.002), history of congestive heart failure (OR 1.73; 95% CI 1.08–2.78; p = 0.02). Small lesion size was inversely associated with mortality or disability (OR 0.20; 95% CI 0.13–0.30; p = 0.0001) while bridging therapy was not associated with this outcome (OR 1.11; 95% CI 0.75–1.65; p = 0.5).
Bridging and nonbridging therapy and the risk of recurrent ischemic events or bleedings associated with the day of initiating anticoagulant treatment
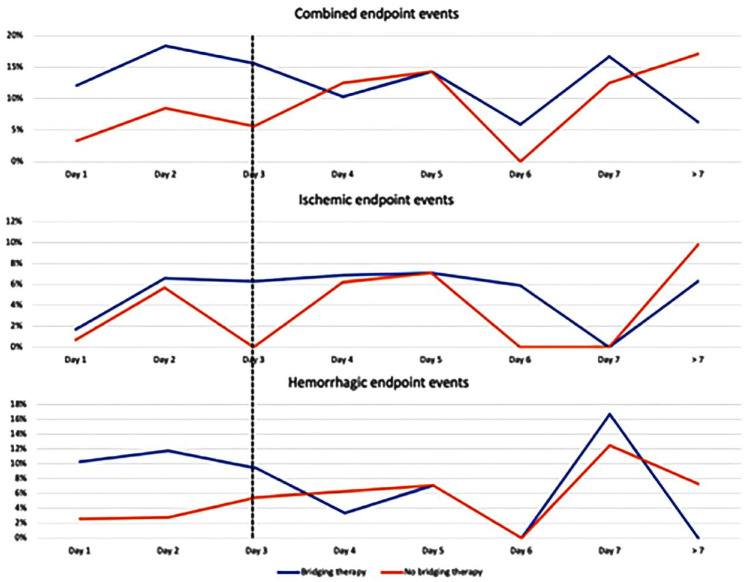
The graphs in Figure 3 show the different risks associated with the day of initiating anticoagulant treatment in patients with or without bridging therapy for ischemic and hemorrhagic outcome events. The results suggest that patients treated with bridging therapy had an increased risk of major bleeding, compared to patients treated without bridging therapy, especially when anticoagulant treatment was initiated within the first 3 days from index event. In fact, 18 (10.8%) out of 166 patients treated with bridging therapy, within 3 days from index stroke, had a hemorrhagic outcome compared to 7 (2.9%) out of 240 patients treated without bridging therapy (OR 4.05; 95% CI 1.65–9.93; p = 0.002). In addition, 8 (4.8%) out of the 166 patients treated with bridging therapy, within 3 days from index stroke, had an ischemic outcome compared to 5 (2.1%) out of the 240 patients treated without bridging therapy (OR 2.38; 95% CI 0.76–7.41; p = 0.1).
Figure 3.
Cumulative risk of combined, ischemic or hemorrhagic endpoint events stratified according to timing of anticoagulant therapy initiation in patients receiving or not bridging therapy.
Discussion
The ESTREM study found that the use of bridging therapy is common among patients with MHV and acute ischemic stroke and may be associated with bleeding risk. Specifically, the risk of bleeding was particularly high when therapy with heparins was initiated early, within the first 3 days from index stroke. In agreement with our data, previous randomized studies included in a meta-analysis had reported that early anticoagulation initiated within 48 h with heparins in patients with cardioembolic stroke was associated with a non-significant reduction in the recurrence of ischemic stroke. However, these studies observed increased intracranial bleeding rates. It should be noted that in these studies the majority of the patients had AF. 3 Another difference, was that in our study, bleedings were located for the most part extracranially while the rates of symptomatic intracranial bleeding were similar between the bridging and no bridging groups. The mechanism as to why bridging therapy was associated, especially with an increased risk of extracranial severe bleedings is not well understood. But other studies, investigating other types of patients reported that early bridging therapy was associated with an overall increase in severe bleedings: specifically, in patients with acute ischemic stroke and AF and also in patients with MHV who had their VKA interrupted for invasive surgical or diagnostic procedures.1,2,14 Whereas, in the abovementioned meta-analysis of randomized controlled clinical trials, the rate of severe extracranial bleeding was not reported. Albeit, the results are in agreement: an early use of heparins was associated with a high risk of bleeding events. Recent results from randomized controlled trials in patients with AF and stroke have reported a very low rates of intracranial and extracranial bleedings in patients started on direct oral anticoagulants early after stroke; which is in contrast to the findings presented in this study.15,16-. This may have been due to the differences between Vitamin K antagonists and direct oral anticoagulants; the latter had not been suggested to be effective and safe in patients with MHV. 17
In our study, following PSM, the observed significant difference in combined outcome events between the two groups emerging in the multivariable model was reduced to a marginally significant trend, most likely due to the reduction in the sample size along with the decrease in the number of outcome events recorded. Bridging therapy with heparin is initiated for sub-acute ischemic stroke, as it is thought to reduce the risk of ischemic recurrence possibly due to a presumed prothrombotic activity attributed to warfarin at treatment initiation if this latter treatment had been interrupted before and re-initiated. 18 However, reliable data on the role of warfarin in blocking endogenous anticoagulants has not been demonstrated. Therein, it is plausible that warfarin alone might be more effective and safer than bridging therapy with heparin in the subacute phase of stroke in reducing recurrence.
In the ESTREM study, patients treated with bridging therapy had more severe strokes on admission and larger-sized lesions compared to patients treated without bridging therapy. Specifically, more than 62% of the patients treated with bridging therapy had medium or large-sized lesions compared to 48% of the patients had not received bridging therapy. We hypothesize that clinicians might have preferred to continue VKA therapy without interruption in less severe patients. Another probable reason that might have lead clinicians to treat patients without bridging therapy and continuing VKAs, might have been the patient’s INR values on admission. In fact, about 30% of the patients without bridging therapy had an INR ⩾ 2.5 on admission compared to about 17% of the patients treated with bridging therapy.
The ESTREM study had several limitations. First, the reported associations in our nonrandomized study were undoubtedly influenced by numerous potential confounders, even though they were adjusted using statistical models. In fact, the two groups differed for several vascular risk factors, stroke severity and lesion size. Second, both central adjudication of the outcome events and centralization of vascular imaging for measurement of the ischemic lesions were not performed. Third, a possible bias in the ascertainment of recurrent strokes versus asymptomatic intracranial hemorrhage depending on antithrombotic status could have been present, given the absence of blinding. Fourth, the study included patients derived from several registries and these registries could have differed in their designs, quality assurances, recruitment strategies, care settings, geographic distributions and their follow-ups. Interpretations of data from these registries must take into account the impact of recall bias and survivorship bias that can be incurred with enrollment. 19 Fifth, the original protocol for this study specified that all of the patients had to be included consecutively and that all centers had to follow guidelines for the management of the patients. We have no objective data to confirm that this was done. However, patients were enrolled from Stroke Units with high expertise on the management of patients with stroke. Finally, we did not collect data on the type of MHV. Several types of MHV exist having different risks of thromboembolism requiring different therapeutic ranges of INR.
The strengths of our study included its adequate sample size and its findings that reflect real-life experiences. Regarding the latter, in view of the complete absence of any randomized data, these findings may provide observational information that could assist stroke physicians in better managing acute cerebral ischemic patients with MHV.
In conclusion, acute ischemic stroke patients with MHV in the bridging therapy group had marginally higher risks of ischemic or hemorrhagic events, compared to the nonbridging therapy group. These results require cautious interpretation because of the substantial potential for confounding.
Supplemental Material
Supplemental material, sj-pdf-1-eso-10.1177_23969873231186863 for Anticoagulation in acute ischemic stroke patients with mechanical heart valves: To bridge or not with heparin. The ESTREM study by Maurizio Paciaroni, Valeria Caso, Michele Romoli, Cecilia Becattini, Alexander Salerno, Costanza Rapillo, Fanny Simonnet, Davide Strambo, Isabella Canavero, Marialuisa Zedde, Rosario Pascarella, Sung-Il Sohn, Simona Sacco, Raffaele Ornello, Kristian Barlinn, Daniela Schoene, Jan Rahmig, Maria Giulia Mosconi, Ilaria Leone De Magistris, Andrea Alberti, Michele Venti, Giorgio Silvestrelli, Alfonso Ciccone, Marina Padroni, Michele Laudisi, Andrea Zini, Luana Gentile, Odysseas Kargiotis, Georgios Tsivgoulis, Rossana Tassi, Francesca Guideri, Maurizio Acampa, Luca Masotti, Elisa Grifoni, Alessandro Rocco, Marina Diomedi, Theodore Karapanayiotides, Stefan T Engelter, Alexandros A Polymeris, Annaelle Zietz, Fabio Bandini, Pietro Caliandro, Giuseppe Reale, Marco Moci, Aurelia Zauli, Manuel Cappellari, Andrea Emiliani, Antonio Gasparro, Valeria Terruso, Marina Mannino, Elisa Giorli, Danilo Toni, Marco Andrighetti, Anne Falcou, Lina Palaiodimou, George Ntaios, Dimitrios Sagris, Efstathia Karagkiozi, Anastasia Adamou, Panagiotis Halvatsiotis, Yuriy Flomin, Umberto Scoditti, Antonio Genovese, Nemanja Popovic, Leonardo Pantoni, Francesco Mele, Nicola Molitierno, Piergiorgio Lochner, Alessandro Pezzini, Massimo Del Sette, Davide Sassos, Sotirios Giannopoulos, Maria Kosmidou, Evangelos Ntais, Enrico Maria Lotti, Vincenzo Mastrangelo, Alberto Chiti, Andrea Naldi, Peter Vanacker, Mario Ferrante, Vera Volodina, Michelangelo Mancuso, Nicola Giannini, Marco Baldini, Kostantinos Vadikolias, Sofia Kitmeridou, Carlo Emanuele Saggese, Tiziana Tassinari, Valentina Saia and Patrik Michel in European Stroke Journal
Acknowledgments
None
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paciaroni received honoraria as a member of the speaker bureau of Sanofi-Aventis, Bristol Meyer Squibb, Daiiki Sankyo and Pfizer. Becattini received honoraria as a member of the speaker bureau of Bristol Meyer Squibb and Bayer. Michel received research grant by Swiss National Science Foundation, Swiss Heart Foundation and University of Lausanne. Tsivgoulis has received funding for travel or speaker’s honoraria from Bayer, Pfizer, and Boehringer Ingelheim. He has served on scientific advisory boards for Bayer, Boehringer Ingelheim, and Daiichi Sankyo. Toni has received personal fees from Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, and Pfizer. Caso received honoraria as a member of the speaker bureau of Boehringer Ingelheim, Bayer, and Daiichi Sankyo (all fees were paid to Associazione Ricerca Stroke, Umbria). She received honoraria as consultant or advisory board member of Boehringer Ingelheim, Bayer, Daiichi Sankyo, and Pfizer. Ntaios reports speaker fees/advisory board/ research support from Bayer, Pfizer, Boehringer Ingelheim and Elpen. All fees are paid to his institution. Sacco has received personal fees as speaker or advisor from Abbott, Allergan, Astra Zeneca, Eli Lilly, Lundbeck, Novartis, NovoNordisk, Teva and research grants from Allergan, Novartis, and Uriach. Vanacker received honoraria as a member of the speaker bureau and as advisory board of Boehringer Ingelheim, Bristol Meyer Squibb, Daiichi Sankyo, Medtronic, EG and Pfizer. Del Sette has received honoraria for speaking from Bayer and Boehringer Ingelheim. Zedde received speaking and consulting fees from Daiichi Sankyo, Amicus Therapeutics, Sanofi Genzyme, Abbott, and Takeda. Cappellari has received consulting fees from Boehringer Ingelheim, Pfizer – Bristol Meyer Squibb, and Daiichi Sankyo. Flomin has received personal fees from Boehringer Ingelheim, Bayer and Takeda, grants, personal fees and nonfinancial support from Pfizer, personal fees and nonfinancial support from Sanofi Genzyme. Ornello has received nonfinancial support from Novartis, Allergan, and Teva. Giannopoulos has received funding for travel from Bayer and speaker’s honoraria from Pfizer. Zini has received consulting fees from Boehringer Ingelheim, CSL Behing and Alexion, Astra Zeneca. The other authors report no conflicts.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent had been obtained according to local requirements.
Ethical approval: The studies had been approved by the pertinent institutional review boards if required.
Guarantor: Maurizio Paciaroni
Contributoship: Maurizio Paciaroni conceived the study and wrote the first draft of the manuscript. All Investigators were involved in protocol development, gaining ethical approval, patient recruitment and data analysis. All Investigators reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Maurizio Paciaroni  https://orcid.org/0000-0002-5483-8795
https://orcid.org/0000-0002-5483-8795
Michele Romoli  https://orcid.org/0000-0001-8009-8543
https://orcid.org/0000-0001-8009-8543
Alexander Salerno  https://orcid.org/0000-0001-8494-5527
https://orcid.org/0000-0001-8494-5527
Marialuisa Zedde  https://orcid.org/0000-0001-7530-818X
https://orcid.org/0000-0001-7530-818X
Simona Sacco  https://orcid.org/0000-0003-0651-1939
https://orcid.org/0000-0003-0651-1939
Maria Giulia Mosconi  https://orcid.org/0000-0002-0456-9160
https://orcid.org/0000-0002-0456-9160
Luana Gentile  https://orcid.org/0000-0002-0311-0630
https://orcid.org/0000-0002-0311-0630
Odysseas Kargiotis  https://orcid.org/0000-0002-8317-6428
https://orcid.org/0000-0002-8317-6428
Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Rossana Tassi  https://orcid.org/0000-0002-5906-8718
https://orcid.org/0000-0002-5906-8718
Maurizio Acampa  https://orcid.org/0000-0003-4149-1785
https://orcid.org/0000-0003-4149-1785
Elisa Grifoni  https://orcid.org/0000-0002-7889-6367
https://orcid.org/0000-0002-7889-6367
Alessandro Rocco  https://orcid.org/0000-0002-8121-3774
https://orcid.org/0000-0002-8121-3774
Alexandros A. Polymeris  https://orcid.org/0000-0002-9475-2208
https://orcid.org/0000-0002-9475-2208
Annaelle Zietz  https://orcid.org/0000-0002-4362-2497
https://orcid.org/0000-0002-4362-2497
Pietro Caliandro  https://orcid.org/0000-0002-1190-4879
https://orcid.org/0000-0002-1190-4879
Marco Moci  https://orcid.org/0000-0002-7893-1660
https://orcid.org/0000-0002-7893-1660
Marina Mannino  https://orcid.org/0000-0001-7683-7235
https://orcid.org/0000-0001-7683-7235
Marco Andrighetti  https://orcid.org/0000-0003-2426-2075
https://orcid.org/0000-0003-2426-2075
Lina Palaiodimou  https://orcid.org/0000-0001-7757-609X
https://orcid.org/0000-0001-7757-609X
George Ntaios  https://orcid.org/0000-0002-0629-9248
https://orcid.org/0000-0002-0629-9248
Anastasia Adamou  https://orcid.org/0000-0003-4701-0858
https://orcid.org/0000-0003-4701-0858
Yuriy Flomin  https://orcid.org/0000-0002-7123-3659
https://orcid.org/0000-0002-7123-3659
Antonio Genovese  https://orcid.org/0000-0003-0490-187X
https://orcid.org/0000-0003-0490-187X
Leonardo Pantoni  https://orcid.org/0000-0001-7357-8530
https://orcid.org/0000-0001-7357-8530
Nicola Molitierno  https://orcid.org/0009-0003-9189-4475
https://orcid.org/0009-0003-9189-4475
Alessandro Pezzini  https://orcid.org/0000-0001-8629-3315
https://orcid.org/0000-0001-8629-3315
Sotirios Giannopoulos  https://orcid.org/0000-0001-7443-5179
https://orcid.org/0000-0001-7443-5179
Evangelos Ntais  https://orcid.org/0000-0001-6739-6108
https://orcid.org/0000-0001-6739-6108
Andrea Naldi  https://orcid.org/0000-0001-7323-0286
https://orcid.org/0000-0001-7323-0286
Valentina Saia  https://orcid.org/0000-0001-9855-8894
https://orcid.org/0000-0001-9855-8894
Supplemental material: Supplemental material for this article is available online.
References
- 1. Altavilla R, Caso V, Bandini F, et al. Anticoagulation after stroke in patients with atrial fibrillation. Stroke 2019; 50: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 2. Yaghi S, Mistry E, Liberman AL, et al. Anticoagulation type and early recurrence in cardioembolic stroke. Stroke 2020; 51: 2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paciaroni M, Agnelli G, Micheli S, et al. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke 2007; 38: 423–430. [DOI] [PubMed] [Google Scholar]
- 4. Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol 1993; 14: 3–13. [PMC free article] [PubMed] [Google Scholar]
- 5. Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008; 39: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 6. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 7. Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 8. Tatu L, Moulin T, Bogousslavsky J, et al. Arterial territories of the human brain: cerebral hemispheres. Neurology 1998; 50: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 9. Tatu L, Moulin T, Bogousslavsky J, et al. Arterial territories of human brain: brainstem and cerebellum. Neurology 1996; 47: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 10. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing. Stroke 2015; 46: 2175–2182. [DOI] [PubMed] [Google Scholar]
- 11. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141: e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 13. Reboldi G, Angeli F, Verdecchia P. Multivariable analysis in cerebrovascular research: practical notes for the clinician. Cerebrovasc Dis 2013; 35: 187–193. [DOI] [PubMed] [Google Scholar]
- 14. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer U, Koga M, Strbian D, et al.; ELAN Investigators. Early versus later anticoagulation for stroke with atrial fibrillation. N Engl J Med 2023; 388: 2411–2421. [DOI] [PubMed] [Google Scholar]
- 16. Oldgren J, Åsberg S, Hijazi Z, et al. Early versus delayed non-vitamin K antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): A registry-based randomized controlled noninferiority study. Circ 2022; 146: 1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369: 1206–1214. [DOI] [PubMed] [Google Scholar]
- 18. Freedman MD. Oral anticoagulants: pharmacodynamics, clinical indications and adverse effects. J Clin Pharmacol 1992; 32: 196–209. [DOI] [PubMed] [Google Scholar]
- 19. Fox KAA, Accetta G, Pieper KS, et al.; GARFIELD-AF Investigators. Why are outcomes different for registry patients enrolled prospectively and retrospectively? Insights from the global anticoagulant registry in the FIELD-Atrial fibrillation (GARFIELD-AF). Eur Heart J Qual Care Clin Outcomes 2018; 4: 27–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-eso-10.1177_23969873231186863 for Anticoagulation in acute ischemic stroke patients with mechanical heart valves: To bridge or not with heparin. The ESTREM study by Maurizio Paciaroni, Valeria Caso, Michele Romoli, Cecilia Becattini, Alexander Salerno, Costanza Rapillo, Fanny Simonnet, Davide Strambo, Isabella Canavero, Marialuisa Zedde, Rosario Pascarella, Sung-Il Sohn, Simona Sacco, Raffaele Ornello, Kristian Barlinn, Daniela Schoene, Jan Rahmig, Maria Giulia Mosconi, Ilaria Leone De Magistris, Andrea Alberti, Michele Venti, Giorgio Silvestrelli, Alfonso Ciccone, Marina Padroni, Michele Laudisi, Andrea Zini, Luana Gentile, Odysseas Kargiotis, Georgios Tsivgoulis, Rossana Tassi, Francesca Guideri, Maurizio Acampa, Luca Masotti, Elisa Grifoni, Alessandro Rocco, Marina Diomedi, Theodore Karapanayiotides, Stefan T Engelter, Alexandros A Polymeris, Annaelle Zietz, Fabio Bandini, Pietro Caliandro, Giuseppe Reale, Marco Moci, Aurelia Zauli, Manuel Cappellari, Andrea Emiliani, Antonio Gasparro, Valeria Terruso, Marina Mannino, Elisa Giorli, Danilo Toni, Marco Andrighetti, Anne Falcou, Lina Palaiodimou, George Ntaios, Dimitrios Sagris, Efstathia Karagkiozi, Anastasia Adamou, Panagiotis Halvatsiotis, Yuriy Flomin, Umberto Scoditti, Antonio Genovese, Nemanja Popovic, Leonardo Pantoni, Francesco Mele, Nicola Molitierno, Piergiorgio Lochner, Alessandro Pezzini, Massimo Del Sette, Davide Sassos, Sotirios Giannopoulos, Maria Kosmidou, Evangelos Ntais, Enrico Maria Lotti, Vincenzo Mastrangelo, Alberto Chiti, Andrea Naldi, Peter Vanacker, Mario Ferrante, Vera Volodina, Michelangelo Mancuso, Nicola Giannini, Marco Baldini, Kostantinos Vadikolias, Sofia Kitmeridou, Carlo Emanuele Saggese, Tiziana Tassinari, Valentina Saia and Patrik Michel in European Stroke Journal