Introduction:
Endovascular repair (EVR) is the most common method of repair for patients with abdominal aortic aneurysms in the United States. In 2013, nearly 80% of Medicare patients treated for abdominal aortic aneurysms underwent EVR.1 EVR is associated with lower perioperative morbidity, mortality, and a shorter hospital stay when compared to open surgical repair. The procedure has disseminated widely, is performed in both community and tertiary settings by interventional cardiologists and radiologists, and cardiac and vascular surgeons.2-5
However, the primary tradeoff between EVR and open surgery is the durability of the repair. EVR is associated with higher rates of reintervention and late aneurysm rupture than open surgical repair.2, 3 In an effort to decrease these events, lifelong annual imaging surveillance after EVR is recommended by the United States Food and Drug Administration, the National Institute for Health and Care Excellence (NICE), the Society for Vascular Surgery, the American College of Cardiology, and the American Heart Association.6-9 Despite these recommendations, fewer than half of EVR patients receive long-term surveillance, and late aneurysm rupture may follow surveillance failure.10-12 Improving surveillance for patients at the highest risk for reintervention and late rupture remains a priority, but this effort faces a key limitation: Determining which patients are at greatest risk for reintervention remains uncertain.6, 7, 13 Published reports describing factors associated with reintervention have been limited in scope, contain few relevant clinical factors, and most importantly lack long-term follow-up.2, 11, 13-15
To address these gaps, we studied a large national cohort of patients treated with EVR in the Vascular Quality Initiative registry linked to Medicare claims, and assessed long-term rates of reintervention using a clinically validated claims-based algorithm.16 By studying national practice patterns in a clinically detailed registry with 100% claims-based follow-up using a clinically validated algorithm, we aimed to better understand the actual rates of reintervention for patients treated with EVR over the course of their remaining lifetime, and identify clinical factors associated with reintervention. This would allow for reintervention risk stratified post-EVR surveillance for patients undergoing abdominal aortic aneurysm repair.
Methods:
Data sources and cohort creation
We studied data from the Vascular Quality Initiative, a national quality improvement registry that captures granular clinical information on demographics, comorbid conditions, and procedural details for vascular procedures performed at participating hospitals and vascular centers across the United States. We linked patients in the registry directly at the patient level to their respective Medicare claims file for long-term outcome assessment with 95.0% matching success.17 Procedural and diagnostic data from Medicare were available from January 1st, 2003 until September 30th, 2015, and billing codes were extracted using validated algorithms.16, 17
Inclusion and exclusion criteria
All patients in the linked registry-claims database who underwent EVR, including both elective procedures and urgent or emergent procedures for symptomatic or ruptured aortic aneurysms were eligible for inclusion in the study (n=13,994). We excluded patients with missing information on baseline clinical and procedural characteristics when that variable was missing for <1.0% of the total cohort (n=1,083). We found no meaningful difference in the rate of reintervention between the two cohorts. We therefore report the rate of reintervention for the cohort where baseline characteristics were known to allow for a consistent cohort across analyses and regression models. Characteristics missing in ≥1.0% of the total cohort are described with a denominator.
Outcomes and definitions
Our primary exposure of interest was EVR. All patients in the cohort had the exposure. Patients who received more than one procedure, such as EVR converted to open repair on the same day, were assigned according to the first procedure they underwent.
Our primary outcome of interest was reintervention. We defined reintervention as any repeat procedure related to the aneurysm or an aneurysm repair related complication after discharge from the initial EVR hospitalization that occurred in the inpatient setting. We identified the outcome using a clinically validated International Classification of Diseases, Ninth Revision (ICD-9) coding algorithm demonstrated to have 92.0% sensitivity and 96.0% specificity (Supplementary Appendix, Supplementary Table 1).16 Reinterventions included procedures such as femoral to femoral artery bypass for EVR graft limb occlusion, and catheter-based coiling of persistent aneurysm side-branch bleeding (such as a Type II endoleak, as outlined in the Supplementary Appendix).16
Secondary outcomes included late aneurysm rupture and all-cause mortality. We defined late aneurysm rupture in Medicare claims as any billing event for abdominal aortic aneurysm rupture that was associated with a reintervention or death within a 14-day window of the admission encounter. We defined all-cause mortality as any death event identified within the Medicare Denominator file during the study interval. Surviving patients were censored at the end of the study period (September 30th, 2015), the most recent Medicare data available for use with our ICD-9 claims-based algorithm.
Statistical analysis
In our descriptive analyses, we report continuous variables as means with standard deviations and categorical variables as percentages. Denominators are reported when missing data were present. The increase in centers participating in the Vascular Quality Initiative also means that more patients are entered into the registry in later years of the study. Therefore, we choose Kaplan-Meier estimation to allow us to handle censoring associated with this phenomenon.
To identify factors associated with reintervention, we created a classification and regression tree (CART) informed Cox-proportional hazard regression model. CART is an algorithm adapted to time-to-event data that is helpful for predictive modeling.18 Its advantage lies in identifying associations of any form between predictors, or functions of multiple predictors (e.g., interaction effects), and the primary outcome. Unlike standard regression models, such associations are not constrained to linear or other parametrically-specified relationships. We used CART to suggest the clinical factors and their interactions involving them likely to be strongly associated with reintervention based on their presence in splits in the regression tree. We then included the CART identified variables and their interaction terms to specify a Cox-proportional hazard regression model of reintervention after EVR with the hospital center included as a random-effect.
We conducted the bundled CART followed by Cox regression analyses using a derivation and validation cohort. We randomly selected 8,616 patients (two-thirds of the study population) for the derivation cohort. We then used the CART algorithm adapted to time-to-event data to generate a predictive model for reintervention. We included all variables in the model for which complete data were present on all 12,911 patients. Next, we applied the predictive model to the validation cohort of the remaining 4,295 patients. We found no meaningful difference in the outcome or the predictor variables between the two cohorts. We performed a sensitivity analysis of the CART model that included variables for which there were missing data. We did not identify any new variables that were more strongly associated with reintervention than those reported in the original model. Therefore, we report the results of the predictive model using the entire cohort of 12,911 patients. Individual results for the derivation and validation cohorts are available as Supplementary Figures 1 and 2 respectively. Statistical analyses were performed with Stata version 15 and R version 3.3.2 software.
Human subjects protection
All data are collected under the auspices of an Agency for Healthcare Research and Quality designated Patient Safety Organization and were de-identified. Our study was approved by the Center for the Protection of Human Subjects at Dartmouth. This study was conducted in accordance with the STROBE reporting guidelines for observational studies.19 To maintain patient confidentiality, the Center for Medicare and Medicaid services requires that any reported estimates with less than 11 observations at risk at the beginning of the study period, at the end of the study period, and failing over the study interval be suppressed. In compliance with this, we have suppressed estimates where this requirement was not met and report shorter Kaplan-Meier estimates as necessary. Complete follow-up was used for all regression modeling.
While summary results are presented herein, patient-level datasets were not made publicly available given restrictions in data sharing outlined in our Data Use Agreement with the Centers for Medicare and Medicaid Services (CMS DUA #23789).
Results:
Patients
We studied 12,911 patients across 168 centers in the United States who underwent EVR between 2003 and 2015. The mean age among the cohort was 75.5 ±7.3 years, 79.9% of patients were male, and 93.0% of patients were of white race (Table 1). Comorbid conditions were common. We found that 28.3% of patients were actively smoking at the time of their index operation, and 25.2% of patients had previously undergone coronary artery revascularization. Aspirin was taken by 65.2% of the cohort, and 68.7% of patients were on a statin at the time of their index operation.
Table 1:
Baseline characteristics of the 12,911 patients.*
| Variable | % (n) | |
|---|---|---|
| Demographics | ||
| Age, mean (SD), years | 75.5 (7.3) | |
| Male | 79.9 (10,310) | |
| Race | ||
| White | 93.0 (12,012) | |
| Black | 3.9 (503) | |
| Other | 3.1 (396) | |
| Comorbid conditions | ||
| Hypertension | 84.0 (10,841) | |
| Smoking history | ||
| Never | 15.2 (1,957) | |
| Former | 56.5 (7,294) | |
| Current | 28.3 (3,660) | |
| Positive stress rest | 10.0 (1,285) | |
| Coronary disease | ||
| History of MI | 22.5 (2,907) | |
| Stable angina | 6.1 (783) | |
| UA or MI <6 months | 1.4 (177) | |
| Prior coronary revascularization | ||
| <5 years prior to surgery | 13.1 (1,694) | |
| ≥5 years prior to surgery | 22.1 (2,856) | |
| Heart failure | ||
| Asymptomatic | 7.0 (903) | |
| Mild | 3.4 (442) | |
| Moderate / Severe | 1.5 (194) | |
| Diabetes | ||
| Diet controlled | 4.8 (613) | |
| Oral medications | 12.2 (1,576) | |
| Insulin | 3.4 (439) | |
| COPD | ||
| Untreated | 10.4 (1,347) | |
| Treated with medications | 17.4 (2,250) | |
| Continuous oxygen | 5.1 (654) | |
| Renal insufficiency | 8.2 (1,053) | |
| BMI (SD), kg/m2 | 27.7 (5.6) | |
| Obesity | 28.8 (3,718) | |
| Preop hemoglobin, mean (SD), g/dL | 13.4 (2.6; 12,314 / 12,911) | |
| Preoperative medications | ||
| Antiplatelet therapy | ||
| Aspirin | 65.2 (8,412) | |
| P2y12 inhibitor | 11.3 (1,458) | |
| Beta-blocker | 60.9 (7,868) | |
| Statin | 68.7 (8,868) | |
Denominators are given when less than 12,911
Legend: SD, standard deviation; MI, myocardial infarction; UA, unstable angina; BMI, body mass index; kg/m2, kilograms per meter squared; g/dL, grams per deciliter.
Detailed operative characteristics were available for the majority of the cohort (Table 2). We found that 89.1% of patients underwent EVR in an elective setting, while the remaining patients presented with a symptomatic (7.3%) or ruptured (3.6%) aortic aneurysm. The size of the aneurysm at the time of the index operation varied, with 15.8% of patients undergoing EVR with an aneurysm <5.0 centimeters, and 30.3% of patients having an aneurysm of 6.0 centimeters or more. Nearly one in four patients had a concomitant iliac artery aneurysm of 2.0 or more centimeters in diameter (23.3%). Complete percutaneous access was common (56.5%, 1,630 / 2,886). The mean amount of contrast used during the operation was 103.0 milliliters (±64.9 milliliters). Supra-renal fixation was used in more than half of patients (55.8%, 7,204 / 11,943). Many different types of endografts were used, but the most common device manufacturer was Medtronic™ (58.9%), followed by Gore™ (18.8%) and Cook™ (17.0%).
Table 2:
Procedural characteristics for the 12,911 patients.*
| Variable | % (n) |
|---|---|
| Any prior aortic surgery | 3.7 (478) |
| Urgency | |
| Elective | 89.1 (11,498) |
| Symptomatic | 7.3 (946) |
| Ruptured | 3.6 (467) |
| Aneurysm size | |
| <5.0 cm | 15.8 (2,040) |
| 5.0 – 5.4 cm | 29.5 (3,809) |
| 5.5 – 5.9 cm | 24.4 (3,151) |
| ≥6.0 cm | 30.3 (3,911) |
| Concomitant iliac artery aneurysm | 23.3 (3,011) |
| Procedure time, mean (SD), min | 13..7 (72.1) |
| Unfit for open repair | 17.3 (2,234) |
| Percutaneous access | 56.5 (1,630 / 2,886) |
| Left endpoint in external iliac artery | 5.3 (148 / 2,817) |
| Right endpoint in external iliac artery | 6.3 (176 / 2,818) |
| Volume of iodinated contrast used, mean (SD), mL | 103.0 (64.9; 12,309 / 12,911) |
| Anesthetic type | |
| General | 90.7 (11,708) |
| Regional | 4.8 (622) |
| Local | 4.5 (581) |
| Aortic neck length <1.5 cm | 15.8 (254 / 1,356) |
| Aortic neck diameter | |
| <2.0 cm | 14.1 (222 / 1,575) |
| 2.0-2.9 cm | 75.0 (1,181 / 1,575) |
| ≥3.0 cm | 10.9 (172 / 1,575) |
| Aorta to neck angle | |
| <45 degrees | 87.0 (1,285 / 1,477) |
| 45-60 degrees | 8.9 (131 / 1,477) |
| >60 degrees | 4.1 (61 / 1,477) |
| Neck to aneurysm angle | |
| <45 degrees | 80.8 (1,181 / 1,462) |
| 45-60 degrees | 12.3 (180 / 1,462) |
| >60 degrees | 6.9 (101 / 1,462) |
| Supra-renal fixation | 55.8 (7,204 / 11,943) |
| Endograft manufacturer | |
| Medtronic | 58.9 (7,607) |
| Gore | 18.8 (2,421) |
| Cook | 17.0 (2,195) |
| Endologix | 2.9 (377) |
| Trivascular | 1.2 (158) |
| Lombard | 0.2 (26) |
| Bolton | 0.1 (15) |
| Other | 0.9 (112) |
Denominators are given when less than 12,911
Legend: SD, standard deviation; min, minute; mL milliliter; cm, centimeter
Rates of reintervention
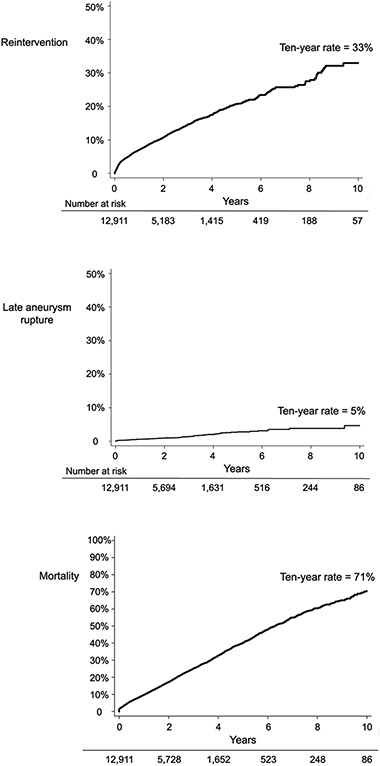
The cumulative rate of reintervention was 15% at three years and 33% at ten years (Figure 1). This rate appeared constant during the study period, and did not appear to plateau during the ten-year follow-up period. Nearly two-thirds, (60% of all reinterventions) were associated with a hospital length of stay of 3 or more days, while the remaining procedures were associated with a hospital length of stay of 2 or less days.
Figure 1:
Cumulative incidence of reintervention, late aneurysm rupture, and all-cause mortality.
Clinical factors predicting reintervention
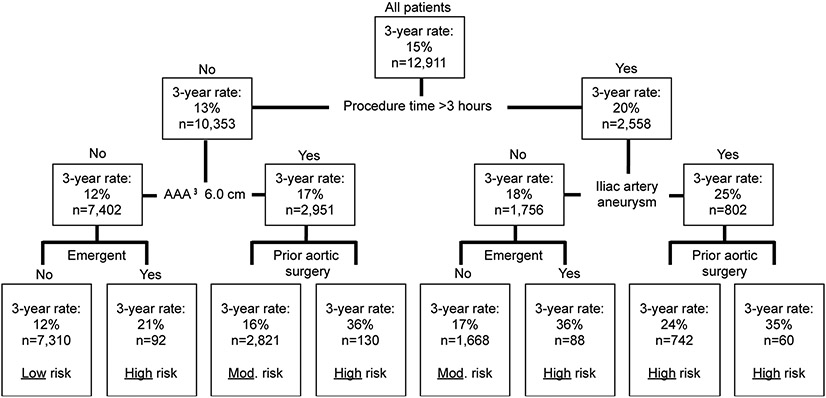
CART modeling revealed five clinical factors associated with a higher risk of reintervention after EVR (Figure 2). These factors were: operative time of 3.0 hours or more, the presence of a concomitant iliac artery aneurysm of 2.0 or more centimeters at the time of EVR, an aortic aneurysm size of 6.0 or more centimeters at the time of repair, those who underwent emergency surgery for a symptomatic or ruptured aneurysm, and those who had a history of any type of aortic surgery prior to undergoing their index EVR.
Figure 2:
Classification and regression tree identified predictors of reintervention.
Note: Three-year Kaplan-Meier rates are reported due to Medicare suppression requirements.
CART modeling created 8 subgroups of patients based on these clinical factors (Figure 2). Patients in these 8 subgroups were then risk-stratified as being low, moderate, or high-risk for reintervention. Patients were at low-risk if they had none of the five clinical predictive factors. Low-risk patients (n=7,310, 56.6%) had three-year Kaplan-Meier rate of reintervention of 15%, and a ten-year rate of 26%. Moderate-risk patients (n=4,489, 34.8%) had a three-year reintervention rate of 16-17%, and an overall ten-year rate of 37%. High-risk patients (n=1,112, 8.6%) had a three-year reintervention rate ranging between 21-36%, and an overall ten-year rate of of 46%. Modifiable procedural factors such as the EVR graft manufacturer or the use of an EVR graft with supra-renal fixation were not associated with higher or lower rates of reintervention (log-rank p=0.76 and 0.79 respectively).
Cox-proportional hazards regression allowed a more granular look at the magnitude of the association between the clinical factors and reintervention (Table 3). Procedure time again demonstrated a strong association with the likelihood of reintervention over time with a hazard ratio (HR) of 1.003 per minute (confidence interval (CI): 1.002 – 1.004). Similarly, emergent surgery for a ruptured aneurysm, aneurysms 6.0 cm or larger, patients with prior aortic surgery, and those with concomitant iliac artery aneurysms showed a high likelihood of reintervention.
Table 3:
CART informed Cox-regression model examining predictors and interaction terms associated with reintervention.
| Predictors | HR | 95% CI |
|---|---|---|
| Emergent surgery for ruptured aneurysm | 2.686 | 0.869-3.268 |
| Aneurysm ≥6.0 centimeters | 1.814 | 1.438-2.291 |
| Prior aortic surgery | 1.694 | 0.959-2.993 |
| Iliac artery aneurysm | 1.205 | 0.940-1.545 |
| Procedure time, per minute | 1.003 | 1.002-1.004 |
| Interaction terms | ||
| Prior aortic surgery and emergent surgery | 2.966 | 1.481-5.942 |
| Prior aortic surgery and aneurysm ≥ 6.0 centimeters | 1.139 | 0.710-1.828 |
| Prior aortic surgery and iliac artery aneurysm | 1.133 | 0.699-1.837 |
| Iliac artery aneurysm and emergent surgery | 1.012 | 0.562-1.822 |
| Procedure time and emergent surgery | 1.004 | 1.001-1.007 |
| Procedure time and iliac artery aneurysm | 1.000 | 0.998-1.001 |
| Procedure time and aneurysm ≥6.0 centimeters | 0.998 | 0.997-0.999 |
| Procedure time and prior aortic surgery | 0.998 | 0.996-1.001 |
| Aneurysm ≥6.0 centimeters and emergent surgery | 0.411 | 0.232-0.732 |
Legend: CART, classification and regression tree, HR, hazard ratio; CI, confidence interval.
The CART informed interaction terms revealed that these predictive estimates were not always additive (Table 3). Patients with both a history of prior aortic surgery who also underwent emergent surgery for a ruptured aneurysm had the highest likelihood of reintervention (HR for interaction: 2.966, CI: 1.481 – 5.942, three-year reintervention rate 72%). Conversely, the presence of an aneurysm of 6.0 centimeters or more at the time of emergent surgery was protective (HR for interaction term: 0.411, CI: 0.232 – 0.732).
Secondary outcomes
The rate of late aortic aneurysm rupture among all surviving patients after EVR was 5% at ten years (Figure 1). All-cause mortality, from both aneurysm-related and other sources, was 41% at five years and 71% at ten years.
We performed a subgroup analysis of late aneurysm rupture and all-cause mortality among patients who would eventually undergo reintervention, versus patients who did not undergo reintervention during follow-up. Patients who underwent reintervention during follow-up had a ten-year mortality rate of 74%, while patients who did not undergo reintervention had a rate of 69% (log-rank p=0.010; Supplementary Figure 3). In addition, patients who underwent reintervention had a ten-year late rupture rate of 20%, while patients who did not undergo reintervention had a late rupture rate of just 1% (log-rank p<0.001; Supplementary Figure 4).
Discussion:
In our observational cohort of 12,911 patients who underwent EVR across 168 centers in the United States, one in three patients underwent reintervention within ten years. The hospitalizations associated with reinterventions were not trivial, as nearly two-thirds represented procedures associated with an inpatient hospital stay of three or more days. Finally, one in twenty EVR patients experienced potentially fatal late aneurysm rupture, a conservative estimate given that a significant proportion of patients with late aneurysm rupture die in the pre-hospital setting and would not be captured in a claims-based dataset. We identified five distinct clinical variables readily measurable at the time of their EVR procedure that predicted patients who were at an increased likelihood of undergoing reintervention during follow up. Given that fewer than half of EVR patients undergoing the recommended long-term imaging surveillance, our study demonstrates that patients with these factors who undergo EVR may be well served by a focused effort emphasizing longitudinal surveillance by their cardiovascular specialist.
While reintervention is common, insights into which patients remain most vulnerable for reintervention after EVR are lacking (Table 4).2, 13, 15, 20-22 Prior studies considering this question have been limited by limited follow-up, incomplete clinical details, or small sample sizes.2, 11, 13-15 We employed clinical registry data linked with generalizable, thorough long-term reintervention assessment via a validated claims-based algorithm to remedy these limitations, allowing us to better understand which patients are at high risk for reintervention after EVR. In doing so, we identified five clinical factors associated with reintervention – a long procedure time, large aortic aneurysms, a concomitant iliac artery aneurysm, urgent or emergent repair, and prior aortic surgery. These factors can be used by clinicians to better inform patients of their expected postoperative course when they are considering repair of an abdominal aortic aneurysm.
Table 4:
Studies examining outcomes after EVR.
| Author | Year | n | Type of data | Primary factors associated with reintervention |
Follow-up |
|---|---|---|---|---|---|
| Hobo20 | 2007 | 5,183 | Clinical registry (EUROSTAR) | Type 1 endoleak | 5 years |
| Schanzer21 | 2011 | 10,228 | Imaging (M2S) | Reintervention not reported | 5 years |
| Schermerhorn2 | 2016 | 39,966 | Medicare claims | No factors studied | 8 years |
| Huang15 | 2017 | 874 | Clinical, single center | Aneurysm ≥6·0 cm | 5 years |
| Grootes22 | 2018 | 785 | RCT (EVAR-1 and EVAR-2) | Aneurysm sac diameter and growth | 5 years |
| Columbo | 2018 | 12,911 | Clinical registry (VQI) and Medicare claims | Current study | 10 years |
Legend: RCT, randomized controlled trial.
Many proponents of EVR hypothesized that most reinterventions after EVAR would be infrequent and minor.23-25 However, our analysis and others suggest that the observed impact of reintervention on patients is not minor, as we found that nearly two-thirds of reinterventions were associated with a hospital stay of 3 or more days.2, 10 These hospital stays for reinterventions often longer than the index procedure itself.26, 27 In addition, late rupture occurred in 5% of patients within 10 years after EVR. While the objective of reintervention is to reduce this risk, our findings suggest that the likelihood of late-rupture is highest among patients who undergo reintervention. Although it is unlikely that reintervention is causing late-rupture, those at highest risk for reintervention may also be those at highest risk for late-rupture and represent a population in whom EVR may not be the preferred treatment modality. Therefore, for younger patients with an aneurysm which has several risk factors for reintervention, their surgeon should carefully review the relative advantages and disadvantages of both open and endovascular approaches, and emphasize the need for high fidelity surveillance over time for those patients who choose EVR.
The recent draft NICE guidelines in the United Kingdom invoke and highlight many of these same concerns.8 With equivalent long-term survival between EVR and open surgical repair, patients at high-risk for reintervention may choose to consider open repair as a primary therapeutic option.2, 3 Despite nearly universal adoption of EVR as a primary treatment strategy for abdominal aortic aneurysm in the United States, many other countries and health systems have adopted this technology differently. For example, a recent study from the International Consortium of Vascular Registries showed threefold variation in the proportion of aneurysm patients receiving EVR, from 28% in Hungary to 79% in the United States.28 Where the ideal rate of EVR lies among those treated for abdominal aortic aneurysms remains an empirical question, and a better understanding of the role that reintervention plays in this decision may help to better align patient preferences and treatments.
Finally, although diligent postoperative surveillance is indicated for all patients after EVR, many patients do not receive imaging at the recommended intervals.6-10, 29 The risk factors described herein may inform targeted surveillance improvement efforts targeting patients at highest risk for reintervention. While improving surveillance for all patients is warranted, these factors can help cardiovascular specialists and their referring physicians more effectively prioritize surveillance efforts for patients who undergo EVR.6
Our study has limitations. Reinterventions were performed at the discretion of the attending surgeon, and there is likely inherent heterogeneity in the reasons for and thresholds to perform these procedures. Anatomic characteristics such as aortic neck angle and aortic neck length, variables linked to reintervention in other studies, were not available in all patients in our registry, although subgroup analyses among patients with this information available suggested the variables we identified were more strongly associated with reintervention risk. Endoleak was not included in our analysis. We are unable to comment on the predictive ability of this variable, as it was only recorded in registry forms at 1-year follow-up, and not reported in our long-term claims-based analytics. We did not compare our results of reintervention after EVR to open surgical repair. The reinterventions that tend to occur between the two types of repairs are fundamentally different (abdominal wall hernia versus endovascular aneurysm related procedures). Therefore, we felt that a thorough analysis of reintervention after EVR was best handled isolation. Finally, our analyses are based on observational data. While this allows an assessment of real world results, the observational nature of the study does not allow us to prove causation between clinical factors and reintervention after EVR.
Conclusions:
One in three surviving patients underwent reintervention after EVR within the first decade after repair, and nearly two-thirds of these reinterventions were associated with a hospital stay of three or more days. In addition, 5% of individuals suffered a late-rupture event. We identified five clinical factors that can be used to risk-stratify patients in their likelihood of reintervention: operative time ≥3.0 hours, aneurysm diameter ≥6.0 cm, an iliac artery aneurysm ≥2.0 cm, emergency surgery, and a history of prior aortic surgery. These factors can assist providers caring for post-EVR patients by informing targeted surveillance efforts for those who are at highest risk for reintervention and may enhance procedure decision making for patients who are candidates for both EVR and open surgical repair.
Supplementary Material
Funding:
This work was supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (ME-1503-28261), the United States Food and Drug Administration (U01-FD005478), the National Institute on Aging (PO1-AG19783), and the American Heart Association (18SFRN33900147). All statements in this paper, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of our funding agencies. Specifically, the funders played no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Declaration of competing interests:
Authors PM, AJO and PPG had support from the Patient-Centered Research Outcomes Institute, the Food and Drug Administration, the National Institute on Aging, and the National Institutes of Health; AS performs case proctoring for Cook Medical™ and MLS performs consulting for Cook Medical™, Endologix™, and Medtronic™; there are no other relationships or activities that could appear to have influenced the submitted work.
Disclaimer:
The views expressed do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
This study was funded in part by the Patient Centered Outcomes Research Initiative, the Food and Drug Administration, the National Institute on Aging, and the American Heart Association.
References:
- 1.Suckow BD, Goodney PP, Columbo JA, et al. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J Vasc Surg 2018; 67(6):1690–1697 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schermerhorn ML, Buck DB, O'Malley AJ, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med 2015; 373(4):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. The Lancet 2016; 388(10058):2366–2374. [DOI] [PubMed] [Google Scholar]
- 4.Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev 2014(1):CD004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qadura M, Pervaiz F, Harlock JA, et al. Mortality and reintervention following elective abdominal aortic aneurysm repair. J Vasc Surg 2013; 57(6):1676–83, 1683 e1. [DOI] [PubMed] [Google Scholar]
- 6.The United States Food and Drug Administration. Safety Alerts for Human Medical Products. Accessed August 1st, 2017. Available from: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm611193.htm
- 7.Zierler RE, Jordan WD, Lal BK, et al. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. Journal of Vascular Surgery 2018; 68(1):256–284. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Abdominal Aortic Aneurysm: Diagnosis and Management. Accessed August 1st, 2018. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-cgwave0769. [PubMed]
- 9.2011 Writing Group Members, 2005 Writing Committee Members, ACCF/AHA Task Force Members. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124:2020–45. [DOI] [PubMed] [Google Scholar]
- 10.Garg T, Baker LC, Mell MW. Postoperative Surveillance and Long-term Outcomes After Endovascular Aneurysm Repair Among Medicare Beneficiaries. JAMA Surg 2015; 150(10):957–63. [DOI] [PubMed] [Google Scholar]
- 11.Hicks CW, Zarkowsky DS, Bostock IC, et al. Endovascular aneurysm repair patients who are lost to follow-up have worse outcomes. J Vasc Surg 2017; 65(6):1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karthikesalingam A, Holt PJ, Vidal-Diez A, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. The Lancet 2014; 383(9921):963–969. [DOI] [PubMed] [Google Scholar]
- 13.Patel SR, Allen C, Grima MJ, et al. A Systematic Review of Predictors of Reintervention After EVAR: Guidance for Risk-Stratified Surveillance. Vascular and Endovascular Surgery 2017; 51(6):417–428. [DOI] [PubMed] [Google Scholar]
- 14.Chang RW, Goodney P, Tucker LY, et al. Ten-year results of endovascular abdominal aortic aneurysm repair from a large multicenter registry. J Vasc Surg 2013; 58(2):324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Gloviczki P, Duncan AA, et al. Maximal aortic diameter affects outcome after endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2017; 65(5):1313–1322 e4. [DOI] [PubMed] [Google Scholar]
- 16.Columbo JA, Kang R, Hoel AW, et al. A comparison of reintervention rates after endovascular aneurysm repair between the Vascular Quality Initiative registry, Medicare claims, and chart review. J Vasc Surg 2019; 69(1):74–79 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoel AW, Faerber AE, Moore KO, et al. A pilot study for long-term outcome assessment after aortic aneurysm repair using Vascular Quality Initiative data matched to Medicare claims. J Vasc Surg 2017; 66(3):751–759 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics 1992; 48(2):411–25. [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4(10):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobo R, Kievit J, Leurs LJ, et al. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J Endovasc Ther 2007; 14(1):1–11. [DOI] [PubMed] [Google Scholar]
- 21.Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011; 123(24):2848–55. [DOI] [PubMed] [Google Scholar]
- 22.Grootes I, Barrett JK, Ulug P, et al. Predicting risk of rupture and rupture-preventing reinterventions following endovascular abdominal aortic aneurysm repair. Br J Surg 2018; 105(10):1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bendermacher BL, Stokmans R, Cuypers PW, et al. EVAR reintervention management strategies in contemporary practice. J Cardiovasc Surg (Torino) 2012; 53(4):411–8. [PubMed] [Google Scholar]
- 24.Roos H, Djerf H, Brisby Jeppsson L, et al. Re-interventions after endovascular aortic repair for infrarenal abdominal aneurysms: a retrospective cohort study. BMC Cardiovasc Disord 2016; 16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White SB, Stavropoulos SW. Management of Endoleaks following Endovascular Aneurysm Repair. Semin Intervent Radiol 2009; 26(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King EG, Farber A, Rybin D, et al. Preoperative Risk Factors Predict Protracted Hospital Length of Stay after Elective Endovascular Abdominal Aortic Aneurysm Repair. Ann Vasc Surg 2017; 43:73–78. [DOI] [PubMed] [Google Scholar]
- 27.Hanley SC, Steinmetz O, Mathieu ES, et al. Safety and feasibility of endovascular aortic aneurysm repair as day surgery. J Vasc Surg 2018; 67(6):1709–1715. [DOI] [PubMed] [Google Scholar]
- 28.Beck AW, Sedrakyan A, Mao J, et al. Variations in Abdominal Aortic Aneurysm Care: A Report From the International Consortium of Vascular Registries. Circulation 2016; 134(24):1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Judelson DR, Simons JP, Flahive JM, et al. Determinants of Follow-Up Failure in Patients Undergoing Vascular Surgery Procedures. Ann Vasc Surg 2017; 40:74–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.