Abstract
Broadly neutralizing antibodies (bNAbs) may provide an alternative to standard antiretroviral treatment (ART) for controlling human immunodeficiency virus (HIV)-1 replication and may have immunotherapeutic effects against HIV-1 reservoirs. We conducted a prospective clinical trial with two HIV-1 bNAbs (VRC01LS and 10–1074) in children (n=25) who had previously initiated small-molecule ART treatment before 7 days of age and who continued treatment for at least 96 weeks. Both bNAbs were dosed intravenously every 4 weeks, overlapping with ART for at least 8 weeks and then continued for up to 24 weeks or until detectable viremia of HIV-1 RNA rose above 400 copies/mL in the absence of ART. Eleven (44%) children maintained HIV-1 RNA below 400 copies/mL through 24 weeks of bNAb-only treatment; fourteen (56%) had detectable viremia above 400 copies/mL at a median of 4 weeks. Archived HIV-1 provirus susceptible to 10–1074, lower birth HIV-1 DNA reservoir in peripheral blood mononuclear cells, sustained viral suppression throughout early life, and combined negative qualitative HIV-1 DNA polymerase chain reaction and negative HIV-1 serology at entry were associated with maintaining suppression on bNAbs alone. This proof-of-concept study suggests that bNAbs may represent a promising treatment modality for infants and children living with HIV-1. Future studies using newer bNAb combinations with greater breadth and potency are warranted.
One-sentence summary:
Treatment with broadly neutralizing antibodies maintained viral suppression in children with HIV-1 with favorable reservoir characteristics at birth.
Editor’s Summary:
bNAbs for Babies. Broadly neutralizing antibodies (bNAbs) have shown promise as a companion or alternative to antiretroviral therapy (ART) for HIV-1, although their efficacy in a pediatric population remains unclear. Here, Shapiro et al. report the results of a prospective clinical trial where children on ART from birth in Botswana were treated with two HIV-1 bNAbs, VRC01LS and 10–1074. The treatment was administered initially in combination with ART, then alone. Eleven of 25 infants maintained viral suppression during the bNAb-only step; the authors found that these infants had more favorable HIV-1 reservoir characteristics, including a smaller initial proviral reservoir and susceptibility of those proviruses to bNAb neutralization. Together, these results highlight the potential of bNAb treatment for infants and children living with HIV-1. –CM
INTRODUCTION
Broadly neutralizing monoclonal antibodies (bNAbs) against human immunodeficiency virus (HIV-1) are an emerging treatment option for people living with HIV-1 with the potential to maintain HIV-1 RNA suppression (1, 2). bNAbs can be administered infrequently, which avoids adherence concerns of daily oral antiretroviral treatment (ART), may limit long-term toxicity from prolonged ART, and may enhance immune responses and deplete residual viral reservoirs, offering a potential pathway to post-treatment viral control in some individuals (3, 4). Children living with HIV-1 who have been treated continuously from birth are an ideal group for bNAb treatment as they have limited viral reservoirs (5) and may be less likely to have pre-existing viral resistance to bNAbs (6). Children are also ideal candidates for ART-sparing strategies that avoid long-term toxicities and adherence considerations with daily dosing.
Long-term HIV-1 RNA suppression has been reported in some adult trials using bNAbs in combination (3, 7), warranting similar studies in pediatric populations. The Tatelo Study was a phase I/II, single arm, multi-site clinical trial to evaluate the combined use of two bNAbs, VRC01LS and 10–1074. VRC01 targets the CD4 binding site and the LS formulation has a modified Fc receptor that increases its half-life. VRC01 had been used in a pediatric trial previously (8), and a pediatric study of VRC01LS was underway at the time of starting Tatelo (9). 10–1074 targets the V3 loop and has been used as combined treatment with CD4 binding agents in prior adult (2) (but not pediatric) studies. The Tatelo Study administered both of these bNAbs as monthly treatment in a cohort of children in Botswana who had received ART since birth in a previous study (5, 10).
RESULTS
Participant Characteristics
Between March 2020 and January 2021, all 28 (70%) Early Infant Treatment (EIT) study participants who were eligible for the Tatelo Study were enrolled (Fig. 1); the remaining 12 (30%) EIT study participants were ineligible for Tatelo because of detectable viremia of HIV-1 RNA ≥40 copies/mL within the preceding 24 weeks. Of the 28 Tatelo enrollees, 14 (50%) had never experienced detectable viremia ≥40 copies/mL during EIT participation, and 14 (50%) had experienced viremia ≥40 copies/mL at least once. Enrolled children had a median age of 3.6 (range 2.4 to 5.6) years, 19 (68%) were female, and the median entry CD4 T cell count was 1198 with an interquartile range (IQR) of 843 to 1684 cells/mm3 (Table 1). All enrolled children were screened for HIV-1 RNA prior to entry into the ART and bNAbs step. HIV-1 RNA was >40 copies/mL in two children on the day of bNAb initiation, and in one child after 4 weeks of ART/bNAbs; thus, bNAb treatment was discontinued in these three children, ART was continued, and adherence was reinforced, leading to subsequent viral re-suppression.
Fig. 1. Flow diagram.
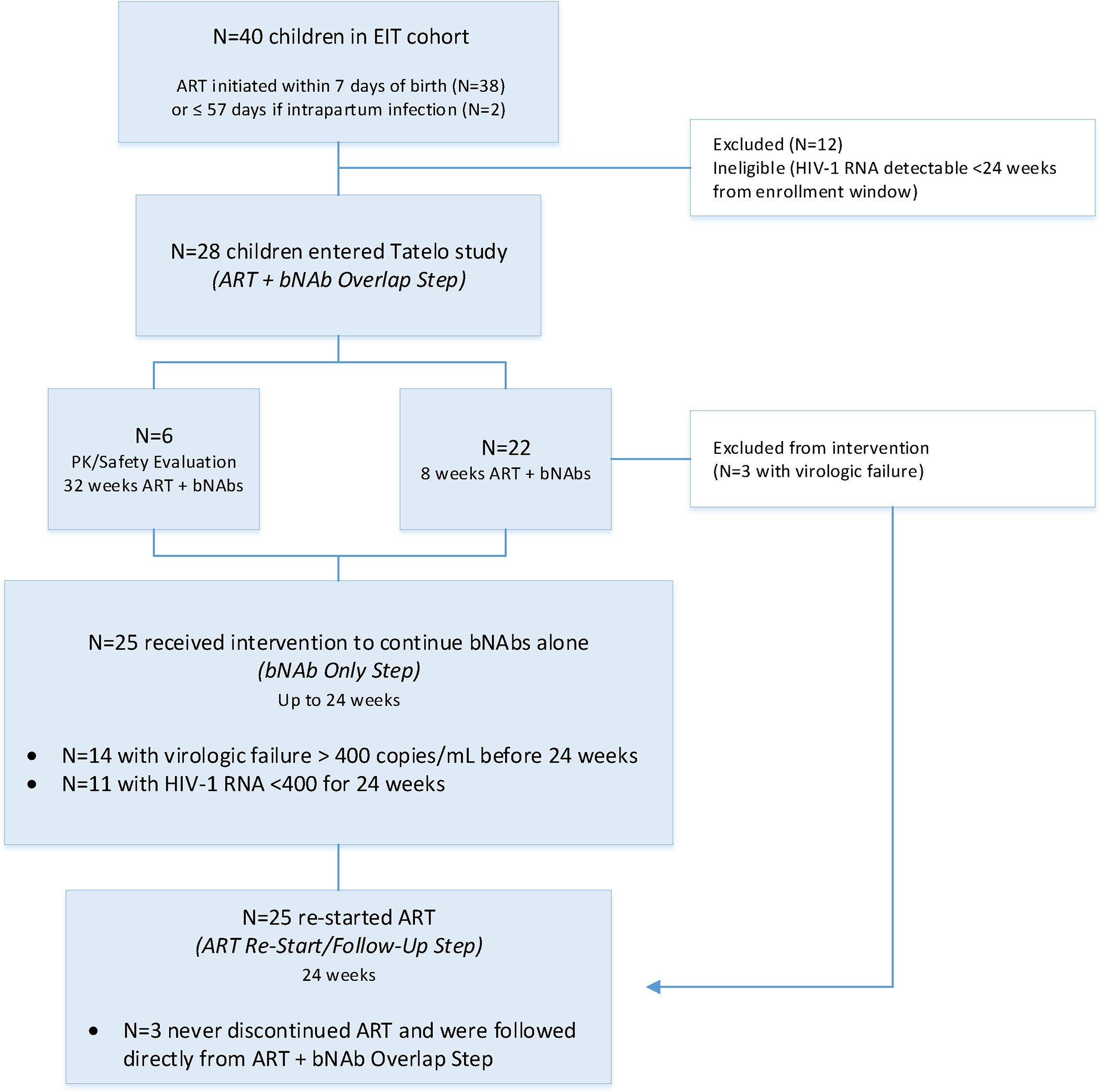
N=40 children in the Early Infant Treatment (EIT) Study were potentially eligible for inclusion in Tatelo; 28 enrolled in the ART + bNAb Overlap Step, and 25 continued into the bNAb Only Step.
Table 1.
Baseline characteristics of enrolled Tatelo participants (N=28).
| Birth Characteristics | |
| Female sex – no. (%) | 19 (68) |
| Gestational age at birtha – no. (%) | |
| 35 weeks | 4 (14) |
| 36 weeks | 6 (21) |
| 37 weeks | 2 (7) |
| 38–41 weeks | 16 (57) |
| Median birth weight – kg (IQR) | 2.95 (2.60–3.20) |
| Median HIV-1 RNA at birth – log10 copies/mL (IQR) | 4.09 (2.54–4.65) |
| Median HIV DNA at birth – copies/million cells (IQR)b | 490 (121–1221) |
| Maternal education, highest level attained – no. (%) | |
| None/Primary | 6 (21) |
| Junior Secondary | 13 (46) |
| Senior Secondary | 4 (14) |
| Tertiary | 5 (18) |
| Maternal employment status – no. (%) | |
| Salaried | 6 (21) |
| Paid domestic work | 2 (7) |
| None/Unemployed | 20 (71) |
| Characteristics at Time of Enrollment to Tatelo | |
| Median age – years (IQR) | 3.60 (3.10–4.46) |
| Median weight – kg (IQR) | 12.90 (11.90–15.20) |
| ART regimenc – no. | |
| ZDV+3TC+LPV/r | 26 |
| ABC+3TC+LPV/r | 1 |
| ZDV+ABC+3TC+LPV/r | 1 |
| Median CD4 count – cells/mm3 (IQR) | 1198 (843–1684) |
| Median HIV DNA (at 84/96 weeks of age) – copies/million cells (IQR) | 35.3 (8.38–102.3) |
IQR, interquartile range.
EIT Study excluded children born before 35 weeks gestational age.
Measured in PBMCs by ddPCR.
ZDV=zidovudine, 3TC=lamivudine, LPV/r=lopinavir/ritonavir, ABC=abacavir
Virologic and Immunologic Outcomes
Twenty-five (89%) children completed the ART plus bNAb step (six for 32 weeks, and 19 for 8 weeks) and advanced to the bNAb-only step of the study. Of these, 11 [44%, 95% confidence interval (CI) 24% to 65%] maintained HIV-1 RNA <400 copies/mL (defined as bNAb successes) and 10 (40%, 95% CI 21% to 61%) maintained <40 copies/mL for 24 weeks (Fig. 2A); one child had a single HIV-1 RNA value of 234 copies/mL at week 16, with <40 copies/mL at all other weeks. Fourteen children (56%) had detectable viremia ≥400 copies/mL at a median of 4 (range 1–20) weeks (defined as bNAb failures), and were re-started on ART at a median of 4 (range 1 to 7) days from first detected viremia. Kaplan-Meier estimates for time to first HIV-1 RNA ≥400 copies/mL are shown in Fig. 2B. Among children with failure, median HIV-1 RNA at ART re-start was 4.42 (range 2.87 to 6.42) log10 copies/mL. After failure, all children had viral re-suppression to <40 copies/mL on their prior ART regimen (lopinavir/ritonavir [LPV/r]-based in all cases), at a median of 4 (range 1 to 20) weeks from ART re-start. CD4 T cell counts in children who experienced detectable viremia ≥400 copies/mL were similar to post-intervention CD4 cell counts in children who did not, with a median above 1000 copies/mm3 in both groups. No child in either group had a concerning pattern of CD4 T cell count decline during the study (Fig. S1).
Fig. 2. Treatment outcomes in the Tatelo Study.
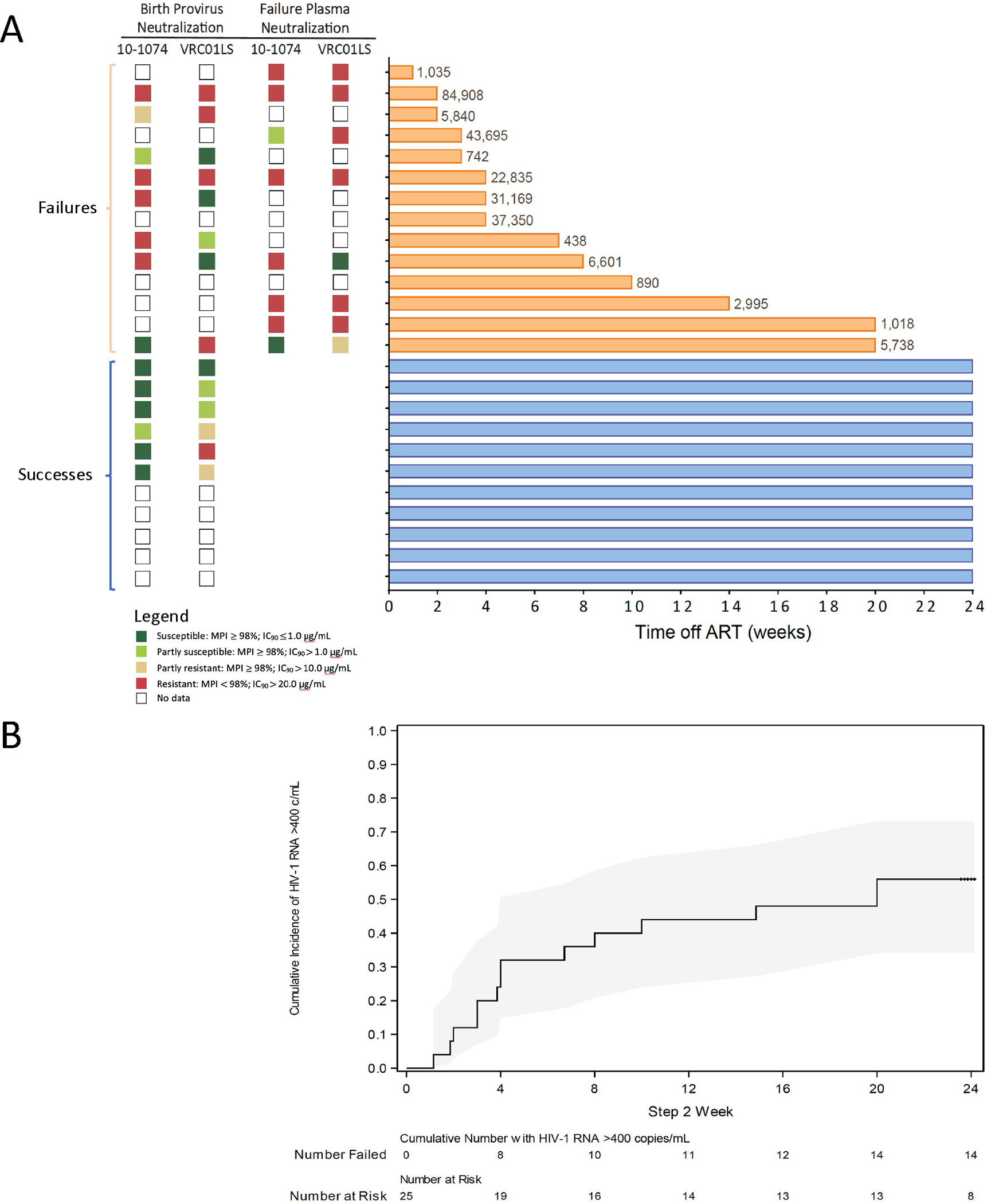
(A) Shown on the left is available participant antibody neutralization assay results for env amplicons of full-length intact provirus near birth and plasma at failure. Proviral samples from PBMCs at baseline were available for all participants, but amplification succeeded in only 14 of 25. Baseline amplicons were from birth (85%), 4 to 24 weeks (13%), or 84 weeks (2%). Plasma samples were available for 14 failures but amplified in only 8. We defined full susceptibility to each bNAb as 90% inhibitory concentration (IC90) ≤1.0 μg/mL and maximum percent inhibition (MPI) ≥98%. The plot on the right shows bNAb-only step HIV-1 RNA outcomes, grouped by failures (top, orange) and successes (bottom, blue). Participant HIV-1 RNA outcomes are shown by bNAb-only week; the bars extend through week of completion of this study step. Values at ends of bars indicate HIV-1 RNA copies/mL at first virologic failure. Each row in (A) indicates the same participant. (B) Shown is the cumulative proportion of participants with HIV-1 RNA detectable viremia ≥400 copies (c)/mL over time during the bNAb-only phase. The shaded area shows the 95% confidence interval.
Safety, pharmacokinetics, and anti-drug antibody analysis
No infusion reactions occurred, and bNAbs were well tolerated. Three children experienced a total of five grade 3 events, with one (neutropenia) during the bNAb/ART step considered possibly related to bNAb treatment (Table S1 and S2). There were no grade 4 events. Pharmacokinetic (PK) troughs prior to each dose in the bNAb-only step revealed adequate 10–1074 and VRC01LS concentrations for all children. Overall pre-dose troughs were in the expected range and were consistently above 100 μg/mL for both bNAbs: median 211.0 (IQR 183.6 to 259.2) μg/mL for 10–1074, and 259.6 (IQR 201.0 to 305.6) μg/mL for VRC01LS. Although fewer values were available for those with bNAb failure who exited the study step early, median trough bNAb values were similar for successes and failures during the bNAb-only step (Fig. S2). No anti-drug antibodies were observed to 10–1074 or VRC01LS.
Characteristics of children with successful bNAb treatment
Children with bNAb-only treatment success had favorable pre-intervention clinical and viral reservoir characteristics (Table 2). Almost all children who succeeded (9, 82%) had sustained viral suppression (<40 copies/mL) at all EIT visits from initial suppression through Tatelo entry, compared with 29% of children who failed. In addition, total HIV DNA in peripheral blood mononuclear cells (PBMCs) near birth was significantly lower in children who succeeded (p=0.02). Most children who succeeded had negative qualitative DNA (9, 82%) or negative EIA (9, 82%), or both (8, 73%), at Tatelo entry; in contrast, none of the children who failed had a “negative/negative” pattern at Tatelo entry (73% versus 0%, p<0.001).
Table 2.
Characteristics by response group for bNAb-only treatment with VRC01LS and 10– 1074 in children living with HIV in Botswana.
| Baseline/Enrollment Characteristics | Total (N=25)a | Treatment Success on bNAbs (N=11) | Treatment Failure on bNAbs (N=14) | P-value |
|---|---|---|---|---|
| Median (IQR) or number (%) | ||||
| Age at ART start (days) | 3 (2–4) | 3 (3–5) | 2 (2–3) | 0.10 |
| Age at bNAb start (years) | 3.70 (3.10–4.40) | 4.20 (3.40–4.60) | 3.45 (2.90–4.40) | 0.26 |
| HIV-1 RNA at birth (copies/mL) | 3145 (310–25507) | 2279 (381–12984) | 20465 (292–33502) | 0.37 |
| HIV-1 RNA undetectable since 24 weeksb | ||||
| Yes | 13 (52%) | 9 (82%) | 4 (29%) | 0.02 |
| No | 12 (48%) | 2 (18%) | 10 (71%) | |
| Total HIV DNA in PBMCs at birth by ddPCR (copies/106)c | 465 (100–1129) | 155 (46–465) | 784 (166–1246) | 0.02 |
| Intact HIV DNA in PBMCs at birth by FLIP-seq (copies/106)c | 2.9 (0.22–280.4) | 1.16 (0.22–38.3) | 4.59 (0.52–280.4) | --d |
| Number with intact HIV DNA in PBMCs at birth by FLIP-seq (for those >L.O.D.) | 18 (72%) | 6 (55%) | 12 (86%) | 0.18 |
| Intact HIV DNA in PBMCs at Tatelo entry by FLIP-seq (copies/106)c | 0.24 (0.04–2) | 0.26 (0.04–2) | 0.22 (0.08–1) | --d |
| Number with intact HIV DNA in PBMCs at Tatelo entry by FLIP-seq (for those >L.O.D.) |
5 (20%) | 2 (18%) | 3 (21%) | >0.99 |
| Negative whole blood qualitative HIV DNA PCR and HIV enzyme immunoassay at Tatelo entry | ||||
| Yes | 8 (32%) | 8 (73%) | 0 | <0.001 |
| No | 17 (68%) | 3 (27%) | 14 (100%) | |
| Amount of bNAb/ART Overlap | ||||
| 32 weeks | 6 (24%) | 5 (45%) | 1 (7%) | 0.06 |
| 8 weeks | 19 (76%) | 6 (55%) | 13 (93%) | |
| CD4 T cell count (cells/mm3) | ||||
| Start of bNAb-only treatment | 1149 (922–1502) | 984 (808–1185) | 1380 (1004–1868) | 0.05 |
| bNAb susceptibilitye | ||||
| 10–1074 susceptible (PBMCs at birth) | 8 (57%) | 6 (100%) | 2 (25%) | 0.01 |
| VRC01LS susceptible (PBMCs at birth) | 7 (50%) | 3 (50%) | 4 (50%) | >0.99 |
Excludes 3 children who began bNAbs but never discontinued ART.
Defined as all visits at or after 24 weeks of age with HIV-1 RNA <40 copies/mL; per protocol, all HIV-1 RNA values in the 24 weeks prior to bNAb initiation must be <40 copies/mL.
An imputed value of 1 provirus in double the number of analyzed cells used if no target identification.
Low sample size and high proportion below the L.O.D. precluded comparison of distributions.
N=14 (8 treatment failures, 6 treatment successes) with amplification. bNAb susceptibility was based on maximum percent inhibition (MPI) ≥98%.
Neutralization and reservoir quantification assays
Neutralization assay data were limited either by amplification failure or failure of the cloning or cell assay step. In all plasma samples with a successful assay at time of detectable viremia ≥400 copies/mL (bNAb failure), some degree of reduced neutralization by 10–1074 or VRC01LS was observed (Fig. 2A). Viral envelope sequences from intact proviruses collected near birth had a largely similar pattern as plasma at bNAb failure when matched results were available. Among virologic successes on bNAbs, archived provirus demonstrated dual bNAb susceptibility for 3 (50%) of 6 with available results, and all had complete (83%) or partial (17%) 10–1074 susceptibility. In contrast, only 2 (29%) of 7 failures with available results had complete or partial susceptibility to 10–1074 in archived provirus (p=0.02).
Digital droplet polymerase chain reaction (ddPCR) and near full-length proviral sequencing were performed at study entry and several follow-up timepoints, but most children had values below the limit of detection (likely due to low viral reservoirs and the limited number of PBMCs that could be safely collected from children). Birth ddPCR in PBMCs predicted successful dual bNAb treatment (Table 2) and a similar pattern was observed for full-length individual proviral sequencing (FLIP-seq) (Fig. 3). At entry to Tatelo, both defective provirus and total provirus DNA (intact plus defective) by FLIP-seq were more commonly above the limit of detection in children who later failed (each 79%) than those who succeeded (each 18%) (p=0.005). Among failures, detectable intact provirus was observed in 3 individuals (21%) at entry, and 7 individuals (50%) after failure. These findings were not attributable to variations in cell numbers used for measurements (Fig. S3).
Fig. 3. Differences in intact, defective, and total HIV-1 by FLIP-seq at birth, 84 to 96 weeks, Tatelo Study entry, and end of bNAb treatment among successes and failures.
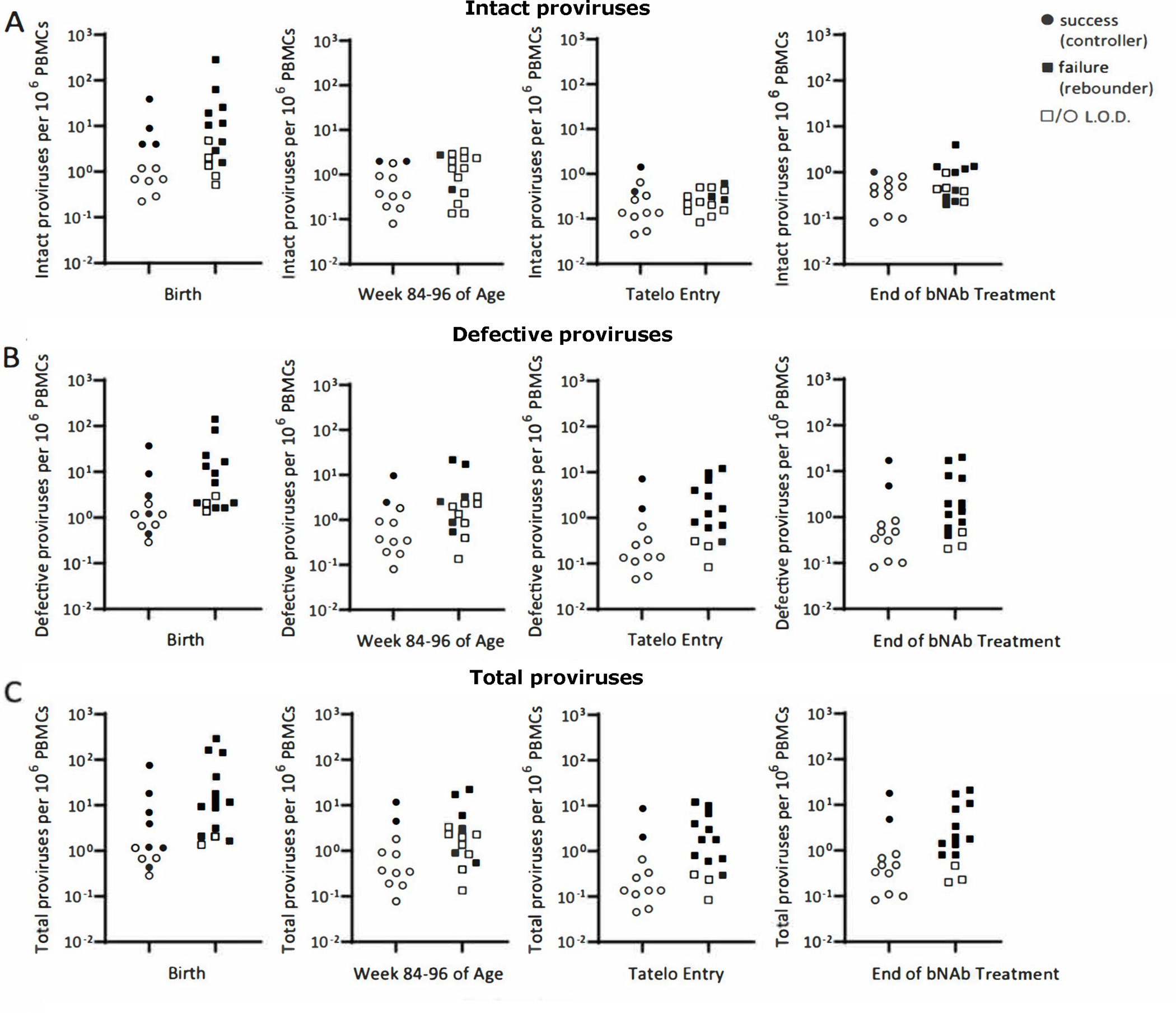
(A to C) Shown is the quantification of the viral reservoir obtained by FLIP-seq. Intact (A), defective (B), and total (C) proviruses per 1 million PBMCs were identified at birth, week 84 to 96 of age, Tatelo entry, and end of bNAb treatment. Study participants were divided into two groups according to success or failure to maintain viral control (HIV1-RNA <400 copies/mL) throughout the bNAb-only step. Open symbols represent the limit of detection (L.O.D.) when no virus was detectable, with an imputed value of 1 provirus in double the number of analyzed cells without target identification. The range of the number of PBMCs across all time points and both groups was 1.51E+06 to 1.11E+07 cells (median = 1.45E+06); for those below L.O.D., the range of the number of PBMCs was 6.06E+04 to 4.31E+06 cells (median = 1.0E+06).
DISCUSSION
As the breadth, potency, and half-lives of bNAbs improve, immune-based HIV treatment may offer advantages for children facing lifelong ART. We found that monthly VRC01LS and 10–1074 dual bNAb infusions were well tolerated and maintained viral suppression <400 copies/mL for 24 weeks in 44% of children who had been treated with ART from birth. This proof-of-concept study provides early evidence that current bNAb combinations may offer an alternative to small molecule ART in selected children with favorable clinical and resistance characteristics, and that future long-acting bNAb combinations may expand the benefits of this strategy to more children living with HIV.
Our results were generally concordant with adult treatment studies using similar bNAb combinations, though virologic success was somewhat lower in our study. Among adults with chronic HIV subtype B, treatment with 10–1074 plus 3BNC117 maintained viral suppression in 76% of participants for 20 weeks after ART discontinuation (3). This same combination maintained viral suppression for all adults with pre-screened susceptible virus in two separate studies (7, 11). Plasma pre-screening for HIV-1 susceptibility to bNAbs in our study was not possible for our virally suppressed cohort, but our data suggest that screening archived provirus stored from pre-ART birth samples might identify the children most likely to maintain viral suppression on bNAbs. Neutralization assay results in our study were limited because of assay failure or inability to amplify full-length intact proviral DNA (in part due to very low viral reservoirs in the cohort), but a pattern of reduced susceptibility to one or both bNAbs was observed for all children with results at the time of virologic failure. This same pattern was also present in archived provirus when matching samples were available and in all but one when only archived provirus was available. In contrast, we found that, for half of children who succeeded, archived proviruses were susceptible to both bNAbs; further, all analyzed proviruses from successes had at least partial susceptibility to 10–1074. Pre-screening may be particularly important for regions where subtype C predominates (including Botswana), as there is less inherent susceptibility to most current bNAbs in these regions (12).
Our study also advances the concept of pre-screening for future pediatric cohorts beyond the use of neutralization assays, as we identified several additional markers for virologic success with bNAb treatment. HIV DNA in PBMCs from birth was significantly lower in those who succeeded on bNAbs alone (p=0.02), suggesting a potential advantage in some children from very early life. Most of the children who succeeded also had sustained viral suppression prior to the bNAb intervention, whereas the majority of those who failed had experienced detectable viremia ≥40 copies/mL at some point in early life. Viremia before bNAb intervention may have therefore led to some bNAb resistance, and its detection served as a useful marker for bNAb failure. Finally, an additional predictor of success was the combination of having reverted to a negative whole blood HIV qualitative DNA test at Tatelo entry, and never developing positive serology by EIA; all eight children with this pattern succeeded. These simple clinical markers are widely available, and we believe this combination of “negative/negative” should be explored as a real-time biomarker for entry into future pediatric bNAb treatment studies. Of the three children who succeeded without the “negative/negative” pattern, two had detectable intact provirus integrated into non-encoding regions of the genome, and this “locked” pattern is being further studied (13); the third was one of a small number of successes with several episodes of detectable viremia prior to Tatelo and had a positive EIA at entry. It is important to note that each of the characteristics for success listed above were closely inter-related, and because of small numbers, multivariable analysis was not possible. Likewise, although five of six children who received 32 weeks of ART/bNAb overlap succeeded, these children all had additional favorable characteristics, limiting our ability to assess whether the longer overlap period improved outcomes.
Whether bNAbs were directly responsible for maintaining viral suppression in our study, or whether some children would have maintained viral suppression without ART or bNAbs, remains an open question. Using data from the CHER study (14, 15), we pre-specified that 24-week success ≥30% would be unlikely to occur by chance, even among low-reservoir children. The neutralization data provided additional support for a causal role of the bNAbs, but were not definitive because of small numbers and because we cannot exclude the possibility that higher bNAb sensitivity tracked with more limited viral diversity. A randomized design comparing bNAbs to an analytic treatment interruption (ATI) was not considered feasible at the time Tatelo was conducted, but for children with low or undetectable reservoirs and a constellation of favorable markers, an ATI component could be considered in future trials. Given the potential vaccinal effect (16, 17) and reservoir-lowering effect (1, 4, 18, 19) previously described for bNAbs, candidates for such a trial may benefit from a period of bNAbs prior to ATI to maximize the chance for success.
There were no safety concerns raised for VRC01LS or 10–1074 in our study, and both were well tolerated, as expected from prior studies (2, 19–21). Few grade 3 (and no grade 4) events were reported, and none resulted in bNAb discontinuation. CD4 T cell counts were largely unaffected between the beginning and end of the study. Trough PK values were sufficient at nearly all timepoints and did not differ between successes and failures. The slow elimination of VRC01LS and frequent (every 4 weeks) administration resulted in trough concentrations substantially higher than prior VRC01 therapy switch (20) and pre-exposure prophylaxis (PrEP) studies (22). Monthly bNAb infusions were also highly acceptable to caregivers of the participants in the study (23).
Limitations of our study were the small sample size, including the limited ability to perform neutralization assays and to quantify changes in intact viral reservoir over time, both of which were unavoidable challenges related to the extraordinarily low reservoirs in this cohort. Children treated with ART from birth and virally suppressed at bNAb initiation may not be representative of other children with HIV. All study participants are now in long-term follow-up, and in-depth profiling of reservoir cells and immune responses are ongoing. Our study benefitted from having complete treatment and reservoir data on a cohort of children followed from birth, from no losses to follow up, and from our ability to ensure that ART could be safely re-started when needed. In conclusion, nearly half of children who had received ART from birth maintained viral suppression for 24 weeks with monthly 10–1074 and VRC01LS alone, and easily identifiable markers predicted successful outcomes. These findings support the use of bNAbs with greater breadth and potency in future pediatric trials, and provide a methodology to easily screen participants with the greatest chance for success.
MATERIALS AND METHODS
Study design
The Tatelo study evaluated dual bNAbs as a treatment alternative in children. The study began with an intensive PK and safety phase for 10–1074 and VRC01LS while ART was continued—first individually among 12 participants (6 per bNAb), and then during dual administration among 6 of the first 12 participants. Results were previously reported from this intensive PK and safety evaluation (24). The main study consisted of a single arm; participants in the main study were followed through 3 steps. The first was an ART/bNAb overlap step, where ART was continued while dual bNAbs were administered every 4 weeks. This step lasted 32 weeks for the first 6 participants (for planned PK and safety analyses and Safety Monitoring Committee approval prior to permitting additional enrollment in the step), and 8 weeks for subsequent participants. The next step was the bNAb-only step, where ART was discontinued for up to 24 weeks (or until any detectable viremia ≥400 copies/mL). At 24 weeks (or upon detectable viremia ≥400 copies/mL), participants discontinued bNAbs and resumed ART, and were followed for an additional 24 weeks in the ART-restart step. ART regimens at study entry and upon re-start were per Botswana guidelines, and consisted of lopinavir-ritonavir-based 3-drug oral ART in all children.
Trial ethics and oversight
The study was approved by Institutional Review Boards in Botswana (Human Research Development Committee, HPDME 13/18/1 X1) and at the Harvard T.H. Chan School of Public Health (Harvard Longwood Campus Institutional Review Board, IRB18–0062). A parent or guardian provided written informed consent for all participants. The study was monitored by an independent Safety Monitoring Committee.
Study population and monitoring
All Tatelo participants had previously taken part in the EIT study, a clinical trial evaluating early infant HIV-1 diagnosis and treatment in Gaborone and Francistown, Botswana (NCT02369406) (5, 10). Children eligible for Tatelo were EIT participants treated since before 7 days of age, on continuous ART for ≥96 weeks, and with HIV-1 RNA <40 copies/mL for at least 24 weeks before entry. Tatelo visits occurred at least every 4 weeks during bNAb administration, and at 1- to 2-week intervals during the bNAb-only step. All adverse events (grade 1 or higher) (25), including any infusion reactions, were recorded.
Laboratory testing
HIV-1 RNA (Abbott m2000sp/m2000rt, Abbott Molecular Inc.) quantified to a threshold of 40 copies/mL and qualitative DNA PCR (Roche Cobas Ampliprep/Cobas Taqman HIV-1 Qualitative Polymerase Chain Reaction) testing were performed at the Botswana Harvard HIV Reference Laboratory, Gaborone, Botswana, every 1 to 2 weeks during the bNAb-only step. Safety and monitoring evaluations included hematology, serum chemistry, CD4 and CD8 T cell count, and enzyme immunoassay (EIA) serologic HIV testing at 4- to 8-week intervals during bNAb administration. EIA was performed in parallel using Murex HIV-1.2.O (DiaSorin), Biorad Genetic Systems HIV-1/HIV-2 Plus O, or Abbott architect i1000SR (Abbott Diagnostics). Trough PK testing occurred before each bNAb dose, and anti-drug antibody (ADA) testing occurred at entry and following final bNAb dosing. bNAb PK testing was performed as described (24). ADA testing was performed by 3-tiered approach for VRC01LS at the Vaccine Research Center (21, 26), and for 10–1074 at Dartmouth College by electrochemiluminescence bridging assay (27, 28).
PBMCs were collected at least monthly during bNAb steps. ddPCR testing for a subset of specimens and FLIP-seq (29, 30) at entry and at last bNAb receipt were performed at the Ragon Institute. ddPCR was performed using the QX100 Droplet Digital PCR System (ddPCR; Bio-Rad) using primers and probes described previously (31) [127-base pair 5’-LTR-gag amplicon; coordinates 684 to 810 in HIV-1 reference strain HXB2] and normalized to the RPP30 gene. When viral copies were undetectable, data were reported as “limit of detection” (L.O.D., calculated as 0.2 copies per maximum number of cells tested without target identification) (5). Based on ddPCR results and Poisson distribution statistics, genomic DNA was diluted to single HIV-1 copies and subjected to HIV-1 near-full-genome amplification using a one amplicon approach with primers described previously (5). When there were no HIV-1 amplification products detectable, results were reported as L.O.D., calculated as 0.5 copies per maximum number of cells analyzed without target identification. Amplification products were subjected to Illumina MiSeq sequencing and our computational pipeline was used to distinguish intact and defective sequences as described before (5, 32). Monogram Biosciences performed neutralization assays for monoclonal antibodies (PhenoSense mAb) on plasma collected at time of virologic failure and env amplicons from full-length intact proviruses at baseline (usually from birth). We defined full susceptibility to each bNAb as 90% inhibitory concentration (IC90) ≤1.0 μg/mL and maximum percent inhibition ≥98% (3).
Study products and dosing
10–1074 was manufactured by Mass Bio under contract to the National Institute of Allergy and Infectious Diseases (NIAID) and dosed at 30 mg/kg intravenously every 4 weeks. VRC01LS was manufactured at the Vaccine Research Center, NIAID, and dosed at 30 mg/kg intravenously at entry and then continued at 15 mg/kg intravenously every 4 weeks. Administration of bNAbs occurred over approximately 60 minutes each. 10–1074 was given first, followed by VRC01LS, with a gap of approximately 10 to 15 minutes between infusions. Post-infusion monitoring for 2 to 4 hours occurred following the first infusion, and one hour with subsequent infusions.
Pre-specified objectives and definitions
Pre-specified primary outcomes were adverse events through study end graded by NIAID Division of AIDS criteria (25) and the proportion of children maintaining HIV-1 RNA in plasma <400 copies/mL through week 24 of the bNAb-only step. The protocol prespecified that ≥30% of children maintaining HIV-1 RNA <400 copies/mL for 24 weeks would likely represent a true effect of dual bNAbs on viral suppression. This percentage excluded overlap in 95% confidence intervals with the CHER Study (14, 15), where the estimated probability of HIV-1 RNA suppression <400 copies/mL at 6 months was 6% (range 3% to 10%) after treatment interruption among children who started ART at younger than 12 weeks of age.
Statistical analysis
All raw, individual-level data for experiments where n<20 are presented in data file S1. The sample size of children eligible for the EIT Study allowed reasonable precision to estimate the proportion able to maintain HIV-1 RNA suppression <400 copies/mL through Week 24 of the bNAb-only step. Study objectives were analyzed under a proof-of-concept framework, without a control arm. Cumulative incidence of detectable viremia ≥400 copies/mL during the bNAb-only step was estimated by Kaplan-Meier method. Characteristics of participants with ongoing viral suppression or viremia ≥400 copies/mL were compared using Wilcoxon Rank-Sum (for continuous variables) or Fisher’s Exact (for categorical variables) tests. Reported two-sided p-values and confidence intervals are presented as nominal, with a significance level set at 0.05. Analyses were conducted in SAS version 9.4.
Supplementary Material
Acknowledgments:
We would like to thank the Tatelo Study participants and their families. We thank the Tatelo and EIT Study teams and collaborators, including Dorcus Babuile, Rachel Bowman, Caroline Brackett, Loveness Bunhu, Alex Carnacchi, Lars Colson, Trevor Cordwell, Jack Disaro, Lorato Esele, Tshepho Frank, Olebile Kgakge, Tsholofelo Kebopetswe, Nametso Kelentse, Bob Lin, Judith Lucas, Kaia Lyons, Abraham Maigwa, Ria Madison, Princess Mapenshi, Mogomotsi Matshaba, Simon Masopa, Sam McMillan, Charlotte Mdluli, Lendsey Melton, Mompati Mmalane, Maureen Mosetlhi, Akanyang Motlhanka, Sarah Mudrak, Sandeep Narpala, Muhammed Naqvi, Thabani Ncube, Sandra Ndongwe, Martha Ngwaca, Maduo Oabona, Salome Othusitse, Gaoele Pelontle, Obonwe Pule, Lynette Purdue, Christina Reding, Marcella Sarzotti-Kelsoe, Tumalano Sekoto, Ngozana Seonyatseng, Dineo Tumagole, and Joshua Weiner; and, at Labcorp-Monogram Biosciences, Christos Petropoulos, Kristi Strommen, Yolanda Lie, Tim Persyn, and the Clinical Reference Lab. We also thank the Botswana Ministry of Health and Wellness and the Tatelo Safety Monitoring Committee, including Grace John-Stewart, Katherine Luzuriaga, Loeto Mazhani, Pablo Tebas, Terry Fenton, and Jane Lindsey.
Funding:
This work was funded by the US National Institute of Allergy and Infectious Diseases cooperative agreement U01 AI135940 (to RLS, DRK, and ML). RLS and ML are members of the PAVE Martin Delaney Collaboratory for HIV Cure Research (UM1 AI164566). ML is a member of the EPIICAL consortium, funded by ViiV Healthcare. SM was supported by the US NIH Fogarty International Center (K43 TW012350). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official positions of the funding agencies.
Footnotes
Competing interests: RLS, MH, SL, and JM serve on the governing boards of the Botswana Harvard Health Partnership. KB consulted with Harvard TH Chan School of Public Health, Massachusetts Eye and Ear Infirmary (MEEI), and University of Alabama at Birmingham. JDR is an employee and stockholder of Labcorp-Monogram Biosciences. DEY was formerly an unpaid technical advisor for the nonprofit organizations Cover the Globe and Maipelo Trust. DRK has served as a consultant for AbbVie, Gilead, GlaxoSmithKline, Janssen, Merck, and ViiV; has received research support from Gilead, Merck, and ViiV; has received speaking honoraria from Gilead and Janssen; and has provided expert testimony for Gilead.
Data availability:
All data associated with this study are in the paper or supplementary materials. Deidentified or partially deidentified data, as appropriate, will be made available after the completion of the study to researchers with an approved protocol who complete a data use agreement. All inquiries should be sent to the corresponding author.
References
- 1.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O’Brien C, Weiland D, Robles A, Kummerle T, Wyen C, Levin R, Witmer-Pack M, Eren K, Ignacio C, Kiss S, West AP Jr., Mouquet H, Zingman BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Klein F, Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 23, 185–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaebler C, Nogueira L, Stoffel E, Oliveira TY, Breton G, Millard KG, Turroja M, Butler A, Ramos V, Seaman MS, Reeves JD, Petroupoulos CJ, Shimeliovich I, Gazumyan A, Jiang CS, Jilg N, Scheid JF, Gandhi R, Walker BD, Sneller MC, Fauci A, Chun TW, Caskey M, Nussenzweig MC, Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature 606, 368–374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, Oliveira T, Lorenzi JC, Parrish EH, Learn GH, West AP Jr., Bjorkman PJ, Schlesinger SJ, Seaman MS, Czartoski J, McElrath MJ, Pfeifer N, Hahn BH, Caskey M, Nussenzweig MC, HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 352, 997–1001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Broncano P, Maddali S, Einkauf KB, Jiang C, Gao C, Chevalier J, Chowdhury FZ, Maswabi K, Ajibola G, Moyo S, Mohammed T, Ncube T, Makhema J, Jean-Philippe P, Yu XG, Powis KM, Lockman S, Kuritzkes DR, Shapiro R, Lichterfeld M, Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci Transl Med 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma P, Zangari P, Alteri C, Tchidjou HK, Manno EC, Liuzzi G, Perno CF, Rossi P, Bertoli A, Bernardi S, Early antiretroviral treatment (eART) limits viral diversity over time in a long-term HIV viral suppressed perinatally infected child. BMC Infect Dis 16, 742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sneller MC, Blazkova J, Justement JS, Shi V, Kennedy BD, Gittens K, Tolstenko J, McCormack G, Whitehead EJ, Schneck RF, Proschan MA, Benko E, Kovacs C, Oguz C, Seaman MS, Caskey M, Nussenzweig MC, Fauci AS, Moir S, Chun TW, Combination anti-HIV antibodies provide sustained virological suppression. Nature 606, 375–381 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham CK, McFarland EJ, Morrison RL, Capparelli EV, Safrit JT, Mofenson LM, Mathieson B, Valentine ME, Perlowski C, Smith B, Hazra R, Purdue L, Muresan P, Harding PA, Mbengeranwa T, Robinson LG, Wiznia A, Theron G, Lin B, Bailer RT, Mascola JR, Graham BS; IMPAACT P1112 team. Safety, Tolerability, and Pharmacokinetics of the Broadly Neutralizing Human Immunodeficiency Virus (HIV)-1 Monoclonal Antibody VRC01 in HIV-Exposed Newborn Infants. J Infect Dis 23, 628–636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland EJ, Cunningham CK, Muresan P, Capparelli EV, Perlowski C, Morgan P, Smith B, Hazra R, Purdue L, Harding PA, Theron G, Mujuru H, Agwu A, Purswani M, Rathore MH, Flach B, Taylor A, Lin BC, McDermott AB, Mascola JR, Graham BS; International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1112 Team. Safety, Tolerability, and Pharmacokinetics of a Long-Acting Broadly Neutralizing Human Immunodeficiency Virus Type 1 (HIV-1) Monoclonal Antibody VRC01LS in HIV-1-Exposed Newborn Infants. J Infect Dis 224,1916–1924 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maswabi K, Ajibola G, Bennett K, Capparelli EV, Jean-Philippe P, Moyo S, Mohammed T, Batlang O, Sakoi M, Lockman S, Makhema J, Lichterfeld M, Kuritzkes DR, Hughes MD, Shapiro RL, Safety and Efficacy of Starting Antiretroviral Therapy in the First Week of Life. Clin Infect Dis 72, 388–393 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, Cohen YZ, Wyen C, Kummerle T, Karagounis T, Lu CL, Handl L, Unson-O’Brien C, Patel R, Ruping C, Schlotz M, Witmer-Pack M, Shimeliovich I, Kremer G, Thomas E, Seaton KE, Horowitz J, West AP Jr., Bjorkman PJ, Tomaras GD, Gulick RM, Pfeifer N, Fatkenheuer G, Seaman MS, Klein F, Caskey M, Nussenzweig MC, Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, Rist M, Rademeyer C, Yoon H, Lapedes A, Gao H, Greene K, Louder MK, Kong R, Karim SA, Burton DR, Barouch DH, Nussenzweig MC, Mascola JR, Morris L, Montefiori DC, Korber B, Seaman MS, Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog 12, e1005520 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niesar A, Lian X, Hua R, Ajibola G, Pretorius Holme M, Maswabi K, Moyo S, Maphorisa NC, Mohammed T, Mosetlhi M, Powis KM, Kuritzkes D, Shapiro RL, Lichterfeld M. Viral reservoir landscape of children with HIV in Botswana treated with dual bNAbs. 30th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle; 19–22 Feb 2023. Abstract 141. [Google Scholar]
- 14.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, Josipovic D, Liberty A, Lazarus E, Innes S, van Rensburg AJ, Pelser W, Truter H, Madhi SA, Handelsman E, Jean-Philippe P, McIntyre JA, Gibb DM, Babiker AG, Team CS, Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 382, 1555–1563 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violari A, Chan M, Otwombe KN, Panchia R, Jean-Philippe P, Gibb D, Cotton M, Babiker A. Time to viral rebound after stopping ART in children treated from infancy in CHER. Conference on Retroviruses and Opportunistic Infections (CROI); Boston; 4–7 March 2018. Abstract 137. [Google Scholar]
- 16.Awan SF, Happe M, Hofstetter AR, Gama L, Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS 17, 247–257 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Noailly B, Yaugel-Novoa M, Werquin J, Jospin F, Drocourt D, Bourlet T, Rochereau N, Paul S, Antiviral Activities of HIV-1-Specific Human Broadly Neutralizing Antibodies Are Isotype-Dependent. Vaccines (Basel) 10, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC, Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352, 1001–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O’Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, Team VRCS, Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7, 319ra206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O’Dell SJ, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, Chun TW, Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 375, 2037–2050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, DeZure A, Lynch RM, Gordon I, Plummer S, Hendel CS, Pegu A, Conan-Cibotti M, Sitar S, Bailer RT, Narpala S, McDermott A, Louder M, O’Dell S, Mohan S, Pandey JP, Schwartz RM, Hu Z, Koup RA, Capparelli E, Mascola JR, Graham BS, Team VRCS, Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 182, 289–301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corey L, Gilbert PB, Juraska M, Montefiori DC, Morris L, Karuna ST, Edupuganti S, Mgodi NM, deCamp AC, Rudnicki E, Huang Y, Gonzales P, Cabello R, Orrell C, Lama JR, Laher F, Lazarus EM, Sanchez J, Frank I, Hinojosa J, Sobieszczyk ME, Marshall KE, Mukwekwerere PG, Makhema J, Baden LR, Mullins JI, Williamson C, Hural J, McElrath MJ, Bentley C, Takuva S, Gomez Lorenzo MM, Burns DN, Espy N, Randhawa AK, Kochar N, Piwowar-Manning E, Donnell DJ, Sista N, Andrew P, Kublin JG, Gray G, Ledgerwood JE, Mascola JR, Cohen MS, Hvtn H, Teams HHS, Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N Engl J Med 384, 1003–1014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosetlhi M, Ajibola G, Haghighat R, Batlang O, Maswabi K, Pretorius Holme M, Powis KM, Lockman S, Makhema J, Lichterfeld M, Kuritzkes D, Shapiro RL. Caregivers of children with HIV in Botswana prefer monthly IV bNAbs to daily oral ART. 30th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle; 19–22 Feb 2023. Abstract 828. [Google Scholar]
- 24.Capparelli EV, Ajibola G, Maswabi K, Holme MP, Bennett K, Powis KM, Moyo S, Mohammed T, Maphorisa C, Hughes MD, Seaton KE, Tomaras GD, Mosher S, Taylor A, O’Connell S, Narpala S, McDermott A, Caskey M, Gama L, Lockman S, Jean-Philippe P, Makhema J, Kuritzkes DR, Lichterfeld M, Shapiro RL, Tatelo Study T, Safety and Pharmacokinetics of Intravenous 10–1074 and VRC01LS in Young Children. J Acquir Immune Defic Syndr 91, 182–188 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Division of AIDS. Table for Grading the Severity of Adult and Pediatric Adverse Events, version 2.1 July 2017. [cited 2021 July 8]. Available from: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. [Google Scholar]
- 26.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, Holman LA, Gordon I, Plummer S, Hendel CS, Conan-Cibotti M, Lorenzo MG, Sitar S, Carlton K, Laurencot C, Bailer RT, Narpala S, McDermott AB, Namboodiri AM, Pandey JP, Schwartz RM, Hu Z, Koup RA, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, Team VRCS, Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 15, e1002493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen YZ, Butler AL, Millard K, Witmer-Pack M, Levin R, Unson-O’Brien C, Patel R, Shimeliovich I, Lorenzi JCC, Horowitz J, Walsh SR, Lin S, Weiner JA, Tse A, Sato A, Bennett C, Mayer B, Seaton KE, Yates NL, Baden LR, deCamp AC, Ackerman ME, Seaman MS, Tomaras GD, Nussenzweig MC, Caskey M, Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10–1074 in healthy adults: A randomized, phase 1 study. PLoS One 14, e0219142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharadwaj P, Riekofski C, Lin S, Seaman MS, Garber DA, Montefiori D, Sarzotti-Kelsoe M, Ackerman ME, Weiner JA, Implementation of a three-tiered approach to identify and characterize anti-drug antibodies raised against HIV-specific broadly neutralizing antibodies. J Immunol Methods 479, 112764 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang C, Lian X, Gao C, Sun X, Einkauf KB, Chevalier JM, Chen SMY, Hua S, Rhee B, Chang K, Blackmer JE, Osborn M, Peluso MJ, Hoh R, Somsouk M, Milush J, Bertagnolli LN, Sweet SE, Varriale JA, Burbelo PD, Chun TW, Laird GM, Serrao E, Engelman AN, Carrington M, Siliciano RF, Siliciano JM, Deeks SG, Walker BD, Lichterfeld M, Yu XG, Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 585, 261–267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee GQ, Orlova-Fink N, Einkauf K, Chowdhury FZ, Sun X, Harrington S, Kuo HH, Hua S, Chen HR, Ouyang Z, Reddy K, Dong K, Ndung’u T, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M, Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J Clin Invest 127, 2689–2696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M, HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 20, 139–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartana CA, Garcia-Broncano P, Rassadkina Y, Lian X, Jiang C, Einkauf KB, Maswabi K, Ajibola G, Moyo S, Mohammed T, Maphorisa C, Makhema J, Yuki Y, Martin M, Bennett K, Jean-Philippe P, Viard M, Hughes MD, Powis KM, Carrington M, Lockman S, Gao C, Yu XG, Kuritzkes DR, Shapiro R, Lichterfeld M, Immune correlates of HIV-1 reservoir cell decline in early-treated infants. Cell Rep 40, 111126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are in the paper or supplementary materials. Deidentified or partially deidentified data, as appropriate, will be made available after the completion of the study to researchers with an approved protocol who complete a data use agreement. All inquiries should be sent to the corresponding author.


