Atherosclerosis is a multi-faceted disease caused by the synergism of cardiovascular risk factors including hyperlipidemia, aging, and disturbed flow pattern. A common pathophysiological exertion of these atherosclerotic risks is redox stress imposed on the vasculature. Research advances in neuron degeneration diseases show that excessive reactive oxygen species increase oxidative modifications of guanine (G), including 8OH-dG–modified DNA and 8OH-G–modified RNA causing a guanine-to-uracil (G-U) transversion.1 If undergoing this G-to-U transversion in their seed sequence, microRNAs (miRs) no longer effectively bind to their cognate mRNAs. Alternatively, 8OH-G–modified miRs may recognize new mRNA targets.2,3 Here, we studied whether and how 8OH-G–modified miRs are involved in endothelial dysfunction, thus aggravating atherosclerosis.
8OH-G–modified RNAs emerged in the cytosol of cultured endothelial cells (ECs) when challenged with H2O2, oxidized-LDL (ox-LDL), or oscillatory flow (OS) (Fig. A). RNA electrophoresis revealed that a large proportion (~30%) of these modified RNAs were 8OH-G–modified miRs (Fig. B). Cross-linking and immunoprecipitation (CLIP) miR-seq analysis of the 8OH-G–modified RNAs revealed that 8OH-G–modified miR-106b, miR-99b, miR-25, miR-1, miR-127, and miR-483 were the most enhanced in ECs induced by ox-LDL (Fig. C). Indeed, H2O2, ox-LDL, and OS significantly altered the ratio of 8OH-G–modified miR-483 (hereafter 8OH-G miR-483) to miR-483 in cultured ECs (Fig. D–E). In C57 mice, the level of 8OH-G miR-483 was ~3-fold higher in the aortic arch (under atheroprone flow) than thoracic aorta (under atheroprotective flow; Fig. D–E). In line with the finding that miR-483 targets proprotein convertase subtilisin/kexin type 9 (PCSK9),4 ratios of 8OH-G miR-483 to miR-483 were higher in serum collected from hypercholesterolemic mice and humans than respective controls [Fig. D–E; Animals were maintained in accordance with NIH guidelines, and the IACUC of UCSD approved all experimental procedures (No. S12263); The study protocol for humans was approved by the ethics committees of Xi’an Jiaotong University. Written informed consent was obtained from all individuals].
Epitranscriptomic modification of miR-483 to 8OH-G miR-483 impairs EC function and increases atherosclerosis susceptibility.
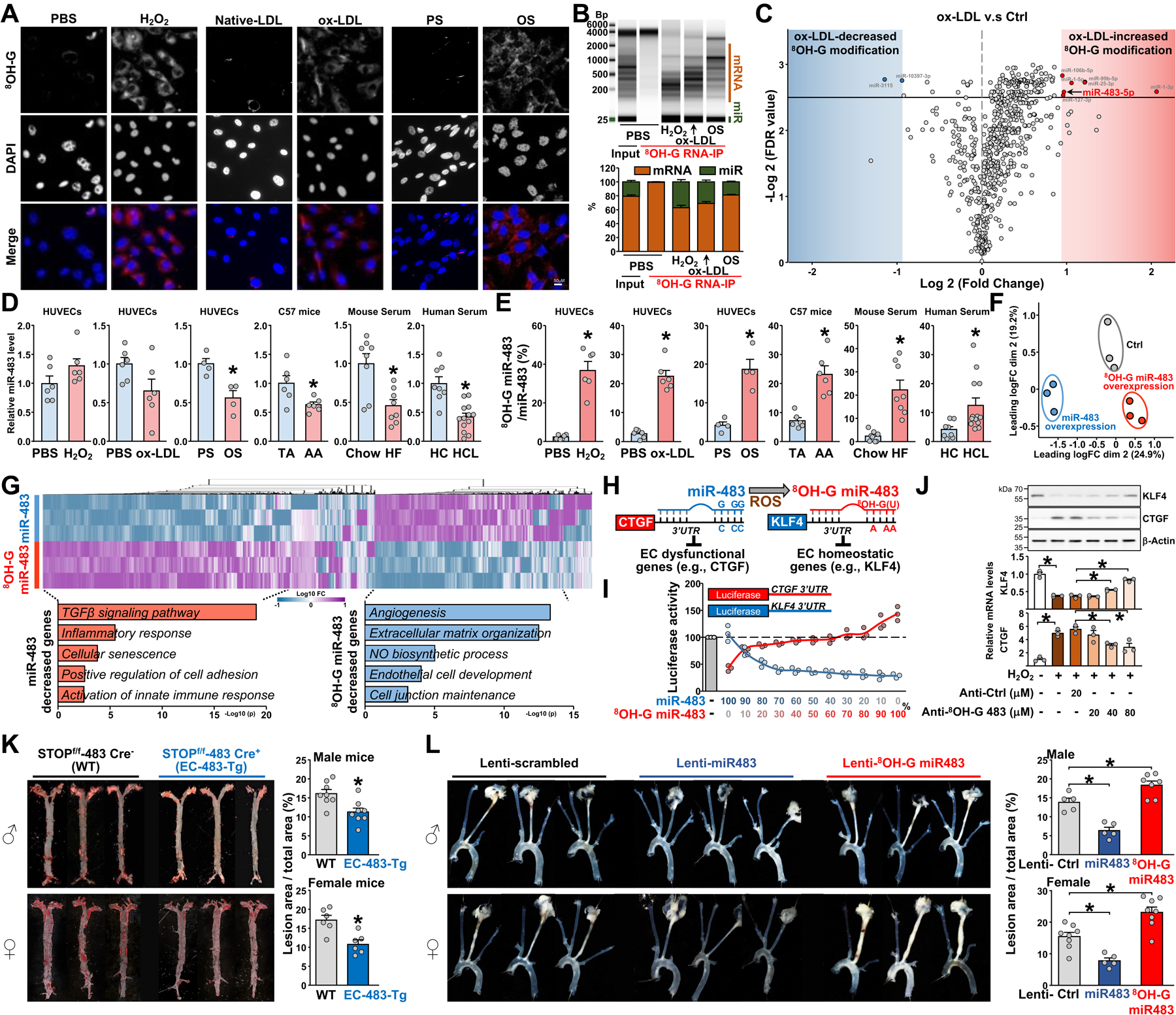
(A-E) Human umbilical vein endothelial cells (HUVECs) were treated with H2O2 (100 μM; with the same volume of PBS as controls) for 4 hr or oxidized-LDL (ox-LDL, 100 μg/mL; with the same amount of native-LDL as controls) for 24 hr or exposed to oscillatory shear stress [OS, 0.5±4 dyn/cm2; with pulsatile shear stress (PS), 12±4 dyn/cm2 as controls] for 16 hr. In (A), cells were stained with an 8OH-G antibody (revealed by red staining; Catalog No, 12501, clone 15A3, QED Bioscience; dilution 1:500) and co-stained with DAPI (Catalog No, D1206, Invitrogen) and images were captured and analyzed by Olympus FV1000 confocal microscopy. Scale bars: 50 μm. In (B), total cell lysates were isolated and immunoprecipitated with an 8OH-G antibody (5 μL per 1 mg protein) or isotype mouse IgG control antibody, then total RNA was isolated by Trizol reagent (Invitrogen). Total RNA was analyzed by electrophoresis by using the TapeStation system (Agilent). In (C), miRs were isolated from the 8OH-G antibody-immunoprecipitated RNAs or input total RNAs by using the mirVana miRNA Isolation Kit (Invitrogen). cDNA sequencing libraries were prepared by using TruSeq Small RNA Library Preparation Kits (Illumina) and then sequenced by using the NovaSeq 6000 system (Illumina) as 50 single-end reads. The x-axis of volcano plot represents the binary logarithm modified fold change of 8OH-G modified miRs (ox-LDL v.s. Ctrl), and the y-axis represents the minus of the binary logarithm modified FDR (false discovery rate) value. In (D, E), the thoracic aorta (TA) or aortic arch (AA) was isolated from 30 C57BL/6 mice (15 males). Each dot represents samples pooled from 5 mice. Serum was isolated from C57Bl/6j mice under an 8-week chow diet (n=8, 4 males) or mice with a single-dose tail vein injection of 1.0×1011 vector genome rAAV8-D377Y-mPCSK9 (Addgene #58376) followed by an 8-week high-fat diet (HF, 40 kcal% fat, 1.25% cholesterol, 0.5% cholic acid, Catalog No. D12109, Research Diets. N=8, 4 males). Serum was collected from hypercholesterolemia individuals (LDL-C>130mg/dL, n=13) or sex- and age-matched ones with normal lipid profile (LDL-C <110 mg/dL, n=8). The lipid profiles were detected by automatic chemical analysis (Hitachi LABOSPECT 008AS). All individuals were enrolled at the First Affiliated Hospital of Xi’an Jiaotong University during 2018 to 2019. Purified RNAs were incubated with an anti-8OH-G antibody for immunoprecipitation (IP). The input, miR-483, and 8OH-G miR-483 were analyzed by qPCR. Mouse IgG was an isotype IP control. The level of miR-483 (D) and ratio of 8OH-G miR-483-to-miR-483 (E) were shown. HC, healthy controls; HCL, hypercholesterolemia. (F, G) HUVECs were transfected with miR-483, 8OH-G miR-483, or scramble control (Ctrl) for 48 hr, then transcriptomes were analyzed by RNA-seq. All miRs were customized in Integrated DNA Technologies. (F), mapping of transcriptomes from each sample for a 2-D PCA analysis. In (G), heatmap comparison of log2 fold change (FC) for differential expressed genes in ECs overexpressing miR-483 or 8OH-G miR-483 (FC >1.5, FDR <0.1). Gene Ontology enrichment analyzed by using Metascape for the top 300 miR-483– or 8OH-G miR-483–downregulated genes. (H) Schematic diagram demonstrating the epitranscriptomic modification of miR-483 to 8OH-G miR-483 and the altered targeting from EC dysfunctional genes (e.g., CTGF) to EC homeostatic genes (e.g., KLF4). (I) Bovine aortic ECs transfected with different molar ratios of miR-483 to 8OH-G miR-483 together with Luc-hCTGF-3’-UTR (shown in red) or Luc-hKLF4–3’-UTR (shown in blue) for 48 hr. Luciferase activity was measured by pRL-TK activity as a transfection control. (J) HUVECs were transfected with anti-sense oligonucleotide against 8OH-G-miR-483 (Anti-8OH-G 483, 5’- dTdTØCAAGUAUØCAAGUAUØCAAGUAUØCAAGUAUØdTdT-3’) or anti-control (scramble sequence) at the dose as indicated, followed by H2O2 (100 μM; with the same volume of PBS as controls) treatment for 4 hr. The protein expression levels of KLF4 (abcam, ab272860; 1:1000 dilution) and CTGF (abcam, ab6992; 1:750 dilution) were assessed by western blotting, with β-Actin (abcam, ab8227; 1:5000 dilution) used as an internal control. mRNA levels were determined through qPCR. (K) Six-week-old EC-483-Tg and wild-type (WT) mice (8 males and 6 females in each group) were administered a single dose of rAAV8-D377Y-mPCSK9 (1.0×1011 vector genomes) via tail vein injection and fed a high-fat diet for 8 weeks. En face oil-red O staining of aortae are shown. (L) Eight-week-old male and female C57BL/6 mice were administered AAV8-PCSK9 and fed western diet to induce atherosclerosis. One week later, the left carotid artery was partially ligated and administered Lentivirus overexpressing scramble miRNA (Lenti-Ctrl, 5 male and 8 female mice), miR-483 (Lenti-miR-483, 5 male and 5 female mice), or the 8OH-G miR-483 mimic oligonucleotide (Lenti-8OH-G miR-483, with G-to-U mutation in the seed sequence of mmu-miR-483, 7 male and 8 female mice) through tail vein injection. The right carotid artery was shamed operated. All mice were sacrificed three weeks later and carotid arteries were isolated. The representative images showed arteries isolated from 3 male and 3 female mice in each groups. In (D), (E), (J), (K), and (L), data are expressed as mean ± SEM. Data were initially tested for normality and equal variance to confirm the appropriateness of parametric tests. Data with normal distribution and equal variance were analyzed by 2-tailed Student t test. If the normality or equal variance test failed, nonparametric data were analyzed by 2-tailed Mann-Whitney U test. * P<0.05 was considered statistically significant.
To explore the effect of 8OH-G miR-483 in EC transcriptomes, we overexpressed miR-483, 8OH-G miR-483, and scramble miR in ECs. Principal component analysis of RNA-seq profiling revealed transcriptomic segregations among the ECs transfected with miR-483, 8OH-G miR-483, and scramble miR (Fig. F; the associated sequencing data have been made publicly available at the NCBI’s GEO as GSE226665). Gene Ontology analysis of the 4,641 differentially expressed genes (fold change >1.5, false discovery rate [FDR] <0.1) suggested that miR-483 suppressed genes involved in transforming growth factor β signaling, inflammatory response, cellular senescence, positive regulation of cell adhesion, and activation of the innate immune response. In contrast, 8OH-G miR-483 downregulated genes involved in angiogenesis, extracellular matrix organization, nitric oxide biosynthetic process, EC development, and cell junction maintenance (Fig. G). Thus, the modification of miR-483 to 8OH-G miR-483 was postulated to cause EC dysfunction.
The G-to-U transversion of miR-483 alters the original mRNA targeting efficacy and allows 8OH-G miR-483 to recognize new mRNA target sites. As such, miR-483 targets connective tissue growth factor (CTGF) mRNA,5 but 8OH-G miR-483, with its G-U transversion, targets krüppel-like factor 4 (KLF4) mRNA (Fig. H). To validate such alterations in mRNA targetomes, we transfected ECs with Luc-hCTGF-3’-UTR or Luc-hKLF4–3’-UTR together with a mixture of miR-483 and 8OH-G miR-483 varying in molar ratios. At higher ratios of miR-483 to 8OH-G miR-483, lower luciferase activity was found for the Luc-hCTGF-3’-UTR reporter, indicative of miR-483 targeting the CTGF mRNA 3’-UTR (Fig. I). However, higher ratios of 8OH-G miR-483 to miR-483 resulted in decreased activity of the Luc-hKLF4–3’-UTR reporter, owing to 8OH-G miR-483 targeting the KLF4 mRNA 3’-UTR. To further elucidate the detrimental effect of 8OH-G-miR-483 in vitro, we overexpressed an anti-sense oligonucleotide (ASO) against 8OH-G-miR-483 in cultured ECs and then treated with H2O2. The anti-8OH-G-miR-483 ASO decreased the mRNA and protein levels of CTGF, while increased those of KLF4 in a dose-dependent manner (Fig. J).
To further investigate the role of the transversion of miR-483 to 8OH-G miR-483 in atherosclerosis in mouse models, we generated an EC-miR-483 transgenic (Tg) line by crossing the VE-Cadherin Cre line with a ROSA26-STOPflox/flox-miR-483 knock-in line. Then mice were administered AAV8-PCSK9 via tail vein injection followed by an 8-week high-fat diet to induce atherosclerosis. Both male and female EC-483-Tg mice showed ameliorated atherosclerotic lesions as compared with their wild-type littermates, and this reduction was seen in the aortic arch and thoracic aorta (Fig. K). These results suggest that higher ratios of miR-483 to 8OH-G miR-483 in endothelium are atheroprotective in rodents. We also performed partial carotid artery ligation experiments in C57Bl/6j mice with lentiviral overexpressing scramble miRNA (Lenti-Ctrl), miR-483 (Lenti-miR-483), or the 8OH-G-miR-483 mimic oligonucleotide (with G-to-U mutation in the seed sequence of mmu-miR-483). As shown in Fig. L, Lenti-miR-483 administration significantly reduced restenosis, whereas 8OH-G-miR-483 administration increased restenosis in both male and female mice.
Collectively, this study demonstrates that the redox burden incurred by cardiovascular risk factors drives the modification of miR-483 to 8OH-G miR-483. Without genetic sequence alteration, this epitranscriptomic modification of miR alters its targetome, thus leading to an atheroprone phenotype. Increased ratios of 8OH-G miR-483 to miR-483 may be common in various endothelial beds under redox stress. Hence, interventions aiming at changing the ratio of miR-483 to 8OH-G miR-483 might be feasible to improve EC health.
Supplementary Material
Acknowledgements
We thank Melody Shyy at UC Santa Barbara for experimental assistance.
Funding Sources
This work was supported in part by American Heart Association Career Development Award 859625 and NIH R21AG075450 (to M.H.), U54AG065141 (to J.S., S.C.), and the National Natural Science Foundation of China 82200455 (to C.W.), the Warshel Institute for Computational Biology funding from Shenzhen City and Longgang District (to H.-Y.H.), Excellent Doctoral Dissertation Incubation Grant of First Clinical School of Guangzhou University of Chinese Medicine YB202001 (to J.W).
Non-standard Abbreviations and Acronyms
- CTGF
connective tissue growth factor
- FDR
false discovery rate
- KLF4
krüppel-like factor 4
- PCSK9
proprotein convertase subtilisin/kexin type 9
- UTR
untranslated region
Footnotes
Disclosures
None.
References
- 1.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JX, Gao J, Ding SL, Wang K, Jiao J-Q, Wang Y, Sun T, Zhou L-Y, Long B, Zhang X-J, et al. Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol Cell. 2015;59:50–61. [DOI] [PubMed] [Google Scholar]
- 3.Seok H, Lee H, Lee S, Ahn SH, Lee H-S, Kim G-WD, Peak J, Park J, Cho YK, Jeong Y, et al. Position-specific oxidation of miR-1 encodes cardiac hypertrophy. Nature. 2020;584:279–285. [DOI] [PubMed] [Google Scholar]
- 4.Dong J, He M, Li J, Pessentheiner A, Wang C, Zhang J, Sun Y, Wang W-T, Zhang Y, Liu J, et al. MicroRNA-483 ameliorates hypercholesterolemia by inhibiting PCSK9 production. JCI Insight. 2020;5:e143812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He M, Chen Z, Martin M, Zhang J, Sangwung P, Woo B, Tremoulet AH, Shimizu C, Jain MK, Burns JC, et al. MiR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: therapeutic implications in Kawasaki disease. Circ Res. 2017;120:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


