Abstract
Loss of control (LOC) eating is the defining feature of binge-eating disorder, and it has particular relevance for bariatric patients. The biomarkers of LOC eating are unclear; however, gut hormones (i.e., ghrelin, cholecystokinin [CCK], peptide YY [PYY], glucagon-like peptide 1 [GLP-1], and pancreatic polypeptide [PP]), adipokines (i.e., leptin, adiponectin), and pro- and anti-inflammatory cytokines/markers (e.g., high-sensitivity C-reactive protein [hsCRP]) are candidates due to their involvement in the psychophysiological mechanisms of LOC eating. This review aimed to synthesize research that has investigated these biomarkers with LOC eating. Because LOC eating is commonly examined within the context of binge-eating disorder, is sometimes used interchangeably with subclinical binge-eating, and is the latent construct underlying disinhibition, uncontrolled eating, and food addiction, these eating behaviors were included in the search. Only studies among individuals with overweight or obesity were included. Among the identified 31 studies, 2 studies directly examined LOC eating and 4 studies were conducted among bariatric patients. Most studies were case-control in design (n=16) and comprised female-dominant (n=13) or female-only (n=13) samples. Studies generally excluded fasting total ghrelin, fasting CCK, fasting PYY, and fasting PP as correlates of the examined eating behaviors. However, there was evidence that the examined eating behaviors were associated with lower levels of fasting acyl ghrelin (the active form of ghrelin) and adiponectin, higher levels of leptin and hsCRP, and altered responses of postprandial ghrelin, CCK, and PYY. The use of GLP-1 analog was able to decrease binge-eating. In conclusion, this review identified potential biomarkers of LOC eating. Future studies would benefit from a direct focus on LOC eating (especially in the bariatric population), using longitudinal designs, exploring potential mediators and moderators, and increased inclusion of the male population.
Keywords: Loss of control eating, binge-eating, gut hormones, adipokines, inflammation, cytokines
1. Introduction
Binge-eating disorder (BED), a mental illness that affects 2.8% of women and 1.0% of men worldwide1, is characterized by recurrent binge-eating episodes in the absence of compensatory behaviors2. Binge-eating consists of two intercorrelated but dissociable features: loss of control (LOC) eating and overeating2,3. LOC eating is a subjective experience of being or feeling out of control when eating, no matter the amount of food consumed. As individuals who report LOC eating with or without overeating present similar psychosocial profiles3,4, LOC eating has been widely recognized as the most salient feature of BED. Notably, emerging evidence has supported the validity of LOC eating as an independent construct3. For example, independent of overeating, LOC eating has unique cross-sectional and prospective associations with various physiological and psychosocial vulnerabilities, such as obesity5, metabolic syndromes6,7, general psychopathology (e.g., depression), and eating-related psychopathology (e.g., body dissatisfaction)8,9.
The clinical relevance of LOC eating is particularly prominent in patients with severe obesity who seek or have undergone bariatric surgery. First, LOC eating is prevalent in this population, affecting 6.6%10-78.6%11 of the preoperative and 5.412-50.7%13 of the postoperative patients. Second, LOC eating at post-surgery predicts adverse surgical outcomes, including less weight loss14, weight regain12,15, and more surgical complications (e.g., dumping, vomiting)16,17. Finally, and most importantly, as bariatric surgery dramatically reduces gastric volume, alters the gastrointestinal environment, and restricts eating capacity, overeating becomes physically difficult or impossible after surgery (at least in the short-term). Therefore, the overeating feature inherent in binge-eating may not apply to postoperative patients, making the importance of LOC eating stand out.
With increasing emphasis being placed on LOC eating, many efforts have been made to identify its physiological and psychosocial mechanisms. Physiologically, LOC eating involves a disruption in the homeostatic and hedonic eating regulation systems. The homeostatic system controls physiological appetite, hunger, and satiety through the hypothalamus, especially the hypothalamic arcuate nucleus18. Individuals with LOC eating have imbalanced expressions of orexigenic and anorexigenic neurons within the arcuate nucleus, reporting increased appetite, increased hunger, and decreased satiety compared to healthy controls19. The hedonic system regulates eating behavior mainly through the dopamine and opioid reward circuits, which modulates the “wanting” (food craving) and “liking” (food enjoyment) components of food reward, respectively. An accumulating body of behavioral and neuroimaging studies has shown that individuals with LOC eating self-report an elevated food craving/enjoyment and exhibit heightened brain reward responsivity for high-energy foods (e.g., fats, sweets)20,21. Consequently, they are more likely to consume high-energy foods than those without LOC eating22,23.
The psychosocial mechanisms of LOC eating have been widely studied both within and outside the bariatric population, and several types of risk factors have been identified. These psychosocial risk factors include negative emotions or affects (e.g., anxiety, depression, distress)24,25, maladaptive emotion regulations strategies (e.g., rumination, suppression)26,27, weight-related dysfunctional behaviors or perceptions (e.g., dietary restraint, weight suppression, and body dissatisfaction)28,29, and deficits in cognitive control, especially inhibitory control30,31.
While the physiological and psychosocial mechanisms of LOC eating have been somewhat uncovered, the biomarkers that are related to LOC eating are less studied in the general or bariatric population. Gut hormones, adipokines, and pro- and anti-inflammatory cytokines/markers are promising candidates because they are involved in homeostatic and hedonic eating regulation. As a result, the alterations of these biomarkers may increase or decrease individuals’ appetite, hunger, satiety, and perceived food reward, thus ultimately influencing their motivations to initiate or stop consuming food and contributing to the experience of difficulty in stopping eating (LOC eating). Additionally, these biomarkers are closely related to the psychosocial risk factors of LOC eating32–37.
Gut hormones include the “hunger” hormone, ghrelin, and “satiety” hormones such as cholecystokinin (CCK), peptide YY (PYY), glucagon-like peptide 1 (GLP-1), and pancreatic polypeptide (PP). Ghrelin activates the orexigenic and dopaminergic neurons, thus promoting appetite, hunger, food craving, and food-seeking behaviors38,39. Ghrelin exists in circulation in two major forms: acylated and des-acylated ghrelin. While the majority of circulating ghrelin is des-acylated, the acylated form is thought to be essential for ghrelin’s biological activity in appetite stimulation38. In contrast, CCK, PYY, GLP-1, and PP inhibit orexigenic neurons, thus promoting satiety and eating termination. Additionally, there is evidence that GLP-1 also suppresses dopaminergic neurons40. Besides participating in hemostatic and hedonic eating regulations, gut hormones also regulate moods and cognitive functions that are related to LOC eating. For example, research has repeated demonstrated the anti-depressant effect of ghrelin32 and the cognitive-enhancing effect of GLP-133.
Leptin and adiponectin are adipokines that are mostly secreted by white adipose tissue. Leptin acts as a negative feedback signal to control energy homeostasis at the hypothalamus41,42, and it also suppresses dopamine signaling to reduce the craving or motivation to seek and consume food42. Although there is no consensus, it has been suggested that adiponectin regulates eating behavior in a glucose-dependent fashion. At low glucose conditions, adiponectin downregulates orexigenic and upregulates anorexigenic neurons to attenuate appetite; at high glucose levels, adiponectin downregulates both orexigenic and anorexigenic activities43,44. In addition to regulating eating behaviors, as the receptors of leptin and adiponectin are widely distributed in hippocampus and neocortex, they are likely to be involved in emotion regulation and cognitive control34,36.
Pro-inflammatory cytokines, including interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are involved in eating regulation possibly through acting on hypothalamus45,46 and dopaminergic neurocircuits47,48, and through interacting with eating-regulation hormones (e.g., ghrelin, GLP-1, leptin)49,50. Despite inconclusive evidence, animal and human studies have shown that IL-1β, IL-6, and TNF-α suppress appetite51,52 and eating motivation53,54, hence reducing meal size and meal duration55,56. Furthermore, pro-inflammatory cytokines are intertwined with emotions and moods, such as depression, anxiety, and stress37, which are all risk factors of LOC eating.
The pro-inflammatory cytokines are positively associated with inflammatory markers: C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). In contrast, the synthesis of pro-inflammatory cytokines is inhibited by anti-inflammatory cytokines, such as interleukin-10 (IL-10) and interleukin-13 (IL-13). Therefore, these inflammatory markers and anti-inflammatory cytokines are potentially related to LOC eating, although their effect on eating regulation is unclear.
Recognizing the role of gut hormones, adipokines, and inflammatory cytokines/markers in eating regulation and psychosocial functioning, prior studies have reviewed their relationships with eating disorders57–59. However, these reviews predominantly focused on bulimia nervosa and anorexia nervosa, of which information cannot be readily applied to LOC eating due to the distinctive disease symptoms among these eating behaviors57–59. To address this gap, the aim of this review was to identify and synthesize quantitative research that has thus far investigated the associations of gut hormones, adipokines, or pro- and anti-inflammatory cytokines/markers with LOC eating in both children and adults. A greater understanding of the biomarkers related to LOC eating could advance the identification, prevention, and treatment of this disordered eating behavior. Additionally, as LOC eating is prevalent and negatively impacts weight loss and metabolic outcomes among bariatric patients, such knowledge could facilitate the development of interventions to promote optimal surgical outcomes.
2. Methods
2.1. Study design
A scoping review was conducted to examine the range and extent of existing evidence available on gut hormones, adipokines, and inflammatory cytokines/markers related to LOC eating. A scoping review instead of a systematic review was chosen because there are few publications on this topic, and the goal of a scoping review is to examine emerging and unclear evidence60. A standard five-stage process of scoping review was followed: (1) identifying the research question, (2) identifying relevant studies, (3) selecting studies, (4) charting the data, (5) collating, summarizing, and reporting the results60.
2.2. Inclusion/exclusion criteria
Although LOC eating has been increasingly accepted as an independent construct and there is a growing call to study LOC eating and overeating separately3,61,62, in most cases, it is examined within the context of BED. Additionally, subclinical binge-eating is sometimes used interchangeably with LOC eating. Therefore, both BED and subclinical binge-eating were included in the search. Moreover, given studies have provided evidence that disinhibition, uncontrolled eating (a loss of control on food intake in response to emotional or external stimuli), and food addiction (a loss of control on food intake accompanied by other symptoms such as tolerance and withdraw) share the latent construct of “loss of control”63,64, these eating behaviors were also included in the review. Studies that examined bulimia nervosa were excluded because although it has the defining feature of binge-eating, the levels of hormones, adipokines, and inflammatory cytokines/markers could be influenced by compensatory behaviors such as purging behaviors and vomiting.
It is known that the levels and functions of gut hormones, adipokines, and inflammatory cytokines/markers are influenced by body weight. For example, in individuals with obesity, levels of ghrelin and adiponectin are generally lower while levels of leptin and pro-inflammatory cytokines/markers are elevated65,66. To eliminate the confounding effect of body weight and increase the potential applicability to bariatric patients, only studies conducted among individuals with overweight or obesity (body mass index [BMI]>25 kg/m2) were included.
In general, studies were included if they 1) reported associations of gut hormones, adipokines, or inflammatory cytokines/markers with LOC eating, subclinical binge-eating, BED, disinhibition, uncontrolled eating, or food addiction among individuals with overweight or obesity, or 2) compared the biomarkers between individuals with these eating behaviors and unaffected controls, where participants in both groups were overweight or obese, or 3) manipulated the biomarkers and reported an effect on these eating behaviors among individuals with overweight or obesity.
Studies published in non-English languages, and animal studies, abstracts, editorials, case studies, book chapters, dissertation work, review papers, or kin studies were excluded.
2.3. Search strategy
Three electronic databases, including PubMed (1947–March 5, 2021), PsycINFO (1967–March 5, 2021), and Embase (1974–March 5, 2021) were searched to obtain relevant studies. The search included the combination of the following keywords: gut hormones, ghrelin, CCK, PYY, GLP-1, PP, leptin, adiponectin, inflammation, IL-1α, IL-1β, IL-6, TNF-α, IL-10, IL-13, CRP, ESR, LOC eating, binge-eating, disinhibition, uncontrolled eating, and food addiction. The database search was complemented by a hand search of the reference lists obtained from the identified articles.
2.4. Study selection
A total of 31 studies were included in the final review. The study selection flow is presented in Figure 1. Author 1 (YY) screened titles and abstracts, both author 1 and author 2 (WZ) reviewed full-text articles.
Figure 1.
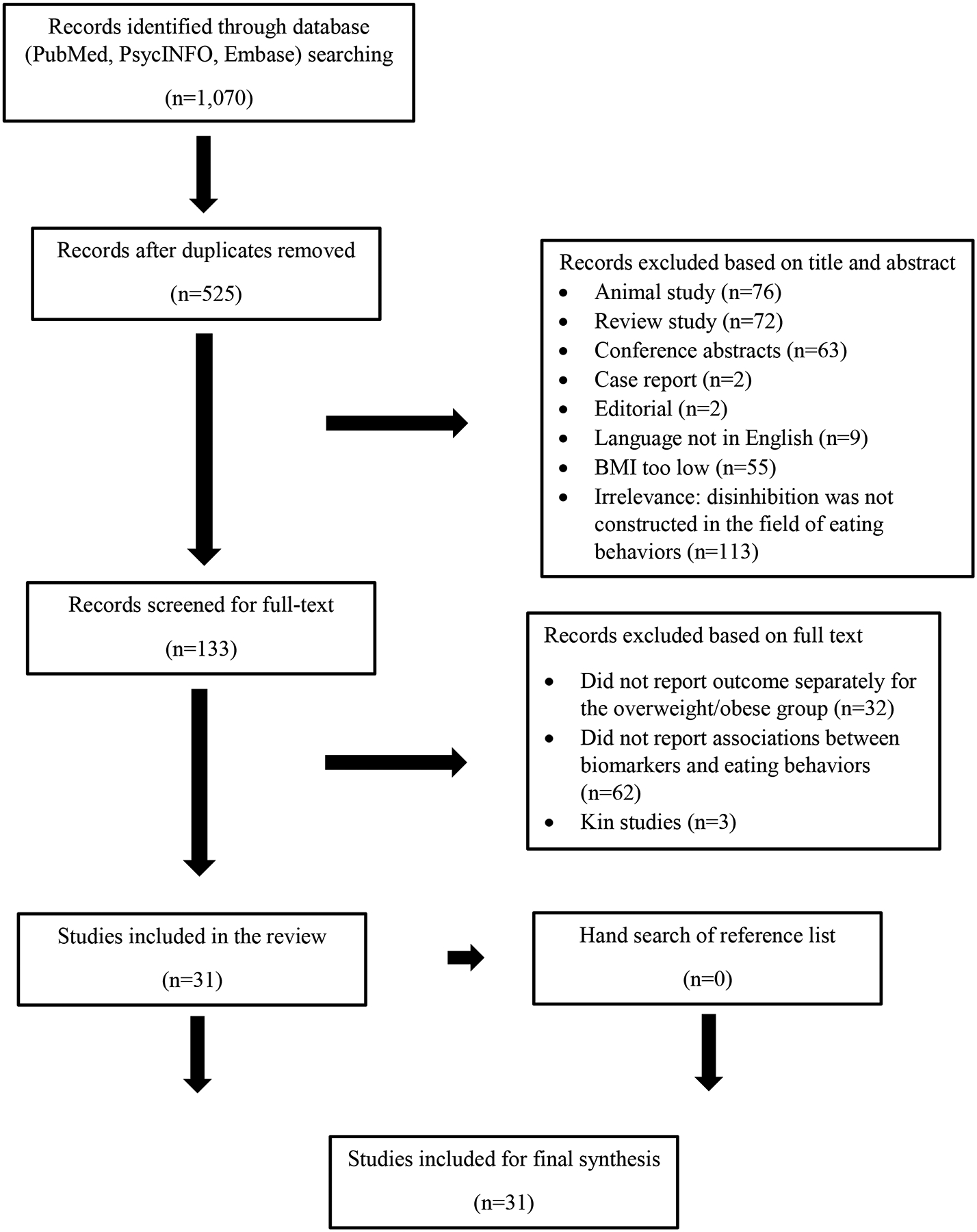
Study Selection Flow
2.5. Data extraction and synthesis
Key components extracted from each paper included: 1) study characteristics of title, first author, country, publication year, and study design; 2) participant characteristics of gender, age, race, BMI, sample size, and inclusion/exclusion criteria; 3) biomarkers assessed; 4) eating behaviors assessed; and 5) main study conclusion.
3. Results
3.1. Study and participant characteristics
The extracted data were organized according to individual biomarkers (Table 1). In terms of study characteristics, the 31 studies were conducted in 13 different countries with Italy (n=9) and US (n=8) accounted for the majority. Eleven studies were published in the last 5 years and 9 in the last 10 years. Most of the studies were case-control in design (n=16), followed by longitudinal (n=6), cohort (n=2), cross-sectional (n=4), randomized controlled trial (RCT, n=2), and randomized cross-over (n=1) designs. For the 16 case-control and 2 cohort studies, except for 1 study67, BMI was either comparable between groups68–80 or was adjusted as a covariate in analysis81–84. Regarding sample characteristics, 4 studies were conducted among pre- or post-bariatric patients75,85–87, and participants from the remaining studies were patients who were seeking eating disorder (n=3) or behavioral weight loss treatment (n=6), were attending pediatric (n=3), endocrinology (n=3), or psychiatric clinics (n=1), non-treatment seeking (n=5), a mix of treatment-seeking and non-treatment seeking (n=2), and not specified (n=4). Six studies were conducted among children or adolescents78,79,83,84,88,89, and the remaining 25 were among adults. Except for 1 study that did not report sex composition90 and 4 studies that comprised comparable proportions of males and females72,84,88,91, studies were female-dominant (n=13) or female-only (n=13). Two studies directly examined LOC eating78,83 and 6 studies examined subclinical binge-eating72–74,84,90,92 (none of these were conducted among bariatric population), others examined BED (n=13), disinhibition or uncontrolled eating (n=8), and food addiction (n=2).
Table 1.
Description of included studies (N=31)
| Study characteristics | Sample description | Biomarker | Eating behavior | Conclusion |
|---|---|---|---|---|
| Ghrelin | ||||
Circulating ghrelin is decreased in non-obese and obese women with binge eating disorder as well as in obese non-binge eating women, but not in patients with bulimia nervosa68
|
|
|
|
|
Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight86
|
|
|
|
|
The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat81
|
|
|
|
|
Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED)71
|
|
|
|
|
Lifestyle intervention discloses an association of the Eating Inventory-51 factors with cardiometabolic health risks94
|
|
|
|
|
Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery87
|
|
|
|
|
Ghrelin and peptide YY increase with weight loss during a 12-month intervention to reduce dietary energy density in obese women95
|
|
|
|
|
Hormonal and dietary characteristics in obese human subjects with and without food addiction69
|
|
|
|
|
Appetite sensations, appetite signaling proteins, and glucose in obese adolescents with subclinical binge eating disorder84
|
|
|
|
|
Plasma ghrelin levels and weight regain after Roux-en-Y Gastric Bypass Surgery85
|
|
|
|
|
CCK, ghrelin, and PYY responses in individuals with binge eating disorder before and after a cognitive behavioral treatment (CBT)70
|
|
|
|
|
Meal-related acyl and des-acyl ghrelin and other appetite-related hormones in people with obesity and binge eating72
|
|
|
|
|
Association between des-acyl ghrelin at fasting and predictive index of muscle derangement, metabolic markers and eating disorders: a cross-sectional study in overweight and obese adults92
|
|
|
|
|
| CCK | ||||
Gastric capacity, test meal intake, and appetitive hormones inbinge eating disorder73
|
|
|
|
|
Glycemic index, cholecystokinin, satiety and disinhibition: is there an unappreciated paradox for overweight women?97
|
|
|
|
|
CCK, ghrelin, and PYY responses in individuals with binge eating disorder before and after a cognitive behavioral treatment (CBT)70
|
|
|
|
|
Meal-related acyl and des-acyl ghrelin and other appetite-related hormones in people with obesity and binge eating72
|
|
|
|
|
| PYY | ||||
Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED)71
|
|
|
|
|
Ghrelin and peptide YY increase with weight loss during a 12-month intervention to reduce dietary energy density in obese women95
|
|
|
|
|
Lifestyle intervention discloses an association of the Eating Inventory-51 factors with cardiometabolic health risks94
|
|
|
|
|
Hormonal and dietary characteristics in obese human subjects with and without food addiction69
|
|
|
|
|
Appetite sensations, appetite signaling proteins, and glucose in obese adolescents with subclinical binge eating disorder84
|
|
|
|
|
CCK, ghrelin, and PYY responses in individuals with binge eating disorder before and after a cognitive behavioral treatment (CBT)70
|
|
|
|
|
Meal-related acyl and des-acyl ghrelin and other appetite-related hormones in people with obesity and binge eating72
|
|
|
|
|
Association between eating behavior, anthropometric and biochemical measurements, and peptide YY (PYY) hormone levels in obese adolescents in outpatient care88
|
|
|
|
|
| GLP-1 | ||||
Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED)71
|
|
|
|
|
Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery87
|
|
|
|
|
Hormonal and dietary characteristics in obese human subjects with and without food addiction69
|
|
|
|
|
Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome100
|
|
|
|
|
Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide — A pilot study90
|
|
|
|
|
Dulaglutide reduces binge episodes in type 2 diabetic patients withbinge eating disorder: A pilot study91
|
|
|
|
|
Meal-related acyl and des-acyl ghrelin and other appetite-related hormones in people with obesity and binge eating72
|
|
|
|
|
| PP | ||||
Lifestyle intervention discloses an association of the Eating Inventory-51 factors with cardiometabolic health risks94
|
|
|
|
|
Hormonal and dietary characteristics in obese human subjects with and without food addiction69
|
|
|
|
|
| Leptin & Adiponectin | ||||
Relationship between dietary restraint, binge eating, and leptin in obese women74
|
|
|
|
|
Serum leptin concentration in obese patients with binge eating disorder75
|
|
|
|
|
Opposite modifications in circulating leptin and soluble leptin receptor across the eating disorder spectrum76
|
|
|
|
|
Gastric capacity, test meal intake, and appetitive hormones inbinge eating disorder73
|
|
|
|
|
Leptin/adiponectin ratio in obese women with and without binge eating disorder67
|
|
|
|
|
Cardiovascular stress reactivity and recovery in bulimia nervosa and binge eating disorder77
|
|
|
|
|
Lifestyle intervention discloses an association of the Eating Inventory-51 factors with cardiometabolic health risks94
|
|
|
|
|
Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery87
|
|
|
|
|
Hormonal and dietary characteristics in obese human subjects with and without food addiction69
|
|
|
|
|
Serum leptin and loss of control eating in children andadolescents78
|
|
|
|
|
The association of serum leptin levels with food addiction is moderated by weight status in adolescent psychiatric inpatients89
|
|
|
|
|
Comparison of endocannabinoids levels, FAAH gene polymorphisms, and appetite regulatory substances in women with and without binge eating disorder: a cross-sectional study101
|
|
|
|
|
Altered regional grey matter volume and appetite-related hormone levels in adolescent obesity with or without binge-eating disorder79
|
|
|
|
|
| Inflammatory markers | ||||
Lifestyle intervention discloses an association of the Eating Inventory-51 factors with cardiometabolic health risks94
|
|
|
|
|
Hormonal and dietary characteristics in obese human subjects with and without food addiction69
|
|
|
|
|
Obese patients with a binge eating disorder have an unfavorable metabolic and inflammatory profile82
|
|
|
|
|
Pediatric loss of control eating and high-sensitivity C-Reactive Protein concentrations83
|
|
|
|
|
Brain-behavior-immune interaction: serum cytokines and growth factors in patients with eating disorders at extremes of the body mass index (BMI) Spectrum80
|
|
|
|
|
3.2. Biomarkers Related to LOC Eating, Subclinical Binge-Eating, BED, Disinhibition, Uncontrolled Eating, or Food Addiction
3.2.1. Fasting ghrelin
Ghrelin is an appetite-stimulating peptide that increases food intake. In a healthy population, circulating levels of ghrelin increase during fasting to promote meal initiation and decrease shortly after meal consumption to terminate an eating episode38. Ghrelin has two major molecular forms (acylated and des-acylated ghrelin)—only the acyl ghrelin is able to bind to the ghrelin receptor and stimulate appetite93.
Twelve studies have examined fasting ghrelin in relation to subclinical binge-eating, BED, disinhibition, uncontrolled eating, or food addiction. The majority (n=9) did not support a role of fasting total ghrelin in these eating behaviors. Specifically, 3 studies reported that fasting total ghrelin did not differ between individuals with subclinical binge-eating84, BED68, or food addiction69 and unaffected controls. Next, 5 studies did not find significant cross-sectional or longitudinal associations between fasting total ghrelin and disinhibition86,94,95 or uncontrolled eating85,87 among patients who sought or had undergone surgical or behavioral weight loss treatment. Finally, 1 longitudinal study70 assessed fasting total ghrelin and BED before and after a cognitive-behavioral therapy (CBT). This study found that fasting total ghrelin did not respond to the intervention, and it failed to predict binge-eating behaviors at pre- or post-intervention.
In contrast to these null findings, an earlier study found that fasting total ghrelin was lower in women with BED relative to non-BED controls, and this difference was normalized after CBT71. However, results should be interpreted with caution because the ghrelin changes from pre- to post-intervention were independent of the CBT treatment and were not related to the self-reported binge-eating days71.
Unlike the above studies that examined total ghrelin, two recent studies72,92 specifically examined its active form—acyl ghrelin—and reported significant findings. One study compared the fasting acyl ghrelin between adults with and without subclinical binge-eating, which revealed that participants with subclinical binge-eating had significantly lower fasting acyl ghrelin concentrations than unaffected controls72. Similarly, a significant negative association was observed between fasting acyl ghrelin and binge-eating behaviors in a cross-sectional study conducted among 88 adults with overweight or obesity92.
Overall, studies appeared to be consistent in indicating that fasting total ghrelin did not have a role in subclinical binge-eating, BED, disinhibition, uncontrolled eating, and food addiction. However, some evidence supports a reduced level of acyl ghrelin in patients with subclinical binge-eating that warrants future investigation.
3.2.2. Postprandial ghrelin
Postprandial ghrelin (total or acylated) has been assessed in 6 studies with conflicting results. In one study71 conducted among women with BED (n=10) and controls (n=9), the authors found that postprandial total ghrelin was lower and showed a blunted decline following meal consumption in the BED group after adjusting for weight change. One recent study72 had similar findings in that they documented that acyl ghrelin (but not des-acyl ghrelin) was significantly lower and declined slower after a meal in adults with subclinical binge-eating (n=20) than in unaffected controls (n=22). However, 3 other studies70,81,84 were unable to replicate this finding, which reported levels and responses of total ghrelin following test meals were comparable between adults or adolescents with and without BED. Additionally, a study that was conducted among bariatric patients did not detect any significant associations between postprandial total ghrelin and uncontrolled eating before or at 12 months following bariatric surgery87.
Two studies of the above 6 studies also tested whether postprandial ghrelin changed following CBT. One study71 reported that the lower level and blunted response of postprandial total ghrelin in the BED group were “normalized” after CBT, although the “normalization” could not be conclusively attributed to the intervention. Conversely, the other study70 did not find any intervention effect on postprandial total ghrelin level or response, despite 50% of the patients achieved BED abstinence at the end of the intervention.
The inconsistent results among these studies could be partially attributed to a lack of differentiation the two forms (acylated and des-acylated) of ghrelin, which may bias the study results. Furthermore, the small sample sizes (ranged from 6 to 20 in the binge-eating group), different participant characteristics (e.g., 5 different countries: US, Italy, UK, Canada, Switzerland; a wide age range: mean age ranged from 13 to 50), and methodological differences across the studies could also contribute to the mixed findings. For example, the 6 studies used 6 different test meals with different total energy (ranged from 300 kcal to 797 kcal) and energy compositions. In addition, they adjusted for different covariates (e.g., age72,81, sex70,72,81,84, BMI70,72,81,87, weight change71, fat mass84), were comprised of treatment-seeking81,84,87 or non-treatment seeking samples70, and adopted different outcome measures (e.g., Eating Disorder Examination70,72,84, Questionnaire on Eating and Weight Patterns71,72, Three Factor Eating Questionnaire87).
In summary, study results are mixed in terms of the association between postprandial ghrelin and LOC eating. However, there is some evidence that the level and response of postprandial ghrelin, especially acyl ghrelin, to meal consumption may be lower and blunted in patients with subclinical binge-eating or BED71,72.
3.2.3. Fasting and postprandial CCK
CCK is a satiety hormone that reduces food intake96. In the general population, the circulating level of CCK increases rapidly in response to a meal to promote meal termination96. Three studies have examined fasting CCK related to subclinical binge-eating or BED. All 3 of them reported that fasting CCK did not differ between adults with and without binge-eating70,72,73. One of these studies was longitudinal in design, in which 18 patients with BED received an 8-week CBT. Neither the fasting CCK was affected by the intervention, nor it was related to the binge-eating behaviors at post-intervention70.
Postprandial CCK was assessed in 4 studies and findings were mixed. Two studies72,73 did not find significant differences in postprandial CCK between adults with subclinical binge-eating or BED and unaffected controls. However, in one study70, patients with BED relative to controls exhibited an augmented CCK secretion within 60 minutes following meal ingestion, which was not corrected by an 8-week CBT. The authors interpreted the heightened CCK stimulation as an initial effort of the central nervous system to prevent individuals with BED from bingeing. In contrast, in another study conducted among a cohort of women with overweight, the authors observed a positive association between blunted CCK secretion and high disinhibition, but the association was significant only among women who had a high level of dietary restraint97. This result suggested potential interactions among dietary restraint, CCK responses, and disinhibition.
The samples across the 4 studies that examined postprandial CCK tended to share similar characteristics. For example, they were mostly female, middle-aged, from US, and non-treatment seeking. However, all of the studies had small sample sizes (ranged from 11 to 20 in the binge-eating group). Additionally, there were other variations among studies, including various test meals (4 different meals), different covariates controlled in the analysis (e.g., age72, sex70,72, BMI70,72, and weight change97), and varied outcome measurements (e.g., Questionnaire on Eating and Weight Patterns, Eating Disorder Examination).
To sum up, despite the limited number of studies that have been done, fasting CCK has been consistently found to be unrelated to subclinical binge-eating or BED. The association of postprandial CCK with BED70 or disinhibition97 was found in two studies. However, the responses of postprandial CCK were controversial between these studies70,97. Additionally, there is preliminary evidence suggesting the interactions among dietary restraint, disinhibition, and CCK secretion97.
3.2.4. Fasting and postprandial PYY
PYY is a hormone that increases satiety and suppresses food intake. In the general population, PYY levels increase within 15 minutes in response to food intake and contribute to termination of food intake98. Eight studies have examined fasting PYY with subclinical binge-eating, BED, uncontrolled eating, disinhibition, or food addiction, and all of them reported null findings. In detail, 5 studies documented that fasting PYY did not differ between adults or adolescents with subclinical binge-eating72,84, BED70,71, or food addiction69 and unaffected controls. Additionally, 2 of these studies did not find any intervention effect of CBT on fasting PYY among patients with BED70,71. Finally, 3 studies did not find significant cross-sectional or longitudinal associations between fasting PYY and uncontrolled eating88 or disinhibition94,95 among adolescents or adults with obesity.
Postprandial PYY has been assessed in 4 studies, and 3 of them reported that the PYY secretion in response to test meals was comparable between adolescents or adults with subclinical binge-eating72,84 or BED71 and controls. Additionally, postprandial PYY did not respond to a 6-week CBT among patients with BED71. In contrast, 1 study observed a higher increase in PYY within the first 80 minutes following meal ingestion in patients with BED vs non-BED controls70. It was interpreted that the more intense stimulation of the PYY secretion after food intake was an adaptive response from the central nervous system and the gut to counteract the initiation of binge-eating.
In summary, current studies did not support fasting PYY as a significant correlate of subclinical binge-eating, BED, uncontrolled eating, disinhibition, or food addiction. While most studies did not detect postprandial PYY alterations in subclinical binge-eating or BED, one study found an augmented response that deserves further investigation70.
3.2.5. Fasting and postprandial GLP-1
GLP-1 suppresses appetite, promotes satiety, and reduces energy intake99. In the general population, GLP-1 increases rapidly after meal ingestion to prevent overeating99. Four observational studies have examined fasting GLP-1 with subclinical binge-eating, BED, uncontrolled eating, and food addiction, and they consistently reported null findings. Among these 4 studies, 3 studies did not find significant differences in fasting GLP-1 between adults with subclinical binge-eating72, BED71, or food addiction69 and controls, and the fasting GLP-1 did not change after BED treatment71. In the remaining study, fasting GLP-1 was not related to uncontrolled eating among 12 patients at pre-, 2 and 12 months post-bariatric surgery87. Three of the above 4 studies also assessed postprandial GLP-1. They found that postprandial GLP-1 was comparable between adults with and without subclinical binge-eating72 or BED71, was nonresponsive to CBT among patients with BED71, and was not significantly related to uncontrolled eating among bariatric patients87.
In contrast to the above observational studies that reported null findings, 3 intervention studies that tested the effect of GLP-1 analog—liraglutide—on subclinical binge-eating, BED, and uncontrolled eating reported significant findings. One randomized controlled trial90 assigned non-diabetic patients with subclinical binge-eating to either a 12-week lifestyle intervention (n=21) or lifestyle intervention plus liraglutide (n=21), and results revealed that at the end of the intervention, participants who received liraglutide showed significant improvement in binge-eating. The other 2 studies reported similar results by demonstrating significant reductions of BED among adults with diabetes91 and reductions of uncontrolled eating among women with polycystic ovary syndrome100 after 12-week treatment of liraglutide.
Taken together, while observational studies consistently reported null findings, intervention studies suggested the possible relevance of GLP-1 to subclinical binge-eating, BED, and uncontrolled eating.
3.2.6. Fasting PP
PP is a gut hormone that reduces food intake99. In the general population, PP is released in response to food ingestion to prevent overconsumption of food99. Only 2 studies have examined PP, with one study that reported fasting PP did not differ between adults with and without food addiction69 and the other study reported no significant association between fasting PP and disinhibition among a cohort of women with obesity94.
3.2.7. Leptin
Thirteen studies have examined leptin in relation to LOC eating, subclinical binge-eating, BED, disinhibition, uncontrolled eating, and food addiction, and 5 studies identified significantly higher levels of leptin in these eating behaviors. In detail, 4 studies found significantly higher levels of leptin in adolescents with LOC eating78, bariatric candidates or women with BED75,101, and adolescents with food addiction89 relative to controls. Among these 4 studies, 1 study also reported that higher levels of leptin predicted higher odds of binge-eating behaviors among women with BED101, and another study demonstrated a positive association between leptin and disinhibition among bariatric candidates (regardless of BED diagnosis)75. In line with these findings, 1 study reported that leptin levels went up in parallel with the increase of uncontrolled eating among bariatric candidates87.
There was one study that reported LOC eating as an independent outcome. In that study the authors explored the mediating or moderating effects of dietary restraint and/or sex in the relationship between leptin and LOC eating. Results revealed that the positive relationship between leptin and LOC eating was significant for females only. Moreover, the relationship was partially mediated by higher dietary restraint after controlling for age, race, sex, socioeconomic, fat mass, height, treatment-seeking status, and pubertal status78.
Unlike the above studies that supported significantly higher levels of leptin in LOC eating, BED, uncontrolled eating, and food addiction, 6 studies reported null findings. However, 4 of them found that although lacking statistical significance, the leptin levels were higher in adults or adolescents with subclinical binge-eating74, BED76,79, or food addiction69 compared to unaffected controls. Importantly, it was found that leptin levels increased linearly along with the increase of binge-eating severity among women with obesity74. It should be noted that all these 4 studies had a relatively small sample size (ranged from 18 to 35 in the binge-eating or food addiction group), which may be underpowered to detect any statistical significance. There were other 2 studies that did not detect differences of leptin between women with BED73 or disinhibition94 and controls; yet, statistics were not provided in these 2 studies.
Two studies found lower leptin levels in BED vs non-BED groups. Specifically, in 1 case-control study, leptin levels were found to be significantly lower in women with BED than non-BED controls67. However, it should be noted that BMI was significantly higher in the non-BED group than the BED group and it was not controlled as a covariate in the analysis, which may explain the contrasting finding in this study. The lower leptin levels in women with vs. without BED were observed in another study, but the difference was minor (46.4 vs. 50.7 μg), and the authors did not provide a level of statistical significance77.
Overall, with some exceptions, studies generally supported higher leptins levels in LOC eating, subclinical binge-eating, BED, disinhibition, uncontrolled eating, and food addiction (with or without statistical significance). Additionally, there is preliminary evidence that the relationship is mediated by dietary restraint and moderated by sex.
3.2.8. Adiponectin
Three studies have examined adiponectin in relation to BED, disinhibition, and food addiction, and they consistently demonstrated lower adiponectin levels in individuals with these eating behaviors. Specifically, 1 study67 observed significantly lower adiponectin levels in women with BED compared to non-BED women; a second study69 reported lower adiponectin levels (statistically nonsignificant) in adults with food addition compared with age-, sex-, BMI-, and physical activity-matched controls; and a third study94 detected a negative association between adiponectin and disinhibition among 67 women who participated in a 4-week lifestyle intervention.
Taken together, despite the limited number of studies, it appeared that the adiponectin levels were lower in individuals with BED, disinhibition, or food addiction.
3.2.9. Pro- and anti-inflammatory cytokines/markers
Five studies have examined pro- (i.e., IL-1α, IL-1β, IL-6, TNF-α, CRP, ESR) and anti-inflammatory (i.e., IL-10) cytokines/markers with LOC eating, BED, disinhibition, and food addiction. CRP has been assessed in 3 studies: 2 of them reported that high-sensitivity CRP (hsCRP) was significantly higher in adolescents with LOC eating83 or adults with BED82 than unaffected controls; the remaining study did not find a significant association between CRP and disinhibition among a cohort of women with obesity94. Notably, the latter study examined CRP instead of hsCRP and did not adjust for covariates in analysis, which may introduce bias and lack sensitivity in capturing the differences in inflammatory status. ESR has been assessed in 1 study82, in which significantly higher ESR levels were observed in the BED vs non-BED group.
TNF-α has been examined in 2 studies: 1 study showed a significantly lower level of TNF-α in individuals with food addiction compared to those without food addiction69; and the other study showed no difference in levels between adults with BED and non-BED controls (statistics not provided)80. The different findings may be due to the sample characteristics (e.g., Canadian69 vs Italian80, non-treatment seeking69 vs treatment seeking80), outcome measures (food addiction69 vs BED80), and covariates controlled in analysis (non-specified69 vs depressive symptoms, sex, and age80). The latter study also examined other pro- and anti-inflammatory cytokines such as IL-1α, IL-1β, IL-6, and IL-10. While no significant differences were detected in IL-1α, IL-1β, or IL-6 between the BED and control groups, IL-10 was found to be significantly lower in the BED group80.
In summary, limited studies have assessed the relationship between inflammatory cytokines/markers and LOC eating. There may be potential associations of LOC eating with elevated inflammatory status, including higher hsCRP, higher ESR, and lower IL-10.
4. Discussion
This scoping review synthesized current evidence regarding gut hormones, adipokines, and pro- and anti-inflammatory cytokines/markers related to LOC eating, subclinical binge-eating, BED, disinhibition, uncontrolled eating, and food addiction, among children or adults with overweight or obesity. Results can be summarized as follows: 1) only 2 studies directly examined LOC eating and 6 examined subclinical binge-eating, and only 4 studies were conducted among bariatric patients; 2) fasting total ghrelin, fasting CCK, fasting PYY, and fasting PP did not appear to be related to the eating behaviors as mentioned above; 3) although studies were scarce and findings were inconsistent, there was evidence supporting lower levels of fasting acyl ghrelin and adiponectin, higher levels of leptin and pro-inflammatory markers (e.g., hsCRP, ESR), and altered responses of postprandial ghrelin (blunted), CCK (blunted or amplified), and PYY (amplified) in the aforementioned eating behaviors; and 3) using GLP-1 analog decreased binge-eating.
The lower fasting acyl ghrelin and blunted responses of postprandial ghrelin (total or acylated) observed in individuals with subclinical binge-eating or BED suggested that ghrelin, especially acyl ghrelin, is a potential correlate of LOC eating. Considering that the acyl ghrelin acts to stimulate appetite and promote eating, the lower fasting acyl ghrelin is unlikely to cause binge-eating or LOC eating; instead, it may represent a secondary change or an adaption aiming to counteract repeated binge-eating or LOC eating. The blunted responses of postprandial ghrelin imply that the normal suppression of hunger and food craving after meal consumption is impaired, which may contribute to the initiation or maintenance of binge-eating or LOC eating. Studies in patients with bulimia nervosa have similarly observed an attenuated decrease of postprandial ghrelin102–104 following a meal, although it is not clear whether the attenuation is due to the binge behavior or purging behavior. Furthermore, animal studies have provided additional evidence supporting the involvement of ghrelin in binge-eating or LOC eating. For example, there have been observations that ghrelin receptor-deficient mice failed to induce binge-eating105,106 and central ghrelin infusion enhanced binge-like behaviors in palatable schedule fed rats107.
It is worth emphasizing that the lower levels of fasting ghrelin and blunted responses of postprandial ghrelin observed in subclinical binge-eating or BED are not universal findings. In addition to reasons like small sample sizes and methodological differences across studies, the failure to distinguish acylated and des-acylated ghrelin may bias and undermine the validity of findings based on total ghrelin concentration. Given recent evidence that des-acylated ghrelin can impair the orexigenic actions of acyl ghrelin108,109, future studies should examine the separate forms rather than the total concentration of ghrelin.
Included studies consistently showed that fasting CCK, PYY, and PP did not differ between individuals with and without subclinical binge-eating, BED, uncontrolled eating, disinhibition, or food addiction, indicating that these fasting hormones may not be significant corelates of LOC eating. However, 2 studies reported altered postprandial CCK (amplified70 or blunted97) and PYY responses (amplified70) in those with BED or disinhibition compared to unaffected controls, suggesting that the alterations may be relevant to LOC eating. The blunted CCK increase post-meal has also been observed in the BN population110–112, and it is speculated that the blunted response may play a role in the diminished satiety observed in BN and contribute to the initiation, perpetuation, or relapse of this eating disorder. The finding that individuals with BED had amplified increases of postprandial CCK and PYY as reported in 1 study70 was not replicated anywhere else, although the interpretation that the augmented responses reflect an effort to prevent binge initiation is plausible. Given that current studies produced inconsistent findings of whether and in which direction the postprandial CCK and PYY responses were altered, further investigations are needed on this topic.
All of the observational studies did not support fasting or postprandial GLP-1 as a significant indicator of subclinical binge-eating, BED, uncontrolled eating, and food addiction. However, the interventional drug trials utilizing GLP-1 analog have demonstrated effectiveness in reducing binge-eating and uncontrolled eating, suggesting there is potential GLP-1 involvement in LOC eating. Several studies conducted in the BN population have established a rationale to consider GLP-1 as an indicator of LOC eating. With that said, these studies have noted that among patients with BN, GLP-1 was positively associated with bingeing behaviors113 but not purging behaviors114, implying that GLP-1 may be a unique indicator of binge-eating or LOC eating.
The leptin level was found to be higher in patients with LOC eating, subclinical binge-eating, BED, disinhibition, or uncontrolled eating compared with unaffected controls, albeit a few exceptions. Since participants included in this review were all overweight or obese, the higher levels of leptin suggest a specific link between this adipokine and LOC eating that is not simply explained by the enhanced fat stores or leptin resistance associated with extra weight. Furthermore, the study that reported a strong association between leptin and LOC eating after adjusting for adiposity provides direct evidence to consider leptin as a significant indicator of LOC eating. Besides, studies conducted in the BN population have also detected higher leptin levels115 and a positive association between leptin and bingeing behaviors75,116 among patients with BN, which adds support to the possibility that higher leptin levels may play a role in the development or maintenance of LOC eating.
Adiponectin is less investigated than leptin, and the few studies consistently reported lower levels of adiponectin in individuals with BED, disinhibition, or food addiction compared to unaffected controls. As mentioned, the effect of adiponectin on eating regulation is glucose-dependent. However, one limitation of these few studies is that they did not consider glucose levels, which preclude clear conclusions about the role that adiponectin plays in these eating behaviors. Despite this limitation, the lower levels of adiponectin are synchronized with the behavioral manifestations of LOC eating. For example, individuals with LOC eating show a trend of eating faster and consuming more high-energy foods, both of which are negatively associated with adiponectin levels117–119.
The inflammation markers (hsCRP, ESR) have been found to be elevated in adolescents with LOC eating and adults with BED relative to unaffected controls, indicating that LOC eating may be associated with an elevated inflammatory status. Additional support for this association comes from the Avon Longitudinal Study of Parents and Children (ALSPAC), which followed 3480 nationally representative children from birth to 18 years of age. Using the ALSPAC data, one study found that children with higher levels of IL-6 and CRP had greater odds of binge-eating in adolescence, although these associations were weak120. It is worth mentioning that one study included in this review reported opposite findings, in which pro-inflammatory cytokine (TNF-α) decreased in individuals with food addiction. Beyond differences in sample characteristics and methodologies, the discrepancy may be due to the complex interaction between inflammation and gut hormones and adipokines. For example, research has shown that the gut hormones (e.g., ghrelin, CCK) and adiponectin suppress the production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β121,122. In contrast, leptin acts in opposition facilitating the secretion of these cytokines123. Therefore, without evaluating these interactions, it is difficult to conclude on the relationships between the inflammatory cytokines/markers and LOC eating.
Regardless of biomarker types, 4 common limitations were identified across all of the reviewed studies: studies that directly examine LOC eating are limited in the general population and are absent in bariatric population; there is an insufficient number of longitudinal studies; there is a lack of examination of potential mediators and moderators; and lastly, an underrepresentation of males.
This review only identified 2 studies that directly examined LOC eating78,83 and 6 studies that examined subclinical binge-eating72–74,84,90,92, all of which were conducted among non-bariatric children and adolescents. Although all other eating behaviors (e.g., BED, disinhibition) chosen for review share the core feature of “loss of control”, they are broader constructs that embrace other disordered eating behaviors. For example, BED additionally includes overeating, and disinhibition overlaps with emotional eating and external eating. Even for the studies that examined LOC eating or subclinical binge-eating, measurement limitations exist—they involved only a dichotomous assessment, which failed to reflect current evidence that LOC eating should be studied on a continuum of severity124,125. As LOC eating has been increasingly acknowledged as an independent construct3 and two specific, continuous scales (Eating Loss of Control Scale, Loss of Control over Eating Scale) have been developed and validated in individuals with obesity125–128, future research is needed that makes LOC eating a more explicit focus. Additionally, studies on bariatric patients are warranted because LOC eating is of particular importance for this population.
A few of the studies included in this review longitudinally examined the associations of gut hormones and leptin with BED, disinhibition, or uncontrolled eating following CBT70,71, behavioral weight loss intervention88,94,95, or surgical weight loss intervention86,87. However, these studies did not include patients at various eating behavior stages throughout the follow-up, including new-onset, maintenance, and remission. Consequently, it is impossible to conclude whether the biomarker alterations occur first or after these disordered eating behaviors, and whether the alterations represent state or trait markers. Bariatric surgery, in which LOC eating has particular clinical relevance, offers a unique opportunity to clarify if the biomarker alterations cause or are secondary to LOC eating. From before surgery, four longitudinal patterns of LOC eating have been observed at 6–12 months after surgery (proportion of patients in each pattern): new-onset (17–40%), maintenance (25–38%), remission (27–60%), and LOC eating-free (40–70%)10,129,130. Meanwhile, gut hormones, adipokines, and inflammatory cytokines/markers also change as a result of surgery, although the direction and degree of change depends on the surgical techniques. Taking sleeve gastrectomy as an example, extensive studies among adults and adolescents have shown that fasting and postprandial ghrelin (total and acylated)131–133, leptin133,134, and pro-inflammatory cytokines/markers (e.g., IL-1α, IL-1β, IL-6, TNF-α, CRP, ESR) decrease, while adiponectin and anti-inflammatory cytokines increase after surgery134. The different patterns of LOC eating and the parallel changes of biomarkers offers great value to delineation of the role of these biomarkers in LOC eating.
It has been widely acknowledged that beyond regulating homeostatic and hedonic food consumption, gut hormones, adipokines, and pro- and anti-inflammatory cytokines are closely tied to an individual’s psychosocial functioning. For example, human and animal studies indicate that ghrelin, GLP-1, and leptin have anti-depressant effects32,34,35,135, while levels of CCK and pro-inflammatory cytokines increase with elevated anxiety and depressive symptoms136,137. Furthermore, postprandial CCK and PYY are blunted138,139 but leptin levels are increased140,141 in individuals with dietary restraint. The intrinsic link between biomarkers and psychosocial functioning highlights the importance to examine the indirect associations (through the moderating or mediating effect of psychosocial functioning) between biomarkers and LOC eating. However, among the included studies, only two studies examined dietary restraint as a moderator of relationship between postprandial CCK and disinhibition97, or as a mediation of the relationship between leptin and LOC eating78, signaling an area that needs further research.
As commonly seen in eating disorder research, most studies included in this review consisted of female-only or female-dominant samples. Unlike BED that has a higher prevalence in women than men, the distribution of LOC eating is far less skewed, with reports of comparable prevalence between males and females both in the non-bariatric142 and bariatric population143. Furthermore, given the knowledge that reproductive hormones (e.g., estradiol, progesterone) and females’ menstrual cycle influence the symptoms of binge-eating144, the relationship between biomarkers and LOC eating may differ for males and females. Therefore, future studies should increase the presentation of males and examine the gender differences when investigating biomarkers with LOC eating.
This scoping review has several limitations. First, because studies that directly report LOC eating are too scarce in number, several overlapping but broader eating behaviors were also included. The inclusion of these eating behaviors is necessary to provide richer and more comprehensive information, but it introduces heterogeneity and creates difficulty to isolate the construct of LOC eating. Second, only published full articles were searched; therefore, relevant information presented in the gray literature (e.g., unpublished manuscripts) may have been missed. Third, the literature search was limited to 3 databases. Additional literature may exist that was not included within the scope of this search. Fourth, this review only focused on the significance testing results when reporting the relationships between biomarkers and LOC eating. The lack of an examination of the effect sizes precludes the understanding of the strength of these relationships. Given that the sample size in most of the included studies was small and this scoping review provides initial evidence that several gut hormones (e.g., postprandial ghrelin), adipokines (e.g., leptin), and pro-inflammatory markers (e.g., hsCRP) may be related to LOC eating, future studies would benefit from conducting a meta-analysis and pooling the effect sizes across studies.
5. Conclusion
Despite the limited number of studies and conflicting results, there is evidence that supports the associations of lower levels of fasting acyl ghrelin and adiponectin, higher levels of leptin, hsCRP, and ESR, and altered responses of postprandial ghrelin (blunted), CCK (blunted or amplified), and PYY (amplified) to meal ingestion with the eating behaviors including LOC eating, subclinical binge-eating, BED, disinhibition, uncontrolled eating, and food addiction. Additionally, using GLP-1 analog may reduce binge-eating. Future studies would benefit from a direct examination of LOC eating especially in the bariatric patients, a greater focus on longitudinal studies, an in-depth exploration of the interactions between biomarkers and psychosocial functioning, and an investigation of gender differences that may shape the relationship between biomarkers and LOC eating. Other considerations include examining acyl ghrelin instead of total ghrelin, reporting glucose levels with adiponectin, and investigating the interactions among inflammation, gut hormones, and adipokines.
Acknowledgement
We thank Darcey H. Mulligan for developing the searching strategies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations that are not Standard in the Field
- LOC eating1
Loss of control eating
Abbreviations:
- BED
binge-eating disorder
- BN
bulimia nervosa
- AN
anorexia nervosa
- BMI
body mass index
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- CBT
cognitive behavioral therapy
- CCK
cholecystokinin
- PYY
peptide YY
- GLP-1
glucagon-like peptide 1
- PP
pancreatic polypeptide
- IL-1α
interleukin-1α
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor-α
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- IL-10
interleukin-10
- IL-13
interleukin-13
Footnotes
Declarations of Interest
None.
References
- 1.Galmiche M, Déchelotte P, Lambert G, Tavolacci MP. Prevalence of eating disorders over the 2000–2018 period: a systematic literature review. Am J Clin Nutr. 2019;109(5):1402–1413. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- 3.Goldschmidt AB. Are loss of control while eating and overeating valid constructs? A critical review of the literature. Obes Rev. 2017;18(4):412–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palavras MA, Hay PJ, Lujic S, Claudino AM. Comparing symptomatic and functional outcomes over 5 years in two nonclinical cohorts characterized by binge eating with and without objectively large episodes. Int J Eat Disord. 2015;48(8):1158–1165. [DOI] [PubMed] [Google Scholar]
- 5.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord. 2009;42(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shank LM, Tanofsky-Kraff M, Radin RM, et al. Remission of loss of control eating and changes in components of the metabolic syndrome. Int J Eat Disord. 2018;51(6):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radin RM, Tanofsky-Kraff M, Shomaker LB, et al. Metabolic characteristics of youth with loss of control eating. Eat Behav. 2015;19:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonneville KR, Horton NJ, Micali N, et al. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA Pediatr. 2013;167(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldschmidt AB, Jones M, Manwaring JL, et al. The clinical significance of loss of control over eating in overweight adolescents. Int J Eat Disord. 2008;41(2):153–158. [DOI] [PubMed] [Google Scholar]
- 10.Conceicao EM, Mitchell JE, Pinto-Bastos A, Arrojado F, Brandao I, Machado PPP. Stability of problematic eating behaviors and weight loss trajectories after bariatric surgery: a longitudinal observational study. Surg Obes Relat Dis. 2017;13(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 11.Williams-Kerver GA, Steffen KJ, Smith KE, Cao L, Crosby RD, Engel SG. Negative affect and loss of control eating among bariatric surgery patients: an ecological momentary assessment pilot investigation. Obes Surg. 2020;30(6):2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devlin MJ, King WC, Kalarchian MA, et al. Eating pathology and associations with long-term changes in weight and quality of life in the longitudinal assessment of bariatric surgery study. Int J Eat Disord. 2018;51(12):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivezaj V, Kessler EE, Lydecker JA, Barnes RD, White MA, Grilo CM. Loss-of-control eating following sleeve gastrectomy surgery. Surg Obes Relat Dis. 2017;13(3):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colles SL, Dixon JB, O’Brien PE. Grazing and loss of control related to eating: two high-risk factors following bariatric surgery. Obesity (Silver Spring). 2008;16(3):615–622. [DOI] [PubMed] [Google Scholar]
- 15.Mauro MFFP, Papelbaum M, Brasil MAA, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obes Rev. 2019;20(10):1413–1425. [DOI] [PubMed] [Google Scholar]
- 16.Kalarchian MA, King WC, Devlin MJ, et al. Surgery-related gastrointestinal symptoms in a prospective study of bariatric surgery patients: 3-year follow-up. Surg Obes Relat Dis. 2017;13(9):1562–1571. [DOI] [PubMed] [Google Scholar]
- 17.Kalarchian MA, King WC, Devlin MJ, et al. Surgery-related gastrointestinal symptoms in a prospective study of bariatric surgery patients: 3-year follow-up. Surg Obes Relat Dis. 2017;13(9):1562–1571. [DOI] [PubMed] [Google Scholar]
- 18.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36(2):199–211. [DOI] [PubMed] [Google Scholar]
- 19.Kelly-Weeder S, Willis DG, Mata Lopez L, Sacco B, Wolfe BE. Binge eating and loss of control in college-age women. J Am Psychiatr Nurses Assoc. 2019;25(3):172–180. [DOI] [PubMed] [Google Scholar]
- 20.Lowe MR, Arigo D, Butryn ML, Gilbert JR, Sarwer D, Stice E. Hedonic hunger prospectively predicts onset and maintenance of loss of control eating among college women. Health Psychology. 2016;35(3):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie M, Turton R, Burgess E, Nazar BP, Treasure J. Testing the addictive appetite model of binge eating: The importance of craving, coping, and reward enhancement. Eur Eat Disord Rev. 2018;26(6):541–550. [DOI] [PubMed] [Google Scholar]
- 22.Kurz S, Schoebi D, Dremmel D, Kiess W, Munsch S, Hilbert A. Satiety regulation in children with loss of control eating and attention-deficit/hyperactivity disorder: A test meal study. Appetite. 2017;116:90–98. [DOI] [PubMed] [Google Scholar]
- 23.Theim KR, Tanofsky-Kraff M, Salaita CG, et al. Children’s descriptions of the foods consumed during loss of control eating episodes. Eat Behav. 2007;8(2):258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson BL, Dvorak RD, Wonderlich SA, Crosby RD, Gordon KH. Emotions before and after loss of control eating. Eat Disord. 2018;26(6):505–522. [DOI] [PubMed] [Google Scholar]
- 25.Goldschmidt AB, Crosby RD, Cao L, et al. Ecological momentary assessment of eating episodes in obese adults. Psychosom Med. 2014;76(9):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KE, Mason TB, Lavender JM. Rumination and eating disorder psychopathology: A meta-analysis. Clin Psychol Rev. 2018;61:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prefit AB, Cândea DM, Szentagotai-Tătar A. Emotion regulation across eating pathology: A meta-analysis. Appetite. 2019;143:104438. [DOI] [PubMed] [Google Scholar]
- 28.Lawson JL, LeCates A, Ivezaj V, Lydecker J, Grilo CM. (in press) Internalized weight bias and loss-of-control eating following bariatric surgery. Eat Disord. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldschmidt AB, Lavender JM, Hipwell AE, Stepp SD, Keenan K. Examining two prevailing models of loss of control eating among community-based girls. Obesity (Silver Spring). 2018;26(2):420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manasse SM, Goldstein SP, Wyckoff E, et al. Slowing down and taking a second look: Inhibitory deficits associated with binge eating are not food-specific. Appetite. 2016;96:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svaldi J, Naumann E, Trentowska M, Schmitz F. General and food-specific inhibitory deficits in binge eating disorder. Int J Eat Disord. 2014;47(5):534–542. [DOI] [PubMed] [Google Scholar]
- 32.Bali A, Jaggi AS. An integrative review on role and mechanisms of ghrelin in stress, anxiety and depression. Curr Drug Targets. 2016;17(5):495–507. [DOI] [PubMed] [Google Scholar]
- 33.McClean PL, Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76:57–67. [DOI] [PubMed] [Google Scholar]
- 34.Cao B, Chen Y, Brietzke E, et al. Leptin and adiponectin levels in major depressive disorder: A systematic review and meta-analysis. J Affect Disord. 2018;238:101–110. [DOI] [PubMed] [Google Scholar]
- 35.Lawson EA, Miller KK, Blum JI, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf). 2012;76(4):520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funahashi H, Yada T, Suzuki R, Shioda S. Distribution, function, and properties of leptin receptors in the brain. Int Rev Cytol. 2003;224:1–27. [DOI] [PubMed] [Google Scholar]
- 37.Ambrosio G, Kaufmann FN, Manosso L, et al. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology. 2018;91:132–141. [DOI] [PubMed] [Google Scholar]
- 38.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. [DOI] [PubMed] [Google Scholar]
- 39.Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. Journal of neuroendocrinology. 2015;27(6):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domingos AI, Vaynshteyn J, Voss HU, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14(12):1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khanh DV, Choi YH, Moh SH, Kinyua AW, Kim KW. Leptin and insulin signaling in dopaminergic neurons: relationship between energy balance and reward system. Front Psychol. 2014;5:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suyama S, Maekawa F, Maejima Y, Kubota N, Kadowaki T, Yada T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci Rep. 2016;6:30796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suyama S, Lei W, Kubota N, Kadowaki T, Yada T. Adiponectin at physiological level glucose-independently enhances inhibitory postsynaptic current onto NPY neurons in the hypothalamic arcuate nucleus. Neuropeptides. 2017;65:1–9. [DOI] [PubMed] [Google Scholar]
- 45.Chaskiel L, Bristow AD, Bluthé RM, Dantzer R, Blomqvist A, Konsman JP. Interleukin-1 reduces food intake and body weight in rat by acting in the arcuate hypothalamus. Brain Behav Immun. 2019;81:560–573. [DOI] [PubMed] [Google Scholar]
- 46.Benrick A, Schele E, Pinnock SB, et al. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol. 2009;21(7):620–628. [DOI] [PubMed] [Google Scholar]
- 47.van Heesch F, Prins J, Korte-Bouws GA, et al. Systemic tumor necrosis factor-alpha decreases brain stimulation reward and increases metabolites of serotonin and dopamine in the nucleus accumbens of mice. Behav Brain Res. 2013;253:191–195. [DOI] [PubMed] [Google Scholar]
- 48.Yan Y, Nitta A, Koseki T, Yamada K, Nabeshima T. Dissociable role of tumor necrosis factor alpha gene deletion in methamphetamine self-administration and cue-induced relapsing behavior in mice. Psychopharmacology (Berl). 2012;221(3):427–436. [DOI] [PubMed] [Google Scholar]
- 49.Hunschede S, Schwartz A, Kubant R, Thomas SG, Anderson GH. The role of IL-6 in exercise-induced anorexia in normal-weight boys. Appl Physiol Nutr Metab. 2018;43(10):979–987. [DOI] [PubMed] [Google Scholar]
- 50.Anesten F, Gasull AD, Richard JE, et al. Interleukin-6 in the central amygdala is bioactive and co-localised with glucagon-like peptide-1 receptor. Journal of Neuroendocrinology. 2019;31(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen Ø, Laird B, Aass N, et al. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS One. 2017;12(5):e0177620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunschede S, Kubant R, Akilen R, Thomas S, Anderson GH. Decreased appetite after high-intensity exercise correlates with increased plasma interleukin-6 in normal-weight and overweight/obese boys. Curr Dev Nutr. 2017;1(3):e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yohn SE, Arif Y, Haley A, et al. Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology (Berl). 2016;233(19–20):3575–3586. [DOI] [PubMed] [Google Scholar]
- 54.Treadway MT, Admon R, Arulpragasam AR, et al. Association between Interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol Psychiatry. 2017;82(8):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8(1):75–79. [DOI] [PubMed] [Google Scholar]
- 56.Romanatto T, Cesquini M, Amaral ME, et al. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient--effects on leptin and insulin signaling pathways. Peptides. 2007;28(5):1050–1058. [DOI] [PubMed] [Google Scholar]
- 57.Smitka K, Papezova H, Vondra K, Hill M, Hainer V, Nedvidkova J. The role of “mixed” orexigenic and anorexigenic signals and autoantibodies reacting with appetite-regulating neuropeptides and peptides of the adipose tissue-gut-brain axis: relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa. Int J Endocrinol. 2013;2013:483145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalton B, Bartholdy S, Robinson L, et al. A meta-analysis of cytokine concentrations in eating disorders. J Psychiatr Res. 2018;103:252–264. [DOI] [PubMed] [Google Scholar]
- 59.Prince AC, Brooks SJ, Stahl D, Treasure J. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 2009;89(3):755–765. [DOI] [PubMed] [Google Scholar]
- 60.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 61.Berg KC, Crosby RD, Cao L, et al. Negative affect prior to and following overeating-only, loss of control eating-only, and binge eating episodes in obese adults. Int J Eat Disord. 2015;48(6):641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldschmidt AB, Smith KE, Crosby RD, et al. Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. Int J Eat Disord. 2018;51(6):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vainik U, Garcia-Garcia I, Dagher A. Uncontrolled eating: a unifying heritable trait linked with obesity, overeating, personality and the brain. Eur J Neurosci. 2019;50(3):2430–2445. [DOI] [PubMed] [Google Scholar]
- 64.Racine SE, Hagan KE, Schell SE. Is all nonhomeostatic eating the same? Examining the latent structure of nonhomeostatic eating processes in women and men. Psychol Assess. 2019;31(10):1220–1233. [DOI] [PubMed] [Google Scholar]
- 65.Field BCT, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6(8):444–453. [DOI] [PubMed] [Google Scholar]
- 66.Perry B, Wang Y. Appetite regulation and weight control: the role of gut hormones. Nutrition & Diabetes. 2012;2(1):e26–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandao PP, Garcia-Souza EP, Neves FA, et al. Leptin/adiponectin ratio in obese women with and without binge eating disorder. Neuro Endocrinol Lett. 2010;31(3):353–358. [PubMed] [Google Scholar]
- 68.Monteleone P, Fabrazzo M, Tortorella A, Martiadis V, Serritella C, Maj M. Circulating ghrelin is decreased in non-obese and obese women with binge eating disorder as well as in obese non-binge eating women, but not in patients with bulimia nervosa. Psychoneuroendocrinology. 2005;30(3):243–250. [DOI] [PubMed] [Google Scholar]
- 69.Pedram P, Sun G. Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients. 2014;7(1):223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munsch S, Biedert E, Meyer AH, Herpertz S, Beglinger C. CCK, ghrelin, and PYY responses in individuals with binge eating disorder before and after a cognitive behavioral treatment (CBT). Physiol Behav. 2009;97(1):14–20. [DOI] [PubMed] [Google Scholar]
- 71.Geliebter A, Hashim SA, Gluck ME. Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED). Physiol Behav. 2008;94(5):696–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernandez D, Mehta N, Geliebter A. Meal-related acyl and des-acyl ghrelin and other appetite-related hormones in people with obesity and binge eating. Obesity (Silver Spring). 2019;27(4):629–635. [DOI] [PubMed] [Google Scholar]
- 73.Geliebter A, Yahav EK, Gluck ME, Hashim SA. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. 2004;81(5):735–740. [DOI] [PubMed] [Google Scholar]
- 74.d’Amore A, Massignan C, Montera P, Moles A, De Lorenzo A, Scucchi S. Relationship between dietary restraint, binge eating, and leptin in obese women. Int J Obes Relat Metab Disord. 2001;25(3):373–377. [DOI] [PubMed] [Google Scholar]
- 75.Adami GF, Campostano A, Cella F, Scopinaro N. Serum leptin concentration in obese patients with binge eating disorder. Int J Obes Relat Metab Disord. 2002;26(8):1125–1128. [DOI] [PubMed] [Google Scholar]
- 76.Monteleone P, Fabrazzo M, Tortorella A, Fuschino A, Maj M. Opposite modifications in circulating leptin and soluble leptin receptor across the eating disorder spectrum. Mol Psychiatry. 2002;7(6):641–646. [DOI] [PubMed] [Google Scholar]
- 77.Messerli-Burgy N, Engesser C, Lemmenmeier E, Steptoe A, Laederach-Hofmann K. Cardiovascular stress reactivity and recovery in bulimia nervosa and binge eating disorder. Int J Psychophysiol. 2010;78(2):163–168. [DOI] [PubMed] [Google Scholar]
- 78.Miller R, Tanofsky-Kraff M, Shomaker LB, et al. Serum leptin and loss of control eating in children and adolescents. Int J Obes (Lond). 2014;38(3):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turan S, Sarioglu FC, Erbas IM, et al. (in press) Altered regional grey matter volume and appetite-related hormone levels in adolescent obesity with or without binge-eating disorder. Eat Weight Disord. 2021. [DOI] [PubMed] [Google Scholar]
- 80.Caroleo M, Carbone EA, Greco M, et al. Brain-behavior-immune interaction: serum cytokines and growth factors in patients with eating disorders at extremes of the body mass index (BMI) spectrum. Nutrients. 2019;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rouach V, Bloch M, Rosenberg N, et al. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology. 2007;32(6):693–702. [DOI] [PubMed] [Google Scholar]
- 82.Succurro E, Segura-Garcia C, Ruffo M, et al. Obese patients with a binge eating disorder have an unfavorable metabolic and inflammatory profile. Medicine (Baltimore). 2015;94(52):e2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shank LM, Tanofsky-Kraff M, Kelly NR, et al. Pediatric loss of control eating and high-sensitivity C-reactive protein concentrations. Child Obes. 2017;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adamo KB, Wilson SL, Ferraro ZM, Hadjiyannakis S, Doucet E, Goldfield GS. Appetite sensations, appetite signaling proteins, and glucose in obese adolescents with subclinical binge eating disorder. ISRN obesity. 2014;2014:312826–312826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abu Dayyeh BK, Jirapinyo P, Thompson CC. Plasma ghrelin levels and weight regain after Roux-en-Y gastric bypass surgery. Obes Surg. 2017;27(4):1031–1036. [DOI] [PubMed] [Google Scholar]
- 86.Schindler K, Prager G, Ballaban T, et al. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur J Clin Invest. 2004;34(8):549–554. [DOI] [PubMed] [Google Scholar]
- 87.Bryant EJ, King NA, Falkén Y, et al. Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery. Surg Obes Relat Dis. 2013;9(5):802–808. [DOI] [PubMed] [Google Scholar]
- 88.Fernandes SP, Alessi J, Santos ZEA, de Mello ED. Association between eating behavior, anthropometric and biochemical measurements, and peptide YY (PYY) hormone levels in obese adolescents in outpatient care. J Pediatr Endocrinol Metab. 2020;33(7):873–877. [DOI] [PubMed] [Google Scholar]
- 89.Peters T, Antel J, Föcker M, et al. The association of serum leptin levels with food addiction is moderated by weight status in adolescent psychiatric inpatients. Eur Eat Disord Rev. 2018;26(6):618–628. [DOI] [PubMed] [Google Scholar]
- 90.Robert SA, Rohana AG, Shah SA, Chinna K, Wan Mohamud WN, Kamaruddin NA. Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide - A pilot study. Obes Res Clin Pract. 2015;9(3):301–304. [DOI] [PubMed] [Google Scholar]
- 91.Da Porto A, Casarsa V, Colussi G, Catena C, Cavarape A, Sechi L. Dulaglutide reduces binge episodes in type 2 diabetic patients with binge eating disorder: A pilot study. Diabetes Metab Syndr. 2020;14(4):289–292. [DOI] [PubMed] [Google Scholar]
- 92.Perna S, Spadaccini D, Gasparri C, et al. Association between des-acyl ghrelin at fasting and predictive index of muscle derangement, metabolic markers and eating disorders: a cross-sectional study in overweight and obese adults. Nutr Neurosci. 2020:1–7. [DOI] [PubMed] [Google Scholar]
- 93.Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo). 2013;2013:518909–518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aldhoon Hainerová I, Zamrazilová H, Hlavatá K, et al. Lifestyle intervention discloses an association of the Eating Inventory-51 factors with cardiometabolic health risks. Eat Weight Disord. 2013;18(1):83–86. [DOI] [PubMed] [Google Scholar]
- 95.Hill BR, Rolls BJ, Roe LS, De Souza MJ, Williams NI. Ghrelin and peptide YY increase with weight loss during a 12-month intervention to reduce dietary energy density in obese women. Peptides. 2013;49:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silver AJ, Morley JE. Role of CCK in regulation of food intake. Prog Neurobiol. 1991;36(1):23–34. [DOI] [PubMed] [Google Scholar]
- 97.Burton-Freeman BM, Keim NL. Glycemic index, cholecystokinin, satiety and disinhibition: is there an unappreciated paradox for overweight women? Int J Obes (Lond). 2008;32(11):1647–1654. [DOI] [PubMed] [Google Scholar]
- 98.Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. [DOI] [PubMed] [Google Scholar]
- 99.Baynes KC, Dhillo WS, Bloom SR. Regulation of food intake by gastrointestinal hormones. Curr Opin Gastroenterol. 2006;22(6):626–631. [DOI] [PubMed] [Google Scholar]
- 100.Jensterle M, Kocjan T, Kravos NA, Pfeifer M, Janez A. Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocrine Research. 2015;40(3):133–138. [DOI] [PubMed] [Google Scholar]
- 101.Yagin NL, Aliasgari F, Alizadeh M, Aliasgharzadeh S, Mahdavi R. Comparison of endocannabinoids levels, FAAH gene polymorphisms, and appetite regulatory substances in women with and without binge eating disorder: a cross-sectional study. Nutr Res. 2020;83:86–93. [DOI] [PubMed] [Google Scholar]
- 102.Monteleone P, Martiadis V, Fabrazzo M, Serritella C, Maj M. Ghrelin and leptin responses to food ingestion in bulimia nervosa: implications for binge-eating and compensatory behaviours. Psychological medicine. 2003;33(8):1387–1394. [DOI] [PubMed] [Google Scholar]
- 103.Monteleone P, Martiadis V, Rigamonti AE, et al. Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol Psychiatry. 2005;57(8):926–931. [DOI] [PubMed] [Google Scholar]
- 104.Kojima S, Nakahara T, Nagai N, et al. Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clinical endocrinology. 2005;62(1):74–78. [DOI] [PubMed] [Google Scholar]
- 105.Valdivia S, Cornejo MP, Reynaldo M, De Francesco PN, Perello M. Escalation in high fat intake in a binge eating model differentially engages dopamine neurons of the ventral tegmental area and requires ghrelin signaling. Psychoneuroendocrinology. 2015;60:206–216. [DOI] [PubMed] [Google Scholar]
- 106.King SJ, Rodrigues T, Watts A, Murray E, Wilson A, Abizaid A. Investigation of a role for ghrelin signaling in binge-like feeding in mice under limited access to high-fat diet. Neuroscience. 2016;319:233–245. [DOI] [PubMed] [Google Scholar]
- 107.Bake T, Hellgren KT, Dickson SL. Acute ghrelin changes food preference from a high-fat diet to chow during binge-like eating in rodents. J Neuroendocrinol. 2017;29(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fernandez G, Cabral A, Cornejo MP, et al. Des-acyl ghrelin directly targets the arcuate nucleus in a ghrelin-receptor independent manner and impairs the orexigenic effect of ghrelin. J Neuroendocrinol. 2016;28(2):12349. [DOI] [PubMed] [Google Scholar]
- 109.Delhanty PJ, Neggers SJ, van der Lely AJ. Mechanisms in endocrinology: Ghrelin: the differences between acyl- and des-acyl ghrelin. Eur J Endocrinol. 2012;167(5):601–608. [DOI] [PubMed] [Google Scholar]
- 110.Geracioti TD Jr., Liddle RA. Impaired cholecystokinin secretion in bulimia nervosa. N Engl J Med. 1988;319(11):683–688. [DOI] [PubMed] [Google Scholar]
- 111.Devlin MJ, Walsh BT, Guss JL, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;65(1):114–120. [DOI] [PubMed] [Google Scholar]
- 112.Keel PK, Wolfe BE, Liddle RA, De Young KP, Jimerson DC. Clinical features and physiological response to a test meal in purging disorder and bulimia nervosa. Arch Gen Psychiatry. 2007;64(9):1058–1066. [DOI] [PubMed] [Google Scholar]
- 113.Brambilla F, Monteleone P, Maj M. Glucagon-like peptide-1 secretion in bulimia nervosa. Psychiatry Res. 2009;169(1):82–85. [DOI] [PubMed] [Google Scholar]
- 114.Dossat AM, Bodell LP, Williams DL, Eckel LA, Keel PK. Preliminary examination of glucagon-like peptide-1 levels in women with purging disorder and bulimia nervosa. Int J Eat Disord. 2015;48(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakai Y, Hamagaki S, Takagi R, Taniguchi A, Kurimoto F. Plasma concentrations of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in patients with bulimia nervosa. Clinical endocrinology. 2000;53(3):383–388. [DOI] [PubMed] [Google Scholar]
- 116.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85(12):4511–4514. [DOI] [PubMed] [Google Scholar]
- 117.Reddy NL, Peng C, Carreira MC, et al. Enhanced thermic effect of food, postprandial NEFA suppression and raised adiponectin in obese women who eat slowly. Clin Endocrinol (Oxf). 2015;82(6):831–837. [DOI] [PubMed] [Google Scholar]
- 118.Guillermo C, Boushey CJ, Franke AA, et al. Diet quality and biomarker profiles related to chronic disease prevention: the multiethnic cohort study. J Am Coll Nutr. 2020;39(3):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monfort-Pires M, Folchetti LD, Previdelli AN, Siqueira-Catania A, de Barros CR, Ferreira SR. Healthy Eating Index is associated with certain markers of inflammation and insulin resistance but not with lipid profile in individuals at cardiometabolic risk. Appl Physiol Nutr Metab. 2014;39(4):497–502. [DOI] [PubMed] [Google Scholar]
- 120.Solmi F, Bulik CM, De Stavola BL, Dalman C, Khandaker GM, Lewis G. Longitudinal associations between circulating interleukin-6 and C-reactive protein in childhood, and eating disorders and disordered eating in adolescence. Brain Behav Immun. 2020;89:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202(8):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iikuni N, Lam QLK, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berner LA, Sysko R, Rebello TJ, Roberto CA, Pike KM. Patient descriptions of loss of control and eating episode size interact to influence expert diagnosis of ICD-11 binge-eating disorder. J Eat Disord. 2020;8(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blomquist KK, Roberto CA, Barnes RD, White MA, Masheb RM, Grilo CM. Development and validation of the eating loss of control scale. Psychol Assess. 2014;26(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Latner JD, Mond JM, Kelly MC, Haynes SN, Hay PJ. The Loss of Control Over Eating Scale: development and psychometric evaluation. Int J Eat Disord. 2014;47(6):647–659. [DOI] [PubMed] [Google Scholar]
- 127.Bodell LP, Forney KJ, Chavarria J, Keel PK, Wildes JE. Self-report measures of loss of control over eating: Psychometric properties in clinical and non-clinical samples. Int J Eat Disord. 2018;51(11):1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vannucci A, Ohannessian CM. Psychometric properties of the brief loss of control over eating scale (LOCES-B) in early adolescents. Int J Eat Disord. 2018;51(5):459–464. [DOI] [PubMed] [Google Scholar]
- 129.Smith KE, Orcutt M, Steffen KJ, et al. Loss of control eating and binge eating in the 7 years following bariatric surgery. Obes Surg. 2019;29(6):1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM. Loss of control over eating predicts outcomes in bariatric surgery patients: a prospective, 24-month follow-up study. J Clin Psychiatry. 2010;71(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dardzińska JA, Kaska Ł, Proczko-Stepaniak M, Szymańska-Gnacińska M, Aleksandrowicz-Wrona E, Małgorzewicz S. Fasting and postprandial acyl and desacyl ghrelin and the acyl/desacyl ratio in obese patients before and after different types of bariatric surgery. Wideochir Inne Tech Maloinwazyjne. 2018;13(3):366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu R, Zhu C, Pierre JF, Yin DP. Gastric bypass improves obesity and glucose tolerance independent of gastric pouch size. Obes Surg. 2020;30(5):1635–1641. [DOI] [PubMed] [Google Scholar]
- 133.Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257(4):647–654. [DOI] [PubMed] [Google Scholar]
- 134.Lopez-Nava G, Negi A, Bautista-Castaño I, Rubio MA, Asokkumar R. Gut and metabolic hormones changes after endoscopic sleeve gastroplasty (ESG) Vs. laparoscopic sleeve gastrectomy (LSG). Obes Surg. 2020;30(7):2642–2651. [DOI] [PubMed] [Google Scholar]
- 135.Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology. 2016;65:54–66. [DOI] [PubMed] [Google Scholar]
- 136.Desai SJ, Borkar CD, Nakhate KT, Subhedar NK, Kokare DM. Neuropeptide Y attenuates anxiety- and depression-like effects of cholecystokinin-4 in mice. Neuroscience. 2014;277:818–830. [DOI] [PubMed] [Google Scholar]
- 137.Raison CL, Miller AH. Is depression an inflammatory disorder? Current psychiatry reports. 2011;13(6):467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burton-Freeman B Sex and cognitive dietary restraint influence cholecystokinin release and satiety in response to preloads varying in fatty acid composition and content. J Nutr. 2005;135(6):1407–1414. [DOI] [PubMed] [Google Scholar]
- 139.Scheid JL, Birch LL, Williams NI, Rolls BJ, De Souza MJ. Postprandial peptide YY is lower in young college-aged women with high dietary cognitive restraint. Physiol Behav. 2013;120:26–33. [DOI] [PubMed] [Google Scholar]
- 140.Cassioli E, Rossi E, Squecco R, et al. Reward and psychopathological correlates of eating disorders: The explanatory role of leptin. Psychiatry Res. 2020;290:113071. [DOI] [PubMed] [Google Scholar]
- 141.Eddy KT, Lawson EA, Meade C, et al. Appetite regulatory hormones in women with anorexia nervosa: binge-eating/purging versus restricting type. J Clin Psychiatry. 2015;76(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lydecker JA, Grilo CM. Comparing men and women with binge-eating disorder and co-morbid obesity. Int J Eat Disord. 2018;51(5):411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ivezaj V, Fu E, Lydecker JA, Duffy AJ, Grilo CM. Racial comparisons of postoperative weight loss and eating-disorder psychopathology among patients following sleeve gastrectomy surgery. Obesity (Silver Spring). 2019;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baker JH, Girdler SS, Bulik CM. The role of reproductive hormones in the development and maintenance of eating disorders. Expert Rev Obstet Gynecol. 2012;7(6):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]


