Abstract
Lynch syndrome (LS) is the most common cause of inherited colorectal cancer and the increases risk of developing extracolonic cancers. We present the first case of pediatric-onset LS with recurrent adenomatous colonic polyps presenting with rectal prolapse. This case highlights the importance of considering polyposis syndromes such as LS as possible diagnoses for pediatric patients who present with colorectal adenomatous polyps, as well as the need to consider immunohistochemical staining of polyps for mismatch repair protein expression in pediatric populations to rule out LS as a diagnosis. We demonstrate the need to consider pediatric patients in LS guidelines.
Keywords: adolescent, polyposis, screening
INTRODUCTION
Lynch Syndrome (LS) is the most common cause of inherited colorectal cancer (CRC) and increases the risk of developing extracolonic cancers (1). The mean age at CRC diagnosis in LS is 44–61 years (1), but rare pediatric-onset LS has been reported, generally with CRC present at diagnosis (2–5). We present the first case of pediatric-onset LS with recurrent adenomatous colonic polyps presenting with rectal prolapse.
CASE REPORT
A 12-year-old girl presented to the emergency department with subacute diarrhea and with bloody stool on the day of presentation. She was diagnosed with rectal prolapse, followed by manual reduction. After reduction, sloughed tissue was observed and sent for pathology, which demonstrated a tubulovillous adenoma without high-grade dysplasia.
Her presentation was preceded by 4 years of intermittent distinct epigastric and left upper quadrant abdominal pains. Physical examination was unremarkable with a normal digital rectal examination. Laboratory investigations were all normal (Table 1).
TABLE 1.
Laboratory results of the patient upon the first presentation to the emergency department
| Laboratory investigation | Result (normal range) |
|---|---|
| Hemoglobin | 143 g/L (120–160) |
| Hematocrit | 0.41 L/L (0.36–0.48) |
| MCV | 88 fL (82–100) |
| Platelets | 345 × 109/L (150–400) |
| Leukocytes | 5.8 × 109/L (4.0–11.0) |
| Ferritin | 44 µg/L (10–110) |
| Iron | 14 µmol/L (5–25) |
| TIBC | 60 µmol/L (40–77) |
| Transferrin saturation | 0.22 (0.20–0.55) |
| C-reactive protein | <1.0 mg/L (0.0–8.0) |
| Albumin | 39 g/L (33–48) |
| Alanine aminotransferase | 11 U/L (1–35) |
| Total bilirubin | 8 µmol/L (0–19) |
| INR | 1.1 (0.9–1.1) |
| APTT | 30.2 seconds (27.0–37.0) |
| Stool bacterial culture | Negative |
| Stool C. Difficile test | Negative |
APTT = activated partial thromboplastin time; INR = international normalized ratio; MCV = mean corpuscular volume; TIBC = total iron-binding capacity.
On esophagogastroduodenoscopy and colonoscopy, a large polyp was found in the sigmoid colon (Fig. 1A), requiring piecemeal resection by snare electrocautery. Histopathology again revealed a tubulovillous adenoma. Given the highly unusual presentation of an isolated adenomatous polyp at this age, immunohistochemical staining for Mismatch Repair (MMR) gene proteins was performed and demonstrated isolated mutS homolog (MSH) 6 deficiency (Fig. 2). Referral was made to medical genetics for assessment and counseling, and germline testing was ordered.
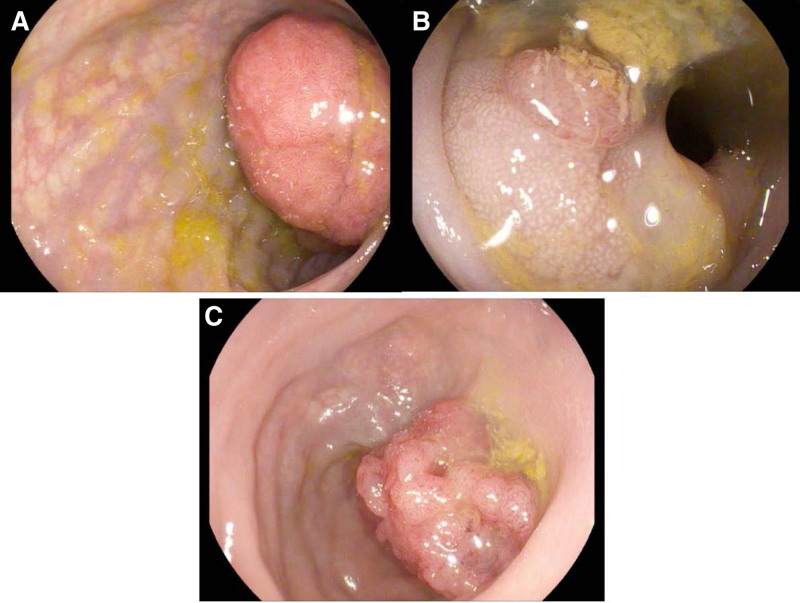
FIGURE 1.
Findings on colonoscopy. A) 0-Ip (pedunculated) polyp estimated 25–30 mm maximal diameter in the sigmoid colon, initial endoscopy. B) 0-Is (sessile) polyp estimated 5–7 mm maximal diameter, repeat sigmoidoscopy. C) 0-Is (sessile) polyp estimated 30 mm maximal diameter in the sigmoid colon, a follow-up colonoscopy.
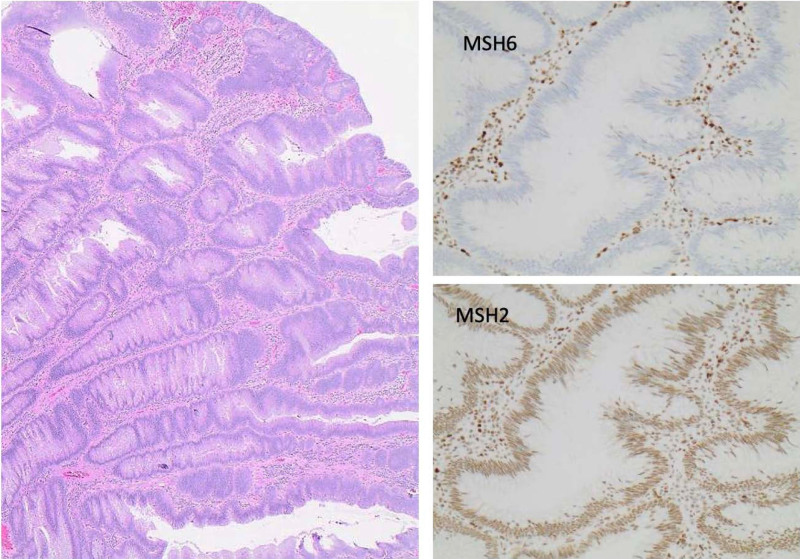
FIGURE 2.
A) Microscopic examination shows a tubulovillous adenoma without high-grade dysplasia (H&E staining, original magnification ×10), (B) loss of MSH6 expression in epithelial cells, while (C) MSH2 expression is intact.
Repeat sigmoidoscopy was arranged 1 month later to tattoo the polypectomy site for future screening, but polyp recurrence was noted (Fig. 1B) and resection was repeated. A pathogenic MSH6 gene variant (c.3996_4000dupATTTC) was subsequently found, confirming the diagnosis of LS. However, 9 months later, a colonoscopy performed by an advanced therapeutic endoscopist detected recurrence of a large sessile polyp that could not be safely resected (Fig. 1C). The lesion was at least 25 cm from the anal verge, so the transanal endoscopic microsurgery was not an option, and endoscopic mucosal resection was also not an option given inadequate visualization of the polyp. Given that current guidelines did not address this patient scenario, stakeholders in the case ultimately agreed to move forward with laparoscopic anterior resection of the sigmoid colon with end-end anastomosis.
Pathology confirmed an MSH6-deficient 30 mm tubulovillous adenoma, negative for high-grade dysplasia, with ample margins. Surveillance endoscopic appearances 1 and 3 years postoperatively were normal, with normal histology from multiple random biopsies.
DISCUSSION
Adenomatous polyps in pediatrics are very uncommon and typically associated with hereditary polyposis syndromes such as familial adenomatous polyposis. Patients with familial adenomatous polyposis generally develop >100 colorectal adenomatous polyps, with or without extracolonic manifestations. Other adenomatous polyposis syndromes, such as LS are rare and typically present in adulthood. When adenomatous polyps occur in pediatric patients, they are very rarely solitary, as was observed with our patient. In this case, a microscopic examination of the fragmented sigmoid polyp confirmed a tubulovillous adenoma. The lesion appeared completely adenomatous, which excluded the possibility that this was a dysplastic or adenomatous lesion arising from a nonadenomatous polyp (eg, serrated adenoma and juvenile polyp).
Thus, immunohistochemical staining of the adenoma for MMR proteins, not otherwise routine, was performed to consider a rare early presentation of LS. LS is an autosomal dominant condition caused by inactivating mutations affecting one of the MMR genes, MLH1, MSH2, MSH6, or PMS2 (1). MMR proteins conserve the DNA sequence by correcting nucleotide base mismatches and inadvertent insertions or deletions by DNA polymerase during DNA replication (1).
While LS traditionally affects adults, rarely reported cases of pediatric CRC occur and may be associated with genetic anticipation (3–6). However, this is only the second reported pediatric case of LS presenting with colonic adenomas (2), and the earliest and first presentation involving rectal prolapse and recurrent polyps. While in LS CRCs are typically proximal to the splenic flexure and precursor adenomas tend to be flatter (1,7,8), our patient’s initial polyp was in the sigmoid colon and pedunculated. Interestingly, though, left-sided polyps have been more commonly reported in females (9). Further, the MSH6 mutation carries the lowest cumulative risk of CRC in females of the 4 genes affected in LS (1), making her early onset of polyposis more unexpected. Therefore, constitutional MMR deficiency was considered, but other clinical features and genetic testing were inconsistent with this diagnosis (10).
Current guidelines recommend colectomy with ileorectal anastomosis for patients with CRC or endoscopically unresectable colorectal neoplasia, due to a high cumulative risk of metachronous CRC even with endoscopic surveillance after partial resection (16% at 10 years, 41% at 20 years) (1). Additionally, the adenoma-carcinoma sequence is more rapid in LS, estimated at 35 months (1). However, our patient underwent segmental resection for an endoscopically unresectable polyp despite these recommendations. They are based largely on studies in significantly older patient populations after CRC diagnosis (9,11), of uncertain applicability to our patient. Further, she did not have additional risk factors for CRC, such as age >40 years, male gender, or MLH1/MSH2 mutation. We also considered patient preference and other potential implications of a colectomy on function and future fertility given her young age.
LS screening recommendations include colonoscopy every 1–2 years starting at age 20–25, or age 30 years for MSH6 mutation carriers (1), which seemed inapplicable to a then 14-year-old with this mutation after low anterior resection for adenomatous polyp. In the absence of pediatric screening guidelines for LS, we opted for a colonoscopy every 2 years for surveillance pending that she remained asymptomatic. This was a shared decision with the patient and family, with earlier screening favored because of her earlier onset of colorectal pathology and because she underwent a less extensive colonic resection for her neoplasia. Screening for extracolorectal cancer was deferred to age 30–35 years as per guidelines, considering the lower risk of these cancers and less well-established evidence to support screening.
Our patient’s family history was negative for CRC; however, this clearly did not preclude a diagnosis of LS, highlighting its variable penetrance. Notably, further testing led to an asymptomatic diagnosis of LS in the patient’s mother, allowing for timely screening and prophylactic measures.
This case demonstrates the need to consider polyposis syndromes in pediatric patients with colorectal adenomatous polyps, even if solitary, with the recommended immunohistochemical staining of polyps for MMR protein expression. LS has classically been considered an adult-onset disease whose major clinical consequence is CRC. However, pediatric-onset colorectal pathology is rare but well-described, with this case of LS presenting as rectal prolapse secondary to a large pedunculated polyp being previously unreported. LS guidelines for screening and management should consider pediatric populations.
ACKNOWLEDGMENTS
Informed Consent was obtained by the parents of this patient prior to the completion of this article, as well as for submission of the article for publication.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US multi-society task force on colorectal cancer. Am J Gastroenterol. 2014;109:1159–1179. [DOI] [PubMed] [Google Scholar]
- 2.Bodas A, Perez-Segura P, Maluenda C, et al. Lynch syndrome in a 15-year-old boy. Eur J Pediatr. 2008;167:1213–1215. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez RS, Shulman SC, Katzenstein HM, et al. Colorectal adenocarcinoma: a pediatric case review with a focus on mismatch repair gene mutations and E-cadherin expression. Pediatr Dev Pathol. 2012;15:192–198. [DOI] [PubMed] [Google Scholar]
- 4.Huang SC, Lavine JE, Boland PS, et al. Germline characterization of early-aged onset of hereditary non-polyposis colorectal cancer. J Pediatr. 2001;138:629–635. [DOI] [PubMed] [Google Scholar]
- 5.Niessen RC, Berends MJW, Wu Y, et al. Identification of mismatch repair gene mutations in young patients with colorectal cancer and in patients with multiple tumors associated with hereditary non-polyposis colorectal cancer. Gut. 2006;55:1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozzao C, Lastella P, Stella A. Anticipation in lynch syndrome: where we are where we go. Curr Genomics. 2011;12:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinicrope FA. Lynch syndrome–associated colorectal cancer. NEJM. 2018;379:764–773. [DOI] [PubMed] [Google Scholar]
- 8.Kalady MF, Kravochuck SE, Heald B, et al. Defining the adenoma burden in Lynch syndrome. Dis Colon and Rectum. 2015;58:388–392. [DOI] [PubMed] [Google Scholar]
- 9.Edelstein DL, Axilbund J, Baxter M, et al. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol. 2011;9:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Durno CA, Erdman SH. Lynch syndrome: a pediatric perspective. J Pediatr Gastroenterol Nutr. 2014;58:144–152. [DOI] [PubMed] [Google Scholar]
- 11.Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers – the advantage of more extensive colon surgery. Gut. 2011;60:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]