Abstract
Mycetoma is a neglected tropical disease commonly caused by the fungus Madurella mycetomatis. Standard treatment consists of extensive treatment with itraconazole in combination with surgical excision of the infected tissue, but has a low success rate. To improve treatment outcomes, novel treatment strategies are needed. Here, we determined the potential of manogepix, a novel antifungal agent that targets the GPI-anchor biosynthesis pathway by inhibition of the GWT1 enzyme. Manogepix was evaluated by determining the minimal inhibitory concentrations (MICs) according to the CLSI-based in vitro susceptibility assay for 22 M. mycetomatis strains and by in silico protein comparison of the target protein. The synergy between manogepix and itraconazole was determined using a checkerboard assay. The efficacy of clinically relevant dosages was assessed in an in vivo grain model in Galleria mellonella larvae. MICs for manogepix ranged from <0.008 to >8 mg/l and 16/22 M. mycetomatis strains had an MIC ≥4 mg/ml. Differences in MICs were not related to differences observed in the GWT1 protein sequence. For 70% of the tested isolates, synergism was found between manogepix and itraconazole in vitro. In vivo, enhanced survival was not observed upon admission of 8.6 mg/kg manogepix, nor in combination treatment with 5.7 mg/kg itraconazole. MICs of manogepix were high, but the in vitro antifungal activity of itraconazole was enhanced in combination therapy. However, no efficacy of manogepix was found in an in vivo grain model using clinically relevant dosages. Therefore, the therapeutic potential of manogepix in mycetoma caused by M. mycetomatis seems limited.
Keywords: mycetoma, manogepix, Madurella, in vitro susceptibility, synergism, Galleria mellonella
Introduction
Recognized as a neglected tropical disease by the World Health Organization in 2016, mycetoma remains a major health concern in regions of Africa, Latin America, and Asia.1,2 Mycetoma can be of either bacterial (actinomycetoma) or fungal (eumycetoma) origin. Globally, the fungus Madurella mycetomatis is the most common causative agent and is reported in over 70% of all eumycetoma cases.3 Eumycetoma is considered an implantation mycosis, in which the causative agent is implanted into the subcutaneous tissue via a minor trauma. The disease starts as a localized infection in the subcutaneous tissue, eventually forming debilitating masses, draining sinuses, and grains harboring the infectious agent. Mycetoma generally affects the feet, legs, and hands.4
Treatment of fungal eumycetoma remains challenging due to the resilient nature of fungal infections and the limited therapeutic options associated with the close resemblance of the fungal and human cells.5,6 The recommended treatment relies on treatment with antifungal agents belonging to the class of azoles, such as itraconazole, fosravuconazole, posaconazole, voriconazole, or terbinafine.7–11 At the moment, itraconazole is considered the drug of choice for mycetoma. Generally, antifungal treatment is started 6 months prior to surgical intervention. Then the lesion is surgically removed, and at least another 6 months of post-operative antifungal treatment are given. However, the post-operative recurrence rate varies from 25% to 50%.12 In addition to high recurrence rates, loss of follow-up frequently occurs due to dissatisfaction with the therapeutic outcome, side effects, and the severe financial burden of the therapy on an average household.6 Therefore, to improve the M. mycetomatis therapy outcome, other promising antifungal agents should be explored.
Manogepix, the active moiety of the prodrug fosmanogepix, is a new first-in-class antifungal agent currently in phase II clinical trials for the treatment of invasive fungal infections.13,14 Manogepix targets the glycosylphosphatidylinositol (GPI) anchor biosynthesis pathway by inhibition of the GWT1 protein, a conserved enzyme that catalyzes the inositol acylation.15 In turn, the maturation of GPI-anchored proteins is prevented, compromising the cell wall integrity, germ tube formation, and biofilm formation.15 Studies have shown that manogepix is active against different clinically relevant moulds, including azole- and echinocandin-resistant Aspergillus spp. and Candida spp.14,16,17 Given the potential of manogepix, the aim of our study was threefold. First, we wanted to establish whether the growth of M. mycetomatis could be inhibited. Second, we tried to determine if manogepix could enhance the activity of itraconazole. Third, we wanted to determine if manogepix showed in vivo efficacy in a Galleria mellonella mycetoma model.
Methods
Drugs and fungal isolates
Manogepix (APX001A) was provided by Pfizer (formerly Amplyx Pharmaceuticals, Inc.), and itraconazole was obtained from Janssen Pharmaceutical Products, Belgium. A total of 22 clinical isolates of M. mycetomatis were included in this study. The isolates originated from Sudan, Mali, Peru, Somalia, Ivory Coast, Algeria, and France. The origin of three isolates is unknown (Table 1). All isolates were previously identified based on morphology, PCR, and sequencing of the internal transcribed spacer (ITS).18,19 Furthermore, genetic variability was determined by MmySTR analysis, as described by Nyuykonge and associates.20
Table 1.
Overview of included Madurella mycetomatis isolates and the respective minimum inhibitory concentrations of manogepix and itraconazole.
| Isolate | ITS ascension number | GWT1 ascension number | Origin | Itraconazole (mg/l) | Manogepix 50% (mg/l) | Manogepix 80% (mg/l) |
|---|---|---|---|---|---|---|
| MM55 | JN573181.1 | LCTW02000523.1 | Sudan | 0.125 | 4 | 4 |
| SO1 | MW493233 | OR134903 | Somalia | 0.063 | 4 | 4 |
| MM13 | JX280866.1 | Sudan | 0.125 | 4 | 4 | |
| MM14 | MW513510 | Sudan | 0.063 | 8 | 8 | |
| MM25 | MW494400 | Sudan | 0.016 | 4 | 4 | |
| MM30 | MW520456 | Sudan | 0.063 | 4 | 4 | |
| MM35 | MW513693 | Sudan | 0.125 | 2 | 4 | |
| MM36 | MW520454 | Sudan | 0.25 | 4 | 4 | |
| MM41 | MW520455 | Sudan | 0.063 | ≤0.008 | ≤0.008 | |
| MM52 | JN573179.1 | Sudan | 0.25 | 4 | 8 | |
| MM54 | JN573180.1 | Sudan | 0.063 | 8 | 8 | |
| MM71 | ON319059.1 | Sudan | 0.125 | 0.5 | 8 | |
| MM83 | ON319061.1 | Sudan | 0.125 | 0.25 | 2 | |
| I1 | JX280864.1 | India | 0.063 | 0.5 | 8 | |
| I11 | MW541890 | OR134900 | India | 0.25 | ≤0.008 | 0.031 |
| CBS116298 | MW542679 | Ivory Coast | 0.063 | 8 | 8 | |
| Peru72012 | ON319062.1 | OR134902 | Peru | 0.063 | 0.5 | 1 |
| P1 | MW520453 | OR134901 | Mali | 0.063 | ≤0.008 | ≤0.008 |
| AL1 | MW541888 | Algeria | 0.016 | 2 | 2 | |
| T606931 | MW541889 | Unknown | 0.031 | 8 | 8 | |
| CBS247.48 | JX280745.1 | Unknown | 0.063 | 4 | 4 | |
| Pasteur 10.757 | OR095764 | France | 0.25 | 8 | 16 | |
| MIC range | 0.016–0.250 | ≤0.008–8 | ≤0.008–16 | |||
| MIC50 | 0.063 | 4 | 4 | |||
| MIC90 | 0.25 | 8 | 8 |
GWT1 protein comparison
The GWT1 encoding sequence was extracted from GenBank from the annotated M. mycetomatis MM55 genome (BioProject: PRJNA267680, Accession: LCTW00000000.2).21 The reference sequence (Accession: LCTW02000523.1) was used to retrieve the GWT1 sequences of four M. mycetomatis isolates (SO1, Peru72012, P1, and I11) from whole genome sequence data (data not published). The coding sequences were used to generate protein sequences using the built-in translation function in Molecular Evolutionary Genetics Analysis (MEGA, version X), and a multiple sequence alignment (MSA) was constructed of the five M. mycetomatis GWT1 sequences using Clustal Omega V1.2.4.22,23
In vitro susceptibility testing
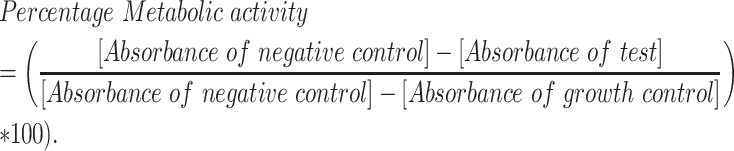
The antifungal susceptibility of M. mycetomatis against manogepix was determined according to a modified CLSI method using resazurin as a viability dye, as previously described.24,25 Within this modification, we generate hyphal fragments as inoculum instead of using conidia as stated in the guideline. In short, all isolates were cultured on Sabouraud Dextrose Agar (SDA) at 37°C for 2–3 weeks prior to susceptibility testing. Mycelium was harvested and sonicated at 10 microns for 10 s (Soniprep 150 Plus, MSE), transferred into RPMI 1640 medium containing 0.35 gr/l l-glutamine and 1.98 m m 4-Morpholinepropanesulfonic acid (MOPS), and further incubated for 7 days at 37°C. Mycelium was harvested by centrifugation, washed, and sonicated using the same settings described above. A standardized hyphal suspension of 70% ± 2% was prepared in RPMI 1640 medium containing 0.35 gr/l l-glutamine and 1.98 m m MOPS. A twofold dilution series for both manogepix and itraconazole was prepared in sterile dimethyl sulfoxide (DMSO) (Merck, Germany). The drugs were transferred to round bottom plates (Corning Fisher, the Netherlands) and further diluted in standardized hyphal suspension and resazurin. Final concentrations ranged from 0.008 mg/l to 16 mg/l for manogepix, 0.008 mg/l to 4 mg/l for itraconazole, and a final concentration of 37.5 mg/l for resazurin. The cultures were incubated for 7 days at 37°C under 5% CO2 conditions. After incubation, the absorbance of the supernatant was determined spectrophotometrically using the EPOCH 2 microplate reader (BioTek, USA), and the MIC was calculated according to the formula below, in which the reduction of resazurin to resorufin is determined by measuring the decrease in absorbance of resazurin. The MIC was determined as the lowest concentration where the metabolic activity was ≥80%.
 |
The minimal effective concentration (MEC) could not be determined because a hyphal inoculum was used as a starting inoculum. Instead, the recently reported surrogate maker of ≥50% was used.26
Analysis of synergism between manogepix and itraconazole
Synergism between manogepix and itraconazole was evaluated by checkerboard microdilution as previously described, using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) as a viability dye.27 Manogepix and itraconazole concentrations ranged from 0.0025 to 8 mg/l and 0.002 to 0.5 mg/l, respectively. The MIC was determined by calculating the percentage of metabolic inhibition using the formula below:
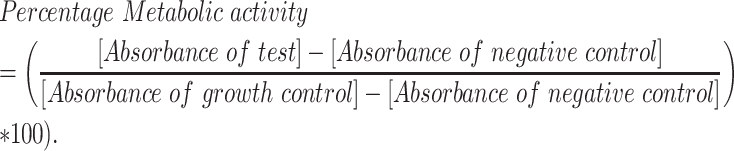 |
The MIC was considered the first value in each row and column in which ≥80% reduction of fungal metabolic activity was found. To determine synergism between manogepix and itraconazole, the fractional inhibitory concentration (FIC) was determined. The FIC was calculated according to the formula below, determining up to three decimal places:
 |
The FIC was determined for all wells of the microtitration plates that correspond to an MIC in combinations, reporting both the minimum and maximum FIC values.28 A minimum FIC value <0.5 indicates synergism, a maximum FIC >4 indicates antagonism, and an FIC between 0.5 and 4 is interpreted as indifferent. Three biological replicates were performed for each respective isolate, and the median FIC min and FIC max were used to determine synergism or antagonism.
Galleria mellonella grain model
The in vivo efficacy of manogepix monotherapy and in combination with itraconazole was determined as described by Eadie and associates.29 In brief, M. mycetomatis strain MM55 was cultured on SDA at 37°C for 3 weeks. Mycelium was harvested and sonicated at 10 microns for 30 s, transferred into RPMI 1640 medium containing 350 mg/l l-glutamine, 1.98 m m MOPS, and 100 mg/l chloramphenicol, and further incubated for 2 weeks at 37°C. The fungal mycelium was harvested, sonicated at 10 microns for 2 min, and diluted to an inoculum size of 4 mg per larvae. Next, the larvae were injected with the fungal inoculum in the left lower proleg using an insulin 29-G U-100 needle (BD Diagnostics, Sparks, USA). At 4 h, 28 h, and 52 h after infection, larvae were treated with 20 μl of 0.21 mg/ml manogepix, 0.14 mg/ml itraconazole, or a combination of both. To reach this concentration, manogepix and itraconazole were first dissolved in DMSO and further diluted in PBS, so that the final concentration of DMSO did not exceed 5%, a concentration well tolerated by the larvae. This resulted in a final concentration of 8.57 mg/kg manogepix and 5.71 mg/kg itraconazole in the larvae. Dosages were based on clinically relevant dosages of 600 mg and 400 mg of manogepix and itraconazole, respectively, based on an average of 70 kg person.30 The survival of the larvae was monitored for 10 days after infection. The toxicity of the compounds was assessed separately by administering treatment to healthy, uninfected larvae equal to the infected groups. The toxicity of the drugs was monitored for up to 5 days after the initial treatment. Pupa formed during the monitored period were excluded from the analysis. Three biological replicates were performed.
Results
Manogepix inhibits M. Mycetomatis growth in vitro
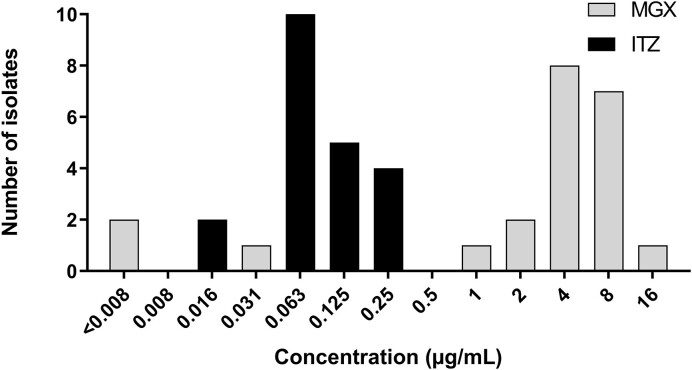
We determined the in vitro susceptibility of itraconazole and manogepix against 22 M. mycetomatis isolates. As shown in Figure 1, the MIC of itraconazole ranged between 0.016 and 0.25 mg/l and of manogepix between <0.008 and 16 mg/l. No minimal effective concentration could be observed due to the use of a hyphal inoculum as the starting method; therefore, a 50% reduction in metabolic activity was used as a surrogate marker (Table 1). Using this surrogate marker, the same range of inhibitory concentrations was noted for manogepix, and also the same MIC50 was obtained. A complete overview of the included isolates and the corresponding MIC values for both manogepix and itraconazole are provided in Table 1. As can be seen in this table, 16 of the 22 isolates had MICs for manogepix of 4 mg/l or higher.
Figure 1.
Antifungal susceptibility, expressed as MICs, of 22 Madurella mycetomatis isolates to manogepix and itraconazole. MIC distribution as determined by the resazurin assay for manogepix (MGX) and itraconazole (ITZ). The MIC is depicted as calculated using an 80% inhibition cutoff.
No genetic link between high and low MICs for manogepix
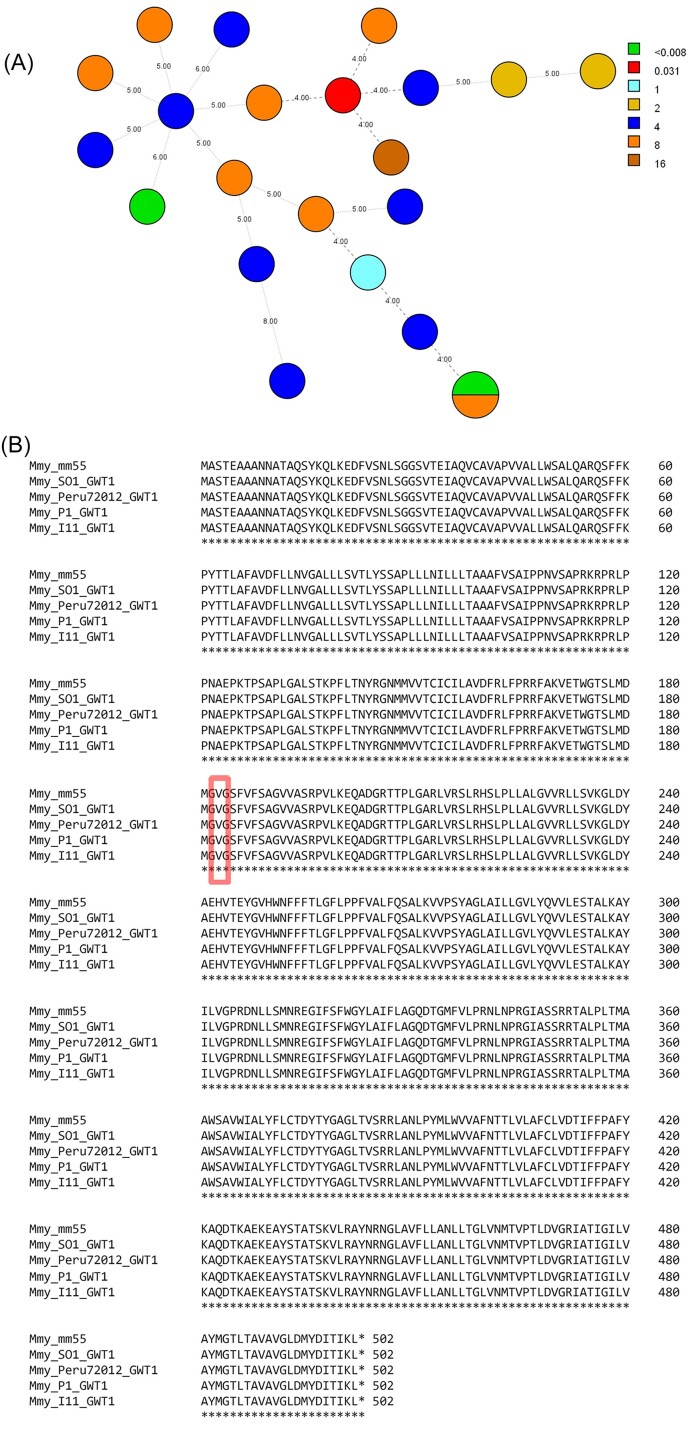
To determine if there was a genetic link for the high diversity in MICs obtained for manogepix, the 22 included M. mycetomatis isolates were typed with the MmySTR assay, and the translated DNA sequence of GWT1 of five isolates with diverse MICs was compared. All 22 isolates had a unique MmySTR profile (Fig. 2A). No apparent clusters are observed between the genotype and the susceptibility to manogepix. For five M. mycetomatis isolates for which whole genome sequence data was available, the GWT1 protein sequence was compared. As shown in Figure 2B, the GWT1 protein was conserved among these different isolates, and no differences in amino acids were observed. Of these five isolates, I11 and P1 had a very low MIC of <0.008 mg/l, Peru72012 had an MIC of 1 mg/l, and MM55 and SO1 had a relatively high MIC of 4 mg/l. The Val-168 residue corresponding to the valine residue that has been linked to resistance in different isolates of C. albicans, C. glabrata, and S. cerevisiae is conserved among all isolates31 (Fig. 2B).
Figure 2.
(A) Minimum spanning tree (MST) showing the genetic diversity of the panel of Madurella mycetomatis isolates included for in vitro susceptibility testing. Each circle represents a unique genotype, and the size is proportional to the number of isolates of the respective genotype. The numbers on the connecting lines represent the number of different STR markers between the genotypes. Each color represents the MIC of manogepix against the respective isolate in mg/l. (B) Multiple sequence alignment of the GWT1 protein from M. mycetomatis isolates MM55, SO1, Peru72012, P1, and I11. The Val-168 residue previously identified in resistant isolates of C. albicans, C. glabrata, and S. cerevisiae is highlighted by the red box30.
Manogepix and itraconazole are synergistic against M. Mycetomatis
To determine if manogepix and itraconazole act synergistically and could potentially be combined in therapy, a checkerboard assay was performed on a smaller subset of 10 different M. mycetomatis isolates. As shown in Table 2, combined exposure of manogepix and itraconazole for MM14, MM25, and P1 was found to be indifferent, with median FIC min values of 0.501, 0.547, and 0.563, respectively. This indicates that against these three isolates, manogepix and itraconazole do not act synergistically. For the other seven isolates, median FIC min values of ≤0.5 were found, indicating that manogepix and itraconazole act synergistically.
Table 2.
Overview checkerboard outcome. Synergism is indicated by Synergy (S) or Indifferent (I).
| Isolate | Median FIC min | FIC min range | Median FIC max | FIC max range | Synergism |
|---|---|---|---|---|---|
| MM14 | 0.501 | 0.500–0.750 | 0.750 | 0.625–1.000 | I |
| MM25 | 0.547 | 0.504–1.000 | 1.141 | 1.031–2.001 | I |
| MM54 | 0.126 | 0.070–0.127 | 0.625 | 0.563–0.625 | S |
| MM55 | 0.258 | 0.156–0.258 | 0.750 | 0.620–0.750 | S |
| MM83 | 0.344 | 0.250–0.375 | 0.816 | 0.563–1.016 | S |
| SO1 | 0.141 | 0.127–0.188 | 0.594 | 0.563–0.625 | S |
| AL1 | 0.195 | 0.133–0.250 | 0.688 | 0.625–0.750 | S |
| P1 | 0.563 | 0.500–1.063 | 1.500 | 0.750–2.063 | I |
| CBS247.48 | 0.227 | 0.188–1.000 | 0.625 | 0.625–1.000 | S |
| Peru72012 | 0.174 | 0.078–0.504 | 1.377 | 0.563–2.500 | S |
Manogepix in mono- and combination therapy does not enhance larval survival
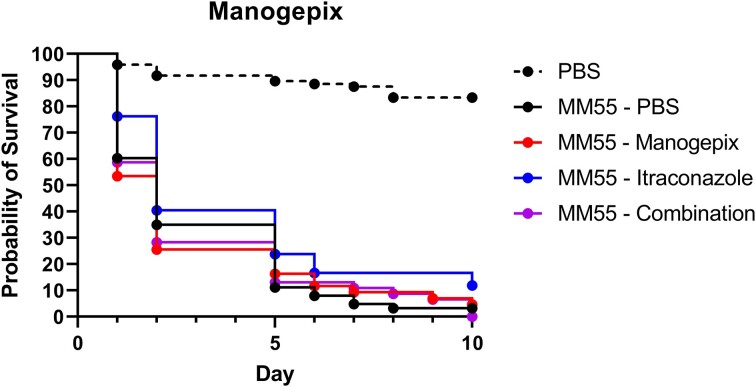
Although relatively high MICs were obtained for manogepix, synergy was obtained with itraconazole. We, therefore, determined the in vivo efficacy of a clinically relevant dosage of manogepix as monotherapy and in combination with itraconazole, the current drug of choice for mycetoma therapy, in our M. mycetomatis G. mellonella grain model. For these selected dosages, no toxicity was observed in G. mellonella larvae (data not shown). As shown in Figure 3, both 8.57 mg/kg manogepix as monotherapy and in combination with 5.71 mg/kg itraconazole did not significantly prolong the survival of M. mycetomatis-infected larvae.
Figure 3.
In vivo efficacy of 8.57 mg/kg manogepix, 5.71 mg/kg itraconazole, a combination of manogepix and itraconazole (8.57 and 5.71 mg/kg, respectively), or a 5% DMSO in PBS control within Galleria mellonella larvae. The full lines indicated infected larvae treated with the corresponding dosage at 4-, 28-, and 52 h post-infection. Dotted lines indicate uninfected larvae treated with a 5% DMSO in PBS, which were treated at the same time points as the infected groups. All larvae were followed up until 10 days after infection, and no significant difference was observed between any of the test groups compared to the infected PBS (5% DMSO) control.
Discussion
With the discovery of manogepix, numerous studies have been conducted on its effectiveness in treating invasive fungal diseases. In this study, we demonstrate growth inhibition of M. mycetomatis upon exposure to manogepix. According to the established method for susceptibility testing for M. mycetomatis, we report a colorimetric MIC50 of 4 mg/l and an MIC90 of 8 mg/l for manogepix, respectively, using both a 50% and an 80% inhibition cutoff vs. an itraconazole MIC50 and MIC90 of 0.063 mg/l and 0.25 mg/l. The broad range of MICs ranging from ≤0.008 to >4 mg/l could not be explained by differences in the drug target enzyme, as this was conserved within the M. mycetomatis isolates analyzed.
Manogepix has been reported to be effective in inhibiting the yeasts Candida spp. and Cryptococcus spp.30–32 For Candida spp., reported MIC90 values ranged from 0.008 mg/l (for C. albicans) to 0.5 mg/l (for C. kefyr), with the exception of C. krusei (MIC90 > 0.5 mg/l reported).31,32 The higher MICs observed for M. mycetomatis were in the same range as those reported for other filamentous fungi. For Aspergillus spp., MIC values for manogepix were above 8 mg/l when read with the standard CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) methods for in vitro susceptibility testing of filamentous fungi.33 Using 2,3-Bis(2-Methoxy-4-Nitro-5- Sulfophenyl)-5-[(Phenylamino)Carbonyl]-2H- Tetrazolium Hydroxide (XTT) as a viability dye, MICs >0.5 mg/l were obtained for A. fumigatus. However, similar to the echinocandins, alteration in growth for filamentous fungi was observed at a much lower concentration, and for A. fumigatus, only 0.06 mg/l manogepix was needed to alter the fungal growth.26 Therefore, similar to the echinocandins, for filamentous fungi, it is recommended to determine the concentration at which growth is visibly altered, defined as the MEC. The MEC90 values for manogepix against A. flavus, A. fumigatus, A. niger, and A. terreus ranged between 0.03 mg/l and 0.125 mg/l.16 For other rare moulds, among which the genetically more closely related Fusarium spp. and Scedosporium spp., similar MEC90 values between 0.03 mg/l and 0.25 mg/l are reported.34–38 MEC values for filamentous fungi are determined visually as the first concentration where growth is altered. This is clearly seen when conidia or spores are used as a starting inoculum, although lab-to-lab differences in interpretation are noted. Since M. mycetomatis only sporulates on rare occasions, hyphal fragments are used as starting inoculum in standard in vitro susceptibility assays.25 Unfortunately, when hyphal fragments are used, it is not possible to reliably determine an MEC since no clear morphological differences are noted for manogepix and echinocandins.39 Recently, a colorimetric alternative for visual MEC determination was described for Aspergillus spp. In this alternative method, a lower starting inoculum and the viability dye XTT are used, and a 50% reduction in metabolic activity corresponded to the visual MEC.26 Upon implementing this 50% reduction threshold in our methodology, a 50% reduction in metabolic activity with our standard hyphal starting inoculum did not result in a significantly lower MIC value. A possible explanation may be the number of fungal cells within the starting inoculum, as this effect for Aspergillus was also only observed when a 1:1000 diluted starting inoculum was used or because hyphal fragments were used instead of conidia.16 Altogether, the determination of the MIC, and especially the MEC, of manogepix against M. mycetomatis provides limited insights due to the starting conditions used in this methodology, regardless of the inhibition threshold, and should be interpreted with care.
Although for most strains, a concentration of 4 mg/l manogepix was needed to inhibit M. mycetomatis growth, we did observe synergy when manogepix was combined with itraconazole. This synergy was also observed for Candida, Cryptococcus, and Aspergillus.40,41 We, therefore, evaluated the in vivo efficacy of manogepix alone and in combination in an M. mycetomatis G. mellonella grain model. Unfortunately, neither for manogepix alone nor for the combination of manogepix and itraconazole prolonged larval survival was noted. This was in contrast to the efficacy shown for other fungal infections. In murine models of invasive candidiasis, 78 and 104 mg/kg fosmanogepix significantly reduced the fungal burden in the kidney, lung, and brain, resulting in enhanced murine survival.42,43 The same was found when mice with invasive pulmonary Aspergillosis, Scedosporiosis, Mucormycosis, or disseminated Fusariosis were treated with fosmanogepix.44–46 Fosmanogepix alone was ineffective in a C. neoformans meningitis mice model, but in combination with fluconazole, the fungal burden in the brain was significantly reduced.40 This difference in efficacy for manogepix against M. mycetomatis might be due to a difference in formulation or dosage used. In most studies, the prodrug fosmanogepix is used instead of manogepix. Fosmanogepix is cleaved into manogepix by the activity of host phosphatases. Since G. mellonella is not a mammalian model and there was no data on the activity of the G. mellonella phosphatases in comparison to those in mouse models, we used the active moiety manogepix in the G. mellonella model instead of the prodrug. Furthermore, the concentration we used in our study, 8.57 mg/kg, was much lower than the concentrations used in other studies in mice. In mice, the concentrations ranged from 26 mg/kg to 264 mg/kg. Dosages used in humans in ongoing clinical trials are 600 mg/dosage, which, based on an average 70 kg adult, translates to 8.57 mg/kg.30 Another factor may be the difference in virulence and biofilm formation, as manogepix inhibits glycophosphatidylinositol biosynthesis. This pathway is directly linked to A. fumigatus morphogenesis and virulence and has been described to suppress biofilm formation in C. albicans.15,47 For M. mycetomatis, grains (biofilm-like structures), in particular, are challenging as these remain viable for extended periods of time, even upon prolonged treatment with itraconazole.11 In our grain model, grains are observed after 4 h, at which time point the first dosage is administered, meaning that inhibition of grain formation could not be assessed.48 Altogether, both the treatment time, selected dosage, and additional unknown factors on the clearance and bioavailability of manogepix could contribute to the contradictory efficacy results compared to other studies and thus be seen as shortcomings in our study. For a thorough in vivo evaluation of fosmanogepix in the treatment of eumycetoma, additional studies are needed, including dose-finding studies as well as studies in mammalian models.
To conclude, we determined the potential of manogepix as a treatment for eumycetoma caused by M. mycetomatis. We found that relatively high concentrations of manogepix are needed to completely inhibit the growth of M. mycetomatis, which was similar to other filamentous fungi. In vitro, combining manogepix and itraconazole was synergistic, a finding not documented in vivo. Based on these findings, manogepix seems inferior to itraconazole in mycetoma treatment.
Contributor Information
Mickey Konings, Department of Medical Microbiology and Infectious Diseases, ErasmusMC University Medical Center Rotterdam, Dr. Molewaterplein 40, 3015GD, Rotterdam, The Netherlands.
Kimberly Eadie, Department of Medical Microbiology and Infectious Diseases, ErasmusMC University Medical Center Rotterdam, Dr. Molewaterplein 40, 3015GD, Rotterdam, The Netherlands.
Nikolaos Strepis, Department of Medical Microbiology and Infectious Diseases, ErasmusMC University Medical Center Rotterdam, Dr. Molewaterplein 40, 3015GD, Rotterdam, The Netherlands.
Bertrand Nyuykonge, Department of Medical Microbiology and Infectious Diseases, ErasmusMC University Medical Center Rotterdam, Dr. Molewaterplein 40, 3015GD, Rotterdam, The Netherlands.
Ahmed H Fahal, Mycetoma Research Center, University of Khartoum, Khartoum, Sudan.
Annelies Verbon, Department of Medical Microbiology and Infectious Diseases, ErasmusMC University Medical Center Rotterdam, Dr. Molewaterplein 40, 3015GD, Rotterdam, The Netherlands; Department of Internal Medicine, UMC Utrecht, Utrecht, The Netherlands.
Wendy W J van de Sande, Department of Medical Microbiology and Infectious Diseases, ErasmusMC University Medical Center Rotterdam, Dr. Molewaterplein 40, 3015GD, Rotterdam, The Netherlands.
Author contributions
Mickey Konings (Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing), Kimberly Eadie (Data curation, Investigation, Validation, Writing – review & editing), Nikolaos Strepis (Data curation, Formal analysis, Investigation, Software, Validation, Writing – review & editing), Bertrand Nyuykonge (Formal analysis, Investigation, Writing – review & editing), Ahmed H. Fahal (Resources, Writing – review & editing), Annelies Verbon (Supervision, Writing – review & editing), and Wendy W.J. van de Sande (Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing)
Funding
No financial support was obtained for this study. Manogepix was provided by Pfizer. Pfizer had no role in study design, data collection, and analysis, nor in the decision to publish.
Declaration of interest
None of the authors had any potential conflicts of interest.
Ethical approval
Not required.
References
- 1. World Health Organization . Sixty-Ninth World Health Assembly - addressing the burden of mycetoma. WHA69.21. 2016: Agenda item 15.3:1-2.
- 2. Zijlstra EE, van de Sande WW, Welsh O, Mahgoub El S, Goodfellow M, Fahal AH. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016; 16: 100–112. [DOI] [PubMed] [Google Scholar]
- 3. van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013; 7: e2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watts CJ, Wagner DK, Sohnle PG. Fungal infections, cutaneous. In: Schaechter M, ed. Encyclopedia of Microbiology, 3rd edn.Cambridge, MA: Academic Press, 2009: 382–388. [Google Scholar]
- 5. Matthew CF, Sarah JG, Christina AC, et al. Threats posed by the fungal Kingdom to humans, wildlife, and agriculture. MBio. 2020; 11: e00449–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van de Sande WWJ, Maghoub ES, Fahal AH, Goodfellow M, Welsh O, Zijlstra E. The mycetoma knowledge gap: identification of research priorities. PLoS Negl Trop Dis. 2014; 8: e2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacroix C, de Kerviler E, Morel P, Derouin F, de Chavin MF. Madurella mycetomatis mycetoma treated successfully with oral voriconazole. Br J Dermatol. 2005; 152: 1067–1068. [DOI] [PubMed] [Google Scholar]
- 8. Negroni R, Tobón A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005; 47: 339–346. [DOI] [PubMed] [Google Scholar]
- 9. N’Diaye B, Dieng MT, Perez A, Stockmeyer M, Bakshi R. Clinical efficacy and safety of oral terbinafine in fungal mycetoma. Int J Dermatol. 2006; 45: 154–157. [DOI] [PubMed] [Google Scholar]
- 10. DNDi . Clinical Trial for a Better Treatment for Mycetoma Starts in Sudan. Drugs for Neglected Diseases Intiative, 2017. [Google Scholar]
- 11. Fahal AH, Rahman IA, El-Hassan AM, Rahman ME, Zijlstra EE. The safety and efficacy of itraconazole for the treatment of patients with eumycetoma due to Madurella mycetomatis. Trans R Soc Trop Med Hyg. 2011; 105: 127–132. [DOI] [PubMed] [Google Scholar]
- 12. Suleiman SH, Wadaella ES, Fahal AH. The surgical treatment of mycetoma. PLoS Negl Trop Dis. 2016; 10: e0004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pharmaceuticals A. Open-label study of APX001 for treatment of patients with invasive mold infections caused by Aspergillus or rare molds (AEGIS). (ClinicalTrials.gov Identifier: NCT04240886).https://clinicaltrials.gov/ct2/show/study/NCT04240886
- 14. Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. Antimicrobial activity of manogepix, a first-in-class antifungal, and comparator agents tested against contemporary invasive fungal isolates from an international surveillance programme (2018–2019). J Glob Antimicrob Resist. 2021; 26: 117–127. [DOI] [PubMed] [Google Scholar]
- 15. Nao-aki W, Mamiko M, Takaaki H, Koji S, Kappei T, Katsura H. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother. 2012; 56: 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jørgensen KM, Astvad KMT, Arendrup MC. In vitro activity of manogepix (APX001A) and comparators against contemporary molds: MEC comparison and preliminary experience with colorimetric MIC determination. Antimicrob Agents Chemother. 2020; 64: e00730–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maiken Cavling A, Anuradha C, Karin Meinike J, Joseph M. Manogepix (APX001A) in vitro activity against Candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother. 2020; 64: e00656–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmed AO, Mukhtar MM, Kools-Sijmons M, et al. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J Clin Microbiol. 1999; 37: 3175–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van de Sande WW, Gorkink R, Simons G, et al. Genotyping of Madurella mycetomatis by selective amplification of restriction fragments (amplified fragment length polymorphism) and subtype correlation with geographical origin and lesion size. J Clin Microbiol. 2005; 43: 4349–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nyuykonge B, Eadie K, Zandijk WHA, et al. A short-tandem-repeat assay (MmySTR) for studying genetic variation in Madurella mycetomatis. J Clin Microbiol. 2021; 59: e02331–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smit S, Derks MF, Bervoets S, et al. Genome sequence of Madurella mycetomatis mm55, isolated from a human mycetoma case in Sudan. Genome Announc. 2016; 4: e00418–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis software for microcomputers. Bioinformatics. 1994; 10: 189–191. [DOI] [PubMed] [Google Scholar]
- 23. Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abd Algaffar SO, Verbon A, van de Sande WWJ, Khalid SA. Development and validation of an in vitro resazurin-based susceptibility assay against Madurella mycetomatis. Antimicrob Agents Chemother. 2021; 65: e01338–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed AOA, van de Sande WWJ, van Vianen W, et al. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob Agents Chemother. 2004; 48: 2742–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meletiadis J, Siopi M, Kanioura L, et al. A multicentre study to optimize echinocandin susceptibility testing of Aspergillus species with the EUCAST methodology and a broth microdilution colorimetric method. J Antimicrob Chemother. 2020; 75: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmed SA, Kloezen W, Fahal AH, de Hoog GS, van de Sande WW. In vitro interaction of currently used azoles with terbinafine against Madurella mycetomatis. Antimicrob Agents Chemother. 2015; 59: 1373–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meletiadis J, Verweij PE, Te Dorsthorst DTA, Meis JFGM, Mouton JW. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol. 2005; 43: 133–152. [DOI] [PubMed] [Google Scholar]
- 29. Eadie K, Parel F, Helvert-van Poppel M, Fahal A, van de Sande W. Combining two antifungal agents does not enhance survival of Galleria mellonella larvae infected with Madurella mycetomatis. Trop Med Int Health. 2017; 22: 696–702. [DOI] [PubMed] [Google Scholar]
- 30. Shaw KJ, Ibrahim AS. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel). 2020; 6: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arendrup MC, Jørgensen KM. Manogepix (APX001A) displays potent in vitro activity against human pathogenic yeast, but with an unexpected correlation to fluconazole MICs. Antimicrob Agents Chemother. 2020; 64: e00429–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. In vitro activity of APX001A (Manogepix) and comparator agents against 1,706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother. 2019; 63: e00840–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pfaller MA, Duncanson F, Messer SA, Moet GJ, Jones RN, Castanheira M. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Aspergillus spp. Determined by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother. 2011; 55: 5155–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mariana C, Frederick PD, Daniel JD, Josep G, Ronald NJ, Michael AP. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob Agents Chemother. 2012; 56: 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of APX001A against rare moulds using EUCAST and CLSI methodologies. J Antimicrob Chemother. 2019; 74: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 36. Lim W, Nyuykonge B, Eadie K, et al. Screening the pandemic response box identified benzimidazole carbamates, olorofim and ravuconazole as promising drug candidates for the treatment of eumycetoma. PLoS Negl Trop Dis. 2022; 16: e0010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van de Sande WWJ. Phylogenetic analysis of the complete mitochondrial genome of Madurella mycetomatis confirms its taxonomic position within the order Sordariales. PLoS One. 2012; 7: e38654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badali H, Patterson HP, Sanders CJ, et al. Manogepix, the active moiety of the investigational agent Fosmanogepix, demonstrates in vitro activity against members of the Fusarium oxysporum and Fusarium solani species complexes. Antimicrob Agents Chemother. 2021; 65: e02343–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van de Sande WW, Fahal AH, Bakker-Woudenberg IA, van Belkum A. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob Agents Chemother. 2010; 54: 2738–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shaw KJ, Schell WA, Covel J, et al. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob Agents Chemother. 2018; 62:e00523–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watanabe NA, Horii T, Miyazaki M, Hata K. In vitro activity of E1210 and in vivo activity of E1211: a water-soluble prodrug of E1210, in combination with other antifungals. Intersci Conf Antimicrob Agents Chemother. 2011. abstract F1-1377 [Google Scholar]
- 42. Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother. 2018; 62: e02319–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiederhold NP, Najvar LK, Shaw KJ, et al. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother. 2019; 63: e01120–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gebremariam T, Alkhazraji S, Alqarihi A, et al. APX001 is effective in the treatment of murine invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2019; 63: e01713–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alkhazraji S, Gebremariam T, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Chemother. 2020; 64: e01735–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gebremariam T, Alkhazraji S, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother. 2020; 64: e00178–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li H, Zhou H, Luo Y, Ouyang H, Hu H, Jin C. Glycosylphosphatidylinositol (GPI) anchor is required in Aspergillus fumigatus for morphogenesis and virulence. Mol Microbiol. 2007; 64: 1014–1027. [DOI] [PubMed] [Google Scholar]
- 48. Kloezen W, van Helvert-van Poppel M, Fahal AH, van de Sande WWJ. A Madurella mycetomatis grain model in Galleria mellonella larvae. PLoS Negl Trop Dis. 2015; 9: e0003926. [DOI] [PMC free article] [PubMed] [Google Scholar]