ABSTRACT
Isolation of triplet pnictinidenes, which bear two unpaired electrons at the pnictogen centers, has long been a great challenge due to their intrinsic high reactivity. Herein, we report the syntheses and characterizations of two bismuthinidenes MsFluindtBu-Bi (3) and MsFluind*-Bi (4) stabilized by sterically encumbered hydrindacene ligands. They were facilely prepared through reductions of the corresponding dichloride precursors with 2 molar equivalents of potassium graphite. The structural analyses revealed that 3 and 4 contain a one-coordinate bismuth atom supported by a Bi–C single σ bond. As a consequence, the remaining two Bi 6p orbitals are nearly degenerate, and 3 and 4 possess triplet ground states. Experimental characterizations with multinuclear magnetic resonance, magnetometry and near infrared spectroscopy coupled to wavefunction based ab initio calculations concurred to evidence that there exist giant and positive zero field splittings (>4300 cm–1) in their S = 1 ground states. Hence even at room temperature the systems almost exclusively populate the lowest-energy nonmagnetic Ms = 0 level, which renders them seemingly diamagnetic.
Keywords: bismuth, bismuthinidene, triplet state, zero field splitting, theoretical calculation
Two triplet bismuthinidenes have been isolated and structurally characterized, but both behave as if they possessed diamagnetic ground states in magnetometric measurements due to the exceedingly strong relativistic effect.
INTRODUCTION
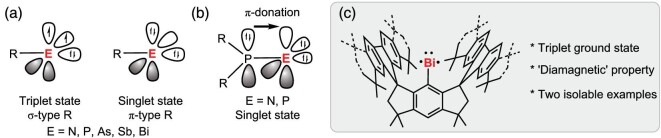
Low-valent main group compounds are intriguing species thanks to their unique physical and chemical properties [1–3]. Apart from being often used as supporting ligands in transition metal chemistry, they have been shown to play important roles in small molecule activation and catalysis [1–3]. Among them, pnictinidenes R-E (E = P, As, Sb or Bi; R = monoanionic ligands), a group of 15 compounds containing a one-coordinate central atom in the oxidation state of + 1, have attracted a great deal of attention, which is ascribed to their interesting electronic structures and significant uses in synthetic chemistry [4]. They can adopt either a singlet or a triplet electronic ground state, depending on the nature of the attached substituent R (Fig. 1a, where R is an anionic monosubstituted ligand) [5]. The preparation of free pnictinidenes is, however, highly challenging, which lies in the fact such species have a high propensity to undergo self-aggregation leading to dipnictenes or higher oligomers [6–12]. To date, only a limited number of free pnictinidenes have been isolated under standard experimental conditions. A singlet phosphinidene reported by Bertrand and coworkers in 2016 was kinetically stabilized by a bulky π-type diaminophosphino ligand [13], and the same group used a similar approach to access a singlet nitrene [14]. The stabilization of both species was found to be largely accomplished by the π-donation from the phosphinyl center to an empty p orbital at the terminal nitrogen or phosphorus center (Fig. 1b). It is noteworthy that carbyne anions [15] are isoelectronic to pnictinidenes, and a singlet copper(I) carbyne anion complex has recently been reported by Liu et al. [16]. In 2020, a transient triplet metallonitrene was characterized by Schneider, Holthausen et al. through in situ X-ray crystallography at low temperatures, but its high thermal sensitivity hinders the isolation [17].
Figure 1.
(a) Possible electronic structures of nitrenes and pnictinidenes. (b) The strategy used to stabilize singlet nitrene [14] and phosphinidene [13] reported by Bertrand et al. (c) An isolable bismuthinidenes reported in this work.
In comparison to nitrenes and phosphinidenes, the isolation of heavier pnictinidenes is more difficult attributed to more diverging ns and np orbitals when descending the periodic table of elements [18]. Therefore, stibinidenes and bismuthinidenes are typically stabilized by coordination with Lewis bases leading to two- or three-coordinate metal centers, which, however, significantly changes their ground-state electronic structures from a triplet to a singlet [19–23]. Cornella et al. showed that chelating N, C, N-ligated bismuthinidenes could act as efficient catalysts through Bi(I)/Bi(III) redox couples [24–26].
Triplet bismuthinidenes, BiX (X = H, F, Cl, Br, I, Me and AlCl4), have been studied in the gas phase at elevated temperatures (> 400 K) since the 1960s [27–29]. Very recently, using our own developed sterically encumbered hydrindacene ligands, we succeeded in accessing base-stabilized germylidenylpnictinidenes [30], free germylyne [31] and stannylyne radicals [32], and more strikingly, a triplet stibinidene [33]. As our ongoing research targeted heavier free pnictinidenes, here we report the syntheses, characterizations and reactivity studies of two highly thermally stable bismuthinidenes, 3 and 4 (Fig. 1c). However, during the preparation of this manuscript, exactly the same compound 3 was reported by Cornella and coworkers [34]. Combined spectroscopic and computational studies demonstrate that 3 and 4 feature triplet electronic ground states with the two unpaired electrons dominantly residing in the two energetically near-degenerate Bi 6px and 6py orbitals. Remarkably, spectroscopic and magnetometric measurements revealed that their paramagnetic ground states feature gigantic, positive zero-field splittings (>4300 cm–1), which render them seemingly diamagnetic up to room temperature.
RESULTS AND DISCUSSION
The Bi(III) precursors MsFluindtBu-BiCl2 (1) and MsFluind*-BiCl2 (2) were readily synthesized through the reactions of the corresponding lithium salts and bismuth trichloride in THF at room temperature (Scheme 1). Compounds 1 and 2 were isolated in moderate yields as colorless solids. The molecular structures determined by single-crystal X-ray diffraction (SC-XRD) analyses are depicted in Figures S1 and S2 in Supplementary Information (SI), respectively. We then carried out the reduction reactions of 1 and 2 in THF with 2 molar equivalents of KC8 in THF at room temperature. Upon workup, bismuthinidenes 3 and 4 were isolated as yellow crystals in 42% and 46% yields, respectively. Compound 3 is marginally soluble in n-hexane, but moderately soluble in toluene and benzene, while 4 has a good solubility in all these solvents. Although highly air-sensitive, they can be kept at room temperature under a N2 atmosphere for at least 1 month, and can even be heated to 80oC for 6 hours in benzene solutions without noticeable decomposition.
Scheme 1.
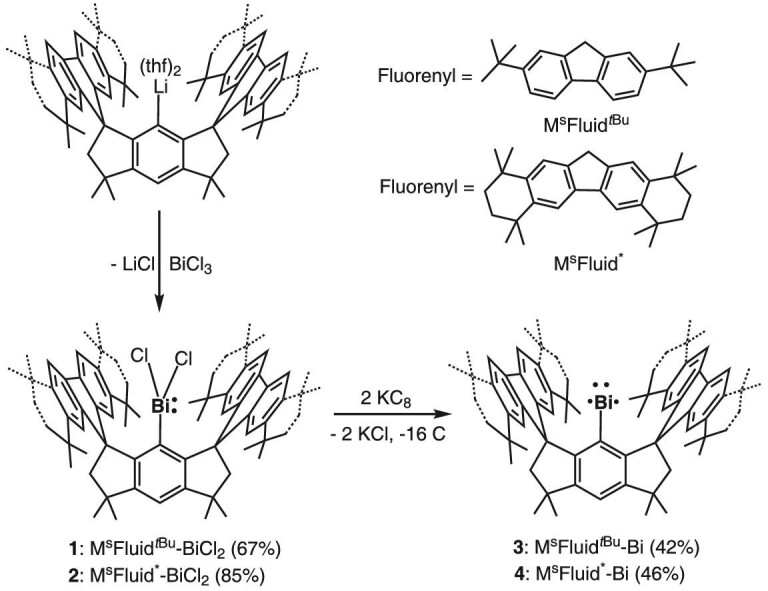
Syntheses of bismuthinidenes through the reductions of Bi(III) precursors with potassium graphite.
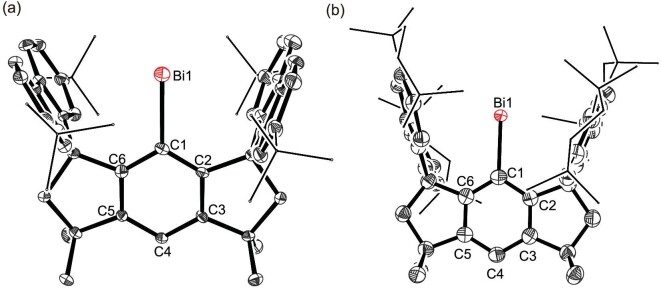
Molecular structures of 3 and 4 were unambiguously determined by SC-XRD analyses (Fig. 2). Both crystallize in the triclinic space group P-1, and feature similar geometric parameters; therefore, we mainly discuss those of 3 here. The Bi atom is only bonded to the C atom of the ligand central phenyl (Ph) ring with a Bi–C distance of 2.284(4) Å, which is comparable to those in 1 (2.278(6) Å) and a dibismuthene (2.290(7) Å) reported by Tokitoh et al. [9], indicative of a single bond character. The Bi atom resides at the center of the ligand pocket as suggested by the almost identical angles of Bi1-C1-C6 (121.3(3)o) and Bi1-C1-C2 (121.6(3)o). Moreover, the shortest distance of ∼3.4 Å from the Bi atom to the flanking fluorenyl substituents in 3 indicates the absence of noticeable covalent bonding interactions between them [35], but the presence of van der Waals interactions [36,37]. The two neighboring molecules of 3 exhibit a face-to-face interaction with an intermolecular Bi•••Bi distance of 4.405 Å (Supplementary Figure S3). This long distance, which substantially exceeds the Bi–Bi single bond length in {[(SiMe3)2CH]2Bi}2 (3.0053(4) Å) [38], and, more importantly, the sum of the van der Waals radii of two Bi atoms (4.14 Å) [36,37], evidences that there exists no Bi–Bi bonding interaction. Analogously, the Bi center is well-separated by the ligand skeleton in 4 (Supplementary Figure S4), and the shortest Bi•••Bi distance of 13.627 Å significantly surpasses that in 3, which is attributed to the presence of two bulkier flanking fluorenyl substituents in 4. Therefore, 3 and 4 represent the first isolable examples of free bismuthinidenes containing one-coordinated Bi atoms. In fact, the isolation of one-coordinate main-group species remains a formidable task, and very recently one-coordinate Al(I) compounds have been reported by Power et al. [39] and Zhang and Liu [40].
Figure 2.
Thermal ellipsoid drawings of the molecular structures of 3 (a) and 4 (b). All hydrogen atoms are omitted, and the substituents at the fluorenyl moieties are shown in a wireframe style for clarity. Selected bond lengths (Å) and angles (o): 3: Bi1–C1 2.284(4), C1–C2 1.397(5), C1–C6 1.408(5), C2–C3 1.394(5), C3–C4 1.399(5), C4–C5 1.376(5), C5–C6 1.404(5); C2-C1-Bi1 121.6(3), C6-C1-Bi1 121.3(3). 4: Bi1–C1 2.275(3), C1–C2 1.399(5), C1–C6 1.398(5), C2–C3 1.397(5), C3–C4 1.388(5), C4–C5 1.390(5), C5–C6 1.402(5); C2-C1-Bi1 121.8(2), C6-C1-Bi1 122.0(2).
All features in the 13C and 1H NMR spectra of 3 and 4 recorded in solutions are in the typical diamagnetic region and similar to those of the Bi(III) precursors, except those for atoms in the vicinity of the Bi center (Supplementary Figures S23–S26). For 3, the most remarkable feature is that the 13C resonance for the carbon connected to the Bi atom has a chemical shift of δ –204.8 ppm at room temperature, whereas the corresponding 13C signals in 1 and 2,6-Trip2C6H3-BiCl2 (Trip = 2,4,6-iPr3C6H2) [8] appear at δ 213.0 and 214.6 ppm, respectively. Similarly, an upfield shift was also found for the resonance of the Ph proton at the para-position to the bismuth atom at δ –1.06 ppm in 3 relative to δ 7.58 ppm observed for 1. The heteronuclear single quantum correlation (HSQC) spectrum clearly shows the correlation of this signal with the carbon feature at δ 160.2 ppm (Supplementary Figure S9). In analogy to 3, the corresponding C and H atoms in 4 exhibit abnormally upfield shifted NMR signals at δ –189.5 and –0.63 ppm in comparison to those at δ 219.3 and 7.50 ppm in 2, respectively. Moreover, the absence of any absorption at around v of 1700 cm–1 in the infra-red spectrum (Supplementary Figure S10) rules out the possibility that there is any hydride bonded to the bismuth center [41]. Based on all findings, we surmised that 3 and 4 have an S = 1 ground state. As integer spin systems are usually silent for conventional X-band EPR spectrometers, we thus carried out variable-temperature (T) magnetic susceptibility (χ) measurements with a superconducting quantum interference device (SQUID). However, as shown in Fig. 3a, the χT value thus obtained varies linearly with respect to T. Consequently, χT can be reasonably modelled by a moderate temperature independent paramagnetism (TIP) term of 194 × 10−6 emu for an S = 0 ground state, because after subtraction of the TIP, the χT value is essentially independent of T. These findings seem to be incompatible with the ground-state spin multiplicity deduced from multinuclear NMR investigations and necessitates further scrutiny.
Figure 3.
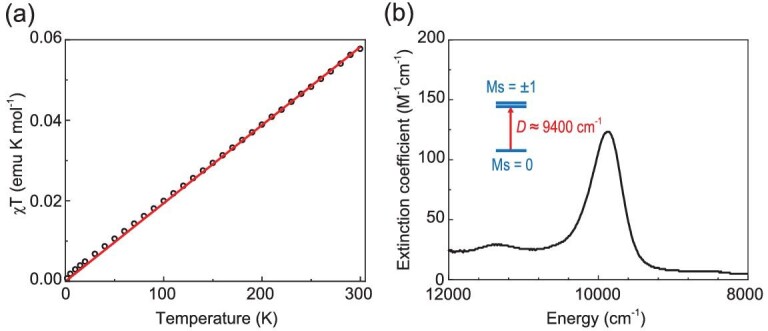
(a) Variable temperature magnetization measurements of a solid sample of 3. The dots represent experimental measurements and the solid line (red) represents fitting with χTIP = 194 × 10−6 emu. (b) Near infrared absorption spectrum of 3 in THF (1.014 mM) at room temperature. (See online supplementary material for a color version of this figure.)
To elucidate the electronic structure of bismuthinidene 3, we undertook detailed theoretical computations. Noncovalent interaction (NCI) [42] analyses at the PBE level of theory employing the scalar relativistic second-order Douglas-Kroll-Hess Hamiltonian [43] (Supplementary Figure S12) show that, except for van der Waals interactions, there is no other considerable bonding between the Bi center and the flanking fluorenyl functionalities. Therefore, we hereafter focus on the covalent bonding between the Bi atom and the central Ph group. To this end, wavefunction based multireference CASSCF [44]/NEVPT2 [45] (CASSCF = the complete active space self-consistent field, NEVPT2 = N-electron valence perturbation theory up to the 2nd order) computations were performed, for which a simplified model (3ʹ) was employed where all methyl substituents of 3 were replaced by hydrogen atoms. An active space was chosen to distribute 12 electrons into 11 orbitals including Bi–C σ and σ*, Bi 6s and 6p orbitals as well as the six π orbitals of the Ph ring. Theoretical results suggested that 3ʹ possesses a triplet ground state that is 18.4 and 18.5 kcal/mol lower in energy than the open- and closed-shell singlet states, respectively. As shown in Fig. 4a, the dominant electron configuration of the triplet ground state is (Bi 6s)2(Ph π1,2,3)6(Bi–C σz)2(Bi 6px)1(Bi 6py)1(Ph π*4,5,6)0(Bi–C σ*z)0 and accounts for 86% of the wavefunction. Because of the exceedingly large energy separation between Bi 6p and C 2p atomic orbitals, one hardly identifies any discernible π-bonding between Bi 6py and Ph C 2p orbitals. The Bi–Ph interaction is thus best described as a single σ bond, consistent with the computed Mayer bond order of 0.77. In other words, the Bi 6px and 6py orbitals are nearly degenerate. In line with this notion is that both orbitals make nearly identical contributions to the spin population, thereby resulting in donut-like positive spin density around the Bi center (Fig. 4b). As such, 3 ought to have a triplet ground state on the grounds of Hund's rule. On the other hand, if it possessed a diamagnetic ground state with a nominal vacant Bi 6py orbital, for the same reason discussed above, the π donation of the occupied Ph π orbitals into Bi 6py would not be strong enough to stabilize such an S = 0 ground state.
Figure 4.
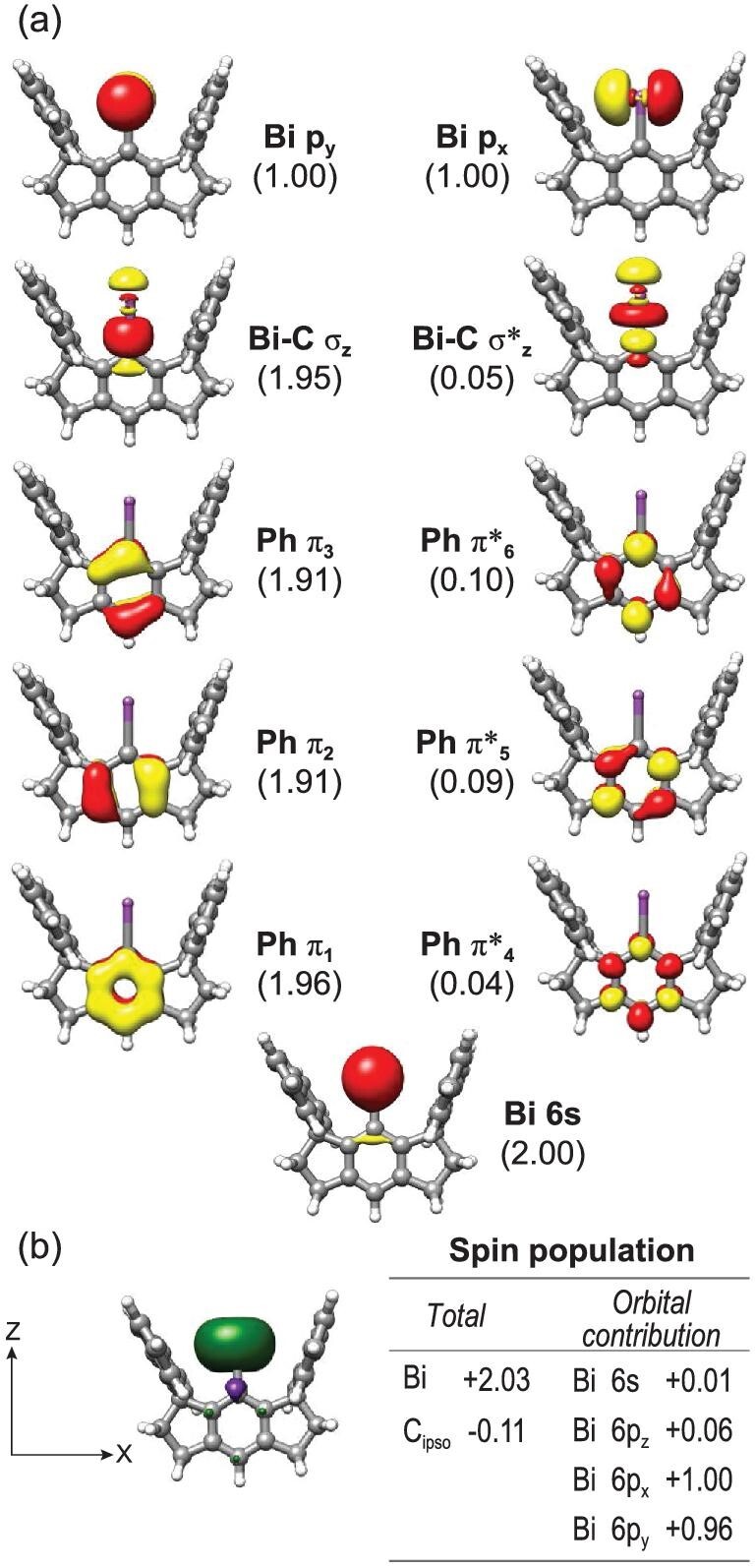
(a) Natural orbitals of the ground-state electronic structure of 3ʹ obtained from CASSCF(12,11) calculations with the corresponding occupation number in parentheses listed below each orbital label. (b) Computed spin density, spin population and orbital contributions thereof.
CASSCF(12,11)/NEVPT2 calculations predicted that 3ʹ possesses an axial zero-field splitting (ZFS) D >4300 cm–1 (Supplementary Table S1). Due to such a huge, positive ZFS, the system almost exclusively populates at the lowest-energy non-magnetic Ms = 0 level even at room temperature, whereas the populations of the excited magnetic Ms = ±1 levels are negligible. As a consequence, the precise ground state of 3 cannot be readily determined by SQUID measurements up to 300 K (Supplementary Figure S14). As elaborated in the Supporting Information, this unprecedented giant D value originates from the exceptionally strong spin-orbit coupling (SOC) between the triplet ground state and low-lying closed-shell singlet excited states, which gets accentuated by the effective SOC constant of Bi reaching as high as 12 000 cm–1 [46]. Bi22– dianion, which is isoelectronic to triplet dioxygen, also exhibited a diamagnetic nature owing to the strong SOC effect of the Bi atom [47,48]. Consistent with the theoretical prediction, the near infrared spectrum of 3 registers a broad absorption peak at 9870 cm–1 with a band width of 570 cm–1 (Fig. 3b and Supplementary Figure S11). This feature has a low intensity (ϵ = 120 M–1 cm–1), which likely reflects its formally spin-forbidden nature; thus, we tentatively attributed it to Ms = 0 → Ms = ±1 transitions, an assignment that requires further experimental verification.
We further performed reactivity studies of 3 to have a better understanding of its chemical properties. Facile oxidative addition of PhChChPh (Ch' = S and Se) to the Bi center in 3 was observed to afford Bi(III) compounds MsFluidtBu-Bi(Ch’Ph) (5: Ch' = S; 6: Ch' = Se) (Scheme 2), consistent with the + 1 oxidation state of the Bi atom in 3. Moreover, the reaction of 3 with PhSSPh in a C6D6 solution was tested in a NMR tube, but did not show the formation of H2, further confirming that no hydride is attached to the Bi atom in 3 (Supplementary Figure S29). The molecular structures of 5 and 6 were unambiguously determined by SC-XRD analyses as shown in Supplementary Figures S5 and S6, respectively.
Scheme 2.
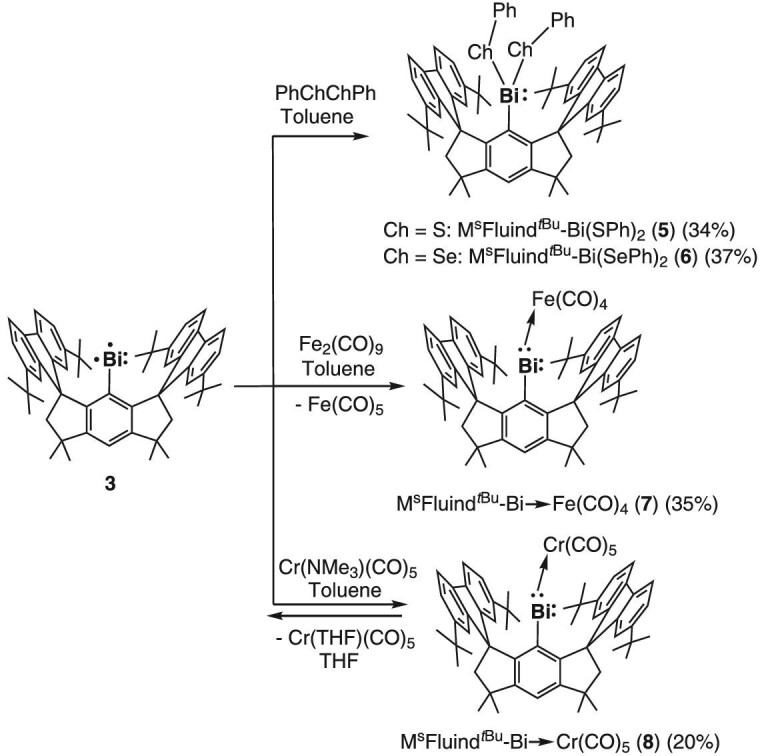
Reactivity studies of 3.
Upon treatment of 3 with Fe2(CO)9 and Cr(NMe3)(CO)5, complexes MsFluidtBu-Bi→Fe(CO)4 (7) and MsFluidtBu-Bi→Cr(CO)5 (8) were isolated as purple and green crystals, respectively. Interestingly, 7 and 8 feature singlet ground states as shown by NMR spectroscopy. It is noteworthy that the Cr(CO)5 unit is only weakly coordinated to the bismuth atom in 8, and readily dissociates in a THF-D8 solution to convert back to 3. The molecular structures of 7 (Supplementary Figure S7) and 8 (Supplementary Figure S8) show that the Bi–Fe (2.5501(9) Å) and Bi–Cr (2.7263(14) Å) distances are shorter than the corresponding Bi–M bonds (2.6705 and 2.8144(19) Å, respectively) in N, C, N-chelated bismuthinidene-metal carbonyl complexes [49]. This is most probably attributed to the low-coordinate nature of the Bi atoms in 7 and 8, which strengthens π-back-donation interactions with transition metal centers. To the best of our knowledge, 7 and 8 are the first bismuthinidene complexes containing two-coordinate Bi atoms.
Theoretical calculations predicted that the triplet-singlet gaps of 7 and 8 are as high as 16.3 and 38.9 kcal/mol, respectively, and hence confirmed their diamagnetic ground states. Both systems feature considerable π-back-donation from the transition metal moieties to the unoccupied pπ acceptor orbital of the Bi center (Supplementary Figures S15 and S16), an analogous stabilizing effect was also observed for singlet phosphinidene complexes [50]. Upon closer inspection, the π-back-donation in 7 is much stronger than that in 8, presumably due to the attenuated π donation ability of the Cr(CO)5 unit having a relatively electron deficient Cr0 center compared to Fe0, which accounts for their distinct Bi–Fe and Bi–Cr bond lengths and bonding strengths.
CONCLUSION
In summary, this work demonstrated that bismuthinidenes 3 and 4 featuring essentially one-coordinate Bi atoms can be isolated in the condensed phase at room temperature by utilizing the sterically congested hydrindacene ligands. Theoretical analyses revealed that the one-coordinate Bi atom features two nearly degenerate Bi 6px and 6py orbitals; thus, the bismuthinidenes favor a triplet, instead of a singlet, ground state with a leading electron configuration of (Bi–C σz)2(Bi 6px)1(Bi 6py)1. They represent the first isolable examples of Lewis base-free bismuthinidenes. Multinuclear NMR, SQUID and near infrared measurements revealed that the positive, gigantic ZFSs (>4300 cm–1) in their S = 1 ground states render them to behave as if they had S = 0 ground states. The unique electronic structures of the bismuthinidenes may lead to interesting reactivity, which is currently under investigation in our laboratory.
ADDITIONAL INFORMATION
X-ray data are available from the Cambridge crystallographic Data Centre under reference numbers CCDC 2227156 (1), 2227157 (2), 2227158 (3), 2227159 (4), 2227160 (5), 2227161 (6), 2227162 (7), 2227163 (8). These data can be obtained free of charge from the CCDC at http://www.ccdc.cam.ac.uk/data_request/cif. All other experimental, spectroscopic, crystallographic, and computational data are included in the Supplementary Information.
Supplementary Material
Contributor Information
Mengyuan Wu, Innovation Center for Chemical Sciences, Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China.
Wang Chen, State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Dongmin Wang, Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, IGCME, Sun Yat-sen University, Guangzhou 510275, China; Innovation Center for Chemical Sciences, Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China.
Yizhen Chen, Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, IGCME, Sun Yat-sen University, Guangzhou 510275, China; Innovation Center for Chemical Sciences, Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China.
Shengfa Ye, State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China.
Gengwen Tan, Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, IGCME, Sun Yat-sen University, Guangzhou 510275, China; Innovation Center for Chemical Sciences, Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China; State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China.
FUNDING
The experimental work was supported by the National Natural Science Foundation of China (22071164), Fundamental Research Funds for the Central Universities, Sun Yat-sen University (23qnpy35) and the Suzhou Science and Technology NOVA Program (ZXL2022445). The magnetic studies and theoretical calculations were supported by the National Natural Science Foundation of China (92161204).
AUTHOR CONTRIBUTIONS
G.T. and S.Y. conceived and supervised the project. M.W. synthesized and characterized the compounds. D.W. and Y.C. collected the single-crystal X-ray diffraction data. W.C. carried out SQUID and UV-vis-NIR measurements and theoretical calculations. G.T. and S.Y. wrote the manuscript, which was reviewed and edited with input from all authors.
Conflict of interest statement. None declared.
REFERENCES
- 1. Asay M, Jones C, Driess M. N-heterocyclic carbene analogues with low-valent group 13 and group 14 elements: syntheses, structures, and reactivities of a new generation of multitalented ligands. Chem Rev 2011; 111: 354–96. 10.1021/cr100216y [DOI] [PubMed] [Google Scholar]
- 2. Power PP. Main-group elements as transition metals. Nature 2010; 463: 171–7. 10.1038/nature08634 [DOI] [PubMed] [Google Scholar]
- 3. Mizuhata Y, Sasamori T, Tokitoh N. Stable heavier carbene analogues. Chem Rev 2009; 109: 3479–511. 10.1021/cr900093s [DOI] [PubMed] [Google Scholar]
- 4. Dostál L. Quest for stable or masked pnictinidenes: emerging and exciting class of group 15 compounds. Coord Chem Rev 2017; 353: 142–58. 10.1016/j.ccr.2017.10.009 [DOI] [Google Scholar]
- 5. Nguyen MT, Van Keer A, Vanquickenborne LG. In search of singlet phosphinidenes. J Org Chem 1996; 61: 7077–84. 10.1021/jo9604393 [DOI] [PubMed] [Google Scholar]
- 6. Weber L, Ebeler F, Ghadwal RS. Advances and recent trends in dipnictenes chemistry. Coord Chem Rev 2022; 461: 214499. 10.1016/j.ccr.2022.214499 [DOI] [Google Scholar]
- 7. Yoshifuji M, Shima I, Inamoto Net al. Synthesis and structure of bis(2,4,6-tri-tert-butylphenyl)diphosphene: isolation of a true phosphobenzene. J Am Chem Soc 1981; 103: 4587–9. 10.1021/ja00405a054 [DOI] [Google Scholar]
- 8. Twamley B, Sofield CD, Olmstead MMet al. Homologous series of heavier element dipnictenes 2,6-Ar2H3C6E = EC6H3-2,6-Ar2 (E = P, As, Sb, Bi; Ar = Mes = C6H2-2,4,6-Me3; or Trip = C6H2-2,4,6-iPr3) stabilized by m-terphenyl ligands. J Am Chem Soc 1999; 121: 3357–67. 10.1021/ja983999n [DOI] [Google Scholar]
- 9. Tokitoh N, Arai Y, Okazaki Ret al. Synthesis and characterization of a stable dibismuthene: evidence for a Bi-Bi double bond. Science 1997; 277: 78–80. 10.1126/science.277.5322.78 [DOI] [Google Scholar]
- 10. Tuscher L, Ganesamoorthy C, Bläser Det al. A gallium-substituted distibene and an antimony-analogue bicyclo[1.1.0]butane: synthesis and solid-state structures. Angew Chem Int Ed 2015; 54: 10657–61. 10.1002/anie.201502827 [DOI] [PubMed] [Google Scholar]
- 11. Dange D, Davey A, Abdalla JABet al. Utilisation of a lithium boryl as a reducing agent in low oxidation state group 15 chemistry: synthesis and characterisation of an amido-distibene and a boryl-dibismuthene. Chem Commun 2015; 51: 7128–31. 10.1039/C5CC01772F [DOI] [PubMed] [Google Scholar]
- 12. Tokitoh N, Arai Y, Sasamori Tet al. A unique crystalline-state reaction of an overcrowded distibene with molecular oxygen: the first example of a single crystal to a single crystal reaction with an external reagent. J Am Chem Soc 1998; 120: 433–4. 10.1021/ja973295y [DOI] [Google Scholar]
- 13. Liu L, Ruiz DA, Munz Det al. A singlet phosphinidene stable at room temperature. Chem 2016; 1: 147–53. 10.1016/j.chempr.2016.04.001 [DOI] [Google Scholar]
- 14. Dielmann F, Back O, Henry-Ellinger Met al. A crystalline singlet phosphinonitrene: a nitrogen atom–transfer agent. Science 2012; 337: 1526–8. 10.1126/science.1226022 [DOI] [PubMed] [Google Scholar]
- 15. Feng E, Yu ZJ, Jiang Het al. Gas-phase reactivity of phenylcarbyne anions. J Am Chem Soc 2022; 144: 8576–90. 10.1021/jacs.1c13714 [DOI] [PubMed] [Google Scholar]
- 16. Wei R, Wang X-F, Hu Cet al. Synthesis and reactivity of copper carbyne anion complexes. Nat Synth 2023; 2: 357–63. 10.1038/s44160-022-00225-y [DOI] [Google Scholar]
- 17. Sun J, Abbenseth J, Verplancke Het al. A platinum(II) metallonitrene with a triplet ground state. Nat Chem 2020; 12: 1054–9. 10.1038/s41557-020-0522-4 [DOI] [PubMed] [Google Scholar]
- 18. Wedler HB, Wendelboe P, Power PP. Second-order Jahn–Teller (SOJT) structural distortions in multiply bonded higher main group compounds. Organometallics 2018; 37: 2929–36. 10.1021/acs.organomet.8b00382 [DOI] [Google Scholar]
- 19. Krüger J, Wölper C, Auer AAet al. Formation and cleavage of a Sb−Sb double bond: from carbene-coordinated distibenes to stibinidenes. Eur J Inorg Chem 2022; 2022: e202100960. 10.1002/ejic.202100960 [DOI] [Google Scholar]
- 20. Dorsey CL, Mushinski RM, Hudnall TW. Metal-free stabilization of monomeric antimony(I): a carbene-supported stibinidene. Chem Eur J 2014; 20: 8914–7. [DOI] [PubMed] [Google Scholar]
- 21. Šimon P, deProft F, Jambor Ret al. Monomeric organoantimony(I) and organobismuth(I) compounds stabilized by an NCN chelating ligand: syntheses and structures. Angew Chem Int Ed 2010; 49: 5468–71. 10.1002/anie.201002209 [DOI] [PubMed] [Google Scholar]
- 22. Vránová I, Dušková T, Erben Met al. Trapping of the N,C,N-chelated organobismuth(I) compound, [2,6-(Me2NCH2)2C6H3]Bi, by its coordination toward selected transition metal fragments. J Organomet Chem 2018; 863: 15–20. 10.1016/j.jorganchem.2018.03.024 [DOI] [Google Scholar]
- 23. Wang G, Freeman LA, Dickie DAet al. Isolation of cyclic(alkyl)(amino) carbene–bismuthinidene mediated by a beryllium(0) complex. Chem Eur J 2019; 25: 433539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang X, Reijerse EJ, Bhattacharyya Ket al. Radical activation of N–H and O–H bonds at bismuth(II). J Am Chem Soc 2022; 144: 16535–44. 10.1021/jacs.2c05882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pang Y, Leutzsch M, Nöthling Net al. Catalytic hydrodefluorination via oxidative addition, ligand metathesis, and reductive elimination at Bi(I)/Bi(III) centers. J Am Chem Soc 2021; 143: 12487–93. 10.1021/jacs.1c06735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moon HW, Cornella J. Bismuth redox catalysis: an emerging main-group platform for organic synthesis. ACS Catal 2022; 12: 1382–93. 10.1021/acscatal.1c04897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukhopadhyay DP, Schleier D, Wirsing Set al. Methylbismuth: an organometallic bismuthinidene biradical. Chem Sci 2020; 11: 7562–8. 10.1039/D0SC02410D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corbett JD, Lynde RA. Bismuth(I) tetrachloroaluminate. Spectrophotometric study of its equilibrium formation in the gas phase. Inorg Chem 1971; 10: 1746–9. 10.1021/ic50102a042 [DOI] [Google Scholar]
- 29. Cubicciotti D. The equilibrium 2/3Bi(l) + 1/3BiCl(g) = BiCl(g) and the thermodynamic properties of BiCl gas. J Phys Chem 1960; 64: 791–4. 10.1021/j100835a022 [DOI] [Google Scholar]
- 30. He Y, Dai C, Wang Det al. Phosphine-stabilized germylidenylpnictinidenes as synthetic equivalents of heavier nitrile and isocyanide in cycloaddition reactions with alkynes. J Am Chem Soc 2022; 144: 5126–35. 10.1021/jacs.2c00305 [DOI] [PubMed] [Google Scholar]
- 31. Wang D, Zhai C, Chen Yet al. An isolable germylyne radical with a one-coordinate germanium atom. Nat Chem 2023; 15: 200–5. 10.1038/s41557-022-01081-1 [DOI] [PubMed] [Google Scholar]
- 32. Wang D, Chen W, Zhai Cet al. Monosubstituted doublet Sn(I) radical featuring substantial unquenched orbital angular momentum. J Am Chem Soc 2023; 145: 6914–20. 10.1021/jacs.3c00421 [DOI] [PubMed] [Google Scholar]
- 33. Wu M, Li H, Chen Wet al. A triplet stibinidene. Chem 2023; 9: doi: 10.1016/j.chempr.2023.05.005 10.1016/j.chempr.2023.05.005. [DOI] [Google Scholar]
- 34. Pang Y, Nöthling N, Leutzsch Met al. A “non-magnetic” triplet bismuthinidene enabled by relativity. ChemRxiv 2022; doi: 10.26434/chemrxiv-2022-d3jl7 10.26434/chemrxiv-2022-d3jl7. [DOI] [Google Scholar]
- 35. Pyykkö P, Atsumi M. Molecular single-bond covalent radii for elements 1–118. Chem Eur J 2009; 15: 186–97. 10.1002/chem.200800987 [DOI] [PubMed] [Google Scholar]
- 36. Bondi A. Van der Waals volumes and radii. J Phys Chem 1964; 68: 441–51. 10.1021/j100785a001 [DOI] [Google Scholar]
- 37. Mantina M, Chamberlin AC, Valero Ret al. Consistent van der Waals radii for the whole main group. J Phys Chem A 2009; 113: 5806–12. 10.1021/jp8111556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balázs G, Breunig HJ, Lork E. Synthesis and characterization of R2SbH, R2BiH, and R2Bi−BiR2 [R = (Me3Si)2CH]. Organometallics 2002; 21: 2584–6. 10.1021/om020202z [DOI] [Google Scholar]
- 39. Queen JD, Lehmann A, Fettinger JCet al. The monomeric alanediyl: AlAriPr8 (AriPr8 = C6H-2,6-(C6H2-2,4,6-Pir3)2-3,5-Pir2): an organoaluminum(I) compound with a one-coordinate aluminum atom. J Am Chem Soc 2020; 142: 20554–9. 10.1021/jacs.0c10222 [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Liu LL. A free aluminylene with diverse σ-donating and doubly σ/π-accepting ligand features for transition metals. Angew Chem Int Ed 2021; 60: 27062–9. 10.1002/anie.202111975 [DOI] [PubMed] [Google Scholar]
- 41. Hardman NJ, Twamley B, Power PP. (2,6-Mes2H3C6)2BiH, a stable, molecular hydride of a main group element of the sixth period, and its conversion to the dibismuthene (2,6-Mes2H3C6)BiBi(2,6-Mes2C6H3). Angew Chem Int Ed 2000; 39: 2771–3. [DOI] [PubMed] [Google Scholar]
- 42. Johnson ER, Keinan S, Mori-Sánchez Pet al. Revealing noncovalent interactions. J Am Chem Soc 2010; 132: 6498–506. 10.1021/ja100936w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jansen G, Hess BA. Revision of the Douglas-Kroll transformation. Phys Rev A 1989; 39: 6016–7. 10.1103/PhysRevA.39.6016 [DOI] [PubMed] [Google Scholar]
- 44. Roos BO. The complete active space self-consistent field method and its applications in electronic structure calculations. In: Lawley K.P. (Ed.). Advances in Chemical Physics. New York: John Wiley & Sons, Inc, 1987, 399–445. [Google Scholar]
- 45. Angeli C, Cimiraglia R, Evangelisti Set al. Introduction of N-electron valence states for multireference perturbation theory. J Chem Phys 2001; 114: 10252–64. 10.1063/1.1361246 [DOI] [Google Scholar]
- 46. Martin WC. Table of spin-orbit energies for p-electrons in neutral atomic (core)np configurations. J Res Natl Bur Stan Sect A 1971; 75A: 109–11. 10.6028/jres.075A.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dai D, Whangbo M-H, Ugrinov Aet al. Analysis of the effect of spin−orbit coupling on the electronic structure and excitation spectrum of the Bi22− anion in (K-crypt)2Bi2 on the basis of relativistic electronic structure calculations. J Phys Chem A 2005; 109: 1675–83. 10.1021/jp044675q [DOI] [PubMed] [Google Scholar]
- 48. Xu L, Bobev S, El-Bahraoui Jet al. A naked diatomic molecule of bismuth, [Bi2]2−, with a short Bi−Bi bond: synthesis and structure. J Am Chem Soc 2000; 122: 1838–9. 10.1021/ja992422i [DOI] [Google Scholar]
- 49. Vránová I, Kremláček V, Erben Met al. A comparative study of the structure and bonding in heavier pnictinidene complexes [(ArE)M(CO)n] (E = As, Sb and Bi; M = Cr, Mo, W and Fe). Dalton Trans 2017; 46: 3556–68. 10.1039/C7DT00095B [DOI] [PubMed] [Google Scholar]
- 50. Ehlers AW, Lammertsma K, Baerends EJ. Phosphinidene complexes M(CO)5−PR: a density functional study on structures and electronic states. Organometallics 1998; 17: 2738–42. 10.1021/om980057i [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.