Abstract
Respiratory problems are a major cause of morbidity and mortality in patients with congenital myasthenic syndromes, a rare heterogeneous group of neuromuscular disorders caused by genetic defects impacting the structure and function of the neuromuscular junction. Recurrent, life-threatening episodic apnoea in early infancy and childhood and progressive respiratory failure requiring ventilation are features of certain genotypes of congenital myasthenic syndromes. Robb et al. published empirical guidance on respiratory management of the congenital myasthenic syndromes, but other than this workshop report, there are little published longitudinal natural history data on respiratory outcomes of these disorders. We report a retrospective, single-centre study on respiratory outcomes in a cohort of 40 well characterized genetically confirmed cases of congenital myasthenic syndromes, including 10 distinct subtypes (DOK7, COLQ, RAPSN, CHAT, CHRNA1, CHRNG, COL13A1, CHRNE, CHRNE fast channel syndrome and CHRNA1 slow channel syndrome), with many followed up over 20 years in our centre. A quantitative and longitudinal analysis of key spirometry and sleep study parameters, as well as a description of historical hospital admissions for respiratory decompensation, provides a snapshot of the respiratory trajectory of congenital myasthenic syndrome patients based on genotype.
Keywords: congenital myasthenic syndromes, respiratory, sleep study, spirometry
Poulos et al. report a retrospective cohort study of 40 patients with congenital myasthenic syndrome characterizing the natural history focused on respiratory outcomes. They provide key respiratory markers and useful insights that may help early diagnosis, surveillance and interventions in respiratory management of congenital myasthenic syndrome thereby improving outcomes.
Graphical Abstract
Graphical Abstract.
Introduction
The congenital myasthenic syndromes (CMSs) are a group of rare, genetically heterogeneous disorders that result in impaired neuromuscular transmission and fatigable weakness.1
Respiratory problems are a major cause of morbidity and mortality in CMS, with many presenting with respiratory difficulties at birth and most at risk of ventilatory failure with intercurrent illness.2,3 In the long term, progressive respiratory failure may occur in a small subgroup of patients.2 The suspected respiratory phenotype for each of the 10 CMS subtypes this study reports on, based on existing literature, includes episodic respiratory phenotypes in which apnoeas and crises predominate (RAPSN, CHAT, DOK7, COLQ, CHRNE FCS, COL13A1, CHRNG), those that follow a more progressive course with chronic hypoventilation (DOK7, COLQ, CHRNA1 SCS) and those that have a limited impact on respiratory outcomes (CHRNA1 SCS, CHRNE).3-12
Published long-term natural history data for CMS patients are limited and tend to be descriptive rather than systematic. Specifically, quantitative and longitudinal data on respiratory function and outcomes are yet to be reported. The largest published cohorts with genetically confirmed CMS have been from France (n = 79),13 Turkey (n = 69)14 and UK (n = 46).2 These studies reported the prevalence of genotypes and associated phenotypic features as well as treatment response. None of these studies focused specifically on respiratory outcomes. Published data from sleep studies are even rarer, with only one study in infants (n = 5).15
The benefits of surveillance with cardiorespiratory sleep studies, as well as the impact of interventions such as adenotonsillectomy, tracheostomy and long-term ventilation on respiratory outcomes, in relation to CMS, have not yet been systematically reported either.
The aim of the study was to produce a description of the various respiratory phenotypes and outcomes i.e. long-term evolution of sleep and respiratory problems in CMS, through analysis of various sleep study parameters, spirometry outcomes and historical hospital admissions for respiratory decompensation in relation to genetic subtypes. These data could help improve the respiratory morbidity and possibly the survival of these patients by highlighting relevant early diagnostic markers, urgent respiratory intervention and best practices to prevent respiratory crises.
Materials and methods
Study type
This is a retrospective cohort study using clinical records and recorded respiratory assessment(s) data.
Participants
All CMS patients with a confirmed genetic diagnosis receiving care at GOSH (2000–2020) were identified by a database search, with 40 patients selected for inclusion based on the following criteria.
Eligibility criteria
Inclusion criteria
Patients were selected if they had a genetically confirmed diagnosis of CMS and at least 2 years of follow-up data available over 20 years.
Exclusion criteria
Patients were excluded if they had a primary respiratory or sleep disorder unrelated to CMS or lacked a genetic diagnosis. Spirometry recordings prior to 1998 and sleep study recordings prior to August 2009 were excluded in the interests of standardized equipment use.
Study outcomes
Primary outcome(s)
The primary outcome measure was an accurate description of the respiratory morbidity and mortality in relation to the genetic subtypes of CMS.
Secondary outcome(s)
The secondary outcome measure was the characterization of the respiratory phenotype of the genetic subtypes of CMS, based on as follows:
age of genetically confirmed diagnosis,
requirement for tracheostomy and age at decannulation/presence at last follow-up,
requirement for adenotonsillectomy and age at surgery,
requirement of emergency or long-term ventilation whether continuous or intermittent at last follow-up,
reports of neonatal stridor at birth,
reports of episodic apnoeas, and ages when these occurred, and
number of intensive care admissions related to respiratory decompensation, and ages when these occurred
And serial measurements collected on as follows:
spirometry: forced vital capacity (FVC); cough peak expiratory flow (PEF) and
cardiorespiratory sleep studies: apnoea/hypopnoea index (AHI), partial pressure of carbon dioxide (pCO2), and oxygen desaturation index (ODI).
Data collection
A total of 164 spirometry recordings from 26 of the CMS patients receiving routine respiratory care at GOSH, recorded from 2003–2019, contributed to this analysis. A total of 161 episodes occurred at GOSH using equipment standardized since 1993. The remaining three, conducted in the neuromuscular respiratory clinic of Belfast Hospital, using the same equipment, were included for completeness. To calculate percentage predicted values for FVC and PEF, cohort results were compared to the fifth percentile of Global Lung Function Initiative reference equations for spirometry for FVC and mean predicted values of the European Respiratory Study for PEF, controlling for gender, age, height and ethnicity, respectively.16,17 Ten FVC measurements and four PEF measurements were excluded because the age at which spirometry recordings were performed was outside of the range of ages for the reference values used.
The analysis also includes the results of 97 cardiorespiratory sleep studies from 19 of the CMS patients receiving routine respiratory care at GOSH, recorded from 2009–2019. All 97 of these recordings were conducted in the respiratory sleep unit of GOSH, using equipment installed in the unit since August 2009 [Embla S4000, 4500, Stowood Scientific Instruments (SSI, UK) and TOSCA500/TCM5, transcutaneous carbon dioxide TcCO2 (Radiometer, Den)]. Sleep study parameters, including AHI, were scored manually, and classified using the American Academy of Sleep Medicine (AASM) manual version 2.6.18 Oxygen desaturation index 3% (dips of ≥3%, ODI3%) was analysed from oximetry with a 2 s averaging time (Nonin, Plymouth, MN).
The ventilation history, tracheostomy status, adenotonsillectomy status, reports of neonatal stridor and evidence of historic intensive care and hospital admissions were acquired from patient clinical records.
Statistical analysis
All statistical analyses were carried out using IBM SPSS Statistics 25.0© software.19
Results
Primary outcome(s)
Respiratory mortality and morbidity
We identified 40 patients (18 male, 22 female) belonging to 37 families, with a genetically confirmed diagnosis of CMS (Tables 1 and 2). The cohort were affected with 10 distinct genetic subtypes as follows: 3 affected transmitter release or the synaptic cleft (CHAT, COLQ, COL13A1) and 7 affected postsynaptic function through primary AChR deficiency (CHRNE, CHRNA1, CHRNG); 2 with impact on AChR kinetics (CHRNE fast channel syndrome, CHRNA1 slow channel syndrome) or defects in the synaptic structure (DOK7) or AChR clustering (RAPSN). There were 12 DOK7 patients, 10 COLQ patients, 6 RAPSN patients, 4 CHRNE patients, 2 COL13A1 patients, 2 CHAT patients, one CHRNE FCS patient, one CHRNG patient, one CHRNA1 patient and one CHRNA1 SCS patient in the cohort. Patients 24 and 25 are siblings, as are patients 4, 5 and 14.
Table 1.
Summary of cohort (ID 1–19)
| ID | Sex | CMS subtype | Age at diagnosis | Age at time of study | Respiratory morbidity | Number of historical PICU admissions for respiratory decompensation | Respiratory interventions and treatments | Number of sleep studies | Number of lung function tests |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | CHRNE | 1 year | 2 years | N | - | - | 1 | - |
| 2 | M | DOK7 | 2 years | 5 years | N | - | - | - | - |
| 3 | M | CHRNE | 10 months | 4 years | N | - | - | - | - |
| 4 | M | COLQ | 1.5 years | 8 years | Y | 3 | - | 2 | 3 |
| 5 | F | COLQ | 2 years | 10 years | Y | 1 | - | 2 | 5 |
| 6 | F | COLQ | 9 years | 15 years | N | - | - | 4 | 11 |
| 7 | M | DOK7 | 9.5 years | 15 years | N | - | - | - | - |
| 8 | M | COL13A1 | 5.5 years | 8 years | Y | 2 | - | 1 | - |
| 9 | F | COLQ | 1.5 years | 8 years | Y | 3 | Daily NIV | - | - |
| 10 | F | COLQ | 12 years | 19 years | Y | - | - | 3 | 5 |
| 11 | M | COL13A1 | 13.5 years | 21 years | Y | - | - | - | 4 |
| 12 | F | CHRNA1 | 10 years | 16 years | N | - | - | - | 3 |
| 13 | F | CHRNE | 8 years | 17 years | N | - | - | 1 | 11 |
| 14 | M | COLQ | 5 years | 11 years | Y | 3 | - | 4 | 7 |
| 15 | F | CHRNG | 7 months | 20 years | Y | 1 | Daily NIV | 21 | - |
| 16 | M | DOK7 | 7 months | 12 years | Y | 13 | Daily NIV | - | 1 |
| 17 | F | DOK7 | 10 years | 13 years | Y | - | Daily NIV | 5 | 5 |
| 18 | F | DOK7 | 4 months | 24 years | Y | 3 | Daily ventilation, tracheostomy | 17 | 1 |
| 19 | F | CHRNE FCS | 11 months | 21 years | Y | 4 | Emergency NIV, adenotonsillectomy | - | 7 |
For patients 1–19 in the cohort. Includes sex, age at diagnosis, age at time of study, evidence of respiratory morbidity after review of patient record, the number of historical paediatric intensive care admissions, the details of respiratory interventions and treatments including tracheostomy, adenotonsillectomy and non-invasive ventilation and the number of cardiorespiratory sleep studies and lung function tests undertaken at Great Ormond Street Hospital. For age of genetically confirmed diagnosis, ages were rounded to the nearest month if in the first year of life, and from thereon rounded to the nearest half year. ‘-' refers to areas where there is no evidence for, or missing data for the outcome in question. ‘Respiratory morbidity’ refers to any respiratory difficulty related to congenital myasthenic syndrome (CMS), inclusive of stridor, respiratory difficulties at birth, non-invasive ventilation use and episodes of respiratory decompensation throughout life.
Table 2.
Summary of cohort (ID 20–40)
| ID | Sex | CMS subtype | Age at diagnosis | Age at time of study | Respiratory morbidity | Number of historical PICU admissions for respiratory decompensation | Respiratory interventions and treatments | Number of sleep studies | Number of lung function tests |
|---|---|---|---|---|---|---|---|---|---|
| 20 | M | DOK7 | 10 years | 16 years | N | - | - | - | 2 |
| 21 | F | DOK7 | 8 years | 18 years | N | - | - | 5 | 6 |
| 22 | M | CHRNE | 4 years | 17 years | N | - | - | - | 4 |
| 23 | F | DOK7 | 4.5 years | 20 years | Y | - | - | 2 | 5 |
| 24 | M | COLQ | 3 years | 17 years | Y | - | - | - | 1 |
| 25 | M | COLQ | 7 years | 20 years | N | - | - | - | 3 |
| 26 | F | RAPSN | 7 years | 17 years | Y | 1 | Emergency NIV | - | 10 |
| 27 | F | RAPSN | 10 months | 18 years | Y | 1 | - | - | 6 |
| 28 | F | DOK7 | 6 years | 18 years | Y | - | - | 4 | 20 |
| 29 | F | RAPSN | 7 years | 21 years | Y | 2 | Emergency NIV | 7 | 22 |
| 30 | M | CHRNA1 SCS | 2 years | 22years | N | - | - | 2 | 4 |
| 31 | M | RAPSN | 13 years | 24 years | N | - | - | 3 | 4 |
| 32 | F | COLQ | 11.5 years | 22 years | Y | 4 | Daily NIV | 7 | 8 |
| 33 | M | DOK7 | 11 years | 23 years | Y | - | Daily NIV | 6 | 6 |
| 34 | F | RAPSN | 10 years | 25 years | Y | 1 | Daily NIV | 0 | 0 |
| 35 | M | CHAT | - | 25 years | Y | 1 | Daily ventilation, tracheostomy | 0 | 0 |
| 36 | F | COLQ | - | 26 years | Y | - | Daily NIV | 0 | 0 |
| 37 | F | DOK7 | - | 20 years | Y | - | Tracheostomy | 0 | 0 |
| 38 | M | CHAT | 3 months | 1 year | Y | 1 | Continuous ventilation, tracheostomy | 0 | 0 |
| 39 | F | RAPSN | 3 months | 1 year | Y | - | Daily NIV | 0 | 0 |
| 40 | M | DOK7 | - | 24 years | Y | - | Tracheostomy | 0 | 0 |
For patients 20–40 in the cohort. Includes sex, age at diagnosis, age at time of study, evidence of respiratory morbidity after review of patient record, the number of historical paediatric intensive care admissions, the details of respiratory interventions and treatments including tracheostomy, adenotonsillectomy and non-invasive ventilation and the number of cardiorespiratory sleep studies and lung function tests undertaken at Great Ormond Street Hospital. For age of genetically confirmed diagnosis, ages were rounded to the nearest month if in the first year of life, and from thereon rounded to the nearest half year. ‘-‘refers to areas where there is no evidence for, or missing data for the outcome in question. ‘Respiratory morbidity’ refers to any respiratory difficulty related to congenital myasthenic syndrome (CMS), inclusive of stridor, respiratory difficulties at birth, non-invasive ventilation use and episodes of respiratory decompensation throughout life.
There was no mortality in this cohort. Most patients (27/40) suffered from some form of respiratory morbidity in their lifetime.
Secondary outcome(s)
Respiratory symptoms at birth
A total of 35% (14/40) of patients experienced respiratory difficulties at birth. Seven of these patients had neonatal stridor. The genotypes affected by neonatal stridor were DOK7 (4/12), CHAT (1/2) CHRNE FCS (1/1) and RAPSN (1/6).
Historic paediatric intensive care unit admissions
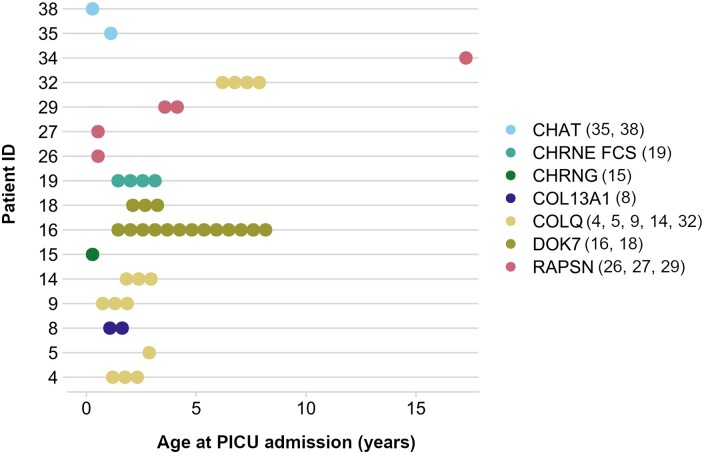
40% of patients (16/40) had at least one historic admission to intensive care related to respiratory decompensation, resulting in intubation (Fig. 1). The 44 total admissions from these patients broken down by genotype were as follows: 16 DOK7 (n = 2), 14 COLQ (n = 5), 4 CHRNE FCS (n = 1), 5 RAPSN (n = 4), 2 CHAT (n = 2), 2 COL13A1 (n = 1) and 1 CHRNG (n = 1). Only one of these patients (ID 5, COLQ) had a genetically confirmed diagnosis of CMS prior to their first intensive care admission. Notably, the majority of COLQ (5/10) and RAPSN (4/6) patients in our cohort had at least one paediatric intensive care unit (PICU) admission related to respiratory decompensation. DOK7 patients had the highest number of PICU admissions per patient, with a mean of eight historic admissions per patient. The majority of PICU admissions occurred between birth and 2 years of age, with COLQ and RAPSN being the only subtypes with any admissions after 4 years of age. Admission notes from historic PICU admissions are summarized in Supplementary Table 1.
Figure 1.
Age at paediatric intensive care (PICU) admission by congenital myasthenic syndrome (CMS) genotype. Each circle represents one admission. The number of PICU admissions was calculated after a thorough review of all inpatient notes from the cohort.
Symptoms
Tables 3 and 4 demonstrate the prevalence of CMS symptoms in the cohort, with respiratory insufficiency affecting many patients (21/40). Stridor at birth was present in a number of patients either secondary to vocal cord palsy or laryngomalacia. Some of these patients underwent tracheostomy whilst in others, this improved over time. When we attempted to co-relate the severity of respiratory involvement and the intensity of muscle weakness, we found that those affected with bulbar weakness needing PEG and scoliosis were much weaker in limb and axial muscles compared to those without.
Table 3.
Symptoms at presentation in 40 children with congenital myasthenic syndrome (CMS)
| Symptom | Percentage of cohort affected | Genotypes affected |
|---|---|---|
| Respiratory insufficiency | 52.5% (21/40) | CHAT (2/2), CHRNG (1/1), COL13A1 (2/2), COLQ (5/10), DOK7 (6/12), CHRNE FCS (1/1), RAPSN (4/6) |
| Feeding difficulties | 37.5% (15/40) | CHAT (2/2), CHRNE (2/4), COL13A1 (1/2), COLQ (3/10), DOK7 (4/12), RAPSN (3/6) |
| Fatigue | 27.5% (11/40) | CHAT (1/2), CHRNA1 (1/1), CHRNE (2/4), COLQ (3/10), DOK7 (3/12), RAPSN (1/6) |
| Muscle weakness | 27.5% (11/40) | CHAT (1/2), COLQ (3/10), DOK7 (4/12), SCS (1/1), RAPSN (2/6) |
| Motor delay | 27.5% (11/40) | CHAT (2/2), DOK7 (4/12), COLQ (2/10), RAPSN (3/6) |
| Ptosis | 25.0% (10/40) | CHAT (1/2), CHRNE (2/4), COLQ (4/10), DOK7 (2/12), CHRNA SCS (1/1) |
| Hypotonia | 17.5% (7/40) | COL13A1 (1/2), COLQ (1/10), DOK7 (2/12), RAPSN (3/6) |
Ordered by decreasing prevalence and broken down by genotypes affected.
Table 4.
Symptoms at presentation in 40 children with congenital myasthenic syndrome (CMS)
| Symptom | Percentage of cohort affected | Genotypes affected |
|---|---|---|
| Gait abnormality | 17.5% (7/40) | CHAT (1/2), COLQ (2/10), DOK7 (3/12), RAPSN (1/6) |
| Vocal cord palsy | 12.5% (5/40) | DOK7 (4/12), CHRNE FCS (1/1) |
| Status epilepticus | 5.0% (2/40) | COLQ (2/10) |
| Frequent falls | 5.0% (2/40) | COLQ (1/10), DOK7 (1/12) |
| Scoliosis | 32% (13/40) | COL13A1(1/2), COLQ (2/10) DOK7(6/12), CHAT (1/2), FCS (1/1) RAPSN (1/6), CHRNG (1/1) |
| Arthrogryposis | 12.5% (5/40) | RAPSN (4/6) CHRNG (1/1) |
| Gastro-oesophageal reflux disease | 12.5% (5/40) | DOK7(2/12), CHRNE (1/4), FCS (1/1), CHRNG (1/1) |
| Episodic apnoeas | 37.5% (15/40) | COLQ (3/10), DOK7(2/12), FCS (1/1), CHAT (2/2), RAPSN (6/6) COL13A1 (1/2) |
Ordered by decreasing prevalence and broken down by genotypes affected.
Tracheostomy
Five individuals in the cohort [DOK7 (n = 3), CHAT (n = 2)] received a tracheostomy for the purposes of long-term ventilation.
For the DOK7 patients, tracheostomies were performed at 5 months (ID 18), 1 month (ID 37) and at 7 years (ID 40). Decannulation was only possible for one patient (ID 37). This patient was decannulated aged 3 years and has not experienced any subsequent respiratory difficulties, required ventilation or been admitted to PICU since last follow-up aged 19. ID 18, now aged 6, has not been admitted to PICU for the past 3 years, suffers from 1–2 chest infections a year and receives nocturnal ventilation via their tracheostomy. ID 40 is still recorded as tracheostomy dependent, however there is no record of ventilation use via tracheostomy for this patient.
For the CHAT patients, tracheostomies were performed at 4 years (ID 35) and 7 months (ID 38). Decannulation has not been possible for either patient, with ID 35 receiving intermittent nocturnal ventilation via tracheostomy until last review aged 17 years, and ID 38 remains full time ventilator dependent at 11 months.
Adenotonsillectomy
Only one patient in the cohort (ID 19, CHRNE FCS) had adenotonsillectomy, at 4 years of age for airway obstruction. The patient suffered from repeated severe respiratory crisis prior to adenotonsillectomy, resulting in three PICU admissions. After an emergency adenotonsillectomy was performed, the patient no longer suffered from sleep disordered breathing. This patient was last seen aged 13 years and has not suffered from a respiratory crisis or had a PICU admission since the adenotonsillectomy.
Ventilation history
Fourteen of the 40 patients (35%) required long-term ventilation. Ventilation requirements were categorized into daily nocturnal ± diurnal use, or ventilation only for emergency use, in which a home ventilator is made available to be used only in the event of a respiratory crises requiring ventilation. The genotypes that had a record of either daily nocturnal or diurnal long-term ventilation use were as follows: CHAT (1/2), CHRNG (1/1), COLQ (3/10), DOK7 (4/12) and RAPSN (2/6). The genotypes that had a record of intermittent emergency ventilation use (at home) were as follows: RAPSN (2/6) and CHRNE FCS (1/1). One patient in the cohort (ID 38, CHAT) is on 24-hour invasive ventilation via a tracheostomy.
The long-term ventilation history (Table 5) varied by genotype: those with progressive synaptopathy such as COLQ and DOK7 patients were associated with commencing long-term nocturnal ventilation in adolescence, and there was a widespread requirement for an emergency home ventilation (non-invasive) provision amongst RAPSN patients.
Table 5.
Type of non-invasive ventilation (NIV) used amongst congenital myasthenic syndrome (CMS) patients with a history of NIV use (n = 15)
| ID | CMS subtype | Type of ventilation | Further information |
|---|---|---|---|
| 35 | CHAT | Nocturnal | Intermittent use of nocturnal NIV via tracheostomy commenced at 10 months and continued up to the point of discharge aged 17 |
| 15 | CHRNG | Nocturnal | Continuous nocturnal and diurnal NIV via a facemask from birth until aged 3 months. Switched to nocturnal NIV that was poorly tolerated. Aged 3 years moved to nightly Optiflow |
| 9 | COLQ | Nocturnal | Diurnal and nocturnal NIV for ∼20 hours per day from birth-1 years old. Reducing regimen every two weeks by age 2. Currently on nocturnal NIV |
| 32 | COLQ | Nocturnal and diurnal | Nocturnal NIV from 11 to 14 years old, with 2 hours of diurnal NIV |
| 36 | COLQ | Nocturnal | Continued nocturnal NIV use commenced at 11 years up to at the point of discharge aged 19 |
| 16 | DOK7 | Nocturnal | Nocturnal NIV regime commenced at 6 months of age and discontinued at 3 years |
| 17 | DOK7 | Nocturnal | Nocturnal NIV commenced at age 15 |
| 18 | DOK7 | Nocturnal | Commenced nocturnal and diurnal ventilation via tracheostomy age 1, with regimen altered to solely nocturnal ventilation via tracheostomy aged 2 |
| 33 | DOK7 | Nocturnal | Nocturnal ventilation commenced at age 17 |
| 19 | CHRNE FCS | Emergency non-invasive ventilation | Moved to emergency NIV availability after diurnal NIV regime aged 3 to 8 years |
| 26 | RAPSN | Emergency non-invasive ventilation | Moved to emergency NIV availability after diurnal NIV regime from 6 to 7 years |
| 29 | RAPSN | Emergency non-invasive ventilation | Moved to emergency NIV availability after poor tolerance of nocturnal NIV aged 16 |
| 34 | RAPSN | Nocturnal | Nocturnal NIV commenced aged 16, and continued nocturnal NIV use at the point of discharge aged 18 |
| 39 | RAPSN | Nocturnal | Nocturnal NIV from birth |
Summary of ventilation history after a review of patient records included.
Spirometry
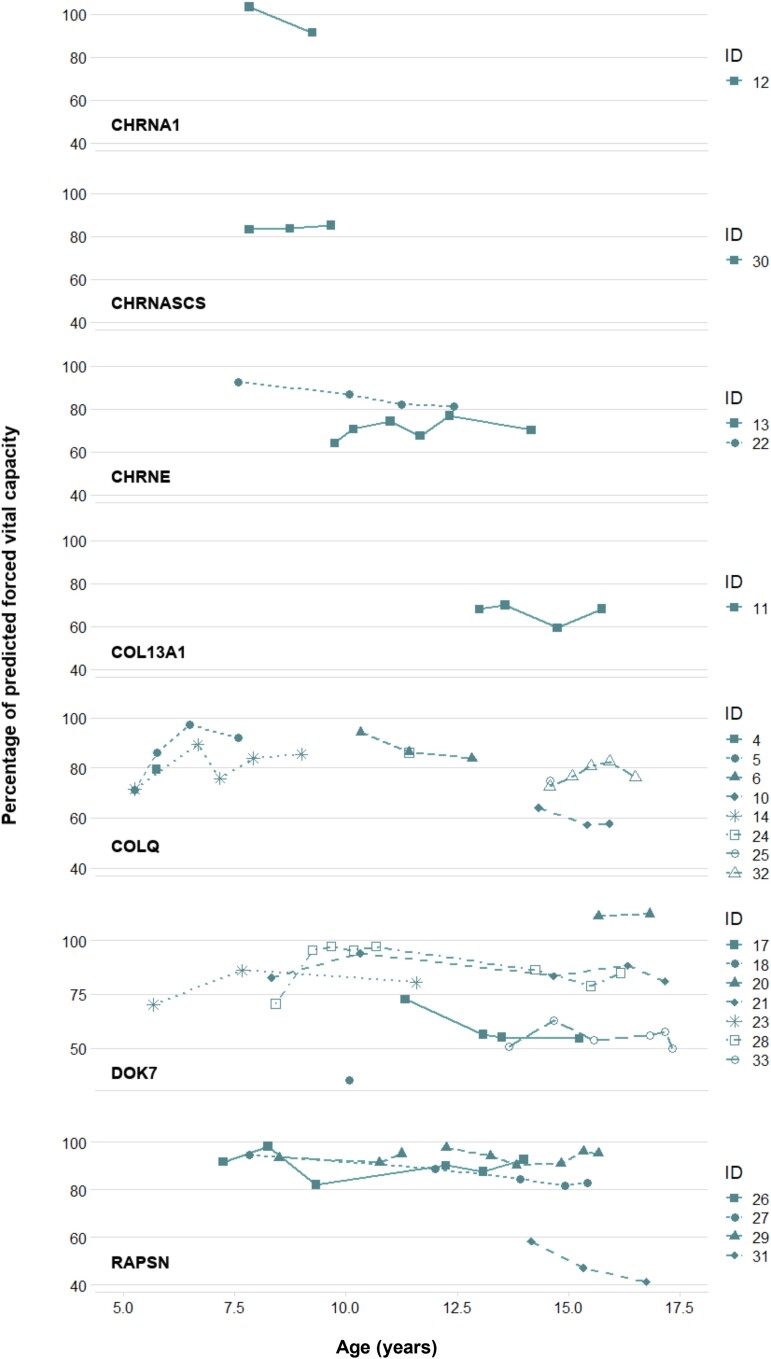
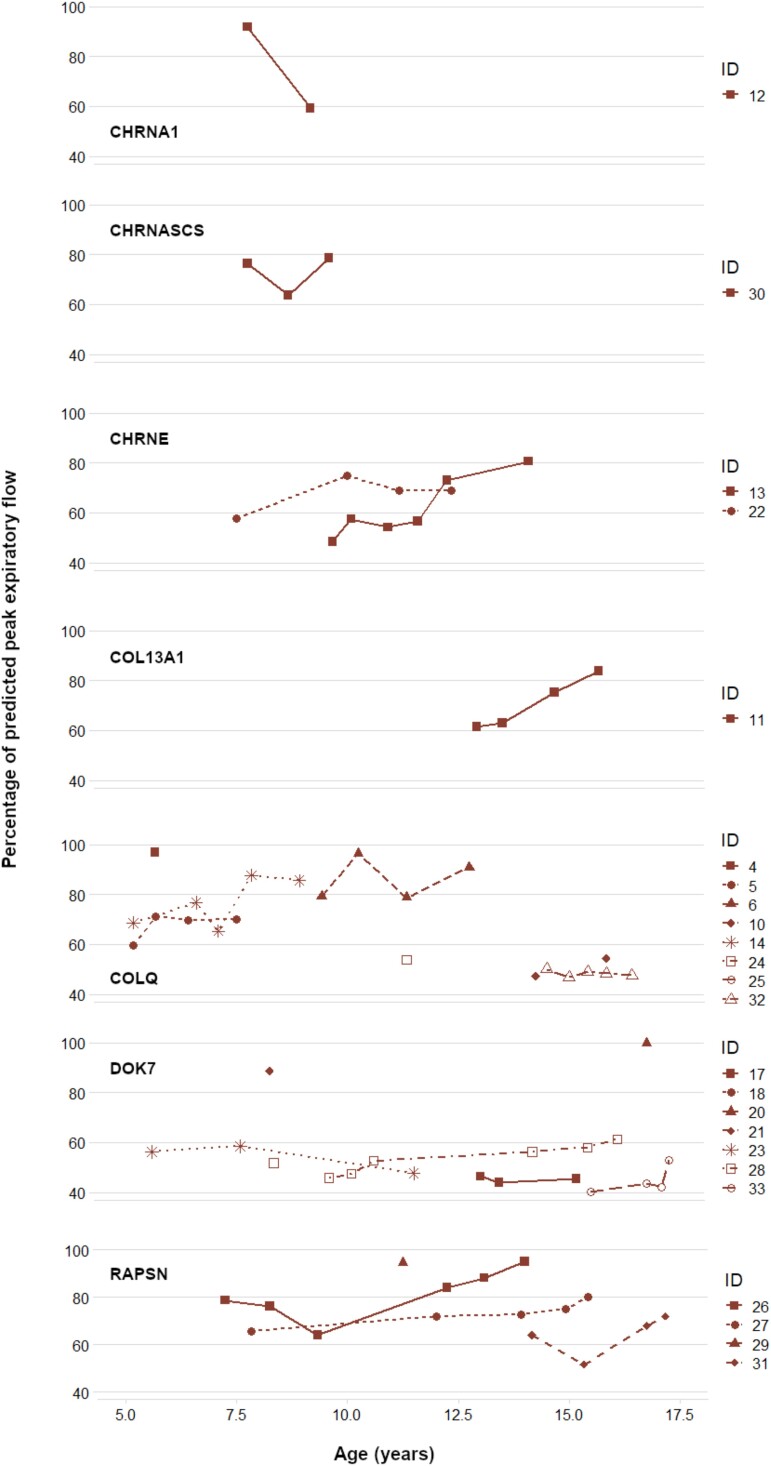
Our analysis of lung function tests focused on the prognosis of the CMS genotype based on percentage of predicted peak expiratory flow (PEF%) and percentage of predicted forced vital capacity (FVC%) (Figs 2 and 3).
Figure 2.
Predicted forced vital capacity (FVC%) by congenital myasthenic syndrome (CMS) genotype. Each data point represents the percentage of forced vital capacity (FVC) on spirometry when compared to the fifth percentile of Global Lung Function Initiative (GLI) reference equations for spirometry for FVC controlling for gender, age, height and ethnicity, respectively.
Figure 3.
Predicted peak expiratory flow (PEF%) by congenital myasthenic syndrome (CMS) genotype. Each data point represents the percentage of peak expiratory flow on spirometry when compared to mean predicted values of the European Respiratory Study (ERS) for PEF, controlling for gender, age, height and ethnicity, respectively.
FVC data were derived from a total of 154 episodes of spirometry from patients with eight CMS genotypes. In many cases, there was an incomplete respiratory trajectory over time due to an absence of data, however a clearer picture of the respiratory impact of RAPSN, COLQ and DOK7 subtypes throughout childhood and adolescence can be defined. Two patients (IDs 29 and 27) with RAPSN mutations had a stable FVC% over time, with no FVC% < 80% from ages 7 to 16. The FVC% of another RAPSN patient (ID 31) significantly deteriorated during adolescence to 40% by the age of 17. DOK7 and COLQ patients demonstrated a progressive worsening of lung function over time, with both genotypes relatively stable in terms of FVC% prior to early adolescence.
PEF data were derived from a total of 160 lung function tests from patients with eight CMS genotypes. COLQ patients during adolescence and the majority of DOK7 patients (6/8) exhibited a consistently poor expiratory cough with minimal fluctuations. CHRNE patients, as well as the COL13A1 patients, demonstrated an overall improvement in PEF% over time. RAPSN patients varied in PEF%, but all showed stability over time. Original spirometry data and details of FVC% and PEF% calculations are documented in Supplementary Table 2A and B.
Cardiorespiratory sleep studies
Referral for polysomnography is a common practice for clinicians to assess the respiratory impact of CMS and to guide management in certain genotypes that need provision of ventilation for emergency or regular use.
A total of 47.5% of the patients (19/40) had attended at least one sleep study, resulting in a total of 97 studies across the cohort.
Cardiorespiratory sleep studies undertaken whilst on ventilatory support usually carry the objective of assessing the adequacy of respiratory support to guide ventilator adjustments. Studies performed at baseline, however, reflect the natural respiratory patterns during sleep for CMS patients and are considered key to forming a diagnosis. AHI, tCO2 and ODI 3% levels were analysed from a total of 49 sleep studies from seven genotypes (n = 18) undergoing a sleep study at baseline.
Only a limited number of patients at baseline recorded an AHI > 5, with COL13A1 being the only genotype with a mean AHI above this threshold at 7.4 events per hour (evs/hour) (n = 1).
The genotypes with mean ODI 3% levels at baseline above threshold20 were as follows: 5.27 evs/hour (95% CI: 1.05, 9.49) COLQ (n = 6), 14.8 evs/hour (95% CI: −1.50, 31.10) DOK7 (n = 6) and 21 evs/hour COL13A1 (n = 1).
The only genotype with a mean tCO2 above threshold for hypoventilation for a study at baseline was CHRNG (n = 1), at 56.22 mmHg (95% CI: 49.40, 63.04).
The only genotype with a mean AHI above the threshold of significance was COL13A1 (n = 1). This was a single recording from one baseline sleep study.
CHRNG, COLQ, DOK7 and COL13A1 patients averaged an ODI 3% above the threshold of significance of 5 evs/hour. RAPSN and CHRNE patients averaged consistently low AHI and ODI levels.
Original cardiorespiratory sleep study data can be accessed in Supplementary Table 3.
Discussion
Our findings will be discussed in three areas as follows: the impact on clinical practice for CMS patients, using clinical markers from the respiratory phenotype of an undiagnosed child to aid diagnosis and the natural history and respiratory prognosis for CMS patients of each genotype once the diagnosis has been given.
CHRNE mutations are the most common CMS subtype, affecting 30–50% of patients but varying widely by ethnicity.1,21,22 In our cohort, only 10% (4/40) are affected with CHRNE. One possible explanation could be that these patients are often milder and thus under-represented in a tertiary centre cohort. These patients infrequently have a history of respiratory problems. FVC% slightly deteriorated over time for CHRNE patients in our cohort but numbers are small, and this did not fall below 70% by adolescence. PEF% was shown to improve over time, and these patients did not require cough assist technology.
In our study, a history of severe respiratory crises shortly after birth as well as neonatal stridor was reported in several genotypes, including CHAT, CHRNG, CHRNE FCS, COL13A1, COLQ, DOK7 and RAPSN.
Upper airway obstruction may have been a contributing factor to the early respiratory difficulties experienced by the CHRNE FCS patient in our cohort, who demonstrated significant clinical improvement after an adenotonsillectomy. The importance of this intervention in CMS has been highlighted by Robb et al.3
In concordance with evidence in the literature,4-6 several COLQ (4/10) and DOK7 (2/12) patients in our cohort presented with respiratory distress at birth, as well as repeated respiratory crises between the ages of 0 and 5 years old requiring PICU admissions. One DOK7 patient (ID 16) and one COLQ patient (ID 32) also experienced severe respiratory crises across childhood resulting in multiple admissions to intensive care in addition to progressive adolescent respiratory insufficiency and progressive scoliosis. Long-term nocturnal ventilation was required for COLQ (3/10) and DOK7 (4/12) patients in the cohort. Progressive decline in respiratory function in patients with DOK7 and COLQ has been reported in the literature and this was also seen in our cohort as a number of patients with these genes remain on long-term ventilation in the second decade compared to none with CHRNE or RAPSN.
It is well recognized that RAPSN patients are likely to suffer from episodic apnoeas and respiratory crises across infancy and childhood that may require intensive care support.7 Most of the RAPSN patients (4/6) in our cohort exhibited respiratory insufficiency during the first few months of life, and four patients had either one or two admissions to PICU. The majority of RAPSN patients (4/6) required either a daily or emergency non-invasive ventilation (NIV) provision. Three of the RAPSN patients (IDs 26, 29, 34) were on an ‘emergency NIV only’ provision by the point of last follow-up in late adolescence, and none of these patients reported using their emergency NIV. One RAPSN patient (ID 39), aged 2 years at last follow-up, has remained on a nocturnal NIV provision since birth. RAPSN patients in our study did not demonstrate evidence of deteriorating lung function over time.
Both CHAT patients in our cohort suffered from severe apnoeic and respiratory crises, with one patient with neonatal onset on continuous ventilation, and the other, with infantile onset, suffering from recurrent respiratory crises, though these decreased in frequency across childhood, but the patient remained on intermittent ventilation use at the point of transition to adult services. The literature suggests that neonatal onset may present as a more severe phenotype, whilst infantile onset is more often characterized by variable symptoms, a decrease in the frequency of apnoea and some resolution of symptoms into adulthood.23
The CHRNG patient in our cohort was genetically diagnosed shortly after birth, due to severe arthrogryposis. We are unable to comment on lung function for the CHRNG patient due to a lack of data. Sleep studies at baseline for the CHRNG patient demonstrated ODI and tCO2 levels above the threshold of significance for both desaturation events and hypoventilation across several studies. This patient was managed with daily nocturnal NIV since birth and is clinically stable with minimal respiratory crises.
The CHRNA1 SCS patient in the cohort suffered from generalized weakness but exhibited only minimal respiratory symptoms throughout childhood and had no requirement for ventilation. The wider literature suggests that there can be some decline in lung function for CHRNA1 SCS patients in adolescence, however we are unable to comment on this due to a lack of lung function testing for the CHRNA1 SCS patient for this period.21
Respiratory crises and hypoventilation requiring long-term ventilation are well described in COL13A1-related CMS,11 and this was also seen in one of our COL13A1 patients with two PICU admissions during infancy and respiratory crisis shortly after birth. The other COL13A1 patient had frequent respiratory infections but did not require NIV and did not experience crises. Lung function was stable in adolescence, with FVC% at 70% and an improving predicted peak cough flow over time. Additional long-term follow-up data are needed for COL13A1 patients.
Implications for clinical practice
Cardiorespiratory sleep studies are unhelpful in predicting the episodic apnoea/crises phenotype of CMS patients and may even be falsely reassuring. However, they are essential for detecting chronic hypoventilation, as well as for the establishment and adjustment of ventilator settings.
Management of respiratory symptoms in CMS includes optimization or introduction of medications targeting the neuromuscular junction (for example pyridostigmine, 3,4-diaminopyridine and salbutamol). These drugs are most helpful in the treatment of the respiratory problems in CMS particularly in a ventilator-dependent neonate or infant. Response to treatment with pyridostigmine is often prompt and may allow weaning of the pressures and duration of ventilation; although caution is needed with higher doses that may increase secretions. Response to salbutamol may take several weeks to months, nevertheless this may also help in the management of respiratory muscle weakness. Acetyl cholinesterase inhibitors are not helpful and should be avoided in certain suspected or confirmed forms of CMS (example DOK7, COLQ and slow channel syndrome), as muscle weakness may be exacerbated. These forms are best treated with salbutamol or ephedrine.
As recommended by Robb et al.,3 clinicians should seek to pre-empt severe events of respiratory decompensation by recommending patients to have immediate availability of their own device for home ventilation and providing resuscitation training for all parents of patients with neonatal stridor or severe respiratory difficulties at birth, and if genetically diagnosed, in the high-risk genotypes for PICU admissions (CHAT, COLQ, DOK7, CHRNE FCS, RAPSN, CHRNG and COL13A1). Emergency home ventilation should be considered for all patients diagnosed with a genotype linked to episodic and recurrent crises (RAPSN, CHRNE FCS, CHAT). Early access to ventilation at home may not only be lifesaving but also crucial to avoiding PICU admissions in these subtypes characterized by episodic respiratory crises. The use of home ventilation in adolescence was often indicated for patients demonstrating poor FVC% in later childhood, such as DOK7 and COLQ patients.
Cough assist technology has only recently come into use for patients with CMS, and so its effectiveness could not be assessed in this study. However, cough assist may be warranted for CMS patients of genotypes in which worsening PEF is anticipated over time, particularly during adolescence (DOK7, COLQ).
An assessment by an ear, nose and throat specialist for microlaryngoscopy and bronchoscopy surgery should be considered for CMS patients with stridor at high risk of vocal cord palsy (CHRNE FCS, DOK7). Tracheostomy may need to be considered for patients presenting with bilateral vocal cord palsy and persistent respiratory symptoms.
Limitations
Due to the rarity of CMS, our study was limited by the small number of patients in the cohort (n = 40) and even smaller number in different genetic subgroups with single cases with certain genotypes. It is therefore difficult to generalize these results. Furthermore, not all patients in the cohort received routine respiratory care at GOSH, with only 26 patients attending for regular lung function tests, and 19 patients for cardiorespiratory sleep studies. This results in an even more limited sample size in these areas of discussion. CMS patients with more severe respiratory problems are also more likely to be followed creating a further bias. Regular spirometry and cardiorespiratory sleep studies for these patients results in a greater amount respiratory data and a bias towards the more severe phenotypes. Although no mortality has been reported in this cohort of patients, we are aware of the sudden death of a previous sibling of one of the children in our cohort with a retrospective diagnosis of CMS. This has also been discussed in other cohort studies that have documented the deaths in infancy of undiagnosed prior siblings of children with CMS.2,3 There are missing and incomplete data in the reporting of certain outcomes, due to the retrospective nature of the study. In our cohort, there are no data on CMS outcomes in adulthood, with the entire cohort of patients aged 25 years or below. Further study is warranted on the CMS subtypes not included in this cohort, rates of mortality associated with CMS and longer-term respiratory outcomes for CMS patients in adulthood. A larger longitudinal international prospective CMS cohort study may reduce some of the biases and limitations encountered in this research.
In conclusion, this retrospective cohort study sets out to characterize the natural history and respiratory outcomes in 10 subtypes of congenital myasthenic syndromes and provides some key respiratory markers that may help with an early diagnosis and management of CMS. It also provides a useful insight into the long-term respiratory course of the reported genotypes and will help clinicians in managing these patients with effective surveillance and early interventions, with the aim of improving the respiratory outcomes, and indeed survival in this patient population.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We thank Aidan Laverty for providing access to the database of spirometry and cardiorespiratory sleep study recordings, as well as the members of the respiratory team for their additional support.
Contributor Information
Jordan Poulos, Paediatrics, University College London Medical School, London WC1E 6BT, UK.
Martin Samuels, Respiratory Medicine, Great Ormond Street Hospital, London WC1N 3JH, UK.
Jacqueline Palace, University of Oxford and Department of Neurology, Oxford Radcliffe Hospitals, Oxford OX3 9DU, UK.
David Beeson, Neurology, Nuffield Department of Clinical Neurosciences, Oxford OX3 7BN, UK.
Stephanie Robb, Dubowitz Neuromuscular Centre, Great Ormond Street Hospital, London WC1N 3JH, UK.
Sithara Ramdas, Neurology, MDUK Neuromuscular Centre, Oxford University Hospitals, Oxford OX3 9DU, UK.
Samantha Chan, Dubowitz Neuromuscular Centre, Great Ormond Street Hospital, London WC1N 3JH, UK; Neurosciences, University College London and Institute of Child Health, London WC1N 1EH, UK.
Pinki Munot, Dubowitz Neuromuscular Centre, Great Ormond Street Hospital, London WC1N 3JH, UK; Neurosciences, University College London and Institute of Child Health, London WC1N 1EH, UK.
Funding
This research was supported by the National Institute of Health research Great Ormond Street Hospital Biomedical Research Centre. S.C. is supported by a University of London Chadburn Lectureship.
Competing interests
J.P. has received support for advisory work and received a grant from Amplo Biotechnology.
Data availability
Data from this study are available as supplementary information, and the authors encourage its use for wider cohort studies of CMS patients.
This research was approved by the Joint Research and Development Office of UCL Institute of Child Health and Great Ormond Street Hospital for Children (Project ID 18BI04).
References
- 1. Finsterer J. Congenital myasthenic syndromes. Orphanet J Rare Dis. 2019;14(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kinali M, Beeson D, Pitt MC, et al. Congenital myasthenic syndromes in childhood: Diagnostic and management challenges. J Neuroimmunol. 2008;201–202:6–12. [DOI] [PubMed] [Google Scholar]
- 3. Robb SA, Muntoni F, Simonds AK. Respiratory management of congenital myasthenic syndromes in childhood: Workshop 8th December 2009, UCL Institute of Neurology, London, UK. Neuromuscul Disord. 2010;20(12):833–838. [DOI] [PubMed] [Google Scholar]
- 4. Palace J. DOK7 congenital myasthenic syndrome. Ann N Y Acad Sci. 2012;1275:49–53. [DOI] [PubMed] [Google Scholar]
- 5. Ammar AB, Petit F, Alexandri N, et al. Phenotype genotype analysis in 15 patients presenting a congenital myasthenic syndrome due to mutations in DOK7. J Neurol. 2010;257(5):754–766. [DOI] [PubMed] [Google Scholar]
- 6. Wargon I, Richard P, Kuntzer T, et al. Long-term follow-up of patients with congenital myasthenic syndrome caused by COLQ mutations. Neuromuscul Disord. 2012;22(4):318–324. [DOI] [PubMed] [Google Scholar]
- 7. Natera-de Benito D, Töpf A, Vilchez J, et al. Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscul Disord. 2017;27(12):1087–1098. [DOI] [PubMed] [Google Scholar]
- 8. McMacken G, Whittaker RG, Evangelista T, Abicht A, Dusl M, Lochmüller H. Congenital myasthenic syndrome with episodic apnoea: Clinical, neurophysiological and genetic features in the long-term follow-up of 19 patients. J Neurol. 2018;265(1):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaouch A, Müller JS, Guergueltcheva V, et al. A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol. 2012;259(3):474–481. [DOI] [PubMed] [Google Scholar]
- 10. Webster R, Liu W-W, Chaouch A, Lochmüller H, Beeson D. Fast-channel congenital myasthenic syndrome with a novel acetylcholine receptor mutation at the α–ɛ subunit interface. Neuromuscul Disord. 2014;24(2):143–147. [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez Cruz PM, Cossins J, Estephan EP, et al. The clinical spectrum of the congenital myasthenic syndrome resulting from COL13A1 mutations. Brain. 2019;142(6):1547–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenzoni PJ, Scola RH, Kay CS, Werneck LC. Congenital myasthenic syndrome: A brief review. Pediatr Neurol. 2012;46(3):141–148. [DOI] [PubMed] [Google Scholar]
- 13. Eymard B, Stojkovic T, Sternberg D, et al. Syndromes myasthéniques congénitaux: difficultés diagnostiques, évolution et pronostic, thérapeutique L’expérience du réseau national « Syndromes Myasthéniques Congénitaux ». Rev Neurol (Paris). 2013;169:S45–S55. [DOI] [PubMed] [Google Scholar]
- 14. Durmus H, Shen XM, Serdaroglu-Oflazer P, et al. Congenital myasthenic syndromes in Turkey: Clinical clues and prognosis with long term follow-up. Neuromuscul Disord. 2018;28(4):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caggiano S, Khirani S, Verrillo E, et al. Sleep in infants with congenital myasthenic syndromes. Eur J Paediatr Neurol. 2017;21(6):842–851. [DOI] [PubMed] [Google Scholar]
- 16. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respiratory Soc. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quanjer PH, Tammeling G, Cotes J. Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J. 1993;6(Suppl 16):1–100. [PubMed] [Google Scholar]
- 18. AASM , Manual for the Scoring of Sleep and Associated Events. 2020.
- 19. IBM Corp, N.J.V. , IBM SPSS statistics for windows. 2013.
- 20. Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: A sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesthesia Analgesia. 2012;114(5):993–1000. [DOI] [PubMed] [Google Scholar]
- 21. Finlayson S, Beeson D, Palace J. Congenital myasthenic syndromes: An update. Pract Neurol. 2013;13(2):80–91. [DOI] [PubMed] [Google Scholar]
- 22. Chang T, Cossins J, Beeson D. A rare c.183_187dupCTCAC mutation of the acetylcholine receptor CHRNE gene in a South Asian female with congenital myasthenic syndrome: A case report. BMC Neurol. 2016;16(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schara U, Christen HJ, Durmus H, et al. Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur J Paediatr Neurol. 2010;14(4):326–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available as supplementary information, and the authors encourage its use for wider cohort studies of CMS patients.
This research was approved by the Joint Research and Development Office of UCL Institute of Child Health and Great Ormond Street Hospital for Children (Project ID 18BI04).