Abstract
Summary
Federated learning enables collaboration in medicine, where data is scattered across multiple centers without the need to aggregate the data in a central cloud. While, in general, machine learning models can be applied to a wide range of data types, graph neural networks (GNNs) are particularly developed for graphs, which are very common in the biomedical domain. For instance, a patient can be represented by a protein–protein interaction (PPI) network where the nodes contain the patient-specific omics features. Here, we present our Ensemble-GNN software package, which can be used to deploy federated, ensemble-based GNNs in Python. Ensemble-GNN allows to quickly build predictive models utilizing PPI networks consisting of various node features such as gene expression and/or DNA methylation. We exemplary show the results from a public dataset of 981 patients and 8469 genes from the Cancer Genome Atlas (TCGA).
Availability and implementation
The source code is available at https://github.com/pievos101/Ensemble-GNN, and the data at Zenodo (DOI: 10.5281/zenodo.8305122).
1 Introduction
Machine learning and deep learning offer new opportunities to transform healthcare, and have been used in many different areas, including oncology (Bibault et al. 2016), pathology (Coudray et al. 2018), diabetes (Spänig et al. 2019), or infectious diseases (Riemenschneider et al. 2016, Ren et al. 2022). However, clinical datasets are typically rather small and need to be aggregated over different hospitals. Often, data exchange over the internet is perceived as insurmountable, posing a roadblock hampering big data-based medical innovations. The most pressing problem in training powerful AI is that all the data usually distributed over various hospitals needs to be accessible in a central cloud. Due to recent data leaks, public opinion, and patient trust (Holzinger 2021, Holzinger et al. 2021) demand more secure ways of storing and processing data. As the E.U. GDPR (General Data Protection Regulations) and as the E.U. NISD (Network and Information Security Directive) entered into force in 2018 and 2016, respectively, data providers, researchers, and IT solution providers are challenged to find ways of providing hospitals complete control over how the patient data is processed. Federated AI enables collaborative AI without sharing the data and, thus, is a promising approach toward GDPR compliance. Federated AI implies that each participant securely stores its data locally and only shares some intermediate parameters computed on local data (Hauschild et al. 2022, Näher et al. 2023). It should be noted, that using federated learning alone does not automatically fulfill all GDPR requirements. For instance, federated learning does not protect against attacks such as model inversion.
Graph neural networks (GNNs) are widely adopted within the biomedical domain (Muzio et al. 2021). Biological entities such as proteins do not function independently and thus must be analyzed on a systems level. GNNs provide a convenient way to model such interactions using e.g prior knowledge defined by a protein–protein interaction network (PPI). The PPI can be used as the GNN’s input graph, while the nodes can be enriched by patient-specific omics profiles. These knowledge-enriched deep learning models might be more interpretable compared to standard approaches when used to predict patient outcomes. However, due to the GDPR mentioned above, the challenge remains how to enable federated learning on GNNs. Here, we present a software tool and Python package for federated ensemble-based learning with GNNs. The implemented methodology enables federated learning by decomposing the input graph into relevant subgraphs based on which multiple GNN models are trained. The trained models are then shared by multiple parties to form a global, federated ensemble-based deep learning classifier.
2 Materials and methods
2.1 Input data
The input data for our software package consists of patient omics data on a gene level and a PPI network reflecting the interaction of the associated proteins. In order to perform graph classification using GNNs, each patient is represented by a PPI network, and its nodes are enriched by the patient’s individual omics features. We call these networks patient-specific PPI networks. Given that specific data representation, it is possible to classify patients based on their genomic characteristics while incorporating the knowledge about the functional relationships between proteins. It should be noted, that the topology of the network is the same for all patients.
2.2 Ensemble learning with graph neural networks
The proposed algorithm for graph-based ensemble learning consists of three steps:
Decomposition of the PPI network into communities using explainable AI.
Training of an ensemble GNN graph classifier based on the inferred communities.
Predictions via Majority Voting.
In the first step, the Python package GNNSubNet (Pfeifer et al. 2022) is used to build a GNN classifier and to infer relevant PPI network communities (disease subnetworks). In detail, GNNSubNet utilizes the Graph Isomorphism Network (GIN) (Xu et al. 2018) to derive a graph classification model and implements a modification of the GNNExplainer (Ying et al. 2019) program such that it computes model-wide explanations. This is done by randomly sampling patient-specific networks while optimizing a single-node mask. From this node mask, edge relevance scores are computed and assigned as edge weights to the PPI network. A weighted community detection algorithm finally infers disease subnetworks. In the second step, an ensemble classifier based on the inferred disease subnetworks is created, and predictions are accommodated via Majority Voting. The ensemble members are predictive GNN models, that are based on the detected disease subnetworks, which overall makes the deep learning model more interpretable. High performing members of the ensembles may consist of a subnetwork biologically important for a specific disease or disease subtype.
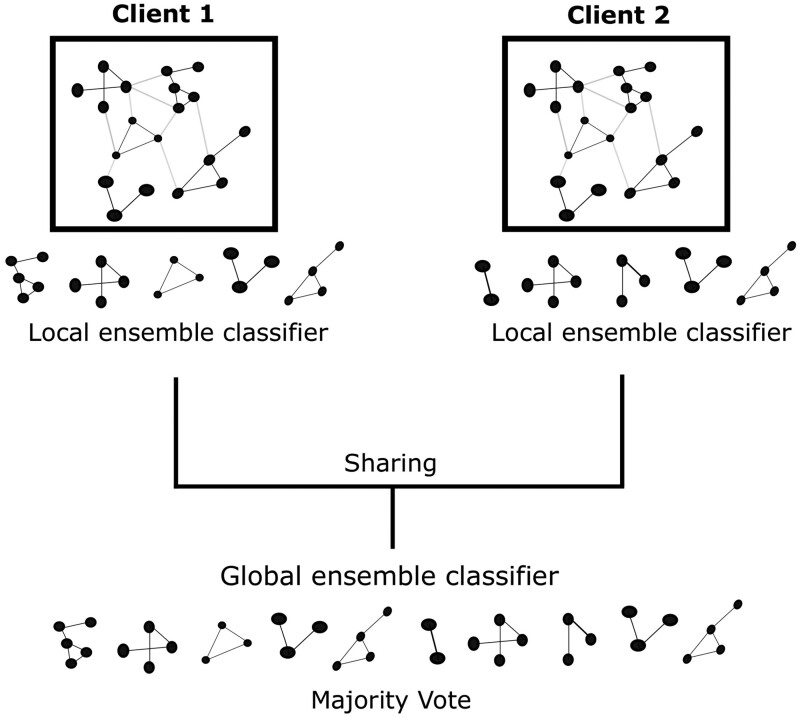
In the federated case, each client has its dedicated data based on which GNN models of the ensemble are trained. These models are shared among all clients creating a global ensemble model, and predictions are again accomplished via Majority Vote (see Fig. 1).
Figure 1.
Federated ensemble learning with GNNs. Each client builds its dedicated ensemble classifier based on relevant subnetworks. The models trained on these subnetworks are shared and a global ensemble classifier is created. Final predictions are based on Majority Voting
3 Results and discussion
We used the gene expression data of human breast cancer patient samples for an experimental evaluation of the herein proposed methodologies. The Cancer Genome Atlas (TCGA) provided the data preprocessed as described in (Chereda et al. 2021b). The data was structured by the topology of Human Protein Reference Database (HPRD) PPI network (Keshava Prasad et al. 2009). The resulting dataset comprises 981 patients and 8469 genes. The binary prediction task was to classify the samples into a group of patients with the luminal A subtype (499 samples) and patients with other breast cancer subtypes (482 samples).
3.1 Performance of Ensemble-GNN in nonfederated case
We assessed the performance of our algorithm using 10-fold cross-validation (see Table 1). Ensemble-GNN, initially using GIN as a base learner, showed the average balanced accuracy (Brodersen et al. 2010) of 0.86 (fourth row of Table 1). As a comparison, a Random Forest (RF) classifier, not guided and restricted by any PPI knowledge graph, demonstrated 0.90 of average balanced accuracy on the same dataset. The slight decrease in performance can be explained by the following reason: The GIN method (Xu et al. 2018) shows worse convergence during training on gene expression modality, compared to data with combined gene expression and DNA methylation modalities [see also Pfeifer et al. (2022), Table 2]. Since transcriptomics is one of the most common omics types (Athieniti and Spyrou 2023), we improved the performance of Ensemble-GNN specifically on gene expression modality using the ChebNet approach from Defferrard et al. (2016). ChebNet models have been successfully applied in Chereda et al. (2021a) to classify patients based on gene expression profiles structured by a PPI network. The corresponding predictions were further explained on a patient level (Chereda et al. 2021a). Ensemble-GNN employing ChebNet as a base learner achieved as good classification performance as RF (see Table 1).
Table 1.
Performance within 10-fold CV (mean balanced accuracy ± standard error of the mean) of GNNSubNet and Ensemble-GNN utilizing TCGA-BRCA data, depending on a base learner (GIN or ChebNet).
| Method (base learner) | Balanced accuracy |
|---|---|
| RF (decision tree) | 0.90 ± 0.0109 |
| GNNSubNet (ChebNet) | 0.90 ± 0.0094 |
| Ensemble-GNN (ChebNet) | 0.90 ± 0.0105 |
| Ensemble-GNN (GIN) | 0.86 ± 0.0120 |
| GNNSubNet (GIN) | 0.52 ± 0.0121 |
Table 2.
Performance (mean balanced accuracy) of Ensemble-GNN based on TCGA-BRCA data, depending on the base learner GIN and ChebNet.a
| Method (base learner) | Federated setup with client-specific test data |
Federated setup with global test data |
||
|---|---|---|---|---|
| Local model | Federated model | Local model | Federated model | |
| Ensemble-GNN (ChebNet) | 0.85 | 0.87 | 0.87 | 0.88 |
| Ensemble-GNN (GIN) | 0.81 | 0.85 | 0.78 | 0.83 |
For both federated setups, the data was distributed across three clients within five Monte-Carlo iterations.
For the GIN architecture we could observe that decomposing the PPI into subnetworks has a positive effect on the predictive performance. For the ChebNet architecture, however, no such effect can be reported. We explain this difference by the fact that the employed GIN classifier applies global pooling to the whole graph. In a classical graph classification set-up pooling from an entire graph, comprising a small set of vertices, is more efficient than pooling from a graph with thousands of vertices. In our case the graph topology is the same across all patients, which might intensify the aforementioned effect. Furthermore, it has been reported that GNNs are susceptible to an effect called “oversmoothing” in multilayered architectures (Rusch et al. 2023). In our case, GIN had five message-passing layers while ChebNet comprised one graph convolutional layer and one fully connected layer, without global pooling. GNNs trained on smaller subnetworks could be less affected by the “oversmoothing” phenomenon. Our reported results on gene expression data using the GIN architecture support this observation; however, additional investigation is required to draw definitive conclusions.
3.2 Performance of Ensemble-GNN in federated case
In the federated case, we evaluated the performance of Ensemble-GNN using the two implemented base learners: GIN and ChebNet. We utilized five Monte Carlo iterations, in which the data was equally distributed across three clients. Each client-specific data was then split into a train (80%) and test dataset (20%). The trained models of the client-specific Ensemble-GNNs were combined into a global federated model. The performance of the global model was estimated using client-specific test sets.
For Ensemble-GNN (GIN), the mean local client-specific test accuracy from five Monte Carlo iterations was [0.84, 0.83, 0.78, 0.79, 0.81] with an overall mean value of 0.81 (see Table 2). For the global, federated model we obtained [0.85, 0.86, 0.83, 0.84, 0.87] with a mean value of 0.85. Mean performance values for Ensemble-GNN using the ChebNet architecture were higher, 0.85 and 0.87 respectively.
We have conducted an additional experiment, where we initially split the data into a global train set and a global test dataset. The train set was then equally distributed across three clients. The average performance of the local classifier to predict the global test set was [0.78, 0.80, 0.77, 0.77, 0.76] with an overall mean value of 0.78. The accuracy of the federated model was [0.82, 0.83, 0.82, 0.80, 0.86] with an overall mean value of 0.83. The performance of Ensemble-GNN could be improved using ChebNet. In this case, we obtained mean values of 0.87 and 0.88 respectively (see Table 2).
Note, we report on balanced accuracy in all cases to account for a possible unbalanced sample distribution caused by the data splits. However, whether the federated model can compensate for possible batch effects and/or non-IID (independent and identically distributed) data is uncertain. In our future work, we could use the concepts of federated domain adaptation as proposed by Peng et al. (2019) and transfer learning (Park et al. 2021) to make a global model adaptable to a specific client.
Finally, the overall accuracy could be further improved using aggregation techniques beyond Mayority Voting. For instance, the inferred subnetworks could be weighted by their relevances to obtain the final labels. Alternative voting rules were also developed and discussed by Werbin-Ofir et al. (2019).
4 Conclusion
We present Ensemble-GNN, a Python package for ensemble-based deep learning with interpretable disease subnetworks as ensemble members. The implemented methodology is especially suited, but not limited to, the federated case, where sensitive data is distributed across multiple locations. We could show that the models trained on subnetworks locally, and shared across multiple parties/clients globally, can improve client-specific predictive performance.
Contributor Information
Bastian Pfeifer, Institute for Medical Informatics, Statistics and Documentation, Medical University Graz, Graz 8036, Austria.
Hryhorii Chereda, Medical Bioinformatics, University Medical Center Göttingen, Göttingen 37077, Germany.
Roman Martin, Data Science in Biomedicine, Department of Mathematics and Computer Science, University of Marburg, Marburg 35043, Germany.
Anna Saranti, Institute for Medical Informatics, Statistics and Documentation, Medical University Graz, Graz 8036, Austria; Human-Centered AI Lab, University of Natural Resources and Life Sciences, Vienna 1190, Austria.
Sandra Clemens, Data Science in Biomedicine, Department of Mathematics and Computer Science, University of Marburg, Marburg 35043, Germany.
Anne-Christin Hauschild, Institute for Medical Informatics, University Medical Center Göttingen, Göttingen 37075, Germany.
Tim Beißbarth, Medical Bioinformatics, University Medical Center Göttingen, Göttingen 37077, Germany.
Andreas Holzinger, Institute for Medical Informatics, Statistics and Documentation, Medical University Graz, Graz 8036, Austria; Human-Centered AI Lab, University of Natural Resources and Life Sciences, Vienna 1190, Austria.
Dominik Heider, Data Science in Biomedicine, Department of Mathematics and Computer Science, University of Marburg, Marburg 35043, Germany.
Conflict of interest
None declared.
Funding
This work has received funding from the European Union’s Horizon 2020 research and innovation programme [826078] (Feature Cloud). HC was supported by the German Ministry of Education and Research (BMBF) FAIrPaCT project [01KD2208A]. This publication reflects only the authors’ view and the European Commission is not responsible for any use that may be made of the information it contains. Parts of this work have been funded by the Austrian Science Fund (FWF), Project: P-32554 explainable Artificial Intelligence.
Data availability
The Python package Ensemble-GNN, including comprehensive documentation of its usage is freely available from our GitHub repository (https://github.com/pievos101/Ensemble-GNN) as well as the data at Zenodo (DOI: 10.5281/zenodo.8305122).
References
- Athieniti E, Spyrou GM.. A guide to multi-omics data collection and integration for translational medicine. Comput Struct Biotechnol J 2023;21:134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibault J-E, Giraud P, Burgun A.. Big data and machine learning in radiation oncology: state of the art and future prospects. Cancer Lett 2016;382:110–7. [DOI] [PubMed] [Google Scholar]
- Brodersen KH, Ong CS, Stephan KE. et al. The balanced accuracy and its posterior distribution. In: 2010 20th International Conference on Pattern Recognition, Istanbul, Turkey. IEEE, 2010, 3121–3124.
- Chereda H, Bleckmann A, Menck K. et al. Explaining decisions of graph convolutional neural networks: patient-specific molecular subnetworks responsible for metastasis prediction in breast cancer. Genome Med 2021a;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereda H, Leha A, Beissbarth T. Stability of feature selection utilizing graph convolutional neural network and layer-wise relevance propagation. bioRxiv, 10.1101/2021.12.26.474194, 2021b, preprint: not peer reviewed. [DOI] [PubMed]
- Coudray N, Ocampo PS, Sakellaropoulos T. et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 2018;24:1559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defferrard M, Bresson X, Vandergheynst P. Convolutional Neural Networks on Graphs with Fast Localized Spectral Filtering. arXiv, arXiv:1606.09375 [cs, stat], 2016, preprint: not peer reviewed.
- Hauschild A-C, Lemanczyk M, Matschinske J. et al. Federated random forests can improve local performance of predictive models for various health care applications. Bioinformatics 2022;38:2278–86. [DOI] [PubMed] [Google Scholar]
- Holzinger A. The next frontier: AI we can really trust. In: Kamp M (ed.), Proceedings of the ECML PKDD 2021, CCIS 1524. Bilbao, Spain: Springer Nature, 2021, 427–440. [Google Scholar]
- Holzinger A, Dehmer M, Emmert-Streib F. et al. Information fusion as an integrative cross-cutting enabler to achieve robust, explainable, and trustworthy medical artificial intelligence. Inf Fusion 2021;79:263–78. [Google Scholar]
- Keshava Prasad TS, Goel R, Kandasamy K. et al. Human protein reference database-2009 update. Nucleic Acids Res 2009;37:D767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio G, O'Bray L, Borgwardt K.. Biological network analysis with deep learning. Brief Bioinform 2021;22:1515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher A-F, Vorisek CN, Klopfenstein SAI. et al. Secondary data for global health digitalization. Lancet Digit Health 2023;5:e93–101. [DOI] [PubMed] [Google Scholar]
- Park Y, Hauschild A-C, Heider D.. Transfer learning compensates limited data, batch effects and technological heterogeneity in single-cell sequencing. NAR Genom Bioinform 2021;3:lqab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Huang Z, Zhu Y. et al. Federated adversarial domain adaptation. arXiv, arXiv:1911.02054 [cs.CV], 2019, preprint: not peer reviewed.
- Pfeifer B, Saranti A, Holzinger A.. GNN-SubNet: disease subnetwork detection with explainable graph neural networks. Bioinformatics 2022;38:ii120–6. [DOI] [PubMed] [Google Scholar]
- Ren Y, Chakraborty T, Doijad S. et al. Prediction of antimicrobial resistance based on whole-genome sequencing and machine learning. Bioinformatics 2022;38:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider M, Hummel T, Heider D.. SHIVA—a web application for drug resistance and tropism testing in HIV. BMC Bioinformatics 2016;17:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch TK, Bronstein MM, Mishra S. A survey on oversmoothing in graph neural networks. arXiv, arXiv:2303.10993, 2023, preprint: not peer reviewed.
- Spänig S, Emberger-Klein A, Sowa J-P. et al. The virtual doctor: an interactive clinical-decision-support system based on deep learning for non-invasive prediction of diabetes. Artif Intell Med 2019;100:101706. [DOI] [PubMed] [Google Scholar]
- Werbin-Ofir H, Dery L, Shmueli E.. Beyond majority: label ranking ensembles based on voting rules. Expert Syst Appl 2019;136:50–61. [Google Scholar]
- Xu K, Hu W, Leskovec J. et al. How powerful are graph neural networks? arXiv, arXiv:1810.00826, 2018, preprint: not peer reviewed.
- Ying R, Bourgeois D, You J. et al. Gnnexplainer: generating explanations for graph neural networks. Adv Neural Inf Process Syst 2019;32:9240–51. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Python package Ensemble-GNN, including comprehensive documentation of its usage is freely available from our GitHub repository (https://github.com/pievos101/Ensemble-GNN) as well as the data at Zenodo (DOI: 10.5281/zenodo.8305122).