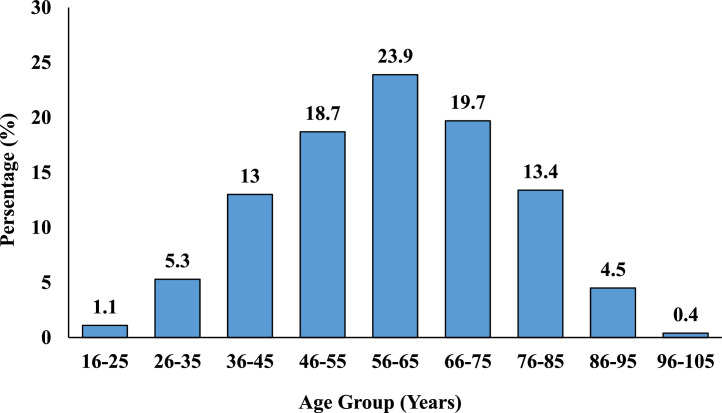Highlights
-
•
The study aims to assess the risk factors associated with COVID-19 mortality in Nepal.
-
•
A total of 1459 COVID-19 hospital-based deaths were collected.
-
•
Individuals with comorbidities were at greater risk of developing mortality.
Keywords: Comorbidities, Coronavirus disease, COVID-19 pneumonia, Mortality
Abstract
Objectives
Reports from other countries have indicated that severe forms and fatal cases of COVID-19 in older adults and people with underlying comorbidities. The aim of this study was to assess the risk factors associated with COVID-19 mortality in Nepal.
Methods
A cross-sectional study was conducted from April 12 to July 23, 2021 to identify the underlying factors associated with COVID-19 deaths. Our sample included all cases diagnosed and registered as COVID-19–related deaths at 30 hospitals of Nepal.
Results
A total of 1459 COVID-19 hospital-based death records were collected from 30 hospitals. Mean age at death was 60.2 (±15.6) years. One-third of cases were admitted with fever, cough, and shortness of breath. The computerized tomography Severity Score showed that 7.3% of the individuals who underwent high-resolution computerized tomography chest had a severe form of lung involvement, and 3.6% had mild to moderate involvement. The most common comorbidities were hypertension (43.7%) followed by diabetes mellitus (25.8%). Among the deceased, 37.7% were diagnosed as cases of COVID-19 pneumonia. The most common recorded causes of death were respiratory failure followed by cardio-pulmonary arrest.
Conclusions
Individuals with comorbidities including hypertension and diabetes mellitus were at greater risk of developing complications and had a higher rate of mortality.
Introduction
COVID-19 was first reported in Wuhan, China, in December 2019 and spread rapidly to become a pandemic as declared by WHO on January 23, 2020 [1]. According to the WHO estimates, as of May 20, 2022, the pandemic witnessed 521,920,560 cases and 6,274,323 deaths globally and 58,056,402 cases and 787,989 deaths in South East Asia [2]. The first COVID-19 case was identified in Nepal on January 23, 2020 [3]. According to the Ministry of Health and Population of Nepal, as of April 3, 2023, the total number of confirmed COVID-19 cases reached up to 1,001,470 and 12,020 deaths [4]. Previous studies have shown that the older people and patients with comorbidities including chronic lung diseases, liver disease, diabetes mellitus, chronic kidney disease, immunosuppressed, and overweight are likely to have a more severe illness [5]. In Nepal, the majority of the COVID-19 deaths were associated with people having comorbid conditions including hypertension, diabetes, respiratory illness, and chronic kidney patients [6], [7], [8]. The objective of this study was to identify the risk factors associated with COVID-19 deaths in Nepal. The results of this study would assist in developing evidence-based policies and prevention strategies to reduce COVID-19–related mortality.
Methods
A cross-sectional study was conducted to identify the underlying risk factors associated with COVID-19 deaths. We collected data of polymerase chain reaction (PCR)-diagnosed cases that were registered as COVID-19–related deaths between May 2020 and July 2021. Of the total hospitals requested to participate in this study only 30 hospitals agreed for data collection. The in-hospital mortality data was provided by the Ministry of Population and Health of Nepal. Those hospitals which were not willing to share their data were excluded from the study. Data were collected from hospital's medical records and from the death certificates of the patients. The socio-demographic profile and clinical details such as clinical presentation, risk factors, treatment history, and outcomes of the deceased were explored.
The sample size was calculated using the formula, N/ (1+Ne2), where “e” (margin of error) is taken at 0.05, and “N” refers to the population [9]. Hence, the final sample size was calculated as 351. All the data were entered in a Microsoft Excel spreadsheet from the paper-based hospital records. The collected data were analyzed using SPSS (Statistical Package for Social Science), version 16. Various descriptive and inferential data analyses were used.
Results
Between May 2020 and July 2021, there were 1459 COVID-19–related deaths in 30 hospitals of seven provinces in Nepal. These cases were tested positive for COVID-19 by reverse transcriptase (RT)-PCR. Almost half of the deaths (735 cases or 50.4%) occurred in the hospitals in Bagmati province, whereas Karnali province had the least number of deaths (52 cases or 3.6%). The mean age was 60.2 ±15.6 years, and the median age was 60 years with interquartile range (17-102). The age distribution of the cases showed that highest rate of in-hospital COVID-19–related mortality (23.9%) occurred in the age group 56-65 years (Figure 1). Among all COVID-19 deaths, 952 (65.3%) were male while 507 (34.7%) were female. Majority of the COVID-19–infected individuals 1217 (83.4%) who presented to the healthcare center had shortness of breath as the common presenting symptom followed by cough in 1077 (73.8%) patients. The mean (± SD) duration of hospital stay before the event (death) was 6.8 (± 6 days). The mean (± SD) duration of illness on presentation was 5.2 (± 4.3) days and median days were 4 (interquartile range 1-39). Among the total COVID-19–related deaths, 826 (56.6%) were smokers.
Figure 1.
Age distribution of COVID-19 deaths (n = 1459).
Oxygen saturation status on admission to the hospital
At the time of admission, out of all individuals who had COVID-19–related death, 139 (24.5%) had oxygen saturation of <90% in room air, 450 (31.5%) had oxygen saturation of <90% even with oxygen supplementation, and 18 (1.2 %) had oxygen saturation of <90% even under Bilevel Positive Airway Pressure support (Figure 2).
Figure 2.
Oxygen saturation of COVID-19–infected individuals on admission (n = 1459). BiPAP, Bilevel Positive Airway Pressure
Of the 311 patients who had done chest X-ray, 71 (4.9%) individuals showed homogenous patchy opacities with lower zone infiltration in bilateral lungs. Similarly, among the 203 patients who had HRCT (High Resolution Computed Tomography) chest, 70 (4.8%) showed diffuse ground glass opacities in bilateral lungs as the most common finding. The computerized tomography Severity Score showed that 107 (7.3%) of the individuals who underwent an HRCT chest had a severe form of lung involvement and 52 (3.6%) had a mild to moderate form of the disease.
Comorbidities on admission to the hospital
The most commonly known comorbidities on admission to the hospital among the COVID-19 mortality cases were found to be hypertension (638 cases or 43.7%) followed by diabetes mellitus (376 cases or 25.8%), acute and chronic renal disorders, (180 cases or 12.3%) and chronic obstructive pulmonary disease (100 cases or 6.9%).
Diagnosis upon hospital admission
Among all deceased, the most common diagnosis at admission to the hospital were COVID-19 pneumonia (619 cases or 42.4%) without comorbidities followed by COVID-19 pneumonia with hypertension (210 cases or 14.4%). In addition to chronic liver disease and coronary artery disease, interestingly, hypothyroidism was also seen in a significant number of cases (27 or 1.9%) who had COVID-19–related deaths. There were COVID-19 pneumonia with complications like acute respiratory distress syndrome (56 cases or 3.9%). There were few cases of COVID-19 PCR positive with Myocardial Infarction (16 cases or 1.1%) and road traffic accident with the accidental finding of COVID-19 PCR positive (5 cases or 0.3%) (Table 1).
Table 1.
Diagnosis upon admission to the hospital in the order of frequency (n = 1459).
| Diagnosis | Frequency (N) | Percent (%) |
|---|---|---|
| COVID-19 PCR positive with Pneumonia | ||
| Without comorbidities | 619 | 42.4 |
| With comorbidities | 521 | 35.8 |
| HTN | 210 | 14.4 |
| HTN and DM | 90 | 6.2 |
| CKD | 58 | 4.0 |
| AKI | 28 | 2.0 |
| DM/ DKA | 42 | 2.9 |
| CLD | 10 | 0.6 |
| Other Comorbidities | ||
| Hypothyroidism | 27 | 1.9 |
| Coronary Artery Disease | 21 | 1.4 |
| Carcinoma | 35 | 2.4 |
| COVID-19 Pneumonia with complications | ||
| ARDS | 56 | 3.9 |
| Sepsis | 9 | 0.6 |
| COVID-19 PCR positive without Pneumonia | ||
| RT-PCR positive for COVID-19 only | 136 | 9.3 |
| Acute exacerbation of COPD | 72 | 4.9 |
| MI | 16 | 1.1 |
| Ischemic stroke | 13 | 0.9 |
| RTA | 5 | 0.3 |
| Fall injury with multiple fractures | 3 | 0.2 |
| OP poisoning | 1 | 0.1 |
| Other infections with RT-PCR positive | ||
| PTB | 3 | 0.2 |
| Scrub Typhus | 3 | 0.2 |
| Dengue fever | 2 | 0.1 |
AKI, Acute Kidney Injury; ARDS, Acute Respiratory Distress Syndrome; CKD, Chronic Kidney Disease; CLD, Chronic Liver Disease; COPD, Chronic Obstructive Pulmonary Disease; DKA, Diabetic Ketoacidosis; DM, Diabetes Mellitus; HTN, Hypertension; MI, Myocardial Infarction; OP, Organophosphorus Poisoning; PCR, Polymerase Chain Reaction, PTB, Pulmonary Tuberculosis; RTA, Road Traffic Accident.
Treatment received by patients for the illness
The majority of deceased, 99.4% who visited the hospital for their symptoms were admitted in the healthcare facilities for further treatments. A total of Nine patients died in the emergency room, 97.3% had oxygen therapy and 85.3% needed intensive care unit care. More than 80% had taken steroids and 40.3% got intubated. Similarly, more than 40% had received heparin and azithromycin for treatment (Table 2).
Table 2.
Treatment received for the illness (n = 1459).
| Treatment | Yes |
No |
||
|---|---|---|---|---|
| N | % | N | % | |
| Oxygen therapy | 1,420 | 97.3 | 39 | 2.7 |
| Symptomatic treatmenta | 1,240 | 85.5 | 219 | 15.0 |
| Steroids (Dexamethasone) | 1,186 | 81.3 | 273 | 18.7 |
| Vitamin C | 809 | 55.4 | 650 | 44.6 |
| Non- invasive ventilationb | 732 | 50.2 | 727 | 49.8 |
| Zinc | 699 | 47.9 | 760 | 52.1 |
| Heparin | 664 | 45.5 | 795 | 54.5 |
| Azithromycin | 610 | 41.8 | 849 | 58.2 |
| Invasive ventilationc | 588 | 40.3 | 871 | 59.7 |
| Antiviral (Remdesivir) | 543 | 37.2 | 916 | 62.8 |
| Vitamin D | 470 | 32.2 | 989 | 67.8 |
| Ivermectin | 241 | 16.5 | 1,218 | 83.5 |
| Hydroxychloroquine | 169 | 11.6 | 1,290 | 88.4 |
| Convalescent plasma therapy | 152 | 10.4 | 1,307 | 89.6 |
| Warfarin | 144 | 9.9 | 1,315 | 90.1 |
Paracetamol, Oral rehydration solution, Ringer's lactate
Non-invasive ventilation include CPAP, Bi-PAP
Invasive ventilated patients included mechanical ventilator
Final Diagnoses of the COVID-19 deaths
Among a total of 1459 COVID-19–positive deceased cases, 550 (37.6%) had a diagnosis of COVID-19 pneumonia without known comorbidities, 214 (14.6%) with COVID-19 pneumonia with hypertension, and 163 (11.2%) with COVID-19 pneumonia induced acute respiratory distress syndrome and/or sepsis (Table 3).
Table 3.
Final Diagnoses (n = 1459).
| Diagnoses | Frequency (N) | Percent (%) |
|---|---|---|
| COVID-19 pneumonia without known comorbidities | 550 | 37.6 |
| COVID-19 pneumonia with comorbidities | 699 | 48 |
| Hypertension | 214 | 14.6 |
| Kidney disease | 115 | 7.9 |
| Hypertension with Diabetes mellitus | 100 | 6.9 |
| Chronic obstructive pulmonary disease | 62 | 4.2 |
| Diabetic ketoacidosis | 57 | 4 |
| Thyroid disorder | 53 | 3.6 |
| Carcinoma | 35 | 2.6 |
| Coronary Heart Disease | 28 | 1.8 |
| Chronic liver disease | 10 | 0.7 |
| Rheumatoid arthritis | 7 | 0.5 |
| Other Comorbiditiesa | 18 | 1.2 |
| COVID-19 pneumonia with complications | ||
| COVID-19 pneumonia-induced ARDS and/or sepsis | 163 | 11.2 |
| COVID-19 pneumonia with Atrial fibrillation | 14 | 1.0 |
| Other diagnosis | ||
| COVID-19 pneumonia with MI with HTN | 22 | 1.5 |
| COVID-19 pneumonia with Ischemic Stroke with HTN | 6 | 0.4 |
| RTA with SAH with RT-PCR positive for COVID-19 | 5 | 0.3 |
Other Comorbidities include: Pulmonary Tuberculosis, Hepatitis C, Crohn's disease, Idiopathic Thrombocytopenic Purpura, Scrub Typhus, Schizophrenia, Cushing's syndrome, Leprosy, Organophosphorus Poisoning.
ARDS, Acute Respiratory Distress Syndrome; HTN, Hypertension; MI, Myocardial Infarction; RTA, Road Traffic Accident; RT-PCR, Reverse Transcriptase Polymerase Chain Reaction, SAH, Subarachnoid Hemorrhage.
Cause of death
As per death certificates, the most commonly recorded immediate causes of deaths were respiratory failure, 635 (43.5%) followed by cardio-pulmonary arrest 298, (20.4%). Other common terminal events leading to deaths included acute respiratory distress syndrome, 210 (14.4%), septic shock, 156 (10.7%), and cardiac arrhythmias, 155 (10.6%).
Discussion
Our review of COVID-19–related deaths among hospitalized patients in Nepal showed that the majority of severe diseases leading to deaths occurred among the older (over 55 years old) and patients with multiple comorbidities. In this study, the mean age of COVID-19 deceased was 60.2 (SD±15.6) years. In comparison to our findings one of the study in Nepal revealed that the mean age of death was 50 years, in contrast to another study by Sanyaolu et al. where the mean age of death was 41 years [10,11]. Moreover, the median age of death in this study was 60.0 years which was almost similar to the publication by Patan Academy of Health Sciences, Nepal [12]. According to our study, nearly one-fourth of the COVID-19 deaths occurring in the hospitals were commonly distributed in the age group of 56-65 years, and almost two-thirds of deaths occurred in patients over 55 years. A systematic review and meta-analysis of 42 studies showed the average age of individuals with COVID-19 deaths occurred in the age range from 48.9-77 years [13]. The majority of mortality occurred among males (65.3% males, 34.7% females) in this study. These results are similar to the findings of a meta-analysis performed in China as well as two other studies done in two different hospitals of Nepal [11,12,14].
In this study, the most common presenting symptoms at the time of hospital admission were shortness of breath (83.4%) and cough (73.8%), which is similar to findings from other studies [12,14,15]. Other studies from Nepal and Bangladesh have also shown fever as the most frequent symptom [10,16]. The median duration of hospital stay in this study before reporting of an event (death) was five days and ranged from 0 to 43 days. Other studies have reported longer duration of hospital admissions prior to death from COVID-19. This outcome was discordant with the findings of Shrestha et al., another retrospective study conducted at Sukraraj Tropical and Infectious Diseases Hospital, Nepal, where the median duration of hospital stay was 10.5 days (range 1-35 days). A German study reported the average length of hospital stay as 16 days before death [12,14,17].
Out of total COVID-19 deaths, 31.5% were hypoxemic (oxygen saturation of <90% in room air) at presentation to the hospital which is similar to other studies [16]. The most common comorbidities among the COVID-19 mortality cases were hypertension, diabetes mellitus, chronic kidney disease, and acute renal insufficiency among patients who presented with COVID-19 pneumonia, whereas exacerbation of chronic obstructive pulmonary disease was the most common problem among those without pneumonia. These findings were similar to various studies conducted in Nepal, Brazil, USA, and Italy and meta-analysis performed by Peishan et al [10,[18], [19], [20], [21]. Similar findings were reported in various systematic reviews and meta-analysis, and comparable publications from Nepal, Wuhan (China), and France [11], [12], [13], [14],17,[22], [23], [24]. These would help to know the associated comorbidities of COVID-19 infection. In the current study, more than 80% of the patients had received steroids and 40.3% got invasive ventilation. Other medications received by the patients during the course of therapy included heparin, azithromycin, remdesivir, hydroxychloroquine, convalescent plasma therapy and warfarin. The usage of these medications were also reported by Bastola et al. in his study from Nepal, the study done by Koirala et al. and similar other studies from Bangladesh and Germany [14,16,17,25].
The most commonly documented final causes of deaths in the current study were respiratory failure followed by cardio-pulmonary arrest, acute respiratory distress syndrome, and septic shock. This was comparable to the reports from similar other studies from Nepal and other parts of the world including India and USA [14,[26], [27], [28], [29]. In this study, there were 1.5% cases of Myocardial Infarction with a single case of Organophosphorus poisoning. This might conclude that all of the associated factors may not be the actual cause of COVID-19 death.
Although we had planned to trace all cases of COVID-19–related mortality, we were unable to acquire detailed data because of the inadequate documentation of patient details, inaccessibility to complete data, and unwillingness to share the details of COVID-19 mortality cases by many medical institutions. These could be considered as the limitations of the study.
Conclusions
This analysis of COVID-19 related mortality among hospitalized patients revealed the highest death rates among the age group over 55 years. This study concludes that the patients with comorbidities including hypertension, diabetes mellitus, renal insufficiency, and COPD (Chronic Obstructive Pulmonary Disease) were associated with COVID-19 deaths.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval statement
Ethical clearance was obtained from the Ethical Review Board of Nepal Health Research Council with a reference number of 2915. All the information collected was kept confidential and anonymity of the participants was assured.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2023.08.002.
Appendix. Supplementary materials
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; 2021. WHO coronavirus (COVID-19) dashboard. [Google Scholar]; https://covid19.who.int/; [accessed 20 September 2021].
- 3.Bastola A, Sah R, Rodriguez-Morales AJ, Lal BK, Jha R, Ojha HC, Shrestha B, Chu DKW, Poon LLM, Costello A, Morita K, Pandey BD. The first 2019 novel coronavirus case in Nepal. Lancet Infect Dis. 2020;20:279–280. doi: 10.1016/S1473-3099(20)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health and Population. COVID-19-Dashboard. Nepal Government. 2021 https://covid19.mohp.gov.np/ [accessed 20 September 2021] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basnet BB, Pant RR, Bishwakarma K, Paudel S, Pandey N, Adhikari SK, et al. A year trend analysis and spatial distribution of COVID-19 cases in Nepal. Asia Pac J Public Health. 2021;33:641–644. doi: 10.1177/10105395211012233. [DOI] [PubMed] [Google Scholar]
- 7.Rayamajhee B, Pokhrel A, Syangtan G, Khadka S, Lama B, Rawal LB, et al. How well the Government of Nepal is responding to COVID-19? An experience from a resource- limited country to confront unprecedented pandemic. Front Public Heal. 2021;9 doi: 10.3389/fpubh.2021.597808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamichhane DK, Shrestha S, Kim HC. District-level risk factors for COVID-19 incidence and mortality in Nepal. Int J Environ Res Public Health. 2022;19:2659. doi: 10.3390/ijerph19052659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaokromthong K, Sintao N. Sample size estimation using Yamane and Cochran and Krejcie and Morgan and green formulas and Cohen statistical power analysis by G* Power and comparisions. Apheit international journal. 2021;10:76–86. https://so04.tci-thaijo.org/index.php/ATI/article/view/254253 [Google Scholar]
- 10.Panthee B, Dhungana S, Panthee N, Gyawali S, Paudel A, Panthee S. Clinical and epidemiological features of COVID-19 deaths in Nepal. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha A, Bajracharya S, Sharma R, Chhetri Budhathoki AC. Descriptive analysis of COVID-19 death at Patan Academy of Health Sciences. J Patan Acad Health Sci;8:18–25. doi: 10.3126/jpahs.v8i1.36856. [DOI]
- 13.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastola A, Shrestha S, Nepal R, Maharjan K, Shrestha B, Chalise BS, Thapa P, Balla P, Sapkota A, Shah P. Clinical mortality review of COVID-19 patients at Sukraraj tropical and infectious disease hospital, Nepal; A retrospective study. Trop Med Infect Dis. 2021;6:137. doi: 10.3390/tropicalmed6030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeuffer C, Le Hyaric C, Fabacher T, Mootien J, Dervieux B, Ruch Y, et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.es.2020.25.48.2000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhuyan MA, Al Mahtab M, Ashab E, Haque MJ, Hoque SMM, Faizul Huq AF, Islam MA, Choudhury N, Alia RA, Mahtab M, Khan MSI, Akbar SM. Treatment of COVID-19 patients at a medical college hospital in Bangladesh. Euroasian J Hepatogastroenterol. 2020;10:27–30. doi: 10.5005/jp-journals-10018-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieg S, von Cube M, Kalbhenn J, Utzolino S, Pernice K, Bechet L, et al. COVID-19 in-hospital mortality and mode of death in a dynamic and non-restricted tertiary care model in Germany. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0242127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Souza WM, Buss LF, Candido DDS, Carrera JP, Li S, Zarebski AE, et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat Hum Behav. 2020;4:856–865. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- 19.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SARS-CoV-2 Italy: Surveillance Group Istituto Superiore di Sanit. Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data. 2020 https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths [accessed on 7 September 2020] [Google Scholar]
- 21.Qiu P, Zhou Y, Wang F, Wang H, Zhang M, Pan X, Zhao Q, Liu J. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32:1869–1878. doi: 10.1007/s40520-020-01664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paudel SS. A meta-analysis of 2019 novel corona virus patient clinical characteristics and comorbidities, 08 April 2020, PREPRINT (Version 1) available at Research Square. doi: 10.21203/rs.3.rs-21831/v1. [DOI]
- 23.Tchicaya A, Lorentz N, Leduc K, de Lanchy G. COVID-19 mortality with regard to healthcare services availability, health risks, and socio-spatial factors at department level in France: A spatial cross-sectional analysis. PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0256857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koirala J, Gyanwali P, Gerzoff RB, Bhattarai S, Nepal B, Manandhar R, et al. Experience of treating COVID-19 with Remdesivir and convalescent plasma in a resource-limited setting: a prospective, observational study. Open Forum Infect Dis. 2021;8:ofab391. doi: 10.1093/ofid/ofab391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha P, Deshmukh Y, Tumbe C, Suraweera W, Bhowmick A, Sharma S, Novosad P, Fu SH, Newcombe L, Gelband H, Brown P. COVID mortality in India: national survey data and health facility deaths. Science. 2022;375:667–671. doi: 10.1126/science.abm5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf SH, Chapman DA, Sabo RT, Zimmerman EB. Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. JAMA. 2021;325:1786–1789. doi: 10.1001/jama.2021.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.