Abstract
Alzheimer’s disease (AD) is one of the primary health problems linked to the decrease of acetylcholine in cholinergic neurons and elevation in oxidative stress. Myco-fabrication of ZnO-NPs revealed excellent biological activities, including anti-inflammatory and acetylcholinesterase inhibitory potentials. This study aims to determine if two distinct doses of myco-fabricated ZnO-NPs have a positive impact on behavioral impairment and several biochemical markers associated with inflammation and oxidative stress in mice that have been treated by aluminum chloride (AlCl3) to induce AD. Sixty male mice were haphazardly separated into equally six groups. Group 1 was injected i.p. with 0.5 ml of deionized water daily during the experiment. Mice in group 2 received AlCl3 (50 mg/kg/day i.p.). Groups 3 and 4 were treated i.p. with 5 and 10 mg/kg/day of ZnO-NPs only, respectively. Groups 5 and 6 were given i.p. 5 and 10 mg/kg/day ZnO-NPs, respectively, add to 50 mg/kg/day AlCl3. Results showed that the AlCl3 caused an increase in the escape latency time and a reduction in the time spent in the target quadrant, indicating a decreased improvement in learning and memory. Moreover, acetylcholinesterase enzyme (AChE) activity and malondialdehyde (MDA), tumor necrosis factor-alpha (TNF-α), and interleukin 1β (IL-1β) levels were significantly increased, and the content of glutathione (GSH), activities of superoxide dismutase (SOD), catalase (CAT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), as well as levels of serotonin and dopamine, were decreased in brain tissues only in AlCl3 treated mice. However, treatment of mice with myco-fabrication of ZnO-NPs at doses of 5 or 10 mg/kg improves learning and memory function through ameliorate all the previous parameters in the AD mice group. The low dose of 5 mg/kg is more effective than a high dose of 10 mg/kg. In accordance with these findings, myco-fabricated ZnO-NPs could enhance memory and exhibit a protective influence against memory loss caused by AlCl3.
Keywords: Neurotoxicity, Alzheimer’s disease, ZnO nanoparticles, Mice, Myco-fabrication
Introduction
Alzheimer’s disease (AD) is one of the primary health issues whose prevalence has grown recently throughout the world. In 2015, about 44 million people in the world had AD, and by 2050, it is expected that this number will have doubled (Ngolab et al. 2019). The development of AD was connected with a number of variables, involving oxidative stress-induced neuronal injury, loss of acetylcholine in cholinergic neurons, and the formation of β-amyloid (Aβ) plaques in the cells of the brain (Cheignon et al. 2018). A buildup of particular metals, such as aluminum, can start processes that result in the creation of highly reactive radicals. Its simple entry and maintenance in the brain induce oxidative stress that leads to excessive AchE activity that induces a low level of acetylcholine which is linked to the development of β-amyloid plaques and memory loss in AD patients (Liaquat et al. 2019). Malik et al. (2022) reported that AlCl3 induced mouse model of AD characterized by memory loss, elevated expression of β-amyloid and increased acetylcholinesterase activity. Many drugs used for the treatment of AD depend on the inhibition of acetylcholinesterase, such as galantamine, rivastigmine, and donepezil. This allows for prolonging the action of the deficient neurotransmitter in the brain, but these drugs have side effects with extended use, e.g., hepatotoxicity (Joe and Ringman 2019). Therefore, searching for treatments with a high potential to reverse neuronal dysfunction and little risk of side effects and expense will be beneficial. Several studies suggested that natural or metal nanoparticle supplements with antioxidant and anti-inflammatory characteristics could be used to regulate oxidative stress and inflammation in order to slow or stop the development of AD (Szczechowiak et al. 2019; Ayaz et al. 2020).
There are numerous uses for metal nanoparticles (NPs) and their oxides in the domains of medicine, agriculture, and industry (El-Sayed et al. 2020a, b, 2023b). The applicability of the NPs has been greatly enhanced by their reduced size, special physicochemical features, and surface changes (Hussein et al. 2022). Among metal oxide nanoparticles, ZnO-NPs are widely employed in biomedical uses such as drug delivery, antibacterial, anticancer, antioxidant, and wound healing (Gomaa et al. 2022). Zinc is a neuromodulator that carries out a variety of physiological actions (Blakemore and Trombley 2017; Hatab et al. 2022), and it is vital for controlling cell proliferation. Additionally, it functions as a molecular signal for transcription factors and immune cells join in the generation of inflammatory cytokines. According to literature, zinc giving diminishes infection incidence and inflammatory cytokine generation. The capacity of zinc to bind metals, along with its role in the catalysis of Cu/Zn superoxide dismutase, preservation of the protein’s –SH group, and upregulation of metallothionein (MT) production, make it a well-known antioxidant (Jarosz et al. 2017).
ZnO-NPs are prepared using different methods, including physical, chemical, and biological ones (Abdelhakim et al. 2020; Mousa et al. 2021). The chemical and physical routes have a number of disadvantages, such as high cost, the need for high-yield equipment, and the formation of unsafe by-products that could be harmful to human health or the environment (Suntako 2015). The green synthesis method eliminates all of these issues by offering safer, extra cost-effectiveness, and less harm to the environment (Singh et al. 2018; Anwar et al. 2022). In the literature, gamma rays can be used as a physical mutagen to improve microbial cultures and develop over-producers of bioactive substances with high economic value (Mousa et al. 2021; El-Sayed 2021; El-Sayed et al. 2022a; b, c). Consistent with Mossa and Shameli (2021), Ag-NPs produced using gamma-irradiated synthesis had a stronger antibacterial impact than those created via chemical synthesis.
In this respect, in our previous study by El-Sayed et al. (2023a), in vitro, we found that the myco-fabricated ZnO-NPs revealed excellent in vitro biological activities, including anti-inflammatory and acetylcholinesterase inhibitory potentials, so we need to apply these results in vivo. Thus, the aim of this investigation was to determine whether two distinct doses of ZnO-NPs had any positive effects on biochemical variables related to neurotransmission, oxidative stress, and inflammation in mice that had been given AlCl3 to cause AD.
Materials and methods
Animals
A total of 60 male albino mice, each weighing 50–60 g and being 9–10 weeks old, were used in the tests. The mice were acclimated before the experiment by spending a week living in our animal building, eating a standard mouse meal, and having unlimited access to water. The mice were separated into six groups equally. The research protocol with serial number 52 A/22 for the purpose of overseeing and monitoring experimental animals was approved by the National Centre for Radiation Research and Technology’s Research Ethics Committee.
Chemicals
Aluminum Chloride (AlCl3, 133.34 M.wt) was bought from Sigma-Aldrich Co., Munich, Germany, and dissolved into distilled water. ZnO-NPs were produced using the gamma-irradiated mutant fungus Alternaria tenuissima AUMC10624, according to El-Sayed et al. (2023a). In brief, the was cultured in potato-dextrose broth and the obtained cell-free culture filtrates were mixed with an aqueous solution of the corresponding salt (2 mM zinc sulfate for ZnONPs). Then, the mixture was vigorously stirred for 20 min and kept at room temperature, and the precipitate was separated by ultracentrifugation, washed in deionized water followed by ethanol, and finally dried at 50 °C. Myco-fabricated ZnO-NPs were suspended in deionized water. To prevent particle aggregation, the suspension was well mixed for 1 min before each injection. ZnO nanoparticles mean size of 18.65 nm with a hexagonal crystal structure (El-Sayed et al. 2023a).
Experimental groups
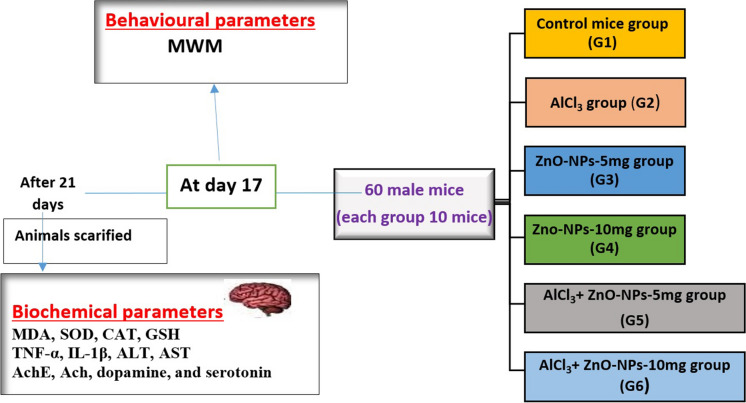
Six groups of mice (Fig. 1) were equally divided as follows:
Fig. 1.
A schematic diagram showing the experimental design
G1:
The control group included mice that were injected i.p. with 0.5 ml of deionized water daily throughout the experiment.
G2:
Mice were injected i.p. with AlCl3 at a dose of 50 mg/kg to induce AD in mice (Abdelazem 2020) for three weeks.
G3 and G4:
Mice were received myco-fabricated ZnO-NPs only (5 and 10 mg/kg /day i.p, respectively) for three weeks daily.
G5 and G6 groups:
Included mice were given AlCl3 (50 mg/kg i.p.) and afterward treated with myco-fabricated ZnO-NPs (5 mg/kg /day and 10 mg/kg i.p., respectively) 1 h after AlCl3 injection for three weeks daily.
Morris water maze (MWM)
With some modifications to the initial technique, the Morris water maze was utilized in the current investigation to test spatial learning and memory (Vorhees and Williams 2006) in the last week of the experiment. The maze was built as a circular tank with dimensions of 180 cm in diameter and 60 cm in height. It was full of water that was kept at a constant temperature of (27 ± 2 °C) and was made opaque by adding a white nontoxic dye. For the objective of the experiment, four equal quadrants were created in the swimming pool: Northeast, Southeast, Northwest, and Southwest, with one of the diagonal lines serving as the starting point. In the target quadrant, a movable circular platform with a 9 cm diameter was erected on a column and placed in the pool 2 cm below the water’s surface. The first four days of training were spent teaching the animals where the platform was so they were able to attempt to find it. For each mouse to swim in the pool, a cut-off time of 120 s was chosen. Mice were allowed 60 s to locate the platform on the test day (the fifth day) when it was taken away. The total time that the animals consumed in the quadrant of the pool known as the “target” on test day can be used to measure spatial memory (D’Hooge and De Deyn 2001).
Samples
Once the experimental period has ended, mice were not eating overnight, euthanized with intraperitoneal injections of sodium pentobarbital, and undergoing a complete necropsy. Samples of brain tissue were gathered and then homogenized in 9 volumes of ice–cold 0.05 mM potassium phosphate buffer (pH 7.4) through a glass homogenizer. The supernatant from centrifuging homogenates at 5000 rpm for 15 min at 4 °C was then utilized to measure biochemical parameters.
Biochemical analysis
Brain neurotransmitter biomarkers
Acetylcholine was measured in brain supernatants using a colorimetric choline/acetylcholine assay kit (BioVision Inc., Waltham, MA, USA). The Elisa kits (BioVision) were used to assess the levels of dopamine and serotonin in brain homogenates in accordance with the manufacturer’s instruction.
Brain acetylcholinesterase (AchE) activities
The activity of the acetylcholinesterase enzyme was determined using quantification ELISA kits purchased from Cusabio company according to the method of Ellman et al. (1961).
Brain lipid peroxidation and antioxidant markers
Using assay kits from Biodiagnostic Co. in Egypt, researchers estimated the levels of glutathione (GSH), malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD) in brain homogenates using the methods of Beutler et al. (1963), Ohkawa et al. (1979), Aebi (1984), and Sun et al. (1988), respectively.
Brain inflammation markers
Tumor necrosis factor- alpha (TNF-α) and interleukin 1β (IL-1β) levels in the brain homogenates were quantified using the Ray Bio mouse ELISA technique (Bio-Techne LTD company) in accordance with the manufacturer’s recommendations.
Brain enzymes
Diagnostic kits from Biodiagnostic Reagent Kits, Dokki, Giza, Egypt, were used to detect the activity of ALT and AST in the brain supernatant.
Statistical analysis
The data were displayed as means ± SD. The analysis of difference (ANOVA) in one direction was used. Using the statistical package program COSTAT 3.03,198, Duncan's test was used to compare the groups statistically. At p < 0.05, differences among the groups were measured as significant. The relationship between escape latency and the measured parameters was assessed using Pearson correlation coefficients.
Results
Effect of ZnO-NPs on the AlCl3 -induced behavioral alterations (memory deficits) in mice by Morris water maze (MWM) test
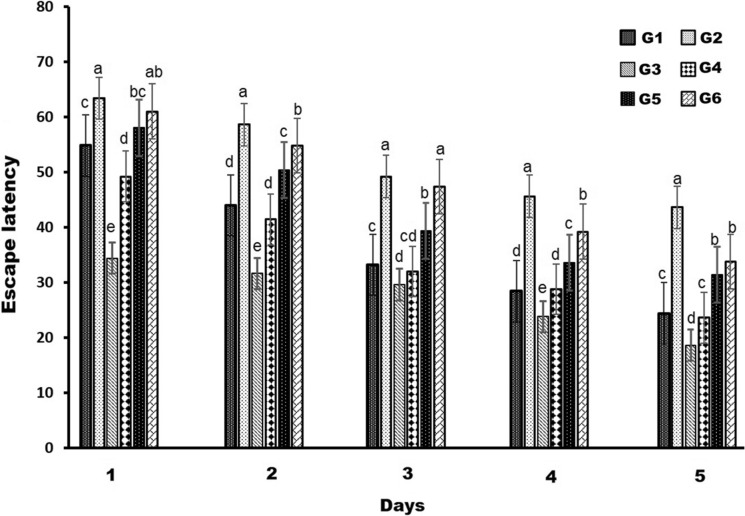
The MWM test was performed to evaluate the memory and learning ability in mice (for all groups) for 5 days. A two-way analysis of variance was applied to test the significance of the difference between the mean values of escape latency (the actual time it took the mice to reach the platform) of different groups representing the effect of various treatments (A) and time intervals (B). A significant value of A (F = 353.86) and B (F = 529.19) at p < 0.001, were obtained indicating that escape latency differed according to 5 days of training (Table 1). Significant interactions between training days and treatments were also observed (F = 6.57). Duncan,s multiple range test revealed AlCl3 caused a significant increase in escape latency between time intervals at p < 0.05. while ZnO-NPs alone at two doses showed a significant decrease in the escape latency from day one compared to the control group.
Table 1.
Effects of ZnO-NPs on escape latency during 5 days intervals in AlCl3-stimulated behavioral changes in AD mice
| Groups | Escape latency | F and p values | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | A | B | A and B | |
| G1 | 54.8 ± 2.3 | 44 ± 2.28 | 33.24 ± 1.18 | 28.4 ± 1.02 | 24.4 ± 1.01 | 353.86 | 529.19 | 6.57 |
| G2 | 63.4 ± 2.6 | 58.6 ± 0.97 | 49.2 ± 3.05 | 45.6 ± 3.14 | 43.6 ± 1.85 | |||
| G3 | 34.4 ± 3.93 | 31.6 ± 1.88 | 29.6 ± 1.95 | 23.8 ± 1.47 | 18.6 ± 1.85 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 |
| G4 | 49.2 ± 1.72 | 41.4 ± 3.07 | 32.0 ± 1.38 | 28.8 ± 1.16 | 23.6 ± 2.94 | |||
| G5 | 58.0 ± 2.09 | 50.4 ± 1.02 | 39.4 ± 1.01 | 33.6 ± 1.62 | 31.4 ± 1.49 | |||
| G6 | 61.0 ± 1.22 | 54.8 ± 3.2 | 47.4 ± 1.85 | 39.2 ± 2.04 | 33.8 ± 1.93 | |||
F to-way analysis of variance, A comparison among the treatment, B comparisons among the time intervals, A and B the interaction between treatment and times
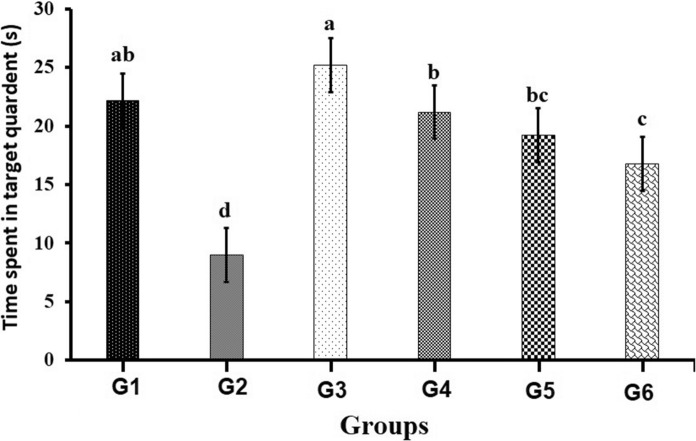
Additional analysis with one–way ANOVA showed that mice given AlCl3 demonstrated a substantial (p < 0.05) increase in escape latency and a decrease in time spent in the target quadrant compared to the control group. In comparison to the AlCl3 group, the mice who were co-treated by ZnO-NPs (5 and 10 mg/kg) had significantly shorter travel times to the platform and spent extra time in the desired quadrant (Figs. 2 and 3). These findings demonstrate that mice given AlCl3 may recover spatial memory by giving ZnO-NPs. Furthermore, in comparison to the control group, mice that were given ZnO-NPs alone showed statistically significant elevations in time spent in the desired quadrant and reductions in escape latency, 5 mg/kg ZnO-NPs being more efficient than 10 mg/kg ZnO-NPs.
Fig. 2.
The MWM test was used to determine the effects of ZnO-NPs on escape latency in AlCl3-stimulated behavioral changes in AD mice. The results were provided as mean ± SD (one-way ANOVA followed by Duncan's test). A distinct superscript is used to denote a significant difference (p ≤ 0.05) in comparison to the control group. a–e means with different superscripts for each data series are considered significantly different
Fig. 3.
Results of the MWM test measuring the effects of ZnO-NPs on the total time spent in the target quadrant in AlCl3-stimulated behavioral changes in AD mice were shown as mean ± SD one-way ANOVA followed by Duncan's test). A distinct superscript is used to denote a significant difference (p ≤ 0.05) in comparison to the control group. a–d means with different superscripts for each data series are considered significantly different
Effect of myco-fabricated ZnO-NPs on variations in lipid peroxidation and antioxidant indicators in the brain of mice
There were significant reductions (p < 0.05) in the levels of GSH (97.2 ± 4.09) in addition to activities of CAT (132.0 ± 1.58) and SOD (143.2 ± 8.53) in the brain tissues of mice treated by AlCl3 compared to control (127.2 ± 4.4, 288.0 ± 13.04 and 216.6 ± 3.85, respectively) (Table 2). Moreover, there was a significant (p ≤ 0.05) elevation in brain levels of MDA (10.04 ± 0.35) in AlCl3-intoxicated mice compared to control values (6.46 ± 0.31). In contrast, all treated animals, notably those given 5 mg/kg of myco-fabricated ZnO-NP alone showed a statistically significant reduction in brain MDA levels (4.19 ± 0.34) and increases in GSH content (176.4 ± 5.03), as well as CAT (489.1 ± 5.83) and SOD (285.0 ± 6.21) activity compared to the control group. These findings imply that ZnO-NPs at a concentration of 5 mg/kg are more effective for reducing oxidative stress in the mouse brain.
Table 2.
Effect of myco-fabricated ZnO-NPs on lipid peroxidation and antioxidant indicators in the brain of mice administrated with AlCl3.
| Parameters | Groups | F value | p value | |||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |||
| MDA (nmol/mg) | 6.46 ± 0.31b | 10.04 ± 0.35a | 4.19 ± 0.34d | 5.08 ± 0.24c | 5.14 ± 0.44c | 6.38 ± 0.39b | 177.80 | ≤ 0.001 |
| % of changes | 55.41 | −35.14 | −21.36 | −20.43 | −1.23 | |||
| SOD (U/mg) | 216.6 ± 3.85c | 143.2 ± 8.53f | 285.0 ± 6.21a | 236.1 ± 3.94b | 173.2 ± 3.63d | 166.4 ± 4.39e | 523.5 | ≤ 0.001 |
| % of changes | −33.89 | 31.57 | 9.0 | −20.04 | −23.18 | |||
| CAT (ng/mg) | 288.0 ± 13.04c | 132.0 ± 1.58f | 489.1 ± 5.83a | 352.2 ± 6.91b | 158.4 ± 6.11d | 143.2 ± 2.59e | 920.8 | ≤ 0.001 |
| % of changes | −54.17 | 69.83 | 22.29 | −45 | −50.28 | |||
| GSH (ng/mg) | 127.2 ± 4.4c | 97.2 ± 4.09e | 176.4 ± 5.03a | 156.6 ± 3.98b | 122.8 ± 2.59c | 102.4 ± 3.05d | 307.13 | ≤ 0.001 |
| % of changes | −23.58 | 38.68 | 23.11 | −3.46 | −19.49 | |||
The mean and standard deviation of ten mice from each group are used to represent the values. Means with different superscripts within the same row differ significantly at (p ≤ 0.05)
Effect of ZnO-NPs on alteration of pro-inflammatory cytokines and transaminases enzymes in the brain of mice
Mice that were given AlCl3 resulted in a statically significant rise (p ≤ 0.05) of the pro-inflammatory cytokines TNF- α (287.0 ± 4.53) and IL-1 β (418.0 ± 23.87) as well as a significant reduction in the activities of AST (11.4 ± 0.14) and ALT (3.6 ± 0.32) in brain cells in comparison to mean values of control mice (74.4 ± 2.51, 282.0 ± 14.83, 34.0 ± 0.36 and 11.86.0 ± 0.85, respectively). In AlCl3-intoxicated mice, a combined treatment with myco-fabricated ZnO-NPs at two doses (5 and 10 mg/kg) resulted in a significant decrease (p ≤ 0.05) in TNF- α (108.0 ± 7.97, 124.8 ± 3.89) and IL-1 β levels (336.0 ± 23.02, 384.0 ± 15.16) and an increase in AST (27.2 ± 0.16, 16.4 ± 0.29) and ALT (8.86 ± 0.98, 5.28 ± 0.77) activities in comparison to animals treated with AlCl3 alone, but these values were still significantly different (p ≤ 0.05) from the control values. The mice treated with 5 mg/kg myco-fabricated ZnO-NPs rather than 10 mg/kg ZnO-NPs showed the best outcomes. Additionally, in myco-fabricated ZnO-NPs alone at the two doses in comparison to the control group, IL-1 β was considerably reduced (p ≤ 0.05). Comparing mice treated with ZnO-NPs alone to control mice, the levels of TNF-α and the activities of ALT and AST did not alter significantly (Table 3).
Table 3.
Effect of myco-fabricated ZnO-NPs on changes in pro-inflammatory cytokines and transaminase enzymes in the brain of mice induced by AlCl3
| Parameters | Groups | F value | p value | |||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |||
| TNF- α (pg/ml) | 74.4 ± 2.51d | 287.0 ± 4.53a | 75.6 ± 4.83d | 76.4 ± 8.21d | 108.0 ± 7.97c | 124.8 ± 3.89b | 1037.02 | ≤ 0.001 |
| % of changes | 285.7 | 1.61 | 2.69 | 45.16 | 67.74 | |||
| IL1B (pg/ml) | 282.0 ± 14.83d | 418.0 ± 23.87a | 225.0 ± 11.18e | 236.8 ± 17.06e | 336.0 ± 23.02c | 384.0 ± 15.16b | 94.92 | ≤ 0.001 |
| % of changes | 48.23 | −20.21 | −16.03 | 19.15 | 36.17 | |||
| AST(U/L) | 34.0 ± 0.36a | 11.4 ± 0.14d | 33.0 ± 0.21a | 34.4 ± 0.32a | 27.2 ± 0.16b | 16.4 ± 0.29c | 74.29 | ≤ 0.001 |
| % of changes | −66.47 | −2.94 | 1.18 | −20 | −51.76 | |||
| ALT (U/L) | 11.86.0 ± 0.85a | 3.6 ± 0.32d | 12.20 ± 1.68a | 10.98 ± 0.58a | 8.86 ± 0.98b | 5.28 ± 0.77c | 70.41 | ≤ 0.001 |
| % of changes | −69.65 | 2.86 | −7.41 | −25.29 | −55.48 | |||
The mean and standard deviation of ten mice from each group are used to represent the values. Means with different superscripts within the same row differ significantly at (p ≤ 0.05)
Effect of myco-fabricated ZnO-NPs on changes in acetylcholinesterase, acetylcholine, dopamine, and serotonin in the brain of mice test
Table 4 shows how ZnO-NPs affect acetylcholinesterase, acetylcholine, dopamine, and serotonin. A statistically significant (p < 0.05) decline in the levels of Ach (0.91 ± 0.14), dopamine (12.4 ± 0.52), and serotonin (49.02 ± 2.35) was found in the AlCl3 group when compared to the normal group (6.0 ± 0.36, 33.46 ± 2.72 and 116.76 ± 4.64, respectively). Additionally, compared to control mice (0.36 ± 0.04), a substantial (p < 0.05) rise in acetylcholinesterase activity was found in mice treated with AlCl3 (1.91 ± 0.14). The values of all the earlier parameters almost reverted to the control value when mice were treated with ZnO-NPs at two doses (5, 10 mg/kg). The results from the lower dose of ZnO-NPs (5 mg/kg) are better to those from the higher dose (10 mg/kg).
Table 4.
Effect of myco-fabricated ZnO-NPs on changes in acetylcholinesterase, acetylcholine, dopamine, and serotonin in the brain of mice induced by AlCl3
| Parameters | Groups | F value | p value | |||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |||
| AchE (pg/mg) | 0.36 ± 0.04d | 1.91 ± 0.14a | 0.39 ± 0.02d | 0.38 ± 0.03d | 0.53 ± 0.02c | 0.62 ± 0.04b | 436.08 | ≤ 0.001 |
| % of changes | 430.56 | 8.33 | 5.56 | 47.22 | 72.22 | |||
| Ach (pg/mg) | 6.0 ± 0.36a | 0.91 ± 0.14d | 6.34 ± 0.21a | 5.76 ± 0.32a | 5.0 ± 0.16b | 4.46 ± 0.29c | 288.9 | ≤ 0.001 |
| % of changes | −84.83 | 5.67 | −4 | −16.67 | −25.67 | |||
| Dopamine (ng/mg) | 33.46 ± 2.72a | 12.4 ± 0.52c | 35.0 ± 1.19a | 33.6 ± 0.37a | 34.12 ± 3.69a | 21.76 ± 0.97b | 107.94 | ≤ 0.001 |
| % of changes | −62.94 | 4.60 | 0.42 | 1.97 | −34.96 | |||
| Serotonin (ng/mg) | 116.76 ± 4.64a | 49.02 ± 2.35c | 105.7 ± 7.11a | 96.0 ± 3.54ab | 93.0 ± 5.09ab | 69.66 ± 5.38bc | 121.06 | ≤ 0.001 |
| % of changes | −58.02 | −9.47 | −17.78 | −20.35 | −40.34 | |||
The mean and standard deviation of ten mice from each group are used to represent the values. Means with different superscripts within the same row differ significantly at (p ≤ 0.05)
The correlation between escape latency and some brain biochemical parameters
Table 5 shows correlation coefficient (r) values between escape latency and some brain biochemical parameters. There was a significant positive correlation between escape latency and MDA (r = 0.7346, p < 0.01), AchE (r = 0.8552, p < 0.01), TNF-α (r = 0.8837, p < 0.01), IL 1ß (r = 0.9171, p < 0.01). Moreover, there was a negative correlation between escape latency and GSH (r = − 0.7018, p < 0.01), CAT (r = − 0.7041, p < 0.01), SOD (r = − 0.800, p < 0.01), ST (r = − 0.7112, p < 0.01), DA (r = − 0.9056, p < 0.01), Ach (r = − 0.9075, p < 0.01), ALT (r = − 0.9023, p < 0.01) and AST (r = − 0.8905, p < 0.01).
Table 5.
Correlation coefficient between escape latency and biochemical parameters in the brain of mice
| Brain parameters | Escape latency |
|---|---|
| GSH | − 0.7018 |
| CAT | − 0.7041 |
| SOD | − 0.80 |
| MDA | 0.73463 |
| ST | − 0.7112 |
| DA | − 0.9056 |
| AchE | 0.8552 |
| Ach | − 0.9075 |
| ALT | − 0.9023 |
| AST | − 0.8905 |
| TNF-α | 0.88375 |
| IL-1β | 0.91712 |
Discussion
The present study proved the ameliorative action of myco-fabricated ZnO-NPs against AlCl3-induced AD in mice. Aluminum cross into the brain through the specific great affinity receptors for transferrin expressed in the blood–brain barrier (Roskams and Connor 1990). The hippocampus and cortex are crucial for cognitive functions including learning and memory, and these areas are the most susceptible to AD and Al poisoning (Malik et al. 2022). In the current research, supplementation of Myco-fabricated ZnO-NPs significantly ameliorated the neural, behavioral, and biochemical abnormality in AlCl3-induced AD in mice, which means the beneficial and neuroprotective action of Myco-fabricated ZnO-NPs against the AD. Baydar et al. (2003) stated that the measurements of behavioral alterations are more sensitive than neurochemical variations as signs of neurotoxicity through AlCl3 exposure. According to our current findings, mice treated with AlCl3 had poorer spatial memory and accuracy, as shown by higher escape latencies to the platform and less time spent on the target (platform) quadrant during the MWM test. This may be due to the accumulation of aluminum in the brain, which induces increasing AchE activity, inflammation, and the accumulation of beta-amyloid, as well as reducing the antioxidant activity that affects learning and memory (Thenmozhi et al. 2015). This behavioral alteration is confirmed by the biochemical changes in the AlCl3 groups compared with the normal group. Our study findings were in agreement with the previous article stated by Ekundayo et al. (2022). However, myco-fabricated ZnO-NPs treatment at two different doses significantly enhanced this diminished spatial learning and memory adjacent to the control group, and the dose of 5 mg/kg/bw was more powerful than 10 mg/kg/bw. This may be related to zinc having antioxidant, and anti-inflammatory action (Jarosz et al. 2017). These findings suggest that myco-fabricated ZnO-NPs has a memory improving function and a protective effect against AlCl3-induced memory loss through antioxidant, anti-inflammatory, and inhibitory actions for AchE.
In the current investigation, mice treated with AlCl3 displayed substantial changes in brain MDA concentration, GSH content, SOD, and CAT activity, all of which are markers of enhanced oxidative damage and lipid peroxidation caused by aluminum accumulation in brain tissues. Due to its high oxygen consumption and insufficient antioxidant system, the brain is particularly vulnerable to oxidative stress (Parashar and Udayabanu 2017). Thus, neurotoxicity caused by AlCl3 might be due to the overproduction of ROS resulting in considerable neuronal injury arising from disorders in the antioxidant defense system. It is widely known that aluminum can enter the blood-brain barrier and build up in many brain tissues, promoting the production of free radicals, which in turn raises protein and DNA oxidation and lipid peroxidation. Aluminum has also been demonstrated to interfere with iron homeostasis by displacing iron from the iron transport protein transferrin, increasing the amount of redox-active iron in brain tissues (Vieelien et al., 2022). This causes significant oxidative damage and may result in brain injury, particularly in regions of the brain associated with memory and learning (Saba et al. 2017). Khan et al. (2011) found that the significant decrease in GSH in the AlCl3-treated group may have resulted from aluminum attaching to the SH group of GSH, which can be excreted, reducing GSH’s ability to act as a neutrophilic scavenger. Nehru and Anand (2005) observed that decreased activities of SOD and CAT in rat’s brains exposed to AlCl3 may be attributed to a decrease in the synthesis of enzyme proteins. Similarly, numerous studies stated that declines in activities of SOD and CAT are connected with AD (Jadhav and Kulkarni 2022; Ekundayo et al. 2022; Ojha 2023 ; Chen et al. 2021).
One of the potential components that can stop AD from starting and progressing is antioxidants. The mice treated with myco-fabricated ZnO-NPs alone had higher levels of GSH as well as activities of SOD and CAT than the other groups in the current study. This may be attributable to an increase in Zn concentration in the brain tissue as a result of Zno nanoparticle dissociation. According to Sidhu and Garg (2005), zinc reduces the action of pro-oxidant enzymes, inhibits lipid peroxidation, and promotes the production of proteins and enzymes such as antioxidant proteins, GSH, CAT, and SOD. According to Abd Elmonem et al. (2021) ZnO-NPs can decrease MDA levels, improve antioxidant enzyme activities, and protect cell membrane integrity from oxidative stress damage. Furthermore, Zhao et al. (2014) verified that Cu-Zn-SOD activity is stimulated by a suitable concentration of ZnO-NPs, and this improvement will reduce ROS production. Hence, myco-fabricated ZnO-NPs (5 or 10 mg/kg) given to the AlCl3 group revealed a significant reduction in the brain MDA and significantly improved SOD and CAT activities as well as GSH levels compared with the AlCl3 treated group. Additionally, a dose of 5 mg/kg of myco-fabricated ZnO-NPs was more efficient than a dose of 10 mg/kg, meaning that a low dose of these particles had powerful antioxidant effects by increasing antioxidant activity and lowering free radical levels.
Numerous authors have studied the connection between oxidative stress and inflammation, and they discovered that high levels of pro-inflammatory cytokines are associated with low antioxidant levels and insufficient antioxidant enzyme activity (Salim et al. 2012). Our findings indicated an increase in brain TNF-α and IL-1β, which may be related to an increase in oxidative stress induced by aluminum in the AlCl3 group. According to Popa-Wagner et al. (2013), ROS produced in brain cells can alter synaptic and non-synaptic transmission among neurons, leading to neuro-inflammation, cell death, neuro-degeneration, and memory loss. Previous studies have shown that neuro-inflammatory cytokines reduce the efflux transfer of amyloid (Aβ), which results in increased Aβ concentrations in the brain (Blasko et al. 1999). Amyloid β plaque formation in the brain is one of the primary causes of AD (Murphy and LeVine 2010). Additionally, it is shown that TNF-α plays an essential role in Aβ made destruction of LTP, a kind of synaptic plasticity directly related to memory and learning (Wang et al. 2005).
In the current investigation, it was found that myco-fabricated ZnO-NPs co-treatment with AlCl3 decreased the rise levels of TNF-α and IL-1β in mice’s brains in comparison with AlCl3 alone, which significantly raised these cytokines. This may be attributed to the elevated Zn content in the brain tissue. Zinc improves the up-regulation of A20 protein (TNF-α-induced protein 3). It is a highly conserved protein that has seven zinc finger (ZnF) domains in its C-terminus, which decline NF-kappaB activation, causing reduced gene expression and the generation of TNF-α, IL-1 ß, and IL-8 (Dardenne 2002; Prasad 2008). This result confirms our previous study in vitro, which showed that myco-fabricated ZnO-NPs have wound healing, anti-inflammatory action (El-Sayed et al. 2023a, b). Our findings concur with earlier research that showed the ability of ZnO-NPs to reduce inflammation (Ekundayo et al. 2022; Chen et al. 2021; Abdulmalek et al. 2021).
Aspartate aminotransferase and alanine aminotransferase are active brain enzymes that are found in cytosolic and mitochondria and they have a role in glutamate metabolism (Palailogos et al. 1989). The significant decline in brain AST and ALT activities in the AlCl3 group could be related to oxidative stress formed from the buildup of aluminum in brain tissues, which disturbs protein synthesis and causes a decrease in ALT and AST activities (Netopilová et al. 2001). The decline in transaminase enzymes indicates a decrease in glutamate metabolism, causing neurological dysfunction. Glutamate plays a part in synaptic plasticity, one of the key neurochemical bases of memory and learning, which is important for cognitive processes like memory and learning in the brain (Meldrum 1994). Bartos et al. (2019) reported that oxidative stress induced by exposure to fluoride caused a decline in ALT and AST enzymes in brain offspring rats, which led to a decrease in glutamate, a possible mechanism of neurotoxicity and memory impairment. Moreover, Amel et al. (2016) found that giving rats 1000 ppm lead acetate in drinking water decreased brain ALT and AST. Moreover, myco-fabricated ZnO-NPs ameliorated these enzymes, which may be related to the antioxidant effects of myco-fabricated ZnO-NPs.
The current investigation found that AlCl3 significantly increased AchE activity and decreased Ach levels. This might be related to aluminum’s allosteric interaction with the peripheral anionic site of the enzyme molecule (Pohanka 2011), producing variation of its secondary structure and so increasing its activity (Zatta et al. 1994). An additional explanation for the elevated AchE could be related to IL-1ß overproduction, which stimulates the activity and expression of AchE through the interaction of IL-1β by muscarinic acetylcholine receptors (Schliebs et al. 2006). Also, an increase in AchE may be due to increased oxidative stress and lipid peroxidation and a decrease in antioxidant capacity induced by aluminum in brain tissues (Kumar and Gill 2014). Kaizer et al. (2005) suggested that changes in the lipid membrane might be responsible for an alteration in the structural form of the AchE molecule, that make induction of AchE activity after prolonged exposure to aluminum. Additionally, the levels of the neurotransmitter dopamine and serotonin (5-hydroxytryptamine, 5-HT) in the mice’s brain tissue significantly decreased in the AlCl3-exposed animals. This could be linked to the oxidative stress caused by AlCl3, which makes the oxidation of tryptophan a precursor to 5-HT. It is true that both ROS and pro-inflammatory cytokines can convert tryptophan to kynurenine. According to Bakunina et al. (2015), this molecule might be further metabolized to the pro-oxidant substances 3-hydroxykynurenine and quinolinic acid, which are linked to the causing of depression. Also, Cunnington and Channon (2010) illustrated that an increase in ROS can lead to reduced availability of tetrahydrobiopterin (BH4). BH4 is a cofactor that uses the three aromatic amino acid hydroxylase enzymes to produce the precursors of the major monoamine neurotransmitters dopamine and serotonin from aromatic amino acids like phenylalanine, tyrosine, and tryptophan. This reduced synthesis of dopamine and serotonin results from the availability of these precursors (Kappock et al. 1996). The results of our investigation concurred with those of the earlier study mentioned by Ekundayo et al. (2022).
We found that the AchE activity in brain tissues was significantly suppressed in both doses of myco-fabricated ZnO-NP (5 and 10 mg/kg)-treated mice, which confirms our previous study in vitro by El-Sayed et al. (2023a,) we found that myco-fabricated Zno-Ps have a stronger inhibitory influence on AchE through direct interaction with AchE by molecular docking. Inhibited AchE activity can prevent Ach from being degraded in the synaptic cleft, resulting in a buildup of Ach, that augments cholinergic neurotransmission and improves memory and cognition in animals. This result suggested that myco-fabricated ZnO-NPs has an ameliorating effect on neurodegenerative symptoms in AD through its antioxidant, anti-inflammatory, and inhibiting AchE. Thus, ZnO-NPs can be enhancing cognition in experimental animals by elevating acetylcholine at synapses. The anti-cholinesterase activities ZnO-NPs detected in our research are in alignment with previous studies (Guo et al. 2020; Hamza et al. 2019). According to Lu et al. (2013), zinc was found to mitigate the negative effects of aluminum exposure on AchE activity, dopamine and serotonin levels, and brain redox status.
Longer escape latencies to arrive at the platform and less time spent in the target quadrant during the MWM test in AlCl3-treated mice indicate deteriorating spatial memory, which is indicative of poor learning and memory. This may be due to the accumulation of aluminum in the brain, which induces increasing AchE activity, inflammation, and the accumulation of beta-amyloid, as well as reducing the antioxidant activity that affects learning and memory (Thenmozhi et al. 2015). These behavioral results supported the previous biochemical finding. Myco-fabricated ZnO-NP treatment at two different doses significantly enhanced this diminished spatial learning and memory adjacent to the control group, and the dose of 5 mg/kg/bw was more powerful than 10 mg/kg/bw. These findings suggest that myco-fabricated ZnO-NPs have a memory-improving function and a protective effect against AlCl3-induced memory loss through antioxidant, anti-inflammatory, and inhibitory actions for AchE.
Conclusion
The findings of this work demonstrated that myco-fabricated ZnO-NPs provide neuro-amelioration against an experimental AD model caused by AlCl3 through reducing IL1 β, TNF-ἀ, MDA, and activity of AChE and increasing production of GSH, SOD, and CAT. Also, myco-fabricated ZnO-NPs can enhance behavioral alterations by reducing escape latency and increasing time spent in the target quadrant. This indicates that the myco-fabricated ZnO-NPs (especially 5 mg/kg b.w) have antioxidant, anti-inflammatory, and inhibitory action for AChE, and these in vivo results confirm our previous study in vitro.
Abbreviations
- Ach
Acetylcholine
- AchE
Acetylcholinesterase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CAT
Catalase
- DA
Dopamine
- GSH
Glutathione
- IL-1β
Interleukin-β
- MDA
Malondialdehyde
- SOD
Superoxide dismutase activity
- ST
Serotonin
- TNF-α
Tumor necrosis factor-alpha
Author contributions
ERE research topic suggestion, experimental methodology design, data analysis, and manuscript revision. DSM, RMM, and HAA experimental methodology design, experimental, data analysis, original draft writing, manuscript revision, and proofreading. All authors read and approved the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The research protocol with (Serial Number 52 A/22) for the purpose of overseeing and monitoring experimental animals was approved by the National Centre for Radiation Research and Technology’s Research Ethics Committee.
Consent for publication
All authors agreed to publish this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelazem H. Effect of Moringa oleifera on antioxidant enzymes and oxidative stress induced by aluminum exposure in male albino rat testes. Int J Cancer Biomed Res. 2020;3:34–41. [Google Scholar]
- Abdelhakim HK, El-Sayed ER, Rashidi FB. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J Appl Microbiol. 2020;128:1634–1646. doi: 10.1111/jam.14581. [DOI] [PubMed] [Google Scholar]
- Abd Elmonem HA, Mahmoud AH, Abbas MM. Ameliorative effect of zinc oxide nanoparticles and vitamin E on some biochemical and histological changes in irradiated albino rats. Egypt J Rad Sci Appl. 2021;34:1–10. [Google Scholar]
- Abdulmalek S, Nasef M, Awad D, Balbaa M. Protective effect of natural antioxidant, curcumin nanoparticles, and zinc oxide nanoparticles against type 2 diabetes-promoted hippocampal neurotoxicity in rats. Pharmaceutics. 2021;13:1937. doi: 10.3390/pharmaceutics13111937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Amel B, Omar K, Faiza F, Miloud S, Abdelkader A. Behavior and glutamate transaminase changes in rat exposed to lead and treated by wormwood extract. Int J Pharm Pharm Sci. 2016;8:208–213. [Google Scholar]
- Anwar MM, Aly SSH, Nasr EH, El-Sayed ER. Improving carboxymethyl cellulose edible coating using ZnO nanoparticles from irradiated Alternaria tenuissima. AMB Express. 2022;12:116. doi: 10.1186/s13568-022-01459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz M, Ovais M, Ahmad I, Sadiq A, Khalil AT, Ullah F. Metal nanoparticles for drug delivery and diagnostic applications. Amsterdam: Elsevier; 2020. Biosynthesized metal nanoparticles as potential Alzheimer’s disease therapeutics; pp. 31–42. [Google Scholar]
- Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015;144:365–373. doi: 10.1111/imm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Gumilar F, Gallegos CE, Bras C, Dominguez S, Cancela LM, Minetti A. Effects of perinatal fluoride exposure on short-and long-term memory, brain antioxidant status, and glutamate metabolism of young rat pups. Int J Toxicol. 2019;38:405–414. doi: 10.1177/1091581819857558. [DOI] [PubMed] [Google Scholar]
- Baydar T, Papp A, Aydin A, Nagymajtenyi L, Schulz H, Isimer A et al. (2003) Accumulation of aluminum in rat brain: does it lead to behavioral and electrophysiological changes. Biol Trace Elem Res 2003;92:231–44 [DOI] [PubMed]
- Beutler E, Duron O, Kefly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Blakemore LJ, Trombley PQ. Zinc as a neuromodulator in the central nervous system with a focus on the olfactory bulb. Front Cell Neurosci. 2017;11:297. doi: 10.3389/fncel.2017.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFα plus IFNγ induce the production of Alzheimer β‐amyloid peptides and decrease the secretion of APPs. FASEB J. 1999;13:63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P. Neuroprotective effects of ononin against the aluminum chloride-induced Alzheimer’s disease in rats. Saudi J Biol Sci. 2021;28:4232–4239. doi: 10.1016/j.sjbs.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington C, Channon KM. Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart. 2010;96:1872–1877. doi: 10.1136/hrt.2009.180430. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002;56(3):20–23. doi: 10.1038/sj.ejcn.1601479. [DOI] [PubMed] [Google Scholar]
- Ekundayo BE, Obafemi TO, Afolabi BA, Adewale OB, Onasanya A, Osukoya OA, Adu IA. Gallic acid and hesperidin elevate neurotransmitters level and protect against oxidative stress, inflammation and apoptosis in aluminum chloride-induced Alzheimer’s disease in rats. Pharmacol Res - Mod Chin Med. 2022;5:100193. doi: 10.1016/j.prmcm.2022.100193. [DOI] [Google Scholar]
- El-Sayed ER, Abdelhakim HK, Ahmed AS. Solid-state fermentation for enhanced production of selenium nanoparticles by gamma-irradiated Monascus purpureus and their biological evaluation and photocatalytic activities. Bioproc Biosyst Eng. 2020;43:797–809. doi: 10.1007/s00449-019-02275-7. [DOI] [PubMed] [Google Scholar]
- El-Sayed ER, Abdelhakim HK, Zakaria Z. Extracellular biosynthesis of cobalt ferrite nanoparticles by Monascus purpureus and their antioxidant, anticancer and antimicrobial activities: yield enhancement by gamma irradiation. Mater Sci Eng C. 2020;107:110318. doi: 10.1016/j.msec2019.110318. [DOI] [PubMed] [Google Scholar]
- El-Sayed ER (2021) Discovery of the anticancer drug vinblastine from the endophytic Alternaria alternata and yield improvement by gamma irradiation mutagenesis. J Appl Microbiol 131(6): 2886–2898. 10.1111/jam.15169 [DOI] [PubMed]
- El-Sayed R, El-Sayed Magdia A, Hazaa Magdy M, Shebl Mahmoud M, Amer Samar R, Mahmoud Abeer A, Khattab (2022a) Bioprospecting endophytic fungi for bioactive metabolites and use of irradiation to improve their bioactivities Abstract AMB Express 12(1). 10.1186/s13568-022-01386-x [DOI] [PMC free article] [PubMed]
- El-Sayed R, El-Sayed Joanna, Gach Teresa, Olejniczak Filip, Boratyński (2022b) A new endophyte Monascus ruber SRZ112 as an efficient production platform of natural pigmenta using agro-industrial wastes. Sci Rep 12(1). 10.1038/s41598-022-16269-1 [DOI] [PMC free article] [PubMed]
- El-Sayed ER, Mousa SA, Abdou DAM, Abo El-Seoud MA, Elmehlawy AA, Mohamed SS. Exploiting the exceptional biosynthetic potency of the endophytic aspergillus terreus in enhancing production of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles using bioprocess optimization and gamma irradiation. Saudi J Biol Sci. 2022 doi: 10.1016/j.sjbs.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed ER, Mansour DS, Morsi RM, Abd Elmonem HA. Gamma irradiation mediated production improvement of some myco-fabricated nanoparticles and exploring their wound healing, anti-inflammatory and acetylcholinesterase inhibitory potentials. Sci Rep. 2023;13:1629. doi: 10.1038/s41598-023-28670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed ER, Mohamed SS, Mousa SA, Abo El-Seoud MA, Elmehlawy AA, Abdou DAM. Bifunctional role of some biogenic nanoparticles in controlling wilt disease and promoting growth of common bean. AMB Expr. 2023;13:41. doi: 10.1186/s13568-023-01546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RMA. New and Rapid Colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Gomaa EZ. Microbial mediated synthesis of zinc oxide nanoparticles, characterization and multifaceted applications. J Inorg Organomet Polym. 2022;32:4114–4132. doi: 10.1007/s10904-022-02406-w. [DOI] [Google Scholar]
- Guo Z, Zhang P, Luo Y, Xie HQ, Chakraborty S, Monikh FA, Bu L, Liu Y, Ma Y, Zhang Z, Valsami-Jones E, Zhao B, Lynch I. Intranasal exposure to ZnO nanoparticles induces alterations in cholinergic neurotransmission in rat brain. Nano Today. 2020;35:100977. doi: 10.1016/j.nantod.2020.100977. [DOI] [Google Scholar]
- Hamza RZ, Al-Salmi FA, El-Shenawy NS. Evaluation of the effects of the green nanoparticles zinc oxide on monosodium glutamate-induced toxicity in the brain of rats. PeerJ. 2019;7:e7460. doi: 10.7717/peerj.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatab MH, Rashad E, Saleh HM, El-Sayed ER, Abu Taleb AM. Effects of dietary supplementation of myco-fabricated zinc oxide nanoparticles on performance, histological changes, and tissues Zn concentration in broiler chicks. Sci Rep. 2022;12:18791. doi: 10.1038/s41598-022-22836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein HG, El-Sayed ER, Younis NA, Hamdy AA, Easa SM. Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Expr. 2022;12:68. doi: 10.1186/s13568-022-01408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav R, Kulkarni YA. Neuroprotective effect of Quercetin and Memantine against AlCl3-Induced neurotoxicity in albino Wistar rats. Molecules. 2023;28:417. doi: 10.3390/molecules28010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe E, Ringman JM. Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ. 2019;367:l6217. doi: 10.1136/bmj.l6217. [DOI] [PubMed] [Google Scholar]
- Justin Thenmozhi A, Raja TR, Janakiraman U, Manivasagam T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem Res. 2015;40:767–776. doi: 10.26717/BJSTR.2022.42.006771. [DOI] [PubMed] [Google Scholar]
- Kaizer RR, Corrêa MC, Spanevello RM, Morsch VM, Mazzanti CM, Gonçalves JF, Schetinger MR. Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem. 2005;99:1865–1870. doi: 10.1016/j.jinorgbio.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Kappock TJ, Caradonna JP. Pterin-dependent amino acid hydroxylases. Chem Rev. 1996;96:2659–2756. doi: 10.1021/cr9402034. [DOI] [PubMed] [Google Scholar]
- Khan H, Khan MF, Jan SU, Ullah N. Effect of aluminium metal on glutathione (GSH) level in plasma and cytosolic fraction of human blood. Pak J Pharm Sci. 2011;24:13–18. [PubMed] [Google Scholar]
- Kumar V, Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41:154–166. doi: 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Liaquat L, Sadir S, Batool Z, Tabassum S, Shahzad S, Afzal A, Haider S. Acute aluminum chloride toxicity revisited: study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci. 2019;217:202–211. doi: 10.1016/j.lfs.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Lu H, Hu J, Li J, Pang W, Hu Y, Yang H, Li W, Huang C, Zhang M, Jiang Y. Optimal dose of zinc supplementation for preventing aluminum-induced neurotoxicity in rats. Neural Regen Res. 2013;8:2754–2762. doi: 10.3969/j.issn.1673-5374.2013.29.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N, Amber S, Zahid S. Rosmarinus officinalis and methylphenidate exposure improves cognition and depression and regulates anxiety-like behavior in AlCl3-induced mouse model of Alzheimer’s Disease. Front Pharmacol. 2022;13:943163. doi: 10.3389/fphar.2022.943163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS. The role of glutamate in epilepsy and other CNS disorder. Neurology. 1994;44:S14–S23. [PubMed] [Google Scholar]
- Mossa S, Shameli K. Gamma Irradiation-Assisted synthesis of silver nanoparticle and their antimicrobial applications: a review. J Res Nanosci Nanotechnol. 2021;3:53–75. doi: 10.37934/jrnn.3.1.5375. [DOI] [Google Scholar]
- Mousa SA, El-Sayed ER, Mohamed SS, Abo El-Seoud MA, Elmehlawy AA, Abdou DAM. Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl Microbiol Biotechnol. 2021;105:741–753. doi: 10.1007/s00253-020-11046-4. [DOI] [PubMed] [Google Scholar]
- Murphy MP, LeVine H. Alzheimer’s disease and the amyloid-β peptide. J Alzheimers Dis. 2010;19:311–323. doi: 10.3233/jad-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehru B, Anand P. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol. 2005;19:203–208. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Netopilová M, Haugvicová R, Kubová H, Drsata J, Mares P. 36. Influence of convulsant on rat brain activities of alanine aminotransferases and aspartate aminotransferases. Neurochem Res. 2001;26:1285–1291. doi: 10.1023/a:1014386416109. [DOI] [PubMed] [Google Scholar]
- Ngolab J, Honma P, Rissman RA. Reflections on the utility of the retina as a biomarker for Alzheimer’s disease: a literature review. Neurol Ther. 2019;8:57–72. doi: 10.1007/s40120-019-00173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ojha PS, Biradar PR, Tubachi S, Patil VS. Evaluation of neuroprotective effects of Canna indica L against aluminium chloride induced memory impairment in rats. ADV TRADIT MED (ADTM) 2023;23:539–556. doi: 10.1007/s13596-021-00627-x. [DOI] [Google Scholar]
- Palailogos G, Hertz L, Schousboe A. Role of aspartate aminotransferase and mitochondrial dicarboxylate transport for release of endogenously and exogenously supplied neurotransmitter in glutamatergic neurons. Neurochem Res. 1989;14:359–366. doi: 10.1007/bf01000039. [DOI] [PubMed] [Google Scholar]
- Parashar A, Udayabanu M. Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:1–7. doi: 10.1016/j.parkreldis.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:219–229. doi: 10.5507/bp.2011.036. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013:963520. doi: 10.1155/2013/963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams AJ, Connor JR. Aluminum access to the brain: a role for transferrin and its receptors. Proc Natl Acad Sci USA. 1990;87:9024–9027. doi: 10.1073/pnas.87.22.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba K, Rajnala N, Veeraiah P, Tiwari V, Rana RK, Lakhotia SC, Patel AB. Energetics of excitatory and inhibitory neurotransmission in aluminum chloride model of Alzheimer’s disease: reversal of behavioral and metabolic deficits by Rasa Sindoor. Front Mol Neurosci. 2017;10:323. doi: 10.3389/fnmol.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. doi: 10.1016/b978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Heidel K, Apelt J, Gniezdzinska M, Kirazov L, Szutowicz A (2006) Interaction of interleukin-1β with muscarinic acetylcholine receptor-mediated signaling cascade in cholinergically differentiated SH-SY5Y cells. Brain res 1122(1): 78-85. 10.1016/j.brainres.2006.09.014 [DOI] [PubMed]
- Sidhu P, Garg ML. Dhawan DK Protective effects of zinc on oxidative stress enzymes in liver of protein-deficient rats. Drug Chem Toxicol. 2005;28:211–230. doi: 10.1081/dct-52551. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh N, Afzal S, Singh T, Hussain I. Zinc oxide nanoparticles: a review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J Mater Sci. 2018;53:185–201. doi: 10.1007/s10853-017-1544-1. [DOI] [Google Scholar]
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. doi: 10.1093/clinchem/34.3.497. [DOI] [PubMed] [Google Scholar]
- Suntako R. Effect of synthesized ZnO nanograins using a precipitation method for the enhanced cushion rubber properties. Mater Lett. 2015;158:399–402. doi: 10.1016/j.matlet.2015.06.061. [DOI] [Google Scholar]
- Szczechowiak K, Diniz BS, Leszek J. Diet and Alzheimer’s dementia–nutritional approach to modulate inflammation. Pharmacol Biochem Behav. 2019;184:172743. doi: 10.1016/j.pbb.2019.172743. [DOI] [PubMed] [Google Scholar]
- Vieželienė D, Beekhof P, Naginienė R, Baranauskienė D, Baltušnikienė A. Aluminium induces iron-mediated oxidative stress in brain tissue. J Sci Tech Res. 2022;42:33762–33767. doi: 10.26717/BJSTR.2022.42.006771. [DOI] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wu J, Rowan MJ, Anwyl R. Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci. 2005;22:2827–2832. doi: 10.1111/j.1460-9568.2005.04457.x. [DOI] [PubMed] [Google Scholar]
- Zatta P, Zambenedetti P, Bruna V, Filippi B. Activation of acetylcholinesterase by aluminium (III): the relevance of the metal species. NeuroReport. 1994;5:1777–1780. doi: 10.1097/00001756-199409080-00023. [DOI] [PubMed] [Google Scholar]
- Zhao L, Peralta-Videa JR, Rico CM, Hernandez-Viezcas JA, Sun Y, Niu G, Servin A, Nunez JE, Duarte-Gardea M, Gardea-Torresdey JL. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus) J Agric Food Chem. 2014;62:2752–2759. doi: 10.1021/jf405476u. [DOI] [PubMed] [Google Scholar]