Abstract
Purpose
To evaluate benefits and harms of transcutaneous electrical nerve stimulation (TENS) for chronic primary low back pain (CPLBP) in adults to inform a World Health Organization (WHO) standard clinical guideline.
Methods
We searched for randomized controlled trials (RCTs) from various electronic databases from July 1, 2007 to March 9, 2022. Eligible RCTs targeted TENS compared to placebo/sham, usual care, no intervention, or interventions with isolated TENS effects (i.e., combined TENS with treatment B versus treatment B alone) in adults with CPLBP. We extracted outcomes requested by the WHO Guideline Development Group, appraised the risk of bias, conducted meta-analyses where appropriate, and graded the certainty of evidence using GRADE.
Results
Seventeen RCTs (adults, n = 1027; adults ≥ 60 years, n = 28) out of 2010 records and 89 full text RCTs screened were included. The evidence suggested that TENS resulted in a marginal reduction in pain compared to sham (9 RCTs) in the immediate term (2 weeks) (mean difference (MD) = -0.90, 95% confidence interval -1.54 to -0.26), and a reduction in pain catastrophizing in the short term (3 months) with TENS versus no intervention or interventions with TENS specific effects (1 RCT) (MD = -11.20, 95% CI -17.88 to -3.52). For other outcomes, little or no difference was found between TENS and the comparison interventions. The certainty of the evidence for all outcomes was very low.
Conclusions
Based on very low certainty evidence, TENS resulted in brief and marginal reductions in pain (not deemed clinically important) and a short-term reduction in pain catastrophizing in adults with CPLBP, while little to no differences were found for other outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10926-023-10121-7.
Keywords: Low back pain, Systematic review, Meta-analysis, Transcutaneous electrical nerve stimulation
Introduction
Electrical stimulation therapies are therapeutic adjuncts used in the management of chronic pain conditions such as osteoarthritis, fibromyalgia, and chronic primary low back pain (CPLBP) [1–3]. They include a range of non-invasive peripheral stimulation techniques to relieve pain. Among these therapies, transcutaneous electrical nerve stimulation (TENS) and interferential therapy are the most used low volatage electrical stimulation therapies [1, 2].
Both are reported to have similar mechanisms of action, namely acting through segmental inhibition or activation of descending pain-inhibitory systems [1]. TENS units are widely available and accessible globally [4]. A TENS unit is a battery-powered device that can be self-administered and delivers electrical impulses through electrodes placed on the intact skin surface near the source of maximal pain. Interferential therapy involves a different form of electrical stimulation than TENS, and treatment is administered using two pairs of electrodes usually in a clinical setting. Compared to interferential therapy, TENS is used more frequently as a self-delivered intervention given that it is inexpensive and easily accessible.
In 2008, Khadilkar and colleagues published a Cochrane systematic review to assess the effectiveness of TENS versus placebo for the management of CPLBP (4 randomized controlled trials [RCTs], 585 patients) [4]. Their outcomes of interest were pain, functional status, generic health status, work disability, participant satisfaction, treatment side effects, physical examination measures (e.g., range of motion), medication use, and use of medical services. The authors concluded that the evidence did not support the use of TENS for the routine management of CPLBP. Similarly, Resende et al. (2018) found low-quality evidence that TENS did not improve function immediately after therapy when compared to placebo [5]. However, little is known about the effects of TENS versus other interventions and benefits and harms in people with CPLBP – pain between the lower costal margin and the gluteal fold with no specific underlying cause of more than three months duration. Therefore, to develop clinical practice guideline recommendations for the management of CPLBP in adults, the World Health Organization (WHO) commissioned the current systematic review to update the evidence and expand the aims of the Cochrane review [4] by assessing additional comparators (e.g., no intervention, usual care), important outcomes (e.g., psychological functioning, social participation including work), and conducting additional subgroup analyses (e.g., gender/sex, race/ethnicity).
The objectives of this systematic review of RCTs were to determine: (1) the benefits and harms (as reported in RCTs) of TENS compared to placebo/sham, usual care, or no intervention for the management of CPLBP in adults, including older adults (aged ≥ 60 years); and (2) whether the benefits and harms of TENS vary by age, gender/sex, presence of leg pain, race/ethnicity, or national economic development of the countries where the RCTs were conducted.
Methods
This systematic review was conducted as part of a series of reviews to inform the WHO guideline on the management of CPLBP in adults. The development of this guideline was ongoing at the time of submission of this manuscript. The methods are detailed in the methodology article of this series [3, 6].
Briefly, we updated and expanded the scope of the previously published high-quality Cochrane systematic review by Khadilkar et al. (2008) [4]. We registered our review protocol with Prospero (CRD42022314817) on 7 March 2022. We searched MEDLINE (Ovid), CINAHL (EBSCO), Embase (Ovid), Cochrane Central Register of Controlled Trials (Wiley), PEDRO, and the WHO International Clinical Trials Registry Platform (ICTRP) from the period of 1 July 2007 (end date of previous Cochrane review) to 9 March 2022 (see Online Resource 1). We also searched the reference lists of systematic reviews and included RCTs.
We included RCTs that compared TENS to placebo/sham, usual care, and no intervention (including comparison interventions where the attributable effect of TENS could be isolated, e.g., TENS + medication vs. same medication alone) in adults (aged ≥ 20 years) with CPLBP. TENS interventions could be applied using device settings of any of the stimulation parameters including pulse intensity, frequency, duration, and type (burst or continuous). RCTs of electrical stimulation administered percutaneously using needles were excluded. In addition to the main critical outcomes requested by the WHO Guideline Development Group (GDG) and assessed for all reviews in this series (pain, function, health-related quality of life, harms, psychological functioning, and social participation including work), we also assessed additional critical outcomes requested by the WHO GDG for this review – the change in use of medications and falls in older adults (aged ≥ 60 years). We reported outcomes based on post-intervention follow-up intervals including: (1) immediate term (closest to 2 weeks after the intervention period); (2) short term (closest to 3 months after the intervention period); (3) intermediate term (closest to 6 months after the intervention period); (4) long term (closest to 12 months after the intervention period); and (5) extra-long term (more than 12 months after the intervention period).
We assessed between-group differences to determine the magnitude of the effect of an intervention and to assess its effectiveness [7, 8] (details in the methodology article in this series) [6]. Briefly, we considered a mean difference (MD) of ≥ 10% of the scale range (e.g., MD = 1 on visual analogue scale 0 to 10) or ≥ 10% difference in risk for dichotomous outcomes to be a minimally important difference (MID) [9, 10]. If the standardized mean difference (SMD) was calculated, SMD ≥ 0.2 was considered a MID [11].
Pairs of reviewers independently screened studies for eligibility, and critically appraised risk of bias using the Cochrane ROB 1 tool [12], modified from the Cochrane Back and Neck Methods Guidelines [13]. One reviewer extracted data for all included RCTs, which was then verified by a second reviewer. Any disagreements were resolved by consensus between paired reviewers or with a third reviewer when necessary. Forms and guidance for screening, risk of bias assessment, and data extraction were adapted from those used by Hayden et al. in the conduct of the ‘exercise for chronic low back pain’ collaborative review, in which members of our team participated [14]. The forms were modified and completed using a web-based electronic systematic review software DistillerSR Inc. [15].
In addition to the main sub-group analyses conducted for all reviews in this series (age, gender/ sex, presence of leg pain, race/ethnicity, national economic development of country where RCT was conducted), we conducted the following pre-specified sub-group and sensitivity analyses: number of treatment sessions (i.e., ≥ 10 sessions vs. <10 sessions) and removal of RCTs rated as high risk of bias.
We conducted random-effects meta-analyses and narrative synthesis where meta-analysis was not appropriate [16], and graded the certainty of evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE) [17]. The comparisons no intervention and interventions where the specific effects of TENS could be isolated were combined in meta-analyses. Meta-analyses were conducted using R [18, 19], and GRADE Evidence Profiles and GRADE Summary of Findings tables were developed using GRADEpro software [20].
Results
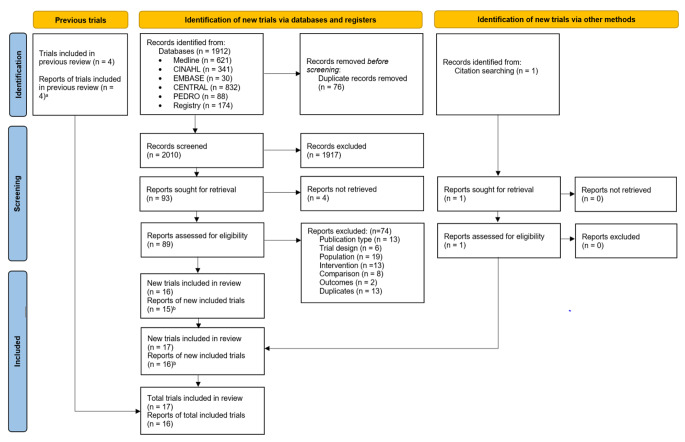
We screened 2010 records and 89 full-text reports (Fig. 1). We identified five unpublished RCTs in the WHO ICTRP, of which we contacted the authors with contact information listed (four of the five). One author responded to inform us that the RCT was ongoing, and therefore was not included in our review. Thus, none of the five unpublished RCTs identified in the WHO ICTRP were included (because the other three authors did not reply). We included 17 published RCTs (16 reports) [2, 21–35] with a total of 1027 adults (ranging from 11 to 134 adults per RCT) from predominantly healthcare settings (see Online Resources 2, 3). The RCTs were conducted in high-income economies [36]: Canada (1 RCT) [23], Greece (1 RCT) [31], Japan (1 RCT) [2], and the United States (3 RCTs) [22, 29, 32]; upper-middle income economies: Brazil (3 RCTs) [24–26], China (1 RCT) [23], and Turkey (3 RCTs) [30, 34, 35]; and lower-middle income economies: Egypt (1 RCT) [27], Iran (1 RCT) [21], and Nigeria (2 RCTs) [28, 33]. The mean age ranged from 22 to 64 years; two RCTs (both included in one report) assessed older adults (n = 28) [2]. The percentage of females within the RCTs ranged from 13 to 100%. In eight RCTs, adults had CPLBP without leg pain [21, 23, 24, 27, 28, 31, 32, 35], in four RCTs (three reports) adults had CPLBP either with or without leg pain (radicular or non-radicular) [2, 29, 30], in a single RCT, adults had CPLBP with radiculopathy [22], and presence of leg pain was not reported in four RCTs [25, 26, 33, 34]. Where reported by authors, CPLBP duration ranged from 31 weeks to 13 years.
Fig. 1.
Flow diagram of literature search
a4 RCTs from previous review were also identified from current search; thus, they did not add to the total
b1 report contained 2 RCTs [2]
The TENS interventions in the included RCTs involved electrode placement over the paravertebral lumbosacral area and sometimes to the affected leg, using conventional continuous or burst pulse types. TENS was compared to either sham TENS (11 RCTs) [2, 22–24, 26, 28, 30–32, 34, 35], no intervention (1 RCT) [29], or interventions where the specific effects of TENS were isolated (9 RCTs) [2, 21, 24, 25, 27, 30–33] (Table 1). Sham TENS used similar electrode placements as the intervention groups, except the current was switched off. We did not find any RCTs comparing TENS to usual care. The outcomes were assessed in the immediate term (closest to 2 weeks after the intervention period) (14 RCTs in 13 reports) [2, 21–28, 30, 32–34], or short term (closest to 3 months after the intervention period) (1 RCT) [29], or both (2 RCTs) [31, 35]. None of the included RCTs assessed outcomes in the intermediate (closest to 6 months after the intervention period), long (closest to 12 months after the intervention period) or extra long (> 12 months after the intervention period) term. The RCTs were rated as overall high (14, 82%), or unclear (3, 18%) risk of bias (Online Resource 4). The agreement on overall ROB ratings was high (weighted overall kappa score 0.95).
Table 1.
Number of included RCTs by comparison and outcome
| Outcome | Follow-up | ||||
|---|---|---|---|---|---|
| Immediate (2 weeks) |
Short (3 months) |
Intermediate (6 months) | Long (12 months) |
Extra-long (> 12 months) | |
| TENS versus sham | |||||
| Pain | 10a | 2 | - | - | - |
| Function | 4 | 2 | - | - | - |
| HRQoL | 2 | - | - | - | - |
| Fear avoidance | - | - | - | - | - |
| Catastrophizing | - | - | - | - | - |
| Depression | - | 1 | - | - | - |
| Anxiety | - | - | - | - | - |
| Self-efficacy | - | - | - | - | - |
| Social participation | - | - | - | - | - |
| Medication use | - | - | - | - | - |
| Falls | - | - | - | - | - |
| Harms | 1 | - | - | - | - |
| TENS versus no intervention or interventions where effects of TENS were isolated | |||||
| Pain | 8 | 2 | - | - | - |
| Function | 6 | 2 | - | - | - |
| HRQoL | 1 | - | - | - | - |
| Fear avoidance | - | - | - | - | - |
| Catastrophizing | - | 1 | - | - | - |
| Depression | - | 1 | - | - | - |
| Anxiety | - | - | - | - | - |
| Self-efficacy | - | - | - | - | - |
| Social participation | - | - | - | - | - |
| Medication use | - | - | - | - | - |
| Falls | - | - | - | - | - |
| Harms | 1 | - | - | - | - |
| TENS versus usual care | |||||
| All outcomes | - | - | - | - | - |
HRQoL: health-related quality of life.
a2 RCTs included older adults (aged ≥ 60 years)
Certainty of Evidence
The certainty of the evidence for all outcomes was very low, and was downgraded due to risk of bias, inconsistency, indirectness, and imprecision of the effect estimates (see Online Resources 5, 6 and 7).
TENS Versus Sham
All Adults
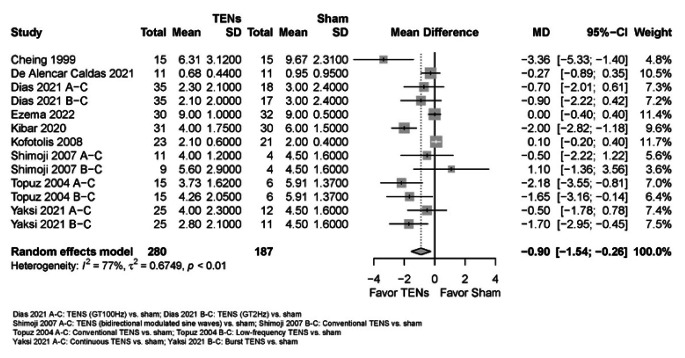
Due to very low certainty evidence, it is uncertain whether TENS reduces pain (scale 0 to 10, 0 = no pain) in the immediate term (9 RCTs; mean difference (MD) = -0.90, 95% confidence interval (CI) -1.54 to -0.26) (see Online Resource 7, plot 1.1.1) [2, 23, 24, 26, 28, 30, 31, 34, 35]. The effect estimate did not reach the threshold for what we considered to be a minimally important between-group difference (MD = -1) [6]. It is uncertain whether TENS makes little or no difference to pain in the short term (2 RCTs; MD = -0.40, 95% CI -2.21 to 1.41) (plot 1.1.2) [31, 35].
Fig. 2.
TENS versus sham for pain in the immediate term (closest to 2 weeks); scale range is 0 to 10
Due to very low certainty evidence, it is uncertain whether TENS makes little or no difference to function in the immediate (4 RCTs; standardized mean difference (SMD) = -0.96, 95% CI -3.20 to 1.28; benefit indicated by lower values) (plot 1.2.1) [24, 30, 31, 32], or short term (2 RCTs; MD = -0.24, 95% CI -4.30 to 3.81; scale 0 to 50, 0 = no disability) (plot 1.2.2) [31, 35]. It is uncertain whether TENS makes little or no difference to health-related quality of life (scale 0 to 100, 0 = poor quality of life; PCS: physical component summary, MCS: mental component summary) in the immediate term (2 RCTs; PCS: MD = 3.21, 95% CI -21.17 to 27.59; plot 1.3.1; MCS: MD = 3.57, 95% CI -30.06 to 37.20; plot 1.4.1) [24, 34]. It is uncertain whether TENS makes little or no difference to depression (scale 0 to 63, 0 = no depression) in the short term (1 RCT; MD = 3.04, 95% CI -19.15 to 25.22) (plot 1.5.1) [35]. It is uncertain whether TENS makes little or no difference to adverse events/harms (1 RCT) (no plot, narrative synthesis) [35]. None of the other RCTs assessed adverse events.
Older Adults (aged ≥ 60 Years)
Due to very low certainty evidence, in older adults, it is uncertain whether TENS makes little or no difference to pain (scale 0 to 10, 0 = no pain) in the immediate term (1 RCT; MD = 0.13, 95% CI -9.80 to 10.06) (plot 1.6.1.1) [2].
TENS Versus no Intervention or Interventions where the Attributable Effect of TENS could be Isolated
All Adults
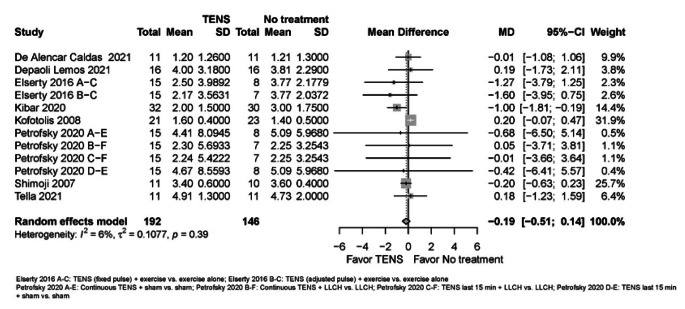
Due to very low certainty evidence, it is uncertain whether TENS makes little or no difference to pain (scale 0 to 10, 0 = no pain) in the immediate (8 RCTs; MD = -0.19, 95% CI -0.51 to 0.14) (see Online Resource 7, plot 2.1.1) [2, 24, 25, 27, 30–33], or short term (2 RCTs; MD = -0.98, 95% CI -16.83 to 14.88) (plot 2.1.2) [29, 31].
Fig. 3.
TENS versus no intervention or interventions where the effects of TENS were isolated for pain in the immediate term (closest to 2 weeks); scale range is 0 to 10
Due to very low certainty evidence, it is uncertain whether TENS makes little or no difference to function (benefit indicated by lower values) in the immediate (6 RCTs; SMD = -0.32, 95% CI -0.71 to 0.07) (plot 2.2.1) [21, 24, 25, 27, 30, 31], or short term (2 RCTs; SMD = 1.05, 95% CI -18.51 to 20.61) (plot 2.2.2) [29, 31]. It is uncertain whether TENS makes little or no difference to health-related quality of life (scale 0 to 100; 0 = poor health-related quality of life) in the immediate term (PCS: MD = -6.82, 95% CI -27.06 to 13.42; plot 2.3.1; MCS: MD = -2.91, 95% CI -10.25 to 4.43; plot 2.4.1) [24]. It is uncertain whether TENS makes little or no difference to depression (scale 0 to 21; 0 = no depression) in the short term (1 RCT; MD = -1.40, 95% CI -5.57 to 2.77) (plot 2.5.1) [29]. It is uncertain whether TENS reduces pain catastrophizing (scale 0 to 52; 0 = no catastrophizing) in the short term (1 RCT; MD = -11.20, 95% CI -17.88 to -3.52) (plot 2.6.1) [30]. It is uncertain whether TENS makes little or no difference to adverse events/harms in the immediate term (1 RCT) (no plot, narrative synthesis) [31].
Subgroup and Sensitivity Analyses
The results of the subgroup analyses did not substantially alter our main findings; however, the subgroups require further investigation. For all comparisons, the subgroups were small (consisting of 1–3 RCTs with sample sizes ranging from 11 to 134 adults per group) and yielded small, pooled effects with marked imprecision (wide 95% CIs) and unclear clinical implications. Therefore, subgroup differences could not be explained and/or the differences between subgroups would likely not result in different recommendations for different subgroups (see Online Resource 7). This is due to the very low certainty evidence and little or no differences between TENS and comparisons for all outcomes.
Discussion
The evidence regarding the benefits and harms of TENS for CPLBP in adults is based on 17 RCTs with a total of 1027 adults. Of these, two RCTs (one report, n = 28) assessed older adults (aged ≥ 60 years) [2]. Most of the RCTs (14, 82%) were rated as having a high overall risk of bias, and three (18%) were rated as unclear overall risk of bias. The certainty of the evidence for all outcomes was very low. For most outcomes there was little or no difference between TENS and sham, no intervention, or interventions where the attributable effect of TENS could be isolated (i.e., combined TENS with treatment B versus treatment B alone). In the comparison of TENS with sham, in the immediate term, evidence suggested a marginal reduction in pain that did not meet our MID (MD = -1) [6] and was not found in the RCTs exclusively assessing older adults. Additionally, no harms were reported, but this was assessed in only one RCT. In the comparison of TENS with no intervention, evidence suggested a reduction in pain catastrophizing in the short term.
Our findings are similar to those in the review by Khadilkar et al. (2008) [4] concluding that the evidence does not support the use of TENS in the routine management of CPLBP. There are, however, some notable methodological differences between these two reviews. First, Khadilkar et al. exclusively included RCTs comparing TENS to sham. We expanded the breadth of the comparisons and identified nine RCTs comparing TENS to no intervention or interventions where the effects of TENS could be isolated. Our review differed in our assessment of the risk of bias. Aside from using a different risk of bias tool, the interpretation of each type of bias differed between reviews. Khadilkar et al. used an arbitrary cut-off score to determine the level of quality (high versus low quality) attributed to each RCT; whereas we based our assessment on the inherent impact that each type of bias could have contributed to the RCT and its interpretation of results [37]. Interestingly, one RCT relevant to both the Khadilkar and current review [34] was judged to be high quality by Khadilkar et al. and high risk of bias (low quality) by our review team.
Since the publication of Khadilkar et al. in 2008, we identified one related systematic review assessing the effectiveness of TENS versus placebo for CLBP, the findings of which aligned with ours. Resende et al. (2018) [5] found low-quality evidence that TENS did not improve function immediately after therapy. No other results were available that specifically assessed the effects of TENS versus placebo in people with CLBP. Our review differed by specifically assessing the effects of TENS (not in combination with IFC) on multiple additional outcomes such as pain, psychological functioning, and social participation including work.
Our systematic review has strengths. To begin with, our team consisted of clinical and methodological experts from around the world, specializing in LBP, systematic reviews, and evidence syntheses. Secondly, our review process involved conducting thorough and peer-reviewed literature searches without any language restrictions. Third, during the screening and ROB assessments, a core team member (with the most expertise and reliability in screening and ROB evaluations) was involved in each screening and ROB pair. Fourth, our ROB assessments did not rely on summary scores or the number of items at risk of bias, as some other systematic reviews have done. Instead, we created supplementary guidance forms based on the ROB1 criteria [12, 13], which allowed reviewers to consider critical flaws in the studies [6]. Our use of these forms resulted in high agreement on overall ROB ratings. Lastly, we maintained transparency throughout the review process, providing detailed ROB assessments and footnotes for grading the certainty of the evidence (see Online Resources 4, 5). These notes give readers a better understanding of our judgements and allow them to reach their own conclusions.
Our review has potential limitations. One limitation is the possibility that we missed relevant RCTs. However, we attempted to address this by using comprehensive and peer-reviewed literature search strategies developed with the assistance of experienced health sciences librarians. Additionally, we searched the reference lists of included RCTs and related systematic reviews. Another limitation is that we did not search the grey literature, which could introduce publication bias as studies published in peer-reviewed journals tend to report larger intervention effects than those in the grey literature [38]. Nonetheless, we believe our review was not impacted by publication bias. We searched for unpublished RCTs in the WHO ICTRP registry and contacted authors of unpublished RCTs. Responses from authors indicated that incomplete RCTs were the main reason for non-publication. Moreover, unpublished studies are known to represent a small proportion of studies and rarely impact results and conclusions [39]. However, it may be important to include such studies in limited scenarios or where there are potential conflicts of interest in published research [39].
Of note, the WHO GDG sought a homogeneous population comprising adults with CPLBP [6]. That is, we excluded RCTs that included post-surgical adults within 12 months post-surgery, adults who had undergone fusion or disc replacement surgery at any time, pregnant individuals, and adults with a clearly determined specific cause for their LBP (e.g., vertebral fracture, malignancy, inflammatory disease). Some reviews use a majority criterion, deeming eligible studies whereby most participants (e.g., ≥ 80%) do not have certain conditions or characteristics. We excluded a highly cited RCT by Deyo et al. (1990) [40] for two reasons. First, it included adults who had surgery or chymopapain therapy (TENS group: 7/65, 11%; sham group: 6/60, 10%), and the results were not stratified based on whether surgery was received, or type of treatment received. Further, they provided no information on the timing of surgery or type of surgery. Second, Deyo et al. included adults with spondylolysis, spondylolisthesis, compression fracture, or scoliosis (TENS group: 7/65, 11%; sham group: 7/60, 12%), and did not stratify their results based on type of LBP (i.e., primary vs. secondary CLBP). While authors were contacted in most cases to clarify study eligibility, this RCT was published 32 years ago; and for pragmatic reasons, authors were not contacted. Nonetheless, we conducted a sensitivity analysis including the results of this RCT and our findings did not change (i.e., we are uncertain if TENS makes little or no difference to pain, function, and adverse events, since the certainty of the evidence is very low). In the current review, no other RCTs were excluded solely for the inclusion of post-surgical participants or participants with secondary CLBP.
We identified several gaps in the evidence that applied across all comparisons involving TENS. First, we did not identify any RCTs reporting on the benefits or harms of TENS beyond the short term (i.e., 3 months after the intervention period). Second, we did not identify any RCTs assessing the effects of TENS on fear avoidance, anxiety, self-efficacy, or social participation including work. Finally, we were unable to assess whether benefits or harms of TENS vary by race/ethnicity. For the comparison of TENS to sham in all adults, we found no RCTs assessing the effects of treatment on catastrophizing behaviours. In older adults (aged ≥ 60 years), two small RCTs assessed pain; we found no RCTs assessing function, health-related quality of life, depression, fear avoidance, change in medication use, falls, or harms. We were also unable to assess whether the benefits or harms of TENS in older adults vary by gender, presence of leg pain/symptoms, or in people from higher versus lower income countries. For the comparison of TENS to no intervention or interventions where the effects of TENS could be isolated, we found no RCTs reporting on fear avoidance behaviours, anxiety, self-efficacy, or social participation including work, and no RCTs in older adults. Finally, we found no RCTs comparing TENS to usual care. These identified gaps may be an avenue for further research, as well as comparing the effects of different doses, frequencies, and durations of TENS. Harms, including those that may be associated with persistent long-term use, should also be investigated systematically.
Conclusion
Based on very low certainty evidence, adults with CPLBP experienced brief and marginal reductions in pain (not deemed clinically important) and a short-term reduction in pain catastrophizing with the use of TENS. Harms from TENS are unclear, as only one included RCT assessed adverse events. The remaining evidence showed little to no difference in benefits between TENS and the comparison interventions for a range of other outcomes (e.g., function, health-related quality of life, depression). Care plans for patients should be created through shared decision making, considering the scientific data and other contextual factors, such as patient preferences and values.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author Contributions
CC (Carol Cancelliere), LV, CAH, DS, HY, AB, DPG, PP, SM, ACT, CC, GB, MN, GC, HMS, JJW, and KM contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by LV and CC (Carol Cancelliere) and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by University of Zurich. This work was funded by the World Health Organization (Switzerland/Ageing and Health Unit). We thank Rahim Lalji for critically reviewing the manuscript.
Open access funding provided by University of Zurich
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics
Ethical approval was not required for this systematic review of previously published studies.
Competing Interests
Competing Interests: All team members provided DOI forms to WHO for evaluation at inception. CC (Carol Cancelliere), LV, DS, HY, GC, JJW, HMS report funding from the Canadian Chiropractic Guideline Initiative. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. JJW is funded by a Banting Postdoctoral Fellowship from the Canadian Institutes of Health Research (CIHR). CAH reports grants to the University of Zurich from the Foundation for the Education of Chiropractors in Switzerland, the Swiss National Science Foundation (SNSF), and the European Centre for Chiropractic Research Excellence (ECCRE) outside the submitted work. SM reports grants from Canadian Chiropractic Association, Canadian Chiropractic Research Foundation, and Canadian Institute of Health Research outside of submitted work. JJW reports grants from the Canadian Institutes of Health Research (CIHR) and Canadian Chiropractic Research Foundation (paid to university), and travel reimbursement for research meetings from the Chiropractic Academy of Research Leadership outside the submitted work. JML reports a grant from the European Cooperation in Science and Technology (COST) outside the submitted work. AB reports grants from the Canadian Chiropractic Association, Canadian Chiropractic Research Foundation, and Health Canada outside the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cesar A. Hincapié, Email: cesar.hincapie@uzh.ch
Carol Cancelliere, Email: carolina.cancelliere@ontariotechu.ca.
References
- 1.Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17(1):33–46. doi: 10.1097/00002508-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Shimoji K, Takahashi N, Nishio Y, Koyanagi M, Aida S. Pain relief by transcutaneous electric nerve stimulation with bidirectional modulated sine waves in patients with chronic back pain: a randomized, double-blind, sham-controlled study. Neuromodulation. 2007;10(1):42–51. doi: 10.1111/j.1525-1403.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 3.Verville L, Hincapié CA, Southerst D, Yu H, Bussières A, Gross DP, et al. Systematic review to inform a World Health Organization (WHO) clinical practice guideline: benefits and harms of transcutaneous electrical nerve stimulation (TENS) for chronic primary low back pain in adults. J Occup Rehabil. 2023. 10.1007/s10926-023-10121-7. [DOI] [PMC free article] [PubMed]
- 4.Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database Syst Rev. 2008(4):CD003008. [DOI] [PMC free article] [PubMed]
- 5.Resende L, Merriwether E, Rampazo EP, Dailey D, Embree J, Deberg J, et al. Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain. Eur J Pain. 2018;22(4):663–78. doi: 10.1002/ejp.1168. [DOI] [PubMed] [Google Scholar]
- 6.Cancelliere C, Verville L, Southerst D, Yu H, Hayden JA, Ogilvie R, Bussières A, Gross DP, Pereira P, Mior S, Tricco AC, Cedra[1]schi C, Brunton G, Nordin M, Wong JJ, Shearer HM, Connell G, Hincapié CA. Systematic review procedures for the World Health Organization (WHO) evidence syntheses on benefts and harms of structured and standardized education/advice, structured exercise programs, transcutaneous electrical nerve stimulation (TENS), and needling therapies for the management of chronic low back pain in adults. J Occup Rehabil. 2023. 10.1007/s10926-023-10156-w. [DOI] [PubMed]
- 7.Herbert RD. How to estimate treatment effects from reports of clinical trials. I: continuous outcomes. Aust J Physiother. 2000;46(3):229–35. doi: 10.1016/S0004-9514(14)60334-2. [DOI] [PubMed] [Google Scholar]
- 8.Herbert RD. How to estimate treatment effects from reports of clinical trials. II: dichotomous outcomes. Aust J Physiother. 2000;46(4):309–13. doi: 10.1016/S0004-9514(14)60292-0. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein SM, Terwee CB, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for acute low-back pain. Cochrane Database Syst Rev. 2012(9):CD008880. [DOI] [PMC free article] [PubMed]
- 10.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey (NJ): Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 12.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976) 2015;40(21):1660–73. doi: 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, Group BEC-CRW. Commentary: collaborative systematic review may produce and share high-quality, comparative evidence more efficiently. J Clin Epidemiol. 2022;152:288–94. doi: 10.1016/j.jclinepi.2022.09.013. [DOI] [PubMed] [Google Scholar]
- 15.DistillerSR . Version 2023.2.1. 2023.2.1 ed. Ottawa, Canada: DistillerSR; 2023. [Google Scholar]
- 16.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. 2022 [cited January 5, 2022]. In: Cochrane Handbook for Systematic Reviews of Interventions version 63 (updated February 2022) [Internet]. Cochrane, [cited January 5, 2022]. Available from: www.training.cochrane.org/handbook.
- 18.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna; 2022.
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softwe. 2010;36(3):1–48. [Google Scholar]
- 20.GRADEpro GDT. GRADEpro Guideline Development Tool (Software]. McMaster University and Evidence Prime; 2021.
- 21.Alizadeh MH, Ahmadizad S. A comparison of exercise therapy and transcutaneous electrical nerve stimulation for the treatment of chronic low back pain. World J Sport Sci. 2009;2(1):43–7. [Google Scholar]
- 22.Bloodworth DM, Nguyen BN, Garver W, Moss F, Pedroza C, Tran T, et al. Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. Am J Phys Med Rehabil. 2004;83(8):584–91. doi: 10.1097/01.PHM.0000133439.28817.51. [DOI] [PubMed] [Google Scholar]
- 23.Cheing GL, Hui-Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch Phys Med Rehabil. 1999;80(3):305–12. doi: 10.1016/S0003-9993(99)90142-9. [DOI] [PubMed] [Google Scholar]
- 24.de Alencar Caldas VV, Maciel DG, Cerqueira MS, Barboza JAM, Neto JBV, Dantas G, et al. Effect of pain education, cryotherapy, and transcutaneous electrical nerve stimulation on the pain, functional capacity, and quality of life in patients with nonspecific chronic low back pain: a single-blind randomized controlled trial. Am J Phys Med Rehabil. 2021;100(3):243–9. doi: 10.1097/PHM.0000000000001552. [DOI] [PubMed] [Google Scholar]
- 25.Depaoli Lemos VJ, Selau RC, Blos C, Baptista Dohnert M, Boff Daitx R, de Almeida Brito V. Electroacupuncture and transcutaneous electrical nerve stimulation in chronic nonspecific low back pain: a blind randomized clinical trial. Muscles, ligaments. tendons J. 2021;11(4):719–27. [Google Scholar]
- 26.Dias LV, Cordeiro MA, Schmidt de Sales R, Bieberbach Rodrigues dos Santos MM, Korelo RIG, Said Vojciechowski A, et al. Immediate analgesic effect of transcutaneous electrical nerve stimulation (TENS) and interferential current (IFC) on chronic low back pain: randomised placebo-controlled trial. J Bodyw Mov Ther. 2021;27:181–90. doi: 10.1016/j.jbmt.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Elserty N, Kattabei O, Elhafez H. Effect of fixed versus adjusted transcutaneous electrical nerve stimulation amplitude on chronic mechanical low back pain. J Altern Complement Med. 2016;22(7):557–62. doi: 10.1089/acm.2015.0063. [DOI] [PubMed] [Google Scholar]
- 28.Ezema CI, Onyeso OK, Nna EO, Awosoga OA, Odole AC, Kalu ME et al. Transcutaneous electrical nerve stimulation effects on pain-intensity and endogenous opioids levels among chronic low-back pain patients: a randomised controlled trial. J Back Musculoskelet Rehabil. 2022. [DOI] [PubMed]
- 29.Jamison RN, Wan L, Edwards RR, Mei A, Ross EL. Outcome of a high-frequency transcutaneous electrical nerve stimulator (hfTENS) device for low back pain: a randomized controlled trial. Pain Pract. 2019;19(5):466–75. doi: 10.1111/papr.12764. [DOI] [PubMed] [Google Scholar]
- 30.Kibar S, Konak HE, Ay S, Doganay Erdogan B, Evcik D. The effectiveness of combined transcutaneous electrical nerve stimulation and interferential current therapy on chronic low back pain: a randomized, double-blind, sham-controlled study. Fiziksel Tip ve Rehabilitasyon Bilimleri Dergisi [J Phys Med Rehabil Sci] 2020;23(1):32–40. doi: 10.31609/jpmrs.2019-71464. [DOI] [Google Scholar]
- 31.Kofotolis ND, Vlachopoulos SP, Kellis E. Sequentially allocated clinical trial of rhythmic stabilization exercises and TENS in women with chronic low back pain. Clin Rehabil. 2008;22(2):99–111. doi: 10.1177/0269215507080122. [DOI] [PubMed] [Google Scholar]
- 32.Petrofsky J, Laymon M, Lee H. The effect of transcutaneous electrical nerve stimulation and low-level continuous heat on non-specific low back pain: a randomized controlled trial. Gazz Med Ital. 2020;179(6):419–27. [Google Scholar]
- 33.Tella BA, Oghumu SN, Gbiri CAO. Efficacy of transcutaneous electrical nerve stimulation and interferential current on tactile acuity of individuals with nonspecific chronic low back pain. Neuromodulation 2021 Aug 17:Epub ahead of print. 2021. [DOI] [PubMed]
- 34.Topuz O, Ozfidan E, Ozgen M, Ardic F. Efficacy of transcutaneous electrical nerve stimulation and percutaneous neuromodulation therapy in chronic low back pain. J Back Musculoskelet Rehabil. 2004;17:127–33. doi: 10.3233/BMR-2004-173-407. [DOI] [Google Scholar]
- 35.Yaksi E, Ketenci A, Baslo MB, Orhan EK. Does transcutaneous electrical nerve stimulation affect pain, neuropathic pain, and sympathetic skin responses in the treatment of chronic low back pain? A randomized, placebo-controlled study. Korean J Pain. 2021;34(2):217–28. doi: 10.3344/kjp.2021.34.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Bank Country and Lending Groups. [cited 2022 May 1, 2022]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 37.Viswanathan M, Patnode C, Berkman ND, Bass EB, Chang S, Hartling L et al. Assessing the risk of bias in systematic reviews of health care interventions. Methods guide for comparative effectiveness reviews. (Prepared by the Scientific Resource Center under Contract No. 290-2012-0004-C)December 2017 March 20, 2023 [cited 2023 March 20, 2023]; AHRQ Publication No. 17(18)-EHC036-EF. Available from: Posted final reports are located on the Effective Health Care Program search page. DOI: https://doi.org/10.23970/AHRQEPCMETHGUIDE2.
- 38.Hopewell S, McDonald S, Clark MJ, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007(2):Art. No.: MR000010. [DOI] [PMC free article] [PubMed]
- 39.Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-english reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Med Res Methodol. 2017;17(1):64. doi: 10.1186/s12874-017-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322(23):1627–34. doi: 10.1056/NEJM199006073222303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.