Abstract
Traumatic brain injury (TBI) is a leading worldwide cause of disability, and there are currently no medicines that prevent, reduce, or reverse acute or chronic neurodegeneration in TBI patients. Here, we review the target-agnostic discovery of nicotinamide adenine dinucleotide (NAD+)/NADH-stabilizing P7C3 compounds through a phenotypic screen in mice and describe how P7C3 compounds have been applied to advance understanding of the pathophysiology and potential treatment of TBI. We summarize how P7C3 compounds have been shown across multiple laboratories to mitigate disease progression safely and effectively in a broad range of preclinical models of disease related to impaired NAD+/NADH metabolism, including acute and chronic TBI, and note the reported safety and neuroprotective efficacy of P7C3 compounds in nonhuman primates. We also describe how P7C3 compounds facilitated the recent first demonstration that chronic neurodegeneration 1 year after TBI in mice, the equivalent of many decades in people, can be reversed to restore normal neuropsychiatric function. We additionally review how P7C3 compounds have facilitated discovery of new pathophysiologic mechanisms of neurodegeneration after TBI. This includes the role of rapid TBI-induced tau acetylation that drives axonal degeneration, and the discovery of brain-derived acetylated tau as the first blood-based biomarker of neurodegeneration after TBI that directly correlates with the abundance of a therapeutic target in the brain. We additionally review the identification of TBI-induced tau acetylation as a potential mechanistic link between TBI and increased risk of Alzheimer’s disease. Lastly, we summarize historical accounts of other successful phenotypic-based drug discoveries that advanced medical care without prior recognition of the specific molecular target needed to achieve the desired therapeutic effect.
Keywords: Traumatic brain injury; Alzheimer’s disease, P7C3; Neurodegeneration; Neuroprotection; Tau, Drug discovery; Neurogenesis; NAD; Phenotypic screening
Introduction
Traumatic brain injury (TBI) is a leading cause of disability that currently affects upwards of 75 million people worldwide [1]. Acutely, TBI presents with a range of symptoms, including headache, mood disruption, cognitive impairment, anxiety, and vertigo, as well as occasionally loss of consciousness [2]. It is reported that 22% of people in the USA have sustained at least one TBI with loss of consciousness at some point in their lifetime [3, 4], and there are approximately 5 million people living with TBI-related disabilities at an estimated annual cost of ~ 80 billion USD [5]. Although most patients’ symptoms resolve within the first few months, a growing body of evidence demonstrates that patients often transition to chronic neurodegeneration with persisting symptoms after their initial injury, which can last for decades [6]. Furthermore, a history of TBI greatly increases one’s risk of developing other neurodegenerative disorders, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [7–12]. Strikingly, severe TBI is associated with a 4–fivefold increased risk for developing AD, and even a moderate TBI is associated with a twofold increased risk [6, 10, 12]. TBI also elevates the risk for developing other chronic neuropsychiatric conditions, such as panic disorder, psychotic disorders, mood disorders, and substance-use disorders [6], as well as chronic seizures, sleep disorders, neuroendocrine dysregulation, and systemic metabolic changes [9, 11].
TBI is currently treated in the clinic primarily as an acute emergency, and therapeutic management of chronic sequelae is largely limited to rehabilitation efforts, such as physical and cognitive therapy. Currently, there are no treatments that prevent transition of acute TBI into chronic neurodegenerative disease in patients, or that reverse chronic symptoms and restore normal neuropsychiatric function [13–15]. Thus, there is a tremendous need to identify new therapeutics. The lack of consensus on any single driving force behind neurodegeneration, however, underscores the need for innovative approaches to drug discovery. Here, we review the discovery and development of the nicotinamide adenine dinucleotide (NAD+)/NADH-stabilizing P7C3-class of neuroprotective compounds. P7C3 compounds originated through an in vivo screen based on postnatal hippocampal neurogenesis in mice and have emerged to show protective efficacy across a wide range of preclinical models of disease. We focus here on recent work demonstrating efficacy of P7C3 compounds in acute and chronic TBI, including the first reported reversal of chronic neurodegeneration and restoration of normal cognitive function an entire year after TBI in mice, which is the equivalent of decades in people.
Postnatal Hippocampal Neurogenesis and Its Role in Neurodegenerative Disease
Postnatal hippocampal neurogenesis in laboratory rodents involves the division, differentiation, migration, and maturation of neuronal precursor cells in the dentate gyrus of the hippocampus over a regular pattern of approximately 4 weeks [16]. Newborn neurons, once matured and integrated into hippocampal circuitry, contribute to learning and memory [16–19]. Though it is not fully understood why, under homeostatic conditions approximately half of newly divided neuronal precursor cells die within the first couple of weeks. Those that survive past the first month are typically integrated into long-term hippocampal circuitry, with the vast majority becoming neurons and a smaller fraction becoming glial cells [20, 21]. Thus, the net magnitude of postnatal hippocampal neurogenesis results from the balance between rate of proliferation of neuronal precursor cells and rate of death of maturing newborn neurons.
Many neurodegenerative and neuropsychiatric disorders, including TBI and AD, are characterized by diminished postnatal hippocampal neurogenesis, primarily through an increased rate of cell death during the maturation process [16, 22]. With TBI, for example, while increased proliferation of neural precursor cells occurs in the acute stages immediately after injury [23, 24], this effect is outweighed by an elevated rate of death during the later stages [25, 26]. Behaviorally, animals with ablation of hippocampal neurogenesis after TBI show poorer outcomes on spatial memory tests [27], suggesting the importance of newborn neurons in preserving memory after brain injury. As aberrantly elevated hippocampal neurogenesis may also impair some neurobehavioral measures [28], however, it is important to recognize that intact function may rely on preserving a normal homeostatic balance of hippocampal neurogenesis.
Discovery of a Proneurogenic, Neuroprotective Compound
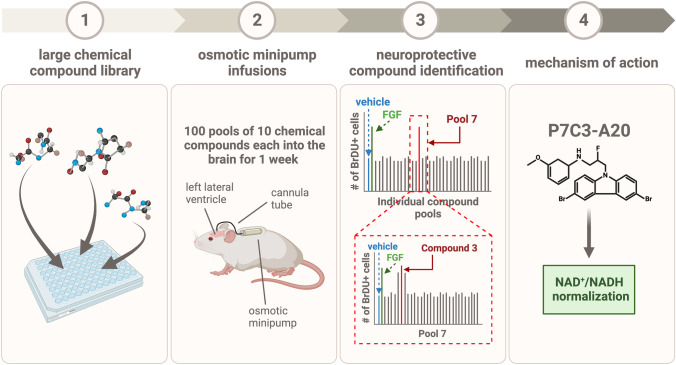
Given the evidence that impaired hippocampal neurogenesis underlies cognitive dysfunction across many conditions, it was reasoned that identifying pharmacologic agents capable of increasing the net magnitude of hippocampal neurogenesis could provide novel biological insights and a starting point for new neurotherapeutics. As such, a target-agnostic, unbiased in vivo screen in living mice to identify small drug-like molecules that could either promote the proliferation of neuronal precursor cells or the survival of newborn hippocampal neurons was undertaken (Fig. 1) [21, 29, 30]. This non-traditional approach of phenotypic screening is advantageous when there is no clear consensus on what specific molecular target will drive a physiologic process in a therapeutically beneficial direction. Additionally, the use of an in vivo phenotypic screen takes into consideration the complexity of a desirable phenotype, such as neurogenesis, in the mature organism. Simply put, the goal was to identify a new small molecule that could safely and potently increase the net magnitude of postnatal hippocampal neurogenesis, without preconceived bias about the mechanism of action.
Fig. 1.
Schematic representation of the in vivo phenotypic screening process by which the neuroprotective NAD+/NADH-normalizing series of P7C3 compounds was discovered, as described in the text
Briefly, 1000 drug-like compounds were selected for screening from a library of over 200,000 small molecules in a manner designed to maximize diversity of chemistry and complexity, while avoiding any clear predictors of potential toxicity. Compounds were then randomly pooled into groups of 10 and delivered via intracerebroventricular infusion to the left lateral ventricle of the brain via an osmotic minipump on a continual basis for 7 days. During this time, animals were intraperitoneally injected daily with bromodeoxyuridine (BrdU), a thymidine analog that incorporates into the DNA of mitotic cells. In this way, hippocampal tissues stained and analyzed at the end of the 1-week period of infusion reflected the relative number of proliferating and surviving new cells in the hippocampus.
Using this systematic approach, pools were narrowed down to those that yielded a BrdU signal equal in magnitude to direct infusion of fibroblast growth factor (FGF), which is made endogenously in the brain and augments postnatal hippocampal neurogenesis. Those pools were then disambiguated, and each molecule of each active pool was tested individually for efficacy in the same in vivo assay. Ultimately, 8 structurally distinct small molecules were identified that generated equivalent BrdU signal as direct infusion of FGF. Of these, the aminopropyl carbazole compound #3, derived from pool #7 (and hence named “P7C3”), was predicted by in silico modeling to have the most favorable likelihood of crossing the blood–brain barrier (BBB). Upon further analysis, P7C3 was found to be readily bioavailable to the brain by either oral or intraperitoneal delivery [21, 30]. Notably, elevation of the magnitude of basal hippocampal neurogenesis with P7C3 compounds in normal healthy wild-type mice did not impair or enhance any neurobehavioral measures.
Since BrdU labels all actively dividing cells, additional experiments were conducted to confirm neuronal specificity. Indeed, P7C3-mediated upregulation of BrdU staining did not colocalize with glial cells and was found exclusively in young mature neurons. A careful multi-time point BrdU pulse-chase experiment with P7C3 treatment then revealed that the resulting increase in magnitude of neurogenesis was due to increased survival of young hippocampal neurons, rather than increased proliferation of neuronal precursor cells [21, 30].
For initial evaluation of therapeutic efficacy, P7C3 was tested in a mouse model with genetically impaired neurogenesis. These mice, missing the neuronal PAS domain 3 (NPAS3) gene, do not properly express the gene for fibroblast growth factor receptor 1 (FGFR1) [31], which is needed for FGF-mediated neurogenesis. Npas3−/− mice show significantly impaired neuronal precursor cell proliferation and survival in the dentate gyrus [31], reduced proliferation of ganglionic eminence precursors, and fewer interneurons in the cortex [32]. These mice also demonstrate significant memory deficits in the novel object recognition task [33]. Both npas3−/− mice and humans with mutations in NPAS3 additionally show reduced hippocampal volume, neuronal activity, and granule layer thickness [33]. Npas3 mutants possess important clinical relevance, because aberrations in this gene in humans are associated with schizophrenia, bipolar disorder, major depression, attention-deficit/hyperactivity disorder, and intellectual disability [34], all of which are associated with various degrees of cognitive impairment. Indeed, NPAS3 and its highly related transcription factor NPAS1 together reciprocally control postnatal hippocampal neurogenesis and more generally synergize as master regulators of expression of neuropsychiatric risk genes [32, 34].
Prenatal treatment of npas3−/− mice with P7C3 highly augmented the survival of newborn hippocampal neurons, as measured by both BrdU pulse-chase and doublecortin expressing cells in the dentate gyrus, and fully restored hippocampal morphology and function [21]. Since the Morris water maze is a well-characterized tool for evaluating hippocampal memory and learning, it was used to further test whether the hippocampal neuroprotective properties of P7C3 could restore memory decline in aging Fischer 344 rats. Here, 18-month-old rats were treated daily for 2 consecutive months with either P7C3 or vehicle. Those that received P7C3 showed significantly improved spatial memory that correlated with augmented magnitude of postnatal hippocampal neurogenesis [21].
After the neuroprotective potency of P7C3 was confirmed, significant work was done to optimize its molecular scaffold to improve its efficacy. Each individual moiety was modified and evaluated in vivo [29]. In time, P7C3-A20 was developed, which involved the fluorination of an existing hydroxyl group, and (−)-P7C3-S243, which also possessed improved physicochemical properties [35, 36]. In addition, to evaluate the safety profile and neuroprotective efficacy of the P7C3 class of compounds more thoroughly, P7C3-A20 was tested in nonhuman primates by daily oral administration for 38 weeks. Compared to vehicle-treated animals, P7C3-A20 dramatically augmented the net magnitude of hippocampal neurogenesis, through increasing survival of newborn hippocampal neurons in nonhuman primates. Importantly, necropsy studies conducted by an independent contract research organization across 35 organs (eyes, lung, heart, aorta, tongue, spleen, liver, kidney, adrenal gland, thyroid, parathyroid, pancreas, stomach, testis, small intestine, large intestine, skeletal muscle, vesicular gland, spinal cord, peripheral nerve, prostate, salivary gland, gall bladder, bone marrow, epididymis, optic nerve, lymph node, mammary gland, larynx, skin, trachea, ureter, bone, joint, and pituitary gland) revealed no toxicology from this extended P7C3 treatment. Thus, it was established that P7C3 compounds safely exert their neuroprotective effect over many months in nonhuman primates without overt toxicity [37].
NAD+/NADH Dysregulation in Traumatic Brain Injury
Maintaining balance between NAD+ and its reduced counterpart NADH is critical for energy homeostasis within cells, especially neurons [38]. After TBI, NAD+/NADH levels in the brain are depleted [39–42], and this energy failure causes axonal degeneration [38, 41]. To this end, many groups have investigated WldS mice, which possess a spontaneous mutation conferring elevated activity of the nicotinamide mononucleotide adenylyltransferase (NMNAT) enzyme. NMNAT is involved in generation of NAD+ in cells [38], and WldS mice are inherently resistant to axonal degeneration by virtue of maintaining normal NAD+/NADH levels within the axon in the face of injury. These animals have shown protection in several models of axonal injury, including TBI [38, 43]. WldS mice are also protected from TBI-mediated elevation and cellular mislocalization of acetylated tau, a newly established mechanism of axonal degeneration in TBI that was discovered though application of P7C3 compounds in the laboratory [44]. Alternatively, some researchers have investigated the sterile alpha and TIR motif containing 1 (Sarm1) knockout mouse as another way to test the protective effect of increasing endogenous NAD+/NADH levels by tempering cellular consumption of NAD+ [38]. For example, these mice show significant protection in the weight drop model of TBI [45].
Importantly, the P7C3 class of compounds stabilizes NAD+/NADH levels within cells and the brain under conditions of injury and disease, without elevating NAD+/NADH above normal [44, 46, 47]. Given the importance of NAD+ metabolism in postnatal neurogenesis [48–53], this is consistent with the discovery of P7C3 in the target-agnostic postnatal neurogenesis phenotypic screen [21]. Maintaining normal levels of NAD+/NADH, without elevating above normal homeostatic levels, is a favorable property of P7C3 compounds, as aberrantly high NAD+ levels may support cancer cell growth [54, 55]. In fact, abnormally elevated NAD+ through administration of the commonly used NAD+ precursor nicotinamide riboside has recently been shown to significantly increase the rate of cancer and tumor metastases to the brain in cancer-prone mice [56]. Protective treatments to prevent NAD+ depletion in the human brain therefore need to specifically normalize NAD+ without elevating it above normal homeostatic levels. Currently, the NAD+/NADH-stabilizing property of P7C3 compounds is considered to be largely responsible for the broad protective efficacy. Importantly, P7C3 compounds do not directly inhibit either the poly ADP ribose polymerase (PARP) enzymes or the SARM1 enzyme, which are major NAD+-consuming enzymes in the brain that are activated in TBI and suggested as neuroprotective targets [57–68]. Furthermore, specific protection from axonal degeneration by P7C3 occurs independently of Wallerian degeneration [69], illustrating the novelty of P7C3-mediated neuroprotection.
P7C3 Compounds Are Broadly Protective Across Preclinical Models of Neurodegeneration and Disease Associated with Diminished NAD+/NADH
To determine whether P7C3 compounds might also exert neuroprotective efficacy for mature neurons outside of the hippocampus, these agents were next tested in preclinical models of PD characterized by neurodegeneration in the striatum and substantia nigra. In an MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) toxin model of PD, P7C3-A20 substantially increased the number of dopaminergic neurons that survived after toxic exposure [70]. Potent neuroprotective efficacy, including preservation of normal motor behavior, of the later P7C3-S243 derivative was also established in the 6-OHDA (6-hydroxydopamine) toxin rat model of PD [71]. Furthermore, in a cell culture model, the conditioned media of reactive microglia, which is known to be toxic to dopaminergic neurons, could be made benign by treating the microglial culture with P7C3 [72], suggesting an additional immune-mediated mechanism of neuroprotection by P7C3. Notably, when the same dopaminergic cell line was exposed to MPP+, a toxic derivative of MPTP, dopaminergic degeneration was prevented with P7C3 [73].
P7C3 compounds are also neuroprotective in the spinal cord. For example, in a rat model of spinal cord injury comprised of laminectomy at the T10 level, daily treatment with P7C3 compounds for 2 weeks following injury significantly improved locomotor function. This also reduced lesion size and neuronal death, increased proliferation of oligodendrocytes, stabilized NAD+/NADH levels, and suppressed microglial reactivity [74]. The same group additionally demonstrated that using P7C3 compounds alongside a collagen hydrogel further bolstered therapeutic efficacy after spinal cord injury [75]. P7C3 compounds were additionally tested in the G93A-SOD1 mutant mouse model of amyotrophic lateral sclerosis (ALS). When treatment was initiated at disease onset, degeneration of cholinergic neurons in the spinal cord was significantly prevented and motor function was preserved [76].
P7C3 compounds have also shown efficacy in preclinical models of Alzheimer’s disease (AD). In TgF344-AD rats, for example, animals were treated with P7C3-243 daily for 9 and 18 months, beginning at 6 months of age. This treatment prevented cognitive decline and depressive-like symptoms, as well as neurodegeneration and neuroinflammation, without affecting amyloid pathology [77]. This reinforces the concept that pursuing novel therapeutic mechanisms, such as maintaining normal NAD+/NADH homeostasis in the brain, may be a uniquely effective approach to treating AD.
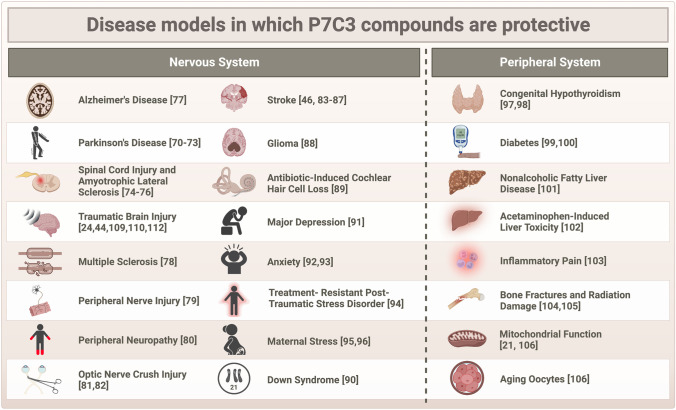
P7C3 compounds have additionally shown protective efficacy in a number of other neurological disease models, including multiple sclerosis [78], peripheral nerve injury [79], chemotherapy-induced peripheral neuropathy [80], optic nerve crush injury [81, 82], stroke [46, 83–87], glioma [88], and antibiotic-induced cochlear hair cell loss [89]. P7C3 compounds have also shown efficacy in preclinical models of neuropsychiatric disorders characterized by hippocampal neurodegeneration, including Down syndrome [90], major depression [91], anxiety [92, 93], treatment-resistant post-traumatic stress disorder [94], and maternal stress [95, 96]. These compounds have even proven beneficial in disorders of energy imbalance related to NAD+ biology outside of the nervous system, including congenital hypothyroidism [97, 98], diabetes [99, 100], nonalcoholic fatty liver disease [101], acetaminophen-induced liver toxicity [102], inflammatory pain [103], physical and radiation bone damage [104, 105], and mitochondrial function in aging oocytes [106]. The most recent list of preclinical models in which P7C3 compounds have been reported to exert protective efficacy is shown in Fig. 2. Notably, no publications to date have reported a lack of efficacy, or worsened outcomes, with P7C3 treatment in any preclinical model. While this offers an optimistic outlook for the therapeutic potential of this novel class of NAD+/NADH-stabilizing agents, it is important to note the existence in the field of publication bias against negative results [107].
Fig. 2.
Overview of the animal disease models to date in which P7C3 compounds have been shown to be protective, as described in the text
P7C3 Compounds Are Neuroprotective After TBI
In 2014, the efficacy of P7C3 compounds in TBI was initially investigated using a fluid percussion injury model in mice. In this paradigm, P7C3-A20-treated mice experienced significant reduction in contusion volume and improved neuronal survival in the cortex. As expected, P7C3-A20 also significantly boosted the number of surviving and maturing hippocampal neural precursor cells after injury, which correlated with preserved cognitive function in the Morris water maze test of hippocampal-dependent spatial learning and memory [24].
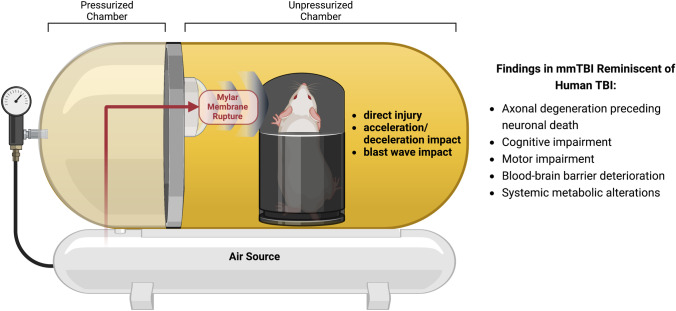
Following this study, the efficacy of P7C3-A20 was further assessed in an alternative model of TBI, multimodal TBI (mmTBI), which has proven useful in laboratory investigation of putative TBI therapies and complex neurophysiological process after TBI. This model uses jet-flow overpressure mechanics to deliver a sudden jet of air to the animal’s exposed, freely moving head. It should be noted that this is not a model of TBI from an isolated pure blast wave [108]. Rather, this apparatus generates a collimated high-speed jet flow with extreme dynamic pressure that delivers well-defined and readily calibratable intensities of global concussive injury, acceleration/deceleration injury, and early blast wave exposure [108]. The apparatus itself is comprised of a large tank divided into two chambers, as shown in Fig. 3. The overpressure chamber on the right side houses the anesthetized mouse, which is placed on a platform with its body contained in a protective shell. This allows the exposed head to move freely without subjecting the body to the injurious force. This chamber is separated from the left-hand chamber by a Mylar membrane designed to rupture reproducibly at a specific pressure of pounds per square inch, which depends on the thickness of the membrane. Pressurized air is pumped into the left side until the membrane ruptures, delivering the multimodal injury to the mouse’s head. Since most TBIs in people involve more than one mode of injury, the combinatorial nature of this model may be uniquely relevant to the human condition. For example, several parameters that are often aberrant in human TBI patients are similarly and consistently altered in this TBI model, including but not limited to early axonal degeneration that impairs normal synaptic transmission and ultimately leads to neuronal cell death, acute and chronic cognitive, motor, and visual impairments, BBB deterioration and dysfunction, chronic neuroinflammation, and systemic metabolic changes in the peripheral blood [43, 44, 108–113].
Fig. 3.
Schematic diagram of mmTBI apparatus, as described in the text
In this model of multimodal TBI, transient treatment with P7C3-S243 prevented neurocognitive decline, as measured by Barnes maze 2 weeks post injury. Interestingly, treatment was effective even when initiated 24 h after injury, which likely relates to the slower neurodegenerative process initiated by this model as compared to more aggressive models, such as fluid percussion injury [110]. These findings on memory retention also correlated with TBI-mediated decline in long-term potentiation (LTP) and paired-pulse facilitation, as measured using electrophysiological slice recordings, and both measures were rescued by P7C3-S243 treatment [110]. This showed that treatment with P7C3 compounds preserves normal synaptic function after TBI. Motor impairment on the foot-slip assay 1 month after injury was also prevented by P7C3-S243 treatment after TBI [110].
This same model was more recently studied at a very late chronic stage after TBI, 17 months post injury when mice continue to show neurocognitive impairment in the Morris water maze. At this very chronic time point, TBI animals continued to display significant axonal degeneration, as measured using silver staining [109, 110]. Notably, axonal degeneration at 2 weeks post injury was not accompanied by loss of neuronal cell bodies [110]. At the chronic 17-month time point, however, axonal degeneration across the brain was associated with clear evidence of neuronal cell loss visualized by loss of NeuN staining. Remarkably, when treatment with P7C3-A20 was initiated 12 months post injury (the equivalent of many decades for a human) for 1 month, axonal degeneration was completely halted and memory function was fully restored, effectively reversing the chronic sequelae of TBI [109]. This protective effect persisted even 4 months after treatment with P7C3-A20 was halted. This was the first report of permanent reversal of chronic neurodegeneration and restoration of cognitive function over an entire year after TBI in mice and challenged the popular dogma that acute treatment of TBI is required to normalize brain function. Indeed, this work showed that even in the face of significant neuronal cell loss, normal cognitive function can be restored in the very chronic neurodegenerative state after TBI when the brain is restored to normal health by normalizing NAD+/NADH [109]. We note that in the same year, Henry et al. reported that microglial depletion by 1 week of treatment with the colony stimulating factor 1 receptor inhibitor Plexxikon 5622, initiated 1 month after controlled cortical impact TBI, reduced neuroinflammation, oxidative stress, and neurodegeneration in the brain and improved motor and cognitive function 11 weeks later [114]. Thus, there appear to be multiple opportunities for treating TBI at various time points across the life span that are beyond the very acute time point after injury.
P7C3 compounds have also been shown to protect regions outside of the brain after TBI as well. For example, at 4 months after injury, mmTBI is associated with significant retinal ganglion cell dysfunction and deterioration. Treatment with P7C3-S243 after injury, however, significantly improved retinal ganglion cell survival and function without affecting other parameters, such as intraocular pressure, highlighting the preferential activity of P7C3 compounds on damaged neurons [112]. Interestingly, transmission electron microscopy experiments revealed that significant myelin structural integrity was disrupted after TBI, and this was accompanied by membrane disorganization of axonal mitochondria. These were also preventable with P7C3-S243 treatment [110]. These findings implicate a role for P7C3-mediated protection of mitochondria and oligodendrocytes. Given that P7C3 was able to restore mitochondrial membrane potential [21], it is likely that mitochondrial integrity is an important component of P7C3-mediated neuroprotection through NAD+/NADH stabilization.
The multimodal model of TBI also displays a variable effect on BBB structure and function. For example, within 3–6 h after TBI, the BBB is impaired, but normal permeability is restored by 24 h [109, 110]. Although the brain appears to possess an intrinsic mechanism for restoring BBB function after acute injury, chronic studies in the mmTBI model have revealed that at 17 months, post-TBI brain capillaries show severe endothelial breakage, as well as leakage of immunoglobulin G (IgG) from the blood into the brain parenchyma [109]. Concordantly, brief P7C3-A20 treatment 1 year after TBI also permanently restored capillary length and pericyte coverage of capillaries, as well as endothelial cell integrity, 17 months post-TBI. These findings were accompanied by significant upregulation of microvascular brain endothelial cell tight-junction proteins by P7C3-A20 [109], suggesting an ability of P7C3 compounds to restore endothelial cell health. Given the high degree of dependence of microvascular endothelial cells of the brain on NAD+/NADH, this protection is consistent with our current understanding of how P7C3 compounds work. Further direct efficacy of P7C3 compounds on the BBB was also demonstrated by dose–response protection of cultured human brain microvascular endothelial cells by treatment with P7C3-A20 [107].
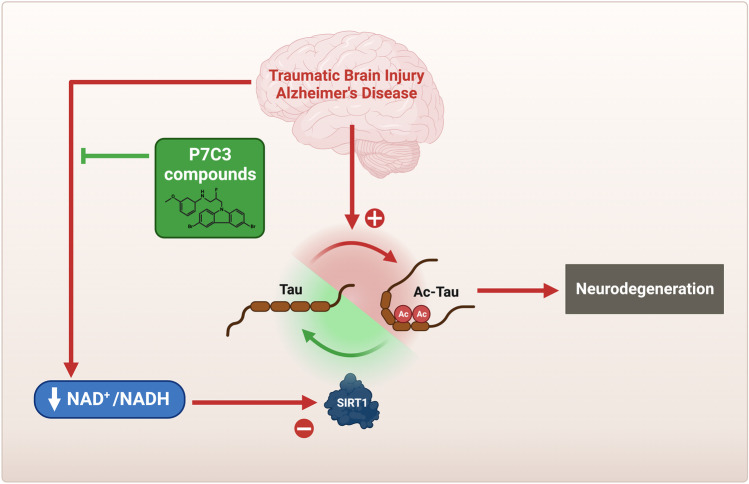
The P7C3 class of NAD+/NADH-stabilizing compounds has facilitated discovery of other aspects of new biology related to neurodegeneration after TBI as well. For example, work with P7C3 enabled the discovery that acetylated tau in the brain drives TBI-mediated axonal degeneration and provided a mechanism-based blood biomarker of TBI in animals and people (Fig. 4) [44]. After TBI, S-nitrosylation of GAPDH initiates a non-canonical signaling cascade that simultaneously activates p300/CBP acetyltransferase (to pathologically acetylate the tau protein) and inhibits NAD+-dependent sirtuin 1 (Sirt1), which normally removes acetyl groups from tau. This inhibition of Sirt1 is achieved by two routes: (1) direct posttranslational modification of Sirt1 to inhibit its activity and (2) general lowering of the brain NAD+/NADH such that Sirt1 activity is lowered. WldS mice, which are significantly protected in the mmTBI model, show reduced acetylated tau levels after TBI, due to preservation of normal brain NAD+/NADH levels, and treatment with P7C3-A20 was shown to have the same effect that was blocked when NAD+ synthesis was experimentally prevented [44]. Increased acetylated tau levels have also been found in people with AD and are even further increased in AD subjects with a history of TBI [44]. Moreover, reducing tau acetylation by other means, such as with a monoclonal antibody, reduces pathological and neurocognitive decline in tauopathy models [115]. Given the protective efficacy of P7C3 compounds in both TBI and AD models, and the striking clinical connection between the two whereby TBI accelerates the onset and severity of AD, P7C3 compounds are proving to be an effective tool for dissecting the mechanisms that link these degenerative disorders together.
Fig. 4.
As described in the text, tau acetylation in traumatic brain injury (TBI) and Alzheimer’s disease (AD) is driven by pathological S-nitrosylation of GAPDH, which triggers axonal degeneration, neuronal cell death, and neuropsychiatric impairment. The NAD+-dependent sirtuin 1 (Sirt1) enzyme mitigates this dangerous accumulation of toxic acetylated tau and thereby helps protect the brain. As brain NAD+/NADH declines in TBI and AD, the ability of Sirt1 to mediate this protective effect is diminished. Treatment with P7C3 compounds preserves the protective efficacy of Sirt1 by normalizing brain NAD+/NADH
Concluding Remarks
With the existing evidence, P7C3 is recognized to stabilize, but not abnormally elevate, NAD+/NADH after TBI and in disease. Work with P7C3 compounds has shown that normalizing NAD+/NADH under conditions of injury or disease in which it is otherwise depleted not only prevents new damage after TBI but also allows the brain to recover from chronic damage. When able to maintain normal NAD+/NADH homeostasis to address the extensive damage inflicted by injury, the brain can effectively combat chronic neurodegeneration and related symptoms. More investigation will be necessary to understand the related downstream mechanisms by which this recovery is achieved. Additionally, it has been well established that TBI can not only increase the risk of developing AD but can also accelerate pathology in patients predisposed to developing AD [12]. Since P7C3 treatment reduces acetylated tau levels, which is a point of pathologic convergence between TBI and AD, an important course of future investigation will be to determine whether P7C3 compounds (or other strategies to reduce accumulation of acetylated tau) can prevent TBI-mediated acceleration of AD.
Although P7C3 was originally discovered in a target-agnostic manner to promote the net magnitude of hippocampal neurogenesis, subsequent investigations revealed that P7C3-mediated neuroprotection acts on several pathogenic pathways after TBI, as well as other models of neurodegeneration and peripheral disease. Modern medicine and science have allowed us to study molecular and cellular pathways at such a granular level that much of the field has collectively shifted focus to pursue only specific mechanisms and well-defined targets in pursuit of drug discovery. However, this is not historically how many impactful medicines have been identified.
For example, metformin was originally found as a traditional herbal treatment from medieval Europe, used to treat symptoms of frequent thirst and urination [116]. Today, we know that those were likely symptoms of diabetes, and metformin was later re-discovered and implemented in modern medicine for its glucose-lowering properties. To this day, we are still discovering new targets, mechanisms, and properties for metformin, and it is being investigated for uses in polycystic ovarian syndrome, neurodegeneration, and cancers [116]. As another example, aspirin was used for thousands of years to alleviate pain without knowing its specific target or mechanism of action. In the 1940s, though the mechanism was still unknown at the time, it was discovered that aspirin also possessed anticoagulant properties, and it became a staple for prophylactic treatment of cardiovascular disease [117]. Equally impressive is Gerhard Domagk, who tested hundreds of chemical compounds in animals for phenotypic resistance to bacterial infection, even when some of those chemicals were ineffective against bacteria in a dish, because he reasoned that there were likely to be currently unidentified immune mechanisms in the animal that could be favorably manipulated. Through this effort, Domagk discovered prontosil, the precursor to the sulfonamide class of antibiotics [118]. Furthermore, the discovery of tamoxifen (a breast cancer treatment), artemisinin (an anti-malarial drug), and lumacaftor (a cystic fibrosis treatment) provides additional examples of groundbreaking medical discoveries made using phenotypic drug screens [118], without prior knowledge or bias about mechanism of action or molecular target.
Target-agnostic drug discovery has also been instrumental in the identification of antidepressants, for which the mechanism of action is still not fully understood [119]. For example, imipramine, the first antidepressant medication, was discovered in 1957 by the Swiss psychiatrist Roland Kuhn through a phenotypic screen for antipsychotic agents in patients with schizophrenia when he noticed that this test compound made them hypomanic, despite not resolving their psychotic symptoms [120, 121]. Similarly, the mood-stabilizing efficacy of lithium was first tested and established in patients with bipolar disorder in 1949 after the Australian psychiatrist John Cade observed that it reduced toxicity in guinea pigs that had been abdominally injected with the urine from patients with mania [122]. Today, lithium remains remarkably effective and the standard treatment for patients with bipolar disorder, even though we still do not understand how it works.
When the disorder in question is as mechanistically complex as the multiple forms of neuropsychiatric disease facing us today, it can be valuable to complement traditional target-directed approaches to drug discovery with alternative top-down strategies focused solely on safely achieving biological efficacy in living models of disease, regardless of how that beneficial effect may be achieved. Considering the promising results garnered by the P7C3 series of compounds thus far in addressing neurodegenerative sequelae of a complex and nebulous disorder such as TBI, the target-agnostic approach to drug discovery will likely prove fruitful going forward in identifying new therapeutic compounds for other medical conditions as well. Despite modern advances, the field of medical science continues to rely in large part on empirical discovery to fill gaps in our understanding. As illustrated above, in addition to possible new therapeutic agents, tool compounds generated through phenotypic screens can also lead to discovery of new biological disease mechanisms that advance the field.
Funding
This work was supported by a grant to AAP from the Valour Foundation and a grant to AAP from the AHA/Allen Initiative in Brain Health and Cognitive Impairment (19PABH134580006). AAP was also supported as the Rebecca E. Barchas, MD, Professor in Translational Psychiatry of Case Western Reserve University and as the Morley-Mather Chair in Neuropsychiatry of University Hospitals of Cleveland Medical Center. EM was supported by NIH T32 AG071474. PSS was supported by NIH T32 GM007250 and NIA F30 AG076183.
Declarations
Conflict of Interest
None.
Disclaimer
One of the authors (AAP) holds patents related to P7C3 compounds.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019;130:1080–1097. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 2.Magnuson J, Leonessa F, Ling GSF. Neuropathology of explosive blast traumatic brain injury. Curr Neurol Neurosci [Internet]. 2012;12:570--579. Available from: https://link.springer.com/article/10.1007/s11910-012-0303-6. [DOI] [PubMed]
- 3.Bogner J, Corrigan JD, Yi H, Singichetti B, Manchester K, Huang L, et al. Lifetime history of traumatic brain injury and behavioral health problems in a population-based sample. J Head Trauma Rehab. 2020;35:E43–50. doi: 10.1097/HTR.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 4.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehab. 2013;94:1199–1201. doi: 10.1016/j.apmr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehab. 2014;95:986–995.e1. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haarbauer-Krupa J, Pugh MJ, Prager EM, Harmon N, Wolfe J, Yaffe K. Epidemiology of chronic effects of traumatic brain injury. J Neurotraum. 2021;38:3235–3247. doi: 10.1089/neu.2021.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. Jama Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66:75–80. doi: 10.1016/j.mcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotraum [Internet]. 2010;27:1529--1540. Available from: https://www.liebertpub.com/doi/full/10.1089/neu.2010.1358. [DOI] [PubMed]
- 10.Gilbert M, Snyder C, Corcoran C, Norton MC, Lyketsos CG, Tschanz JT. The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer’s disease: the Cache County Dementia Progression Study*. Int Psychogeriatr. 2014;26:1593–1601. doi: 10.1017/S1041610214000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santopietro J, Yeomans JA, Niemeier JP, White JK, Coughlin CM. Traumatic brain injury and behavioral health: the state of treatment and policy. N C Med J. 2015;76:96–100. doi: 10.18043/ncm.76.2.96. [DOI] [PubMed] [Google Scholar]
- 12.Barker S, Paul BD, Pieper AA. Increased risk of aging-related neurodegenerative disease after traumatic brain injury. Biomedicines. 2023;11:1154. doi: 10.3390/biomedicines11041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marklund N, Bellander B-M, Godbolt AK, Levin H, McCrory P, Thelin EP. Treatments and rehabilitation in the acute and chronic state of traumatic brain injury. J Intern Med. 2019;285:608–623. doi: 10.1111/joim.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin N Am. 2017;97:1015–1030. doi: 10.1016/j.suc.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyfroidt G, Bouzat P, Casaer MP, Chesnut R, Hamada SR, Helbok R, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med. 2022;48:649–666. doi: 10.1007/s00134-022-06702-4. [DOI] [PubMed] [Google Scholar]
- 16.Redell JB, Maynard ME, Underwood EL, Vita SM, Dash PK, Kobori N. Traumatic brain injury and hippocampal neurogenesis: functional implications. Exp Neurol. 2020;331:113372. doi: 10.1016/j.expneurol.2020.113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 19.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 21.Pieper AA, Xie S, Capota E, Estill S, i Jo, Zhong J, et al. Discovery of a pro-neurogenic, neuroprotective chemical. Cell [Internet]. 2010;142:39--51. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2930815/. [DOI] [PMC free article] [PubMed]
- 22.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 23.Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotraum. 2014;31:476–486. doi: 10.1089/neu.2013.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Ishrat S, O’Bryan M, Klein B, Saraswati M, Robertson C, et al. Pediatric traumatic brain injury causes long-term deficits in adult hippocampal neurogenesis and cognition. J Neurotraum. 2020;37:1656–1667. doi: 10.1089/neu.2019.6894. [DOI] [PubMed] [Google Scholar]
- 26.Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, et al. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;32:1912–1920. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaiss CA, Yu T-S, Zhang G, Chen J, Dimchev G, Parada LF, et al. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharfman HE, Hen R. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacMillan KS, Naidoo J, Liang J, Melito L, Williams NS, Morlock L, et al. Development of proneurogenic, neuroprotective small molecules. J Am Chem Soc. 2011;133:1428–1437. doi: 10.1021/ja108211m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieper AA, McKnight SL, Ready JM. P7C3 and an unbiased approach to drug discovery for neurodegenerative diseases. Chem Soc Rev [Internet]. 2014;43:6716--6726. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4237066/. [DOI] [PMC free article] [PubMed]
- 31.Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, et al. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc National Acad Sci. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanco A, Pla R, Vogt D, Chen Y, Mandal S, Walker J, et al. NPAS1 represses the generation of specific subtypes of cortical interneurons. Neuron. 2014;84:940–953. doi: 10.1016/j.neuron.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickard BS, Pieper AA, Porteous DJ, Blackwood DH, Muir WJ. The NPAS3 gene—emerging evidence for a role in psychiatric illness. Ann Med. 2006;38:439–448. doi: 10.1080/07853890600946500. [DOI] [PubMed] [Google Scholar]
- 34.Michaelson JJ, Shin M-K, Koh J-Y, Brueggeman L, Zhang A, Katzman A, et al. Neuronal PAS domain proteins 1 and 3 are master regulators of neuropsychiatric risk genes. Biol Psychiat. 2017;82:213–223. doi: 10.1016/j.biopsych.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naidoo J, Bemben CJ, Allwein SR, Liang J, Pieper AA, Ready JM. Development of a scalable synthesis of P7C3-A20, a potent neuroprotective agent. Tetrahedron Lett. 2013;54:4429–4431. doi: 10.1016/j.tetlet.2013.06.024. [DOI] [Google Scholar]
- 36.Naidoo J, Jesus-Cortes HD, Huntington P, Estill S, Morlock LK, Starwalt R, et al. Discovery of a neuroprotective chemical, (S)-N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-6-methoxypyridin-2-amine [(−)-P7C3-S243], with improved druglike properties. J Med Chem. 2014;57:3746–3754. doi: 10.1021/jm401919s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauman MD, Schumann CM, Carlson EL, Taylor SL, Vázquez-Rosa E, Cintrón-Pérez CJ, et al. Neuroprotective efficacy of P7C3 compounds in primate hippocampus. Transl Psychiat. 2018;8:202. doi: 10.1038/s41398-018-0244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieper AA, McKnight SL. Benefits of enhancing nicotinamide adenine dinucleotide levels in damaged or diseased nerve cells. Cold Spring Harb Sym. 2018;83:207–217. doi: 10.1101/sqb.2018.83.037622. [DOI] [PubMed] [Google Scholar]
- 39.Satchell MA, Zhang X, Kochanek PM, Dixon CE, Jenkins LW, Melick J, et al. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+ depletion and ribosylation of 14-3-3γ. J Neurochem. 2003;85:697–708. doi: 10.1046/j.1471-4159.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 40.Clark RSB, Vagni VA, Nathaniel PD, Jenkins LW, Dixon CE, Szab C. Local administration of the poly(ADP-ribose) polymerase inhibitor INO-1001 prevents NAD depletion and improves water maze performance after traumatic brain injury in mice. J Neurotraum. 2007;24:1399–1405. doi: 10.1089/neu.2007.0305. [DOI] [PubMed] [Google Scholar]
- 41.Blaya MO, Wasserman JM, Pieper AA, Sick TJ, Bramlett HM, Dietrich WD. Neurotherapeutic capacity of P7C3 agents for the treatment of traumatic brain injury. Neuropharmacology. 2019;145:268–282. doi: 10.1016/j.neuropharm.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stovell MG, Mada MO, Helmy A, Carpenter TA, Thelin EP, Yan J-L, et al. The effect of succinate on brain NADH/NAD+ redox state and high energy phosphate metabolism in acute traumatic brain injury. Sci Rep-uk. 2018;8:11140. doi: 10.1038/s41598-018-29255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin TC, Voorhees JR, Genova RM, Davis KC, Madison AM, Britt JK, et al. Acute axonal degeneration drives development of cognitive, motor, and visual deficits after blast-mediated traumatic brain injury in mice. Eneuro. 2016;3:ENEURO.0220–16.2016. [DOI] [PMC free article] [PubMed]
- 44.Shin M-K, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K, Cintrón-Pérez CJ, et al. Reducing acetylated tau is neuroprotective in brain injury. Cell. 2021;184:2715–2732.e23. doi: 10.1016/j.cell.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henninger N, Bouley J, Sikoglu EM, An J, Moore CM, King JA, et al. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain. 2016;139:1094–1105. doi: 10.1093/brain/aww001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loris ZB, Pieper AA, Dietrich WD. The neuroprotective compound P7C3-A20 promotes neurogenesis and improves cognitive function after ischemic stroke. Exp Neurol. 2017;290:63–73. doi: 10.1016/j.expneurol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, et al. P7C3 Neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landry T, Huang H. Mini review: the relationship between energy status and adult hippocampal neurogenesis. Neurosci Lett. 2021;765:136261. doi: 10.1016/j.neulet.2021.136261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saharan S, Jhaveri DJ, Bartlett PF. SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus. J Neurosci Res. 2013;91:642–659. doi: 10.1002/jnr.23199. [DOI] [PubMed] [Google Scholar]
- 51.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Xu T, Li W, Miao C. Targeting nicotinamide phosphoribosyltransferase as a potential therapeutic strategy to restore adult neurogenesis. Cns Neurosci Ther. 2016;22:431–439. doi: 10.1111/cns.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okun E, Marton D, Cohen D, Griffioen K, Kanfi Y, Illouz T, et al. Sirt6 alters adult hippocampal neurogenesis. PLoS ONE. 2017;12:e0179681. doi: 10.1371/journal.pone.0179681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D, Chen N-N, Cao J-M, Sun W-P, Zhou Y-M, Li C-Y, et al. BRCA1 as a nicotinamide adenine dinucleotide (NAD)-dependent metabolic switch in ovarian cancer. Cell Cycle. 2014;13:2564–2571. doi: 10.4161/15384101.2015.942208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol. 2018;8:622. doi: 10.3389/fonc.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maric T, Bazhin A, Khodakivskyi P, Mikhaylov G, Solodnikova E, Yevtodiyenko A, et al. A bioluminescent-based probe for in vivo non-invasive monitoring of nicotinamide riboside uptake reveals a link between metastasis and NAD+ metabolism. Biosens Bioelectron. 2023;220:114826. doi: 10.1016/j.bios.2022.114826. [DOI] [PubMed] [Google Scholar]
- 57.Stoica BA, Loane DJ, Zhao Z, Kabadi SV, Hanscom M, Byrnes KR, et al. PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J Neurotrauma. 2014;31:758–772. doi: 10.1089/neu.2013.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whalen MJ, Clark RSB, Dixon CE, Robichaud P, Marion DW, Vagni V, et al. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 1999;19:835–842. doi: 10.1097/00004647-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 59.LaPlaca MC, Zhang J, Raghupathi R, Li J-H, Smith F, Bareyre FM, et al. Pharmacologic inhibition of poly(ADP-Ribose) polymerase is neuroprotective following traumatic brain injury in rats. J Neurotrauma. 2001;18:369–376. doi: 10.1089/089771501750170912. [DOI] [PubMed] [Google Scholar]
- 60.Lai Y, Chen Y, Watkins SC, Nathaniel PD, Guo F, Kochanek PM, et al. Identification of poly-ADP-ribosylated mitochondrial proteins after traumatic brain injury. J Neurochem. 2008;104:1700–1711. doi: 10.1111/j.1471-4159.2007.05114.x. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi K, Pieper AA, Croul SE, Zhang J, Snyder SH, Greenberg JH. Post-treatment with an inhibitor of poly(ADP-ribose) polymerase attenuates cerebral damage in focal ischemia. Brain Res. 1999;829:46–54. doi: 10.1016/S0006-8993(99)01335-9. [DOI] [PubMed] [Google Scholar]
- 62.Pieper AA, Verma A, Zhang J, Snyder SH. Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci. 1999;20:171–181. doi: 10.1016/S0165-6147(99)01292-4. [DOI] [PubMed] [Google Scholar]
- 63.LaPlaca MC, Raghupathi R, Verma A, Pieper AA, Saatman KE, Snyder SH, et al. Temporal patterns of poly(ADP-Ribose) polymerase activation in the cortex following experimental brain injury in the rat. J Neurochem. 1999;73:205–213. doi: 10.1046/j.1471-4159.1999.0730205.x. [DOI] [PubMed] [Google Scholar]
- 64.Pieper AA, Blackshaw S, Clements EE, Brat DJ, Krug DK, White AJ, et al. Poly(ADP-ribosyl)ation basally activated by DNA strand breaks reflects glutamate–nitric oxide neurotransmission. Proc Natl Acad Sci. 2000;97:1845–1850. doi: 10.1073/pnas.97.4.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marion CM, McDaniel DP, Armstrong RC. Sarm1 deletion reduces axon damage, demyelination, and white matter atrophy after experimental traumatic brain injury. Exp Neurol. 2019;321:113040. doi: 10.1016/j.expneurol.2019.113040. [DOI] [PubMed] [Google Scholar]
- 66.Bradshaw DV, Knutsen AK, Korotcov A, Sullivan GM, Radomski KL, Dardzinski BJ, et al. Genetic inactivation of SARM1 axon degeneration pathway improves outcome trajectory after experimental traumatic brain injury based on pathological, radiological, and functional measures. Acta Neuropathol Commun. 2021;9:89. doi: 10.1186/s40478-021-01193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maynard ME, Redell JB, Zhao J, Hood KN, Vita SM, Kobori N, et al. Sarm1 loss reduces axonal damage and improves cognitive outcome after repetitive mild closed head injury. Exp Neurol. 2020;327:113207. doi: 10.1016/j.expneurol.2020.113207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexandris AS, Lee Y, Lehar M, Alam Z, Samineni P, Tripathi SJ, et al. Traumatic axonopathy in spinal tracts after impact acceleration head injury: ultrastructural observations and evidence of SARM1-dependent axonal degeneration. Exp Neurol. 2023;359:114252. doi: 10.1016/j.expneurol.2022.114252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill CS, Menon DK, Coleman MP. P7C3-A20 neuroprotection is independent of Wallerian degeneration in primary neuronal culture. NeuroReport. 2018;29:1544–1549. doi: 10.1097/WNR.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jesús-Cortés HD, Xu P, Drawbridge J, Estill S, i Jo, Huntington P, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. P Natl Acad Sci Usa [Internet]. 2012;109:17010--17015. Available from: http://www.pnas.org/content/109/42/17010. [DOI] [PMC free article] [PubMed]
- 71.Jesús-Cortés HD, Miller AD, Britt JK, DeMarco AJ, Jesús-Cortés MD, Stuebing E, et al. Protective efficacy of P7C3-S243 in the 6-hydroxydopamine model of Parkinson’s disease. Npj Park Dis. 2015;1:15010. doi: 10.1038/npjparkd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu C, Hu Q, Wu J, Mu C, Ren H, Liu C-F, et al. P7C3 inhibits LPS-induced microglial activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Front Cell Neurosci. 2018;12:400. doi: 10.3389/fncel.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu C, Zhang Y, Hu Q, Wu J, Ren H, Liu C-F, et al. P7C3 inhibits GSK3β activation to protect dopaminergic neurons against neurotoxin-induced cell death in vitro and in vivo. Cell Death Dis. 2017;8:e2858–e2858. doi: 10.1038/cddis.2017.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duan F-X, Shi Y-J, Chen J, Ding S-Q, Wang F-C, Tang J, et al. Neuroprotective effects of P7C3 against spinal cord injury in rats. Exp Biol Med. 2019;244:1680–1687. doi: 10.1177/1535370219888620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Fan Y, Zhang H, Zhang Q, Zhao Y, Xiao Z, et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials. 2021;269:120479. doi: 10.1016/j.biomaterials.2020.120479. [DOI] [PubMed] [Google Scholar]
- 76.Tesla R, Wolf HP, Xu P, Drawbridge J, Estill S, i Jo, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc National Acad Sci [Internet]. 2012;109:17016--17021. Available from: http://www.pnas.org/content/109/42/17016. [DOI] [PMC free article] [PubMed]
- 77.Voorhees JR, Remy MT, Cintrón-Pérez CJ, Rassi EE, Khan MZ, Dutca LM, et al. (−)-P7C3-S243 protects a rat model of Alzheimer’s disease from neuropsychiatric deficits and neurodegeneration without altering amyloid deposition or reactive glia. Biol Psychiat. 2018;84:488–498. doi: 10.1016/j.biopsych.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Zhang Y, Zhang W-F, Xiao D, Ciric B, Rostami A, et al. P7C3 attenuates CNS autoimmunity by inhibiting Th17 cell differentiation. Cell Mol Immunol. 2021;18:1565–1567. doi: 10.1038/s41423-020-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kemp SWP, Szynkaruk M, Stanoulis KN, Wood MD, Liu EH, Willand MP, et al. Pharmacologic rescue of motor and sensory function by the neuroprotective compound P7C3 following neonatal nerve injury. Neuroscience. 2015;284:202–216. doi: 10.1016/j.neuroscience.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 80.LoCoco PM, Risinger AL, Smith HR, Chavera TS, Berg KA, Clarke WP. Pharmacological augmentation of nicotinamide phosphoribosyltransferase (NAMPT) protects against paclitaxel-induced peripheral neuropathy. Elife. 2017;6:e29626. doi: 10.7554/eLife.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oku H, Morishita S, Horie T, Nishikawa Y, Kida T, Mimura M, et al. Protective effect of P7C3 on retinal ganglion cells from optic nerve injury. Jpn J Ophthalmol. 2017;61:195–203. doi: 10.1007/s10384-016-0493-6. [DOI] [PubMed] [Google Scholar]
- 82.Oku H, Morishita S, Horie T, Kida T, Mimura M, Kojima S, et al. P7C3 suppresses neuroinflammation and protects retinal ganglion cells of rats from optic nerve crush. Investigative Opthalmology Vis Sci. 2017;58:4877. doi: 10.1167/iovs.17-22179. [DOI] [PubMed] [Google Scholar]
- 83.Loris ZB, Hynton JR, Pieper AA, Dietrich WD. Beneficial effects of delayed P7C3-A20 treatment after transient MCAO in rats. Transl Stroke Res. 2018;9:146–156. doi: 10.1007/s12975-017-0565-z. [DOI] [PubMed] [Google Scholar]
- 84.Bai J, Zeng S, Zhu J, Fu C, He M, Zhu J, et al. The small molecule P7C3-A20 exerts neuroprotective effects in a hypoxic–ischemic encephalopathy model via activation of PI3K/AKT/GSK3β signaling. Neuroscience. 2020;441:197–208. doi: 10.1016/j.neuroscience.2020.05.051. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y-H, Liou K-T, Tsai K-C, Liu H-K, Yang L-M, Chern C-M, et al. GSK-3 inhibition through GLP-1R allosteric activation mediates the neurogenesis promoting effect of P7C3 after cerebral ischemic/reperfusional injury in mice. Toxicol Appl Pharm. 2018;357:88–105. doi: 10.1016/j.taap.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 86.Wang S, Wang Z, Wang X, Zhang X, Xu T, Miao C. Humanized cerebral organoids-based ischemic stroke model for discovering of potential anti-stroke agents. Acta Pharmacol Sin. 2023;44:513–523. doi: 10.1038/s41401-022-00986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S, Xu T, Wang X, Guan Y, Zhang S, Wang P, et al. Neuroprotective efficacy of an aminopropyl carbazole derivative P7C3-A20 in ischemic stroke. Cns Neurosci Ther. 2016;22:782–788. doi: 10.1111/cns.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen W, Jia W, Wu C, Chen L, Sun K, Wang J, et al. The neurogenic compound P7C3 regulates the aerobic glycolysis by targeting phosphoglycerate kinase 1 in glioma. Frontiers Oncol. 2021;11:644492. doi: 10.3389/fonc.2021.644492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rahman MT, Bailey EM, Gansemer BM, Pieper AA, Manak JR, Green SH. Anti-inflammatory therapy protects spiral ganglion neurons after aminoglycoside antibiotic-induced hair cell loss. Neurotherapeutics. 2023;1–24. [DOI] [PMC free article] [PubMed]
- 90.Latchney SE, Jaramillo TC, Rivera PD, Eisch AJ, Powell CM. Chronic P7C3 treatment restores hippocampal neurogenesis. Neurosci Lett. 2015;591:86–92. doi: 10.1016/j.neulet.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walker AK, Rivera PD, Wang Q, Chuang J-C, Tran S, Osborne-Lawrence S, et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatr [Internet]. 2015;20:500--508. Available from: https://www.nature.com/articles/mp201434. [DOI] [PMC free article] [PubMed]
- 92.Lee AS, Jesús-Cortés HD, Kabir ZD, Knobbe W, Orr M, Burgdorf C, et al. The neuropsychiatric disease-associated gene cacna1c mediates survival of young hippocampal neurons. Eneuro [Internet]. 2016;3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4819284/. [DOI] [PMC free article] [PubMed]
- 93.Jesús-Cortés HD, Rajadhyaksha AM, Pieper AA. Cacna1c: protecting young hippocampal neurons in the adult brain. Neurogenesis. 2016;3:e1231160. doi: 10.1080/23262133.2016.1231160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bavley CC, Kabir ZD, Walsh AP, Kosovsky M, Hackett J, Sun H, et al. Dopamine D1R-neuron cacna1c deficiency: a new model of extinction therapy-resistant post-traumatic stress. Mol Psychiatr. 2020;1–13. [DOI] [PMC free article] [PubMed]
- 95.Schroeder R, Sridharan P, Nguyen L, Loren A, Williams NS, Kettimuthu KP, et al. Maternal P7C3-A20 treatment protects offspring from neuropsychiatric sequelae of prenatal stress. Antioxid Redox Sign. 2021; [DOI] [PMC free article] [PubMed]
- 96.Schroeder R, Nguyen L, Pieper AA, Stevens HE. Maternal treatment with P7C3-A20 protects from impaired maternal care after chronic gestational stress. Behav Brain Res. 2022;416:113558. doi: 10.1016/j.bbr.2021.113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duman G, Alcigir ME, Yavuz H. Necroptosis mediated by receptor interacting protein kinase 3 as critical players in experimental congenital hypothyroidism related neuronal damage. North Clin Ýstanbul. 2021;8:472–478. doi: 10.14744/nci.2021.26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dogan HO, Alcigir ME. The Protective effect of P7C3 against DNA and neuron damage in rat pups with congenital hypothyroidism. Biomed Pharmacother. 2018;99:499–503. doi: 10.1016/j.biopha.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 99.Manickam R, Tur J, Badole SL, Chapalamadugu KC, Sinha P, Wang Z, et al. Nampt activator P7C3 ameliorates diabetes and improves skeletal muscle function modulating cell metabolism and lipid mediators. J Cachexia Sarcopenia Muscle. 2022;13:1177–1196. doi: 10.1002/jcsm.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tur J, Badole SL, Manickam R, Chapalamadugu KC, Xuan W, Guida W, et al. Cardioprotective effects of P7C3 in diabetic hearts via Nampt activation. J Pharmacol Exp Ther. 2022;382:JPET-AR-2022–001122. [DOI] [PMC free article] [PubMed]
- 101.Hua X, Sun D, Zhang W, Fu J, Tong J, Sun S, et al. P7C3-A20 alleviates fatty liver by shaping gut microbiota and inducing FGF21/FGF1, via the AMP-activated protein kinase/CREB regulated transcription coactivator 2 pathway. Brit J Pharmacol. 2021;178:2111–2130. doi: 10.1111/bph.15008. [DOI] [PubMed] [Google Scholar]
- 102.Zhang LQ, Nsumu M, Huang P, Heruth DP, Riordan SM, Shortt K, et al. Novel protective role of nicotinamide phosphoribosyltransferase in acetaminophen-induced acute liver injury in mice. Am J Pathology. 2018;188:1640–1652. doi: 10.1016/j.ajpath.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ryu SW, Kim YO, Kim H-B, Oh SB, Choi JI, Yoon MH. Antinociceptive effect of intrathecal P7C3 via GABA in a rat model of inflammatory pain. Eur J Pharmacol. 2021;899:174029. doi: 10.1016/j.ejphar.2021.174029. [DOI] [PubMed] [Google Scholar]
- 104.Li B, Shi Y, Liu M, Wu F, Hu X, Yu F, et al. Attenuates of NAD+ impair BMSC osteogenesis and fracture repair through OXPHOS. Stem Cell Res Ther. 2022;13:77. doi: 10.1186/s13287-022-02748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei F, Tuong ZK, Omer M, Ngo C, Asiatico J, Kinzel M, et al. A novel multifunctional radioprotective strategy using P7C3 as a countermeasure against ionizing radiation-induced bone loss. Bone Res. 2023;11:34. doi: 10.1038/s41413-023-00273-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuan Q, Li J, Du X, Zhang L, Meng L, Cheng K, et al. Nampt affects mitochondrial function in aged oocytes by mediating the downstream effector FoxO3a. J Cell Physiol. 2022;237:647–659. doi: 10.1002/jcp.30532. [DOI] [PubMed] [Google Scholar]
- 107.Stanley TD. Beyond publication bias J Econ Surv. 2005;19:309–345. doi: 10.1111/j.0950-0804.2005.00250.x. [DOI] [Google Scholar]
- 108.Shin M-K, Vázquez-Rosa E, Cintrón-Pérez CJ, Riegel WA, Harper MM, Ritzel D, et al. Characterization of the jet-flow overpressure model of traumatic brain injury in mice. Neurotrauma Reports. 2021;2:1–13. doi: 10.1089/neur.2020.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vázquez-Rosa E, Shin M-K, Dhar M, Chaubey K, Cintrón-Pérez CJ, Tang X, et al. P7C3-A20 treatment one year after TBI in mice repairs the blood–brain barrier, arrests chronic neurodegeneration, and restores cognition. Proc National Acad Sci. 2020;117:27667–27675. doi: 10.1073/pnas.2010430117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin TC, Britt JK, De Jesús-Cortés H, Lu Y, Genova RM, Khan MZ, et al. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Reports [Internet]. 2014;8:1731--1740. Available from: https://www.cell.com/cell-reports/abstract/S2211-1247(14)00706-2. [DOI] [PMC free article] [PubMed]
- 111.Vázquez-Rosa E, Watson MR, Sahn JJ, Hodges TR, Schroeder RE, Cintrón-Pérez CJ, et al. Neuroprotective efficacy of a sigma 2 receptor/TMEM97 modulator (DKR-1677) after traumatic brain injury. Acs Chem Neurosci. 2018;10:1595–1602. doi: 10.1021/acschemneuro.8b00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dutca LM, Stasheff SF, Hedberg-Buenz A, Rudd DS, Batra N, Blodi FR, et al. Early detection of subclinical visual damage after blast-mediated TBI enables prevention of chronic visual deficit by treatment with P7C3-S243. Invest Ophth Vis Sci. 2014;55:8330–8341. doi: 10.1167/iovs.14-15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wattiez A-S, Castonguay WC, Gaul OJ, Waite JS, Schmidt CM, Reis AS, et al. Different forms of traumatic brain injuries cause different tactile hypersensitivity profiles. Pain. 2020;162:1163–1175. doi: 10.1097/j.pain.0000000000002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, et al. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020;40:2960–2974. doi: 10.1523/JNEUROSCI.2402-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song H-L, Kim N-Y, Park J, Kim MI, Jeon Y-N, Lee S-J, et al. Monoclonal antibody Y01 prevents tauopathy progression induced by lysine280-acetylated tau in cell and mouse models. J Clin Investig. 2023;133. [DOI] [PMC free article] [PubMed]
- 116.Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 117.Montinari MR, Minelli S, Caterina RD. The first 3500 years of aspirin history from its roots – a concise summary. Vasc Pharmacol. 2019;113:1–8. doi: 10.1016/j.vph.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 118.Swinney DC. Phenotypic Drug Discovery. 2020;1–19.
- 119.Pereira VS, Hiroaki-Sato VA. A brief history of antidepressant drug development: from tricyclics to beyond ketamine. Acta Neuropsychiatr. 2018;30:307–322. doi: 10.1017/neu.2017.39. [DOI] [PubMed] [Google Scholar]
- 120.Kuhn R. Treatment of depressive states with an iminodibenzyl derivative (G 22355) Schweiz Med Wochenschr. 1957;87:1135–1140. [PubMed] [Google Scholar]
- 121.Brown WA, Rosdolsky M. The clinical discovery of imipramine. Am J Psychiatry. 2015;172:426–429. doi: 10.1176/appi.ajp.2015.14101336. [DOI] [PubMed] [Google Scholar]
- 122.Cade JFJ. Lithium salts in the treatment of psychotic excitement. Méd J Aust. 1949;2:349–352. doi: 10.5694/j.1326-5377.1949.tb36912.x. [DOI] [PubMed] [Google Scholar]