Abstract
A DNA locus from Bordetella pertussis capable of reconstituting lipopolysaccharide (LPS) O-antigen biosynthesis in Salmonella typhimurium SL3789 (rfaF511) has been isolated, by using selection with the antibiotic novobiocin. DNA within the locus encodes a protein with amino acid sequence similarity to heptosyltransferase II, encoded by waaF (previously rfaF) in other gram-negative bacteria. Mutation of this gene in B. pertussis, Bordetella parapertussis, and Bordetella bronchiseptica by allelic exchange generated bacteria with deep rough LPS phenotypes consistent with the proposed function of the gene as an inner core heptosyltransferase. These are the first LPS mutants generated in B. parapertussis and B. bronchiseptica and the first deep rough mutants of any of the bordetellae.
Bordetella pertussis is a gram-negative pathogen causing whooping cough in children and increasingly being implicated in respiratory infections in adults (9, 21, 24, 27). Bordetella parapertussis is also recognized as a cause of whooping cough in children (14, 47) and also infects ovine species (10, 29–31, 46). Bordetella bronchiseptica has only rarely been associated with human disease (13, 34, 42) and is more commonly known as a pathogen of a range of species, including rabbits, pigs, dogs, and cats, among others (1, 5, 17, 23, 35, 43, 44, 49). In the search for improved modern vaccines directed against B. pertussis, a large body of work has been generated regarding protein virulence factors and targets for protective immunity (8, 32). This has led to a relative lack of research into the lipopolysaccharide (LPS) molecule found on the bordetellae, which is highly biologically active as an endotoxin, an immunomodulator, and an antigen (3, 7, 48). It is probable that this molecule plays a role in the infection process, a role overlooked for the want of molecular genetic analysis and appropriate animal models.
B. pertussis LPS appears to have the simplest structure of those of the three bordetellae considered in this paper. It consists of a lipid A molecule linked via a single ketodeoxyoctulosonic acid (Kdo) residue to a branched oligosaccharide core structure, containing heptose, glucose, glucuronic acid, glucosamine, and galactosaminuronic acid (6, 18, 19). This structure may be identified on a silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel as band B LPS (25). Linked to this core structure is a trisaccharide consisting of N-acetyl-N-methyl-fucosamine (FucNAcMe), 2,3-dideoxy-di-N-acetylmannosaminuronic acid (2,3-diNAcManA), and N-acetylglucosamine (GlcNAc). The complete band B LPS plus this trisaccharide forms band A LPS on SDS-PAGE gels (25). B. bronchiseptica LPS also has band B and band A LPS, but in addition, it synthesizes an O-antigen structure consisting of a polymer of the single sugar residue 2,3-dideoxy-di-N-acetyl-galactosaminuronic acid (12). B. parapertussis LPS is somewhat different from either of the other two molecules described. It lacks band A and has a truncated band B, the structure of which has not been published. It does, however, have an O antigen apparently consisting of the same sugar polymer as in the B. bronchiseptica O antigen (12).
The genetics and molecular biology of LPS biosynthesis in the bordetellae have only recently been studied. The wlb locus (previously called bpl) (2, 33) required for the biosynthesis of band A LPS in B. pertussis has been cloned and sequenced, and mutations have been introduced into genes within the locus with consequent loss of band A structures (2). These mutations affect only a distal structure on the LPS and leave the rest of the molecule intact. To study the role of LPS in pathogenicity and immunity, bordetellae with LPS molecules with the deepest possible rough phenotype would be desirable. The deepest rough LPS mutants of Salmonella and Escherichia coli result from lesions in the waaC (rfaC) gene (22), which encodes the glycosyltransferase responsible for the addition of the first heptose residue to Kdo (38). B. pertussis waaC has been identified, but attempts to mutate this gene have been unsuccessful, probably because waaC is immediately upstream of the waaA (previously kdtA) gene (2), which is essential for cell viability. The gene responsible for the next step in enterobacterial LPS biosynthesis is waaF (rfaF) (39). Consequently, we report here the identification, cloning, and sequencing of a DNA locus containing a candidate for B. pertussis waaF and report the construction of deep rough mutants of B. pertussis, B. parapertussis, and B. bronchiseptica. These are the first mutations in these bacteria that result in a deep rough phenotype, and they are the first mutants of any kind constructed that affect LPS in B. parapertussis and B. bronchiseptica.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bordetellae used in this study were B. pertussis BP536, B. parapertussis CN 2591, and B. bronchiseptica CN 7635E from our culture collection. For cloning experiments and maintenance of plasmids, E. coli XL1-Blue (Stratagene) was used. E. coli HU835 was used to package cosmids in vivo. SM10λpir was used as the donor strain in conjugation experiments. Salmonella typhimurium SL3789 has a mutation in the waaF gene (rfaF511) and was a kind gift from Brian Robertson, St. Mary’s Hospital at Imperial College, London, United Kingdom. S. typhimurium AS68 was a r− m+ strain carrying the E. coli LamB protein, enabling it to be infected by λ phage particles.
All cloning and DNA sequencing experiments used the pT7-Blue or pBluescript II series of plasmids. The vector used in conjugation experiments for the generation of mutants in B. pertussis was pSS2141 (41), which has an s12 allele (rpsL) conferring dominant streptomycin sensitivity on streptomycin-resistant bacteria, allowing selection against maintenance of vector sequences via single-crossover events. pSS2141 is a ColE1 replicon and thus cannot replicate in B. pertussis. It contains an oriT mobilizable by E. coli SM10λpir.
Media, chemicals, and reagents.
B. pertussis was routinely cultured on Bordet-Gengou medium supplemented with 15% horse blood. E. coli was cultured on Luria broth or agar (36). Media were purchased from Difco Ltd. or Oxoid Ltd. Antibiotics were used where appropriate. For the bordetellae, gentamicin at 10 μg/ml, ampicillin at 100 μg/ml, and streptomycin at 200 μg/ml were used. For E. coli and S. typhimurium, ampicillin was used at 100 μg/ml. SL3789 with its waaF lesion complemented by the BP536 waaF gene was selected on novobiocin at 2.5 μg/ml. All antibiotics and routine chemicals were purchased from Sigma Chemical Company. Restriction and modifying enzymes were purchased from Boehringer Mannheim. DNA ligase was purchased from Gibco-BRL. Sequenase sequencing kits were purchased from Amersham International.
Cloning of LPS genes.
A cosmid library was constructed in the vector pHC79 (16) from BP536 chromosomal DNA partially digested with Sau3AI. Size selection of 35- to 45-kb DNA fragments was performed with a 0.8% low-melting-point agarose gel in pulsed-field gel electrophoresis. This DNA was purified from the gel with agarase, then ligated with pHC79, and packaged with Gigapack Gold III packaging mixes (Stratagene). These packaged cosmids were transfected into E. coli XL1-Blue, and 1,000 resultant colonies were maintained as a representative library. The packaged library was also amplified with the in vivo packaging strain E. coli HU835. Before using purified cosmids to infect the S. typhimurium SL3789 waaF selection strain, the cosmids were used to infect S. typhimurium AS68 so that the cosmid DNA became modified for S. typhimurium restriction systems, thus ensuring high efficiency and representative transformation of the library into the selection strain. Selection for AS68-carrying cosmids was with ampicillin. Cosmid DNA from a pool of 4,000 resultant ampicillin-resistant colonies was isolated and used to electroporate SL3789 (0.1-cm cuvette; 1,750 V, 25 μF, and 600 Ω). Resultant colonies were selected for complementation of the waaF mutation in SL3789 by selection on novobiocin and ampicillin. Analysis of the LPS from the resultant colonies was performed by silver-stained SDS-PAGE and agglutination experiments with anti-O4,5 antiserum (Murex Diagnostics, Dartford, United Kingdom).
LPS preparation and SDS-PAGE.
LPS was purified by a modification of the method of Hitchcock and Brown (15). Briefly, B. pertussis was grown on Bordet-Gengou plates for 2 to 3 days and then harvested into phosphate-buffered saline. S. typhimurium was grown in the appropriate antibiotics in Luria broth, and bacteria were pelleted by centrifugation and resuspended in phosphate-buffered saline. Resuspended bacteria were lysed by addition of a one-third volume of lysis solution (0.2 M Tris-HCl [pH 6.8], 3% [wt/vol] SDS, 30% [vol/vol] glycerol), followed by incubation at 100°C for 30 min. After cooling, proteinase K was added to a final concentration of 0.1 mg/ml. This was incubated at 55°C for 60 min. Equal volumes of phenol were added and mixed. This was incubated at 68°C for 15 min for LPS extraction from wild-type bordetellae and salmonellae or at room temperature for 5 min for LPS extraction from mutant bordetellae and salmonellae. The LPS-containing aqueous phase was separated from the phenol phase by centrifugation. LPS was then precipitated by the addition of 0.1 volume of 3 M sodium acetate, pH 5.5, and 4 volumes of ethanol followed by centrifugation. Pelleted LPS was redissolved in lysis solution at one-third of its normal concentration, containing bromophenol blue, and boiled for 5 min prior to electrophoresis. SDS-PAGE gels were run in a Tricine buffer system according to the method of Lesse et al. (20). Silver staining was performed according to the method of Tsai and Frasch (45).
DNA sequencing.
Plasmid DNA was sequenced with Sequenase 2 and an ABI automated sequencer. Sequences were assembled and analyzed with the Genetics Computer Group (GCG) package (11) or the Staden programs (40) running on the Oxford University molecular biology VAX computer.
Southern hybridizations.
Southern hybridizations were performed according to the method of Sambrook et al. (36) with probes labelled with [32P]dCTP with a randomly primed labelling kit (Stratagene, Cambridge, United Kingdom).
Mutagenesis of the bordetellae.
For allelic replacement mutagenesis, waaF was insertionally inactivated in B. pertussis, B. parapertussis, and B. bronchiseptica by introduction of the vector pSS2141 into the gene (single-crossover mutagenesis). A 439-bp PCR product (bases 1134 to 1572 in the published sequence) generated by using as primers oligonucleotides BpwaaF1 (5′-CAACTGGCGGGCATCGACCGCC-3′) and BpwaaF2 (5′-GTCGTGGCACTACCTGACCC-3′), corresponding to the middle of the B. pertussis waaF gene, was amplified with Taq DNA polymerase (Applied Biosystems) and cloned into pT7-Blue. This fragment was then released with XbaI and PaeI and cloned into the equivalent sites in pSS2141 (41). This procedure was performed so that the rpsL gene was removed from the vector, allowing the use of streptomycin to select for transconjugants. Conjugation experiments were performed on Bordet-Gengou plates containing 15% horse blood and 10 mM MgCl2 with the appropriate bordetellae as recipients and E. coli SM10λpir containing the recombinant suicide vector pSS2141 carrying the 439-bp fragment as donor. Single crossovers were selected on gentamicin and ampicillin (resistance to both being encoded by the vector) and streptomycin (to which the recipient bordetellae are resistant). LPS from resultant colonies was purified and analyzed by silver-stained SDS-PAGE. Genomic DNAs from resultant colonies were also analyzed by Southern hybridization for the expected DNA rearrangements and confirmed to be single-crossover mutants with mutated waaF genes (data not shown).
Nucleotide sequence accession number.
The DNA sequence described here is deposited with the EMBL database under accession no. Y13475.
RESULTS AND DISCUSSION
Identification and cloning of waaF.
A cosmid library of B. pertussis BP536 DNA, constructed in the vector pHC79, was amplified and modified as described in Materials and Methods. This cosmid DNA was isolated and used to electroporate S. typhimurium SL3789(rfaF511), which has a deep rough LPS phenotype. Complementation of this genetic lesion would enable the bacteria to synthesize complete, smooth LPS. To select for complementation by recombinant cosmids, bacteria were plated on media containing novobiocin, since this antibiotic selectively kills rough bacteria at much lower concentrations than are needed to kill smooth bacteria (4). Transformants and controls consisting of wild-type S. typhimurium SL3770 (positive) and SL3789 alone (negative) were selected on various concentrations of novobiocin with or without ampicillin. SL3770, being smooth, was capable of growth on novobiocin at 2.5 μg/ml, whereas SL3789 was sensitive to this concentration as a consequence of having rough LPS. Electroporation of SL3789 with the cosmid library produced four colonies resistant to both ampicillin and novobiocin at 2.5 μg/ml. LPS was purified from one of these and analyzed by silver-stained SDS-PAGE, confirming the restoration of the O-antigen phenotype (Fig. 1). The complemented bacteria were also agglutinable with anti-O4,5 antiserum. These data indicate the presence of a functional waaF homolog within the locus. The fact that the deep rough LPS molecule from the S. typhimurium waaF mutant is efficiently restored to the wild-type phenotype by the B. pertussis waaF homolog shows that the bordetella protein can recognize the S. typhimurium waaF mutant LPS as a substrate. This might not be immediately expected, as the inner core structures of Salmonella and Bordetella are different in a number of respects (6, 7, 18, 19, 22). For example, two Kdo residues are present between lipid A and the first heptose in the S. typhimurium core, whereas in the equivalent region of the B. pertussis LPS molecule, only one Kdo residue is observed. This difference does not seem to interfere with the correct functioning of the bordetella enzyme.
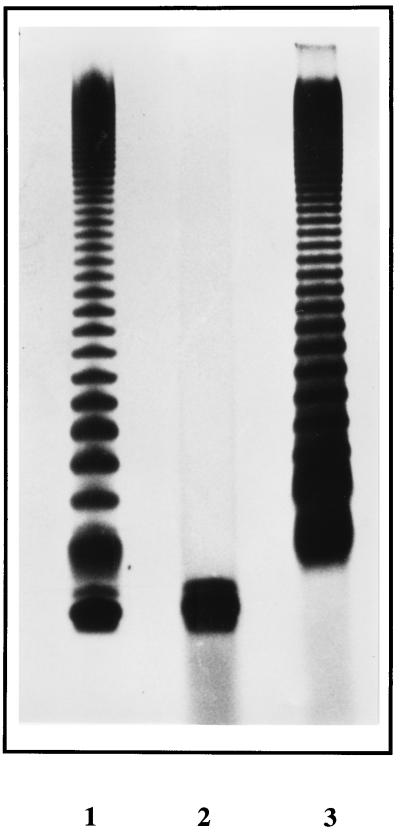
FIG. 1.
Silver-stained Tris-tricine polyacrylamide gel of S. typhimurium LPS isolated from wild type, waaF mutant, and waaF mutant complemented with B. pertussis waaF. Lane 1, S. typhimurium waaF mutant (SL3789) complemented by the BP536 waaF gene; lane 2, SL3789 alone; lane 3, S. typhimurium wild type (SL3770). The waaF mutant displays the deep rough LPS phenotype expected, while the wild-type control shows the ladder pattern expected for a full-length smooth LPS. The complemented mutant also has the O-antigen ladder, but a rough core molecule is also observed in the LPS preparation, suggesting that the complementation is not completely effective.
The cosmid DNA isolated from these four transformants was digested with NarI, revealing several common fragments between the cosmids. One cosmid was partially digested with NarI, then self-ligated, and electroporated into SL3789 with selection again on novobiocin and ampicillin. Plasmids from resultant colonies, when digested with NarI, revealed a minimum insert size of approximately 3.5 kb, with three insert bands in common. These three NarI fragments were separately cloned into pBluescript and sequenced. Analysis of the derived amino acid sequence from a 1-kb NarI fragment revealed an open reading frame (ORF) with similarity to waaF from several bacteria. This NarI fragment was used to reprobe a representative cosmid library, identifying two cosmids (cos4g2 and cos5e6). Restriction enzyme and Southern blot analysis of these showed that they were nearly identical. Several restriction fragments and oligonucleotide primers were used to sequence a 2,258-bp region of the DNA containing waaF. Within the sequenced DNA were three ORFs. Starting from the SacI site which marked the limit of our sequence, a partial ORF was observed, pointing leftward. The proposed start codon for this protein, based on analysis of codon usage in the Staden sequence analysis package and on homology searching, is a TTG codon at position 630. On translation, this ORF has 29% identity and 58% similarity at the amino acid level with the protein encoded by E. coli msbA (Fig. 2), which has recently been proposed to be involved in transport of LPS across cell membranes (28). After a short intergenic region of 113 bp, the next ORF, pointing to the right, starting at an ATG codon at position 742, and extending for 966 bp, encodes a protein with homology to sequences from a number of bacteria corresponding to the ADP-heptose:LPS heptosyltransferase II (encoded by waaF) (Fig. 3). Comparison of the B. pertussis deduced amino acid sequence with that of these proteins demonstrated that the shorter B. pertussis protein does not possess some of the motifs typically associated with these proteins. This is reflected in the lower percentage similarities compared to other WaaF homologs. For example, S. typhimurium and Neisseria gonorrhoeae have 46% amino acid identity and 60% similarity between their WaaF homologs while comparison of these two proteins with the B. pertussis homolog demonstrates 26% identity and 33% similarity and 23% identity and 28% similarity, respectively (26, 37, 39). Within the intergenic region, there are likely to be divergent promoters enabling the waaF and msbA genes to be expressed and suggesting a level of coregulation of core biosynthesis and LPS transport. This arrangement of waaF and msbA together has not been seen in other bacterial genera and raises questions regarding the regulation of LPS biosynthesis in the bordetellae. This is especially interesting given that we have previously observed that there seems to be a divergent promoter for the waaC-waaA operon (required for deep inner core structures) and the wlb locus (required for distal trisaccharide band A structures) (2). A consistent feature is that the genes transcribed from both these sets of divergent promoters are related to different stages of LPS biosynthesis, and this might indicate a role for coordinate regulation between the waaF and waaC-waaA loci in the biosynthesis of the inner core of the B. pertussis LPS molecule. Presumably, the transcription of these two loci must be closely harmonized if the efficient biosynthesis of LPS is to be achieved.
FIG. 2.
BOXSHADE of a PILEUP performed in the GCG DNA analysis package with MsbA protein sequences from E. coli (Ecoli) and H. influenzae (Hin) and the proposed homolog from B. pertussis (BP536). The black shading surrounds blocks of amino acids which are identical, and the grey shading surrounds blocks with conservative substitutions. The B. pertussis sequence is shown as starting with a leucine residue since it has TTG as a start codon. This sequence is truncated at the position of the SacI site where the DNA sequence published here starts. Only the parts of the E. coli and H. influenzae sequences corresponding to the truncated B. pertussis sequence are shown.
FIG. 3.
BOXSHADE of a PILEUP performed in the GCG DNA analysis package with WaaF protein sequences from N. gonorrhoeae (Ngon), Neisseria meningitidis (Nmen), E. coli (Ecoli), S. typhimurium (Salty), H. influenzae (Hin), and Pseudomonas aeruginosa (Psaer) and the proposed B. pertussis (BP536) WaaF protein. See Fig. 2 legend for an explanation of the shading.
The stop codon of B. pertussis waaF is separated by 12 bases from the next partial ORF, which again points rightward. On translation, this is 27% identical and 36% similar with a hypothetical transmembrane protein from Haemophilus influenzae (given the code Yh01_Haein in the Swissprot database).
Identification of waaF in B. parapertussis and B. bronchiseptica.
A 439-bp PCR fragment from the central region of waaF also used to mutagenize the bordetellae (see Materials and Methods) was used to probe Southern blots of restriction digests of B. pertussis, B. parapertussis, and B. bronchiseptica genomic DNA. This identified a single 4-kb SalI fragment in B. parapertussis and B. bronchiseptica, showing that the locus is present in all three bordetellae tested. Further genetic analysis of these waaF loci is ongoing and may allow the differences between B. parapertussis core and the other Bordetella LPS molecules to be addressed at the molecular genetic level.
Construction of waaF mutants in the bordetellae.
To confirm that waaF was required for inner core LPS biosynthesis in the bordetellae, allelic exchange mutants were generated. A single-crossover strategy was chosen to ensure the successful mutagenesis of waaF in all three strains. The same 439-bp PCR product was used to mutagenize the three bordetellae (see Materials and Methods). Following mutagenesis, the LPS phenotypes of resultant colonies were analyzed by silver-stained SDS-PAGE. Each of the waaF mutants had single LPS bands that migrated equally with each other and much faster than band B seen in the controls (Fig. 4). This is consistent with the LPS molecule having a deep rough phenotype. In addition, band A was absent from the B. pertussis and B. bronchiseptica mutants and the characteristic O antigen present in wild-type B. parapertussis and B. bronchiseptica controls was also absent from the waaF mutants. The evidence suggests that the waaF mutation leads to each of the bordetellae biosynthesizing the same deep rough LPS molecule, with concomitant loss of expression of distal structures. This is also consistent with the difference in structure of the B. parapertussis core, compared to B. pertussis and B. bronchiseptica, lying at its nonreducing end. The deeply truncated LPS phenotype observed in the three Bordetella mutants may not have been entirely predictable. Lipid A is linked to a single Kdo which in turn is linked to the first heptose residue. This heptose is then substituted by a glucose residue upon which the rest of the core main chain, the band A trisaccharide, and the O antigen are built. The second heptose, whose transfer is catalyzed by WaaF, forms a branch linked to the first heptose, with this second heptose being substituted by glucosamine and glucuronic acid (6, 7, 18, 19). Thus, a mutation in waaF might have been expected to lead to the removal of the branch structure from the core, leaving the rest of the core intact and potentially allowing the addition of distal structures. The fact that the deep rough phenotype was observed in the waaF mutants indicates that the addition of the rest of the core is dependent on prior addition of the branch structure. Another possibility that cannot be excluded at this stage is that the insert into the waaF gene, being large and complex, may have polar effects on genes downstream of waaF in the locus, which may themselves be required for biosynthesis of the rest of the LPS core molecule. These possibilities are currently being investigated.
FIG. 4.
Silver-stained Tris-tricine polyacrylamide gel of wild type and waaF mutant allelic exchange mutants from B. pertussis, B. bronchiseptica, and B. parapertussis. Lane 1, B. pertussis (BP536) wild type; lane 2, B. pertussis waaF mutant; lane 3, B. bronchiseptica (CN 7635E) wild type; lane 4, B. bronchiseptica waaF mutant; lane 5, B. parapertussis (CN 2591) wild type; lane 6, B. parapertussis waaF mutant.
Conclusions.
In this study we have identified, cloned, and sequenced the waaF gene from Bordetella species and have mutated this gene in B. pertussis, B. parapertussis, and B. bronchiseptica. This has led to these three bordetellae each having a deep rough LPS phenotype. These are the most minimal LPS structures constructed so far in the bordetellae.
ACKNOWLEDGMENT
This work was supported by project grant no. 045666/z/95/z from The Wellcome Trust.
REFERENCES
- 1.Ackermann M R, Register K B, Gentry-Weeks C, Gwaltney S M, Magyar T. A porcine model for the evaluation of virulence of Bordetella bronchiseptica. J Comp Pathol. 1997;116:55–61. doi: 10.1016/s0021-9975(97)80043-6. [DOI] [PubMed] [Google Scholar]
- 2.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 3.Amano K-I, Fukushi K, Watanabe M. Biochemical and immunological comparison of lipopolysaccharides from Bordetella species. J Gen Microbiol. 1990;136:481–487. doi: 10.1099/00221287-136-3-481. [DOI] [PubMed] [Google Scholar]
- 4.Brooke J S, Valvano M A. Biosynthesis of inner core lipopolysaccharide in enteric bacteria identification and characterization of a conserved phosphoheptose isomerase. J Biol Chem. 1996;271:3608–3614. doi: 10.1074/jbc.271.7.3608. [DOI] [PubMed] [Google Scholar]
- 5.Burns E H, Norman J M, Hatcher M D, Bemis D A. Fimbriae and determination of host species-specificity of Bordetella bronchiseptica. J Clin Microbiol. 1993;31:1838–1844. doi: 10.1128/jcm.31.7.1838-1844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caroff M, Chaby R, Karibian D, Perry J, Deprun C, Szabó L. Variations in the carbohydrate regions of Bordetella pertussis lipopolysaccharides: electrophoretic, serological, and structural features. J Bacteriol. 1990;172:1121–1128. doi: 10.1128/jb.172.2.1121-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaby R, Caroff M. Lipopolysaccharide of Bordetella pertussis endotoxin. In: Wardlaw A C, Parton R, editors. Pathogenesis and immunity in pertussis. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 247–271. [Google Scholar]
- 8.Cherry J D. Pertussis: the trials and tribulations of old and new pertussis vaccines. Vaccine. 1992;10:1033–1038. doi: 10.1016/0264-410x(92)90113-x. [DOI] [PubMed] [Google Scholar]
- 9.Cherry J D. Historical review of pertussis and the classical vaccine. J Infect Dis. 1996;174:S259–S263. doi: 10.1093/infdis/174.supplement_3.s259. [DOI] [PubMed] [Google Scholar]
- 10.Connor K M, Porter J F, Quirie M M, Donachie W. Moredun Bordetella medium, an improved selective medium for isolation of Bordetella parapertussis. J Clin Microbiol. 1996;34:638–640. doi: 10.1128/jcm.34.3.638-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Fabio J L, Caroff M, Karibian D, Richards J C, Perry M B. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol Lett. 1992;97:275–282. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 13.Gueirard P, Weber C, Lecoustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals—persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heininger U, Stehr K, Schmittgrohe S, Lorenz C, Rost R, Christenson P D, Uberall M, Cherry J D. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr Infect Dis J. 1994;13:306–309. doi: 10.1097/00006454-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs A A C, Chalmers W S K, Pasman J, Vanvugt F, Cuenen L H. Feline bordetellosis—challenge and vaccine studies. Vet Rec. 1993;133:260–263. doi: 10.1136/vr.133.11.260. [DOI] [PubMed] [Google Scholar]
- 18.Lasfargues A, Caroff M, Chaby R. Structural features involved in the mitogenic activity of Bordetella pertussis lipopolysaccharides for spleen cells of C3H/HeJ mice. FEMS Immunol Med Microbiol. 1993;7:119–130. doi: 10.1111/j.1574-695X.1993.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 19.Lebbar S, Caroff M, Szabó L, Mérienne C, Szilógyi L. Structure of a hexasaccharide proximal to the hydrophobic region of lipopolysaccharides present in Bordetella pertussis endotoxin preparations. Carbohydr Res. 1994;259:257–275. doi: 10.1016/0008-6215(94)84061-x. [DOI] [PubMed] [Google Scholar]
- 20.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel-electrophoresis. J Immunol Methods. 1990;126:109–111. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 21.Mackay D N. Treatment of acute bronchitis in adults without underlying lung disease. J Gen Intern Med. 1996;11:557–562. doi: 10.1007/BF02599608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makela P H, Stocker B A D. Genetics of lipopolysaccharide. In: Rietschel E T, editor. Handbook of endotoxin. 1. Chemistry of endotoxin. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press; 1984. pp. 59–137. [Google Scholar]
- 23.Montaraz J A, Novotny P, Ivanyi J. Identification of a 68-kilodalton protective protein antigen from Bordetella bronchiseptica. Infect Immun. 1985;47:744–751. doi: 10.1128/iai.47.3.744-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nennig M E, Shinefield H R, Edwards K M, Black S B, Fireman B H. Prevalence and incidence of adult pertussis in an urban population. JAMA. 1996;275:1672–1674. [PubMed] [Google Scholar]
- 25.Peppler M S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984;43:224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petricoin E F, Danaher R J, Stein D C. Analysis of the lsi region involved in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1991;173:7896–7902. doi: 10.1128/jb.173.24.7896-7902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichichero M E, Treanor J. Economic impact of pertussis. Arch Pediatr Adolesc Med. 1997;151:35–40. doi: 10.1001/archpedi.1997.02170380039006. [DOI] [PubMed] [Google Scholar]
- 28.Polissi A, Georgopoulos C. Mutational analysis and properties of the msbA gene of Escherichia coli, coding for an essential ABC family transporter. Mol Microbiol. 1996;20:1221–1233. doi: 10.1111/j.1365-2958.1996.tb02642.x. [DOI] [PubMed] [Google Scholar]
- 29.Porter J F, Connor K, Donachie W. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology. 1994;140:255–261. doi: 10.1099/13500872-140-2-255. [DOI] [PubMed] [Google Scholar]
- 30.Porter J F, Connor K, Donachie W. Differentiation between human and ovine isolates of Bordetella parapertussis using pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1996;135:131–135. doi: 10.1111/j.1574-6968.1996.tb07977.x. [DOI] [PubMed] [Google Scholar]
- 31.Porter J F, Connor K, Vanderzee A, Reubsaet F, Ibsen P, Heron I, Chaby R, Leblay K, Donachie W. Characterization of ovine Bordetella parapertussis isolates by analysis of specific endotoxin (lipopolysaccharide) epitopes, filamentous hemagglutinin production, cellular fatty-acid composition and antibiotic sensitivity. FEMS Microbiol Lett. 1995;132:195–201. doi: 10.1111/j.1574-6968.1995.tb07833.x. [DOI] [PubMed] [Google Scholar]
- 32.Rappuoli R, Pizza M, Covacci A, Bartoloni A, Nencioni L, Podda A, de Magistris M T. Recombinant acellular pertussis vaccine—from the laboratory to the clinic: improving the quality of the immune response. FEMS Microbiol Immunol. 1992;105:161–170. doi: 10.1111/j.1574-6968.1992.tb05898.x. [DOI] [PubMed] [Google Scholar]
- 33.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 34.Reina J, Bassa A, Llompart I, Borrell N, Gomez J, Serra A. Pneumonia caused by Bordetella bronchiseptica in a patient with a thoracic trauma. Infection. 1991;19:46–48. doi: 10.1007/BF01643760. [DOI] [PubMed] [Google Scholar]
- 35.Rutter J M. Quantitative observations on Bordetella bronchiseptica infection in atrophic rhinitis of pigs. Vet Rec. 1981;108:451–454. doi: 10.1136/vr.108.21.451. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Schwan E T, Robertson B D, Brade H, van Putten J P M. Gonococcal rfaF mutants express Rd(2) chemotype LPS and do not enter epithelial host cells. Mol Microbiol. 1995;15:267–275. doi: 10.1111/j.1365-2958.1995.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 38.Sirisena D M, Brozek K A, MacLachlan P R, Sanderson K E, Raetz C R H. The rfaC gene of Salmonella typhimurium: cloning, sequencing and enzymatic function in heptose transfer to lipopolysaccharide. J Biol Chem. 1992;267:18874–18884. [PubMed] [Google Scholar]
- 39.Sirisena D M, MacLachlan P R, Liu S L, Hessel A, Sanderson K E. Molecular analysis of the rfaD gene, for heptose synthesis, and the rfaF gene, for heptose transfer, in lipopolysaccharide synthesis in Salmonella typhimurium. J Bacteriol. 1994;176:2379–2385. doi: 10.1128/jb.176.8.2379-2385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staden R. Graphic methods to determine function of nucleic acid sequences. Nucleic Acids Res. 1984;12:521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 42.Tamion F, Girault C, Chevron V, Pestel M, Bonmarchand G. Bordetella bronchiseptica pneumonia with shock in an immunocompetent patient. Scand J Infect Dis. 1996;28:197–198. doi: 10.3109/00365549609049077. [DOI] [PubMed] [Google Scholar]
- 43.Thrusfield M V, Aitken C G G, Muirhead R H. A field investigation of kennel cough—incubation period and clinical signs. J Small Anim Pract. 1991;32:215–220. [Google Scholar]
- 44.Thrusfield M V, Aitken C G G, Muirhead R H. A field investigation of kennel cough—efficacy of different treatments. J Small Anim Pract. 1991;32:455–459. [Google Scholar]
- 45.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 46.Vanderzee A, Groenendijk H, Peeters M, Mooi F R. The differentiation of Bordetella parapertussis and Bordetella bronchiseptica from humans and animals as determined by DNA polymorphism mediated by two different insertion sequence elements suggests their phylogenetic relationship. Int J Syst Bacteriol. 1996;46:640–647. doi: 10.1099/00207713-46-3-640. [DOI] [PubMed] [Google Scholar]
- 47.Vonkonig C H W, Finger H. Role of pertussis toxin in causing symptoms of Bordetella parapertussis infection. Eur J Clin Microbiol Infect Dis. 1994;13:455–458. doi: 10.1007/BF01974634. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe M, Takimoto H, Kumazawa Y, Amano K I. Biological properties of lipopolysaccharides from Bordetella species. J Gen Microbiol. 1990;136:489–493. doi: 10.1099/00221287-136-3-489. [DOI] [PubMed] [Google Scholar]
- 49.Willoughby K, Dawson S, Jones R C, Symons M, Daykin J, Payne Johnson C, Gaskell R M, Bennett M, Gaskell C J. Isolation of B. bronchiseptica from kittens with pneumonia in a breeding cattery. Vet Rec. 1991;129:407–408. doi: 10.1136/vr.129.18.407. [DOI] [PubMed] [Google Scholar]