Abstract
The seven proteins encoded by the comG operon of Bacillus subtilis exhibit similarity to gene products required for the assembly of type 4 pili and for the secretion of certain proteins in gram-negative bacteria. Although polar transposon insertions in comG result in the loss of transformability and in the failure of cells grown through the competence regimen to bind DNA, it was not known whether the ComG proteins are all required for competence. We have constructed strains missing each of these proteins individually and found that they are all nontransformable and fail to bind transforming DNA to the cell surface. The implications of these findings are discussed.
Naturally competent organisms, such as Bacillus subtilis, efficiently bind and internalize transforming DNA. This process requires a set of proteins that was first identified in B. subtilis (reviewed in reference 9) but which has more recently been shown to mediate transformation in a variety of bacterial species, both gram positive and gram negative (8, 11–13, 17, 21, 27). Among these proteins are several possessing hydrophobic N termini with cleavage sites for processing typical of type 4 prepilins. The comG operon of B. subtilis encodes four such proteins (1), which are processed by a mechanism that requires ComC (6, 7), also an essential transformation protein (18). The predicted amino acid sequence of ComC resembles those of a group of proteins known to process the prepilins of gram-negative bacteria (20, 26). In addition to the four prepilin-like ComG proteins (ComGC, ComGD, ComGE, and ComGG), the comG operon encodes three other proteins: ComGF is a small integral membrane protein with no known ortholog, and ComGA and ComGB are predicted to be a nucleotide binding protein and an integral membrane protein, respectively (1). Both ComGA and ComGB resemble morphogenetic proteins, such as PilB and PilC from Pseudomonas aeruginosa (19), which are required for the assembly of type 4 pili.
With the exception of ComGF, the comG and comC gene products all resemble proteins needed for the secretion of certain proteins across the outer membrane of gram-negative organisms (23). Thus, members of the same families of proteins are required for pilus assembly, protein secretion, and competence for genetic transformation. Aside from the facts that the ComC orthologs in the secretory and pilus assembly systems are needed for the processing of their cognate prepilin-like proteins and that one of the prepilin proteins is the structural component of pili, little is known concerning the precise functions of these gene products. Particularly curious is the fact that all three systems (competence, secretion, and pilus assembly) involve multiple members of the prepilin protein family.
Null comC and comG mutants are nontransformable and appear to exhibit no additional phenotype (1, 18). In null comC mutants, competence is reduced at least 107-fold, suggesting that the processing of at least one prepilin-like protein is required for transformation. An in-frame deletion mutation in comGC and a transposon insertion mutation in comGG also completely eliminate transformation. However, since the available transposon insertion mutations in the remaining comG proteins are polar on downstream open reading frames, including comGG, it is not known if the ComGA, -GB, -GD, -GE, and -GF gene products are individually required for competence.
In this study, we have constructed strains in which the comG open reading frames are individually inactivated. These strains are completely noncompetent and are deficient in DNA binding when grown under conditions in which the remaining competence proteins are expressed. We conclude that each of the ComG proteins is needed for DNA binding, and we discuss certain implications of this result.
MATERIALS AND METHODS
Strains and growth conditions.
All of the B. subtilis strains used were derivatives of strain 168 (Table 1). Competent cultures were grown as described previously (3), and extracts for Western blotting were prepared from cultures grown in competence medium (3) to a point 2 h after the transition to stationary phase (T2), at which time the cultures were maximally competent.
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| BD630 | his leu-8 metB5 | 3 |
| BD2685 | his leu-8 metB5 comGΔA | This work |
| BD2686 | his leu-8 metB5 comGΔB | This work |
| BD1770 | his leu-8 metB5 comGΔC | 5 |
| BD1260 | his leu-8 metB5 comGC107 | 1 |
| BD2684 | his leu-8 metB5 comGC107 amyE::comGCDEFG | This work |
| BD2687 | his leu-8 metB5 comGC107 amyE::comGC(ΔD)EFG | This work |
| BD2688 | his leu-8 metB5 comGC107 amyE::comGCD(ΔE)FG | This work |
| BD2689 | his leu-8 metB5 comGC107 amyE::comGCDE(ΔF)G | This work |
| BD1252 | his leu-8 metB5 comGG210 | 1 |
| BD204 | his thyA thyB |
Transformation.
Competent cultures were incubated with transforming DNA (1 μg/ml) for 30 min at 37°C.
DNA manipulations and strain construction.
Molecular cloning and related procedures were carried out by standard methods (24).
The strains carrying in-frame deletions in comGA (BD2685) and comGB (BD2686) were constructed as follows. Plasmid pMA13, carrying comGABC (2), was cut with AvaI (blunt ended with T4 polymerase) and with BglII. The resulting fragment carrying comGABC was cloned into pUCCM18 (15), a pUC18 derivative which carries a chloramphenicol resistance (Cmr) marker. SphI (blunt ended) and BamHI sites were used for this cloning. The resulting plasmid was cut with SphI and NheI, blunt ended, and self-ligated. This removed 486 in-frame base pairs encoding 162 of the 356 amino acid residues of comGA (residues 139 through 300). The plasmid carrying this deletion was used to transform BD630 with selection for Cmr transformants which had received the deleted allele by single reciprocal recombination and also carried an intact copy of comGA. This transformant was then grown in the absence of chloramphenicol selection to allow the loss of one of the copies of comGA. The desired strains were detected by replica plating, and several such colonies were tested by PCR to detect those carrying only the deletion allele of comGA.
To construct the comGB deletion strain, the same AvaI (blunt ended)-BglII fragment from pMA13 was cloned between the BanII and BamHI sites of pUC19. The resulting plasmid was cut with BanII and StuI and self-ligated. This removed 501 in-frame base pairs encoding 167 of the 323 residues of comGB (residues 36 through 202). A fragment carrying the deletion allele in comGB was removed from this plasmid by cutting with EcoRI and XbaI and then cloned into pUCCM18 which had been cut with the same enzymes. The resulting plasmid was then used to construct a strain of B. subtilis carrying the comGB deletion in place of the wild-type comG allele, as described above for the comGA deletion.
To clone a fragment containing comGCDEFG, the plasmid pED19 (1), which carries the entire comG operon, was cut with StuI and KpnI. The latter enzyme cuts at a unique site downstream from comGG, and StuI cuts in comGB. The resulting fragment was cloned into pG67, which had been cut with SmaI and KpnI. The vector pG67 was a derivative of pDR67 (16) into which a fragment containing the comG promoter, isolated by PCR, had been inserted between the EcoRI and BamHI sites (14a). Cutting with EcoRI and BamHI removed the pSPAC promoter from pDR67. pG67 carried genes for chloramphenicol and ampicillin resistance as well as amyE front and back sequences present in pDR67. As a result, fragments under the control of the comG promoter could be readily inserted at the B. subtilis amyE locus. comGCDEFG-carrying derivatives of this plasmid with individual in-frame deletions in comGD, comGE, and comGF were then prepared. These deletions were introduced by using the QuickChange site-directed mutagenesis kit (Stratagene). This involved a limited number of amplification cycles with the high-fidelity Pfu DNA polymerase (Stratagene) and complementary primers, each carrying a desired deletion and about 10 bases flanking each deletion. The mutagenized fragments were then sequenced to document the presence of the expected deletions and to ensure that no unplanned mutations had been introduced. The wild-type and deletant fragments were each integrated at amyE. For comGD, codons 65 through 78 were deleted. For comGE, codons 46 through 60 were deleted. For comGF, codons 83 through 97 were deleted.
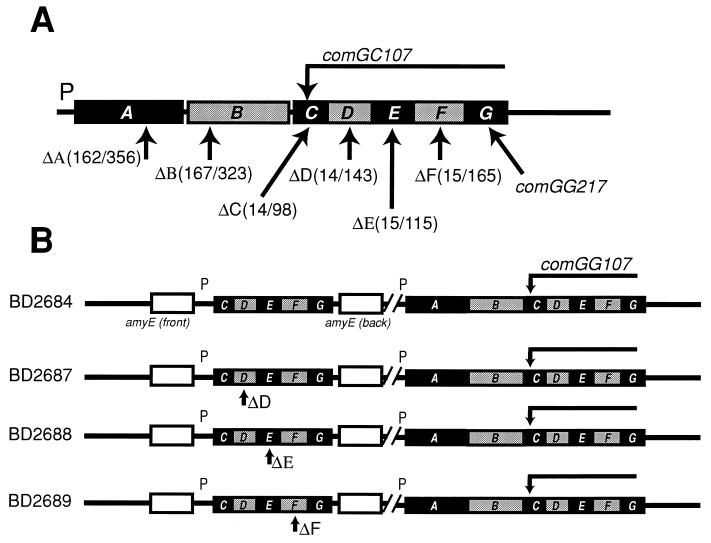
Finally, comG107, a polar Tn917 insertion in comGC, was introduced into the strains carrying comGCDEFG and its deletion derivatives by transduction with phage PBS1, generating strains BD2684 through BD2689 (1) (see Fig. 1).
FIG. 1.
Genotypes of strains used for the complementation experiments. (A) The mutations used in this study are indicated on a map of the comG operon. The numbers in parentheses correspond to the number of deleted amino acid residues followed by the total number of residues in the open reading frame. The horizontal bar extending from the comG107 arrow indicates that this Tn917 insertion is polar on expression of the downstream open reading frames in the operon. ComGG217 is also a Tn917 insertion mutation. (B) Each of the four strains diagrammed carries the comG107 transposon mutation at the comG locus. In addition, each of them carries a copy of comGCDEFG inserted at the amyE locus, either the wild type or one with an in-frame deletion of comGD, comGE, or comGF as indicated. Strain BD2684 therefore exhibits complementation of comGC107, whereas strains BD2687, BD2688, and BD2689 fail to express ComGD, -GE, and -GF, respectively.
DNA binding.
[3H]thymidine-labeled DNA was prepared by metabolic labeling of BD204 (his thyA thyB) as described previously (10). To measure binding, competent cultures were incubated as described above, 0.5-ml samples were withdrawn and washed three times in minimal salts solution (4), and radioactivity was determined by scintillation counting.
Western blotting.
Antibody to ComGG was raised in rabbits against a peptide (CDQKQKKLLRWTE) corresponding to the C-terminal 12 residues of that protein, with the addition of the single C residue used for coupling to maleimide-activated bovine serum albumin (Pierce). Antibody against ComGA was raised in rabbits by injecting the peptide CDHALLKKRDMKKEE-NH2, which had been coupled to maleimide-activated keyhole limpet hemocyanin. This peptide corresponds to ComGA amino acid residues 39 to 52, with the addition of the single C residue. Membranes were purified as described previously (6) and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a Tricine buffer system (25). The gels were blotted to nitrocellulose membranes with a Bio-Rad semidry transfer apparatus and the standard buffer (28). Signals were detected by enhanced chemiluminescence (ECL; Amersham).
RESULTS
A transposon insertion in comGG, the last open reading frame in the comG operon, as well as an in-frame deletion in comGC resulted in transformation-deficient strains that were incapable of binding DNA (5, 14). These studies did not determine whether the remaining five comG open reading frames were required for transformation and for DNA binding.
Phenotypes of comGA and comGB mutants.
In-frame deletions which removed 162 of 336 and 167 of 323 amino acid residues, respectively, were constructed in cloned copies of comGA and comGB. The mutations were introduced into the B. subtilis chromosome, replacing the wild-type copies of comGA and comGB. This was accomplished by transforming a wild-type strain (BD630) with DNA from plasmids carrying the deletion alleles and selecting for the Cmr marker located within the vector. Single reciprocal recombination events resulted in the insertion of the entire plasmid within comG, with duplication of comGA or comGB, so that the transformants each possessed one wild-type and one deleted copy of the comG open reading frame. Growth of the resulting strains in the absence of antibiotic selection permitted the loss of the plasmid and of either the wild-type or deleted comG allele. Cms colonies were detected by replica plating and screened by PCR to identify colonies that carried only the deleted alleles. The nonpolar nature of the comGΔA and comGΔB mutations was verified by Western blotting of membrane preparations. For this, antiserum against the downstream-most comG gene product, ComGG, was used. In both mutant backgrounds, ComGG was produced at levels similar to that of the wild-type strain (data not shown). Western blotting with anti-ComGA antiserum revealed that the ComGA signal was missing from a comGΔA membrane preparation but was present in preparations from the wild-type and comGΔB strains (data not shown).
Transformation of a chromosomal marker was undetectable in either the comGΔA or the comGΔB strain (Table 2), demonstrating that both gene products were essential for transformability.
TABLE 2.
Transformation phenotypes of comG mutants
| Strain | Relevant genotype | Protein affected | Missing residuesa | % Transformationb |
|---|---|---|---|---|
| BD630 | Wild type | None | None | 0.13 |
| BD2685 | comGΔA | ComGA | 162 (356) | <10−6 |
| BD2686 | comGΔB | ComGB | 167 (323) | <10−6 |
| BD1770 | comGΔC | ComGC | 14 (98) | <10−6 |
| BD1260 | comGC107 | ComGCDEFG | None | <10−6 |
| BD2684 | comGC107 amyE::comGCDEFG | None | None | 0.09 |
| BD2687 | comGC107 amyE::comGC(ΔD)EFG | ComGD | 14 (143) | <10−6 |
| BD2688 | comGC107 amyE::comGCD(ΔE)FG | ComGE | 15 (115) | <10−6 |
| BD2689 | comGC107 amyE::comGCDE(ΔF)G | ComGF | 15 (165) | <10−6 |
| BD1252 | comGG210 | ComGG | None | <10−6 |
The numbers in parentheses refer to the total number of residues in the proteins.
Transformation frequency was scored as the number of leucine prototrophs per milliliter times 100 divided by the total CFU per milliliter.
Construction of comGD-, comGE-, and comGF-deficient strains.
A different strategy was adopted for the construction of strains individually lacking comGD, comGE, and comGF, to avoid the time-consuming procedure described above for introducing the comGA and comGB deletions into the chromosome. In-frame deletion derivatives of each gene were constructed in a cloned fragment that carried the comGCDEFG open reading frames, as described in Materials and Methods. The vector used for this cloning carried the comG promoter, so that expression of the cloned genes remained under competence control. The three deletion constructs, as well as the fragment with the wild-type comGCDEFG fragment, were individually integrated at the amyE locus by replacement recombination. The resulting strains were then transformed with a Tn917 insertion mutation in comGC, which is known to be polar. In these transformant strains (Fig. 1), the ComGA and ComGB products should be provided by the intact open reading frames at the comG locus while the other products, with the exception of the deleted one, would be provided by the fragment integrated at amyE. Each strain would therefore be uniquely missing one of the three proteins. To confirm that the in-frame deletions were in fact nonpolar, the presence of ComGG was confirmed by Western blotting (data not shown). ComGG was present in membrane preparations derived from strains carrying the comGC transposon insertion only if the wild-type or mutant fragments with deletions in comGD, -GE, or -GF were present at amyE.
Table 2 contains transformation data obtained with these strains. As expected, no detectable transformation was exhibited by the strain carrying only the Tn917 insertion in comGC. When the intact fragment carrying comGCDEFG was integrated at amyE in this strain, the level of transformation was essentially equal to that of the wild-type control. This demonstrated that the complete fragment, integrated at the ectopic amyE site, was able to completely complement the polar transposon mutation. In contrast, the isogenic strains individually lacking ComGD, ComGE, and ComGF exhibited no detectable transformation (Table 2), demonstrating that each of these gene products was essential for competence.
All of the ComG proteins are required for DNA binding.
Competence mutants can be deficient in DNA binding to the cell surface or in a subsequent step, such as the transport of transforming DNA across the cell membrane. Binding is measured as the association of radioactively labeled DNA with competent cells after centrifugal washing. Transport is determined as the association of the labeled DNA in DNase-resistant form and cannot be measured in binding mutants.
DNA binding was measured in the mutant strains constructed in this study and in the wild-type strains grown through the competence regimen (Table 3). The strain carrying a polar insertion in comGC was, as expected, deficient in binding, and the comGCDEFG fragment inserted in the amyE locus complemented binding activity in this strain nearly to the wild-type level. The in-frame comGA and comGB mutations eliminated binding completely, as did the absence, individually, of ComGD, ComGE, and ComGF. Finally, as reported previously, a Tn917 insertion in comGG likewise resulted in the loss of DNA binding. The transformation deficiency of each of the comG mutants can therefore be explained by the failure of these strains to bind DNA to the cell surface.
TABLE 3.
DNA binding by comG mutants
| Strain | Relevant genotype | Protein affected | Radioactivity bound, cpm (%)a |
|---|---|---|---|
| BD630 | Wild type | None | 389 (100) |
| BD2685 | comGΔA | ComGA | 0 (0) |
| BD2686 | comGΔB | ComGB | 5 (1.3) |
| BD1770 | comGΔC | ComGC | 1 (0.25) |
| BD2684 | comGC107 amyE::comGCDEFG | None | 261 (67) |
| BD2687 | comGC107 amyE::comGC(ΔD)EFG | ComGD | 0 (0) |
| BD2688 | comGC107 amyE::comGCD(ΔE)FG | ComGE | 0 (0) |
| BD2689 | comGC107 amyE::comGCDE(ΔF)G | ComGF | 0 (0) |
| BD1252 | comGG210 | ComGG | 0 (0) |
Radiolabeled DNA was incubated with cultures grown to competence. After washing, the total radioactivity associated with the cells in a fixed volume of culture was determined. The numbers in parentheses refer to percent values normalized to the wild-type level. The zero values indicate that the radioactivity determined was less than background but not by more than 5 cpm.
DISCUSSION
This study, together with previous experiments (5, 14), revealed that each of the seven ComG proteins is essential for the binding of DNA to the competent-cell surface. ComC, a peptidase required for the processing of ComGC, ComGD, ComGE, and ComGG, is also required for DNA binding (14). This is most simply interpreted as indicating a requirement of processing for the function of one or more of these proteins. Since these null mutations result in the complete absence of binding, we cannot exclude the possibility that one or more of the ComG proteins are also required for transport. For instance, ComEA is required for both binding and transport, since a mutation in this protein that eliminates binding has been isolated while another mutation eliminates uptake but not binding (15). We have recently found that ComEA acts as a DNA binding protein in vitro (22).
The ComG proteins must somehow function together with ComEA to bind transforming DNA to the cell surface. The bound DNA must then engage the transport machinery, which consists minimally of ComEA, ComEC, and ComFA. Since ComEA is required for both binding and transport, it may function at the interface of the binding and transport machinery, for instance, by presenting the bound DNA to the exterior of a transport pore. Alternatively or additionally, it may be required to process the bound DNA to a form competent for transport, for instance, by generating a newly cleaved terminus. We have observed that the processed forms of the pilin-like ComG proteins are located on the outer face of the membrane as well as outside the cell membrane (6, 7), possibly in association with cell wall material. ComEA contains a single membrane-spanning segment near its N terminus and extends outward from the membrane surface (15). Thus, these proteins are appropriately located to constitute a DNA binding receptor complex. There is no evidence that the pilin-like proteins themselves bind directly to DNA. They may instead be required for the correct presentation of ComEA at the surface of the cell, for instance, by remodeling cell wall material. Alternatively, by analogy with the part played by the pilin-like proteins in certain protein secretion systems of gram-negative organisms, these proteins may be needed for secretion of an unknown competence protein. However, in the gram-negative systems, transport across the inner membrane is mediated by the sec machinery and the pilin-like proteins are needed for secretion across the outer membrane (23). Since no outer membrane is present in B. subtilis, we favor the first hypothesis.
Like the processing peptidase ComC, ComGA and ComGB are orthologs of proteins required for the assembly of type 4 pili, which are not themselves part of the completed organelle. They are therefore likely to play morphogenetic roles in the assembly or disassembly of a DNA binding structure located at the cell surface. This is certainly true for ComC, whose role has been assigned. An understanding of the parts played by ComGA and ComGB, as well as the roles of the prepilin-like proteins, awaits the characterization of the proposed cell surface complex.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM43756.
We thank Gordon Inamine for useful discussions and members of our lab for their support and for comments on the manuscript.
REFERENCES
- 1.Albano M, Breitling R, Dubnau D A. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989;171:5386–5404. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albano M, Dubnau D A. Cloning and characterization of a cluster of linked Bacillus subtilis late competence mutations. J Bacteriol. 1989;171:5376–5385. doi: 10.1128/jb.171.10.5376-5385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitling R, Dubnau D. A membrane protein with similarity to N-methylphenylalanine pilin is essential for DNA binding by competent Bacillus subtilis. J Bacteriol. 1990;172:1499–1508. doi: 10.1128/jb.172.3.1499-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung Y S, Dubnau D. ComC is required for the processing and translocation of ComGC, a pilin-like competence protein of Bacillus subtilis. Mol Microbiol. 1994;15:543–551. doi: 10.1111/j.1365-2958.1995.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 7.Chung, Y. S., and D. Dubnau. Unpublished data.
- 8.Clifton S W, McCarthy D, Roe B A. Sequence of the rec-2 locus of Hemophilus influenzae: homologies to comE-ORF3 of Bacillus subtilis and msbA of Escherichia coli. Gene. 1994;146:95–100. doi: 10.1016/0378-1119(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 9.Dubnau D. Genetic exchange and homologous recombination. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 555–584. [Google Scholar]
- 10.Dubnau D, Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: size and distribution of the integrated donor segments. J Bacteriol. 1972;111:488–494. doi: 10.1128/jb.111.2.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facius D, Meyer T F. A novel determinant (comA) essential for natural transformation competence in Neisseria gonorrhoeae. Mol Microbiol. 1993;10:699–712. doi: 10.1111/j.1365-2958.1993.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 13.Freitag N E, Seifert H S, Koomey M. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol. 1995;16:575–586. doi: 10.1111/j.1365-2958.1995.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 14.Hahn J, Albano M, Dubnau D. Isolation and characterization of Tn917lac-generated competence mutants of Bacillus subtilis. J Bacteriol. 1987;169:3104–3109. doi: 10.1128/jb.169.7.3104-3109.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Inamine, G., and D. Dubnau. Unpublished data.
- 15.Inamine G S, Dubnau D. ComEA, a Bacillus subtilis integral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J Bacteriol. 1995;177:3045–3051. doi: 10.1128/jb.177.11.3045-3051.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ireton K, Rudner D Z, Jaacks-Siranosian K, Grossman A D. Integration of multiple developmental signals in Bacillus subtilis through the SpoOA transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 17.Lunsford R D, Roble A G. comYA, a gene similar to comGA of Bacillus subtilis, is essential for competence-factor-dependent DNA transformation in Streptococcus gordonii. J Bacteriol. 1997;179:3122–3126. doi: 10.1128/jb.179.10.3122-3126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan S, Aghion J, Guillen N, Dubnau D. Molecular cloning and characterization of comC, a late competence gene of Bacillus subtilis. J Bacteriol. 1989;171:6043–6051. doi: 10.1128/jb.171.11.6043-6051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunn D, Bergman S, Lory S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol. 1990;172:2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunn D N, Lory S. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J Bacteriol. 1993;175:4375–4382. doi: 10.1128/jb.175.14.4375-4382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pestova E V, Morrison D A. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Streptococcus pneumoniae chromosomal loci specifically induced during competence for genetic transformation; p. 293, abstr. H-49. [Google Scholar]
- 22.Provvedi, R., and D. Dubnau. 1997. Unpublished data.
- 23.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 26.Strom M S, Nunn D N, Lory S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc Natl Acad Sci USA. 1993;90:2404–2408. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tønjum T, Freitag N E, Namork E, Koomey M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;16:451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]