Abstract
Populations are ageing worldwide, with considerable time lived in ill-health, putting upwards pressure on healthcare budgets. Healthy ageing is defined as maintaining functional ability, including the ability to: meet basic needs; learn, grow and make decisions; be mobile; build and maintain relationships; and contribute to society. The risk and impact of infectious diseases increase with age due to immunosenescence. Vaccination can help to prevent disease in older adults, promoting healthy ageing and active lives. Herpes zoster (HZ) occurs when the varicella zoster virus is reactivated due to declining immunity. HZ is common, with a lifetime risk of one-third, and increases in incidence with age. HZ is associated with severe and intense pain, substantially affecting the functional status of patients as well as their overall health-related quality of life. HZ and its complications may result in prolonged morbidity, including persistent pain (post-herpetic neuralgia, PHN), hearing impairment, vision loss and increased risk of stroke and myocardial infarction. HZ and PHN are difficult to treat, substantiating the benefits of prevention. Vaccines to prevent HZ include a recombinant zoster vaccine (RZV). RZV has shown efficacy against the HZ burden of disease and HZ burden of interference on activities of daily living of over 90% in immunocompetent adults aged ≥50 years. Vaccine efficacy against HZ was maintained at over 70% at 10 years post-vaccination. Adult vaccination, including against HZ, has the potential to reduce burden of disease, thus helping to maintain functioning and quality of life to support healthy ageing in older adults.
Subject terms: Diseases, Drug discovery
Introduction
Population ageing is a key demographic trend across developed countries globally. In the European Union, life expectancy has increased from 75 years in 1990 to 81 years in 2018, and is predicted to increase further by 20601. In Japan, life expectancy at birth increased from 59.2 years in 1950 to 84.8 years in 20202. By 2050, one person in six worldwide and one person in four in North America and Europe will be aged ≥65 years3.
While life expectancy is rising, considerable time spans are lived in ill health. For example, in 2017 men and women in Singapore were likely to spend the last eight and ten years of life, respectively, in poor health4. Healthcare utilisation tends to increase towards the end of life, with unpredictable and wide variation in the course of disability in the final years4. Disability and comorbidity, rather than age itself, are associated with increased healthcare expenditure. Consequently, maintaining good health in ageing individuals may limit increases in healthcare expenditure5. Maintaining function is key to improving quality of life and preventing the debilitating effects of immobility and inactivity in older adults6.
The prevalence and severity of many infectious diseases increase with increasing age. As well as the immediate effects of an acute episode of infectious disease, many older adults do not fully recover and may experience consequences such as exacerbation of chronic conditions, onset or worsening of frailty, difficulties with activities of daily living and loss of independence7. Vaccine-preventable diseases remain a major public health issue in older adults8. For example, herpes zoster (HZ), pertussis, pneumococcal disease and influenza can all result in reduced quality of life, activity and functioning in older adults, as well as a risk of mortality6. As a result of population ageing and population growth, the annual economic burden of these four diseases in adults aged ≥50 years in the United States (US) is projected to increase from US$35 billion to US$49 billion over the next 30 years9. More recently, coronavirus disease 2019 (COVID-19) has emerged as another vaccine-preventable illness disproportionately affecting older adults10.
HZ is a common and painful condition that occurs due to reactivation of varicella zoster virus (VZV) as a result of a decline in immunity. The incidence of HZ increases with age, together with the risk of complications such as post-herpetic neuralgia (PHN)11. Population ageing is projected to result in a substantial increase in the number of HZ cases in developed countries11.
Vaccines to prevent HZ approved for use in older adults include live-attenuated VZV vaccine (zoster vaccine live, ZVL) and an adjuvanted recombinant zoster vaccine (RZV)3. The potential contribution of HZ vaccination with ZVL to healthy ageing has been discussed in a previously published review5, and therefore the present review focusses on the RZV vaccine. The objectives of this review are to describe the burden of HZ and how vaccination with RZV has the potential to reduce both the risk and severity of disease, thus maintaining functioning and quality of life and supporting healthy ageing in older adults.
Healthy ageing
Healthy ageing is defined as developing and maintaining functional ability to enable well-being in later years. Functional ability refers to the capabilities that allow a person to be and do what they value, including the abilities to: meet their basic needs; learn, grow and make decisions; be mobile; build and maintain relationships; and contribute to society12.
Ageing and impact on Healthcare
The World Health Organization (WHO) Decade of Healthy Ageing report projects that the worldwide number of older adults aged ≥60 years will increase by 34%, from 1 billion in 2019 to 1.4 billion in 203013.
The focus of most healthcare interventions has historically been on adding years to life, and the increase in life expectancy observed across the world in recent decades and projected for the future is an indication of the success of this approach. Healthy ageing focusses on adding quality to these extra years of life14. This is illustrated schematically in Fig. 1. In the current situation, average quality of life and functioning tends to decline with advancing age. The concept of healthy ageing concentrates on maintaining quality of life at a higher level for longer (Fig. 1). Even without further increases in longevity (both curves in Fig. 1 reach the X-axis at the same point), this has the effect of increasing the total time lived in good health (shaded area in Fig. 1).
Fig. 1. Schematic illustration of the potential benefit of healthy ageing, increasing the time lived in good health without extending life expectancy.
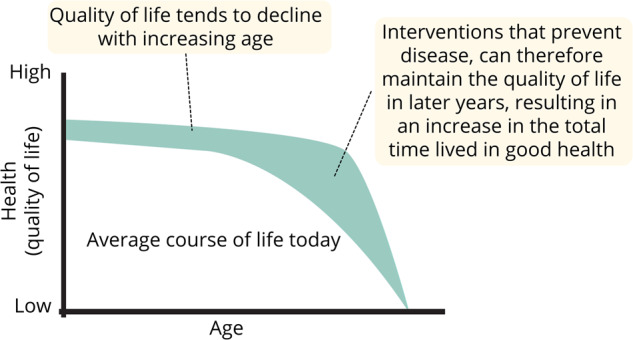
Adapted from McKinsey 202283.
Healthy ageing offers several potential benefits. In addition to the personal benefit of improved quality of life, older adults living additional years in good health can strengthen their societies by continuing to participate as integral parts of families and communities and strengthen healthcare systems by reducing the burden on the healthcare system13. There may also be economic benefits in relation to savings in healthcare costs associated with improved health and functioning, as interventions can be cost-effective or cost-saving5.
Immune System and Ageing
Immunosenescence is the gradual deterioration of the immune system, brought on by natural age advancement15. It is associated with an increased susceptibility to infectious pathogens and may result in diminished response to vaccines in older adults15,16.
Ageing is associated with a chronic sterile inflammatory state called inflammageing15. This low-grade inflammation is predictive of frailty and earlier mortality, is a risk factor for some chronic degenerative diseases, and may be associated with age-related impaired humoral and cellular immune response to vaccination15. Many of the mechanisms involved in inflammageing are also involved in response to infection, and it has been suggested that exposure to infectious agents may promote biological reactions such as inflammation that accelerate ageing17,18.
Impact of vaccination on healthy ageing
The burden of infectious disease is now concentrated in older adults19,20, and more adults than children die from vaccine-preventable disease8,14. The threat of infectious disease to ageing populations, and the gaps in the systems designed to address these risks have been highlighted in the COVID-19 pandemic, which may help to create a sense of urgency in developing adult immunisations and delivery systems21. Prevention of infectious disease by vaccination is part of a triad of interventions to promote healthy ageing, along with healthy diet and exercise19. The immune system retains some plasticity into older age, despite immunosenescence, and it has been suggested that vaccination may contribute to immune fitness, defined as the ability to mount an appropriate immune response to external challenges22. Immune fitness may be seen as a general state that reduces the risk of ill health, and is influenced by genetic factors and external factors such as diet, exercise, presence of chronic infection and avoidance of pollutants such as smoking. Vaccination may also be able to modulate the immune system, contributing to immune fitness in ways that are only beginning to be explored22.
Vaccination as a preventive strategy should span the life course, including adults and older adults as well as children23, and life-course vaccination could be a key tool for supporting healthy ageing22. For decades, most countries have implemented robust vaccination programme for children, but adult immunisation programme have consistently been a lower priority17,19, and vaccine coverage in older adults is lower than for children6,19. Reducing this vaccination gap is important to promote healthy ageing6.
Several vaccines are recommended for older adults, including vaccination against HZ, influenza and pneumococcal disease7. Vaccination in older adults offers substantial health and quality of life benefits17. In the US, for example vaccination programmes against HZ, influenza, pertussis and pneumococcal disease were estimated to avoid 65 million cases of disease over a 30-year period24.
Herpes zoster
VZV lies dormant in nerve cells after a chickenpox infection, and in later life it can reactivate due to age-related immune system decline to cause HZ, also called shingles3. VZV infection is almost universal, and the lifetime risk of HZ is approximately one-third in the US and Asia-Pacific25,26. HZ is common in the US, with over one million cases a year3. A synthesis of HZ incidence rates reported in 61 records from 59 studies worldwide was carried out using meta-regression methods27. HZ incidence increased with increasing age, was higher in females than males, and increased over time27. It was estimated that 14.9 million cases of HZ occurred worldwide in people aged ≥50 years in 2020 and was projected to increase to 17.0 million in 2025 and 19.1 million cases by 2030, due to ageing of populations worldwide27.
The most common complication of HZ is PHN; it has been reported that pain lasting for 90 days or more following HZ rash onset occurs in 10–20% of HZ cases28. The risk of PHN increases with age and although the pain of PHN resolves within a year in the majority of cases, in some it may persist for years28. PHN in older individuals can have a long-term impact on the ability to carry out activities of daily living and can compromise functional status and result in loss of independence3. Other complications of HZ may also occur, including neurological complications such as palsies and stroke, eye complications such as iritis and vision loss, skin complications such as secondary bacterial infection, systemic complications such as pneumonia and myocardial infarction, and zoster-related hospitalisation or death29. HZ ophthalmicus can result in eye damage, pain, light sensitivity and loss of vision30. Symptoms of HZ oticus or Ramsay Hunt syndrome include tinnitus (48% of patients) and unilateral hearing loss (24% of patients), and hearing impairment may be permanent in 5% of cases31.
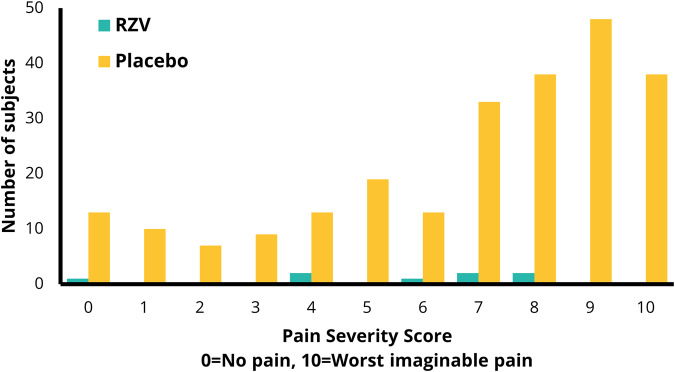
HZ is characterised by localised rash and pain, with itchiness and fatigue also commonly reported32. The pain is frequently reported as severe and intense, with patients choosing language such as a blowtorch or an electric shock to describe their experience of the pain32. In the placebo group of a RZV vaccine trial in individuals aged ≥50 years, 65.2% of patients reported having ‘severe’ HZ pain (Zoster Brief Pain Inventory [ZBPI] score of 7 or above), and 15.8% reported ‘worst pain imaginable’ (ZBPI score of 10) (Fig. 2)33. In a post-hoc analysis of data from the placebo arms of three Phase III trials, increased ZBPI pain scores were significantly associated with worse scores across all domains of quality of life including physical, emotional and social impacts34. For every 1-point increase in ZBPI pain score, a 1.57-point decrease in SF-12 physical score is observed, demonstrating that HZ pain is associated with a loss of physical functioning35.
Fig. 2. Maximum Zoster Brief Pain Inventory (ZBPI) worst pain severity scores by vaccination group in subjects ≥ 50 years of age.
Based on data from Curran et al 201933 with permission from Curran D. RZV, recombinant zoster vaccine.
HZ-associated pain is frequently accompanied by discomfort such as pruritus (itching) and allodynia (pain caused by stimuli that would not normally be painful, such as touch, contact with hot or cold water, or air blowing on the skin)36,37. Allodynia can have a considerable impact on patients’ quality of life, causing difficulties with activities such as bathing, grooming and getting dressed and impairing social activities through fear of pain from being touched by others32. The negative effects of HZ/PHN on quality of life are broad-ranging, affecting multiple domains of quality of life, including impacts on sleep, mood, physical functioning, mental health, ability to undertake activities of daily living, work, and ability to participate in hobbies and social activities28,32,38–43. In a study in Germany, patients said their lives stopped due to the disease, and expressed feelings of helplessness, frustration, depression, sadness or rage, and they never forgot the illness and its impact on their lives44.
In a time trade-off study, individuals were willing to trade a mean of 89 discounted days to avoid the least severe scenario of HZ and a mean of 162 discounted days to avoid the most severe scenario, indicating that individuals place a substantial value on avoiding HZ45. As such there is an apparent paradox that many decision-making bodies on vaccination focus primarily on vaccines that prolong life, whereas individuals are willing to trade off time to avoid disease, thus maintaining their current quality of life. Consequently, vaccines that maintain quality of life should be given equal priority to those that extend length of life.
HZ and PHN are associated with a substantial economic burden in healthcare costs and productivity losses. Approximately 1–4% of patients with HZ require hospitalisation46, and patients with PHN reported an average of 9.5 consultations with healthcare professionals28. In the United Kingdom (UK), two-thirds of patients with HZ who were in employment reported that the condition had affected their work, mainly by having to take time off work38. Similarly, in a Canadian study, 64% of employed subjects reported missing work due to HZ (absenteeism), and 76% reported reduced effectiveness at work (presenteeism)47. Pain severity was associated with higher productivity loss47, and the presence of significant pain in HZ patients was associated with higher productivity losses compared with patients without pain48.
HZ and its complications may result in prolonged morbidity, as HZ is associated with a transient increased subsequent risk of stroke in the immediate period after the HZ episode19. The risk of stroke may be greater for HZ ophthalmicus than for HZ at other sites49. There may also be an increased risk of myocardial infarction, with a greater burden of disease in older adults with zoster17. The mechanisms are unknown, but may include direct effects on blood vessel walls causing inflammation17.
The pain from HZ and PHN is difficult to manage, with significant unmet need in treatment effectiveness and consequent dissatisfaction with treatment among HZ patients, which is presumably driven by experience of inadequate relief of symptoms28,38. Patients with PHN in the UK received multiple medications28, increasing the potential risks of polypharmacy in frail individuals. Given the challenges associated with managing HZ and PHN, preventative strategies for HZ and associated complications should be considered as a means of enabling patients to remain active in old age, minimising the individual patient and societal burden associated with the condition28.
Recombinant zoster vaccine
Two classes of HZ vaccines are available for older adults, ZVL and RZV3. ZVL is a 1-dose vaccine that utilises the vOka live attenuated virus produced by serial passage of a wild-type clinical isolate termed pOka in human and guinea pig cell lines50. It is the same vaccine that is used to prevent varicella in children although with a higher potency (e.g. >14-times the varicella vaccine)5. ZVL is contraindicated in individuals with primary or acquired immunodeficiency5. RZV is a 2-dose non-live recombinant vaccine that combines the VZV glycoprotein E (gE) and the AS01B adjuvant system that helps to improve immunogenicity, especially in older adults, and permits vaccination of immunocompromised individuals46. AS01B is a liposome-based adjuvant comprising 3-O-desacyl-4′-monophosphoryl lipid A (MPL), a Toll-like receptor 4 ligand and QS-21, a saponin extracted from the bark of the Quillaja saponaria Molina tree (50 mg MPL and 50 µg QS-21). RZV has shown higher interleukin-2 and immune memory response than ZVL51,52. The second RZV dose should typically be given 2–6 months after the first; for persons who are or will be immunodeficient or immunosuppressed and who would benefit from a shorter vaccination schedule, the second dose can be administered 1–2 months after the first53.
Some countries, such as Canada and the UK, have preferential recommendations for RZV over ZVL, based on its high efficacy, long duration of protection and greater cost-effectiveness54. Parikh et al., in their overview of national vaccination recommendations for RZV, report much variation in age group recommendations, reflecting evaluations dependent on public funding, and differences with respect to use in immunocompromised and other special populations54.
ZVL has already been the subject of a comprehensive review5, and the present review discusses the RZV vaccine. Table 1 summarises published vaccine efficacy data for RZV. The vaccine efficacy of RZV against HZ was over 90% in immunocompetent adults aged ≥50 years in two phase III trials, ZOE-50 and ZOE-7055,56.
Table 1.
Vaccine efficacy of RZV.
| Vaccine efficacy against: | Vaccine efficacy | 95% CI | Reference |
|---|---|---|---|
| HZ in immunocompetent adults aged 50+ years | 97.2% | 93.7, 99.0 | 55 |
| HZ in immunocompetent adults aged 70+ years | 91.3% | 86.8, 94.5 | 56 |
| PHN in immunocompetent adults aged 70+ years | 88.8% | 68.7, 97.1 | 56 |
| HZ in immunocompetent adults aged 50+, year 10 post-vaccinationa | 73.2% | 46.9, 87.6 | 65 |
| HZ burden of illness (i.e. pain) in immunocompetent adults aged 50+ years | 98.4% | 92.2, 100 | 33 |
| HZ burden of illness in immunocompetent adults aged 70+ years | 92.1% | 90.4, 93.8 | 33 |
| HZ burden of interference with activities of daily living in immunocompetent adults aged 50+ years | 99.1% | 86.2, 100 | 33 |
| HZ burden of interference with activities of daily living in immunocompetent adults aged 70+ years | 90.3% | 88.5, 92.1 | 33 |
| HZ in frail individuals | 90.2% | 75.4, 97.0 | 62 |
| HZ in pre-frail individuals | 90.4% | 84.4, 94.4 | 62 |
| HZ in HSCT recipients | 68.2% | 55.6, 77.5 | 63 |
| HZ in patients with haematological malignancies | 87.2% | 44.3, 98.6 | 64 |
| HZ burden of illness in HSCT recipients | 82.5% | 73.6, 91.4 | 74 |
| HZ burden of interference with activities of daily living in HSCT recipients | 82.8% | 73.3, 92.3 | 74 |
CI confidence interval, HSCT hematopoietic stem cell transplantation, HZ herpes zoster; PHN post-herpetic neuralgia, RZV recombinant zoster vaccine.
aData accrual for year 10 is still ongoing65.
Efficacy was independent of age, sex or ethnicity57, and was maintained in patients with common pre-existing medical conditions such as hypertension and diabetes58–61. Vaccine efficacy was similar in subjects aged 70–79 years and in subjects aged ≥80 years56, indicating that RZV is able to overcome immunosenescence. Furthermore, in a post-hoc analysis of pooled data from the ZOE studies, vaccine efficacy was above 90% in frail, pre-frail and non-frail subgroups62. RZV has also demonstrated efficacy against HZ in phase III trials of immunocompromised adults aged ≥18 years 1) who had recently undergone autologous HSCT63, and 2) with haematological malignancies who had recently undergone immunosuppressive cancer treatments (a post-hoc analysis)64.
An interim analysis of data collected with up to 10 years follow-up after vaccination in the ZOE-50 and ZOE-70 trials estimated vaccine efficacy at 84.2% for year 8, 72.7% for year 9 and 73.2% for year 10 post-vaccination65. Data accrual beyond year 10 is still ongoing and will be reported in a final analysis65. By reducing HZ, RZV also protected against PHN56.
No safety concerns have been identified with RZV, although transient injection site and systemic reactions were more common than with placebo. Most reactions were mild to moderate in intensity and transient (median duration of 1–3 days)55,56. Second dose completion rates of RZV were approximately 95% in clinical trials and approximately 70% to 80% in real world studies, similar to what has been observed for other adult vaccines suggesting vaccine reactions is one of many factors that influence completion rates55,56,66–68. Except for these reactions, the safety profile of RZV was comparable with placebo, regardless of age, gender or race69,70. A self-controlled case series analysis indicated a slight increase in the risk of Guillain-Barré syndrome in the 6 weeks after vaccination (approximately 3 excess cases per million vaccinations); the risk-benefit balance remained in favour of vaccination71. The available information is insufficient to determine a causal relationship72. The safety profile of RZV remained clinically acceptable at year 10 post-vaccination65.
Data from the ZOE-70 study indicated that RZV significantly reduced HZ-associated pain medication use and duration of pain medication use in participants with confirmed HZ73. Figure 2 presents the distribution of the individual maximal ZBPI “worst-pain” scores experienced over the entire HZ episode for the ZOE-50 study33. In addition to preventing HZ episodes (and the pain associated with those episodes), RZV also attenuated the severity of pain in individuals with breakthrough disease33,74. More importantly from a healthy ageing perspective, RZV reduced the burden of HZ pain and the impact of HZ on activities of daily living by greater than 90% in adults aged ≥50 years33. Both “burden of illness” and “burden of interference” were composite endpoints incorporating incidence, duration, and severity (or interference) of HZ.
Modelling studies have indicated that RZV could reduce projected healthcare resource use, such as hospitalisations, outpatient visits and general practitioner visits75,76, and reduce work losses due to HZ77. Vaccination with RZV was reported to be cost-effective in 15 of 18 studies in a recent review, and cost-effective or cost-saving compared with ZVL in the subset of these studies where a comparison to ZVL was made78. In a modelling study in Germany, based on long-term efficacy data up to 8 years post-vaccination, the number needed to vaccinate (NNV) with RZV to prevent one HZ case was estimated at 6–10, and to prevent one PHN case it was 34–4879. The NNV to prevent one HZ case was 6 for individuals aged 50–69 years of age compared with 10 in individuals aged ≥70 years of age79. As such, vaccinating individuals who are younger (and potentially healthier) may provide the best public health impact, given the long-term efficacy observed for the vaccine. Vaccination of healthy individuals ensures that individuals are vaccinated when the immune response is likely to be most robust.
Recent reports suggest that HZ vaccination may reduce the risk of dementia80,81. These findings were based on analyses of retrospective databases where matched cohorts of vaccinated and unvaccinated individuals were compared. Further research is required to 1) better understand the mechanisms that may lead to this association and 2) confirm these associations via prospective clinical studies.
Summary and conclusion
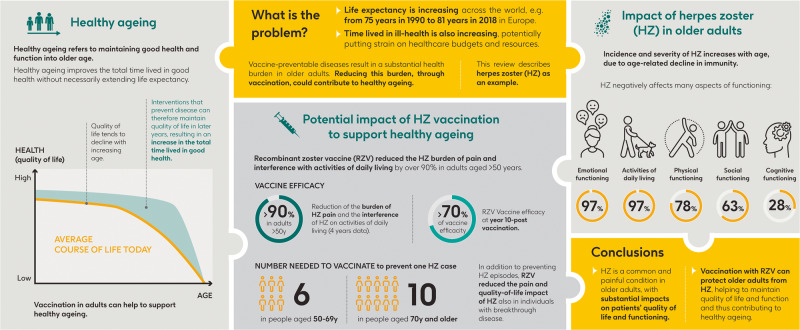
A plain language summary of our findings and their relevance is presented in Fig. 3. The main findings are also represented visually in a graphical abstract in Fig. 4. Population ageing is an established demographic trend worldwide3. With older adults making up an increasing proportion of the population, age-related increases in disability and morbidity have the potential to overburden healthcare budgets and systems. Healthy ageing is defined as developing and maintaining functional ability to enable well-being in later years12. Health interventions need to focus on improving the quality of these longer lifespans, promoting healthy ageing that maintains good health and function into older age.
Fig. 3.
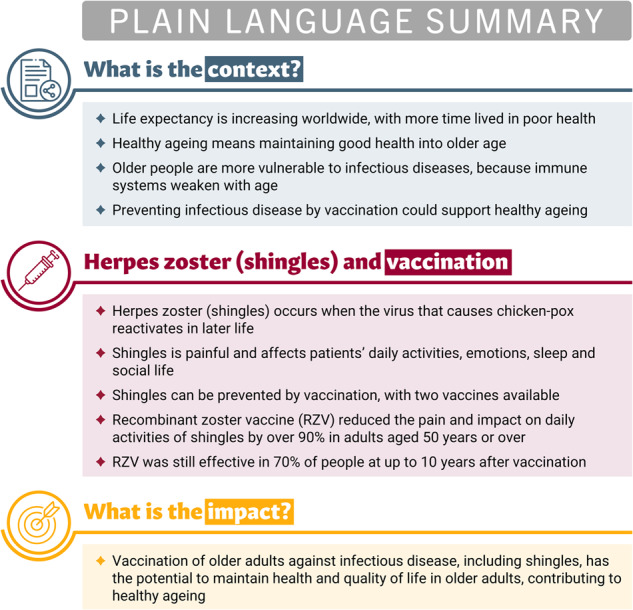
Plain language summary.
Fig. 4. Graphical abstract.
y years.
Immune function characteristically declines with age, resulting in higher prevalence and severity of infectious disease. The burden of vaccine-preventable infectious diseases is now higher in adults rather than children in developed countries such as the US20, due to established vaccination programmes for children with overall reasonably high vaccination rates. However, adult vaccination is less established with much poorer coverage than childhood vaccination20.
HZ and complications such as PHN, HZ ophthalmicus and HZ oticus occur mainly in older adults. HZ and its complications are associated with prolonged morbidity such as vision loss, hearing impairment, increased risk of stroke, impaired functional status and loss of health-related quality of life3,19,30,31.
In addition, a substantial burden in healthcare costs such as hospitalisation46 and lost work time47 is observed for affected individuals. HZ and PHN are difficult to manage with current treatments28. Prevention of HZ through adult vaccination could therefore offer substantial benefits.
RZV has been shown to be effective in reducing HZ incidence55,56, the HZ burden of illness (i.e. pain), and the interference of HZ with activities of daily living in older adults33. Modelling studies also indicate that RZV would be expected to substantially reduce healthcare visits and hospitalisations75,76, and reduce work losses due to HZ77. As social connections and relationships contribute to the life satisfaction of individuals82, reducing the negative effects of HZ on social functioning offers the potential to improve life satisfaction and well-being in older adults.
Adult vaccination programmes, including against HZ, have the potential to reduce morbidity in older adults, maintaining quality of life and providing important social and economic benefits. Adult vaccination is a key component of healthy ageing. The goal for adult vaccination programmes should be to reach the level of maturity currently observed for paediatric vaccines, with the necessary infrastructure to achieve high coverage. This would contribute towards promoting healthy ageing, adding not only years to life but more importantly enhancing the quality of those remaining years.
Acknowledgements
The authors would like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Carole Nadin (Fleetwith Ltd, on behalf of GSK) provided writing support. GlaxoSmithKline Biologicals SA funded this literature review and took in charge all costs associated with the development and publication of this manuscript. AS01 is a trademark owned by or licensed to GSK. AS01B is an MPL, QS-21 and liposome based Adjuvant System (50 µg MPL and 50 µg QS-21). A poster was presented at the 16th European Public Health Conference in Berlin, Germany, from November 09, 2022 to November 12, 2022.
Author contributions
D.C., N.L. and T.B. were involved in the design of the study. D.C., M.D. and N.L. collected or generated the data. All authors analyzed and/or interpreted the data. All authors participated to the development of this manuscript and in its critical review with important intellectual contributions. All authors critically reviewed the paper for important intellectual content. All authors had full access to the data and gave approval of the final manuscript before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
All data included in this review were obtained from publicly available sources and cited accordingly. The references for each study or publication included in this review are provided for further reading and verification. No additional datasets were generated or analyzed specifically for this manuscript.
Competing interests
D.C., M.D., N.L. and T.B. are employed by/hold shares in GSK. N.L. received grants and declared patents from GSK. All authors declare no other financial and non-financial relationships and activities.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Bank Group. Life expectancy at birth, total (years) - European Union. https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=EU (2021).
- 2.World Health Organization. The Global Health Observatory. Life Expectancy at Birth (years). Japan. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-expectancy-at-birth-(years) (2020).
- 3.Harbecke R, Cohen JI, Oxman MN. Herpes Zoster Vaccines. J. Infect. Dis. 2021;224:S429–S442. doi: 10.1093/infdis/jiab387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho V, et al. Healthcare utilisation in the last year of life in internal medicine, young-old versus old-old. BMC Geriatr. 2020;20:495. doi: 10.1186/s12877-020-01894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggi S, et al. Preventing and managing herpes zoster: key actions to foster healthy aging. Aging Clin. Exp. Res. 2015;27:5–11. doi: 10.1007/s40520-015-0314-7. [DOI] [PubMed] [Google Scholar]
- 6.Raina MacIntyre C, et al. Equity in disease prevention: Vaccines for the older adults - a national workshop, Australia 2014. Vaccine. 2016;34:5463–5469. doi: 10.1016/j.vaccine.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger B. Vaccination of older adults: Influenza, pneumococcal disease, herpes zoster, COVID-19 and beyond. Immun. Ageing. 2021;18:38. doi: 10.1186/s12979-021-00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang PO, Aspinall R. Vaccination in the elderly: what can be recommended? Drugs Aging. 2014;31:581–599. doi: 10.1007/s40266-014-0193-1. [DOI] [PubMed] [Google Scholar]
- 9.Talbird SE, et al. Impact of population aging on the burden of vaccine-preventable diseases among older adults in the United States. Hum. Vaccin Immunother. 2021;17:332–343. doi: 10.1080/21645515.2020.1780847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect. Dis. 2021;21:203–212. doi: 10.1016/S1473-3099(20)30769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17:30. doi: 10.1186/s12877-017-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Healthy ageing and functional ability. https://www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability (2020).
- 13.World Health Organization. UN Decade of Healthy Ageing 2021-2030. https://www.who.int/initiatives/decade-of-healthy-ageing (2022).
- 14.Baeyens JP, Michel J-P. Immunization as a preventive healthcare strategy in older adults. Expert Rev. Vaccines. 2010;9:1. doi: 10.1586/erv.10.25. [DOI] [Google Scholar]
- 15.Pereira B, Xu XN, Akbar AN. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Front Immunol. 2020;11:583019. doi: 10.3389/fimmu.2020.583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun. Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty TM, Del Giudice G, Maggi S. Adult vaccination as part of a healthy lifestyle: moving from medical intervention to health promotion. Ann. Med. 2019;51:128–140. doi: 10.1080/07853890.2019.1588470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista MA, et al. Inflammaging in Endemic Areas for Infectious Diseases. Front Immunol. 2020;11:579972. doi: 10.3389/fimmu.2020.579972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Gomensoro E, Del Giudice G, Doherty TM. Challenges in adult vaccination. Ann. Med. 2018;50:181–192. doi: 10.1080/07853890.2017.1417632. [DOI] [PubMed] [Google Scholar]
- 20.Williams WW, et al. Surveillance of Vaccination Coverage Among Adult Populations - United States, 2014. MMWR Surveill. Summ. 2016;65:1–36. doi: 10.15585/mmwr.ss6501a1. [DOI] [PubMed] [Google Scholar]
- 21.Privor-Dumm L, Vasudevan P, Kobayashi K, Gupta J. Archetype analysis of older adult immunization decision-making and implementation in 34 countries. Vaccine. 2020;38:4170–4182. doi: 10.1016/j.vaccine.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laupèze B, Del Giudice G, Doherty MT, Van der Most R. Vaccination as a preventative measure contributing to immune fitness. NPJ Vaccines. 2021;6:93. doi: 10.1038/s41541-021-00354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevalier-Cottin EP, et al. Communicating Benefits from Vaccines Beyond Preventing Infectious Diseases. Infect. Dis. Ther. 2020;9:467–480. doi: 10.1007/s40121-020-00312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrico J, et al. Cost-benefit analysis of vaccination against four preventable diseases in older adults: Impact of an aging population. Vaccine. 2021;39:5187–5197. doi: 10.1016/j.vaccine.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. About Shingles (Herpes Zoster). https://www.cdc.gov/shingles/about/index.html (2019).
- 26.Chen LK, et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect. Dis. 2017;17:213. doi: 10.1186/s12879-017-2198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curran D, et al. Meta-Regression of Herpes Zoster Incidence Worldwide. Infect. Dis. Ther. 2022;11:389–403. doi: 10.1007/s40121-021-00567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serpell M, et al. Burden of post-herpetic neuralgia in a sample of UK residents aged 50 years or older: findings from the Zoster Quality of Life (ZQOL) study. Health Qual. Life Outcomes. 2014;12:92. doi: 10.1186/1477-7525-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes HJ, et al. Incidence of acute complications of herpes zoster among immunocompetent adults in England: a matched cohort study using routine health data. Br. J. Dermatol. 2021;184:1077–1084. doi: 10.1111/bjd.19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF. Eye and Periocular Skin Involvement in Herpes Zoster Infection. Med. Hypothesis Discov. Innov. Ophthalmol. 2015;4:142–156. [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18:2331216514541361. doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Oorschot D, et al. A Cross-Sectional Concept Elicitation Study to Understand the Impact of Herpes Zoster on Patients’ Health-Related Quality of Life. Infect. Dis. Ther. 2022;11:501–516. doi: 10.1007/s40121-021-00581-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran D, et al. Quality of Life Impact of an Adjuvanted Recombinant Zoster Vaccine in Adults Aged 50 Years and Older. J. Gerontol. A Biol. Sci. Med Sci. 2019;74:1231–1238. doi: 10.1093/gerona/gly150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews S, et al. An Analysis of how Herpes Zoster Pain affects Health Related Quality of Life of Placebo subjects from Three Randomized Phase III Studies. Clin. J. Pain. 2023;39:386–393. doi: 10.1097/AJP.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmader KE, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin. J. Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 36.Coplan PM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J. Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Bouhassira D, et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain. 2012;153:342–349. doi: 10.1016/j.pain.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Gater A, et al. Burden of herpes zoster in the UK: findings from the zoster quality of life (ZQOL) study. BMC Infect. Dis. 2014;14:402. doi: 10.1186/1471-2334-14-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curran D, et al. Impact of herpes zoster and postherpetic neuralgia on the quality of life of Germans aged 50 or above. BMC Infect. Dis. 2018;18:496. doi: 10.1186/s12879-018-3395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Díez-Domingo J, Curran D, Cambronero MDR, Garcia-Martinez JA, Matthews S. Economic Burden and Impact on Quality of Life of Herpes Zoster in Spanish Adults Aged 50 Years or Older: A Prospective Cohort Study. Adv. Ther. 2021;38:3325–3341. doi: 10.1007/s12325-021-01717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bricout H, et al. Burden of herpes zoster-associated chronic pain in Italian patients aged 50 years and over (2009–2010): a GP-based prospective cohort study. BMC Infect. Dis. 2014;14:637. doi: 10.1186/s12879-014-0637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toniolo-Neto J, et al. Measuring herpes zoster disease burden in Sao Paulo, Brazil: a clinico-epidemiological single-center study. Clin. (Sao Paulo) 2018;73:e243. doi: 10.6061/clinics/2018/e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizukami A, et al. Impact of Herpes Zoster and Post-Herpetic Neuralgia on Health-Related Quality of Life in Japanese Adults Aged 60 Years or Older: Results from a Prospective, Observational Cohort Study. Clin. Drug Investig. 2018;38:29–37. doi: 10.1007/s40261-017-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinke T, Glogger A, Bertrand I, Lukas K. The societal impact of herpes zoster and postherpetic neuralgia on patients, life partners, and children of patients in Germany. ScientificWorldJournal. 2014;2014:749698. doi: 10.1155/2014/749698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieu TA, et al. Community and patient values for preventing herpes zoster. Pharmacoeconomics. 2008;26:235–249. doi: 10.2165/00019053-200826030-00006. [DOI] [PubMed] [Google Scholar]
- 46.Lecrenier N, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev. Vaccines. 2018;17:619–634. doi: 10.1080/14760584.2018.1495565. [DOI] [PubMed] [Google Scholar]
- 47.Drolet M, et al. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine. 2012;30:2047–2050. doi: 10.1016/j.vaccine.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 48.van Wijck AJM, Aerssens YR. Pain, Itch, Quality of Life, and Costs after Herpes Zoster. Pain. Pr. 2017;17:738–746. doi: 10.1111/papr.12518. [DOI] [PubMed] [Google Scholar]
- 49.Warren-Gash C. Herpes Zoster: Epidemiological Links With Stroke and Myocardial Infarction. J. Infect. Dis. 2018;218:S102–S106. doi: 10.1093/infdis/jiy385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerboni L, et al. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology. 2005;332:337–346. doi: 10.1016/j.virol.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg A, et al. Comparative Immune Responses to Licensed Herpes Zoster Vaccines. J. Infect. Dis. 2018;218:S81–S87. doi: 10.1093/infdis/jiy383. [DOI] [PubMed] [Google Scholar]
- 52.Johnson MJ, et al. Cell-Mediated Immune Responses After Administration of the Live or the Recombinant Zoster Vaccine: 5-Year Persistence. J. Infect. Dis. 2022;225:1477–1481. doi: 10.1093/infdis/jiab580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson TC, et al. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. Morbidity Mortal. Wkly. Rep. 2022;71:80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parikh R, Widenmaier R, Lecrenier N. A practitioner’s guide to the recombinant zoster vaccine: review of national vaccination recommendations. Expert Rev. Vaccines. 2021;20:1065–1075. doi: 10.1080/14760584.2021.1956906. [DOI] [PubMed] [Google Scholar]
- 55.Lal H, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham AL, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 57.Willer DO, et al. Efficacy of the adjuvanted recombinant zoster vaccine (RZV) by sex, geographic region, and geographic ancestry/ethnicity: A post-hoc analysis of the ZOE-50 and ZOE-70 randomized trials. Vaccine. 2019;37:6262–6267. doi: 10.1016/j.vaccine.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 58.Oostvogels L, et al. Medical conditions at enrollment do not impact efficacy and safety of the adjuvanted recombinant zoster vaccine: a pooled post-hoc analysis of two parallel randomized trials. Hum. Vaccin. Immunother. 2019;15:2865–2872. doi: 10.1080/21645515.2019.1627818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JH, et al. The adjuvanted recombinant zoster vaccine is efficacious and safe in Asian adults ≥ 50 years of age: a sub-cohort analysis of the ZOE-50 and ZOE-70 randomized trials. Hum. Vaccin Immunother. 2021;17:2050–2057. doi: 10.1080/21645515.2020.1859321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunningham AL, et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J. Infect. Dis. 2018;217:1750–1760. doi: 10.1093/infdis/jiy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lal H, et al. Immunogenicity, reactogenicity and safety of 2 doses of an adjuvanted herpes zoster subunit vaccine administered 2, 6 or 12 months apart in older adults: Results of a phase III, randomized, open-label, multicenter study. Vaccine. 2018;36:148–154. doi: 10.1016/j.vaccine.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Curran D, et al. Recombinant zoster vaccine is efficacious and safe in frail individuals. J. Am. Geriatr. Soc. 2021;69:744–752. doi: 10.1111/jgs.16917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bastidas A, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322:123–133. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dagnew AF, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect. Dis. 2019;19:988–1000. doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- 65.Strezova A, et al. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: interim efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect. Dis. 2022;9:ofac485. doi: 10.1093/ofid/ofac485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghaswalla PK, et al. Hepatitis A, B, and A/B vaccination series completion among US adults: A claims-based analysis. Hum. Vaccines Immunotherapeutics. 2018;14:2780–2785. doi: 10.1080/21645515.2018.1489189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cubanski, J., Neuman, T. & Damico, A. Who Didn’t Get a Second Shingrix Shot? Implications for Multidose COVID-19 Vaccines. https://www.kff.org/medicare/issue-brief/who-didnt-get-a-second-shingrix-shot-implications-for-multidose-covid-19-vaccines/ (2020).
- 68.Dooling, K. Herpes zoster vaccines: update. https://stacks.cdc.gov/view/cdc/78105 (2019).
- 69.Lopez-Fauqued M, et al. Safety profile of the adjuvanted recombinant zoster vaccine: Pooled analysis of two large randomised phase 3 trials. Vaccine. 2019;37:2482–2493. doi: 10.1016/j.vaccine.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 70.Ocran-Appiah J, et al. Safety of the adjuvanted recombinant zoster vaccine in adults aged 50 years or older. A phase IIIB, non-randomized, multinational, open-label study in previous ZOE-50 and ZOE-70 placebo recipients. Vaccine. 2021;39:6–10. doi: 10.1016/j.vaccine.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 71.Goud R, et al. Risk of Guillain-Barré syndrome following recombinant zoster vaccine in medicare beneficiaries. JAMA Intern Med. 2021;181:1623–1630. doi: 10.1001/jamainternmed.2021.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shingrix. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/shingrix-epar-product-information_en.pdf (2022).
- 73.Kim JH, et al. Adjuvanted recombinant zoster vaccine decreases herpes zoster-associated pain and the use of pain medication across 3 randomized, placebo-controlled trials. Pain. 2023;164:741–748. doi: 10.1097/j.pain.0000000000002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curran D, et al. Recombinant Zoster Vaccine Significantly Reduces the Impact on Quality of Life Caused by Herpes Zoster in Adult Autologous Hematopoietic Stem Cell Transplant Recipients: A Randomized Placebo-Controlled Trial (ZOE-HSCT) Biol. Blood Marrow Transpl. 2019;25:2474–2481. doi: 10.1016/j.bbmt.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 75.Patterson BJ, et al. Incremental clinical and economic impact of recombinant zoster vaccination: real-world data in a budget impact model. J. Manag Care Spec. Pharm. 2020;26:1567–1575. doi: 10.18553/jmcp.2020.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee C, et al. Potential public health impact of the adjuvanted recombinant zoster vaccine among people aged 50 years and older in Beijing. Hum. Vaccin. Immunother. 2021;17:3735–3746. doi: 10.1080/21645515.2021.1932216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curran D, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States who have been previously vaccinated with zoster vaccine live. Hum. Vaccin. Immunother. 2019;15:765–771. doi: 10.1080/21645515.2018.1558689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giannelos N, Ng C, Curran D. Cost-effectiveness of the recombinant zoster vaccine (RZV) against herpes zoster: An updated critical review. Hum. Vaccin. Immunother. 2023;19:2168952. doi: 10.1080/21645515.2023.2168952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curran D, et al. Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany. Hum. Vaccin. Immunother. 2021;17:5296–5303. doi: 10.1080/21645515.2021.2002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnier C, Janbek J, Lathe R, Haas J. Reduced dementia incidence after varicella zoster vaccination in Wales 2013–2020. Alzheimer’s Dement.: Transl. Res. Clin. Interventions. 2022;8:e12293. doi: 10.1002/trc2.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris K, et al. The impact of routine vaccinations on alzheimer’s disease risk in persons 65 years and older: a claims-based cohort study using propensity score matching. J. Alzheimer’s Dis. 2023;95:703–718. doi: 10.3233/JAD-221231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sustainable Development Solutions Network. World Happiness Report. https://worldhappiness.report/ed/2021/ (2021).
- 83.McKinsey Health Institute. Adding years to life and life to years. https://www.mckinsey.com/mhi/our-insights/adding-years-to-life-and-life-to-years#/ (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this review were obtained from publicly available sources and cited accordingly. The references for each study or publication included in this review are provided for further reading and verification. No additional datasets were generated or analyzed specifically for this manuscript.