Abstract
Bacillus anthracis, the etiological agent of anthrax, is a gram-positive spore-forming bacterium. Fully virulent bacilli are toxinogenic and capsulated. Two abundant surface proteins, including the major antigen, are components of the B. anthracis surface layer (S-layer). The B. anthracis paracrystalline S-layer has previously only been found in noncapsulated vegetative cells. Here we report that the S-layer proteins are also synthesized under conditions where the poly-γ-d-glutamic acid capsule is present. Structural and immunological analyses show that the capsule is exterior to and completely covers the S-layer proteins. Nevertheless, analysis of single and double S-layer protein mutants shows that the presence of these proteins is not required for normal capsulation of the bacilli. Similarly, the S-layer proteins assemble as a two-dimensional crystal, even in the presence of the capsule. Thus, both structures are compatible, and yet neither is required for the correct formation of the other.
Bacillus anthracis, a gram-positive spore-forming bacterium, is the causative agent of anthrax. This disease, to which many animals, including humans, are susceptible, involves toxemia and septicemia. In the mammalian host, B. anthracis bacilli synthesize two toxins (lethal and edema toxins) (31) and a capsule (18) encoded by two large plasmids, pXO1 and pXO2, respectively (12, 21). The capsule is composed of poly-γ-d-glutamic acid and has antiphagocytic properties (13, 31, 37). Although unusual, a similar capsule is also found on Bacillus licheniformis bacilli (9). In the absence of pXO2 or the inducer bicarbonate, the cell does not produce a capsule and the cell wall appears layered. These layers are composed of fragments displaying a highly patterned ultrastructure (10, 16). This type of cell surface is now referred to as the surface layer (S-layer).
S-layers are present on the surfaces of many archaea and bacteria (for reviews, see references 29 and 30). Most are formed by noncovalent, entropy-driven assembly of a single (glyco)protein protomer on the bacterial surface, giving rise to proteinaceous paracrystalline layers. Generally, a single S-layer is present, constituting 5 to 10% of total cell protein. Its synthesis is thus presumably energy consuming for the bacterium. Numerous bacteria have S-layers, suggesting that they play important roles in the interaction between the cell and its environment. Various functions have been proposed for S-layers, including shape maintenance and molecular sieving, and they can serve in phage fixation. The S-layer may be a virulence factor, protecting pathogenic bacteria against complement killing, facilitating binding of bacteria to host molecules, or enhancing their ability to associate with macrophages (for reviews, see references 27 and 29).
Some bacteria, such as cyanobacteria or Azotobacter spp., possess both a capsule and an S-layer; however, to our knowledge, their structural relationships have not been analyzed through simultaneous genetic and cytologic studies. Both of these features have been independently described for the surface of the pathogenic bacterium B. anthracis. The components of the B. anthracis S-layer are two abundant surface proteins, EA1 and Sap (6, 20). Previous analyses of the B. anthracis S-layer used plasmid-cured strains; consequently, the interaction, if any, between the capsule and the S-layer could not be studied. Temporal or environmental regulation could be such that only one or the other structure is ever present at the cell surface. However, we show that S-layer proteins are synthesized under conditions where the bacilli are capsulated. We determined the localizations of capsule and S-layer components and analyzed whether the S-layer is necessary for proper capsulation. Finally, the assembly of the S-layer proteins in a two-dimensional crystal was examined in the presence of the capsule.
MATERIALS AND METHODS
Plasmids, bacterial strains, mating experiments, and culture conditions.
The plasmids used to disrupt sap (encoding Sap), eag (encoding EA1), and both genes, i.e., pEAI207, pSAL322, and pSAL303, respectively, were described previously (6, 20) and are listed in Table 1. The construction of B. anthracis CAF10, a pXO2 transductant of plasmidless strain 9131, and its regulation of capsule synthesis have already been reported (8). Escherichia coli JM83 harboring pRK24 was used for mating experiments (34, 35). Allelic exchange was carried out as previously described (26) with the spectinomycin resistance cassette as a selectable marker (24). sap, eag, and both genes were disrupted in CAF10 by heterogramic conjugation, giving CBA91, CSM91, and CSM11, respectively (Table 1). E. coli cells were grown in Luria broth or on L agar plates (22). Capsule synthesis was induced by growing B. anthracis cells in brain heart infusion medium (Difco Laboratories) in the presence of 0.6% sodium bicarbonate or on CAP plates (28) in a 5% CO2 atmosphere for electron microscopy. Antibiotics were used at the following concentrations: kanamycin, 40 μg/ml for E. coli; erythromycin, 5 μg/ml for B. anthracis; and spectinomycin, 60 μg/ml for both E. coli and B. anthracis.
TABLE 1.
B. anthracis strains and relevant plasmids used in this study
| Strain or plasmid | Relevant characteristics | Phenotype or markera | Reference or source |
|---|---|---|---|
| B. anthracis strains | |||
| CAF10 | pXO1− pXO2+ | Cap+ EA1+ Sap+ | 8 |
| CBA91 | Δsap derivative of CAF10 via allelic exchange between pEAI207 and CAF10 chromosome | Cap+ EA1+ | This study |
| CSM91 | Δeag derivative of CAF10 via allelic exchange between pSAL322 and CAF10 chromosome | Cap+ Sap+ | This study |
| CSM11 | Δsap Δeag derivative of CAF10 via allelic exchange between pSAL303 and CAF10 chromosome | Cap+ | This study |
| Plasmids | |||
| pEAI207 | Conjugative suicide plasmid carrying deleted sap gene | Err Knr Spr | 6 |
| pSAL322 | Conjugative suicide plasmid carrying deleted eag gene | Err Knr Spr | 20 |
| pSAL303 | Conjugative suicide plasmid carrying deleted eag and sap genes | Err Knr Spr | 20 |
Err, Knr, and Spr, resistance to erythromycin, kanamycin, and spectinomycin, respectively.
Protein analysis.
To test the in vivo expression of EA1 and Sap, the synthesis of antibodies was assayed. The rationale of this experiment is that antibodies are produced only if the antigen is synthesized in vivo. Seven Swiss mice were injected with 106 spores of strain CAF10 and sacrificed after 30 days. Their sera were pooled. The gel loading samples were obtained as follows. B. anthracis cells were harvested at an optical density at 600 nm of ≅2. Pellets were washed in 25 mM Tris-HCl (pH 8.0), sonicated until complete clarification, and resuspended in Laemmli buffer (19). Samples (3 μg of pellet protein and 20 μl of trichloroacetic acid-precipitated supernatant protein) were loaded on sodium dodecyl sulfate–10% polyacrylamide gels. Separated proteins were transferred to nitrocellulose sheets by use of the Bio-Rad Trans-Blot system. The sera were used at a 1/200 dilution. Western blots were developed with the ECL Western blotting analysis system (Amersham), with a 1/10,000 dilution of the second antibody.
Capsule observation.
The aspect and homogeneity of capsulation were checked by India ink exclusion (4).
Electron microscopy. (i) Thin sections.
Cells were fixed with 2% formaldehyde (made freshly from paraformaldehyde) and 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) containing 5 mM CaCl2 (14, 17). After being washed, the cells were postfixed for 2 h with 2% OsO4 in the same buffer. The pelleted bacteria were embedded in 2% low-melting-point agar (type IX; Sigma) (36). The samples were then treated for 16 h with 0.5% uranyl acetate in water. After extensive washing, small blocks were dehydrated with alcohol and embedded in Spurr’s medium (Ladd Inc.) (32). Thin sections were stained conventionally and observed with a Philips CM12 electron microscope.
(ii) Immunocytochemistry with thin sections.
B. anthracis cells were fixed with 2% formaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate-buffered saline (14 mM Na2HPO4, 7 mM NaH2PO4, 150 mM NaCl) (PBS) (pH 7.4) for 1 h, rinsed in the same buffer, and embedded in 2% low-melting-point agar (36). Small blocks containing bacteria were embedded in Lowicryl HM20 (Polysciences Ltd.) at −50°C following the progressively lower temperatures protocol of Carlemalm et al. (3) as described by Newman and Hobot (25). Thin sections were collected onto Formvar-carbon-coated nickel grids and incubated successively at room temperature with the following solutions: PBS–50 mM NH4Cl for 10 min; PBS–1% bovine serum albumin (BSA)–1% normal goat serum–0.1% Tween 20 for 10 min; specific anti-EA1 or anti-Sap antibodies diluted 1/50 in PBS–1% BSA–1% normal goat serum–0.1% Tween 20 for 1 h; PBS–0.1% BSA three times for 5 min each time; goat immunoglobulin G (heavy and light chains) anti-rabbit immunoglobulin–gold conjugate diluted 1/20 in PBS–0.01% gelatin for 1 h; PBS three times for 5 min each time; PBS–1% glutaraldehyde for 5 min; and five times with water. The thin sections were then stained by incubation with 2% uranyl acetate in water for 35 min and then in lead tartrate for 2 min (23).
(iii) Immunocytochemistry with whole-mount cells.
Immunocytochemistry with whole-mount cells was carried out as previously described (20).
(iv) Negative staining experiments.
B. anthracis cells were resuspended in a 1/10 volume of 25 mM Tris-HCl (pH 8.0)–10 mM MgCl2 with 0.25 or 0.5% glutaraldehyde for EA1 or Sap, respectively, in the presence of approximately 30 μl of 425- to 600-μm glass beads (Sigma) and disrupted by vortexing for 30 s. This treatment disintegrated the capsule. Negative staining was performed as previously described (20). Micrographs were recorded with a Philips CM12 electron microscope under low-dose (17 electrons/Å/s) transmission electron microscopy conditions.
RESULTS
Cosynthesis and respective localization of the capsule and the S-layer components.
All reported data on the B. anthracis S-layer is from noncapsulated strains (6, 7, 10, 16, 20). We therefore investigated whether the capsule and the S-layer components, EA1 and Sap, could all be simultaneously present. The genes for EA1 and Sap are chromosomal and have been well characterized for the plasmid-free strain 9131 (20). We therefore used strain CAF10, a pXO2 transductant of strain 9131 (8), to address the question.
CAF10 was grown in the presence of bicarbonate to induce capsule synthesis, stained with India ink, and examined by phase-contrast microscopy. The vast majority of the bacteria were capsulated (Fig. 1A). The presence of EA1 or Sap in the pellets or the supernatants was verified by Western blot analyses and suggested that, in vitro in a given population, the poly-γ-d-glutamate capsule, EA1, and Sap could be simultaneously present (data not shown).
FIG. 1.
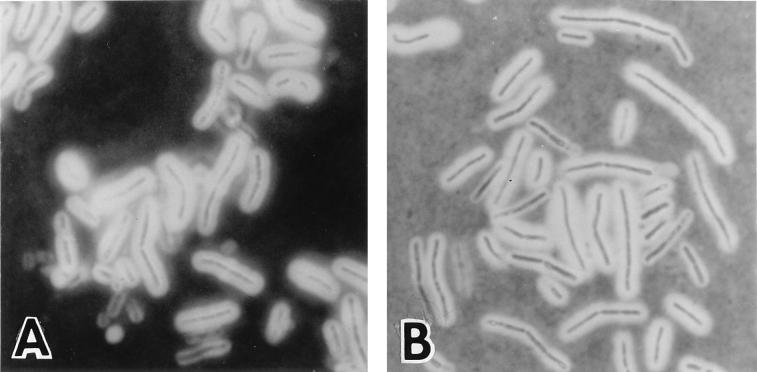
Homogeneity of the capsulation state of B. anthracis cells. Cultures of CAF10 (A) or of its derivative, CSM11, with deletions of both S-layer genes (B), grown in capsule synthesis-inducing conditions were incubated in the presence of India ink. The capsule appears as a bright halo surrounding the cells under the light microscope. Magnification, ×1,600.
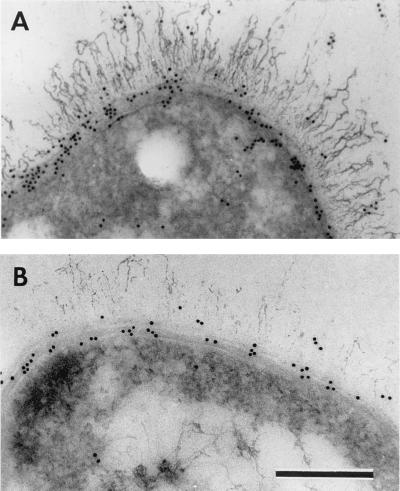
These prior experiments showed that a population of cells could synthesize both capsule and S-layer proteins but could not definitively prove that a single cell possessed both at the same time. To confirm that individual bacterial cells harbored these three components simultaneously, we analyzed capsulated CAF10 bacteria by immunoelectron microscopy (Fig. 2A and B and Fig. 3A). Optimized capsule visualization (Fig. 3) and immunolabeling (Fig. 2) are not compatible. However, despite these technical limitations, the capsule was visible in thin sections where EA1 (Fig. 2A) and Sap (Fig. 2B) were highlighted by the corresponding antibodies. This result indicated that on the surface of CAF10 bacilli grown in the presence of bicarbonate, EA1, Sap, and the capsule were present simultaneously. Moreover, the sites of antibody binding indicated that the corresponding antigens were localized under the capsule (Fig. 2A and B). In thin sections (Fig. 3A), EA1 and Sap were found between the capsule and the peptidoglycan. A complementary immunoelectron microscopy approach was used to examine whole-mount cells incubated with specific antibodies. In noncapsulated strains, i.e., 9131 (20) and noninduced CAF10 bacilli (Fig. 4A and C), the anti-Sap and anti-EA1 antibodies covered the entire bacterial surface, whereas no antibody was detected on the cell surface of CAF10 grown in conditions inducing capsule synthesis (Fig. 4B and D). Labeling, such as in Fig. 4B, was infrequently observed in preparations of capsulated cells and presumably corresponded to the leakage of S-layer components from the bacteria through the capsule. This result indicated that the capsule is distal to the S-layer components and that it completely masks access of the specific antibodies to EA1 and Sap. Capsule and S-layer components can therefore coexist, and the S-layer proteins are localized between the peptidoglycan and the capsule.
FIG. 2.
Immunolabeling of S-layer proteins in capsulated CAF10 bacteria. Thin sections were incubated with anti-EA1 (A) or anti-Sap (B) antibodies and then with 10-nm gold-conjugated anti-rabbit antibodies. The capsule is visible in these micrographs but was only weakly stained with the protocol used. Bar, 200 nm.
FIG. 3.
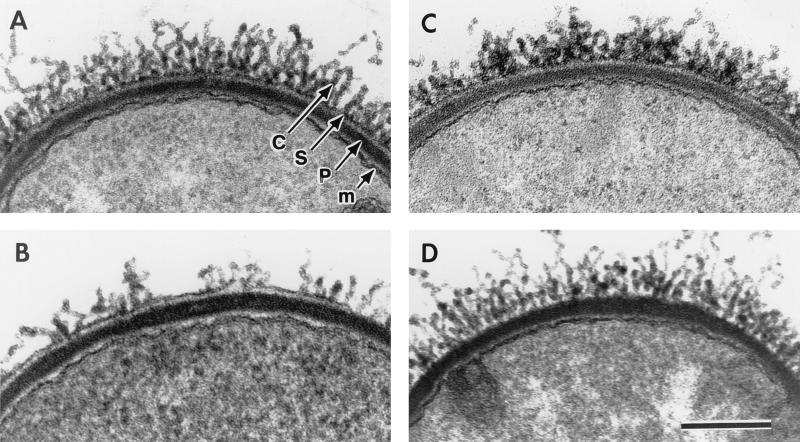
Capsule visualization in CAF10 and its various S-layer gene deletion derivatives. (A) CAF10 (EA1+ Sap+). (B) CBA91 (EA1+). (C) CSM91 (Sap+). (D) CSM11. Thin sections of cultures grown in capsule synthesis-inducing conditions were treated during fixation and embedding to highlight the peptidoglycan (p), the S-layer (s), and the fibrillar capsule (c) (see Materials and Methods). The cytoplasmic membrane is also indicated (m). The cell surface components (p, s, c, and m) are indicated in panel A. Note that the S-layer is absent from the bacterium in panel D. Bar, 250 nm.
FIG. 4.
Whole-mount noncapsulated (A and C) and capsulated (B and D) CAF10 bacteria immunolabeled with anti-Sap (A and B) and anti-EA1 (C and D) antibodies. Preparations of cultures were incubated with anti-Sap or anti-EA1 antibodies, and binding was revealed with 10-nm gold-conjugated anti-rabbit antibodies. Bar, 1 μm.
Analysis of the state of capsulation of strains with a deletion of S-layer component genes.
We determined whether the S-layer proteins were required for normal capsulation of the bacteria. Mutants with deletions of the EA1 gene (CSM91), the Sap gene (CBA91), or both eag and sap (CSM11) were constructed as previously described (20) (Table 1). The pellet and supernatant fractions of CAF10 derivatives grown in capsule synthesis-inducing conditions were analyzed by polyacrylamide gel electrophoresis and immunoblotting with anti-EA1 and anti-Sap antibodies (data not shown). The results indicated that EA1 and Sap are expressed similarly in the presence and the absence of the capsule and also that, in both cases, Sap is shed into the supernatant.
Each strain was grown on CAP plates. The colonies were smooth, suggesting that a capsule was synthesized. The presence of the capsule around the bacteria was confirmed by optical microscopy (Fig. 1A and B). Bacilli from wild-type and S-layer mutants were all capsulated, and no obvious difference in the aspect of capsulation could be seen. The capsule was studied in more detail by electron microscopy (Fig. 3A to D). The micrographs showed that similar amounts of capsule were found around all bacteria tested. This result indicated that the S-layer components, EA1 and Sap, are not required for normal capsulation of B. anthracis bacilli.
Coexistence of the capsule and of the structured S-layer.
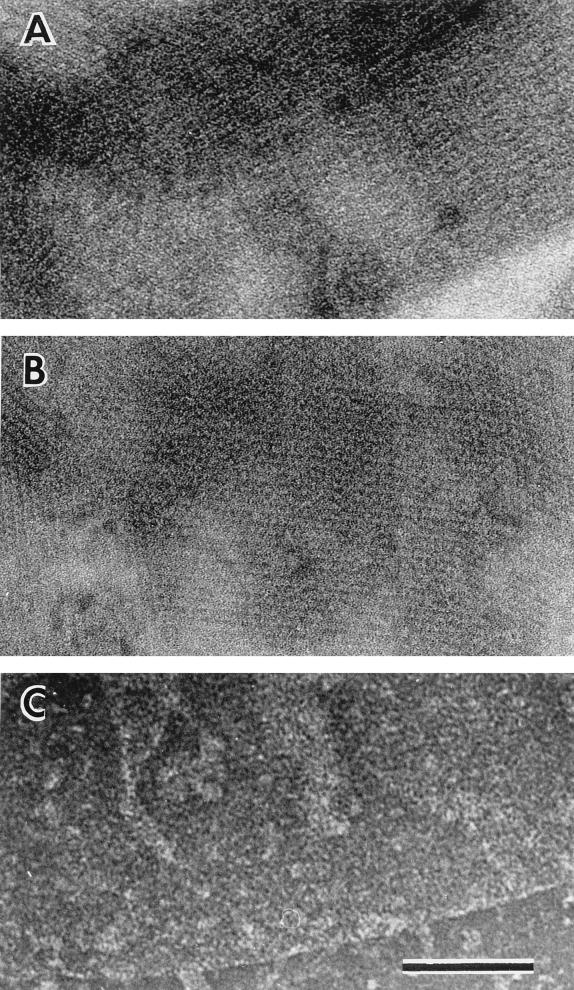
We tested whether EA1 and Sap are organized in a two-dimensional crystalline array when covered by the capsule. Thin sections (Fig. 3A to C) suggested that the S-layer proteins were organized in sheaths. The surfaces of strains CAF10 (EA1+ Sap+), CBA91 (EA1+), CSM91 (Sap+), and CSM11 grown in capsule synthesis-inducing conditions were further analyzed for the presence of structured layers by negative staining (Fig. 5). The cells were vortexed in the presence of glass beads (20). This treatment disrupted the bacteria and tore off the capsule, thus unmasking the S-layers. As expected, no crystalline array was present on the surface of CSM11 cells (data not shown). Conversely, structured layers were clearly visible on CAF10, CBA91, and CSM91 cells (Fig. 5A, B, and C, respectively). This result suggested that in the presence of the capsule, the S-layer components, EA1 and Sap, were able to form structured surface arrays. However, the lattices on these various strains appeared different. The EA1 array was more stable than the Sap array, which was only observed at glutaraldehyde concentrations higher than those required for EA1. In addition, this is the first time that a Sap array has been visualized. Our observations are consistent with the previous suggestion that Sap forms its own, more fragile, structure (20). They also show that the S-layer and capsule structures coexist on the same cell surface.
FIG. 5.
Negative staining of B. anthracis S-layer fragments. Fragments were obtained from disrupted capsulated CAF10 (A), CBA91 (B), or CSM91 (C) cells. Bar, 200 nm.
In vivo production of the capsule and the S-layer.
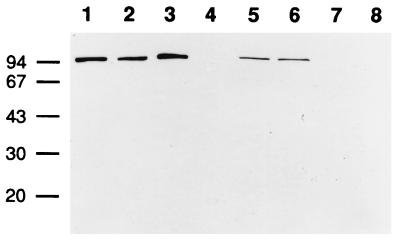
To determine whether both the capsule and the S-layer could be produced in vivo, the presence of anti-EA1 and anti-Sap antibodies was tested in sera from mice infected with strain CAF10, which is capsulated in vivo (Fig. 6). Sera from infected mice recognized EA1 and Sap but no other bacterial protein under the conditions used. The specificities of the antibodies were confirmed with sera adsorbed onto either EA1 or Sap (data not shown). EA1 and Sap were found to be major surface antigens, showing that both EA1 and Sap can be synthesized by a capsulated strain in vivo.
FIG. 6.
In vivo expression of the two B. anthracis S-layer components. Immunoblotting of pellet fractions (odd-numbered lanes) and supernatant fractions (even-numbered lanes) of S-layers from wild-type (CAF10) (lanes 1 and 2), Δsap (CBA91) (lanes 3 and 4), Δeag (CSM91) (lanes 5 and 6), and Δeag Δsap (CSM11) (lanes 7 and 8) strains was carried out with pooled sera from mice infected with the CAF10 strain. Molecular masses are indicated in kilodaltons on the left.
DISCUSSION
Although various bacteria from natural environments possess both a capsule and an S-layer, their cosynthesis or structural relationship has rarely been studied (15).
B. anthracis has a rather unusual capsule: it is composed not of polysaccharide but of poly-γ-d-glutamic acid (13). This bacterium also has an S-layer, previously evidenced only in capsule-free strains (10, 16). Another gram-positive bacterium, B. licheniformis, apparently shares these features, namely, a poly-γ-d-glutamic acid capsule and an S-layer (9, 33). However, this B. licheniformis strain, in which the S-layer component is very similar to EA1 (20), seems to lack a capsule. We therefore investigated whether these two structures, the capsule and the S-layer, were exclusive. We found that B. anthracis bacilli synthesize EA1, Sap, and the capsule both in vivo and in vitro. Furthermore, the capsule and a structured S-layer were found to be simultaneously present on the bacterial surface, the capsule covering the S-layer. Thus, B. anthracis displays a highly complex ultrastructural cell wall architecture.
The coexistence of the capsule and the S-layer could have indicated a structural dependence. Such was not the case, as the S-layer was found in noncapsulated strains and the capsule was present on the EA1-Sap double deletion mutant. These results further suggest that the capsule is anchored either to the peptidoglycan-containing sacculus or to the cytoplasmic membrane, independently of the S-layer. However, the fine structure of the capsule may depend on the presence of the underlying S-layer: the S-layer may modify the arborescence of the poly-γ-d-glutamic acid fibers. That these structures can be independently synthesized and formed does not exclude functional interactions.
Pathogenic organisms have various strategies to escape host recognition. One such strategy, which is widespread, is antigenic variation of exposed proteins, including S-layer proteins. For example, in Campylobacter fetus, genetic rearrangements enable the bacterium to change S-layer components (2, 5). The variants can therefore multiply before the antibody response has developed against the new protein. No gene rearrangement between the B. anthracis S-layer genes has been observed (data not shown). The absence of immunolabeling on B. anthracis whole cells (Fig. 4B and D) in the presence of the capsule suggests that the cell surface is inaccessible to antibodies. The presence of anti-EA1 and anti-Sap antibodies in the sera of mice inoculated with strain CAF10 (Fig. 6) indicates that these proteins are synthesized in vivo by the capsulated strain. The presence of these antibodies could be due to the synthesis of these proteins prior to the complete coverage of the surface by the capsule or to leakage or bacterial lysis. Interestingly, the capsule seems to function as a “one-way” filter. EA1 and Sap are not accessible to antibodies from the outside, whereas the three toxin components (protective antigen, lethal factor, and edema factor) and Sap are found in culture supernatants of capsulated strains, suggesting that they diffuse from the cell through the capsule to the extracellular medium.
The S-layer may have a protective role in the absence of the capsule. It could also be a molecular sieve or could have a still more structural role, delimiting the periplasm, as recently described for gram-positive bacteria (1, 11).
ACKNOWLEDGMENTS
We are grateful to A. L. Sonenshein for critical reading of the manuscript. We thank B. Chavinier-Jove and C. Rolin for excellent technical assistance with electron microscopy experiments and photographic prints, respectively.
S.M. was supported by the Ministère de l’Enseignement Supérieur et de la Recherche.
REFERENCES
- 1.Beveridge T J. The periplasmic space and the periplasm in gram-positive and gram-negative bacteria. ASM News. 1995;61:125–130. [Google Scholar]
- 2.Blaser M J, Wang E, Tummuru M K R, Washburn R, Fujimoto S, Labigne A. High frequency S-layer protein variation in Campylobacter fetus revealed by sapA mutagenesis. Mol Microbiol. 1994;14:521–532. doi: 10.1111/j.1365-2958.1994.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 3.Carlemalm E, Garavito R M, Villiger W. Resin development for electron microscopy and an analysis of embedding at low temperature. J Microsc. 1982;126:123. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 4.Duguid J P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951;63:673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin J, Blaser M J. Nested DNA inversion as a paradigm of programmed gene rearrangement. Proc Natl Acad Sci USA. 1997;94:985–990. doi: 10.1073/pnas.94.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etienne-Toumelin I, Sirard J-C, Duflot E, Mock M, Fouet A. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J Bacteriol. 1995;177:614–620. doi: 10.1128/jb.177.3.614-620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farchaus J W, Ribot W J, Downs M B, Ezzell J W. Purification and characterization of the major surface array protein from the avirulent Bacillus anthracis Delta Sterne-1. J Bacteriol. 1995;177:2481–2489. doi: 10.1128/jb.177.9.2481-2489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouet A, Mock M. Differential influence of the two Bacillus anthracis plasmids on regulation of virulence gene expression. Infect Immun. 1996;64:4928–4932. doi: 10.1128/iai.64.12.4928-4932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner J M, Troy F A. Chemistry and biosynthesis of the poly (γ-d-glutamyl) capsule in Bacillus licheniformis. Activation, racemization, and polymerization of glutamic acid by a membranous polyglutamyl synthetase complex. J Biol Chem. 1979;254:6262–6269. [PubMed] [Google Scholar]
- 10.Gerhardt P. Cytology of Bacillus anthracis. Fed Proc. 1967;26:1504–1517. [PubMed] [Google Scholar]
- 11.Graham L L, Beveridge T J, Nanninga N. Periplasmic space and the concept of periplasm. Trends Biochem Sci. 1991;16:328–329. doi: 10.1016/0968-0004(91)90135-i. [DOI] [PubMed] [Google Scholar]
- 12.Green B D, Battisti L, Koehler T M, Thorne C B, Ivins B E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun. 1985;49:291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanby W E, Rydon H N. The capsular substance of Bacillus anthracis. Biochem J. 1946;40:297–309. [PubMed] [Google Scholar]
- 14.Hayat M A. Fixation for electron microscopy. New York, N.Y: Academic Press, Inc.; 1981. pp. 110–111. [Google Scholar]
- 15.Hoiczyk E, Baumeister W. Envelope structure of four gliding filamentous cyanobacteria. J Bacteriol. 1995;177:2387–2395. doi: 10.1128/jb.177.9.2387-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt S C, Leadbetter E R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969;33:346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karnovsky M J. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137A. [Google Scholar]
- 18.Keppie J, Smith H, Harris-Smith P W. The chemical basis of the virulence of Bacillus anthracis. II. Some biological properties of bacterial products. Br J Exp Pathol. 1953;34:486–496. [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Mesnage S, Tosi-Couture E, Mock M, Gounon P, Fouet A. Molecular characterization of the Bacillus anthracis main S-layer component: evidence that it is the major cell-associated antigen. Mol Microbiol, 1997;23:1147–1155. doi: 10.1046/j.1365-2958.1997.2941659.x. [DOI] [PubMed] [Google Scholar]
- 21.Mikesell P, Ivins B E, Ristroph J D, Dreier T M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983;39:371–376. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Millonig G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3")(9) Mol Gen Genet. 1985;200:33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- 25.Newman G R, Hobot J A. Resin microscopy and on-section immunocytochemistry. Berlin, Germany: Springer-Verlag KG; 1993. pp. 62–65. [Google Scholar]
- 26.Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sara M, Egelseer E M. Functional aspects of S-layers. In: Sleytr U W, Messner P, Pum D, Sara M, editors. Crystalline bacterial cell surface proteins. Austin, Tex: Academic Press, Inc., and R. G. Landes Co.; 1996. pp. 103–131. [Google Scholar]
- 28.Sirard J C, Mock M, Fouet A. Molecular tools for the study of transcriptional regulation in Bacillus anthracis. Res Microbiol. 1995;146:729–737. doi: 10.1016/0923-2508(96)81069-2. [DOI] [PubMed] [Google Scholar]
- 29.Sleytr U B, Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- 30.Sleytr U B, Messner P, Pum D, Sara M. Occurrence, location, ultrastructure and morphogenesis of S-layers. In: Sleytr U W, Messner P, Pum D, Sara M, editors. Crystalline bacterial cell surface proteins. Austin, Tex: Academic Press, Inc., and R. G. Landes Co.; 1996. pp. 5–33. [Google Scholar]
- 31.Smith H, Keppie H S, Stanley J I. The chemical basis of the virulence of Bacillus anthracis. I. Properties of bacteria grown in vivo and preparation of extracts. Br J Exp Pathol. 1953;34:477–485. [PMC free article] [PubMed] [Google Scholar]
- 32.Spurr A R. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–33. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 33.Tang M, Owens K, Pietri R, Zhu X, McVeigh R, Ghosh B K. Cloning of the crystalline cell wall protein gene of Bacillus licheniformis NM105. J Bacteriol. 1989;171:6637–6648. doi: 10.1128/jb.171.12.6637-6648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trieu-Cuot P, Carlier C, Martin P, Courvalin P. Plasmid transfer by conjugation from Escherichia coli to Gram-positive bacteria. FEMS Microbiol Lett. 1987;48:289–294. [Google Scholar]
- 35.Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P. An integrative vector exploiting the transposition properties of Tn1545 for insertional mutagenesis and cloning of genes from Gram-positive bacteria. Gene. 1991;106:21–27. doi: 10.1016/0378-1119(91)90561-o. [DOI] [PubMed] [Google Scholar]
- 36.Wood J I, Klomparens K L. Characterization of agarose as an encapsulation medium for particulate specimens for transmission electron microscopy. Microsc Res Tech. 1993;25:267–275. doi: 10.1002/jemt.1070250402. [DOI] [PubMed] [Google Scholar]
- 37.Zwartouw H T, Smith H. Polyglutamic acid from Bacillus anthracis grown in vivo: structure and aggressin activity. Biochem J. 1956;63:437–454. doi: 10.1042/bj0630437. [DOI] [PMC free article] [PubMed] [Google Scholar]