Abstract
Food waste and food loss has been a growing concern in the manufacturing industry with a gap between identifying the problem and implementing a solution. The manufacturing process of chicken is largely automated by conveyor belts and machines in which initial application of either peroxyacetic acid (PAA) or sodium hypochlorite (chlorine) solution is utilized to reduce the microbial load and prevent food borne illnesses on the chicken products as they are processed and packaged for distribution. However, during this automated process whole chickens can drop from the manufacturing line and become contaminated leading to the disposal and waste of the product. A solution to reduce food waste was to analyze a reconditioning procedure within the manufacturing process. The study evaluated the aerobic microbial growth on salvaged marinated deli raw whole chickens without giblets (WOGs) from conveyor belt loss reconditioned in either PAA or sodium hypochlorite (chlorine) solution to undropped chicken WOGs. Chicken rinsate and segmented samples were collected from each parameter and tested for microbial growth using Petrifilm aerobic plate count (APC) plates and converting results into log colony forming units (CFU). A difference (P < 0.05) was observed with the reconditioning of the WOGs in PAA (0.71 log10 CFU/mL) compared to the control (1.45 ± 0.26 log10 CFU/mL), for rinses. Of the segmented samples, the trussing strings displayed a significant decrease in APC counts for both chlorine (2.30 ± 0.49 log10 CFU/g) and PAA (2.3 ± 0.49 log10 CFU/g) reconditioning compared to the control (2.72 ± 0.39 log10 CFU/g). Reconditioning of salvaged deli chicken WOGs in chlorine or PAA is comparable to or better than the conventional process for the reduction of APC, it is an effective strategy to reintroduce dropped marinated deli chicken WOGs to the manufacturing line and can reduce food waste at a manufacturing level.
Key words: reconditioned, aerobic plate count, food loss and food waste
INTRODUCTION
Food loss and waste is a growing concern all over the world. The concern occurs both at the business and retail levels as well as in our own homes. It is estimated, globally, that around 14% of food produced is lost between harvest and retail, and 17% of total global food production is wasted (United Nations, 2022). In the United States, it is estimated that 30 to 40% of the food supply is wasted with 31% food loss at the retail and consumer levels (USDA, 2022; U.S. FDA, 2022). Reducing food loss and waste can significantly impact households, companies, and the environment.
The gap between knowing the food loss and waste problem and implementing a solution resides in education and communication (USDA, 2022; U.S. FDA, 2022). In 2018 the U.S. Department of Agriculture (USDA), U.S. Food and Drug Administration (FDA), and U.S. Environmental Protection Agency (EPA) approved a joint agency formal agreement aimed to improve coordination and communication across federal agencies to better educate the impacts and importance of reducing food loss and waste (USDA, 2022; U.S. EPA, 2022; U.S. FDA, 2022). The 3 agencies prioritized 6 action areas to coordinate efforts: 1) Enhance interagency coordination, 2) Increase consumer education and outreach efforts, 3) Improve coordination and guidance on food loss and waste measurement, 4) Clarify and communicate information on food safety, food date labels, and food donations, 5) Collaborate with private industry to reduce food loss and waste across the supply chain and, 6) Encourage food waste reduction by federal agencies in their respective facilities (USDA, 2022; U.S. EPA, 2022; U.S. FDA, 2022). Consumer awareness of food loss and waste has improved in households and retail businesses (i.e., grocery stores), however food manufacturing companies are sometimes in the dark about where food loss occurs during production and the financial impact it may cause on their operation (Beurteaux, 2022). An estimated 20% revenue loss can be attributed to food loss during production due to inefficient processes (Beurteaux, 2022).
Reducing food waste and loss in most businesses achieve positive returns, such as tax incentives and being part of the U.S. Food Loss and Waste 2030 Champions, which is to reduce food loss and waste by 50% in their operations by the yr 2030 (ReFed, 2022; USDA, 2022; U.S. EPA, 2022). If done correctly, it is estimated that an annual investment of $14 billion over the next 10 yr can reduce food waste by 45 million tons each year which would result in a $73 billion in annual net financial benefit (ReFed, 2022). This challenge would help to significantly reduce greenhouse gas emissions, conserve resources such as water, save money, and create more jobs (ReFed, 2022; United Nations, 2022; USDA, 2022; U.S. FDA, 2022;).
Manufacturers can reduce food waste by developing a business model to utilize excess food or waste as part of their operations (Senanayake, 2021; Ciccullo et al., 2022). Different models can be categorized by the Food Waste Recovery (and Mitigation) Hierarchy which includes Prevention which is to monitor and manage excess food and food waste, Redistribution, Resell, or Reuse such as to donate to local charities, Recovery methods include transforming excess food and food waste into food and nonfood products, and Recycling through compositing (Senanayake, 2021; Ciccullo et al., 2022). The success of initiating a business model to reduce food loss and waste requires education, training, and determining if the model is helpful or harmful both internally (for the company) and externally (the community/environment) (Senanayake, 2021).
The automated process in the manufacturing of chicken is carried out mainly by machines and conveyor belts with minimal human interaction with the raw chicken (Doughman, 2021). The chicken is processed with the application of peroxyacetic acid (PAA) or sodium hypochlorite (chlorine) to reduce the microbial load and microbial contaminates that have the potential to cause foodborne illnesses (Cano, 2021). However, during this automated process at a poultry manufacturing company it was observed that whole chickens are capable of dropping from the manufacturing line and becoming contaminated leading to the disposal and waste of the chicken. The poultry business has purposed a solution to this food waste problem by reconditioning the salvaged marinated chicken whole bird without giblets (WOG) in a chemical solution to achieve a comparable product to unfallen chicken WOG. A validation study was performed to evaluate the reconditioning of salvaged marinated deli chicken WOG products from conveyor belt loss and control (nondropped) marinated deli chicken WOGs sampled just prior to packaging. The goal of the study was to determine if the reconditioning process would allow dropped marinated deli chicken WOGs to be reintroduced into the manufacturing process for packaging and distribution. APC was used as an indicator for total microbial level.
MATERIALS AND METHODS
Marination of Whole Birds Without Giblets
Postchill, after the application of cleaning interventions, 4.05 to 5.05 lb. A-grade birds were manually trussed, then injected with a liquid marinade spice solution. The injector system is a multineedle head which punctures the WOG at a depth of approximately 5.5 inches. The birds enter the injection apparatus breast up, the needles inject the bird with the liquid marinade, then the WOG continues down the production line to the metal detector.
Collection of Marinated Deli WOGs
The manufacturing facility does not allow the reconditioning of products from high traffic areas, standing water, grates, drains, or if the product has come in direct contact with footwear or pallet jacks. During the manufacturing process, if the product falls in one of those areas, the product is disposed of properly. At the point in manufacturing between the metal detector and final packaging (approximately 5 ft), birds can fall from the production line and land in a salvageable location. A total of 165 marinated deli WOG samples were collected from this section over the course of 5 d, across 3 production lines, during both first and second shift. Fifty-five marinated deli WOGs were collected directly from the line and designated as control samples (n = 55).
Treatment of Marinated Deli WOGs
To replicate the whole birds falling from the production line, the remaining (n = 110) marinated deli WOGs were aseptically removed from the line, dropped from the height of the conveyor belt, and allowed to bounce to a natural stopping point. The birds were allowed to rest on the floor for approximately 1-min, then aseptically collected and placed in a plastic tub to be transported to the designated reconditioning sink, which is separate from routine wash stations to prevent cross contamination. Carcasses were visually inspected for signs of foreign material which was removed if present.
Sink Concentrations—PAA and Chlorine
The production facility utilizes PAA and chlorine for antimicrobial interventions. The concentrations of the antimicrobial interventions have a required range of 100 to 2,000 ppm PAA according to USDA recommendations (USDA FSIS, 2022) or chlorine concentration 5 to 50 ppm, according to their internal HACCP plan. These solutions can fluctuate within that range during poultry production. The sink concentrations were monitored periodically throughout the day and recorded by a trained quality assurance technician. The concentrations taken throughout the time of the study were within the acceptable range and if the chemicals needed adjustment, chemical operators adjusted the sink concentrations using an in-house chemical control system to meet the requirements. The average PAA (Zee Company, Chattanooga, TN) concentration value for the time frame of the study was 250 ppm (minimum 130 ppm, maximum 400 ppm). The average chlorine (Zee Company, Chattanooga, TN) concentration value for the time frame of the study was 25 ppm (minimum 5 ppm, maximum 40 ppm).
Reconditioning of Marinated Deli WOGs
The marinated deli WOGs were reconditioned by submerging the carcass in the sink with either chlorine (n = 55) or PAA (n = 55). Only 1 bird was reconditioned at a time, for a duration of approximately 5 to 10 s in PAA or chlorine. After chemical treatment, birds were aseptically transferred to 15 in. × 20 in. sterile plastic bags for further processing. Whole marinated deli WOG samples were stored under refrigerated conditions (2°C–8°C) until packaged in insulated coolers with ice and delivered to Midwest Laboratories in Fremont, NE.
Preparation of Whole Birds Rinses
A portion of the control (n = 25), PAA reconditioned (n = 25) and chlorine reconditioned (n = 25) marinated deli WOGs were designated as rinse samples. Neutralizing buffered peptone water (400 mL; Edge Biologicals, Memphis, TN) was poured aseptically into the carcass cavity in the bag. Excess air was expelled from the bag, twisted close, and the twist folded over. The rinse solution was mixed through the carcass cavity and outside of the carcass for 1 min by gently flipping the bag from one side to the other while keeping the bag closed. After homogenization, the rinse solution was aseptically poured back into the original container and refrigerated prior to testing.
Segmentation of Whole Birds
The deli WOGs are marinated by the poultry manufacturer using an injection system which is accomplished by a needle-like injector that marinates the interior of the chicken. Therefore, the whole birds were segmented into parts to determine that the reconditioning process was effective on every segment of the bird. The remaining control (n = 30), PAA reconditioned (n = 30), and chlorine (n = 30) marinated deli WOGs were broken down into 6 sample types: trussing strings, wings, drums, thighs, breasts, and tenderloins. Segmenting the whole bird was performed aseptically by the laboratory analyst. Analyst gloves were frequently changed and sanitized using 70% ethanol. The cutting board and knife were sterilized using 99% ethanol and flaming between samples. Following the bird breakdown, the skin and meat from each respective sample type was removed from the bones and collected in a sterile Whirl-Pak bag (Nasco, Fort Atkinson, WI). Random portions from each bag were selected for sample weighing.
Preparation and Dilution of Chicken Segments
The chicken segments were aliquoted in 25 ± 0.5 g portions and placed in individual sterile Whirl-Pak (Nasco, Atlanta, GA) bags. A total of 30 samples were weighed per sample part for the Marinated control, PAA Reconditioned, and chlorine Reconditioned. Weigh stations were sanitized, and utensils sterilized prior to weighing the sample. The sample aliquots designated for APC enumeration were diluted at a 1:10 ratio using phosphate buffer (EMD Millipore, Burlington, MA), placed in a stomacher at normal speed or hand massaged for 30 to 60 s (Midwest Laboratories MI 328, 2022; Midwest Laboratories MI 368, 2022).
Microbiological Analysis
Once the sample was weighed and prepared at a 1:10 initial dilution, the sample was serially diluted by taking 1 mL of the previous dilution and adding it to a 9 mL phosphate buffer tube. Each dilution was vortexed immediately before plating, and 1 mL of each dilution was pipetted on the Petrifilm Rapid Aerobic Count plate (Neogen, St. Paul, MN), spread with a 3M Flat Spreader, and allowed to sit for 1-min to allow the gel to form. Petrifilm plates were incubated at 35°C ± 1°C for 24 ± 2 h. All microbial growth was enumerated, and the results were reported as CFU/g or mL. The limit of detection was established as <10 CFU/g or mL based on the dilution factor (Midwest Laboratories MI 293, 2022).
Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2019. The aerobic plate count data were normalized using log transformation. The average log CFU and standard deviation for each sample type was assessed and a t test was performed to determine if the mean value for the calculated Reconditioned marinated deli WOG samples was comparable to the marinated deli WOG control samples. Significance was determined at P < 0.05.
RESULTS AND DISCUSSION
Rinsed Whole Birds
The log CFU/mL of the 25 poultry rinse samples collected from the reconditioned WOGs in PAA, chlorine, and the control WOGs is displayed in Figure 1. The APC result for the marinated control was 1.45 ± 0.26 log10 CFU/mL, the PAA reconditioned was 0.41 ± 0.71 log10 CFU/mL, and the chlorine reconditioned was 1.44 ± 0.54 log10 CFU/mL. There was no difference (P = 0.91) observed between the chlorine reconditioned and the control samples, indicating that the processes are comparable. A significant difference (P = 3.46 × 10−7) was observed between the control and the PAA reconditioned samples. The PAA samples had a significant reduction in APC levels compared to both the chorine reconditioned samples and the marinated controls. The significant reduction could be due to the efficacy of the PAA compared to the chlorine specifically for rinsate samples. Various researchers have observed PAA to be more effective in reducing the microbial load in comparison to chlorine (Bauermeister, 2008a,b; Nagel, 2013). One such study, observed a similar trend analyzing Enterococcus faecium and Salmonella comparing PAA treated chicken rinsate to chlorine treated chicken rinsate after carcass samples were treated with the respective treatments for 20 s (Lemonakis, 2017; Cano, 2021). The efficacy of PAA is due to its antimicrobial properties and a slower decomposition rate that is not affected by pH, temperature, and organic matter unlike chlorine (Buncic, 2012; Cano, 2021, 2022). Overall, the data for the rinsate samples demonstrate that the reconditioning of marinated deli WOGs in either chlorine or PAA is comparable to or better than the control process.
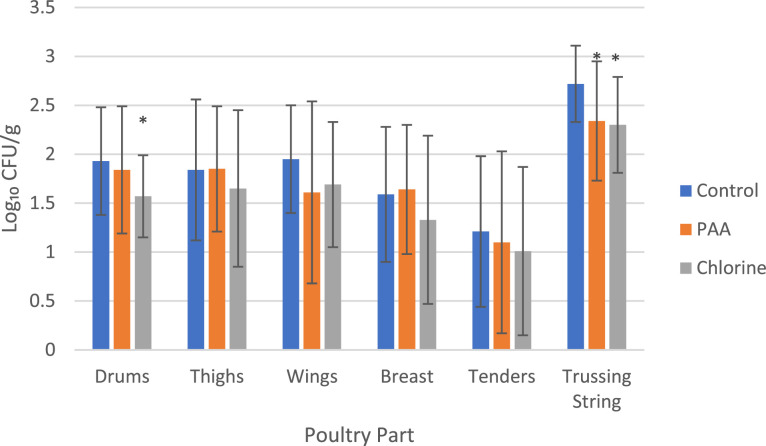
Figure 1.
Aerobic plate counts (log10 CFU/mL) of marinated deli whole bird rinsate samples collected prior to packaging (control; n = 25) and marinated deli WOGs dropped on the floor and reconditioned in either PAA (n = 25) or chlorine (n = 25). Error bars indicate standard deviations.
Whole Birds—Segmented
The log CFU/g of the marinated deli WOG segmented samples reconditioned in PAA, or chlorine was compared to the control segmented samples in Figure 2. There was no statistical difference between the PAA reconditioned deli WOG drum (P = 0.60), thigh (P = 0.96), wing (P = 0.10), breast (P = 0.77), or tender (P = 0.55) samples and the control samples. The only sample type observed to have a statistical difference (P = 0.01) was the trussing string sample (control = 2.72 ± 0.39 log10 CFU/g, PAA = 2.3 ± 0.49 log10 CFU/g) where the reconditioning in PAA resulted in reduced levels of APC. There was no statistically significant difference between the chlorine reconditioned marinated deli WOG thigh (P = 0.37), wing (P = 0.19), breast (P = 0.15), or tender (P = 0.42) samples. A significant difference (P = 0.01) was observed in the drum (control = 2.72 ± 0.39 log10 CFU/g; chlorine = 1.57 ± 0.42 log10 CFU/g) and trussing string (P = 8 × 10−4) (chlorine = 2.30 ± 0.49 log10 CFU/g) samples reconditioned in chlorine compared to the control samples. Both chlorine and PAA reconditioning process was observed to be comparable to the control treated condition for APC counts in the various sample types.
Figure 2.
Aerobic plate counts (log10 CFU/g) of whole birds collected prior to packaging (control; n = 30) and marinated deli WOGs after they had been dropped on the floor and reconditioned in either PAA (n = 30) or chlorine (n = 30), and then segmented. Error bars indicate standard deviations.
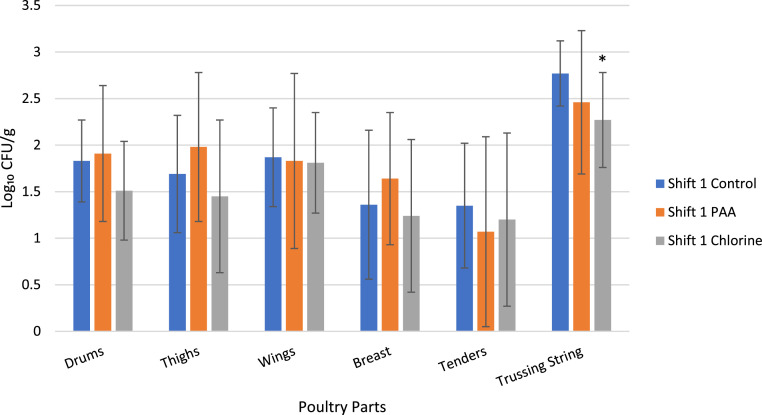
Comparing the 2 reconditioning chemicals, no significant difference (P > 0.05), was identified for any of the segmented sample types (Figure 2). The samples were collected between 2 different shifts, with a total of 15 samples per reconditioning parameter per shift (n = 30). Analyzing the samples between the 2 shifts resulted in no significant difference (P > 0.05) in comparing the 2 shifts for the segmented samples (Figure 3). There was a significant difference in APC levels for the trussing string samples for chlorine reconditioning in the first shift and for both reconditioning chemicals in the second shift compared to the control samples within the respective shifts (Figure 4). There was a reduction in APC levels in the second shift for whole birds collected and segmented for wings (P = 0.03) reconditioned in PAA compared to the control samples (Figure 5). Overall, the data reveal that the processing procedure is consistent in the collection of whole birds between the 2 working shifts.
Figure 3.
Aerobic plate counts (log10 CFU/g) of marinated deli WOGs collected during the first shift and second shift after they had been dropped on the floor and reconditioned in either PAA (n = 15) or chlorine (n = 15), and then segmented. Error bars indicate standard deviations.
Figure 4.
Aerobic plate counts (log10 CFU/g) of whole birds collected during the first shift prior to packaging (control; n = 15) and marinated deli WOGs after they had been dropped on the floor and reconditioned in either PAA (n = 15) or chlorine (n = 15), and then segmented. Error bars indicate standard deviations.
Figure 5.
Aerobic plate counts (log10 CFU/g) of whole birds collected during the second shift prior to packaging (control; n = 15) and marinated deli WOGs after they had been dropped on the floor and reconditioned in either PAA (n = 15) or chlorine (n = 15), and then segmented. Error bars indicate standard deviations.
The data demonstrate that chicken salvaged from the floor and treated with either PAA or chlorine had comparable APC levels to chicken sampled directly from second-processing production line. The concentrations and time the whole birds were washed was found to be comparable or better than the automated process. There was consistency in the data from the whole birds collected between the 2 shifts. Any slight variations with the concentrations or between the shifts did not lead to discrepancies in the data collected.
The use of a second processing step in the poultry manufacturing facility was successful in reducing food loss. The number of chickens salvaged from conveyor belt loss is minimal but can add up to a significant loss in food waste and resources over time. The implementation of the second processing in chlorine or PAA is a possible solution to the food loss and waste at the manufacturing level in which the benefits may extend to other poultry or similar manufacturing companies.
ACKNOWLEDGMENTS
We would like to acknowledge and thank all those involved in conducting this study, from the technicians at the poultry manufacturing facility to the scientists conducting the testing and writing the manuscript. We appreciate all your hard work and dedication that you put forth every day.
REFERENCES
- Bauermeister L.B. The microbial and quality properties of poultry carcasses treated with peracetic acid as an antimicrobial treatment. Poult. Sci. 2008;87:2390–2398. doi: 10.3382/ps.2008-00087. [DOI] [PubMed] [Google Scholar]
- Bauermeister L.B. Validating the efficacy of peracetic acid mixture as an antimicrobial in poultry chillers. J. Food Prot. 2008;71:1119–1122. doi: 10.4315/0362-028x-71.6.1119. [DOI] [PubMed] [Google Scholar]
- Beurteaux, D. 2022. Holding the line on food loss. Accessed Dec. 2022.https://www.ift.org/news-and-publications/food-technology-magazine/issues/2022/july/features/holding-the-line-on-food-loss.
- Buncic S.S. Interventions to control Salmonella contamination during poultry, cattle, and pig slaughter. Food Res. Int. 2012;45:641–655. [Google Scholar]
- Cano C.M. Application of peroxyacetic acid for decontamination of raw poultry products and comparison to other commonly used chemical antimicrobial interventions: a review. J. Food Prot. 2021;4:1772–1783. doi: 10.4315/JFP-21-107. [DOI] [PubMed] [Google Scholar]
- Cano C.S. Peroxyacetic acid effectiveness against Salmonella on raw poultry parts is not affected by organic matter. J. Food Prot. 2022;85:1446–1451. doi: 10.4315/JFP-22-123. [DOI] [PubMed] [Google Scholar]
- Doughman, E. 2021. Automation could future-proof poultry processing. Accessed Sept. 2023.https://www.wattagnet.com/poultry-future/new-technologies/article/15534216/automation-could-future-proof-poultry-processing.
- Ciccullo F., Fabbri M., Abdelkafi N., Pero M. Exploring the potential of business models for sustainability and big data for food waste reduction. J. Cleaner Prod. 2022;340 [Google Scholar]
- Lemonakis L., Li K.-W., Adler J.M., Shen C. Microbiological quality assessment and validation of antimicrobials against unstressed or cold-stress adapted Salmonella and surrogate Enterococcus faecium on broiler carcasses and wings. Poult. Sci. 2017;96:4038–4045. doi: 10.3382/ps/pex195. [DOI] [PubMed] [Google Scholar]
- Midwest Laboratories MI 293. 2022. Rapid Aerobic Plate Count by 3M Petrifilm. Revision 2022. Accessed Dec. 2022.
- Midwest Laboratories MI 328. 2022. Microbiology Sample Weighing. Revision 2022. Accessed Dec. 2022.
- Midwest Laboratories MI 368. 2022. Initial Sample Dilution Procedure for Quantitative Microbiology Methods. Accessed Dec. 2022.
- Nagel G.B. Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. Int. J. Food Microbiol. 2013;165:281–286. doi: 10.1016/j.ijfoodmicro.2013.05.016. [DOI] [PubMed] [Google Scholar]
- ReFed. 2022. Food waste is a solvable problem – here's how to do it. Accessed Dec. 2022. https://refed.org/food-waste/the-solutions.
- Senanayake D., Reitemeier M., Thiel F., Drechsel P. International Water Management Institute (IWMI); Colombo, Sri Lanka: 2021. Business Models for Urban Food Waste Prevention, Redistribution, Recovery and Recycling. CGIAR Research Program on Water, Land and Ecosystems. [Google Scholar]
- United Nations. 2022. Stop food loss and waste, for the people, for the planet. Accessed Dec. 2022. https://www.un.org/en/observances/end-food-waste-day.
- U.S. Department of Agriculture (USDA). 2022. Food Loss and Waste. Accessed Dec. 2022. https://www.usda.gov/foodlossandwaste.
- U.S. Department of Agriculture Food Safety and Inspection Service. 2022. FSIS Directive Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products. Accessed May 2023. https://www.fsis.usda.gov/policy/fsis-directives/7120.1.
- U.S. Environmental Protection Agency. 2022. United States 2030 Food Loss and Waste Reduction Goal. Accessed Dec. 2022.https://www.epa.gov/sustainable-management-food/united-states-2030-food-loss-and-waste-reduction-goal.
- U.S. Food & Drug Administration. 2022. Food Loss and Waste. Accessed Dec. 2022. https://www.fda.gov/food/consumers/food-loss-and-waste.
- U.S. Food and Drug Administration. 2023. Peroxyacids 21 CFR 173.370. Accessed Jan. 2023.https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-173/subpart-D/section-173.370.