Highlights
-
•
Spatially Fractionated Radiation Therapy (SFRT).
-
•
Microvascular alterations.
-
•
‘RadScopal’ effect.
-
•
Microbeam irradiation and showed significant abscopal effects in their bladders.
-
•
Radiation-Induced Bystander Effect (RIBE).
Keywords: SFRT, RIBE, Abscopal effect, Immune checkpoint inhibitors (ICIs)
Abstract
Spatially Fractionated Radiation Therapy (SFRT) is a form of radiotherapy that delivers a single large dose of radiation within the target volume in a heterogeneous pattern with regions of peak dosage and regions of under dosage. SFRT types can be defined by how the heterogeneous pattern of radiation is obtained. Immune checkpoint inhibitors (ICIs) have been approved for various malignant tumors and are widely used to treat patients with metastatic cancer. The efficacy of ICI monotherapy is limited due to the “cold” tumor microenvironment. Fractionated radiotherapy can achieve higher doses per fraction to the target tumor, and induce immune activation (immodulate tumor immunogenicity and microenvironment). Therefore, coupling ICI therapy and fractionated radiation therapy could significantly improve the outcome of metastatic cancer. This review focuses on both preclinical and clinical studies that use a combination of radiotherapy and ICI therapy in cancer.
Introduction
Spatially Fractionated Radiation Therapy (SFRT) is a form of radiotherapy that delivers a single large dose of radiation within the target volume in a heterogeneous pattern with regions of peak dosage and regions of under dosageunderdosing. SFRT types can be defined by how the heterogeneous pattern of radiation is obtained. There are many ways to deliver heterogeneous radiation based on this concept. GRID therapy is the oldest form of SFRT and was introduced by Kohler in 1909 [1], [2]. This involves delivering a relatively high but heterogeneous radiation dose to the tumor through a perforated screen with blocked areas called a GRID. In the 1990s this was shown to be feasible with bulky and refractory tumors [3], [4], [5]. Other variations to this include the multileaf collimator (MLC) based GRID therapy where instead of a block, an MLC consisting of several sets of metallic leaves to shape the beam into GRID pattern when it exits the linear accelerator [7].
Novel SFRT Techniques
The 3D LATTICE radiotherapy (LRT) [8], [9] is an extension of the GRID therapy that delivers a spatially fractionated radiotherapy in three dimensions.
Microplanar beam radiation therapy is another form of spatially fractionated radiotherapy. In Microbeam Radiation Therapy (MRT) [10], [11], the beam thickness ranges from 500 to 700 µm while in while in Minibeam Radiation Therapy (MBRT) [12], [13], [14]the thickness is of the order of 25–50 µm.
Microbeam Radiotherapy is another Novel Therapeutic Approach that has been investigated to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma [15].
Abscopal effect refers to disappearance or reduction of tumor lesions outside of the field of irradiation [117].
A novel SBRT-based PArtial Tumor irradiation of HYpoxic clonogenic cells (SBRT-PATHY) has been developed to enhance the radiotherapy therapeutic ratio of advanced bulky tumors by sparing the peritumoral immune microenvironment and regional circulating lymphocytes. It has been shown SBRT-PATHY can mediate the bystander (BE) and abscopal effects (AE).
Hence, SBRT PATHY is another novel form of SFT that has been pioneered by Tubin et al in recent years [16], [17], [18], [19]. Tubin et al reported excellent clinical outcomes of inducing bystander and abscopal effects by exclusively targeting the hypoxic segment of the tumor with a high, single dose of SFRT.
SCART is another novel approach for induction of tumor immunogenicity that has been pioneered by [20], [21]. Yan et al describes this new treatment methodology 'Stereotactic Centralized Ablative Radiation Therapy' (SCART) for large tumors, which is based on the principles of SFRT, by using SBRT methods to deliver an ablative high radiation dose to central portion/hypoxic necrotic of target while keeping the dose to surrounding normal tissue to relatively low level.
Radiobiological effects: Radiobiological mechanisms of SFRT radiation action proceed through a number of processes with non-linear dose–response curves at low doses. These can be broadly classified as Non-immune and Immune effects:
Non-immune effects: Dose-volume effects: At its simplest, this is the relationship between the radiation doses that cause the same probability of a certain acute or late normal tissue damage and the irradiated proportion or the irradiated volume of the investigated tissue or organ [22], [23]. But the anatomical volume is not homogenous within organs. The relationship between anatomical/structural radiation damage and failure of organ function is different for different organs, and more related to organ physiology than to basic radiobiological concepts of cell survival.
DNA damage: The primary method of cell killing and damage done by radiation is based on the ability of ionizing radiation to damage DNA. This DNA damage can create irreparable base pair mismatches within the nucleus of the cell. There are two main methods in which DNA is damaged by ionizing radiation, direct and indirect damage. Direct damage occurs when the radiation interacts directly with the DNA strand. Indirect damage occurs when the radiation first interacts with the water in the nucleus of the cell creating a free radical. That free radical can then go forward and damage DNA. Indirect damage is more common in low linear energy transfer (LET) forms of radiation like photons or x-rays. Oxygen plays a role in the ability of a cell to repair DNA damage. When DNA damage occurs, Oxygen has the ability to react with the DNA strand at the location of the damage preventing the repair, one of the many cellular responses to DNA damage. While this occurrence in healthy tissue is an unfortunate reality, it plays a large role in the effectiveness of radiation therapy in tumor cells. The oxygen enhancement ratio or (OER) is a concept that proves the importance of oxygen concentration in cell kill with radiation. [24].
Microvascular alterations: Vascular, transvascular, and interstitial transport are affected by fractionated radiation, but that modulation is not consistent. Total doses above 45 Gy can damage tumor microvessels, while doses up to 40 Gy tend to have inconsistent effects on microvasculature. [25], [26], [27], [28], [29], [30], [31] Garcia-Barros et al in a recent communication have postulated that “high dose” radiation of 15 Gy causes an environment of Potential Lethal Damage that makes these cells sensitive to further doses of radiation, especially the endothelial cells of the tumor microvasculature, and this effect is the primary cause of tumor cell death.[32].
Immune effects: Findings from several preclinical studies using various animal models have suggested that the highly heterogeneous dose deposition achieved with SFRT is associated with a superior immunological response in tumor tissue as compared to homogenous radiation doses [33]. Tumor cell ablation from areas of peak dose is thought to discharge tumor antigen material that enables dendritic priming of T-cells. Lymphatic cells are then able to enter tumor tissue via the conserved perfusion of the low-dose areas [34] & [35].
Local effects: Radiation-induced bystander effects: the phenomenon in which non irradiated cells exhibit effects as a result of cell communication [36] and secreted soluble molecules [37] received from nearby irradiated cells. These effects could include DNA damage, chromosomal instability, mutation, and apoptosis.[38], [39], [40], [41], [42], [43], [44], [45]. GRID therapy can evoke significant bystander cytotoxic killing in “shielded” unirradiated tumor cells located nearby the high-dose radiated regions [41]. Immune-related phenomena are involved in mediating the bystander effect via the secretion of inflammatory mediators, interferons (IFNs) and appropriate chemokines, which attract T cells. Moreover, radiotherapy might enhance T-cell trafficking to primary tumors through local vascular endothelial inflammation [46].
Radiation therapy causes a type of sterile tissue injury, and radiation-induced inflammation is regulated by complex interactions among a variety of immune mediators which can durably reshape the immune response [47], [48], [49]. The cytokine profile of tumor cell lines that have been exposed to fractional radiation is different from acute radiation [50]. Radiation therapy can bring about cell death through different mechanisms like mitotic catastrophe, apoptosis, necrosis, autophagy and senescence. The extent of the curative role of these processes is likely to be cell, tissue, tumor and radiation dose-dependent.
Radiation can modify the response of the immune system by immune stimulation or immunosuppression [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]. SFRT induces a greater-immunomodulatory gene expression than regular radiation therapy [55], [59], [63], [64], [65], [67], [68], [69].
Abscopal effects and bystander effects are hypothesized [66]to be immune- or cytokine-based. Spatially-fractionated radiation therapy approaches have been shown to induce these effects [16], [70], [30], [20]. Stereotactic Body RadioTherapy (SBRT)-based PArtial Tumor irradiation targeting HYpoxic segment (SBRT-PATHY) is a model of partial tumor irradiation purposefully developed to spare the peritumoral immune-microenvironment from radiation and in doing so it induces immune-mediated bystander effects and abscopal effects [18].
Immune suppressive effects: Radiation therapy can induce various immune suppressive effects.[71], [72], [73], [74]. It kills irradiated effector immune cells and upregulates immunosuppressive molecules in the tumor microenvironment. Clinical data show that the conventional radiotherapy predominantly generates immune-suppressive effects [70].
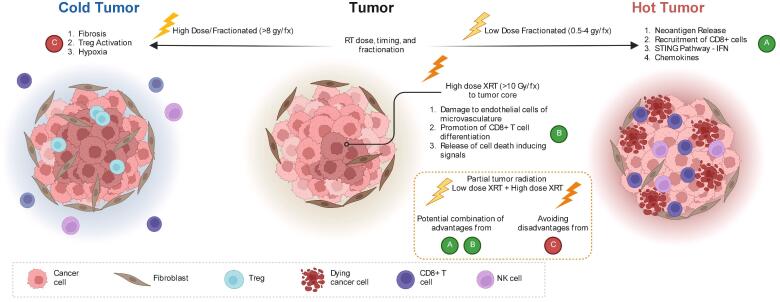
Immune stimulatory effects: Radiation therapy can also evoke immune stimulatory effects [18], [34], [72], [76], [77]. Radiation therapy can induce dendritic cell maturation and antigen release from tumors [75], [46], [79], [80]. It can enhance priming of antigen-specific T cells [78], [81], [82], and increase -T cell trafficking and infiltration [83], [84]. CD8 + T cell proliferation [82], [85], [86], [87], [88] and tumor immunogenicity [89], [90], [91] can be enhanced by radiation therapy. This brings about immunogenic cell death [74], [92], [93], [94] (Fig. 1).
Fig. 1.
Schematic overview of local effects triggered by partial tumor irradiation. At the heart of the primary tumor that is irradiated with high dose (in the middle), three effects can be distinguished: endothelial cells death and vasculature damage; induction of CD8 T cells and cell death signals release. Current evidence suggests that high dose radiation per fraction can induce immunosuppression on tumor edge while low dose radiation can induce immunomodulatory effect via neoantigen release, recruitment of CD8+ T cells to tumor edge and activation of IFN pathway genes.
As a result, partial tumor radiation is primed to attack tumors with high dose in the core and achieve a low dose radiation to tumor edge with rapid dose fall off. This strategy would potentially combine the inherent advantages of various fractionation schemes while avoiding the potential pitfalls of immunosuppressive effects rendered by high dose radiation to the whole tumor.
Distant effect: The ‘RadScopal’ effect arises as a consequence of radiation directed to part of the primary tumor, and may be accompanied by low-dose RT towards secondary lesions. It has been proposed that this double form of radiation, combined with ICIs, might be able to modulate the TME of both primary and secondary tumors, maximizing the anti-tumor immune responses [95], [96]. Barsoumian et al. investigated the priming of T cells in the Lewis lung carcinoma (LLC) mouse model using high-dose RT to a higher tumor burden. They further investigated the combination of low-dose RT to the metastatic sites, in order to modulate the stroma. Interestingly low-dose RT enhanced the anti-tumor responses to ICIs (anti-PD-1 and anti-CTLA-4), promoting M1-polarization of macrophages, NK infiltration, and reduction in transforming growth factor beta (TGF-β) levels. These data suggest that low-dose RT can reprogram the TME, improve the infiltration and function of effector immune cells in the secondary tumors, and are able to overcome the inhibitory effects of the tumor stroma. Finally, the combination with the two ICIs could further increase and prolong the systemic effects of RT, via T regs blocking and attenuation of T cell exhaustion [95], [96].
Anti-Tumor Immunity Elicited by SFRT in Pre-clinical Studies: The radiation scheme of SFRT results in the administration of ablative- and low-dose irradiation in different regions. In conventional radiation therapy the combination of a single, high ablative dose followed by subsequent lower doses can convert the immunosuppressive microenvironment to a more immunogenic one with increased infiltration of immune effector cells and a downregulation of regulatory T cells [94]. To the authors’ best knowledge, detailed studies comparing differences in immune responses between SFRT and conventional RT have not been performed yet in patients. Studies on C57BL/6 mice implanted with Lewis lung carcinoma 1 cells and then treated with a three-dimensional volume-based lattice radiation therapy (LRT) have shown that it can elicit both local and metastatic/distant tumor control through modulation of the tumor immune microenvironment. It increased secretion of inflammatory cytokines and elevated immune cell infiltration compared to un-irradiated tumors [98]. This shows that high-dose partial volume irradiation can cause an improved distant effect than the effect induced by total tumor volume irradiation through activating the host immune system.
Microbeam radiation therapy (MRT) has been compared against broad beam radiation therapy (BBRT) in murine mammary carcinoma models. MRT treated mice had a general decrease in tumor-associated-macrophages (TAMs) while the BBRT group had increased TAMs relative to the un-irradiated controls[63]. EMT6.5 tumors that received MRT vs BBRT differentially regulated immunity-related genes [59], [65]. An antitumor immune response was evoked by Partial-Volume Single-Dose Radiation in 67NR murine orthotopic breast tumors [64]. There are other reviews[96]that have extensively discussed the combinations of immunotherapy and local radiotherapy in preclinical tumor models.
Athymic nude mice injected with F98 glioma cells into their right cerebral hemisphere were exposed to pencil beam or microbeam irradiation and showed significant abscopal effects in their bladders [97]. Murine tumor models have demonstrated that partial tumor irradiation is responsible for an increased immune-mediated tumor cell death in unirradiated distant tumors compared to the whole tumor irradiation [98]. Despite this preclinical evidence of abscopal effects, it remains controversial in clinical settings [66]. Interestingly, when the exodus of CD8 + T cells from LNs was inhibited, there were no effects on tumor response, suggesting that the source of infiltrating T lymphocytes were not the DLNs, but most likely the targeted section of the hemi-irradiated tumor. Overall, this study suggests that partial irradiation alone can be immune-stimulatory and indirectly control tumor growth via immune activation, inducing an abscopal effect in the contralateral tumor. Preclinical data with LRT radiation in mice model showed that LRT can elicit both local and metastatic/distant tumor control [99].
The Radiation Induced Abscopal Effect (RIAE) might be due to modulation of the tumor immune environment, or through triggering systemic immune responses. Single-fraction spatially fractionated radiation therapy (SFRT) significantly delayed unirradiated distant tumor growth in a mice Xenograft lung tumor model. LRT induced increased secretion of inflammatory cytokines in mice serum. Interestingly, infiltration of CD3 + T cells increased significantly in the right-sided unirradiated tumor after irradiation with LRT to 50 % tumor volume in the left-sided leg tumor. This finding suggested that cellular immunity might play a role in LRT-triggered AR. Furthermore, What is more interesting was that high-dose partial volume irradiation with LRT resulted in increased CD3 T-cell infiltration in the unirradiated tumor, compared with whole tumor volume irradiation. Thus, partial tumor irradiation might activate the host immune system in a different manner compared with whole tumor volume irradiation [100].
Fractionated but not single-dose radiotherapy has been shown to induce an immune-mediated abscopal effect when combined with anti-CTLA-4 antibodies in TSA mouse breast carcinoma and MCA38 mouse colon carcinoma models [101]. Immunocompetent mouse models with a triple negative breast tumor (4T1) when treated with GRID and antibodies against immune checkpoints PD1 and CTLA-4 showed that a systemic immune activation to a primary tumor, can promote anti-tumor immune responses outside the radiation field [102]. These studies show that combined radio-immunotherapy where the radiation therapy is spatially fractionated could augment both local and metastatic disease responses. In the next section we will explore clinical studies that combine SFRT and immune-therapies together to get better cancer treatment.
Anti-Tumor Immunity Elicited by SFRT in Clinical Studies: Hypofractionated radiotherapy (HFRT) can raise inflammatory cytokine levels more than compared to conventional fractionated radiotherapy [103]. When combined with immune checkpoint blockade, a higher tumor control effect was observed in HFRT treated mice compared to those treated with conventional radiotherapy schemes [104]. There is a case report of combining of High-Dose LATTICE radiation therapy with immune checkpoint blockade in a patient with non-small cell lung cancer with multiple metastases. The metastatic lesion that received both spatially fractionated radiation therapy combined with anti-PD1 immunotherapy showed complete local response five months after treatment [35]. Stereotactic body radiotherapy on a single tumor site preceding pembrolizumab treatment has been shown to enhance overall response rate in patients with advanced non-small cell lung cancer [106]. In a clinical trial, a fractionated radiation scheme combined with pembrolizumab showed safe antitumor activity in patients with advanced solid tumors [105]. In a case study [6], a patient presented with metastatic melanoma and was refractory to pembrolizumab (anti-PD1). The patient received spatially fractionated GRID radiation therapy with pembrolizumab for five months, and his neck mass was completely resolved. In a prospective single-institution trial (NCT02303990) pembrolizumab was tested in combination with HFRT in patients with metastatic cancers (NSCLC, melanoma, pancreas, breast, others). This showed that combining anti-PD1 with HFRT was well tolerated by patients and led to prolonged and complete response despite previous progression on anti-PD-1 therapy alone [108]. In another clinical study (NCT02383212) patients with advanced solid tumors were treated with cemiplimab (anti-PD-1). When this was combined with HFRT, it showed an augmented response suggesting abscopal effects [106]. In contrast to these studies, a randomized phase I/II trial showed that pembrolizumab when concurrently given with SBRT or HFRT showed no benefits in median progression-free survival or overall survival when compared with pembrolizumab without radiation therapy [107]. This suggests that larger trials are required to address which patients benefit most from combining SBRT/HFRT with immune checkpoint blockade, or a different strategy with HFRT is needed. It has been hypothesized that proteins are able to withstand freezing and thawing, might be responsible for transmitting the bystander signal from irradiated to naïve bystander cells [111], [112], [101], [36], [37], [39], [40], [41], [42], [43], [44], [45], [16], [17], [18], [19]. Reactive oxygen species, growth factors, and cytokines have been implicated in the maintenance of the bystander signal. Their Our results suggest that secreted factors that lead to reactive oxygen species are a very likely candidate for the effects observed in vitro, since the authors we observed the greatest increase in expression of antioxidant genes immediately following GRID treatment. One mechanism for Radiation-Induced Bystander Effect (RIBE) might be through radiation-induced cytokines. Indeed, TNF-α and TRAIL are directly involved in apoptosis and have been suggested to play a role in bystander effects. These are induced in cells that are under the high-dose open GRID areas. Plus, increased therapeutic efficacy of GRID therapy is associated with TNF-α induction in the serum obtained from patients treated [113], [114], [115], [116], [16], [17], [18], [19].
Mohiuddin et al. [6] reported a case of a locally advanced melanoma tumor that initially responded to ipilimumab, but then progressed and became refractory to multiple systemic, immunological agents, including ipilimumab, high-dose IL-2, and pembrolizumab. The patient's tumor was then introduced to high-dose radiation along with the anti-PD-1 agent, pembrolizumab, and the patient had a rapid, complete response to treatment in the neck with minimal side effects. The patient’s tumor had previously progressed on five cycles of pembrolizumab alone prior to radiation. Conventional radiation of 50 Gy alone would not generate the dramatic, rapid response seen here, either. That case is important because it suggests that high-dose radiation can be used as an immunological primer for re-introducing sensitivity to biological agents.
Formenti and Demaria [99] suggested that the local radiation on the patient’s tissue acts as an in-situ vaccine. Radiation targeted to the intact primary tumor can release radiation-specific antigens and induce attracting chemokines to activated T-cells, thus engaging the patient’s innate immune response against the tumor. If the tumor-specific immune response is strong enough, it can also enable systemic regression in areas outside of the local radiation field, known as the abscopal effect.
In SFRT using microbeam therapy, GRID or LATTICE in animal models, a strong bystander effect has been shown in unirradiated tumor cells adjacent to cells exposed to high dose radiation [41]. This is not seen in conventional radiotherapy. It is likely that these abscopal effects in SFRT are due to immunogenic pathways. Indeed, in clinical studies partial bulky-tumor irradiation shows abscopal effects in patients [19]. SBRT-PATHY is a fractionated radiation therapy developed for unresectable bulky tumors and it exploits radiation-hypoxia-induced non-targeted effects like bystander effects and the abscopal effects [17]. In a clinical trial “metabolism-guided” lattice radiotherapy using radiation dosages that produce bystander, abscopal, and immunological effects yielded a clinical response of 89 %, including 23 % of complete remission in stage IV bulky tumor disease patients [118]. Preclinical studies suggest that these bystander effects are mediated by cytokines [109], such as TRAIL [111] and TNF [116].
Since the immune system plays an important role as the mediator/effector of RIBEs/RIAEs, SBRT-PATHY planning considers peritumoral immune microenvironment and the circulating regional lymphocytes as the new organ at risk that should be spared by radiation and ‘protected’ by its own dose constraints. In this published study, the bulky tumor control rate (RIBE response rate) among patients with unresectable NSCLC treated exclusively with SBRT-PATHY was 95 %. RIAEs were seen in 45 % of patients. The authors reported on possible relationship between RIBE and RIAE, stating that a significantly higher AR rate has been seen in patients in which more intense RIBE occurred (i.e., partially treated tumors reduced for >50 %). SBRT-PATHY concept implied that for successful therapeutic immune modulation, the entire tumor volume may not need to be irradiated. This should initiate more optimal antigen shedding, increase effector T-cell activation, and lead to favorable alterations in radiation-spared peritumoral immune microenvironment. Recently, an Italian group also confirmed the efficacy of SBRT-PATHY in their initial experience. Results from preclinical mouse models from Memorial Sloan Kettering Cancer Center also support the hypothesis behind SBRT-PATHY. The authors investigated tumor response to partial irradiation in 67 NR murine orthotopic breast tumors in both immunocompetent and nude mice. Partial tumor irradiation with a single dose of 10 Gy delivered to half of the tumor led to reproducibly inducible antitumor immune responses that eliminates the entire tumor in immunocompetent mice, but not in nude mice. In addition, a significant RIAE was observed after partial irradiation with a single dose of 10 Gy. The results of this study were comparable to those previously achieved in the clinic by using SBRT-PATHY [16], [17], [18], [19].
The studies highlighted in this section show that spatially fractionated radiation renews or enhances an immunological response: The timing for combining radiotherapy and immune checkpoint inhibition therapy could also affect the response from of the combination therapy. Preclinical studies on this are not conclusive about the optimal timing from with studies that suggesting concurrent therapy is better [119] to studies that show no significant difference [120] in treatment outcomes based on sequential or concurrent delivery of the two treatment modalities. The issue of timing is also not well resolved in clinical studies. Several clinical trials suggest that patients receiving immune checkpoint inhibitors immediately after radiotherapy might have better clinical outcomes [113], [110], but there are also clinical trials that show that immune checkpoint inhibition prior to SBRT has better outcomes [115], or that there are no significant differences in sequential vs concurrent treatment [121]. These studies suggest that optimization of the combined combining radiotherapy and immune checkpoint inhibition therapy is likely to be dependent on the type of cancer, number of lesions, and kind of fractionated radiotherapy being used.
SFRT and Immunomodulation/Immunotherapy in Clinical Studies: Conventional radiotherapy and fractionated radiotherapy by itself can function as an immunomodulator [9], [122]. In prostate cancer patients, significant CD68, and CD163 macrophages increase and decrease respectively in CD8 T cells has been observed within two weeks following prostate Stereotactic Body Radiotherapy (SBRT). Differences in radiotherapy fractionation can result in distinct immune-modulatory effects [120], so future clinical studies could test different radiation therapy regimens to see how radiation itself functions as an immunomodulator.
We have presented evidence in the previous sections for immune activation with high dose radiation and for preclinical studies where various SFRT is combined with immunotherapy. Other reviews [114], [121], [123], [124], [125], [126], [127] have described in detail the synergism and potential clinical implications of combining immune checkpoint inhibitors with radiotherapy. It is therefore the logical leap to consider that SFRT coupled with immune checkpoint blockade would be effective and could subsequently lead to improved local tumor control without added toxicities.). A case report describes the case of a patient treated with a novel form of immune-sparing partially ablative irradiation (ISPART) for a bulky peritoneal metastasis from renal cell cancer, refractory to anti-PD-1 therapy (nivolumab) as third-line therapy after sequential therapy with sunitinib and cabozantinib. The observed response suggests that there may be a synergistic effect between ISPART and immunotherapy. This case report supports the inclusion of ISPART in patients presenting with bulky lesions treated with checkpoint inhibitors [128].
Summary: SFRT has the potential to overcome tumor radio resistance and modulate the local immune response, which could lead to significant improvement in tumor control locally, regionally, and distantly while preserving healthy tissues. Combining this with immune checkpoint inhibitors is a promising strategy for treating metastatic cancers and can trigger abscopal effects. We have discussed here the anti-tumor immunity elicited by fractionated radiotherapy and its role in immunomodulation. Wide acceptance of this combinatorial treatment will require further research to optimize the fractional radiotherapy and immune checkpoint therapy parameters that would allow for maximizing an immune response while still reducing the adverse effects of radiotherapy. It is reasonable to hypothesize that radiation could modulate immune functions in its applications such as dose, fractionation size, tumor site and size, and location of nearby organs at risk.
If the hypothesis is proved, radiomics and genomics studies would be valuable to elucidate the effect of radiation in human body in a systematic manner. On occasions, it is difficult or impractical to deliver the higher dose per fraction ideal for eliciting an anti-tumor immune response due to tumor size or location. Under these circumstances, RT may be delivered by irradiating a fractional tumor volume. Spatially fractionated radiation therapy (SFRT) is a way to deliver radiation to a whole or partial tumor with inhomogeneous radiation dose. We suspect that anti-PD-1/anti-PD-L1 combination with SFRT will be translated into meaningful Phase II and Phase III trials and lead to improvement in outcomes for metastatic patients. Knowledge of radiotherapy causing increased antigen shedding and increased expression of neoantigens paved the pathway for combination approaches with anti-CTLA-4. Similarly, advances in checkpoint agonists and cytokines may also provide additional avenues of combination trials with radiotherapy. Future trials should incorporate these immunotherapy agents with modern radiotherapy and use a more biologically targeted, organs at risk sparing approach, which may include the peri-tumor microenvironment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kohler A. Zur röntgentiefentherapie mit massendosen. MMW. 1909;56:2314–2316. [Google Scholar]
- 2.Laissue J.A., Blattmann H., Slatkin D.N. Alban Kohler (1874–1947): Inventor of grid therapy.“. Zeitschrift für Medizinische Physik. 2012;22(2):90–99. doi: 10.1016/j.zemedi.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Mohiuddin M., Curtis D.L., Grizos W.T., Komarnicky L. Palliative treatment of advanced cancer using multiple nonconfluent pencil beam radiation. A pilot study. Cancer. 1990;66(1):114–118. doi: 10.1002/1097-0142(19900701)66:1<114::aid-cncr2820660121>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin M., Stevens J.H., Reiff J.E., Huq M.S., Suntharalingam N. Spatially fractionated (GRID) radiation for palliative treatment of advanced cancer. Radiation Oncology Investigations. 1996;4(1):41–47. [Google Scholar]
- 5.Mohiuddin M., Fujita M., Regine W.F., Megooni A.S., Ibbott G.S., Ahmed M.M. High-dose spatially-fractionated radiation (GRID): a new paradigm in the management of advanced cancers. Int J Radiat Oncol Biol Phys. 1999;45(3):721–727. doi: 10.1016/s0360-3016(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 6.Mohiuddin M, Park H, Hallmeyer S, et al (2015). High-Dose Radiation as a Dramatic, Immunological Primer in Locally Advanced Melanoma. Cureus. 2015 Dec 18;7(12):e417. [DOI] [PMC free article] [PubMed]
- 7.Neuner G., Mohiuddin M.M., Vander Walde N., et al. High-dose spatially fractionated GRID radiation therapy (SFGRT): a comparison of treatment outcomes with Cerrobend vs. MLC SFGRT. International Journal of Radiation Oncology, Biology, Physics. 2012;82(5):1642–1649. doi: 10.1016/j.ijrobp.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 8.Wu X., Ahmed M.M., Wright J., et al. On modern technical approaches of three-dimensional high-dose lattice radiotherapy (LRT) Cureus. 2010;2(3) [Google Scholar]
- 9.Pellizzon, A. C. A. (2020). “Lattice radiation therapy - its concept and impact in the immunomodulation cancer treatment era.” Rev Assoc Med Bras (1992)66(6): 728-731. [DOI] [PubMed]
- 10.Baker C., Curtis H.J., Zeman W., Woodley R. The design and calibration of a deuteron microbeam for biological studies. Radiation Research. 1961;15(4):489–495. [PubMed] [Google Scholar]
- 11.Slatkin D.N., Spanne P., Dilmanian F.A., Gebbers J.O., Laissue J.A. Subacute neuropathological effects of microplanar beams of x-rays from a synchrotron wiggler. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(19):8783–8787. doi: 10.1073/pnas.92.19.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Marzi L., Nauraye C., Lansonneur P., et al. Spatial fractionation of the dose in proton therapy: Proton minibeam radiation therapy. Cancer Radiothérapie. 2019;23(6–7):677–681. doi: 10.1016/j.canrad.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Lansonneur P., Mammar H., Nauraye C., et al. First proton minibeam radiation therapy treatment plan evaluation. Scientific Reports. 2020;10(1):7025. doi: 10.1038/s41598-020-63975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider T. Technical aspects of proton minibeam radiation therapy: Minibeam generation and delivery. Physica Medica. 2022;100:64–71. doi: 10.1016/j.ejmp.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Trappetti V, Fazzari JM, Fernandez-Palomo C et al. (2021) Microbeam Radiotherapy-A Novel Therapeutic Approach to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma. Int J Mol Sci. Jul 20;22(14):7755. [DOI] [PMC free article] [PubMed]
- 16.Tubin S., Raunik W. Hunting for abscopal and bystander effects: clinical exploitation of non-targeted effects induced by partial high-single-dose irradiation of the hypoxic tumour segment in oligometastatic patients. Acta Oncologica. 2017;56(10):1333–1339. doi: 10.1080/0284186X.2017.1346385. [DOI] [PubMed] [Google Scholar]
- 17.Tubin S., Popper H.H., Brcic L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiation Oncology. 2019;14(1):21. doi: 10.1186/s13014-019-1227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tubin S., Gupta S., Grusch M., Popper H.H., Brcic L., Ashdown M.L., et al. Shifting the Immune-Suppressive to Predominant Immune-Stimulatory Radiation Effects by SBRT-PArtial Tumor Irradiation Targeting HYpoxic Segment (SBRT-PATHY) Cancers (basel) 2020;13(1):50. doi: 10.3390/cancers13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tubin S., Fossati P., Carlino A., Martino G., Gora J., Stock M., et al. Novel Carbon Ion and Proton Partial Irradiation of Recurrent Unresectable Bulky Tumors (Particle-PATHY): Early Indication of Effectiveness and Safety. Cancers (basel) 2022;14(9):2232. doi: 10.3390/cancers14092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan W., Khan M.K., Wu X., et al. Spatially fractionated radiation therapy: History, present and the future. Clin Transl Radiat Oncol. 2020;20:30–38. doi: 10.1016/j.ctro.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan W., Wu X., Yang J. SCART, A Multi-Center Phase I Trial of Stereotactic Central Ablative Radiation Therapy for Bulky Tumor. Cureus. 2022 [Google Scholar]
- 22.Hopewell J.W., Trott K.R. Volume effects in radiobiology as applied to radiotherapy. Radiotherapy and Oncology. 2000;56(3):283–288. doi: 10.1016/s0167-8140(00)00236-x. [DOI] [PubMed] [Google Scholar]
- 23.Prasanna A., Ahmed M.M., Mohiuddin M., et al. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. Journal of Thoracic Disease. 2014;6(4):287–302. doi: 10.3978/j.issn.2072-1439.2014.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santivasi W.L., Xia F. Ionizing Radiation-Induced DNA Damage, Response, and Repair. Antioxidants & Redox Signaling. 2014;21(2):251–259. doi: 10.1089/ars.2013.5668. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan E.C., Krishnan L., Botteron G.W., et al. Effect of irradiation on microvasculature: a quantitative study. Cancer Detection and Prevention. 1987;10(1–2):121–127. [PubMed] [Google Scholar]
- 26.Haimovitz-Friedman, A., C. C. Kan, D. Ehleiter, et al (1994). “Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis.” J Exp Med180(2): 525-535. [DOI] [PMC free article] [PubMed]
- 27.Multhoff G., Vaupel P. Radiation-induced changes in microcirculation and interstitial fluid pressure affecting the delivery of macromolecules and nanotherapeutics to tumors. Frontiers in Oncology. 2012;2:165. doi: 10.3389/fonc.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontanella A.N., Boss M.-K., Hadsell M., Zhang J., Schroeder T., Berman K.G., et al. Effects of high-dose microbeam irradiation on tumor microvascular function and angiogenesis. Radiation Research. 2015;183(2):147. doi: 10.1667/RR13712.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maslennikova A.V., Sirotkina M.A., Moiseev A.A., Finagina E.S., Ksenofontov S.Y., Gelikonov G.V., et al. In-vivo longitudinal imaging of microvascular changes in irradiated oral mucosa of radiotherapy cancer patients using optical coherence tomography. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-16823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billena C., Khan A.J. A Current Review of Spatial Fractionation: Back to the Future? International Journal of Radiation Oncology, Biology, Physics. 2019;104(1):177–187. doi: 10.1016/j.ijrobp.2019.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Possenti L., Mecchi L., Rossoni A., Sangalli V., Bersini S., Cicchetti A., et al. Radiobiological Studies of Microvascular Damage through In Vitro Models: A Methodological Perspective. Cancers (basel) 2021;13(5):1182. doi: 10.3390/cancers13051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Barros M., Paris F., Cordon-Cardo C., Lyden D., Rafii S., Haimovitz-Friedman A., et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science (New York, N.Y.) 2003;300(5622):1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddasi L., Reid P., Bezak E., Marcu L.G. Radiobiological and Treatment-Related Aspects of Spatially Fractionated Radiotherapy. International Journal of Molecular Sciences. 2022;23(6):3366. doi: 10.3390/ijms23063366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang W., Chan C.K., Weissman I.L., Kim B.Y.S., Hahn S.M. Immune Priming of the Tumor Microenvironment by Radiation. Trends Cancer. 2016;2(11):638–645. doi: 10.1016/j.trecan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Jiang L., Li X., Zhang J., Li W., Dong F., Chen C., et al. Combined High-Dose LATTICE Radiation Therapy and Immune Checkpoint Blockade for Advanced Bulky Tumors: The Concept and a Case Report. Frontiers in Oncology. 2020;10 doi: 10.3389/fonc.2020.548132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baskar R. Emerging role of radiation induced bystander effects: Cell communications and carcinogenesis. Genome Integrity. 2010;1(1):1–8. doi: 10.1186/2041-9414-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prise K.M., O'sullivan J.M. Radiation-induced bystander signalling in cancer therapy. Nature Reviews. Cancer. 2009;9(5):351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaspari K.C. The use and misuse of cost-effectiveness analysis. Social science and medicine. 1983;17(15):1043–1046. doi: 10.1016/0277-9536(83)90409-4. [DOI] [PubMed] [Google Scholar]
- 39.Hei, T. K., H. Zhou, V. N. Ivanov, et al (2008). “Mechanism of radiation-induced bystander effects: a unifying model.” J Pharm Pharmacol60(8): 943-950. [DOI] [PMC free article] [PubMed]
- 40.Blyth B.J., Sykes P.J. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiation Research. 2011;176(2):139–157. doi: 10.1667/rr2548.1. [DOI] [PubMed] [Google Scholar]
- 41.Asur R., Butterworth K.T., Penagaricano J.A., Prise K.M., Griffin R.J. High dose bystander effects in spatially fractionated radiation therapy. Cancer Letters. 2015;356(1):52–57. doi: 10.1016/j.canlet.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marín A., Martín M., Liñán O., Alvarenga F., López M., Fernández L., et al. Bystander effects and radiotherapy. Rep Pract Oncol Radiother. 2015;20(1):12–21. doi: 10.1016/j.rpor.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdak-Rothkamm S., Rothkamm K. Radiation-induced bystander and systemic effects serve as a unifying model system for genotoxic stress responses. Mutation Research, Reviews in Mutation Research. 2018;778:13–22. doi: 10.1016/j.mrrev.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Daguenet E., Louati S., Wozny A.-S., Vial N., Gras M., Guy J.-B., et al. Radiation-induced bystander and abscopal effects: important lessons from preclinical models. British Journal of Cancer. 2020;123(3):339–348. doi: 10.1038/s41416-020-0942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jokar S., Marques I.A., Khazaei S., Martins-Marques T., Girao H., Laranjo M., et al. The Footprint of Exosomes in the Radiation-Induced Bystander Effects. Bioengineering (basel) 2022;9(6):243. doi: 10.3390/bioengineering9060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krombach, J., R. Hennel, N. Brix, et al (2019) “Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells.” Oncoimmunology8(1): e1523097. [DOI] [PMC free article] [PubMed]
- 47.Anderson R.E., Warner N.L. Ionizing radiation and the immune response. Advances in Immunology. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- 48.Lumniczky K., Impens N., Armengol G., et al. Low dose ionizing radiation effects on the immune system. Environment International. 2021;149 doi: 10.1016/j.envint.2020.106212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Jiang J., Chen Y., et al. The roles of CC chemokines in response to radiation. Radiat Oncol. 2022;17(1):63. doi: 10.1186/s13014-022-02038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai S., Kumar A., Laskar S., et al. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine. 2013;61(1):54–62. doi: 10.1016/j.cyto.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 51.Burnette B.C., Liang H., Lee Y., et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lumniczky K., Sáfrány G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Letters. 2015;356(1):114–125. doi: 10.1016/j.canlet.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Shaked Y. Balancing efficacy of and host immune responses to cancer therapy: the yin and yang effects. Nature Reviews. Clinical Oncology. 2016;13(10):611–626. doi: 10.1038/nrclinonc.2016.57. [DOI] [PubMed] [Google Scholar]
- 54.Brix N., Tiefenthaller A., Anders H., Belka C., Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunological Reviews. 2017;280(1):249–279. doi: 10.1111/imr.12573. [DOI] [PubMed] [Google Scholar]
- 55.Diegeler S., Hellweg C.E. Intercellular Communication of Tumor Cells and Immune Cells after Exposure to Different Ionizing Radiation Qualities. Frontiers in Immunology. 2017;8:664. doi: 10.3389/fimmu.2017.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvalho H.A., Villar R.C. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics (São Paulo, Brazil) 2018;73(suppl 1):e557s. doi: 10.6061/clinics/2018/e557s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Zubiaurre I., Chalmers A.J., Hellevik T. Radiation-Induced Transformation of Immunoregulatory Networks in the Tumor Stroma. Frontiers in Immunology. 2018;9:1679. doi: 10.3389/fimmu.2018.01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forrester H.B., Lobachevsky P.N., Stevenson A.W., Hall C.J., Martin O.A., Sprung C.N. Abscopal gene expression in response to synchrotron radiation indicates a role for immunological and DNA damage response genes. Radiation Research. 2020;194(6) doi: 10.1667/RADE-19-00014.1. [DOI] [PubMed] [Google Scholar]
- 59.Korbecki J., Kojder K., Simińska D., Bohatyrewicz R., Gutowska I., Chlubek D., et al. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. International Journal of Molecular Sciences. 2020;21(21):8412. doi: 10.3390/ijms21218412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monjazeb A.M., Schalper K.A., Villarroel-Espindola F., Nguyen A., Shiao S.L., Young K. Effects of Radiation on the Tumor Microenvironment. Seminars in Radiation Oncology. 2020;30(2):145–157. doi: 10.1016/j.semradonc.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou L., Zhang Y., Wang Y., et al. A Dual Role of Type I Interferons in Antitumor Immunity. Advanced Biosystems. 2020;4(11):e1900237. doi: 10.1002/adbi.201900237. [DOI] [PubMed] [Google Scholar]
- 62.Sprung C.N., Yang Y., Forrester H.B., Li J., Zaitseva M., Cann L., et al. Genome-wide transcription responses to synchrotron microbeam radiotherapy. Radiation Research. 2012;178(4):249. doi: 10.1667/RR2885.1. [DOI] [PubMed] [Google Scholar]
- 63.Bouchet A., Sakakini N., Atifi M.E., Le Clec'h C., Bräuer‐Krisch E., Rogalev L., et al. Identification of AREG and PLK1 pathway modulation as a potential key of the response of intracranial 9L tumor to microbeam radiation therapy. International Journal of Cancer. 2015;136(11):2705–2716. doi: 10.1002/ijc.29318. [DOI] [PubMed] [Google Scholar]
- 64.Bouchet A., Sakakini N., El Atifi M., et al. Early gene expression analysis in 9L orthotopic tumor-bearing rats identifies immune modulation in molecular response to synchrotron microbeam radiation therapy. PLoS One1. 2013;8(12):e81874. doi: 10.1371/journal.pone.0081874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y., Crosbie J.C., Paiva P., Ibahim M., Stevenson A., Rogers P.A.W. In vitro study of genes and molecular pathways differentially regulated by synchrotron microbeam radiotherapy. Radiation Research. 2014;182(6):626. doi: 10.1667/RR13778.1. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y., Swierczak A., Ibahim M., et al. Synchrotron microbeam radiotherapy evokes a different early tumor immunomodulatory response to conventional radiotherapy in EMT6.5 mammary tumors. Radiotherapy and Oncology. 2019;133:93–99. doi: 10.1016/j.radonc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Markovsky E., Budhu S., Samstein R.M., Li H., Russell J., Zhang Z., et al. An Antitumor Immune Response Is Evoked by Partial-Volume Single-Dose Radiation in 2 Murine Models. International Journal of Radiation Oncology, Biology, Physics. 2019;103(3):697–708. doi: 10.1016/j.ijrobp.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grotzer M., Schültke E., Bräuer-Krisch E., Laissue J. Microbeam radiation therapy: Clinical perspectives. Physica Medica. 2015;31(6):564–567. doi: 10.1016/j.ejmp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Kaminski J.M., Shinohara E., Summers J.B., Niermann K.J., Morimoto A., Brousal J. The controversial abscopal effect. Cancer Treatment Reviews. 2005;31(3):159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Buchwald Z.S., Wynne J., Nasti T.H., Zhu S., Mourad W.F., Yan W., et al. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Frontiers in Oncology. 2018;8 doi: 10.3389/fonc.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merrick A., Errington F., Milward K., et al. Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. British Journal of Cancer. 2005;92(8):1450–1458. doi: 10.1038/sj.bjc.6602518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen, J., Y. Cao, B. Markelc, et al (2019). “Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation.” J Clin Invest129(10): 4224-4238. [DOI] [PMC free article] [PubMed]
- 73.Ladbury C.J., Rusthoven C.G., Camidge D.R., Kavanagh B.D., Nath S.K. “Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy.” International Journal of Radiation Oncology* Biology*. Physics. 2019;105(2):346–355. doi: 10.1016/j.ijrobp.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe T., Sato G.E., Yoshimura M., et al. The mutual relationship between the host immune system and radiotherapy: stimulating the action of immune cells by irradiation. International Journal of Clinical Oncology. 2022 doi: 10.1007/s10147-022-02172-2. [DOI] [PubMed] [Google Scholar]
- 75.Gandhi S.J., Minn A.J., Vonderheide R.H., Wherry E.J., Hahn S.M., Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Letters. 2015;368(2):185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 76.Pilones, K. A., M. Charpentier, E. Garcia-Martinez, et al (2020). “Radiotherapy Cooperates with IL15 to Induce Antitumor Immune Responses.” Cancer Immunol Res8(8): 1054-1063. [DOI] [PMC free article] [PubMed]
- 77.Golden E.B., Apetoh L. Radiotherapy and immunogenic cell death. Seminars in Radiation Oncology. 2015;25(1):11–17. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Golden E.B., Chhabra A., Chachoua A., Adams S., Donach M., Fenton-Kerimian M., et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. The Lancet Oncology. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 79.McLaughlin M., Patin E.C., Pedersen M., Wilkins A., Dillon M.T., Melcher A.A., et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nature Reviews. Cancer. 2020;20(4):203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 80.Kodet O., Němejcova K., Strnadová K., Havlínová A., Dundr P., Krajsová I., et al. The Abscopal Effect in the Era of Checkpoint Inhibitors. International Journal of Molecular Sciences. 2021;22(13):7204. doi: 10.3390/ijms22137204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharabi, A. B., C. J. Nirschl, C. M. Kochel, et al (2015) “Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen.” Cancer Immunol Res3(4): 345-355. [DOI] [PMC free article] [PubMed]
- 82.Spiotto M., Fu Y.X., Weichselbaum R.R. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Science Immunology. 2016;1(3) doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lugade, A. A., J. P. Moran, S. A. Gerber, et al (2005). “Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor.” J Immunol174(12): 7516-7523. [DOI] [PubMed]
- 84.Prins R.M., Shu C.J., Radu C.G., Vo D.D., Khan-Farooqi H., Soto H., et al. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunology, Immunotherapy. 2008;57(9):1279–1289. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandey R., Shankar B.S., Sharma D., Sainis K.B. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. International Journal of Radiation Biology. 2005;81(11):801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- 86.Lee Y., Auh S.L., Wang Y., Burnette B., Wang Y., Meng Y., et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood, the Journal of the American Society of Hematology. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim J.Y.H., Gerber S.A., Murphy S.P., Lord E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunology, Immunotherapy. 2014;63(3):259–271. doi: 10.1007/s00262-013-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu J., Luo Y., Yuan C., et al. Downregulation of nitric oxide collaborated with radiotherapy to promote anti-tumor immune response via inducing CD8+ T cell infiltration. International Journal of Biological Sciences. 2020;16(9):1563. doi: 10.7150/ijbs.41653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ciernik I.F., Romero P., Berzofsky J.A., Carbone D.P. “Ionizing radiation enhances immunogenicity of cells expressing a tumor-specific T-cell epitope.” International Journal of Radiation Oncology* Biology*. Physics. 1999;45(3):735–741. doi: 10.1016/s0360-3016(99)00226-6. [DOI] [PubMed] [Google Scholar]
- 90.Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akanda Z.Z., Neeson P.J., John T., Barnett S., Hanna G.G., Miller A., et al. A narrative review of combined stereotactic ablative radiotherapy and immunotherapy in metastatic non-small cell lung cancer. Transl Lung Cancer Res. 2021;10(6):2766–2778. doi: 10.21037/tlcr-20-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vandenabeele P., Vandecasteele K., Bachert C., et al. Immunogenic Apoptotic Cell Death and Anticancer Immunity. Adv. Exp. Med. Biol. 2016;930:133–149. doi: 10.1007/978-3-319-39406-0_6. [DOI] [PubMed] [Google Scholar]
- 93.Vaes R.D.W., Hendriks L.E.L., Vooijs M., De Ruysscher D. Biomarkers of Radiotherapy-Induced Immunogenic Cell Death. Cells. 2021;10(4):930. doi: 10.3390/cells10040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bao X., Xie L. Targeting purinergic pathway to enhance radiotherapy-induced immunogenic cancer cell death. Journal of Experimental & Clinical Cancer Research. 2022;41(1):222. doi: 10.1186/s13046-022-02430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barsoumian HB, Ramapriyan R, Younes AI, et al (2020). Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. 2020 Oct;8(2):e000537. [DOI] [PMC free article] [PubMed]
- 96.Barsoumian HB, Sezen D, Menon H, et al (2022). High Plus Low Dose Radiation Strategy in Combination with TIGIT and PD1 Blockade to Promote Systemic Antitumor Responses. Cancers (Basel). 2022 Jan 3;14(1):221. [DOI] [PMC free article] [PubMed]
- 97.Klug F., Prakash H., Huber P., Seibel T., Bender N., Halama N., et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Kanagavelu S., Gupta S., Wu X., Philip S., Wattenberg M.M., Hodge J.W., et al. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiation Research. 2014;182(2):149–162. doi: 10.1667/RR3819.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Formenti S., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. Journal of the National Cancer Institute. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernandez-Palomo C., Schültke E., Bräuer-Krisch E., Laissue J.A., Blattmann H., Seymour C., et al. Investigation of abscopal and bystander effects in immunocompromised mice after exposure to pencilbeam and microbeam synchrotron radiation. Health Physics. 2016;111(2):149–159. doi: 10.1097/HP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 101.Peters M.E., Shareef M.M., Gupta S., et al. Potential utilization of bystander/abscopal-mediated signal transduction events in the treatment of solid tumors. Current Signal Transduction Therapy. 2007;2(2):129–143. [Google Scholar]
- 102.Romano E, Honeychurch J, Illidge TM (2021). Radiotherapy-Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery? Cancers (Basel). 2021 Jan 26;13(3):457. [DOI] [PMC free article] [PubMed]
- 103.Bazyar S, O'Brien ET 3rd, Benefield T, et al (2021). Immune-Mediated Effects of Microplanar Radiotherapy with a Small Animal Irradiator. Cancers (Basel). 2021 Dec 29;14(1):155. [DOI] [PMC free article] [PubMed]
- 104.Dewan, M. Z., A. E. Galloway, N. Kawashima, et al 2009). “Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody.” Clin Cancer Res15(17): 5379-5388. [DOI] [PMC free article] [PubMed]
- 105.Kulzer L., Rubner Y., Deloch L., et al. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. Journal of Immunotoxicology. 2014;11(4):328–336. doi: 10.3109/1547691X.2014.880533. [DOI] [PubMed] [Google Scholar]
- 106.Theelen W.S.M.E., Peulen H.M.U., Lalezari F., van der Noort V., de Vries J.F., Aerts J.G.J.V., et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncology. 2019;5(9):1276. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luke J.J., Lemons J.M., Karrison T.G., Pitroda S.P., Melotek J.M., Zha Y., et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. Journal of Clinical Oncology. 2018;36(16):1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maity A., Mick R., Huang A.C., George S.M., Farwell M.D., Lukens J.N., et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. British Journal of Cancer. 2018;119(10):1200–1207. doi: 10.1038/s41416-018-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Welsh J., Menon H., Chen D., Verma V., Tang C., Altan M., et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8(2):e001001. doi: 10.1136/jitc-2020-001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 111.Ivanov V.N., Hei T.K. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis. 2014;19(3):399–413. doi: 10.1007/s10495-013-0925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Najafi M., Fardid R., Hadadi G., et al. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 2014;4(4):163–172. [PMC free article] [PubMed] [Google Scholar]
- 113.Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S., et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England Journal of Medicine. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Procureur A., Simonaggio A., Bibault J.-E., Oudard S., Vano Y.-A. Enhance the Immune Checkpoint Inhibitors Efficacy with Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments. Cancers (basel) 2021;13(4):678. doi: 10.3390/cancers13040678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sundahl N., Vandekerkhove G., Decaestecker K., Meireson A., De Visschere P., Fonteyne V., et al. Randomized Phase 1 Trial of Pembrolizumab with Sequential Versus Concomitant Stereotactic Body Radiotherapy in Metastatic Urothelial Carcinoma. European Urology. 2019;75(5):707–711. doi: 10.1016/j.eururo.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Veeraraghavan J., Natarajan M., Aravindan S., et al. Radiation-triggered tumor necrosis factor (TNF) alpha-NFkappaB cross-signaling favors survival advantage in human neuroblastoma cells. The Journal of Biological Chemistry. 2011;286(24):21588–21600. doi: 10.1074/jbc.M110.193755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mole R.H. Whole body irradiation; radiobiology or medicine? The British Journal of Radiology. 1953;26(305):234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 118.Ferini G., Parisi S., Lillo S., Viola A., Minutoli F., Critelli P., et al. Impressive Results after “Metabolism-Guided” Lattice Irradiation in Patients Submitted to Palliative Radiation Therapy: Preliminary Results of LATTICE_01 Multicenter Study. Cancers (basel) 2022;14(16):3909. doi: 10.3390/cancers14163909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dovedi S.J., A.L. Adlard, G. Lipowska-Bhalla, et al. (2014). “Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade.” Cancer Res. 74 (19). 5458-5468. [DOI] [PubMed]
- 120.Herter-Sprie G.S., Koyama S., Korideck H., et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 2016;1(9):e87415. doi: 10.1172/jci.insight.87415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bestvina C.M., Pointer K.B., Karrison T., Al-Hallaq H., Hoffman P.C., Jelinek M.J., et al. A Phase 1 Trial of Concurrent or Sequential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients With Stage IV NSCLC Study. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2022;17(1):130–140. doi: 10.1016/j.jtho.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 122.De Martino M., Daviaud C., Vanpouille-Box C. Radiotherapy: An immune response modifier for immuno-oncology. Seminars in Immunology. 2021;52:101474. doi: 10.1016/j.smim.2021.101474. [DOI] [PubMed] [Google Scholar]
- 123.Demaria S., Guha C., Schoenfeld J., Morris Z., Monjazeb A., Sikora A., et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer. 2021;9(4):e002038. doi: 10.1136/jitc-2020-002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Esposito A., Criscitiello C., Curigliano G. Immune checkpoint inhibitors with radiotherapy and locoregional treatment: synergism and potential clinical implications. Current Opinion in Oncology. 2015;27(6):445–451. doi: 10.1097/CCO.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 125.Asna, N., A. Livoff, R. Batash, et al (2018). “Radiation therapy and immunotherapy-a potential combination in cancer treatment.” Curr Oncol25(5): e454-e460. [DOI] [PMC free article] [PubMed]
- 126.Colbert L.E., Jhingran A. Immunotherapy and Radiation. Adv. Exp. Med. Biol. 2020;1244:205–213. doi: 10.1007/978-3-030-41008-7_9. [DOI] [PubMed] [Google Scholar]
- 127.Procureur A., Simonaggio A., Bibault J.E., et al. (2021). Enhance the Immune Checkpoint Inhibitors Efficacy with Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments. Cancers (Basel). 2021 Feb 8. 13 (4). 678. [DOI] [PMC free article] [PubMed]
- 128.Massaccesi M., Boldrini L., Romano A., et al. (2021). Unconventional radiotherapy to enhance immunotherapy efficacy in bulky tumors: a case report. Immunotherapy. 2021 Dec. 13 (18). 1457-1463. [DOI] [PubMed]