Abstract
Introduction
Pathophysiologic pathways of sickle cell disease (SCD) and air pollution involve inflammation, oxidative stress, and endothelial damage. It is therefore plausible that children with SCD are especially prone to air pollution’s harmful effects.
Methods
Patient data were collected from a single center, urban/peri-urban cohort of children with confirmed SCD. Daily ambient concentrations of particulate matter (PM2.5) were collected via satellite-derived remote-sensing technology, and carbon monoxide (CO), nitrogen dioxide (NO2), and ozone from local monitoring stations. We used multivariable regression to quantify associations of pollutant levels and daily counts of emergency department (ED) visits, accounting for weather and time trends. For comparison, we quantified the associations of pollutant levels with daily all-patient (non-SCD) ED visits to our center.
Results
From 2010–2018, there were 17 731 ED visits by 1740 children with SCD (64.8% HbSS/HbSβ0). Vaso-occlusive events (57.8%), respiratory illness (17.1%), and fever (16.1%) were the most common visit diagnoses. Higher three-day (lags 0–2) rolling mean PM2.5 and CO levels were associated with daily ED visits among those with SCD (PM2.5 incident rate ratio (IRR) 1.051 (95% CI 1.010–1.094) per 9.4 μg/m3 increase; CO 1.088 (1.045–1.132) per 0.5 ppm). NO2 showed positive associations in secondary analyses; ozone levels were not associated with ED visits. The comparison, all-patient ED visit analyses showed lower IRR for all pollutants.
Conclusions
Our results suggest short-term air pollution levels as triggers for SCD events and that children with SCD may be more vulnerable to air pollution than those without SCD. Targeted pollution-avoidance strategies could have significant clinical benefits in this population.
Keywords: sickle cell disease, air pollution, pediatrics, vaso-occlusive events, emergency department
Introduction
Air pollution is a major cause of death and disability and is particularly harmful for those with underlying chronic disease, including cardiovascular, cerebrovascular, and lung disease.[1, 2] Pollution exposure is highest among minorities and otherwise marginalized populations.[3] Young children are also especially sensitive to its effects: they breathe more air per bodyweight than adults and their metabolic pathways are unable to rapidly detoxify pollutants.[4, 5] The most well-studied air pollutant with regards to human health is particulate matter with a diameter of 2.5 microns or less (PM2.5) and no safe threshold of PM2.5 has been identified.[6] Carbon monoxide (CO), nitrogen oxides (NOx), and ozone are other key pollutants.[7]
Sickle cell disease (SCD) is one of the most common monogenetic disorders in the United States, with an estimated prevalence of 100 000.[8] Hallmarks of the disease are recurrent, painful, inflammatory vaso-occlusive events (VOE), severe pneumonias/acute chest syndrome, and multi-organ damage.[9, 10] VOE are the main source of morbidity and mortality in SCD, with population-wide studies showing that VOE and fever account for the majority (60–80%) of pediatric emergency department (ED) visits.[11, 12] Hydroxyurea and other disease-modifying therapies such as L-glutamine and crizanlizumab have been proven efficacious in reducing the number of VOE; however, they do not completely eliminate VOE and clinical management during an acute event consists mainly of supportive care.[13] Though there are several well-known causes of VOE (e.g., infection, dehydration), many patients present without a clear trigger.[14] As such, identifying underlying triggers and associated biologic pathways is key in improving patient care.
There are well-known biologic pathways that indicate exposure to air pollution could be an unrecognized yet important trigger for VOE. First, it is well-established that air pollution exposure induces a systemic inflammatory response.[15–17] Additionally, air pollution directly damages the lungs; acute chest syndrome is characterized by acute lung injury, often of unknown etiology.[18, 19] Other pathways that connect air pollution exposure to poor health outcomes in other settings include altered metabolic pathways and direct endothelial injury;[20] SCD is a chronic inflammatory disease with baseline oxidative stress and the ongoing endothelial damage is recognized as contributing to the disease’s substantial morbidity and mortality.[21, 22]
Several studies have examined the associations of daily increases in air pollution with acute SCD complications, with city-wide, retrospective studies demonstrating a positive association between SCD complications and higher levels of daily ambient pollution.[23–25] While representing important first steps, these studies have all had limitations which hamper interpretation. From a pollution standpoint, data was obtained from a single or only a few monitoring stations, which may not account for city-wide pollution variability. From an SCD standpoint, they have relied on ICD-9/10 codes to identify SCD patients, which are subject to error and often do not reliably distinguish between different types of SCD (e.g. HbSS vs HbSβ+ vs HbSC). For example, an analysis of such hospital discharge coding found that 17% of patients with HbSS/HbSβ0 and nearly 77% of patients with HbSC were misclassified by genotype; this is a serious limitation given the clinical, laboratory, and treatment differences across genotypes.[26] Studies to date have also not included a comparison group to investigate the relative impact of air pollution. Finally, prior studies have relied on cross-sectional snapshots of ED visits rather than following a single cohort of patients over time [23–25].
In this study, we aim to measure the effects of ambient (outdoor) air pollution on pediatric SCD. We hypothesized that short-term changes in air pollutant levels are significantly associated with daily burden of ED visits in this population, with the majority of visits due to inflammatory events (e.g., VOE, fever). Furthermore, we hypothesize that children with SCD are especially sensitive to air pollution’s harmful effects as compared to the general pediatric population. This study augments existing literature by a) utilizing data from a large, longitudinal cohort of children with confirmed SCD in the United States, thereby eliminating errors inherent with ICD-9/10 codes, allowing us to analyze patients by specific SCD genotype, and including only patients who live within the area of interest, b) focusing on the pediatric population, a group uniquely vulnerable to air pollution’s effects, c) incorporating satellite-derived PM2.5 data and data from multiple monitoring stations, which incorporates city-wide variability in air pollution levels, and d) including an all-patient (non-SCD) analysis for comparison.
Methods
SCD Patient Database
Patient data were abstracted from electronic medical records of patients in an ongoing cohort of children with SCD at Children’s Healthcare of Atlanta (CHOA), a comprehensive, clinical database and linked to CHOA’s electronic health record database. Specifically, every child with SCD (verified by hemoglobin analysis) with ≥ 1 clinical encounter at CHOA, including the affiliated hospitals and outpatient clinics, is included in this database. Of note, CHOA is the primary pediatric healthcare system in the Atlanta metropolitan area, including three academic hospitals that provide inpatient, outpatient, and ED care; our analysis included data from all three hospitals. Importantly, CHOA accounts for ~95% of pediatric SCD hospitalizations within the Atlanta metropolitan area [27], representing a nearly complete population-based sample. Patient information included sociodemographic (including home address at time of encounter) and clinical information.
Given variable levels of fetal hemoglobin and disease severity under 1 year of age, we limited our analysis to patients 1.0–17.9 years of age at time of visit. To only include patients who would plausibly seek ED care at a CHOA facility, our geographic area of interest (buffer zone) was defined to include those with a home address (and associated pollution levels) within 20 miles of the nearest CHOA ED. Of note, we also performed secondary analyses that further narrowed the buffer 10 and 5-mile radiuses. Finally, patients were excluded if they were coded as lost to follow up, which we defined as patients who went more than 365 days without being seen by a CHOA provider, with the goal of excluding the minority of patients who receive their SCD care elsewhere. Note that our analysis is ED-focused, and thus only includes children who have visited an ED during the study timeframe. We abstracted ICD-9/10 codes to determine cause of ED visit. This study was approved by the CHOA Institutional Review Board.
To test our hypothesis that children with SCD are especially impacted by air pollution, we quantified the associations of pollutant levels with daily all-patient ED visits to our center. Specifically, this comparison analysis included daily counts of ED visits from all children aged 1.0–17.9 years, minus children with SCD, at a CHOA ED (data available June 2013 – December 2018).
Air Pollution Data
Daily air pollution data were acquired from two main sources. For PM2.5, we accessed publically avaliable, remote-sensing data developed by the NASA Socioeconomic Data and Applications Center (SEDAC) [28] to create a database of daily PM2.5 levels in 1km*1km grids covering the Atlanta metropolitan area. We then averaged the grid values over our buffer zones of interest to obtain daily PM2.5 values for buffers around each facility. As the three CHOA EDs are all within 10 miles of each other, we then averaged the values to obtain one daily PM2.5 value for each buffer to apply in our analyses. Remote-sensing data allow for measurement and inclusion of neighborhood-level variations in pollutant levels and have been well-validated and published in other health settings.[29–31] SEDAC data were available for the period January 1, 2001 – December 31, 2016.
For other air pollutants of interest, we did not have such granular data and instead relied on four Environmental Protection Agency (EPA) pollution monitoring stations in the Atlanta area (Supplemental Figure 1). Data from EPA monitoring stations included PM2.5 (for validation of SEDAC remote-sensing data), CO, NO2, and ozone, with data available from January 1, 2010 – December 31, 2018. We used daily averages across all monitoring stations for each pollutant of interest. Weather data came from Atlanta Hartsfield-Jackson International Airport.
Measures and Statistical Analysis
Our primary exposures of interest were individual air pollutant levels for PM2.5, CO, NO2, and ozone. Specifically, we assessed 3-day rolling means (i.e., average of day of ED visit, 1 day prior, 2 days prior) of pollutants. This strategy is consistent with air pollution literature and the clinical course of SCD, which suggests children most often present to the ED 2–4 days after symptom onset.[14, 19] We also analyzed how specific day (relative to ED presentation) pollutant levels impacted ED visits, both for clinical information and as sensitivity analyses/validation of our models. Our outcome of interest was a count variable of the total number of ED visits per day (summed across the three CHOA EDs) by the 1740 patients in our SCD cohort and total daily ED visits summed across the three EDs for the comparison group.
To estimate the effect of air pollution levels on daily ED visits, we created generalized linear models (negative binomial distribution [32, 33]), with the general form
| (1) |
where y is total ED visits/day by our population of interest and β1 is our coefficient of interest on pollutant values (continuous, mean-centered and scaled by 2 times their standard deviation (s.d.) to allow for comparison of effects amongst different pollutants).[34] The model accounts for other factors potentially associated with both air pollution levels and ED visits, including day of week, rain (indicator variable, 1 = rainfall > 0.5 inches/day, on day of visit), temperature (cubic spline of minimum daily temperature with knots at 25th, 75th percentiles, on day of visit), and long-term time trends (cubic spline with knots at changes in season). Days with missing pollutant values were represented as missing. Incident rate ratios (IRR) were obtained by exponentiating the β1 coefficients (eβ = IRR), where IRR is the relative change in ED visits per 2 s.d. change in air pollutant levels. For example, an IRR of 1.04 can be interpreted as, for every 2 s.d. increase in air pollutant level, ED visits increase by 4%.
We performed sensitivity analyses and robustness checks on our model, including quasi-Poisson distribution (which allows for overdispersion), different temperature and time-trend splines and lags, different lag-day models, lead day analyses for identifying model misspecification such as lack of adequate time trend control, and multi-pollutant models (see Supplement Tables S2, S3, S4). Analyses were performed in R, v4.1.1. We followed STROBE reporting guidelines.[35]
Results
Our final sample consisted of 17 731 ED visits by 1740 unique children with SCD (age range 1.0–17.9, Table 1 and Supplemental Figure 1). The patient population self-identified as mainly Black/African American (91.4%) and most patients were hemoglobin type SS/Sβ0 (64.8% patients, 70.9% ED visits). The study population of interest spanned 11 counties in the Atlanta metropolitan area; 28.5% of the cohort lived in 2 counties with the highest annual pollution levels, and only 2.7% lived in the 2 counties with the lowest annual pollution levels. Table 2 shows the primary and secondary diagnoses associated with ED visits among the SCD cohort, with VOE (defined as SCD crisis or pain, 58.7%), respiratory diagnoses (17.1%), and fever (16.1%) as the most common diagnoses. Figure 1 shows the daily pollutant values during the study period, demonstrating significant day-to-day variability and seasonal trends.
TABLE 1.
Sociodemographic Characteristics of Children with Sickle Cell Disease (January 2010 – December 2018).
| By patients (n = 1 740) |
By visits (n = 17 731) |
|
|---|---|---|
| Age at visit | na | 1.0–17.9 |
| Gender (Female) | 49.2% | 49.8% |
| Ethnicity | ||
| Non-Hispanic | 92.8% | 94.9% |
| Hispanic | 2.6% | 3.6% |
| Not answered | 4.6% | 1.5% |
| Race | ||
| Black/African American | 91.4% | 90.1% |
| White | 1.1% | 1.4% |
| Native American | 0.1% | 0.2% |
| Not answered | 7.4% | 7.9% |
| Genotype | ||
| HbSS/HbSβ0 | 64.8% | 70.9% |
| HbSC | 26.3% | 21.9% |
| HbSβ+ | 7.4% | 6.4% |
| Other | 1.5% | 0.8% |
| Insurance at visit | ||
| Medicaid | na | 64.6% |
| Private | na | 23.3% |
| Self-pay | na | 1.7% |
| Not listed | na | 10.3% |
Includes patients who live within 20 miles of nearest CHOA hospital and who had at least 1 emergency department visit from 2010–2018.
Ethnicity, race, and gender are self/parent identified.
TABLE 2:
Most Common Emergency Department Diagnoses Among Cohort
| Reason for Visit | Percentage* |
|---|---|
| Sickle cell disease crisis / pain | 58.7% |
| Respiratory illness** | 17.1% |
| Fever | 16.1% |
| Acute chest syndrome | 6.0% |
| Nausea/Vomiting/Diarrhea | 1.7% |
| Constipation | 1.4% |
| Headache | 1.4% |
| Priapism | 0.8% |
| Gallstone | 0.7% |
| Splenic sequestration | 0.6% |
| Avascular necrosis | 0.5% |
Percentages of primary and secondary diagnoses for ED visit amongst the cohort, by ICD9/10 code (n = 17,731). The above percentages exclude 2325 (13.1%) ED visits for which there was no associated diagnosis code documenting reason of visit. Percentages do not add to 100 due to multiple reasons per visit (e.g., patient may present with both fever and pain).
Most common respiratory diagnoses included: cough, asthma, pneumonia, hypoxemia. Of note, acute chest syndrome is coded separately, and patients can be coded as both respiratory illness and acute chest syndrome.
Figure 1: Daily Pollutant Levels, Atlanta, GA (2010–2018).
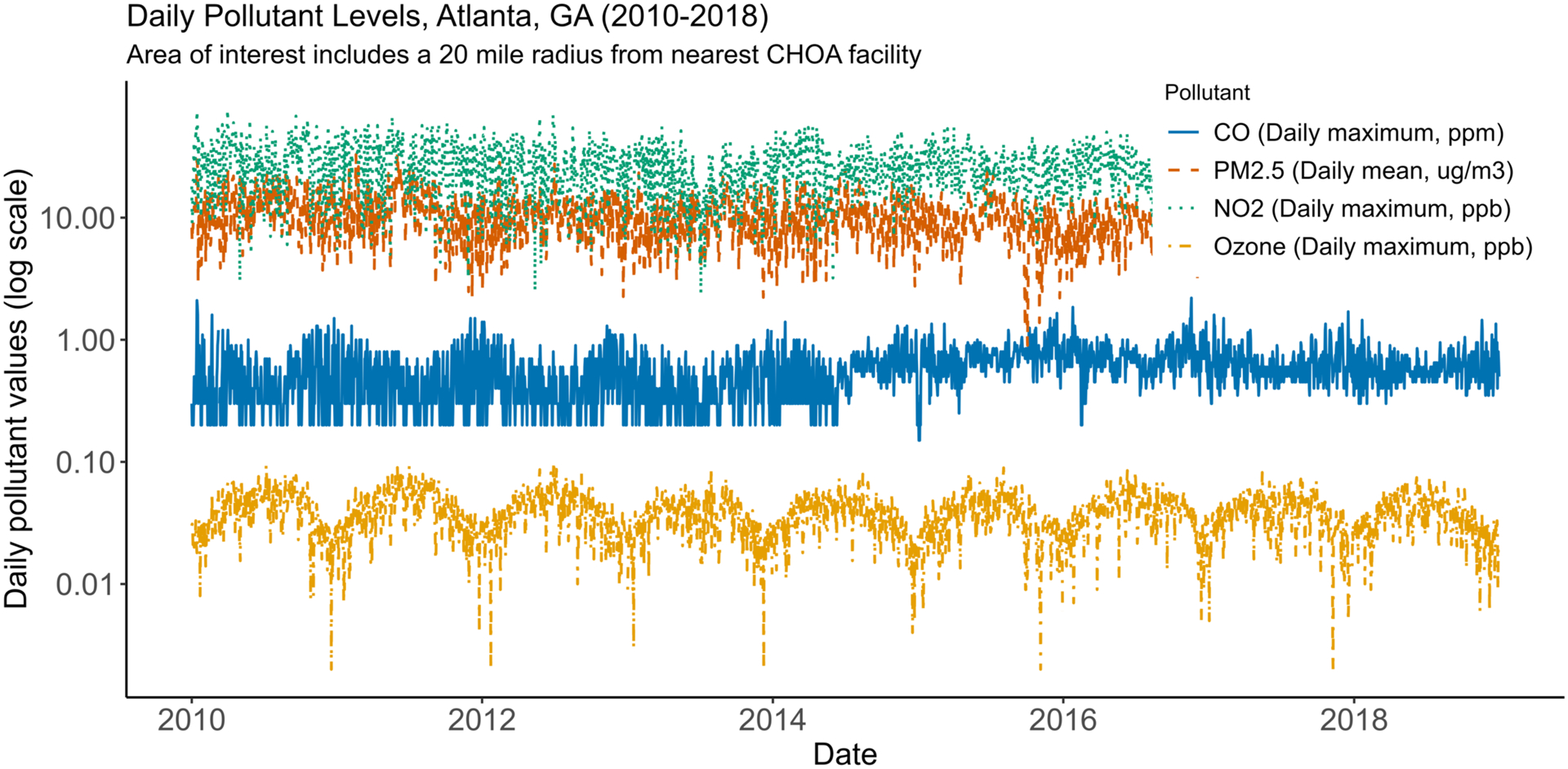
This time series graph shows daily pollutant values during the study period. PM2.5 (SEDAC-derived data, average value of 20-mile buffers around 3 CHOA emergency departments) mean 10.6 (standard deviation (s.d.) 4.7) ug/m3; CO (EPA monitoring station data) mean 0.57 (s.d. 0.25) ppm; NO2 (EPA) mean 26.7 (s.d. 11.4) ppb; ozone (EPA) mean 0.04 (s.d. 0.01) ppb.
Figure 2 shows the results of our primary analyses, focusing on the single pollutant models from Equation (1). Within our a priori primary area of interest (20 miles from nearest CHOA ED), both PM2.5 (IRR 1.051 (95%CI 1.010–1.094) per 2 s.d. (9.4 μg/m3) increase) and CO (IRR 1.087 (1.039–1.138) per 2 s.d. (0.5 ppm) increase) were significantly associated with ED visits (see Supplemental Table S1 for all values). The IRR estimates for our comparison analyses of total daily ED visits (minus patients with SCD) were lower for all pollutants as compared to the estimates for the SCD cohort for all buffer areas. CO and NO2 were both positively and significantly associated with ED visits amongst the comparison group, which is consistent with the broader air pollution literature.
Figure 2: Estimated associations of 3-day rolling mean ambient pollutant levels and ED visits by buffer zone and SCD status.
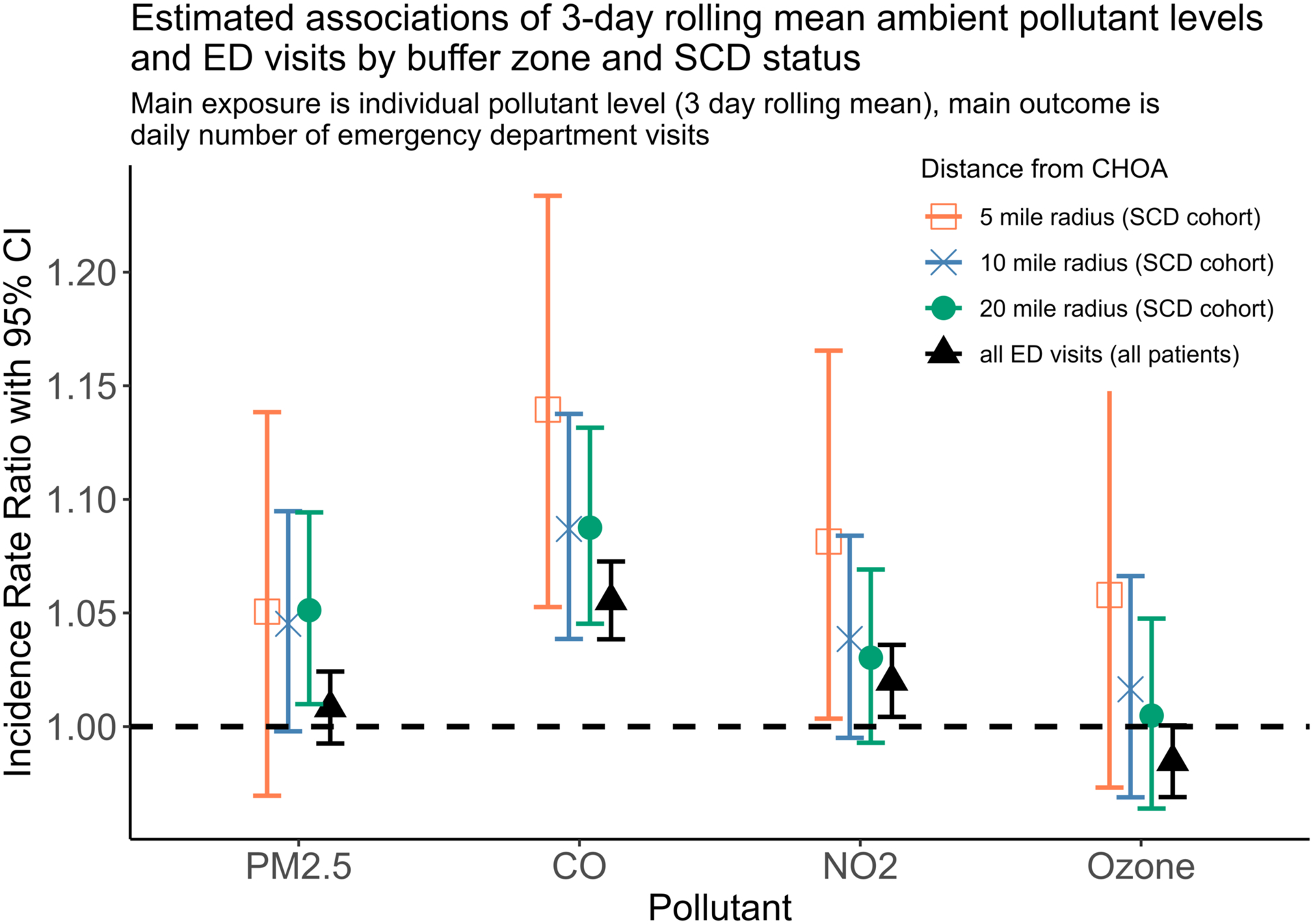
Plot above shows results of 16 separate models (4 pollutants × 4 areas). Pollutant values are standardized (mean centered and divided by 2 times their standard deviation). Thus, PM2.5 20-mile radius incidence rate ratio of 1.051 can be interpreted as for every 2 standard deviation change in 3 day rolling mean PM2.5 levels, the daily number of emergency visits in this cohort increases by 5.1%. All ED visits (all patients) refers to all children 1.0–17.9 years who visited a CHOA emergency department (excluding only patients with SCD), included as comparison analysis. PM2.5 models include data from Jan 1, 2010 - Dec 31, 2016, all patient models from June 1, 2013 - Dec 31, 2018 (due to PM2.5 and all patient data restrictions), all other models from Jan 1, 2010 - Dec 31, 2018.
As secondary analyses (Figure 2), we reduced the buffer area, including only those patients with SCD who live within 10 and 5 miles of the nearest CHOA facility. For all monitoring station-derived pollutants (CO, NO2, ozone), the IRRs were larger for the smaller areas (more urban environments, as all CHOA EDs are within the city of Atlanta) compared to the 20-mile primary area of interest. In contrast, the IRR for PM2.5 was similar across the different areas of interest, except for confidence interval widths reflecting differences in power. For remote-sensing PM2.5 exposure assignment, we were able to account for area of interest size by including only those 1 km × 1 km grids within each area of interest. However, for the other pollutants, we were limited by the few monitoring stations available – 3 of 4 monitoring stations were within 5 miles of the nearest CHOA facility – and as such, our exposure variable did not change with area for CO, NO2, and ozone. Note that the 5 miles estimates for PM2.5, CO, NO2, and ozone are all positive, though only CO and NO2 were statistically significant.
Though our a priori exposure of interest was 3-day rolling mean pollutant levels, we also tested specific day (relative to ED visit) pollutant levels for the SCD analyses (Figure 3). Here, we present results for those pollutants, PM2.5 and CO, that were significantly associated with ED visits in our primary analysis. We find that individual lags 0–2 had the strongest associations, which supports the decision to make lags 0–2 our primary days of interest. Furthermore, that lead days (i.e., days after hospital admission) showed no association with ED visits suggests our models were adequately specified for temporal confounders, adding robustness to the models.
Figure 3: Estimated associations of ambient pollutant levels and ED visits within a 20-mile buffer zone: comparison of pollutant lags relative to day of visit.
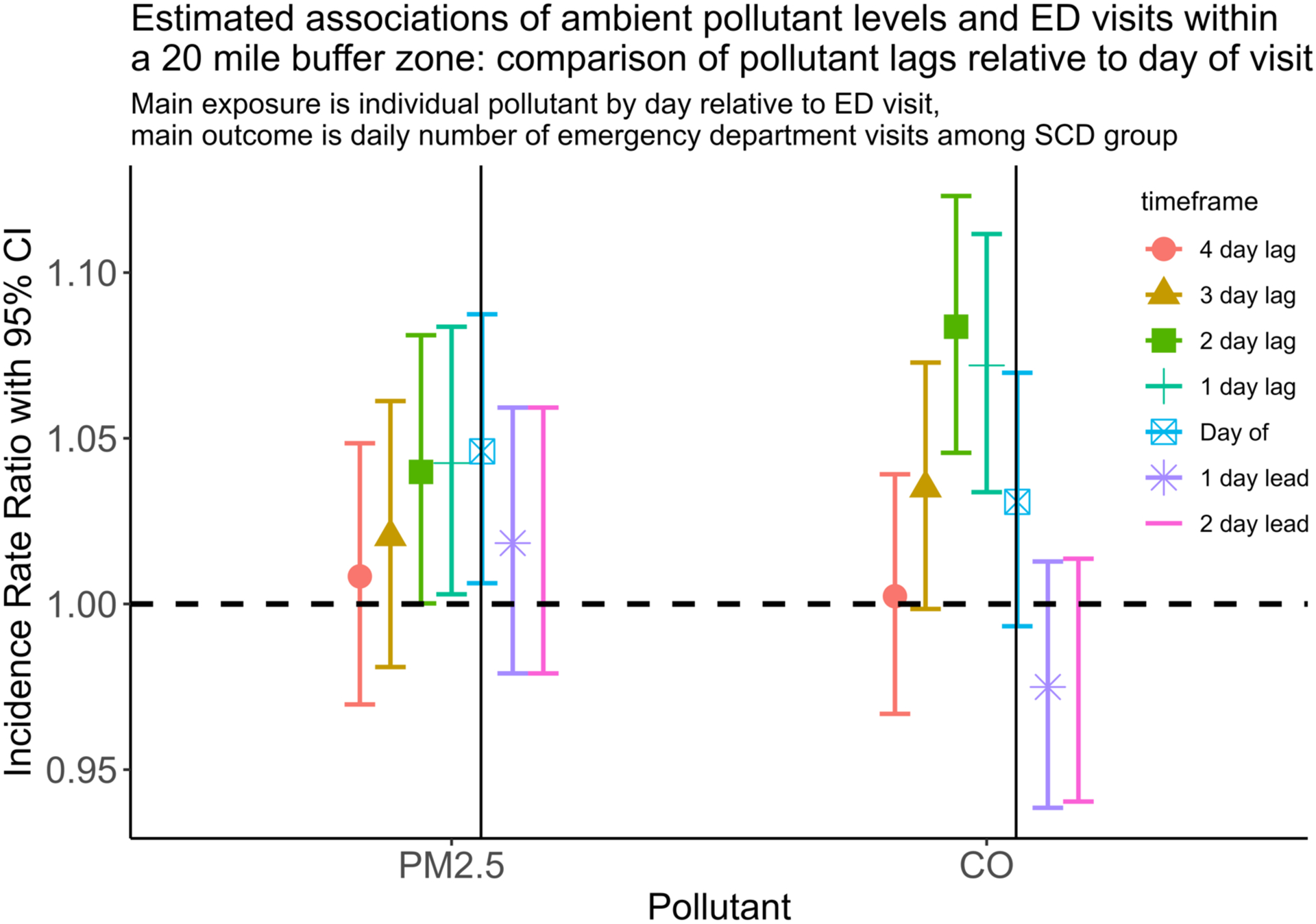
Plot above shows the effect of PM2.5 and CO on daily emergency department visits for the SCD cohort. Lag day means day prior to encounter. For example, 2-day lag refers to the pollution levels 2 days prior to encounter. Solid vertical lines represent day of encounter.
Lastly, we stratified the cohort by severe SCD (i.e. HbSS, HbSβ0) vs. all other genotypes (Figure 4), to determine if there was a differential effect based on hemoglobin type. Although our model showed higher estimates for children with severe SCD, especially in the 5-mile capture area, the confidence intervals significantly overlapped. Our models were robust to the other sensitivity analyses described in the methods section (Supplement).
Figure 4: Estimated associations of 3-day rolling mean ambient pollutant levels and ED visits by SCD genotype (among 5-mile buffer zone cohort).
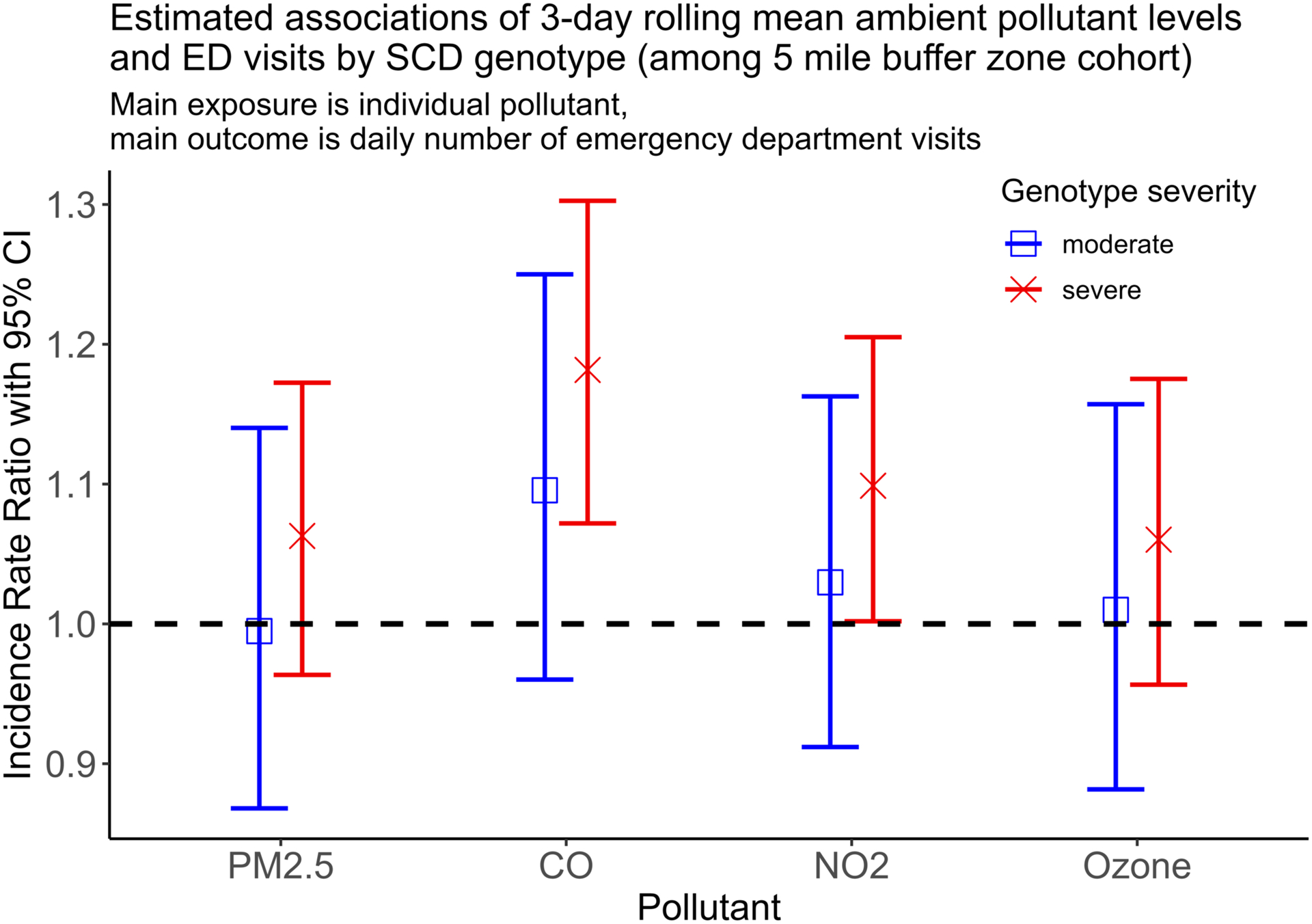
Plot above shows results of 8 separate models (4 pollutants × 2 SCD types). Severe includes patients with HbSS, HbSβ0; moderate includes all other sickle cell disease variants, does not include sickle cell trait. Of note, analysis of 10- and 20-mile buffer zones showed similar, non-significant differences when grouping by genotype.
Discussion
The results of this retrospective study on a cohort of children with SCD in Atlanta, GA show that increases in daily PM2.5, CO (primary analyses) and NO2 levels (secondary analysis) were significantly and positively associated with number of ED visits; ozone levels did not show significant associations. For all pollutants, IRR estimates relating pollution levels to ED visits were higher for the SCD group than the comparison analysis of all non-SCD patient visits. Importantly, our results were robust to a variety of sensitivity analyses, including modifications in distributional assumptions, weather covariates, and inclusion/exclusion criteria.
This study augments the growing literature in air pollution effects on health. First, there are strong pathophysiologic and sociodemographic reasons to suspect that children with SCD are especially prone to harms from air pollution. As previously mentioned, the key pathophysiologic pathways of air pollution and SCD significantly overlap.[8] From an epidemiologic standpoint, much of the pediatric air pollution literature has focused on children with underlying lung disease, such as children with asthma and cystic fibrosis, while adult data has shown pollution worsens disease outcomes in many other health settings, including cardiovascular and cerebrovascular conditions.[36, 37] The pathophysiology of SCD encompasses lung, cardiovascular, and cerebrovascular damage, potentially placing these patients at increased risk. Indeed, for all pollutants modeled, the IRR estimates were higher for the SCD group than the comparison group, which supports our hypothesis that children with SCD are especially prone to harms from air pollution, as compared to the general pediatric population. Furthermore, our results suggest that children with SCD may be even more susceptible to air pollution as compared to other high-risk populations. A meta-analysis of 87 publications that assessed the effect of pollutants on ED visits among children with asthma found increases in ED visits of 2.3% per 10 μg/m3 of PM2.5 (vs. our estimate of IRR 1.051 = 5.1% per 2 s.d. increase, which corresponds to 9.4 μg/m3 in our data), 4.5% for 1 mg/m3 CO (vs. 8.8%), 1.8% for NO2 (vs. 3%, not significant), and 0.9% for ozone (vs. 0.5%, not significant).[38]
Another comparison of effect size can be made within the SCD field. A 2017 analysis, using a subset of our current study’s population, found the IRR for ED visits among patients who started hydroxyurea treatment compared to those not starting this treatment was 0.57 (95%CI 0.49–0.67).[27] To place our study into context, a reduction in daily pollution from the 97.5th to 2nd percentile (i.e., a 4 standard deviation change, similar to comparing the highest and lowest pollution days) results in IRRs of 0.91 (0.84–0.98) for PM2.5 and 0.78 (0.71–0.86) for CO. While these estimates are not as large in magnitude as the change associated with hydroxyurea initiation, they are nonetheless within the same order of magnitude.
Beyond the biologic basis for harm, children with SCD are at potentially higher risk due to their underlying sociodemographic characteristics.[39] Due to a legacy of systemic racism, racial and ethnic minorities are exposed to higher-than-expected levels of air pollution, even when accounting for neighborhood income.[40, 41] Given that SCD overwhelmingly affects the Black population and our findings that 28.5% of the patients in the SCD cohort lived in the two most polluted counties and only 2.7% lived in the two least polluted counties, it is likely that the patients in our cohort are actually exposed to higher than the city-wide averages included here, which could cause our results to under-estimate the true pollution effects. As such, our results suggest that improvements in air quality would disproportionately benefit the SCD population and lessen ongoing health disparities.
From a clinical perspective, our results have important implications. First, they suggest that pollution avoidance strategies could be considered as routine patient counseling for VOE avoidance and prevention. Such strategies, such as those recommended for patients with respiratory conditions, include: limiting outdoor exertion on high pollution days (many smart phones offer pollution warning messages and apps), avoiding physical exertion near major roadways and other sources of pollution, ventilating and isolating cooking areas (especially those with gas stoves), avoidance of indoor fires, and wearing high quality facemasks when near sources of pollution for prolonged or high-intensity periods.[42] High-efficiency particulate air/arresting (HEPA) filters can substantially improve air quality and have been shown to have significant, cost-effective benefits to human health [43, 44]; encouraging routine (every 4–6 months) replacement of school and household air conditioning filters significantly reduces pollution exposure.
There are specific limitations to the study. We have performed an observational study, so causality cannot be verified. Patients may seek care at facilities not included in the database, notably urgent care centers or adult EDs. However, we performed a sub-analysis on patients who lived especially close (within 5 miles) to a CHOA facility and were therefore more likely to seek care at CHOA – that analysis showed similar (and, in fact, slightly larger) IRR estimates as compared to the 20-mile models, adding evidence to support our main model and its assumptions. Specifically, with the underlying assumption that patients’ choice of care location (i.e., CHOA vs outside facility) does not systematically differ with air pollution levels, our results remain unbiased. Another limitation is that we did not distinguish cause of ED visit and our analyses therefore include some visits whose cause are unlikely to be pollution-related, even tangentially (e.g. broken bone). We also did not have reason-for-visit data available for the comparison group; it is likely these children have a different mix of reasons for visit. However, as above, if these other visits are not systematically correlated with air pollution levels, our results remain unbiased. It also warrants mention that respiratory symptoms, headache, viral and other pediatric infections, significant sources of ED visits, are also known to be associated with air pollution, adding validity to our model.[45, 46]
Our patient population comes from an urban/peri-urban environment and caution must be used when extrapolating to a rural environment. Similarly, nearly 10% of our SCD cohort had an address that was unlisted or listed as a PO box. While our database updates a patient’s address at each visit which helps account for housing instability, families experiencing homelessness are an especially vulnerable population and those patients with unlisted or PO box addresses were not included in our analyses. Furthermore, our comparison analysis of daily visits by all children minus children with SCD likely includes different sociodemographic characteristics than the SCD group. Given the association of poverty, race and other sociodemographic variables with air pollution exposure, a combination of these factors, as opposed to air pollution alone, could contribute to the difference between the SCD and non-SCD analyses; due to data limitations in the non-SCD analysis, we were unable to explore individual contributions. However, this study’s focus was to describe associations between ambient air pollution levels and population-wide ED visits and we made no predictions on how patient-level characteristics, such as neighborhood poverty level, medication usage, or tobacco smoke, interact with pollution exposure; additional research is needed in this area.
In conclusion, we find that increases in daily PM2.5, CO, and NO2 levels are associated with significantly higher ED utilization amongst our cohort of 1740 children with SCD. Furthermore, the IRRs obtained in the comparison analyses were lower than those of the SCD cohort, which supports the hypothesis that children with SCD are at especially high-risk for air pollution’s harms. These results provide a potential trigger and underlying pathophysiologic pathway for VOE in patients with SCD. Further research is needed to identify children with SCD most at-risk from air pollution’s harms, as this risk factor is modifiable via targeted clinical counseling, personal and family-level pollution avoidance strategies, and pollution reduction via home and school air filtration systems.
Supplementary Material
Acknowledgements
Paul E. George reports that financial support for this work was provided by National Institutes of Health grant T32HL139443-3.
Glossary
- CHOA
Children’s Healthcare of Atlanta
- CO
Carbon monoxide
- ED
Emergency department
- EPA
Environmental Protection Agency
- HbXX
Hemoglobin with subtype XX (e.g., SS, SC)
- IRR
Incident rate ratio
- NO2
Nitrogen dioxide
- PM2.5
Fine particulate matter 2.5 microns in diameter or less
- Ppb/ppm
Parts per billion / parts per million
- SCD
Sickle cell disease
- s.d.
Standard deviation
- SEDAC
NASA Socioeconomic Data and Applications Center
- VOE
Vaso-occlusive events
- μg/m3
Micrograms/meter cubed
Footnotes
Conflict of Interest Statement
The authors have no competing interests to declare.
References
- 1.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N (Nil), Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potočnik, Preker AS, Ramesh J, Rockström, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella, Zhong M. The Lancet Commission on pollution and health. The Lancet , 2018. 391: 462–512. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fraser MS, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Hay SI, Huynh C, Johnson CO, Kassebaum NJ, Kinfu Y, Kulikoff XR, Kutz M, Kyu HH, Larson HJ, Leung J, Liang X, Lim SS, Lind M, Lozano R, Marquez N, Mensah GA, Mikesell J, Mokdad AH, Mooney MD, Nguyen G, Nsoesie E, Pigott DM, Pinho C, Roth GA, Salomon JA, Sandar L, Silpakit N, Sligar A, Sorensen RJD, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, VanderZanden A, Vollset SE, Wanga V, Whiteford HA, Wolock T, Zoeckler L, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Abreu DMX, Abu-Raddad LJ, Abyu GY, Achoki T, Adelekan AL, Ademi Z, Adou AK, Adsuar JC, Afanvi KA, Afshin A, Agardh EE, Agarwal A, Agrawal A, Kiadaliri AA, Ajala ON, Akanda AS, Akinyemi RO, Akinyemiju TF, Akseer N, Lami FHA, Alabed S, Al-Aly Z, Alam K, Alam NKM, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet , 2016. 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratt GC, Vadali ML, Kvale DL, Ellickson KM. Traffic, Air Pollution, Minority and Socio-Economic Status: Addressing Inequities in Exposure and Risk. International Journal of Environmental Research and Public Health , 2015. 12: 5355–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landrigan PJ, Fuller R, Fisher S, Suk WA, Sly P, Chiles TC, Bose-O’Reilly S. Pollution and children’s health. Science of The Total Environment , 2019. 650: 2389–2394. [DOI] [PubMed] [Google Scholar]
- 5.Brumberg HL, Karr CJ, Bole A, Ahdoot S, Balk SJ, Bernstein AS, Byron LG, Landrigan PJ, Marcus SM, Nerlinger AL, Pacheco SE, Woolf AD, Zajac L, Baum CR, Campbell CC, Sample JA, Spanier AJ, Trasande L, COUNCIL ON ENVIRONMENTAL HEALTH. Ambient Air Pollution: Health Hazards to Children. Pediatrics , 2021. 147: e2021051484. [DOI] [PubMed] [Google Scholar]
- 6.Ambient (outdoor) air pollution [Internet][cited 2021 Dec 23] Available from: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health
- 7.World Health Organization. WHO global air quality guidelines: particulate matter (PM2. 5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Health Organization, 2021 [PubMed] [Google Scholar]
- 8.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. The Lancet , 2010. 376: 2018–2031. [DOI] [PubMed] [Google Scholar]
- 9.Dampier C, Barry V, Gross HE, Lui Y, Thornburg CD, DeWalt DA, Reeve BB. Initial Evaluation of the Pediatric PROMIS® Health Domains in Children and Adolescents With Sickle Cell Disease. Pediatric Blood & Cancer , 2016. 63: 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: Past, present, and future. Pediatric Blood & Cancer , 2012. 59: 377–385. [DOI] [PubMed] [Google Scholar]
- 11.Shah N, Bhor M, Xie L, Paulose J, Yuce H. Sickle cell disease complications: Prevalence and resource utilization. PLOS ONE , 2019. 14: e0214355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency Department Visits Made by Patients with Sickle Cell Disease: A Descriptive Study, 1999–2007. American Journal of Preventive Medicine , 2010. 38: S536–S541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavanagh PL, Fasipe TA, Wun T. Sickle Cell Disease: A Review. JAMA , 2022. 328: 57–68. [DOI] [PubMed] [Google Scholar]
- 14.Jang T, Poplawska M, Cimpeanu E, Mo G, Dutta D, Lim SH. Vaso-occlusive crisis in sickle cell disease: a vicious cycle of secondary events. J Transl Med , 2021. 19: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang K-J, Chan C-C, Su T-C, Lee C-T, Tang C-S. The Effect of Urban Air Pollution on Inflammation, Oxidative Stress, Coagulation, and Autonomic Dysfunction in Young Adults. Am J Respir Crit Care Med , 2007. 176: 370–376. [DOI] [PubMed] [Google Scholar]
- 16.Feng S, Gao D, Liao F, Zhou F, Wang X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicology and Environmental Safety , 2016. 128: 67–74. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JO, Thundiyil JG, Stolbach A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J Med Toxicol , 2012. 8: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatric Respiratory Reviews , 2017. 21: 38–46. [DOI] [PubMed] [Google Scholar]
- 19.Khalili R, Bartell SM, Hu X, Liu Y, Chang HH, Belanoff C, Strickland MJ, Vieira VM. Early-life exposure to PM2.5 and risk of acute asthma clinical encounters among children in Massachusetts: a case-crossover analysis. Environ Health , 2018. 17: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamanaka RB, Mutlu GM. Particulate Matter Air Pollution: Effects on the Cardiovascular System [Internet]. Frontiers in Endocrinology , 2018. 9. [cited 2022 Oct 11] Available from: https://www.frontiersin.org/articles/10.3389/fendo.2018.00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood , 2016. 127: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira RS, Terse-Ramos R, Ferreira TA, Machado VR, Perdiz MI, Lyra IM, Nascimento VL, Boa-Sorte N, Andrade BB, Ladeia AM. Associations between endothelial dysfunction and clinical and laboratory parameters in children and adolescents with sickle cell anemia. PLOS ONE , 2017. 12: e0184076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumberg AH, Ebelt ST, Liang D, Morris CR, Sarnat JA. Ambient air pollution and sickle cell disease-related emergency department visits in Atlanta, GA. Environmental Research , 2020. 184: 109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yallop D, Duncan ER, Norris E, Fuller GW, Thomas N, Walters J, Dick MC, Height SE, Thein SL, Rees DC. The associations between air quality and the number of hospital admissions for acute pain and sickle-cell disease in an urban environment. British Journal of Haematology , 2007. 136: 844–848. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa SM de M, Farhat SCL, Martins LC, Pereira LAA, Saldiva PHN, Zanobetti A, Braga ALF. Air pollution and children’s health: sickle cell disease. Cadernos de Saúde Pública , 2015. 31: 265–275. [DOI] [PubMed] [Google Scholar]
- 26.Snyder AB, Lane PA, Zhou M, Paulukonis ST, Hulihan MM. The accuracy of hospital ICD-9-CM codes for determining Sickle Cell Disease genotype. J Rare Dis Res Treat , 2017. 2: 39–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Quarmyne M-O, Dong W, Theodore R, Anand S, Barry V, Adisa O, Buchanan ID, Bost J, Brown RC, Joiner CH, Lane PA. Hydroxyurea effectiveness in children and adolescents with sickle cell anemia: A large retrospective, population-based cohort. American Journal of Hematology , 2017. 92: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Q, Wei Y, Shtein A, Hultquist C, Xing X, Amini H, Shi L, Kloog I, Silvern R, Kelly J, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, Wang Y, Mickley LJ, Schwartz J. Daily and Annual PM2.5 Concentrations for the Contiguous United States, 1-km Grids, v1 (2000 – 2016) [Internet], 2021. [cited 2022 Jul 20] Available from: https://sedac.ciesin.columbia.edu/data/set/aqdh-pm2-5-concentrations-contiguous-us-1-km-2000-2016
- 29.Li J, Lu X, Liu F, Liang F, Huang K, Yang X, Xiao Q, Chen J, Liu X, Cao J, Chen S, Shen C, Yu L, Lu F, Wu X, Zhao L, Wu X, Li Y, Hu D, Huang J, Zhu M, Liu Y, Shen H, Gu D. Chronic Effects of High Fine Particulate Matter Exposure on Lung Cancer in China [Internet]. Am J Respir Crit Care Med , 2020. [cited 2020 Nov 22] Available from: https://www.atsjournals.org/doi/abs/10.1164/rccm.202001-0002OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrasco-Escobar G, Schwalb A, Tello-Lizarraga K, Vega-Guerovich P, Ugarte-Gil C. Spatio-temporal co-occurrence of hotspots of tuberculosis, poverty and air pollution in Lima, Peru. Infectious Diseases of Poverty , 2020. 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y, Jones MR, Ahn JB, Garonzik-Wang JM, Segev DL, McAdams-DeMarco M. Ambient air pollution and posttransplant outcomes among kidney transplant recipients. American Journal of Transplantation , 2021. 21: 3333–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ver Hoef JM, Boveng PL. Quasi-Poisson Vs. Negative Binomial Regression: How Should We Model Overdispersed Count Data? Ecology , 2007. 88: 2766–2772. [DOI] [PubMed] [Google Scholar]
- 33.Pan A, Sarnat SE, Chang HH. Time-Series Analysis of Air Pollution and Health Accounting for Covariate-Dependent Overdispersion. Am J Epidemiol , 2018. 187: 2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelman A Scaling regression inputs by dividing by two standard deviations. Statistics in medicine , 2008. 27: 2865–2873. [DOI] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. International Journal of Surgery , 2014. 12: 1495–1499.25046131 [Google Scholar]
- 36.Shah ASV, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ , 2015. 350: h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadley MB, Vedanthan R, Fuster V. Air pollution and cardiovascular disease: a window of opportunity. Nature Reviews Cardiology , 2018. 15: 193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng X, Ding H, Jiang L, Chen S, Zheng J, Qiu M, Zhou Y, Chen Q, Guan W. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLOS ONE , 2015. 10: e0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan H, Krull M, Hankins JS, Wang WC, Porter JS. Sickle cell disease and social determinants of health: A scoping review. Pediatric Blood & Cancer , 2022. e30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo B, Kravitz-Wirtz N, Sass V, Crowder K, Teixeira S, Takeuchi DT. Residential Segregation and Racial/Ethnic Disparities in Ambient Air Pollution. Race Soc Probl , 2019. 11: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, Post WS, Kaufman JD, Navas-Acien A. Race/Ethnicity, Residential Segregation, and Exposure to Ambient Air Pollution: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health , 2014. 104: 2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlsten C, Salvi S, Wong GWK, Chung KF. Personal strategies to minimise effects of air pollution on respiratory health: advice for providers, patients and the public [Internet]. European Respiratory Journal , 2020. 55. [cited 2022 Oct 11] Available from: https://erj.ersjournals.com/content/55/6/1902056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen RW, Barn P. Individual- and Household-Level Interventions to Reduce Air Pollution Exposures and Health Risks: a Review of the Recent Literature. Curr Envir Health Rpt , 2020. 7: 424–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilraine M Air Filters, Pollution and Student Achievement [Internet]. Annenberg Institute at Brown University, 2020[cited 2020 Oct 11] Available from: https://www.edworkingpapers.com/ai20-188
- 45.Brugha R, Grigg J. Urban Air Pollution and Respiratory Infections. Paediatric Respiratory Reviews , 2014. 15: 194–199. [DOI] [PubMed] [Google Scholar]
- 46.Bowatte G, Tham R, Perret JL, Bloom MS, Dong G, Waidyatillake N, Bui D, Morgan GG, Jalaludin B, Lodge CJ, Dharmage SC. Air Pollution and Otitis Media in Children: A Systematic Review of Literature. International Journal of Environmental Research and Public Health , 2018. 15: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


