Abstract
Traumatic brain injuries (TBIs) are associated with high morbidity and mortality due to both the original insult as well as the destructive biological response that follows. Medical management aims to slow or even halt secondary neurological injury while simultaneously laying the groundwork for recovery. Statins are one class of medications that is showing increased promise in the management of TBI. Used extensively in cardiovascular disease, these drugs were originally developed as competitive inhibitors within the cholesterol production pipeline. They are now used in diverse disease states due to their pleiotropic effects on other biological processes such as inflammation and angiogenesis. Preclinical studies, retrospective reviews, and randomized clinical trials have shown a variety of benefits in the management of TBI, but to date, no large-scale randomized clinical trial has been performed. Despite this limitation, statins’ early promise and well-tolerated side effect profile make them a promising new tool in the management of TBIs. More bench and clinical studies are needed to delineate proper treatment regimens as well as understand their true potential.
Keywords: Traumatic brain injury, Statins, Trauma, Inflammation, Concussion, Inflammation, Cholesterol
Introduction
Traumatic brain injury (TBI) is a leading health problem in both developing and high-income countries. Over 50 million TBIs occur internationally each year, causing one-third of injury-related deaths and costing 0.5% of worldwide GDP [1]. TBI-related disability has an incidence of approximately 4 million people in the USA. This disease disproportionally affects the young, with peaks in the adolescent and the elderly stages of life [2]. These numbers underestimate the true impact of TBIs, with an order of magnitude more going unreported and unnoticed [2, 3].
Traumatic brain injury is a complex heterogeneous pathology with a wide clinical impact, ranging from asymptomatic to a neurologically devastating disease. Severe TBI has been estimated to have a mortality rate of almost 40%. Those who survive are often debilitated with severe physical, emotional, and economic burdens [4]. Nearly half of TBI survivors develop depression and later in life suffer from dementia at five times the average rate [5]. Even mild TBIs can have long-term impacts including increased risk of dementia, seizures [6], functional limitations, disability, mood disorders [7], and reduced quality of life [8].
Crucial to the management of TBI is recognition that it is not an acute condition, but a chronic and evolving disease process. The “second hit” model posits that damage continues even after the original injury, as swelling and inflammation sets in. Additionally, the recovery process is a complex, poorly understood process dependent on the reshaping or reformation of the damaged neural networks [9].
The standard of care for TBI management is a constantly changing field. In the acute period, surgical intervention such as placement of a ventriculostomy or craniectomy can have a significant impact on morbidity and mortality [10, 11]. However, the vast majority of cases are managed conservatively [10]. In addition to long-term support including physical and occupational therapy, many different medical interventions have been tried. Aside from antiepileptics prevention of early-onset seizures [12] and multimodal therapy for intracranial pressure control [13], no other regimen has been codified in the management of TBI sequelae [14, 15].
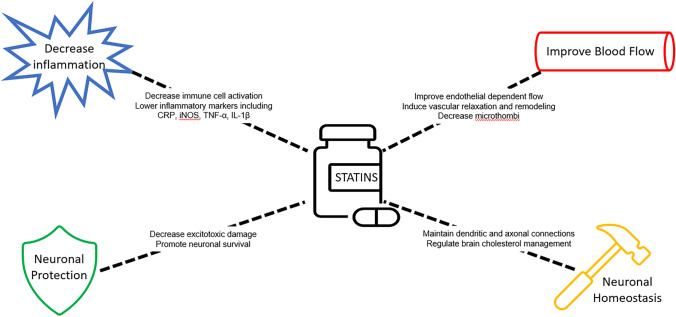
This large divide between a clear clinical need and a lack of solution has driven an enormous amount of research into potential treatments. Clinical trials have investigated a wide variety of known medications [15], such as magnesium [16] and cyclosporine-A [17]. One class of medications receiving increased interest is statins. Statins classically impact physiology as inhibitors of the enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase), the rate-controlled step within the mevalonate pathway. This interrupts the metabolic chain reaction that eventually produces cholesterol and other organic isoprenoid derivates such as steroids and vitamins. However, further investigations have revealed many alternative impacts of statins, including stimulating angiogenesis, anti-inflammatory effects, and influence of neural circuit formation (Fig. 1) [18].
Fig. 1.
The putative beneficial effects and proposed mechanisms of statin therapy for TBI
History
In the late twentieth century, researchers began to elucidate the role of cholesterol in cardiovascular disease and looked for ways to control cholesterol levels. The first statin was actually a byproduct of antibiotic research. Inspired by the discovery of penicillin, researchers were culturing fungi at a large scale to find new compounds. Mevastatin, also known as compactin, was isolated in 1971 from the fungus Penicillium citrinum by researchers looking for an enzyme that might target microbes that depended on sterols or other isoprenoids [19, 20]. Lovastatin was isolated by Merck in 1978 from Aspergillus terreus and in 1987 became the first statin to be approved by the FDA. The incredible results of early trials [21] caused a wave of public interest in statins, leading to the development of many alternative compounds. In the early 2000s, blockbuster drugs such as simvastatin, pravastatin, and atorvastatin had an average annual cost of nearly $25 billion in the USA alone [22].
Mechanism of Action
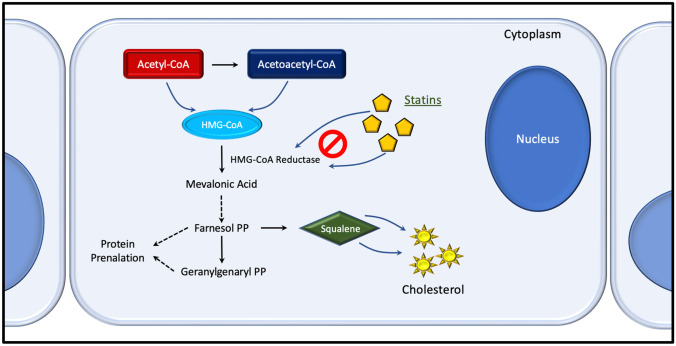
Statins were originally discovered for their ability to competitively inhibit HMG-CoA reductase due to their molecular similarity to HMG-CoA (Fig. 2). This competition for the enzyme’s active site allows it to compete with the native substrative and reduce the rate that mevalonate is produced. The lower availability of mevalonate decreases the body’s ability to generate cholesterol (a downstream molecule). This impact is compounded by the liver, which increases the production of LDL receptors to harvest circulating cholesterol, further lowering bloodstream levels [23].
Fig. 2.
The cholesterol synthetic pathway, highlighting the effects of statins on the mevalonate pathway. PP pyrophosphate, HGM 3-hydroxy-3-methylglutaryl, CoA coenzyme A
Yet as their use became more widespread, new findings began to suggest that this is not the only action of statins in the body. In fact, a large part of its impact may actually be derived from other sources. For example, simply lowering cholesterol by other means does not have the same benefit, and these drugs have been shown to have a benefit in disease processes not classically associated with elevated lipid levels [24].
More than 20 years since statins were put on the market, new findings demonstrated that many of their health benefits may be through their immunomodulatory impact. For example, they are known to inhibit the inductive effect of interferon-γ on major histocompatibility class II (MHC-II), thereby repressing MHC-II-medicated T-cell activation [25]. They have also been shown to lower C reactive protein (CRP) levels by one-third [26], as well as other inflammatory markers such as inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNF-α), and interleukin-1β (IL-1β) [27]. Other studies have shown a disruption of lipid rafts, preventing the organization of proteins necessary for the activation of immune cells [28]. Natural killer cells have lower cytotoxicity in patients on statins, leading to their use in preventing organ rejection [29]. Other autoimmune disorders that have been treated with statins include multiple sclerosis, rheumatoid arthritis, and osteoporosis [25].
Further investigations have also shown a direct impact on vasculature. Endothelial-dependent flow significantly improves after statin treatment [30]. Statins induce endothelial nitric oxide synthase (eNOS), an enzyme that generates nitric oxide (NO) within vessel walls to promote vascular relaxation and decrease interactions with circulating leukocytes and platelets [31]. They also induce the expression of various genetic profiles involved in the remodeling of both endothelial and smooth muscle cells [24].
Lastly, there may be direct neurological impacts both cholesterol and non-cholesterol mediated. Cholesterol is a major component of neural membranes and is a rate-limiting step in synaptogenesis [32]. Compactin has been shown to promote the maintenance of dendritic and axonal connectivity patterns [33]. Statins can protect neurons from excitotoxic damage, such as NMDA-mediate excitotoxic cell death [34]. Simvastatin in particular has been shown to stimulate the Bcl-2 gene, promoting neuronal survival [35] and attenuating axonal injury [36]. Many other enzymes with key roles in the maintenance of neural homeostasis have been shown to be modulated by statins [18].
Pharmacology
Statins can be broadly divided into two categories. Type 1 statins such as lovastatin, pravastatin, and simvastatin are fungal-derived and share structural similarities to the original mevastatin. Type 2 statins such as atorvastatin and rosuvastatin are synthetically derived and have highly variable properties [37]. All statins are absorbed by the intestines and have a bioavailability between 5 and 50%. Most (but not all) are metabolized by cytochrome P-450 (CYP 450) [38]. Lipophilic statins such as atorvastatin and simvastatin passively cross the blood–brain barrier [39]. There is some concern that hydrophilic statins do not have as large of an impact [39], but there is evidence that they also enter the cerebrospinal fluid space, possibly through active transport [40]. The wide diversity of molecular profiles begs the question of the efficacy of each variation. Unfortunately, research is still lacking on how to maximize benefits.
Despite their potency, statins are often very well tolerated. Myopathy has been classically associated with statin use in 1–5% of patients [41], though the incidence is controversial with many studies demonstrating no increased risk relative to placebo [42]. Hepatic dysfunction (as defined by elevated transaminases) has also been reported in up to 3% of patients [43], especially within the first few months of initiation. However, clinically significant liver injury is rare, and regular monitoring is not required [44]. Lastly, there may be a small increased risk in the long-term development of type 2 diabetes [45]. Notably, some of the reported side effects may be due to cognitive biases, with one large-scale study finding discontinuation rates to be similar between statins and placebo [46].
Animal Studies
Early animal models showed significant promise in the role of statins in traumatic neurological injuries. Using simple tasks as proxies for both motor and cognitive function, researchers examined the impact of the administration of statins on TBI outcomes. Table 1 demonstrates select highlighted studies but are not an exhaustive list. Simvastatin was most commonly used, but other statins were tested as well. While not every study showed a definite improvement [47], decades of studies have shown that even a short course of statins can have significant motor [47–52] and cognitive [36, 52–57] benefits. These benefits may have long-term impacts, as one study showed improved functional outcomes up to 3 months with the administration of a variety of statins [58].
Table 1.
Selected animal studies
| Author/date | Study design | Treatment | Clinical outcome | Physiological outcomes |
|---|---|---|---|---|
| Lu et al. 2004 [53, 54, 60, 61] | CCI rats | Atorvastatin × 7–15 days | Improved Corner scores, mNS scores, improved spatial memory | Increased neurogenesis, increased angiogenesis, reduced intracranial hematoma volume, decreased vessel thrombosis |
| Chen et al. 2007 [48] | CCI rats | Lovastatin × 5 days prior to injury | Improved rotarod and adhesive removal tests |
Decreased contusion volume and degenerating neurons Decreased TNF-α, IL-1β |
| Lu et al. 2007 [55] | CCI rats | Atorvastatin and simvastatin × 14 days | Improved spatial learning | Reduced neuronal loss and increased neurogenesis in hippocampus |
| Wang et al. 2007 [49] | CCI mice | Atorvastatin or Simvastatin × 14 prior to injury and 3 days after | Improved rotarod | Reduced neuronal loss, decreased CNS inflammation, improved cerebral hemodynamics |
| Mahmood et al. 2009 [58] | CCI rats | Simvastatin × 14 days | Improved motor function up to 90 days | Increased proliferation in lesional zone |
| Wu et al. 2011 [50] | CCI rats | Simvastatin × 14 days | Decreased falls | Increased angiogenesis |
| Wu et al. 2012 [36] | CCI rats | Simvastatin × 14 days | Improved mNS scores | Decreased axonal injury, increased neurite growth, via mTOR and APC pathways |
| Abrahamson et al. 2013 [62] | CCI Human Amyloid-β mice | Simvastatin × 3 weeks | Decreased Amyloid-β, brain tissues loss, improved blood flow | |
| Darwish et al. 2014 [56] | CCI rats | Simvastatin × 14 days | Decreased memory deficits | |
| Wang et al. 2014 [51] | CCI rats | Simvastatin × 7 days | Improved grip strength | Decreased cerebral vascular endothelial inflammation |
| Xie et al. 2015 [57] | CCI rats | Simvastatin × 35 days | Decreased mNS scores | Increased neurogenesis via Notch-1 |
| Mountney et al. 2016 [59] | PBBI rats | Simvastatin × 10 days | No benefit on rotarod, improved water maze test | Decreased GFAP, IL-1α, I1-17 |
| Mountney et al. 2016 [47] | FPI, CCI, and PBBI rats | Simvastatin × 14 days | Mild benefit on grid walk, balance beam, and rotarod, worsened water maze | Worse cortical loss in FPI model |
| Xu et al. 2017 [52] | CCI mice | Atorvastatin × 3 days | Improved mNS, latency to falls | Decreased neuronal apoptosis, WBC invasion, IFN-γ, IL-6, chemokines |
CCI controlled cortical impact, mNS modified neurological severity, WBC white blood cells, FPI fluid percussion injury, PBBI penetrating ballistic-like brain injury
The use of an animal model allowed these studies to further look into the putative mechanisms of these benefits. Findings supported many of the in vitro and in vivo hypotheses. Many showed a reduced inflammatory profile [48, 49, 52, 59]. Other studies showed that the vascular impact of statins may provide further benefit, with reduced thromboses [53, 60] and intracranial hematoma volumes [61]. A direct neuronal impact was noted in some instances, with increased neurogenesis and reduced neuronal loss [36, 52, 54, 55, 57, 58].
Human Trials
In one of the first large-scale studies, Khokhar et al. looked at the impact of prior statin use on outcomes in TBI in over 100,000 medicare beneficiaries over 65. They showed that statin used was associated with an approximate 0.85 long-term relative risk of dementia, stroke, and depression [63]. While not consistently replicated [64], at least two other studies since then have showed similar outcomes with respect to the risk of dementia even when looking only at mild concussions [65, 66]. Interestingly, this was true even if they were not on a statin prior to their TBI though the effect disappeared if the patient did not continue taking the statin. Additionally, there was no significant difference in the class (lipophilic vs. hydrophilic) or dose of statin prescribed, though rosuvastatin did seem to perform the best [65].
More rigorous studies have been performed, albeit with lower patient numbers. In a randomized control trial of a small population, Sanchez-Aguilar et al. showed that starting rosuvastatin after TBI can reduce disability scores at 3 and 6 months. This study did not show any improvement in amnesia and disorientation, though that could be due to low power or short-term follow-up [67]. In a similar study, Tapia-Perez did find a small reduction in amnesia time but no difference in disability at 3 months [68]. Other metrics of function such as Glasgow Coma Score (GCS) at discharge [69–71], Glasgow Outcomes Scale [72], Disability Rating Scale, and modified Rankin sale [73] are also improved by statins. Data on the impact of statins on mortality is mixed, with some showing a decreased risk [63, 72, 74], others no impact [64, 69, 75], and one demonstrating that stopping statins can increase mortality [76]. However, not all studies are uniformly positive. Robertson et al. performed a phase II clinical trial and found that atorvastatin for 7 days had no difference in the Rivermead score (a post-concussive assessment) at 3 months post-injury [77]. Notably, as with some of the animal studies, these trials have shown the effective time window to be as long as 24 h post-injury, increasing the clinical utility of statins in real-world situations.
Other studies aimed to correlate these outcomes with physiologic parameters of injury. From the inflammatory perspective, statins were shown to reduce levels of tumor necrosis factor- α (TNF-α), with mixed results on interleukin (IL) levels [67, 68]. Similarly, simvastatin has been shown to lower CRP in severe TBIs requiring ICU admission [69]. Interestingly, even in patients that show long-term benefits, it is difficult to notice any immediate difference on cranial imaging, with similar contusion volumes and rates of expansion [73], though one study showed decreased cortical loss [78].
Lastly, atorvastatin will be tested in a multi-arm, multi-stage adaptive platform trial for the acute treatment of TBI by the TRACK-TBI network. This will be a multi-center, double-blind, placebo-controlled adaptive platform, precision medicine trial conducted under a single multi-arm, multi-stage (MAMS) study with parallel groups. Subjects will be randomized to receive one of four possible treatments, being atorvastatin a study drug. It is expected that the findings of this study will assist in clarifying the potential beneficial effect of statins in the management of TBI (Table 2).
Table 2.
Human studies of TBIs
| Author/date | Study design | Patient population | Intervention | Clinical outcome | Physiological outcomes |
|---|---|---|---|---|---|
| Tapia-Perez et al. 2008 [68] | RCT | Age 16–50, GCS 9–13 (n = 21) | Rosuvastatin 20 mg × 10 days | Decreased amnesia. No difference in disability at 3 months | increased IL6. No difference in TNF-α, IL-1β, |
| Schneider et al. 2011 [74] | RCS | Age > 65 head AIS > 2 (n = 523) | Prior statin use | Decreased in-hospital death, improved outcomes at 1 year | |
| Sanchez-Aguilar et al. 2013 [67] | RCT | Moderate to severe TBI (n = 36) | 20 mg rosuvastatin × 10 days | Decreased disability | Decreased TNFα, no effect on IL-1β, IL-6, IL-10 |
| Naghibi et al. 2016 [69] | RCT | Severe TBI (n = 44) | 40 mg simvastatin | Higher GCS at discharge, similar mortality rate and ICU course | Lower CRP at 48 h, no difference in IL6 |
| Nielson et al. 2016 [75] | RCS | Severe TBI | Prior statin use | No decreased mortality at 2 weeks, or changes in GOS at 6 months | |
| Govindarajan et al. 2016 [78] | RCT | Mild TBI (n = 75) | Atorvastatin | Protection from cortical thinning seen in placebo group | |
| Farzanegan et al. 2017 [73] | RCT | Severe TBI (n = 65) | 20 mg atorvastatin × 10 days | Improved mRS, GOS and DRS at 3 months | No difference in contusion volume |
| Robertson et al. 2017 [77] | RCT | Age 18–50, Mild TBI (n = 52) | Atorvastatin 1 mg/kg × 7 days | No difference for the first 3 months in post-concussion syndrome nor many other cognitive tests | |
| Khkohar et al. 2018 [63] | RCS | Age > 65 hospitalized for TBI (n = 100,515) | Prior statin use | Decreased mortality, depression, stroke, dementia | |
| Lokhandwala et al. 2019 [72] | RCS | Age > 18, severe TBI (n = 270) | Prior statin use | Lower mortality, decreased SNF disposition, higher GOS | |
| Redelmeier et al. 2019 [65] | RCS | Age > 66 with concussion (n = 28,815) | Statin prescribed within 90 days of injury | Decreased dementia | |
| Mansi et al. 2020 [64] | RCS | TBI diagnosis (n = 140) | Prior statin use | No difference in neurological outcomes | |
| Li et al. 2020 [66] | RCT | Age 50–89 with TBI (n = 733,920) | Statin prescribed after injury | Decreased dementia | |
| Soltani et al. 2020 [70] | RCT | Age 15–50, moderate to severe TBI (n = 60) | Atorvastatin 40 mg daily during hospitalization | Increased GCS at discharge, decreased ICU stay | Decreased CRP, ESR, WBC at 14 days, |
| Shafiee et al. 2021 [71] | RCT | Severe TBI, GCS < 9 (n = 98) | 40 mg simvastatin × 10 days | Increased GCS scores at discharge and 1 month |
GOS Glasgow Outcome Score, MRS modified Rankin scale, DRS Disabilitiy Rating Scale, SNF skilled nursing facility, AIS Abbreviated Injury Score, RCT randomized control trial, RCS retrospective cohort study, CRP C reactive protein
Conclusion
TBI is a significant and growing public health problem. There is no current standard of care regimen in the medical management of TBI. Statins are a well-studied popular class of medication with minimal side effect profile relative to the proposed benefits. Recent research has demonstrated that their benefits are not limited solely to the cardiovascular outcomes. In vitro, animal, retrospective, and randomized control studies have all demonstrated the potent for multimodal impact on both motor and cognitive outcomes. Further work is needed to clarify how to maximize its impact to ensure that its putative and potential benefits are realized.
Declarations
Conflict of Interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maas AIR, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 2.Frieden TR, Houry D, Baldwin G. Traumatic Brain injury in the United States: epidemiology and rehabilitation. CDC NIH Rep to Congr. 2015;1–74.
- 3.Feigin VL, et al. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 2013;12:53–64. doi: 10.1016/S1474-4422(12)70262-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld Jv, et al. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–1098. [DOI] [PubMed]
- 5.Plassman BL, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/WNL.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 6.Mahler B, et al. Unprovoked seizures after traumatic brain injury: a population-based case-control study. Epilepsia. 2015;56:1438–1444. doi: 10.1111/epi.13096. [DOI] [PubMed] [Google Scholar]
- 7.Hart T, et al. Major and minor depression after traumatic brain injury. Arch Phys Med Rehabil. 2011;92:1211–1219. doi: 10.1016/j.apmr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Riggio S, Wong M. Neurobehavioral sequelae of traumatic brain injury. Mount Sinai Journal of Medicine: J Transl Pers Med. 2009;76:163–172. doi: 10.1002/msj.20097. [DOI] [PubMed] [Google Scholar]
- 9.Rohling ML, Faust ME, Beverly B, Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.’s (2000, 2005) systematic reviews. Neuropsychology. 2009;23:20–39. [DOI] [PubMed]
- 10.Bullock MR, et al. Surgical management of traumatic brain injury. Neurosurgery. 2006;58:16–24. [Google Scholar]
- 11.Timofeev I, et al. Ventriculostomy for control of raised ICP in acute traumatic brain injury. 2008;99–104. 10.1007/978-3-211-85578-2_20. [DOI] [PubMed]
- 12.Chang BS, Lowenstein DH. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60:10–16. [DOI] [PubMed]
- 13.Rangel-Castillo L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26:521. doi: 10.1016/j.ncl.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marehbian J, Muehlschlegel S, Edlow BL, Hinson HE, Hwang DY. Medical management of the severe traumatic brain injury patient. Neurocritical Care. 2017;27(3):430–446. [DOI] [PMC free article] [PubMed]
- 15.Gruenbaum SE, Zlotnik A, Gruenbaum BF, Hersey D, Bilotta F. Pharmacologic neuroprotection for functional outcomes after traumatic brain injury: a systematic review of the clinical literature. CNS Drugs. 2016;30:791–806. doi: 10.1007/s40263-016-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temkin NR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6:29–38. doi: 10.1016/S1474-4422(06)70630-5. [DOI] [PubMed] [Google Scholar]
- 17.Lulic D, Burns J, Bae EC, van Loveren H, Borlongan Cv. A review of laboratory and clinical data supporting the safety and efficacy of cyclosporin A in traumatic brain injury. Neurosurgery. 2011;68:1172–1186. [DOI] [PubMed]
- 18.Farooqui AA, Ong WY, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56:443–471. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg, D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: The discovery of the statins and the end of the controversy. J Lipid Res. 2006;47:1339–1351. [DOI] [PubMed]
- 20.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. doi: 10.1016/S0022-2275(20)41379-3. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen TR, et al. Effect of simvastatin on ischemic signs and symptoms in the scandinavian simvastatin survival study (4S) Am J Cardiol. 1998;81:333–335. doi: 10.1016/S0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 22.Lin SY, et al. Trends in use and expenditures for brand-name statins after introduction of generic statins in the US, 2002–2018. JAMA Netw Open. 2021;4:e2135371–e2135371. doi: 10.1001/jamanetworkopen.2021.35371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 24.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discover 2005;4(12):977–987. [DOI] [PubMed]
- 25.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6. [DOI] [PubMed]
- 26.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5. [DOI] [PubMed]
- 27.Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005;79:340–350. doi: 10.1002/jnr.20345. [DOI] [PubMed] [Google Scholar]
- 28.Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis — as good as it gets? N Engl J Med. 2005;352:73–75. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 29.Katznelson S, et al. The effect of pravastatin on acute rejection after kidney transplantation–a pilot study. Transplantation. 1996;61:1469–1474. doi: 10.1097/00007890-199605270-00010. [DOI] [PubMed] [Google Scholar]
- 30.Anderson TJ, et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 31.Balakumar P, Kathuria S, Taneja G, Kalra S, Mahadevan N. Is targeting eNOS a key mechanistic insight of cardiovascular defensive potentials of statins? J Mol Cell Cardiol. 2012;52:83–92. doi: 10.1016/j.yjmcc.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 33.Fan Q-W, et al. Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J Neurochem. 2002;80:178–190. doi: 10.1046/j.0022-3042.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- 34.Zacco A, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson-Anuna LN, et al. Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem. 2007;101:77–86. doi: 10.1111/j.1471-4159.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Mahmood A, Qu C, Xiong Y, Chopp M. Simvastatin attenuates axonal injury after experimental traumatic brain injury and promotes neurite outgrowth of primary cortical neurons. Brain Res. 2012;1486:121–130. doi: 10.1016/j.brainres.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarland AJ, et al. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15:20607–20637 15, 20607–20637. [DOI] [PMC free article] [PubMed]
- 38.Garcia M, Reinoso R, Sanchez Navarro A, Prous J. Clinical pharmacokinetics of statin. Methods Find Exp Clin Pharmacol. 2003;25:457–481. [PubMed]
- 39.Botti RE, Triscari J, Pan HY, Zayat J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin Neuropharmacol. 1991;14:256–261. doi: 10.1097/00002826-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Johnson-Anuna LN, et al. Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther. 2005;312:786–793. doi: 10.1124/jpet.104.075028. [DOI] [PubMed] [Google Scholar]
- 41.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 42.Ganga Hv, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168:6–15. [DOI] [PubMed]
- 43.Russo MW, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618–624. doi: 10.1016/j.amjmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Swerdlow DI, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–361. doi: 10.1016/S0140-6736(14)61183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riaz H, et al. Meta-analysis of placebo-controlled randomized controlled trials on the prevalence of statin intolerance. Am J Cardiol. 2017;120:774–781. doi: 10.1016/j.amjcard.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 47.Mountney A, et al. Simvastatin treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33:567–580. doi: 10.1089/neu.2015.4130. [DOI] [PubMed] [Google Scholar]
- 48.Chen S-F, et al. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81:288–298. doi: 10.1016/j.lfs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, et al. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, et al. Induction of angiogenesis and modulation of vascular endothelial growth factor receptor-2 by simvastatin after traumatic brain injury. Neurosurgery. 2011;68:1363–1371. doi: 10.1227/NEU.0b013e31820c06b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K-W, et al. Simvastatin combined with antioxidant attenuates the cerebral vascular endothelial inflammatory response in a rat traumatic brain injury. Biomed Res Int. 2014;2014:1–6. doi: 10.1155/2014/408514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14:167. doi: 10.1186/s12974-017-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu D, et al. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004;101:813–821. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- 54.Lu D, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 55.Lu D, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darwish H, Mahmood A, Schallert T, Chopp M, Therrien B. Simvastatin and environmental enrichment effect on recognition and temporal order memory after mild-to-moderate traumatic brain injury. Brain Inj. 2014;28:211–226. doi: 10.3109/02699052.2013.862737. [DOI] [PubMed] [Google Scholar]
- 57.Xie C, et al. The effect of simvastatin treatment on proliferation and differentiation of neural stem cells after traumatic brain injury. Brain Res. 2015;1602:1–8. doi: 10.1016/j.brainres.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Mahmood A, et al. Long-term benefits after treatment of traumatic brain injury with simvastatin in rats. Neurosurgery 2009;65:187–91, discussion 191–2. [DOI] [PMC free article] [PubMed]
- 59.Mountney A, et al. Intravenous administration of simvastatin improves cognitive outcome following severe traumatic brain injury in rats. J Neurotrauma. 2016;33:1492–1500. doi: 10.1089/neu.2015.4139. [DOI] [PubMed] [Google Scholar]
- 60.Lu D, et al. Delayed thrombosis after traumatic brain injury in rats. J Neurotrauma. 2004;21:1756–1766. doi: 10.1089/neu.2004.21.1756. [DOI] [PubMed] [Google Scholar]
- 61.Lu D, et al. Atorvastatin reduction of intracranial hematoma volume in rats subjected to controlled cortical impact. J Neurosurg. 2004;101:822–825. doi: 10.3171/jns.2004.101.5.0822. [DOI] [PubMed] [Google Scholar]
- 62.Abrahamson EE, et al. Cerebral blood flow changes after brain injury in human amyloid-beta knock-in mice. J Cereb Blood Flow Metab. 2013;33:826–833. doi: 10.1038/jcbfm.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khokhar B, et al. Mortality and associated morbidities following traumatic brain injury in older medicare statin users. Journal of Head Trauma Rehabilitation. 2018;33:E68–E76. doi: 10.1097/HTR.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mansi IA, English JL, Alvarez CA, Mortensen EM, Pugh MJ. Statins in survivors of traumatic brain injury: a propensity score-matched analysis. Brain Inj. 2020;34:1367–1374. doi: 10.1080/02699052.2020.1802663. [DOI] [PubMed] [Google Scholar]
- 65.Redelmeier DA, Manzoor F, Thiruchelvam D. Association between statin use and risk of dementia after a concussion. JAMA Neurol. 2019;76:887. doi: 10.1001/jamaneurol.2019.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li M, et al. Beneficial association of angiotensin-converting enzyme inhibitors and statins on the occurrence of possible Alzheimer’s disease after traumatic brain injury. Alzheimers Res Ther. 2020;12:33. doi: 10.1186/s13195-020-00589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sánchez-Aguilar M, et al. Effect of rosuvastatin on cytokines after traumatic head injury. J Neurosurg. 2013;118:669–675. doi: 10.3171/2012.12.JNS121084. [DOI] [PubMed] [Google Scholar]
- 68.Tapia-Perez JH, et al. Effect of rosuvastatin on amnesia and disorientation after traumatic brain injury (NCT003229758) J Neurotrauma. 2008;25:1011–1017. doi: 10.1089/neu.2008.0554. [DOI] [PubMed] [Google Scholar]
- 69.Naghibi T, Madani S, Mazloomzadeh S, Dobakhti F. Simvastatin’s effects on survival and outcome in traumatic braininjury patients: a comparative study. Turk J Med Sci. 2016;46:1–5. doi: 10.3906/sag-1404-125. [DOI] [PubMed] [Google Scholar]
- 70.Soltani F, et al. The effect of low-dose atorvastatin on inflammatory factors in patients with traumatic brain injury: a randomized clinical trial. Arch Neurosci. 2020;7.
- 71.Shafiee S, et al. The effect of oral simvastatin on the clinical outcome of patients with severe traumatic brain injury: a randomized clinical trial. Ethiop J Health Sci. 2021;31:807–816. doi: 10.4314/ejhs.v31i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lokhandwala A, et al. Preinjury statins are associated with improved survival in patients with traumatic brain injury. J Surg Res. 2020;245:367–372. doi: 10.1016/j.jss.2019.07.081. [DOI] [PubMed] [Google Scholar]
- 73.Farzanegan GR, Derakhshan N, Khalili H, Ghaffarpasand F, Paydar S. Effects of atorvastatin on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injury; a randomized double-blind placebo-controlled clinical trial. J Clin Neurosci. 2017;44:143–147. doi: 10.1016/j.jocn.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 74.Schneider EB, et al. Premorbid statin use is associated with improved survival and functional outcomes in older head-injured individuals. J Trauma. 2011;71:815–819. doi: 10.1097/TA.0b013e3182319de5. [DOI] [PubMed] [Google Scholar]
- 75.Neilson SJ, See AAQ, King NKK. Effect of prior statin use on outcome after severe traumatic brain injury in a South-East Asian population. Brain Inj. 2016;30:993–998. doi: 10.3109/02699052.2016.1147599. [DOI] [PubMed] [Google Scholar]
- 76.Orlando A, et al. Unintentional discontinuation of statins may increase mortality after traumatic brain injury in elderly patients: a preliminary observation. J Clin Med Res. 2013;5:168–173. doi: 10.4021/jocmr1333w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robertson CS, et al. Phase II clinical trial of atorvastatin in mild traumatic brain injury. J Neurotrauma. 2017;34:1394–1401. doi: 10.1089/neu.2016.4717. [DOI] [PubMed] [Google Scholar]
- 78.Govindarajan KA, et al. Cortical Thickness in mild traumatic brain injury. J Neurotrauma. 2016;33:1809–1817. doi: 10.1089/neu.2015.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]