Abstract
Medium-chain fatty acids and their derivatives are natural ingredients that support immunological functions in animals. The effects of glycerol monolaurate (GML) on intestinal innate immunity and associated molecular mechanisms were investigated using a chicken embryo model. Sixty-four Arbor Acres broiler embryos were randomly allocated into four groups. On embryonic day 17.5, the broiler embryos were administered with 9 mg of GML, which was followed by a 12-h incubation period and a 12-h challenge with 32 μg of lipopolysaccharide (LPS). On embryonic day 18.5, the jejunum and ileum were harvested. Results indicated that GML reversed the LPS-induced decline in villus height and upregulated the expression of mucin 2 (P < 0.05). GML decreased LPS-induced malondialdehyde production and boosted antioxidant enzyme activity (P < 0.05). GML alleviated LPS-stimulated intestinal secretion of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) (P < 0.05). GML also normalized LPS-induced changes in the gene expression of Toll-like receptor 4, nuclear factor kappa-B p65 (NF-κB p65), cyclooxygenase-2, NOD-like receptor protein 3, IL-18, zonula occludens 1, and occludin (P < 0.05). GML enhanced as well the expression of AMP-activated protein kinase α1 and claudin 1 (P < 0.05). In conclusion, GML improved intestinal morphology and antioxidant status by alleviating inflammatory responses and modulating NF-κB signaling in LPS-challenged broiler embryos.
Keywords: Glycerol monolaurate, Innate immunity, Lipopolysaccharide, Nuclear factor kappa-B, Chicken embryo
1. Introduction
Enteric infections are a constant challenge for the poultry industry and a major cause of economic losses owing to reduced feed efficiency and increased mortality following the ban on the use of antibiotic growth promoters (AGP) (Abd El-Hack et al., 2022). Medium-chain fatty acids and their derivatives as natural alternatives to AGP are gaining attention for controlling enteric diseases of broilers in the post-antibiotic era (Kumar et al., 2021, 2022). The use of medium-chain fatty acids such as caprylic, capric, and lauric acids shows potent effects against necrotic enteritis and benefits the integrity and homeostasis of intestinal health in broilers (Gomez-Osorio et al., 2021). Glycerol monolaurate (GML) is a naturally occurring 12-carbon monoester comprising lauric acid and glycerol that is found in coconut oil, palmetto, and human breast milk (Luo et al., 2022). GML possesses antimicrobial, anti-inflammatory, and immunomodulatory activities and is thus extensively used to inhibit potential bacterial pathogens. GML resistance has not been found in any susceptible microorganisms, and GML has been shown to inhibit bacterial peritonitis and viral vaginal inflammation (Fosdick et al., 2021; Manohar et al., 2013; Valentini et al., 2020). Dietary GML is one of the most promising feed additives for poultry, livestock, and aquaculture because of its great potential to improve intestinal health (Fortuoso et al., 2019; Ren et al., 2020; Wang et al., 2021).
Gram-negative bacterial lipopolysaccharide (LPS) is the main cause of intestinal injury through the increased production of chemokines and cytokines (Deng et al., 2019). LPS reportedly induces innate immune responses in chick embryos at embryonic age day 18 (E 18), similar to findings in mature individuals (Bavananthasivam et al., 2019). Owing to its ease of manipulation, chicken embryo is an attractive model in various fields, such as immunology, drug testing, genetics, and cell biology (Serralbo et al., 2020). Chicken embryos also have a low abundance of intestinal microflora. To exclude any possible interference mediated by host microflora, an LPS stimulation model of chicken embryos was established in the present study. This model may resemble the vertical transmission of gram-negative pathogens such as Escherichia coli from breeders to broilers.
Our previous study has shown that dietary GML improves the intestinal health of broilers and ameliorates LPS-induced immune stress and intestinal injury by suppressing inflammation and regulating the intestinal microbiota (Kong et al., 2021, 2022). In the present study, the direct effects and molecular mechanisms of GML on intestinal health were further investigated using an embryonic model to avoid the confounding effects of diet, intestinal microbiota, and experimental heterogeneity. We aimed to evaluate the protective effects of GML on the inflammatory response, intestinal barrier function, and oxidative stress in the jejunum and ileum of broiler embryos subjected to the LPS challenge.
2. Materials and methods
2.1. Animal ethics
All animal-care procedures were reviewed and approved by the Ethics Committee of Shandong Agricultural University (approval No. SDAUA-2022-50).
2.2. Eggs, experimental design, and in ovo injection
A total of 100 fertile eggs (Arbor Acres) with similar weights were purchased on the day they were laid. All embryonated eggs were incubated at 37.8 °C and a relative humidity of 60% in an automatic incubator. At E 16, the eggs (n = 64) were candled and randomly allocated into four groups for in ovo injection (denoted as the CON, LPS, GML, and GML + LPS groups) with 16 eggs per group. GML (Sigma–Aldrich Inc., St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) (Aladdin Biochemical Technology Co., Ltd., Shanghai, China) as described by Sivinski et al. (2020). The experimental design is shown in Fig. 1. At E 17.5, eggs in the GML and GML + LPS groups were injected with 50 μL of DMSO containing 9 mg of GML, and those in the CON and LPS groups received an equivalent injection of DMSO. At E 18, eggs in the LPS and GML + LPS groups were injected with 50 μL of saline containing 32 μg of LPS (L2880, Sigma–Aldrich Inc., St. Louis, MO, USA), and equivalent volumes of saline were injected into the CON and GML groups as vehicle controls for LPS. The injection volume and diluents were selected according to Khaligh et al. (2018).
Fig. 1.
Experimental design. In ovo injection of 9 mg of GML at embryonic day 17.5 (E 17.5) and incubation for 12 h, followed by a challenge with 32 μg of LPS for 12 h at E 18. GML = glycerol monolaurate; LPS = lipopolysaccharide.
The in ovo injection procedure was performed as previously described (Uni et al., 2005). In a typical procedure, the eggs were removed from the incubator at E 17.5. A hole with a diameter of approximately 1 mm was drilled into the air chamber end after sterilization with 75% alcohol. Then, 50 μL of the solution was injected with a 21-gauge needle into the amniotic fluid. All injected solutions were freshly prepared on the day of injection and heated to 37 °C. The hole was sealed with melted paraffin after the injection, and the eggs were placed back into the incubator.
2.3. Sample collection
Eggs were removed from the incubator and opened for sampling at E 18.5. Intestinal segments of two chicken embryos were combined as one sample, and at least six samples were analyzed for each treatment. Approximately 1 cm segments were collected from the midway of the jejunum and ileum and immediately immersed in 4% paraformaldehyde for histological examinations. A section of the mid-jejunum and mid-ileum (approximately 2 cm) was excised, rapidly frozen in liquid nitrogen, and stored at −80 °C for further analysis.
2.4. Determination of inflammatory parameters
Tissues of the jejunum and ileum were homogenized with phosphate-buffered saline at a weight (g)-to-volume (mL) ratio of 1:9. The supernatant was collected to determine the levels of intestinal interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) by using enzyme-linked immunosorbent assay kits (MLBIO Co., Shanghai, China). All analysis procedures were performed in strict accordance with the manufacturer's instructions. The final results were normalized to the protein concentration of each sample.
2.5. Morphology analysis
After being fixed in a 4% paraformaldehyde solution for 24 h, the jejunum and ileum segments were dehydrated and embedded in paraffin. The paraffin-coated tissue was cut into 4 μm-thick sections, fixed on slides, and stained with hematoxylin and eosin. Ten randomly selected fields from each section were acquired at 200× magnification with an Eclipse 80i Nikon microscope (Nikon Inc., Tokyo, Japan). The average of 10 values from individual embryos was used for statistical analysis.
2.6. Oxidative status assay
The malondialdehyde (MDA) levels, total antioxidant capacity (T-AOC), and catalase (CAT) activities in the jejunum and ileum were measured using commercial assay kits (intraassay coefficients of variation < 5%; interassay coefficients of variation < 8%) according to the manufacturer's protocols (Nanjing Jiancheng Biotechnology Institute, Nanjing, China). The final results were normalized to the protein concentration in the homogenized supernatant of each sample.
2.7. RNA isolation and real-time quantitative PCR
Approximately 50 mg of jejunum and ileum tissues were homogenized in 500 μL of RNA-Easy Isolation Reagent (Vazyme Biotech, Nanjing, China), and total RNA was isolated according to the manufacturer's instructions. Following the removal of genomic DNA with gDNA Eraser, 1 μg of total RNA was reverse transcribed using an RNA reverse-transcription kit (AG11728, Accurate Biotechnology Co., Ltd., Hunan, China). A real-time PCR system was used (QuanStudio 5, Applied Biosystems, Foster City, CA, USA) for quantitative PCR with TB Green Premix Ex Taq (RR820A, Takara Bio Inc., Dalian, China). The primer sequences are shown in Table 1. For each pair of primers, amplification efficiency was verified using a standard curve, and the specificity was checked with a melt curve. The PCR amplification conditions included the following: predenaturation at 95 °C for 10 s, followed by 40 cycles of denaturation at 95 °C for 5 s, and annealing at 60 °C for 34 s. All samples were analyzed in triplicate. The relative expression of each target gene was calculated with the 2−ΔΔCt method after normalization against the expression of peptidylprolyl isomerase A.
Table 1.
Primer sequences used for real-time quantitative PCR.
| Gene | Accession number | Primer sequence (5′→3′) | Product size, bp |
|---|---|---|---|
| MUC2 | NM_001318434.1 | AGGAATGGGCTGCAAGAGAC | 77 |
| GTGACATCAGGGCACACAGA | |||
| TLR4 | NM_001030693.1 | AGGCACCTGAGCTTTTCCTC | 96 |
| TACCAACGTGAGGTTGAGCC | |||
| NF-κB p65 | NM_001396038.1 | CAGCCCATCTATGACAACCG | 152 |
| TCAGCCCAGAAACGAACCTC | |||
| AMPKα1 | NM_001039603.1 | TGGCATTTGGGGATACGGAG | 130 |
| GATTCTTCCGTCGAACACGC | |||
| COX-2 | NM_001167718.1 | TGTCCTTTCACTGCTTTCCAT | 84 |
| TTCCATTGCTGTGTTTGAGGT | |||
| NLRP3 | XM_040700804.1 | GCTCCTTGCGTGCTCTAAGACC | 150 |
| TTGTGCTTCCAGATGCCGTCAG | |||
| IL-18 | NM_204608.2 | AGATGATGAGCTGGAATGCGATGC | 97 |
| ATCTGGACGAACCACAAGCAACTG | |||
| ZO-1 | XM_015278981.2 | CTTCAGGTGTTTCTCTTCCTCCTCTC | 131 |
| CTGTGGTTTCATGGCTGGATC | |||
| OCLN | NM_205128.1 | TCATCGCCTCCATCGTCTAC | 142 |
| TCTTACTGCGCGTCTTCTGG | |||
| CLDN1 | NM_001013611.2 | CTGATTGCTTCCAACCAG | 140 |
| CAGGTCAAACAGAGGTACAAG | |||
| PPIA | NM_001166326.2 | CCTGCTTCCACCGGATCAT | 64 |
| CCGTTGTGGCGCGTAAA |
MUC2 = mucin 2; TLR4 = Toll-like receptor 4; NF-κB p65 = nuclear factor kappa-B p65; AMPKα1 = adenosine monophosphate-activated protein kinase α1; COX-2 = cyclooxygenase-2; NLRP3 = NOD-like receptor protein 3; IL-18 = interleukin 18; ZO-1 = zonula occludens 1; OCLN = occludin; CLDN1 = claudin 1; PPIA = peptidylprolyl isomerase A.
2.8. Statistical analysis
All data analyses were conducted with SPSS software (IBM SPSS Statistics 27.0, Armonk, NY, USA), and the results are presented as the mean ± SEM. Two-way ANOVA was performed to evaluate the main factor effects and interactions of the GML and LPS challenge. Significant variations between the treatments were compared using Tukey's multiple comparisons. Differences were considered significantly different at P < 0.05.
3. Results
3.1. Intestinal morphology and mucin 2 (MUC2) expression
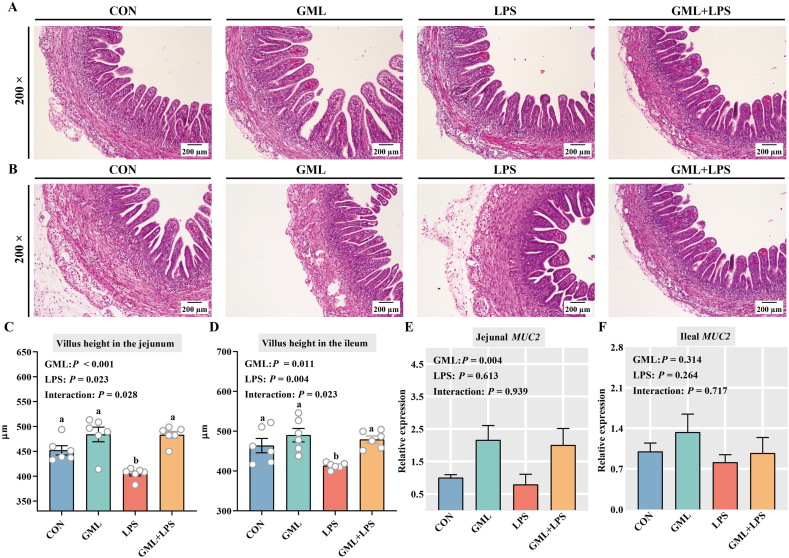
Hematoxylin and eosin staining was performed to observe the effects of GML on the intestinal morphology of challenged embryos (Fig. 2A and B). Significant interactions were observed between GML and LPS groups in the intestinal villus height of chicken embryos (P < 0.05) (Fig. 2C and D). LPS decreased the villus height of the jejunum and ileum in embryos (P < 0.05). Conversely, GML significantly increased the villus height of the small intestine in normal and challenged embryos (P < 0.05), suggesting a protective effect on intestinal morphology. Moreover, GML-treated embryos had higher jejunal MUC2 expression than those in the CON and LPS groups (P = 0.004) (Fig. 2E).
Fig. 2.
Effects of in ovo injection of GML on the intestinal barrier. (A) H&E staining of the jejunum. (B) H&E staining of the ileum. Magnification, 200×; scale bar, 200 μm. Villus height in the (C) jejunum and (D) ileum. Gene-expression levels of MUC2 in the (E) jejunum and (F) ileum. GML = glycerol monolaurate; LPS = lipopolysaccharide; H&E = hematoxylin and eosin; MUC2 = mucin 2. a–b Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 2 chicken embryos per replicate.
3.2. Antioxidant status
The oxidative status of the jejunum and ileum is shown in Fig. 3. Significant interactions occurred between GML and LPS groups in MDA levels (P = 0.003) and T-AOC (P = 0.044) of the jejunum. Increased MDA levels and decreased T-AOC activity were observed in the jejunum of challenged embryos compared with control embryos (P < 0.05) (Fig. 3A and B). However, embryos in the GML + LPS group exhibited decreased MDA levels and upregulated T-AOC activity in the jejunum compared with those in the LPS group (P < 0.05) (Fig. 3A and B). No significant differences were observed in MDA levels, T-AOC, or CAT activity in the ileum (P > 0.05) (Fig. 3D–F).
Fig. 3.
Effects of in ovo injection of GML on oxidative status in LPS-challenged embryos. MDA levels, T-AOC, and CAT activity in the (A–C) jejunum and (D–F) ileum. GML = glycerol monolaurate; LPS = lipopolysaccharide; MDA = malondialdehyde; T-AOC = total antioxidant capacity; CAT = catalase. a–b Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 2 chicken embryos per replicate.
3.3. Inflammatory cytokine production
The effects of the experimental treatments on cytokine levels are shown in Fig. 4. Significant interactions were observed between GML and LPS groups in the intestinal cytokines of embryos (P < 0.05). LPS challenge increased the levels of jejunal IL-1β and TNF-α (P < 0.05). GML significantly reversed the LPS-induced production of these proinflammatory cytokines in the jejunum (Fig. 4A and C). In the ileum, GML injection significantly reduced the levels of ileal IL-6 and TNF-α in challenged embryos (P < 0.05) (Fig. 4E and F). Moreover, GML significantly decreased the contents of jejunal IL-6 (P = 0.004) and ileal IL-1β (P = 0.034) (Fig. 4B and D).
Fig. 4.
Effects of in ovo injection of GML on the secretion of cytokines by LPS-challenged embryos. The levels of IL-1β, IL-6, and TNF-α in the (A–C) jejunum and (D–F) ileum. GML = glycerol monolaurate; LPS = lipopolysaccharide; IL = interleukin; TNF-α = tumor necrosis factor-α. a–b Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 2 chicken embryos per replicate.
3.4. Gene expression in the jejunum and ileum
As shown in Fig. 5, the interaction between GML and LPS notably affected the gene expression of Toll-like receptor 4 (TLR4) (P = 0.003), nuclear factor kappa-B p65 (NF-κB p65) (P = 0.006), cyclooxygenase-2 (COX-2) (P = 0.010), NOD-like receptor protein 3 (NLRP3) (P = 0.013), IL-18 (P = 0.001), and occludin (OCLN) (P = 0.002) in the jejunum of chicken embryos. Embryos treated with LPS had higher jejunal TLR4, NF-κB p65, COX-2, NLRP3, and IL-18 gene expression than control embryos (P < 0.05). However, embryos in the GML + LPS group exhibited significantly downregulated jejunal TLR4, NF-κB p65, COX-2, NLRP3, and IL-18 expression levels compared with those in the LPS group (P < 0.05). The LPS challenge downregulated the expression of jejunal OCLN compared with that in the CON group (P < 0.01). The downregulation of this gene was reversed by GML (P < 0.05). Moreover, a significant main effect of GML was observed in the gene expression of adenosine monophosphate-activated protein kinase α1 (AMPKα1) (P = 0.004) and claudin 1 (CLDN1) (P < 0.001). GML significantly increased AMPKα1 and CLDN1 expression in the jejunum of embryos (P < 0.05).
Fig. 5.
Effects of in ovo injection of GML on the gene-expression levels of TLR4, NF-κB p65, COX-2, NLRP3, IL-18, AMPKα1, ZO-1, OCLN, and CLDN1 in the jejunum. GML = glycerol monolaurate; LPS = lipopolysaccharide; TLR4 = Toll-like receptor 4; NF-κB p65 = nuclear factor kappa-B p65; COX-2 = cyclooxygenase-2; NLRP3 = NOD-like receptor protein 3; IL-18 = interleukin 18; AMPKα1 = adenosine monophosphate-activated protein kinase α1; ZO-1 = zonula occludens 1; OCLN = occludin; CLDN1 = claudin 1. a–b Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 2 chicken embryos per replicate.
Significant interactions were observed between the GML and LPS groups in the gene expression of TLR4 (P = 0.026), NF-κB p65 (P = 0.012), and AMPKα1 (P = 0.043) in the ileum of embryos (Fig. 6). GML significantly increased AMPKα1 expression in challenged embryos and prevented the LPS-induced increase in TLR4 and NF-κB p65 expression (P < 0.05). Notably, the significant main effect of GML was that it affected the gene expression of ileal COX-2 (P = 0.002), IL-18 (P = 0.037), zonula occludens 1 (ZO-1) (P = 0.008), and CLDN1 (P = 0.004).
Fig. 6.
Effects of in ovo injection of GML on the gene-expression levels of TLR4, NF-κB p65, COX-2, NLRP3, IL-18, AMPKα1, ZO-1, OCLN, and CLDN1 in the ileum. GML = glycerol monolaurate; LPS = lipopolysaccharide; TLR4 = Toll-like receptor 4; NF-κB p65 = nuclear factor kappa-B p65; COX-2 = cyclooxygenase-2; NLRP3 = NOD-like receptor protein 3; IL-18 = interleukin 18; AMPKα1 = adenosine monophosphate-activated protein kinase α1; ZO-1 = zonula occludens 1; OCLN = occludin; CLDN1 = claudin 1. a–b Means with no common superscripts differ significantly (P < 0.05). Means are based on 6 replicates per treatment with 2 chicken embryos per replicate.
4. Discussion
Since the ban on AGP in animal feed, intestinal inflammation and compromised mucosal barriers have become major problems in intensive poultry production (Ducatelle et al., 2018). The immunomodulation of innate immunity is a promising alternative to antibiotics to reduce the inflammatory effects of infections and enhance host defense against microbial infections (Lillehoj and Lee, 2012). GML, a dietary immunomodulator, improves the intestinal health of poultry and has thus been suggested as an alternative to AGP (Amer et al., 2021; Liu et al., 2020). However, few studies have examined the mechanisms underlying the specific immunomodulatory effects of GML on broilers. Accordingly, the present study investigated the effects and associated mechanisms of GML on innate immunity in a broiler embryo model.
LPS has been demonstrated to induce innate immune responses in E 18 broiler embryos, including the induction of proinflammatory cytokine expression in multiple organs (Bavananthasivam et al., 2019). This finding was reflected in the current work by the increased levels of IL-1β, IL-6, and TNF-α in the jejunum and ileum of LPS-challenged embryos, similar to the outcomes of previous research (Bhanja et al., 2015). Multiple studies have demonstrated the immunoregulatory properties of GML. Li et al. (2009) found that the secretion of macrophage inflammatory protein 3α and IL-8 is alleviated by GML, which significantly inhibits mucosal signal transduction and the innate immune response. Human milk samples rich in GML inhibit superantigen and bacterial-induced IL-8 production in vitro (Schlievert et al., 2019). In our study, GML normalized the production of IL-1β, IL-6, and TNF-α in the intestines of challenged embryos. These proinflammatory cytokines are considered markers of intestinal inflammation (Xie et al., 2021), suggesting that GML alleviated the LPS-induced inflammatory response in the jejunum and ileum.
Inflammation and oxidative stress are intricately related pathophysiological processes (Biswas, 2016). MDA is considered a biomarker of oxidative stress (Pirinccioglu et al., 2010). Herein, we found that the LPS challenge increased the MDA level, indicating that oxidative stress occurred in challenged embryos. Oxidative stress depends on the balance between pro- and antioxidant factors (Adesso et al., 2018). Thus, the antioxidant response is a pivotal factor in controlling oxidative stress. LPS reportedly decreases the activities of antioxidant enzymes such as T-AOC and CAT, leading to oxidative stress in birds (Zheng et al., 2020). However, dietary GML alleviates LPS-induced oxidative stress by maintaining the delicate equilibrium between oxidants and antioxidants (Liu et al., 2021). In the present study, GML prevented oxidative stress in challenged embryos, as evidenced by the decreased MDA level and increased activities of T-AOC. Oxidative stress is intimately connected with inflammation (Kowalczyk et al., 2016). A vicious cycle occurs when inflammation provokes oxidative stress, which in turn boosts inflammation (Soomro, 2019). Thus, the GML-mediated improvements in oxidative status may be associated with anti-inflammatory effects on LPS-challenged embryos.
A healthy intestinal morphology directly affects nutrient metabolism, disease resistance, and the immune response of the host (Jha et al., 2019). Intestinal morphology plays an essential role in nutrient absorption and provides a protective barrier, which can be reflected by villus height (Xie et al., 2021). GML enhances immune status and intestinal histomorphology in broilers (Amer et al., 2021). Our previous study has demonstrated that dietary GML attenuates the adverse effects of LPS on intestinal morphology in broilers (Kong et al., 2022). The present results indicated that GML rescued the LPS-induced decrease in villus height in the jejunum and ileum of embryos, which was beneficial to the recovery of intestinal function. A crucial part of the intestinal barrier is the mucus layer, which predominantly comprises the mucin glycoprotein MUC2 (Melo-González et al., 2018). GML upregulated the expression of MUC2 in challenged and nonchallenged embryos, indicating enhanced intestinal-barrier function. Improvements in intestinal oxidative stress and the inflammatory response are associated with the recovery of intestinal barrier function (Lu and Wang, 2021). Thus, GML-mediated improvements in intestinal innate immunity and antioxidant status may further promote the recovery of intestinal barrier function in LPS-challenged embryos.
The NF-κB signaling pathway is known to play a crucial role in modulating the immune system and inflammatory responses. This pathway is activated by TLR4 signaling and can subsequently cause the secretion of proinflammatory cytokines (Cario et al., 2000). NF-κB is a master regulator of the inflammatory response and participates in the condition-dependent selective regulation of the expression of specific target genes, including proinflammatory cytokines (such as TNF-α, IL-1β, IL-6, and IL-12), proinflammatory enzymes (COX-2), and chemokines (Rius-Pérez et al., 2019). The current work revealed the stimulatory effect of LPS on TLR4 and NF-κB expression in embryos, consistent with findings in poultry in vitro and in vivo (Surai et al., 2021). Conversely, GML reversed the LPS-induced upregulation of TLR4, NF-κB, and COX-2 expression in the jejunum. These findings indicated that GML attenuated LPS-induced inflammatory responses by suppressing NF-κB transcription. The LPS-induced activation of NF-κB increases the expression of cytokine precursors and is a crucial initial step in activating the NLRP3 inflammasome (Ren et al., 2020). Activation of the NLRP3 inflammasome leads to the maturation and secretion of IL-1β and IL-18, which amplifies the inflammatory cascade and exacerbates the release of inflammatory cytokines (Huang et al., 2020). Our results showed that LPS exposure increased NLRP3 and IL-18 expression, and these effects were significantly reversed by GML pretreatment. Although NF-κB is upstream of NLRP3, NLRP3 overexpression reportedly leads to the activation of NF-κB signaling (Peng et al., 2020). Thus, the modulatory effect of GML on NLRP3 may contribute to the alleviation of inflammatory responses and NF-κB activation in challenged embryos. GML further enhanced the expression of AMPKα1 in the jejunum and ileum of challenged embryos. AMPK is commonly linked to the NF-κB pathway and controls inflammation (Liu et al., 2016). Activated AMPK negatively regulates the nuclear translocation of NF-κB and further alleviates inflammatory responses triggered by LPS (Qing et al., 2019; Salminen et al., 2011). Collectively, GML may attenuate NF-κB signaling through multiple pathways, alleviate the production of proinflammatory cytokines and enzymes, and inhibit inflammatory responses in the intestines of LPS-challenged embryos.
LPS can reduce the expression of tight-junction proteins by triggering proinflammatory cytokines (Toejing et al., 2020). Reduced expression of tight-junction proteins increases intestinal permeability and disrupts intestinal-barrier integrity (Chleilat et al., 2020). In the present study, the decreased expression of ZO-1 in challenged embryos indicated an LPS-induced impairment in the intestinal barrier. However, GML enhanced jejunal OCLN expression in challenged embryos and reversed the LPS-induced downregulation of ileal ZO-1 expression. Defective intestinal tight junctions have been implicated in intestinal inflammation (Al-Sadi et al., 2008). Herein, the alleviation of inflammatory responses by GML attenuated the destruction of tight-junction proteins, thereby protecting intestinal barrier integrity in challenged embryos. The attenuated activation of NF-κB has also been demonstrated to be associated with improved mucosal permeability, mucin expression, and barrier function (Ariyadi et al., 2014). Thus, the GML-mediated suppression of NF-κB signaling may protect against LPS-induced intestinal-barrier disruption.
5. Conclusion
The results of this study confirmed the immunomodulatory properties of GML in a broiler embryo model. GML improved intestinal morphology and antioxidant status by suppressing intestinal inflammatory responses and modulating NF-κB signaling in LPS-challenged broiler embryos.
Author contributions
Linglian Kong designed the study, performed the experiments, and wrote the manuscript. Yuanli Cai, Xue Pan, and Chuanpi Xiao participated in the experiments. Zhigang Song conceived the idea and provided resources. All authors contributed to the article and approved the submitted version.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32272910) and the Natural Science Foundation of Shandong Province (ZR2020MC170).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., et al. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult Sci. 2022;101:101590. doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesso S., Russo R., Quaroni A., Autore G., Marzocco S. Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-κB activation and Nrf2 response. Int J Mol Sci. 2018;19:800. doi: 10.3390/ijms19030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R., Ye D., Dokladny K., Ma T.Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer S.A., A-Nasser A., Al-Khalaifah H.S., AlSadek D.M.M., Abdel fattah D.M., Roushdy E.M., et al. Effect of dietary medium-chain α-monoglycerides on the growth performance, intestinal histomorphology, amino acid digestibility, and broiler chickens' blood biochemical parameters. Animals. 2021;11:57. doi: 10.3390/ani11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyadi B., Isobe N., Yoshimura Y. Toll-like receptor signaling for the induction of mucin expression by lipopolysaccharide in the hen vagina. Poult Sci. 2014;93:673–679. doi: 10.3382/ps.2013-03667. [DOI] [PubMed] [Google Scholar]
- Bavananthasivam J., Alkie T.N., Matsuyama-kato A., Hodgins D.C., Sharif S. Characterization of innate responses induced by in ovo administration of encapsulated and free forms of ligands of toll-like receptor 4 and 21 in chicken embryos. Res Vet Sci. 2019;125:405–415. doi: 10.1016/j.rvsc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Bhanja S.K., Hotowy A., Mehra M., Sawosz E., Pineda L., Vadalasetty K.P., et al. In ovo administration of silver nanoparticles and/or amino acids influence metabolism and immune gene expression in chicken embryos. Int J Mol Sci. 2015;16:9484–9503. doi: 10.3390/ijms16059484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E., Rosenberg I.M., Brandwein S.L., Beck P.L., Reinecker H.-C., Podolsky D. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- Chleilat F., Klancic T., Ma K., Schick A., Nettleton J.E., Reimer R.A. Human milk oligosaccharide supplementation affects intestinal barrier function and microbial composition in the gastrointestinal tract of young Sprague Dawley rats. Nutrients. 2020;12:1532. doi: 10.3390/nu12051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Yuan J., Cha J., Sun X., Bartos A., Yagita H., et al. Endothelial cells in the decidual bed are potential therapeutic targets for preterm birth prevention. Cell Rep. 2019;27:1755–1768. doi: 10.1016/j.celrep.2019.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., et al. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet Res. 2018;49:43. doi: 10.1186/s13567-018-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuoso B.F., dos Reis J.H., Gebert R.R., Barreta M., Griss L.G., Casagrande R.A., et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: impact on health, performance and meat quality. Microb Pathog. 2019;129:161–167. doi: 10.1016/j.micpath.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Fosdick M.G., Chheda P., Tran P.M., Wolff A.J., Peralta R., Zhang M.Y., et al. Suppression of human T cell activation by derivatives of glycerol monolaurate. Sci Rep. 2021;11:8943. doi: 10.1038/s41598-021-88584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Osorio L.-M., Yepes-Medina V., Ballou A., Parini M., Angel R. Short and medium chain fatty acids and their derivatives as a natural strategy in the control of necrotic enteritis and microbial homeostasis in broiler chickens. Front Vet Sci. 2021;8:773372. doi: 10.3389/fvets.2021.773372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.-T., Liu W., Zhou Y., Sun M., Yang H.-H., Zhang C.-Y., et al. Galectin-1 ameliorates lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway in mice. Free Radic Biol Med. 2020;146:222–233. doi: 10.1016/j.freeradbiomed.2019.11.011. [DOI] [PubMed] [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front Vet Sci. 2019;6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaligh F., Hassanabadi A., Nassiri-Moghaddam H., Golian A., Kalidari G.-A. Effects of in ovo injection of chrysin, quercetin and ascorbic acid on hatchability, somatic attributes, hepatic oxidative status and early post-hatch performance of broiler chicks. J Anim Physiol Anim Nutr. 2018;102:e413–e420. doi: 10.1111/jpn.12760. [DOI] [PubMed] [Google Scholar]
- Kong L., Wang Z., Xiao C., Zhu Q., Song Z. Glycerol monolaurate ameliorated intestinal barrier and immunity in broilers by regulating intestinal inflammation, antioxidant balance, and intestinal microbiota. Front Immunol. 2021;12:713485. doi: 10.3389/fimmu.2021.713485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Wang Z., Xiao C., Zhu Q., Song Z. Glycerol monolaurate attenuated immunological stress and intestinal mucosal injury by regulating the gut microbiota and activating AMPK/Nrf2 signaling pathway in lipopolysaccharide-challenged broilers. Anim Nutr. 2022;10:347–359. doi: 10.1016/j.aninu.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A., Jeleń A., Żebrowska M., Balcerczak E., Gorąca A. BQ123 stimulates skeletal muscle antioxidant defense via Nrf2 activation in LPS-treated rats. Oxid Med Cell Longev. 2016;2016:2356853. doi: 10.1155/2016/2356853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kheravii S.K., Li L., Wu S.-B. Monoglyceride blend reduces mortality, improves nutrient digestibility, and intestinal health in broilers subjected to clinical necrotic enteritis challenge. Animals. 2021;11:1432. doi: 10.3390/ani11051432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Toghyani M., Kheravii S.K., Pineda L., Han Y., Swick R.A., et al. Organic acid blends improve intestinal integrity, modulate short-chain fatty acids profiles and alter microbiota of broilers under necrotic enteritis challenge. Anim Nutr. 2022;8:82–90. doi: 10.1016/j.aninu.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Estes J.D., Schlievert P.M., Duan L., Brosnahan A.J., Southern P.J., et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Lee K.W. Immune modulation of innate immunity as alternatives-to-antibiotics strategies to mitigate the use of drugs in poultry production. Poult Sci. 2012;91:1286–1291. doi: 10.3382/ps.2012-02374. [DOI] [PubMed] [Google Scholar]
- Liu T., Li C., Zhong H., Feng F. Dietary medium-chain α-monoglycerides increase BW, feed intake, and carcass yield in broilers with muscle composition alteration. Poult Sci. 2021;100:186–195. doi: 10.1016/j.psj.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Tang J., Feng F. Glycerol monolaurate improves performance, intestinal development, and muscle amino acids in yellow-feathered broilers via manipulating gut microbiota. Appl Microbiol Biotechnol. 2020;104:10279–10291. doi: 10.1007/s00253-020-10919-y. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang N., Fan S., Zheng X., Yang Y., Zhu Y., et al. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci Rep. 2016;6:39735. doi: 10.1038/srep39735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Wang F. Lactobacillus acidophilus and vitamin C attenuate ethanol-induced intestinal and liver injury in mice. Exp Ther Med. 2021;22:1005. doi: 10.3892/etm.2021.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Liu W., Zhao M., Huang Y., Feng F. Glycerol monolaurate beyond an emulsifier: synthesis, in vivo fate, food quality benefits and health efficacies. Trends Food Sci Technol. 2022;127:291–302. [Google Scholar]
- Manohar V., Echard B.W., Perricone N.V., Ingram C., Enig M.G., Bagchi D., et al. In vitro and in vivo effects of two coconut oils in comparison to monolaurin on Staphylococcus aureus: rodent studies. J Med Food. 2013;16 6:499–503. doi: 10.1089/jmf.2012.0066. [DOI] [PubMed] [Google Scholar]
- Melo-González F., Fenton T.M., Forss C., Smedley C., Goenka A., MacDonald A.S., et al. Intestinal mucin activates human dendritic cells and IL-8 production in a glycan-specific manner. J Biol Chem. 2018;293:8543–8553. doi: 10.1074/jbc.M117.789305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Wen L., Shi Q.-F., Gao F., Huang B., Meng J., et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial–mesenchymal transition and inflammation. Cell Death Dis. 2020;11:978. doi: 10.1038/s41419-020-03178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirinccioglu A.G., Gökalp D., Pirinccioglu M., Kizil G., Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin Biochem. 2010;43:1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Qing L., Fu J., Wu P., Zhou Z., Yu F., Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 2019;11:655–668. [PMC free article] [PubMed] [Google Scholar]
- Ren C., Wang Y., Lin X., Song H., Zhou Q., Xu W., et al. A combination of formic acid and monolaurin attenuates enterotoxigenic Escherichia coli induced intestinal inflammation in piglets by inhibiting the NF-κB/MAPK pathways with modulation of gut microbiota. J Agric Food Chem. 2020;68:4155–4165. doi: 10.1021/acs.jafc.0c01414. [DOI] [PubMed] [Google Scholar]
- Rius-Pérez S., Pérez S., Martí-Andrés P., Monsalve M., Sastre J. Nuclear factor kappa B signaling complexes in acute inflammation. Antioxid Redox Signal. 2019;33:145–165. doi: 10.1089/ars.2019.7975. [DOI] [PubMed] [Google Scholar]
- Salminen A., Hyttinen J.M.T., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P.M., Kilgore S.H., Seo K.S., Leung D.Y.M. Glycerol monolaurate contributes to the antimicrobial and anti-inflammatory activity of human milk. Sci Rep. 2019;9:14550. doi: 10.1038/s41598-019-51130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serralbo O., Salgado D., Véron N., Cooper C.A., Dejardin M.-J., Doran T.J., et al. Transgenesis and web resources in quail. Elife. 2020;9 doi: 10.7554/eLife.56312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivinski S.E., Mamedova L.K., Rusk R.A., Elrod C.C., Swartz T.H., McGill J.M., et al. Development of an in vitro macrophage screening system on the immunomodulating effects of feed components. J Anim Sci Biotechnol. 2020;11:89. doi: 10.1186/s40104-020-00497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro S. Oxidative stress and inflammation. Open J Immunol. 2019;9:1–20. [Google Scholar]
- Surai P.F., Kochish I.I., Kidd M.T. Redox homeostasis in poultry: regulatory roles of NF-κB. Antioxidants. 2021;10:186. doi: 10.3390/antiox10020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toejing P., Khat-Udomkiri N., Intakhad J., Sirilun S., Chaiyasut C., Lailerd N. Putative mechanisms responsible for the antihyperglycemic action of Lactobacillus paracasei HII01 in experimental type 2 diabetic rats. Nutrients. 2020;12:3015. doi: 10.3390/nu12103015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R., Tako E., Kedar O. In ovo feeding improves energy status of late-term chicken embryos. Poult Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Valentini J., Da Silva A.S., Fortuoso B.F., Reis J.H., Gebert R.R., Griss L.G., et al. Chemical composition, lipid peroxidation, and fatty acid profile in meat of broilers fed with glycerol monolaurate additive. Food Chem. 2020;330:127187. doi: 10.1016/j.foodchem.2020.127187. [DOI] [PubMed] [Google Scholar]
- Wang J., Jiang H., Alhamoud Y., Chen Y., Zhuang J., Liu T., et al. Integrated metabolomic and gene expression analyses to study the effects of glycerol monolaurate on flesh quality in large yellow croaker (Larimichthys crocea) Food Chem. 2021;367:130749. doi: 10.1016/j.foodchem.2021.130749. [DOI] [PubMed] [Google Scholar]
- Xie W., Song L., Wang Xueying, Xu Y., Liu Z., Zhao D., et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes. 2021;13:1956281. doi: 10.1080/19490976.2021.1956281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.W., Zhang J.Y., Zhou H.B., Guo Y.P., Ma Q.G., Ji C., et al. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poult Sci. 2020;99:5389–5398. doi: 10.1016/j.psj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]